Abstract
A dog adopted as a stray in Spain and then brought to Canada 4 years prior to presentation was evaluated for polyarthritis. An electrophoresis showed a marked polyclonal gammopathy and synovial smears contained leishmanial organisms within macrophages.
A 5-year-old, castrated male, mixed breed dog was presented to the referring veterinarian for a lameness of 1-year duration, which had progressed to a nonambulatory state. The dog had been splinted 1 y earlier by the veterinarian for a suspected left carpal bone fracture. The lameness continued after splint removal and appeared to have spread to several joints. Prednisone therapy was initiated 6 mo prior to presentation. The dog showed a good response initially, but a lesser one over time. According to the owner, the animal was adopted as a stray in Spain 4 y previously. Physical examination indicated swelling of several joints.
Whole blood and serum samples were submitted for analysis. The complete blood cell count revealed a mild leukocytosis consisting of a mature neutrophilia and mild left shift. A moderate, normocytic, normochromic, regenerative anemia was present. These changes were interpreted as being attributable to inflammation and hemorrhage or hemolysis.
Significant changes in the biochemical profile included a marked elevation in alkaline phosphatase (ALP) (523 U/L; normal, 23 to 87 U/L), alanine aminotransferase (ALT) (561 U/L; normal, 5 to 69 U/L), sorbitol dehydrogenase (SDH) (74 U/L; normal, 2 to 20 U/L), and a moderate elevation in aspartate aminotransferase (AST) (238 U/L; normal 20 to 50 U/L) levels. These could all be attributed to a steroid hepatopathy following 6 mo of glucocorticoid therapy. Serum electrophoresis showed that the marked hyperglobulinemia (82 g/L; normal, 22 to 44 g/L) was due to a polyclonal elevation of gammaglobulins. This was presumed to be associated with an antigenic stimulation. The significant but lesser elevation of the α2 and β globulins indicated increased acute phase inflammatory proteins.
Given the dog's previous travel history and the hypergammaglobulinemia, infection by the genera Ehrlichia and Leishmania were included as possible etiologies for the polyarthropathy, as well as immune-mediated disease. The dog was referred to the Atlantic Veterinary College Teaching Hospital for further diagnostic tests.
At the time of referral, the dog was normal on physical examination, except for showing pain on palpation of several joints. There was no lymphadenopathy, splenomegaly, or cutaneous lesion. Detailed orthopedic examination revealed a luxation of the left carpus, increased joint laxity, and pain involving the right carpus and both stifles, elbows, and hocks. Radiographs of the carpi and tarsi showed erosive polyarthritis with subluxation and collapse of the articular spaces. A complete blood profile confirmed the moderate, normocytic, normochromic, regenerative anemia and a mild leukocytosis characterized by a mild neutrophilia and mild regenerative left shift. A urinalysis indicated a urine specific gravity of 1.017 and trace protein. The serum was negative for rheumatoid factor.
Smears of synovial fluid showed a moderately increased cellularity. Cells consisted of 23% nondegenerative neutrophils, 16% mononuclear cells, and 61% small lymphocytes. Many macrophages and a few neutrophils contained numerous magenta-staining organisms, 2 to 3 μm in length with a distinct kinetoplast (Figure 1). The morphological and staining characteristics of these organisms were compatible with Leishmania spp. amastigotes.
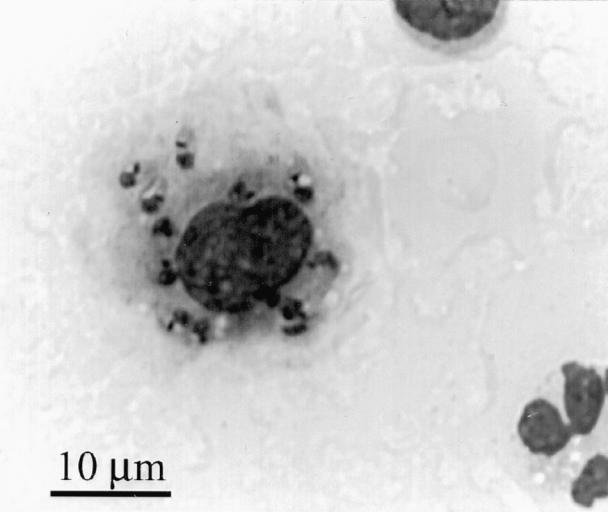
Figure 1. Photomicrograph of synovial fluid showing macrophage containing numerous leishmania; Wright's Giemsa stain; bar = 10 μm.
The owners elected to have the dog euthanized and a complete postmortem examination was done. At necropsy, the dog was found to be in good body condition with abundant subcutaneous fat and pale skeletal musculature. The size of the lymph nodes appeared normal.
Microscopic examination revealed that the bronchoalveolar and perivascular spaces in the lung were filled with eosinophilic edematous fluid. The spleen, liver, lymph nodes, and bone marrow contained numerous macrophages filled with small, Giemsa stain-positive bodies consistent with amastigotes of Leishmania spp. In sections of kidney, Bowman's capsule appeared slightly thickened and the renal interstitium contained focal aggregates of lymphoplasmacytic cells. Most of these renal infiltrates also contain mononuclear cells with amastigotes in the cytoplasm. Sections of skeletal musculature and peripheral nerves showed many well-defined foci of mononuclear infiltrates containing parasites, mainly around blood vessels. The most spectacular microscopic change was seen in the synovial membranes, which appeared thickened with notable villus hyperplasia. Large numbers of plasma cells, lymphocytes, macrophages, and fibroblasts infiltrated the synovium and many of these cells contained parasites. The articular cartilage adjacent to the inflamed synovium showed early stages of fibrillation. The microscopic changes in this dog confirmed the diagnosis of visceral leishmaniasis with marked involvement of joints.
