Abstract
Data from the Food Safety Division, Alberta Agriculture, Food and Rural Development were analyzed to determine the frequency of diagnosis of porcine proliferative enteropathy (PPE) relative to the diagnosis of other porcine enteric infections between 1993 and 1997. Next to colibacillosis, PPE was the most commonly diagnosed enteric disease among those reported.
Proliferative enteropathies of swine are a group of diseases characterized by hyperplasia of crypt enterocytes. These diseases, which include porcine intestinal adenomatosis (PIA), necrotic enteritis (NE), regional ileitis (RI), and proliferative hemorrhagic enteropathy (PHE) (1), are associated with infection by the obligate intracellular bacterium, Lawsonia intracellularis. Although they are associated with significant morbidity, mortality (in the case of PHE), and economic impact in swine herds throughout the world (2,3,4,5,6,7,8), the impact of these diseases in Canada has not been well studied.
Recently, we reported on the frequency of diagnosis of porcine proliferative enteropathy (PPE) relative to other porcine enteric infections recorded in the database of the Animal Health Laboratory (AHL), University of Guelph, (formerly the Veterinary Laboratory Services Branch of the Ontario Ministry of Agriculture, Food and Rural Affairs) between 1992 and 1997 (9). This database contains detailed records on all diagnostic submissions to each of the provincial veterinary laboratories, dating back to 1988. Analysis of these data revealed that, of samples submitted to the Animal Health Laboratory, PPE was among the most frequently diagnosed infectious enteric diseases generally associated with diarrhea in weaner and grower-finisher pigs in Ontario.
The Food Safety Division of the Alberta Ministry of Agriculture, Food and Rural Development also maintains a comprehensive database on past submissions to the provincial veterinary diagnostic laboratories in Alberta, located at Fairview, Airdrie, Lethbridge, and Edmonton. We analyzed case records from this database for the years 1993 to 1997 to determine the relative frequency of diagnosis of PPE in Alberta and to evaluate the consistency of passively collected data from Canadian veterinary diagnostic laboratories for the surveillance of porcine enteric disease.
For the purposes of this study, a submission was defined as a group of one or more pigs submitted from a single source herd on a single day. Pathologic, microbiologic, and virologic examinations were performed at the animal health laboratories in Alberta. As part of routine case management, pathologists reviewed the results of gross and histologic examination of tissues, as well as of ancillary tests, to arrive at a final diagnosis, which was then coded by using a comprehensive hierarchical disease coding system identical to that employed by the AHL.
Data fields containing information on the date of submission, diagnosis, and age for all porcine cases with a gross or histologic diagnosis of either large or small intestinal disease submitted between January 1993 and December 1997 were retrieved after removing personal identifying information. Submissions among the above with a gross or histologic diagnosis of proliferative enteropathy, Escherichia coli enteritis, salmonellosis, swine dysentery, clostridial enteritis, rotaviral enteritis, transmissible gastroenteritis, or coccidiosis were identified by a series of computerized searches for the relevant pathologic codes. Submissions having lesions consistent with infectious enteritis but lacking a specific etiologic diagnosis (nonspecific colitis) were not included in this study. These searches were developed in consultation with the Alberta Animal Health Laboratory veterinary staff who had a detailed understanding of the database structure, as well as extensive experience in its analysis and interpretation of the diagnostic codes.
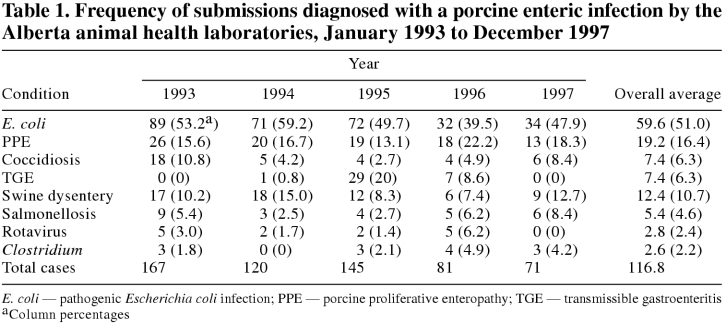
The distribution of annual submissions by diagnosis between 1993 and 1997 is shown in Table 1. The most commonly reported disease was E. coli enteritis (average number of cases/y = 59.6). This was followed, in decreasing order of frequency, by proliferative enteropathy (19.2 cases/y), swine dysentery (12.4 cases/y), coccidiosis (7.4 cases/y), transmissible gastroenteritis (TGE; 7.4 cases/y), salmonellosis (5.4 cases/y), rotaviral enteritis (2.8 cases/y), and clostridial enteritis (2.6 cases/y).
Table 1.
The total annual number of submissions of porcine enteric disease decreased between 1993 and 1997, as did the number of submissions with each individual etiologic diagnosis, with the exception of clostridial enteritis, which remained at a relatively constant, low level, and TGE, which occurred at substantially increased frequency in 1995 and 1996 and at low frequency in 1993, 1994, and 1997. This observed decrease in the reported annual case count over the study period was statistically significant (P < 0.05) by Spearman's rank correlation test only for PPE.
