Heterodimeric complexes of MutS- and MutL-related proteins were identified from studies of DNA mismatch repair (MMR) and have been implicated in processing of recombination intermediates. In a recent issue of PNAS, Wang et al. (1) have reported several observations about the function of MutL-related genes of the yeast Saccharomyces cerevisiae in MMR and recombination in meiosis. First, they have observed that MLH1 is the common subunit in three different MutL-related heterodimeric complexes, MLH1–PMS1, MLH1–MLH2, and MLH1–MLH3. Second, a possible role of MLH1–MLH2 in MMR was detected. Third, and most striking, MLH1–MLH3 was shown to play an important role in promoting crossing-over. This last observation extends the eukaryotic paradigm of combinatorial interactions between MutS- and MutL-related heterodimeric complexes and mispaired bases to other types of DNA structures formed during replication, recombination, and repair.
Genetics of Crossover Regulation.
In most organisms, meiotic crossing-over of homologous chromosomes (homologs) and cohesion between sister chromatids are required for faithful segregation of chromosomes (2, 3). Several yeast genes, including MutS- and MutL-related genes, are required for normal levels of crossing-over but not for gene conversion; these include MSH4, MSH5, MLH1, MLH3, ZIP1, ZIP2, MER3, and EXO1 (refs. 1 and 4–9; H. Tsubouchi and H. Ogawa, personal communication). Mutations in these genes reduce crossing-over by 50% or more, resulting in increased levels of homolog nondisjunction and spore death. Genetic analyses have shown that MSH4, MSH5, and MLH1 are in the same epistasis group with regard to crossing-over, indicating that crossing-over in these mutants is not due to functional redundancy among these genes (5, 6). The formation of MSH4–MSH5 and MLH1–MLH3 heterodimeric complexes has also been established (1, 10–13). The regulation of the distribution of crossovers along a chromosome, crossover interference, is also impaired in msh4, zip1, and mer3 mutants (3, 9, 14). zip1, zip2, and mer3 mutations also cause cell-cycle arrest before meiosis I. Although homolog nondisjunction is a primary cause of missegregation in crossing-over-deficient mutants, precocious separation of sister chromatids has also been observed in msh4, msh5, and zip1 mutants.
ZIP1 localizes continuously along the entire length of meiotic chromosomes when homologs are synapsed, which depends on ZIP2 (7, 8). However, before synapsis, ZIP1 and ZIP2 are colocalized as discrete foci on meiotic chromosomes. ZIP2 also colocalizes with MRE11, a component of the MRE11–RAD50–XRS2 complex, which is required for double-strand break (DSB) formation and resection of the 5′ ends (for a review, see ref. 15). In addition, ZIP2 localization depends on DSB formation. These results suggest that ZIP2 localizes at sites of recombination. MSH4 has also been shown to localize to similar discrete foci on meiotic chromosomes (4). In zip1 and mer3 mutants, DSBs are not completely converted to later intermediates, and progression of meiosis is delayed or blocked (9, 16). Cell-cycle arrest occurs in zip2 mutants, although the kinetics of DSB repair has not been studied (8, 17). The available data suggest that MSH4, MSH5, MLH1, MLH3, ZIP1, ZIP2, MER3, and EXO1 all function at a similar time in meiosis and that defects in these genes cause similar defects in crossing-over.
