Abstract
The title compound, C26H40N5O13P·CH3OH·H2O, crystallizes with one water and one methanol molecule providing important crystal-binding interactions. The compound has the unusual feature of having two butoxycarbonyl groups bound to one N atom. The conventional attractive hydrogen bonds involving hydroxy, amine and water donors include bifurcations at both donors and acceptors with novel R 1 2(6) and R 2 1(6) motifs. These are supplemented by C—H⋯O interactions between adjacent molecules forming chain and R 2 2(10) ring motifs.
Related literature
For related structures, see: Low et al. (1995 ▶, 1998 ▶, 1999 ▶). For background information, see: Veldman et al. (2010 ▶). For ring puckering parameters, see: Cremer & Pople (1975 ▶). For hydrogen-bonding graph-set nomenclature, see: Bernstein et al. (1995 ▶).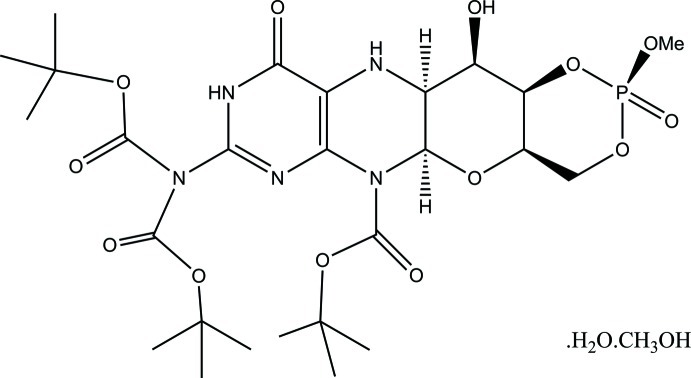
Experimental
Crystal data
C26H40N5O13P·CH4O·H2O
M r = 711.66
Orthorhombic,
a = 10.6435 (4) Å
b = 15.9508 (13) Å
c = 21.1764 (8) Å
V = 3595.2 (3) Å3
Z = 4
Cu Kα radiation
μ = 1.31 mm−1
T = 120 K
0.51 × 0.05 × 0.04 mm
Data collection
Oxford Diffraction SuperNova, Dual, Cu at zero, Atlas diffractometer
Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2007 ▶) T min = 0.853, T max = 1.000
20603 measured reflections
6796 independent reflections
6210 reflections with I > 2σ(I)
R int = 0.040
Refinement
R[F 2 > 2σ(F 2)] = 0.038
wR(F 2) = 0.096
S = 1.04
6796 reflections
452 parameters
2 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.41 e Å−3
Δρmin = −0.48 e Å−3
Absolute structure: Flack (1983 ▶), 2985 Friedel pairs
Flack parameter: −0.02 (2)
Data collection: CrysAlis PRO (Oxford Diffraction, 2007 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP in WinGX (Farrugia, 1999 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S160053681202867X/bh2437sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681202867X/bh2437Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681202867X/bh2437Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N9—H9N⋯O14 | 0.88 | 1.84 | 2.718 (3) | 179 |
| O12—H12O⋯O7i | 0.84 | 2.40 | 3.090 (2) | 139 |
| O12—H12O⋯O9i | 0.84 | 2.32 | 2.997 (2) | 138 |
| O14—H14A⋯O15 | 0.84 (3) | 1.97 (3) | 2.792 (4) | 167 (4) |
| O14—H14B⋯O13ii | 0.83 (4) | 1.97 (4) | 2.780 (3) | 165 (4) |
| O15—H15O⋯O10 | 0.84 | 1.96 | 2.775 (3) | 162 |
| C4—H4B⋯O4iii | 0.99 | 2.31 | 3.142 (3) | 141 |
| C5A—H5A⋯O13iv | 1.00 | 2.34 | 3.307 (3) | 162 |
| C12A—H12A⋯O4iii | 1.00 | 2.38 | 3.200 (2) | 138 |
| C16—H16B⋯O7v | 0.98 | 2.44 | 3.230 (4) | 137 |
| C18—H18B⋯O10vi | 0.98 | 2.38 | 3.339 (4) | 166 |
Symmetry codes: (i) 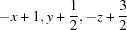 ; (ii)
; (ii) 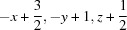 ; (iii)
; (iii) 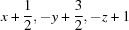 ; (iv)
; (iv) 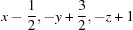 ; (v)
; (v) 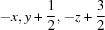 ; (vi)
; (vi) 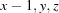 .
.
Acknowledgments
We thank Dr C. Fitchett of the University of Canterbury, New Zealand, for the data collection.
supplementary crystallographic information
Comment
The title compound is an intermediate required for the synthesis of cyclic pyranopterin monophosphate, a molecule whose absence in humans leads to a rare metabolic disorder known as molybdenum cofactor deficiency type A (Veldman et al., 2010).