Of 96 PPE submissions in total, 14 (15%) occurred among weaner pigs (age > 3 to 10 wk), 35 (36%) among growers (> 10 to 18 wk), 13 (14%) among finishers (> 18 to 26 wk), and 15 (16%) among mature animals (> 26 wk). No age was recorded for 19 (20%) submissions. The results of this analysis suggest that PPE is a significant cause of enteric disease of swine in Alberta and, in conjunction with results from Ontario (9), provide evidence of the importance of this disease in swine herds as a whole in Canada. With the exception of E. coli infections, the average annual number of submissions for pathologic examination that were diagnosed with PPE by the Alberta animal health laboratories was higher than that of all of the other swine enteric infections examined. In addition, the absolute number of cases of PPE exceeded that of all of the other enteric diseases in each of the 5 y studied, with the exception of TGE in 1995, and E. coli throughout the study period. The decline in diagnoses of enteric disease over the study period paralleled both an overall reduction in swine submissions for pathologic examination to the Alberta animal health laboratories and a trend toward private swine veterinarians sending diagnostic samples for testing out of the province (unpublished observations; S. Honour). It also coincided with the establishment of a number of private diagnostic pathology laboratories in the province.
The Alberta data show remarkable similarities to those from the AHL, for the same time period (9). The ranking of frequency of diagnosis for each of the diseases examined was identical for the 2 laboratories, with the exception of coccidiosis, which constituted a higher proportion of diagnoses at AHL. The age distribution of PPE cases was also very similar for the 2 groups: 60% of PPE submissions to the Alberta laboratories for which age was recorded occurred in the grower-finisher category compared with 57% at AHL, observations that are consistent with the published literature (1,4,5).
The authors acknowledge that because enteric submissions lacking a specific etiologic diagnosis were not included in this study, their estimates of the relative frequency of diagnoses may differ from those that might have been obtained had the etiology of these cases been known. For example, cases of nonspecific colitis, a condition of unknown etiology that has been attributed to potential infectious and nutritional causes (10), were not included. Since the relevant population at risk is unknown, we also recognize that comparisons of disease counts in this study consist of relative frequencies of submission to diagnostic laboratories, not differences in incidence of disease. Furthermore, it is recognized that the extent of under-reporting of each disease and the extent to which sampling of each population occurs in a representative manner may influence the relative frequency of diagnosis. However, in the absence of a clear bias toward submission of one or more of the enteric diseases studied, we contend that the use of the relative frequencies of these diagnoses as potential indicators of the importance of these diseases in the field is justified, particularly since cost and logistical considerations limit the potential for undertaking extensive random surveys (9,11). Furthermore, the strong similarity in results from studies in 2 distinct populations (Alberta and Ontario) (9), supports this assertion and provides evidence of consistency between provinces in passively collected porcine enteric disease surveillance data from diagnostic laboratories.
Footnotes
Acknowledgment
The authors gratefully acknowledge the assistance of Louise Szaszvari in the extraction and analysis of the data used in this study. CVJ
Support for the study was provided by Alberta Agriculture, Food and Rural Development and Elanco/Provel, Division Eli Lilly Canada Inc.
Address correspondence and reprint requests to Dr. Jeff Wilson.
References
- 1.Barker IK, Van Dreumel AA, Palmer N. The alimentary system. In: Jubb KVF, Kennedy PC, Palmer N, eds. Pathology of Domestic Animals. 4th ed. San Diego: Academic Pr, 1993:1–317.
- 2.Wilson TM, Chang K, Gebhart CJ, Kurtz HJ, Drake TR, Lintner V. Porcine proliferative enteritis: serological, microbiological and pathological studies from three field epizootics. Can J Vet Res 1986;50:217–220. [PMC free article] [PubMed]
- 3.Holyoake PK, Cutler RS, Caple IW. Prevalence of proliferative enteritis on pig farms in Australia. Aust Vet J 1994;71:418–422. [DOI] [PubMed]
- 4.Connor JF. Diagnosis, treatment and prevention of porcine proliferative enteritis. Compend Contin Educ Pract Vet 1991;13: 1172–1176.
- 5.McOrist S, Gebhart CJ. Porcine proliferative enteropathies. In: Straw BE, D'Allaire S, Mengleing WL, Taylor DJ eds. Diseases of Swine. 8th ed. Ames: Iowa State Univ Pr, 1999:521–34.
- 6.Christensen NH, Cullinane LC. Monitoring the health of pigs in New Zealand abattoirs. NZ Vet J 1990;38:136–141. [DOI] [PubMed]
- 7.Holyoake PK, Mullan BP, Cutler RS. Simulation of the economic impact of proliferative enteritis on pig production in Australia. Aust Vet J 1996;73:89–92. [DOI] [PubMed]
- 8.McOrist S, Smith SH, Green LE. Estimate of direct financial losses due to porcine proliferative enteropathy. Vet Rec 1997;140: 579–581. [DOI] [PubMed]
- 9.Wilson JB, Pauling GE, McEwen BJ, Smart N, Carman PS, Dick CP. A descriptive study of the frequency and characteristics of proliferative enteropathy in swine in Ontario by analyzing routine animal health surveillance data. Can Vet J 1999;40:713–717. [PMC free article] [PubMed]
- 10.Hampson DJ, Trott DJ. Spirochetal diarrhea/porcine intestinal spirochetosis. In: Straw BE, D'Allaire S, Mengleing WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames: Iowa State Univ Pr, 1999:553–62.
- 11.Stroup NE, Zack MM, Wharton M. Sources of routinely collected data for surveillance. In: Teutsch SM, Churchill RE, eds. Principles and Practice of Public Health Surveillance. New York: Oxford Univ Pr, 1994:31–85.