MSH4, MSH5, and MLH1 homologs have been found in higher eukaryotes. Human MSH4 and MSH5 proteins form a heterodimeric complex (11, 13). Msh5−/− or Mlh1−/− mutant mice show male and female infertility (18–21). Asynapsis and/or synapsis between nonhomologs followed by apoptotic cell death before or during pachytene are observed in these mutant mice. In Mlh1−/− mice, high levels of univalents are seen as homologs desynapse in the spermatocytes that do not undergo apoptosis, indicating that Mlh1 is required for the formation or stabilization of chiasmata, the cytological manifestation of crossing-over. The MLH1 protein localizes to discrete foci on chromosomes from zygotene (synapsis is started) through pachytene (homologs are fully synapsed) in oocytes (21–23). In contrast, MLH1 is detected only at pachytene in spermatocytes. Although human PMS1 is most closely related to the yeast MLH3 and interacts with MLH1, it is also highly related to yeast MLH2 (12, 24). Interestingly, Pms1−/− mice are fertile, suggesting few, if any, meiotic defects (25). Human PMS2 (the yeast PMS1 homolog) forms a heterodimer with MLH1, and Pms2−/− mice show only male infertility (26, 27). These findings raise questions about the identity of the mammalian MLH3 homolog and possibly suggest some redundancy between mammalian PMS1 and PMS2. However, involvement of murine Msh5 and Mlh1 in chromosome synapsis in meiosis implies a conserved mechanism of crossing-over.
Combinatorial Specificity of MutS and MutL Homologs.
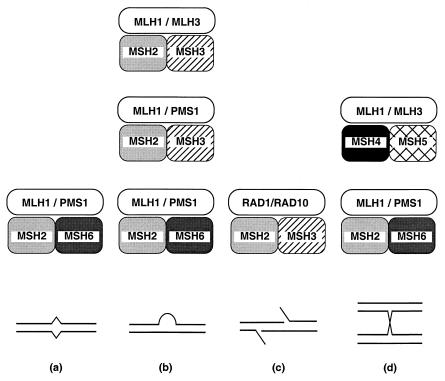
Heterodimeric complexes of MutS- and MutL-related proteins were first observed in studies of eukaryotic MMR (for reviews, see refs. 28–30). Extensive studies have defined at least six different heterodimeric complexes that interact in different combinations (Fig. 1). Combinations of these different complexes apparently direct the processing of different types of DNA structures.
Figure 1.
Combinatorial specificities of MutS- and MutL-related heterocomplexes. (a) Base/base mispairs; (b) insertion/deletion mispairs; (c) 5′ tailed DNA structures generated by single-strand DNA annealing (SSA) recombination; (d) Holliday junctions. Note that the DNA binding specificity for MSH4–MSH5 and its interaction with MLH1–MLH3 have not been shown. MLH1–MLH2 is not indicated, because it is not clear with which MutS-related complex it interacts.
Two MutS-related heterodimeric complexes, MSH2–MSH6 and MSH2–MSH3, have been found to function in the repair of mispaired bases generated during DNA replication and recombination. It seems that MSH2–MSH6 interacts with base/base and insertion/deletion mispairs, whereas MSH2–MSH3 interacts only with insertion/deletion mispairs (for a review, see ref. 30). These complexes function in conjunction with MutL-related heterodimeric complexes and with other interacting proteins such as EXO1 and PCNA (31–36). MLH1–PMS1, a MutL-related complex, plays a major role in both the MSH2–MSH6 and MSH2–MSH3 pathways and interacts with these two complexes (32, 36, 37). MLH1–MLH3, another MutL-related complex, also seems to play a role in the repair of some mispaired bases recognized by MSH2–MSH3 (12).
MutS- and MutL-related proteins have functions in recombination that are independent of MMR. These include the prevention of recombination between divergent DNA sequences, possibly by regulating the extent of heteroduplex formation, and the processing of SSA intermediates. MSH2–MSH6, MSH2–MSH3, MLH1–PMS1, RAD1–RAD10, and EXO1 are involved in preventing recombination between divergent DNA sequences (38–44). The formation of gene conversion polarity gradients during meiotic recombination requires MSH2–MSH6 and MLH1–PMS1 and may involve direct recognition of Holliday junctions by MSH2–MSH6 (45–48). Interestingly, processing of SSA intermediates involves MSH2–MSH3 and RAD1–RAD10, which interact with each other, but does not involve a MutL-related complex (49, 50).
Regulation of crossing-over requires MSH4–MSH5 and MLH1–MLH3 (1, 4–6). However, it is not known whether these protein complexes interact with each other or whether MSH4–MSH5 interacts with DNA. Interactions with other proteins involved in regulating crossing-over have not been investigated, but logical candidates for such proteins include ZIP1, ZIP2, MER3, and EXO1.