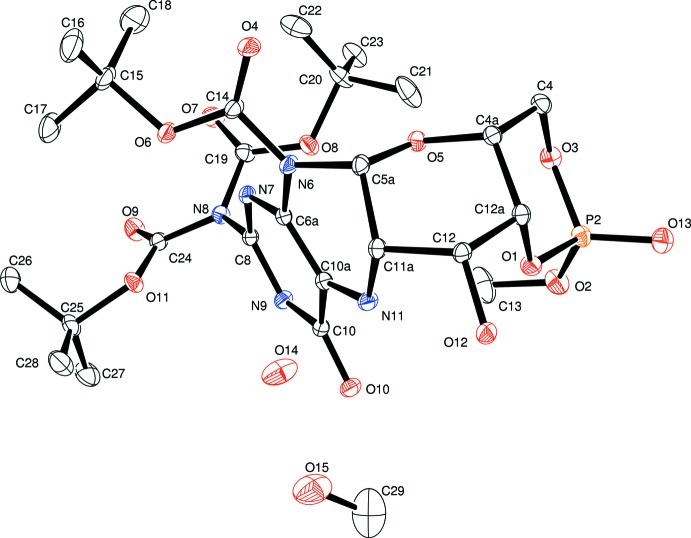
The title compound crystallizes with one independent C26H40N5O13P molecule in the asymmetric unit with one water and one methanol molecule of crystallization (Fig. 1). This and related compounds that were prepared are prone to form merohedrally twinned crystals; this non-twinned structure is the only one successfully solved and refined to date. The absolute configuration was determined using the oxygen anomalous dispersion effect, with the Hooft y parameter value calculated as -0.012 (13) (Spek, 2009). This confirms the expected configurations at C4a (R), C5a (R), C11a (S), C12 (R) and C12a (R).
There are only three closely related structures in the Cambridge Structural Database, namely GODXEC (Low et al., 1999), PUVCIS (Low et al., 1998) and ZENSAM (Low et al., 1995) with none of these involving a fourth fused ring as found here (ring 1, atoms C4a, C12a, O1, P2, O3 & C4). A common feature of all the structures is the buckling of the fused rings progressively away from the near planar ring 4 (C6a, N7, C8, N9, C10 & C10a; see Fig. 1). A comparison of the ring parameters, and their conformational forms (Cremer & Pople, 1975) is given in Table 2 (Ring parameters) with rings 2 & 3 consisting of atoms C5a, C11a, C12, C12a, C4a, O5 and N6, C6a, C10a, N11, C11a, C5a respectively. The similarity of the dihedral angles between the planes for ZENSAM (with the same ring absolute configuration) and PUVCIS (with the inverted configuration) implies that addition of the fourth "capping" ring 1 in the stable chair conformation has not required significant conformational changes to the other rings (see Table 3: Angles between ring planes).
The binding of two BOC groups on nitrogen N8 was slightly unexpected, with the second group expected to bind to the ring nitrogen N9. Indeed, a CSD search (Version 5.33, with Feb. 2012 update) indicates that this occurrence has been reported only 19 times whereas structures with BOC single binding are numbered in the multiple 1000's.
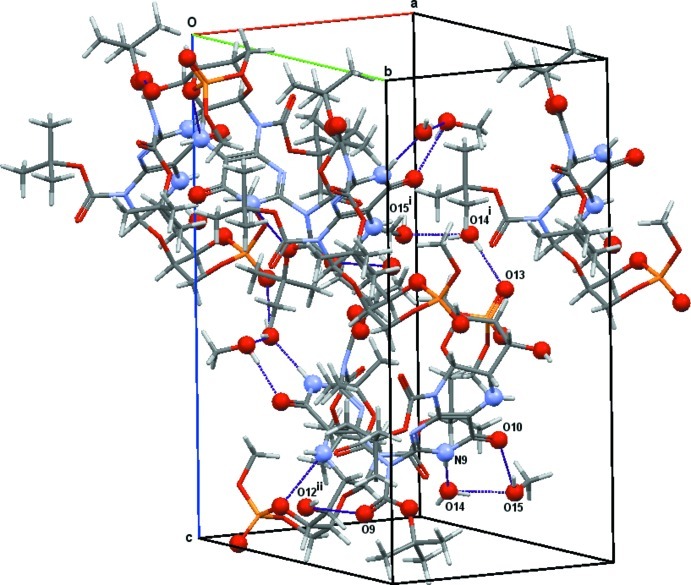
The crystal packing consists of a set of conventional N—H···O(water) and O—H···O(methanol, P, water) hydrogen bonds (see Table 1) which include bifurcations at both donors and acceptors with novel R21(6) and R12(6) motifs. These are supplemented by C—H···O interactions between adjacent molecules forming chain and R22(10) ring motifs (Bernstein et al., 1995).
Experimental
The details of the synthesis of the title compound will be published elsewhere in due course. The title compound was dissolved in methanol at ambient temperature and diethyl ether added. After 2 h the crystals were filtered off and washed with a little diethyl ether and dried at 15 Torr. The title compound was also crystallized from hot ethanol or hot ethanol and heptane mixtures followed by cooling to ambient temperature but the crystals produced in this way were twinned or were only weakly diffracting.