Leishmania spp. are diphasic protozoa found in both the New World, mainly Central and South America, and in the Old World in parts of Africa, India, and the Mediterranean. In the United States, Leishmania spp. are endemic in Ohio, Oklahoma, and Texas (1). The most common species that can affect dogs are L. infantum and L. tropica (both are subspecies of L. donavani (1,2)), L. mexicana, and L. brazilensia (1).
The vectors for Leishmania spp. are sandflies of the genera Phlebotomus in the Old World and Lutzomyia (found in the USA) in the New World. Leishmania spp. circulate between its vertebrate host, as an amastigote, and the sandfly, as a promastigote (flagellate). In vertebrates, the amastigotes, with their telltale kinetoplasts are found predominantly in macrophages, where they multiply by binary fission until the cells rupture, allowing them to escape and spread to new cells (3).
Not all animals exposed to Leishmania spp. develop leishmaniasis. It has been reported that 20% of cases resolve spontaneously (3). Development of clinical disease depends on whether a host mounts a predominant thymus helper (Th) 1 or Th 2 helper T-cell response. The Th 1 lymphocytes are activated by interleukin (IL)-12 and secrete the cytokines IL-2, interferon-γ, and tumor necrosis factor-β. These, in turn, primarily stimulate the cell-mediated immune response with the activation of macrophages (4). This activation enhances the macrophages' ability to phagocytize and destroy the Leishmania organisms.
Clinical signs for leishmaniasis generally develop between 3 mo and 7 y postinfection (2,3). There are 3 major clinical presentations of leishmaniasis: cutaneous, mucocutaneous, and visceral. Clinical features vary with the phase of the disease, state of the animal's immunity, and previous therapy (5). Most cases show dullness, fatigue, pyrexia, weight loss, anorexia, and exercise intolerance, which then culminates in a wasting disease (1,3,5). Lymphadenopathy and splenomegaly are common (3). Articular involvement is also relatively common, and 37.5% of cases may show a reluctance to walk and an abnormal gait. These animals usually have arthralgia with osteolytis or peripheral proliferative periosteal reactions (2,6). Internally, the main histological changes are infiltration of the spleen and lymph nodes by macrophages containing amastigotes (8).
Most dogs with leishmaniasis have a hyperproteinemia due to a hyperglobulinemia. Immunoelectrophoresis shows increases in immunoglobulin G. The antibodies are produced in response to both organism and self (damaged tissue). The gammopathy is usually polyclonal but can appear more monoclonal (5,8), resembling a plasma cell myeloma or ehrlichiosis. There is a decrease in albumin (as it is a negative acute phase protein) in 94% of dogs. Proteinuria is seen in 85% of infected animals due to the glomerulonephritis resulting from the deposition of immune complexes in glomeruli. Other changes in the blood include nonregenerative anemia (60% of dogs), thrombocytopenia, (20%), and leukocytosis (24%) (5).
Definitive diagnosis is usually by demonstration of the protozoon in macrophages in lymph nodes, spleen, liver, or, as in this case, in the synovial fluid. Amastigotes are typically seen in macrophages, but they have also been reported in neutrophils, eosinophils, endothelial cells, and even fibroblasts (9). A serological test can be done if the organisms cannot be found, but the presence of antibodies merely shows exposure to the protozoon and not clinical disease (3). At postmortem, most dogs show cachexia, generalized lymphadenopathy, hepatosplenomegaly, and various skin lesions.
The clinical presentation of this dog was atypical for leishmaniasis, since there was no evidence of cachexia, anorexia, or cutaneous lesions accompanying the polyarthritis (6). Leishmania induced polyarthritis can occur due to an inflammatory reaction to either the organisms or the immune complexes in the synovium.
To our knowledge, this was the first case of canine leishmaniasis to be reported in the Maritimes. It constitutes a good example of the international nature of many diseases because of the increased travel of people and their pets throughout the world.
Footnotes
Acknowledgment
The authors thank Dr. David Sims for assistance with photography. CVJ
Address all correspondence and reprint requests to Dr. Sandra McConkey.
References
- 1.Bravo L, Frank L, Brenneman K. Canine leishmaniasis in the United States. Compend Contin Educ Pract Vet 1993;15:699–708.
- 2.Slappendel RJ. Canine leishmaniasis. A review based on 95 cases in The Netherlands. Vet Q 1988;10:1–16. [DOI] [PubMed]
- 3.Slappendel RJ, Ferrer L. Leishmaniasis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat, 2nd ed. Philadelphia: WB Saunders, 1998:450–458.
- 4.Tizard IR. Veterinary Immunology. 6th ed. Philadelphia: WB Saunders, 2000:98–109.
- 5.Ciaramella P, Oliva G, De Luna R, et al. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet Rec 1997;141:539–543. [DOI] [PubMed]
- 6.Spreng D. Leishmanial polyarthritis in two dogs. J Small Anim Pract 1993;34:559–563.
- 7.Natami A, Sahibi H, Lasri S, Boudouma M, Guessouss-Idrrissi N, Rhalem A. Serological, clinical and histopathological changes in naturally infected dogs with Leishmania infantum in the Khemisset province, Morocco. Vet Res 2000;31:355–363. [DOI] [PubMed]
- 8.Font A, Closa JM, Mascort J. Monoclonal gammopathy in a dog with visceral leishmaniasis. J Vet Intern Med 1994;8:233–235. [DOI] [PubMed]
- 9.Rodriguez JH, Mozos E, Mendez A, Perez J, Gomez-Villamandos JC. Leishmania infection of canine skin fibroblasts in vivo. Vet Pathol 1996;33:469–473. [DOI] [PubMed]