The above data raise the interesting possibility that the different MutS-related complexes reflect either divergent evolution of protein complexes that recognize different DNA structures or a combinatorial mechanism for generating different DNA recognition specificity. Further specificity is generated through interactions with other accessory factors such as the MutL-related complexes and other proteins like RAD1–RAD10 and EXO1. The end result is a diverse set of mechanisms that direct the processing of DNA structures generated during replication, repair, and recombination.
Mechanistic Aspects of Meiotic Crossing-Over.
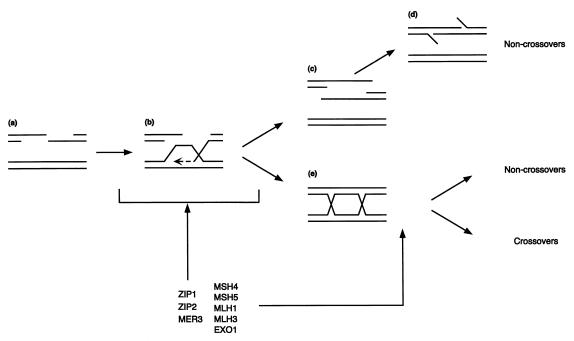
There are both early and late steps during recombination at which proteins like MSH4–MSH5 and MLH1–MLH3 might act (Fig. 2). That an early step might affect crossing-over can be visualized by examining the synthesis-dependent annealing model of DSB repair (for a review, see ref. 51). In this model, the 3′ end of one DNA strand invades the intact DNA partner and primes DNA synthesis. If this intermediate is unwound, single-strand annealing with the other half of the broken chromosome can occur leading to gene conversion not associated with crossing-over. Alternatively, the invaded intermediate can be converted to intermediates containing Holliday junctions, and crossovers or noncrossovers can result depending on how the junction is processed. Crossover control could occur early if the proteins required for crossing-over promote the conversion of the single-end invasion intermediate to intermediates containing Holliday junctions. Alternatively, crossover control could occur at a late step if the protein required for crossing-over increases the Holliday junction cleavage in the orientation for the formation of crossovers (4, 5).
Figure 2.
Steps of recombination and modes of the crossover control. (a) Resected DSBs; (b) strand invasion of one DSB and 3′ to 5′ DNA synthesis (dashed line); (c) Unwound intermediates; (d) 5′ tailed DNA structures generated by SSA recombination; (e) Holliday junction intermediates.
It is unclear whether MSH4–MSH5 and MLH1–MLH3 complexes act at early or late steps in recombination. The timing of recombination and levels of aberrant recombination intermediates in strains lacking these gene products are not affected enough to cause pachytene arrest. However, mutations in other genes encoding proteins that may function along with MSH4–MSH5 and MLH1–MLH3, such as ZIP1 and MER3, cause defects in the transition of DSBs to later intermediates but otherwise cause meiotic defects that are similar to those caused by mutations in MSH4, MSH5, MLH1, and MLH3 (1, 4–7, 9, 16). Both Msh5−/− and Mlh1−/− mutations in mice decrease crossing-over and cause defects in the progression of meiosis (18–23). It is possible that the mouse mutations affect the same step(s) of recombination affected in the corresponding yeast mutants. Overall, these findings are consistent with the idea that MSH4–MSH5 and MLH1–MLH3 promote DSB processing to form Holliday junctions reducing alternative outcomes, such as SSA recombination, rather than to alter the resolution of Holliday junctions. Such an early function could include promoting the formation of specific-types of Holliday junctions (e.g., geometrically distinct types of double Holliday junctions or different distances between two junctions) that are preferentially resolved as crossovers (52, 53). It is also possible that there are two pathways: one that accounts for 50% of crossovers and requires the crossover control proteins discussed herein and another pathway that is required for the rest of the crossovers and noncrossovers. Clearly, additional studies are required to answer this important question.
Links Between Meiotic Recombination and Checkpoint Control.