Refinement
Seven outlier reflections identified by large delta/sigma ratios (> 4.8) were OMITted from the dataset. All methyl H atoms were constrained to an ideal geometry (C—H = 0.98 Å) with Uiso(H) = 1.5Ueq(C), but were allowed to rotate freely about the adjacent C—C bond. All other C bound H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms with C—H distances of 1.00 (methine) or 0.99 Å (methylene) and with Uiso(H) = 1.2Ueq(C). hydroxy H atoms on O12 & O15 were constrained to idealized tetrahedral positions with O—H = 0.84 Å, but were allowed to rotate freely about the C—O bond. H atoms on water atom O14 were refined from their difference Fourier map locations, but O—H bond lengths were constrained to 0.84 Å. All hydroxy H atoms were refined with Uiso(H) = 1.5Ueq(O). Finally, N—H bond lengths were fixed to 0.88 Å. A set of 2985 measured Friedel pairs was used to refine the Flack parameter (Flack, 1983).
Figures
Fig. 1.
ORTEP (Farrugia, 1999) view of the asymmetric unit atoms with 30% ellipsoid probabilities. H atoms are omitted for clarity.
Fig. 2.
Mercury (Macrae et al., 2008) cell contents view down the ab diagonal; contact atoms are shown as balls. Some intermolecular binding contacts are shown as purple dotted lines. Symmetry: (i) 1.5 - x,1 - y, z - 0.5 (ii) 1 - x, y - 0.5, 1.5 - z.
Crystal data
| C26H40N5O13P·CH4O·H2O | F(000) = 1512 |
| Mr = 711.66 | Dx = 1.315 Mg m−3 |
| Orthorhombic, P212121 | Cu Kα radiation, λ = 1.54184 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 8356 reflections |
| a = 10.6435 (4) Å | θ = 2.8–73.7° |
| b = 15.9508 (13) Å | µ = 1.31 mm−1 |
| c = 21.1764 (8) Å | T = 120 K |
| V = 3595.2 (3) Å3 | Needle, colourless |
| Z = 4 | 0.51 × 0.05 × 0.04 mm |
Data collection
| Oxford Diffraction SuperNova, Dual, Cu at zero, Atlas diffractometer | 6796 independent reflections |
| Radiation source: fine-focus sealed tube | 6210 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.040 |
| Detector resolution: 10.6501 pixels mm-1 | θmax = 70.0°, θmin = 3.5° |
| ω scans | h = −12→12 |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2007) | k = −19→19 |
| Tmin = 0.853, Tmax = 1.000 | l = −25→25 |
| 20603 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.038 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.096 | w = 1/[σ2(Fo2) + (0.0524P)2 + 0.416P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max = 0.007 |
| 6796 reflections | Δρmax = 0.41 e Å−3 |
| 452 parameters | Δρmin = −0.48 e Å−3 |
| 2 restraints | Absolute structure: Flack (1983), 2985 Friedel pairs |
| 0 constraints | Flack parameter: −0.02 (2) |
| Primary atom site location: structure-invariant direct methods |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| P2 | 0.80446 (5) | 0.58835 (3) | 0.54460 (3) | 0.02445 (12) | |
| O1 | 0.78734 (14) | 0.68097 (9) | 0.57026 (7) | 0.0244 (3) | |
| O2 | 0.84799 (17) | 0.53670 (10) | 0.60281 (8) | 0.0340 (4) | |
| O3 | 0.66670 (15) | 0.55698 (10) | 0.53112 (7) | 0.0270 (3) | |
| O4 | 0.22697 (14) | 0.79511 (11) | 0.61522 (7) | 0.0297 (4) | |
| O5 | 0.50882 (13) | 0.69304 (9) | 0.57972 (7) | 0.0219 (3) | |
| O6 | 0.24587 (13) | 0.79463 (10) | 0.72208 (7) | 0.0246 (3) | |
| O7 | 0.21893 (16) | 0.46434 (11) | 0.79895 (8) | 0.0340 (4) | |
| O8 | 0.37798 (15) | 0.48589 (10) | 0.72995 (7) | 0.0285 (3) | |
| O9 | 0.29577 (16) | 0.52087 (10) | 0.91740 (7) | 0.0307 (3) | |
| O10 | 0.77295 (13) | 0.67374 (10) | 0.77704 (7) | 0.0256 (3) | |
| O11 | 0.38713 (14) | 0.64782 (10) | 0.89850 (7) | 0.0253 (3) | |
| O12 | 0.81909 (13) | 0.84935 (9) | 0.58518 (7) | 0.0239 (3) | |
| H12O | 0.8140 | 0.8978 | 0.6006 | 0.036* | |
| O13 | 0.89193 (15) | 0.57970 (11) | 0.49161 (8) | 0.0313 (3) | |
| N6 | 0.41153 (16) | 0.76019 (11) | 0.66317 (8) | 0.0215 (3) | |
| N7 | 0.39592 (15) | 0.66294 (11) | 0.74668 (8) | 0.0200 (3) | |
| N8 | 0.38213 (16) | 0.55516 (11) | 0.82093 (8) | 0.0215 (3) | |
| N9 | 0.57866 (16) | 0.61801 (11) | 0.79852 (8) | 0.0215 (3) | |
| H9N | 0.6101 | 0.5847 | 0.8276 | 0.026* | |
| N11 | 0.66929 (16) | 0.76924 (11) | 0.67840 (8) | 0.0227 (4) | |
| H11N | 0.7509 | 0.7731 | 0.6845 | 0.027* | |
| C4 | 0.5850 (2) | 0.61209 (14) | 0.49446 (10) | 0.0248 (4) | |
| H4A | 0.4995 | 0.5879 | 0.4930 | 0.030* | |
| H4B | 0.6168 | 0.6155 | 0.4506 | 0.030* | |
| C4A | 0.57849 (19) | 0.69937 (14) | 0.52219 (9) | 0.0220 (4) | |
| H4AA | 0.5311 | 0.7362 | 0.4923 | 0.026* | |
| C5A | 0.48696 (18) | 0.77318 (13) | 0.60754 (9) | 0.0208 (4) | |
| H5A | 0.4409 | 0.8099 | 0.5771 | 0.025* | |
| C6A | 0.46909 (19) | 0.71540 (13) | 0.71201 (9) | 0.0200 (4) | |
| C8 | 0.45310 (18) | 0.61650 (13) | 0.78714 (9) | 0.0194 (4) | |
| C10 | 0.65855 (18) | 0.67069 (13) | 0.76551 (9) | 0.0201 (4) | |
| C10A | 0.59833 (19) | 0.72028 (13) | 0.71732 (9) | 0.0200 (4) | |
| C11A | 0.61092 (18) | 0.81506 (13) | 0.62689 (9) | 0.0199 (4) | |
| H11A | 0.5922 | 0.8733 | 0.6417 | 0.024* | |
| C12 | 0.69743 (19) | 0.82063 (12) | 0.56888 (9) | 0.0192 (4) | |
| H12 | 0.6600 | 0.8625 | 0.5392 | 0.023* | |
| C12A | 0.70849 (19) | 0.73790 (13) | 0.53355 (9) | 0.0212 (4) | |
| H12A | 0.7496 | 0.7484 | 0.4918 | 0.025* | |
| C13 | 0.7751 (4) | 0.5374 (2) | 0.66060 (13) | 0.0540 (8) | |
| H13A | 0.7075 | 0.5788 | 0.6570 | 0.081* | |
| H13B | 0.8298 | 0.5521 | 0.6962 | 0.081* | |
| H13C | 0.7389 | 0.4817 | 0.6677 | 0.081* | |
| C14 | 0.28548 (18) | 0.78421 (13) | 0.66324 (10) | 0.0219 (4) | |
| C15 | 0.1099 (2) | 0.79637 (19) | 0.73616 (11) | 0.0344 (5) | |
| C16 | 0.0540 (3) | 0.8768 (2) | 0.71126 (14) | 0.0550 (9) | |
| H16A | 0.1050 | 0.9244 | 0.7253 | 0.082* | |
| H16B | −0.0319 | 0.8830 | 0.7273 | 0.082* | |
| H16C | 0.0524 | 0.8751 | 0.6650 | 0.082* | |
| C17 | 0.1096 (3) | 0.7949 (2) | 0.80794 (12) | 0.0453 (7) | |
| H17A | 0.1536 | 0.7447 | 0.8228 | 0.068* | |