Yeast mutants with defective meiotic recombination and/or chromosome synapsis often execute a cell-cycle arrest. The mitotic checkpoint genes RAD17, RAD24, MEC1, MEC3, and DDC1 and the meiosis-specific genes RED1, MEK1, PCH2, and MSC6 are involved in this cell-cycle arrest (17, 54–56). The signals that initiate meiotic checkpoints are unclear but may include DSBs and other recombination intermediates as well as chromosome synapsis. zip1, zip2, and mer3 yeast mutants show reduced crossing-over and pachytene arrest, possibly because of delayed processing of DSBs. msh4, msh5, mlh1, and mlh3 mutants show reduced crossing-over but do not show pachytene arrest. In contrast to yeast, in Mlh1−/− and Msh5−/− mice, pachytene arrest and apoptosis occur. These occurrences could mean that recombination defects in the mutant mice are distinct from those in yeast or that the checkpoint in mice is more sensitive. Alternatively, this difference could reflect different monitoring systems in the two species. For instance, recent observations in mammalian cells have shown that MMR proteins may also serve as DNA damage sensors that trigger an apoptotic response (57). The possible role of proteins like MSH4–MSH5 and MLH1–MLH3 as direct sensors of recombination intermediates will be an interesting area for future investigation.
Acknowledgments
We wish to thank Hideo Tsubouchi, Hideyuki Ogawa, Sue Jinks-Robertson, Gray F. Crouse, and Josef Jiricny for sharing data before publication and Douglas K. Bishop and Alex R. Shoemaker for suggestions and comments on the manuscript. Related work by the authors has been supported by National Institutes of Health Grants GM26017 and GM50006.
Footnotes
See companion article on page 13914 in issue 24 of volume 96.
References
- 1.Wang T-F, Kleckner N, Hunter N. Proc Natl Acad Sci USA. 1999;96:13914–13919. doi: 10.1073/pnas.96.24.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleckner N. Proc Natl Acad Sci USA. 1996;93:8167–8174. doi: 10.1073/pnas.93.16.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roeder G S. Genes Dev. 1997;11:2600–2621. doi: 10.1101/gad.11.20.2600. [DOI] [PubMed] [Google Scholar]
- 4.Ross-Macdonald P, Roeder G S. Cell. 1994;79:1069–1080. doi: 10.1016/0092-8674(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 5.Hollingsworth N M, Ponte L, Halsey C. Genes Dev. 1995;9:1728–1739. doi: 10.1101/gad.9.14.1728. [DOI] [PubMed] [Google Scholar]
- 6.Hunter N, Borts R H. Genes Dev. 1997;11:1573–1582. doi: 10.1101/gad.11.12.1573. [DOI] [PubMed] [Google Scholar]
- 7.Sym M, Engebrecht J A, Roeder G S. Cell. 1993;72:365–378. doi: 10.1016/0092-8674(93)90114-6. [DOI] [PubMed] [Google Scholar]
- 8.Chua P R, Roeder G S. Cell. 1998;93:349–359. doi: 10.1016/s0092-8674(00)81164-2. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa T, Ogawa H. EMBO J. 1999;18:5714–5723. doi: 10.1093/emboj/18.20.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pochart P, Woltering D, Hollingsworth N M. J Biol Chem. 1997;272:30345–30349. doi: 10.1074/jbc.272.48.30345. [DOI] [PubMed] [Google Scholar]
- 11.Bocker T, Barusevicius A, Snowden T, Rasio D, Guerrette S, Robbins D, Schmidt C, Burczak J, Croce C M, Copeland T, et al. Cancer Res. 1999;59:816–822. [PubMed] [Google Scholar]