| H17B | 0.0227 | 0.7941 | 0.8233 | 0.068* | |
| H17C | 0.1523 | 0.8450 | 0.8240 | 0.068* | |
| C18 | 0.0484 (3) | 0.7190 (2) | 0.70969 (16) | 0.0552 (8) | |
| H18A | 0.0465 | 0.7226 | 0.6635 | 0.083* | |
| H18B | −0.0376 | 0.7148 | 0.7258 | 0.083* | |
| H18C | 0.0963 | 0.6694 | 0.7225 | 0.083* | |
| C19 | 0.3147 (2) | 0.49719 (13) | 0.78327 (10) | 0.0244 (4) | |
| C20 | 0.3148 (3) | 0.45903 (14) | 0.67033 (11) | 0.0330 (5) | |
| C21 | 0.4166 (3) | 0.4769 (2) | 0.62155 (12) | 0.0517 (8) | |
| H21A | 0.4359 | 0.5370 | 0.6215 | 0.078* | |
| H21B | 0.3868 | 0.4602 | 0.5796 | 0.078* | |
| H21C | 0.4925 | 0.4451 | 0.6322 | 0.078* | |
| C22 | 0.1987 (3) | 0.51106 (18) | 0.65935 (13) | 0.0475 (7) | |
| H22A | 0.1349 | 0.4966 | 0.6909 | 0.071* | |
| H22B | 0.1657 | 0.4997 | 0.6170 | 0.071* | |
| H22C | 0.2199 | 0.5707 | 0.6630 | 0.071* | |
| C23 | 0.2869 (3) | 0.36592 (15) | 0.67444 (12) | 0.0382 (6) | |
| H23A | 0.3648 | 0.3352 | 0.6828 | 0.057* | |
| H23B | 0.2506 | 0.3467 | 0.6344 | 0.057* | |
| H23C | 0.2270 | 0.3557 | 0.7088 | 0.057* | |
| C24 | 0.34876 (19) | 0.57038 (13) | 0.88421 (10) | 0.0227 (4) | |
| C25 | 0.3793 (2) | 0.68166 (16) | 0.96382 (10) | 0.0301 (5) | |
| C26 | 0.2439 (3) | 0.6844 (2) | 0.98517 (12) | 0.0409 (6) | |
| H26A | 0.1918 | 0.7084 | 0.9515 | 0.061* | |
| H26B | 0.2371 | 0.7193 | 1.0231 | 0.061* | |
| H26C | 0.2151 | 0.6275 | 0.9947 | 0.061* | |
| C27 | 0.4631 (3) | 0.6298 (2) | 1.00673 (12) | 0.0437 (6) | |
| H27A | 0.4300 | 0.5726 | 1.0096 | 0.065* | |
| H27B | 0.4650 | 0.6551 | 1.0489 | 0.065* | |
| H27C | 0.5484 | 0.6283 | 0.9894 | 0.065* | |
| C28 | 0.4294 (3) | 0.77006 (17) | 0.95447 (13) | 0.0410 (6) | |
| H28A | 0.5139 | 0.7674 | 0.9362 | 0.061* | |
| H28B | 0.4329 | 0.7989 | 0.9953 | 0.061* | |
| H28C | 0.3736 | 0.8009 | 0.9259 | 0.061* | |
| O14 | 0.6796 (2) | 0.51634 (16) | 0.88814 (11) | 0.0581 (6) | |
| H14A | 0.742 (3) | 0.547 (2) | 0.895 (2) | 0.087* | |
| H14B | 0.664 (5) | 0.480 (2) | 0.9153 (18) | 0.087* | |
| O15 | 0.8836 (3) | 0.6263 (2) | 0.89018 (13) | 0.0791 (9) | |
| H15O | 0.8575 | 0.6507 | 0.8576 | 0.119* | |
| C29 | 0.9845 (6) | 0.5826 (3) | 0.8763 (3) | 0.0947 (17) | |
| H29A | 1.0130 | 0.5523 | 0.9140 | 0.142* | |
| H29B | 1.0511 | 0.6205 | 0.8619 | 0.142* | |
| H29C | 0.9647 | 0.5424 | 0.8428 | 0.142* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| P2 | 0.0273 (3) | 0.0234 (2) | 0.0227 (3) | 0.0025 (2) | 0.0006 (2) | −0.0004 (2) |
| O1 | 0.0240 (7) | 0.0249 (7) | 0.0243 (7) | 0.0034 (6) | −0.0025 (5) | −0.0016 (6) |
| O2 | 0.0429 (9) | 0.0303 (9) | 0.0288 (8) | 0.0091 (7) | −0.0041 (7) | 0.0011 (7) |
| O3 | 0.0305 (8) | 0.0234 (7) | 0.0271 (8) | −0.0025 (6) | 0.0028 (6) | 0.0003 (6) |
| O4 | 0.0209 (7) | 0.0494 (10) | 0.0187 (7) | 0.0034 (6) | −0.0031 (6) | 0.0043 (7) |
| O5 | 0.0214 (7) | 0.0267 (7) | 0.0177 (7) | −0.0032 (6) | 0.0028 (5) | 0.0018 (6) |
| O6 | 0.0173 (7) | 0.0369 (9) | 0.0195 (7) | 0.0043 (6) | 0.0020 (5) | 0.0023 (6) |
| O7 | 0.0318 (9) | 0.0413 (9) | 0.0288 (8) | −0.0155 (7) | 0.0027 (6) | −0.0010 (7) |
| O8 | 0.0324 (8) | 0.0334 (9) | 0.0197 (7) | −0.0061 (7) | 0.0017 (6) | −0.0031 (6) |
| O9 | 0.0401 (9) | 0.0298 (8) | 0.0222 (7) | −0.0066 (7) | 0.0066 (7) | 0.0043 (6) |
| O10 | 0.0180 (7) | 0.0355 (8) | 0.0233 (7) | −0.0013 (6) | −0.0010 (5) | 0.0048 (6) |
| O11 | 0.0312 (8) | 0.0300 (8) | 0.0147 (7) | −0.0061 (6) | 0.0038 (6) | −0.0010 (6) |
| O12 | 0.0206 (7) | 0.0257 (7) | 0.0252 (7) | −0.0036 (6) | 0.0039 (6) | −0.0030 (6) |
| O13 | 0.0320 (8) | 0.0292 (8) | 0.0326 (8) | 0.0029 (7) | 0.0046 (7) | −0.0019 (7) |
| N6 | 0.0163 (8) | 0.0308 (9) | 0.0175 (8) | 0.0020 (7) | 0.0015 (6) | 0.0044 (7) |
| N7 | 0.0171 (8) | 0.0264 (9) | 0.0164 (8) | −0.0012 (7) | 0.0028 (6) | 0.0012 (7) |
| N8 | 0.0226 (8) | 0.0232 (8) | 0.0186 (8) | −0.0042 (7) | 0.0025 (6) | 0.0028 (7) |
| N9 | 0.0187 (8) | 0.0273 (9) | 0.0184 (8) | 0.0005 (7) | 0.0006 (6) | 0.0048 (7) |
| N11 | 0.0180 (8) | 0.0331 (9) | 0.0171 (8) | −0.0026 (7) | 0.0006 (6) | 0.0042 (7) |
| C4 | 0.0258 (10) | 0.0300 (11) | 0.0185 (10) | −0.0035 (8) | 0.0003 (8) | −0.0001 (8) |
| C4A | 0.0206 (9) | 0.0302 (11) | 0.0153 (9) | −0.0009 (8) | 0.0007 (7) | 0.0017 (8) |
| C5A | 0.0196 (9) | 0.0259 (10) | 0.0169 (9) | 0.0015 (8) | 0.0031 (7) | 0.0025 (8) |
| C6A | 0.0180 (9) | 0.0271 (10) | 0.0148 (9) | −0.0004 (8) | 0.0016 (7) | −0.0019 (8) |
| C8 | 0.0176 (9) | 0.0252 (10) | 0.0154 (9) | −0.0018 (7) | 0.0023 (7) | 0.0005 (8) |
| C10 | 0.0167 (9) | 0.0262 (10) | 0.0175 (9) | 0.0011 (7) | 0.0010 (7) | −0.0008 (8) |
| C10A | 0.0187 (9) | 0.0257 (10) | 0.0157 (9) | −0.0011 (8) | 0.0011 (7) | −0.0009 (8) |
| C11A | 0.0196 (9) | 0.0234 (10) | 0.0165 (9) | −0.0015 (8) | 0.0033 (7) | 0.0012 (7) |
| C12 | 0.0183 (9) | 0.0214 (9) | 0.0178 (9) | −0.0019 (8) | 0.0023 (7) | 0.0027 (7) |
| C12A | 0.0218 (9) | 0.0254 (10) | 0.0165 (9) | −0.0019 (8) | 0.0035 (7) | 0.0026 (7) |
| C13 | 0.085 (2) | 0.0464 (16) | 0.0303 (14) | 0.0231 (16) | 0.0068 (14) | 0.0051 (12) |
| C14 | 0.0151 (9) | 0.0291 (10) | 0.0215 (10) | 0.0003 (8) | 0.0025 (7) | 0.0038 (8) |
| C15 | 0.0171 (10) | 0.0594 (16) | 0.0267 (11) | 0.0071 (10) | 0.0072 (8) | 0.0037 (11) |
| C16 | 0.0445 (16) | 0.085 (2) | 0.0358 (15) | 0.0371 (16) | 0.0060 (12) | 0.0046 (15) |
| C17 | 0.0351 (14) | 0.0730 (19) | 0.0277 (12) | 0.0121 (13) | 0.0130 (10) | 0.0063 (13) |
| C18 | 0.0316 (14) | 0.083 (2) | 0.0508 (17) | −0.0212 (14) | 0.0124 (12) | −0.0025 (16) |
| C19 | 0.0277 (10) | 0.0263 (10) | 0.0193 (10) | −0.0035 (9) | −0.0012 (8) | 0.0038 (8) |
| C20 | 0.0506 (14) | 0.0306 (11) | 0.0178 (10) | −0.0100 (11) | −0.0032 (10) | −0.0012 (9) |
| C21 | 0.082 (2) | 0.0490 (16) | 0.0245 (13) | −0.0280 (16) | 0.0135 (13) | −0.0056 (11) |