- 12.Flores-Rozas H, Kolodner R D. Proc Natl Acad Sci USA. 1998;95:12404–12409. doi: 10.1073/pnas.95.21.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winand N J, Panzer J A, Kolodner R D. Genomics. 1998;53:69–80. doi: 10.1006/geno.1998.5447. [DOI] [PubMed] [Google Scholar]
- 14.Sym M, Roeder G S. Cell. 1994;79:283–292. doi: 10.1016/0092-8674(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 15.Haber J E. Cell. 1998;95:583–586. doi: 10.1016/s0092-8674(00)81626-8. [DOI] [PubMed] [Google Scholar]
- 16.Storlazzi A, Xu L, Schwacha A, Kleckner N. Proc Natl Acad Sci USA. 1996;93:9043–9048. doi: 10.1073/pnas.93.17.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.San-Segundo P A, Roeder G S. Cell. 1999;97:313–324. doi: 10.1016/s0092-8674(00)80741-2. [DOI] [PubMed] [Google Scholar]
- 18.Edelmann W, Cohen P E, Kneitz B, Winand N, Lia M, Heyer J, Kolodner R, Pollard J W, Kucherlapati R. Nat Genet. 1999;21:123–127. doi: 10.1038/5075. [DOI] [PubMed] [Google Scholar]
- 19.de Vries S S, Baart E B, Dekker M, Siezen A, de Rooij D G, de Boer P, te Riele H. Genes Dev. 1999;13:523–531. doi: 10.1101/gad.13.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelmann W, Cohen P E, Kane M, Lau K, Morrow B, Bennett S, Umar A, Kunkel T, Cattoretti G, Chaganti R, et al. Cell. 1996;85:1125–1134. doi: 10.1016/s0092-8674(00)81312-4. [DOI] [PubMed] [Google Scholar]
- 21.Baker S M, Plug A W, Prolla T A, Bronner C E, Harris A C, Yao X, Christie D M, Monell C, Arnheim N, Bradley A, et al. Nat Genet. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- 22.Plug A W, Peters A H, Keegan K S, Hoekstra M F, de Boer P, Ashley T. J Cell Sci. 1998;111:413–423. doi: 10.1242/jcs.111.4.413. [DOI] [PubMed] [Google Scholar]
- 23.Anderson L K, Reeves A, Webb L M, Ashley T. Genetics. 1999;151:1569–1579. doi: 10.1093/genetics/151.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Räschle M, Marra G, Nyström-Lahti M, Schär P, Jiricny J. J Biol Chem. 1999;274:32368–32375. doi: 10.1074/jbc.274.45.32368. [DOI] [PubMed] [Google Scholar]
- 25.Prolla T A, Baker S M, Harris A C, Tsao J L, Yao X, Bronner C E, Zheng B, Gordon M, Reneker J, Arnheim N, et al. Nat Genet. 1998;18:276–279. doi: 10.1038/ng0398-276. [DOI] [PubMed] [Google Scholar]
- 26.Li G M, Modrich P. Proc Natl Acad Sci USA. 1995;92:1950–1954. doi: 10.1073/pnas.92.6.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker S M, Bronner C E, Zhang L, Plug A W, Robatzek M, Warren G, Elliott E A, Yu J, Ashley T, Arnheim N, et al. Cell. 1995;82:309–319. doi: 10.1016/0092-8674(95)90318-6. [DOI] [PubMed] [Google Scholar]
- 28.Kolodner R. Genes Dev. 1996;10:1433–1442. doi: 10.1101/gad.10.12.1433. [DOI] [PubMed] [Google Scholar]
- 29.Modrich P, Lahue R. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 30.Kolodner R D, Marsischky G T. Curr Opin Genet Dev. 1999;9:89–96. doi: 10.1016/s0959-437x(99)80013-6. [DOI] [PubMed] [Google Scholar]
- 31.Johnson R E, Kovvali G K, Guzder S N, Amin N S, Holm C, Habraken Y, Sung P, Prakash L, Prakash S. J Biol Chem. 1996;271:27987–27990. doi: 10.1074/jbc.271.45.27987. [DOI] [PubMed] [Google Scholar]
- 32.Habraken Y, Sung P, Prakash L, Prakash S. J Biol Chem. 1998;273:9837–9841. doi: 10.1074/jbc.273.16.9837. [DOI] [PubMed] [Google Scholar]