| C22 | 0.0617 (17) | 0.0460 (15) | 0.0350 (14) | 0.0003 (14) | −0.0203 (13) | 0.0022 (11) |
| C23 | 0.0573 (16) | 0.0320 (12) | 0.0255 (11) | −0.0112 (11) | 0.0005 (11) | −0.0010 (10) |
| C24 | 0.0211 (9) | 0.0281 (11) | 0.0190 (10) | −0.0004 (8) | 0.0007 (7) | 0.0019 (8) |
| C25 | 0.0361 (12) | 0.0379 (13) | 0.0162 (10) | −0.0013 (10) | 0.0030 (8) | −0.0055 (9) |
| C26 | 0.0391 (14) | 0.0532 (17) | 0.0304 (13) | −0.0011 (11) | 0.0105 (10) | −0.0082 (11) |
| C27 | 0.0549 (16) | 0.0520 (16) | 0.0240 (12) | 0.0032 (13) | −0.0065 (11) | −0.0012 (11) |
| C28 | 0.0504 (15) | 0.0407 (14) | 0.0318 (13) | −0.0084 (11) | 0.0015 (11) | −0.0098 (11) |
| O14 | 0.0417 (11) | 0.0749 (16) | 0.0576 (13) | −0.0065 (10) | −0.0128 (10) | 0.0422 (12) |
| O15 | 0.0619 (15) | 0.116 (2) | 0.0595 (15) | −0.0051 (16) | −0.0190 (13) | 0.0278 (16) |
| C29 | 0.131 (4) | 0.049 (2) | 0.104 (4) | 0.011 (3) | 0.042 (3) | 0.019 (2) |
Geometric parameters (Å, º)
| P2—O13 | 1.4647 (17) | C12A—H12A | 1.0000 |
| P2—O2 | 1.5533 (17) | C13—H13A | 0.9800 |
| P2—O3 | 1.5755 (16) | C13—H13B | 0.9800 |
| P2—O1 | 1.5845 (16) | C13—H13C | 0.9800 |
| O1—C12A | 1.461 (2) | C15—C18 | 1.505 (4) |
| O2—C13 | 1.449 (3) | C15—C16 | 1.509 (4) |
| O3—C4 | 1.460 (3) | C15—C17 | 1.520 (3) |
| O4—C14 | 1.205 (3) | C16—H16A | 0.9800 |
| O5—C5A | 1.427 (3) | C16—H16B | 0.9800 |
| O5—C4A | 1.430 (2) | C16—H16C | 0.9800 |
| O6—C14 | 1.326 (3) | C17—H17A | 0.9800 |
| O6—C15 | 1.478 (3) | C17—H17B | 0.9800 |
| O7—C19 | 1.193 (3) | C17—H17C | 0.9800 |
| O8—C19 | 1.327 (3) | C18—H18A | 0.9800 |
| O8—C20 | 1.493 (3) | C18—H18B | 0.9800 |
| O9—C24 | 1.198 (3) | C18—H18C | 0.9800 |
| O10—C10 | 1.243 (3) | C20—C22 | 1.507 (4) |
| O11—C24 | 1.336 (3) | C20—C23 | 1.517 (3) |
| O11—C25 | 1.487 (3) | C20—C21 | 1.523 (4) |
| O12—C12 | 1.416 (3) | C21—H21A | 0.9800 |
| O12—H12O | 0.8400 | C21—H21B | 0.9800 |
| N6—C14 | 1.395 (3) | C21—H21C | 0.9800 |
| N6—C6A | 1.398 (3) | C22—H22A | 0.9800 |
| N6—C5A | 1.441 (3) | C22—H22B | 0.9800 |
| N7—C8 | 1.286 (3) | C22—H22C | 0.9800 |
| N7—C6A | 1.359 (3) | C23—H23A | 0.9800 |
| N8—C24 | 1.407 (3) | C23—H23B | 0.9800 |
| N8—C19 | 1.416 (3) | C23—H23C | 0.9800 |
| N8—C8 | 1.428 (3) | C25—C26 | 1.511 (3) |
| N9—C8 | 1.358 (3) | C25—C27 | 1.518 (4) |
| N9—C10 | 1.385 (3) | C25—C28 | 1.520 (4) |
| N9—H9N | 0.8800 | C26—H26A | 0.9800 |
| N11—C10A | 1.364 (3) | C26—H26B | 0.9800 |
| N11—C11A | 1.453 (3) | C26—H26C | 0.9800 |
| N11—H11N | 0.8800 | C27—H27A | 0.9800 |
| C4—C4A | 1.513 (3) | C27—H27B | 0.9800 |
| C4—H4A | 0.9900 | C27—H27C | 0.9800 |
| C4—H4B | 0.9900 | C28—H28A | 0.9800 |
| C4A—C12A | 1.533 (3) | C28—H28B | 0.9800 |
| C4A—H4AA | 1.0000 | C28—H28C | 0.9800 |
| C5A—C11A | 1.535 (3) | O14—H14A | 0.83 (2) |
| C5A—H5A | 1.0000 | O14—H14B | 0.829 (19) |
| C6A—C10A | 1.382 (3) | O15—C29 | 1.313 (6) |
| C10—C10A | 1.442 (3) | O15—H15O | 0.8400 |
| C11A—C12 | 1.538 (2) | C29—H29A | 0.9800 |