- 33.Gu L, Hong Y, McCulloch S, Watanabe H, Li G M. Nucleic Acids Res. 1998;26:1173–1178. doi: 10.1093/nar/26.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tishkoff D X, Boerger A L, Bertrand P, Filosi N, Gaida G M, Kane M F, Kolodner R D. Proc Natl Acad Sci USA. 1997;94:7487–7492. doi: 10.1073/pnas.94.14.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umar A, Buermeyer A B, Simon J A, Thomas D C, Clark A B, Liskay R M, Kunkel T A. Cell. 1996;87:65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- 36.Prolla T A, Pang Q, Alani E, Kolodner R D, Liskay R M. Science. 1994;265:1091–1093. doi: 10.1126/science.8066446. [DOI] [PubMed] [Google Scholar]
- 37.Habraken Y, Sung P, Prakash L, Prakash S. Curr Biol. 1997;7:790–793. doi: 10.1016/s0960-9822(06)00337-x. [DOI] [PubMed] [Google Scholar]
- 38.Chambers S R, Hunter N, Louis E J, Borts R H. Mol Cell Biol. 1996;16:6110–6120. doi: 10.1128/mcb.16.11.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saparbaev M, Prakash L, Prakash S. Genetics. 1996;142:727–736. doi: 10.1093/genetics/142.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta A, Adjiri A, New L, Crouse G F, Jinks Robertson S. Mol Cell Biol. 1996;16:1085–1093. doi: 10.1128/mcb.16.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Datta A, Hendrix M, Lipsitch M, Jinks-Robertson S. Proc Natl Acad Sci USA. 1997;94:9757–9762. doi: 10.1073/pnas.94.18.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paques F, Haber J E. Mol Cell Biol. 1997;17:6765–6771. doi: 10.1128/mcb.17.11.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen W, Jinks-Robertson S. Mol Cell Biol. 1998;18:6525–6537. doi: 10.1128/mcb.18.11.6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicholson, A., Hendrix, M., Jinks-Robertson, S. & Crouse, G. F. (1999) Genetics153, in press. [DOI] [PMC free article] [PubMed]
- 45.Alani E, Reenan R A, Kolodner R D. Genetics. 1994;137:19–39. doi: 10.1093/genetics/137.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foss H M, Hillers K J, Stahl F W. Genetics. 1999;153:573–583. doi: 10.1093/genetics/153.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hillers K J, Stahl F W. Genetics. 1999;153:555–572. doi: 10.1093/genetics/153.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marsischky G T, Lee S, Griffith J, Kolodner R D. J Biol Chem. 1999;274:7200–7206. doi: 10.1074/jbc.274.11.7200. [DOI] [PubMed] [Google Scholar]
- 49.Bertrand P, Tishkoff D X, Filosi N, Dasgupta R, Kolodner R D. Proc Natl Acad Sci USA. 1998;95:14278–14283. doi: 10.1073/pnas.95.24.14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugawara N, Paques F, Colaiacovo M, Haber J E. Proc Natl Acad Sci USA. 1997;94:9214–9219. doi: 10.1073/pnas.94.17.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paques F, Haber J E. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwacha A, Kleckner N. Cell. 1995;83:783–791. doi: 10.1016/0092-8674(95)90191-4. [DOI] [PubMed] [Google Scholar]
- 53.Aguilera A, Klein H L. Genetics. 1989;123:683–694. doi: 10.1093/genetics/123.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson D A, Stahl F W. Genetics. 1999;153:621–641. doi: 10.1093/genetics/153.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu L, Weiner B M, Kleckner N. Genes Dev. 1997;11:106–118. doi: 10.1101/gad.11.1.106. [DOI] [PubMed] [Google Scholar]
- 56.Lydall D, Nikolsky Y, Bishop D K, Weinert T. Nature (London) 1996;383:840–843. doi: 10.1038/383840a0. [DOI] [PubMed] [Google Scholar]
- 57.Gong J G, Costanzo A, Yang H Q, Melino G, Kaelin W G, Jr, Levrero M, Wang J Y. Nature (London) 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]