| C11A—H11A | 1.0000 | C29—H29B | 0.9800 |
| C12—C12A | 1.522 (3) | C29—H29C | 0.9800 |
| C12—H12 | 1.0000 | ||
| O13—P2—O2 | 111.63 (10) | O6—C15—C16 | 109.4 (2) |
| O13—P2—O3 | 115.00 (9) | C18—C15—C16 | 113.2 (3) |
| O2—P2—O3 | 104.65 (9) | O6—C15—C17 | 101.75 (18) |
| O13—P2—O1 | 115.07 (9) | C18—C15—C17 | 111.1 (3) |
| O2—P2—O1 | 104.88 (9) | C16—C15—C17 | 111.2 (2) |
| O3—P2—O1 | 104.55 (9) | C15—C16—H16A | 109.5 |
| C12A—O1—P2 | 117.60 (13) | C15—C16—H16B | 109.5 |
| C13—O2—P2 | 120.45 (17) | H16A—C16—H16B | 109.5 |
| C4—O3—P2 | 117.34 (13) | C15—C16—H16C | 109.5 |
| C5A—O5—C4A | 111.91 (15) | H16A—C16—H16C | 109.5 |
| C14—O6—C15 | 120.22 (16) | H16B—C16—H16C | 109.5 |
| C19—O8—C20 | 122.02 (18) | C15—C17—H17A | 109.5 |
| C24—O11—C25 | 121.94 (17) | C15—C17—H17B | 109.5 |
| C12—O12—H12O | 109.5 | H17A—C17—H17B | 109.5 |
| C14—N6—C6A | 124.11 (17) | C15—C17—H17C | 109.5 |
| C14—N6—C5A | 119.81 (17) | H17A—C17—H17C | 109.5 |
| C6A—N6—C5A | 115.74 (16) | H17B—C17—H17C | 109.5 |
| C8—N7—C6A | 116.33 (17) | C15—C18—H18A | 109.5 |
| C24—N8—C19 | 121.39 (17) | C15—C18—H18B | 109.5 |
| C24—N8—C8 | 119.50 (17) | H18A—C18—H18B | 109.5 |
| C19—N8—C8 | 115.65 (16) | C15—C18—H18C | 109.5 |
| C8—N9—C10 | 121.68 (18) | H18A—C18—H18C | 109.5 |
| C8—N9—H9N | 119.2 | H18B—C18—H18C | 109.5 |
| C10—N9—H9N | 119.2 | O7—C19—O8 | 127.6 (2) |
| C10A—N11—C11A | 120.34 (16) | O7—C19—N8 | 124.2 (2) |
| C10A—N11—H11N | 119.8 | O8—C19—N8 | 108.11 (17) |
| C11A—N11—H11N | 119.8 | O8—C20—C22 | 110.0 (2) |
| O3—C4—C4A | 112.04 (16) | O8—C20—C23 | 108.70 (19) |
| O3—C4—H4A | 109.2 | C22—C20—C23 | 112.8 (2) |
| C4A—C4—H4A | 109.2 | O8—C20—C21 | 101.5 (2) |
| O3—C4—H4B | 109.2 | C22—C20—C21 | 112.0 (2) |
| C4A—C4—H4B | 109.2 | C23—C20—C21 | 111.2 (2) |
| H4A—C4—H4B | 107.9 | C20—C21—H21A | 109.5 |
| O5—C4A—C4 | 106.84 (17) | C20—C21—H21B | 109.5 |
| O5—C4A—C12A | 111.27 (16) | H21A—C21—H21B | 109.5 |
| C4—C4A—C12A | 112.84 (17) | C20—C21—H21C | 109.5 |
| O5—C4A—H4AA | 108.6 | H21A—C21—H21C | 109.5 |
| C4—C4A—H4AA | 108.6 | H21B—C21—H21C | 109.5 |
| C12A—C4A—H4AA | 108.6 | C20—C22—H22A | 109.5 |
| O5—C5A—N6 | 107.44 (16) | C20—C22—H22B | 109.5 |
| O5—C5A—C11A | 111.11 (16) | H22A—C22—H22B | 109.5 |
| N6—C5A—C11A | 108.87 (16) | C20—C22—H22C | 109.5 |
| O5—C5A—H5A | 109.8 | H22A—C22—H22C | 109.5 |
| N6—C5A—H5A | 109.8 | H22B—C22—H22C | 109.5 |
| C11A—C5A—H5A | 109.8 | C20—C23—H23A | 109.5 |
| N7—C6A—C10A | 124.14 (19) | C20—C23—H23B | 109.5 |
| N7—C6A—N6 | 117.59 (18) | H23A—C23—H23B | 109.5 |
| C10A—C6A—N6 | 117.86 (18) | C20—C23—H23C | 109.5 |
| N7—C8—N9 | 125.02 (19) | H23A—C23—H23C | 109.5 |
| N7—C8—N8 | 118.57 (18) | H23B—C23—H23C | 109.5 |
| N9—C8—N8 | 116.33 (18) | O9—C24—O11 | 128.4 (2) |
| O10—C10—N9 | 121.75 (19) | O9—C24—N8 | 124.3 (2) |
| O10—C10—C10A | 123.58 (19) | O11—C24—N8 | 107.33 (17) |
| N9—C10—C10A | 114.67 (17) | O11—C25—C26 | 110.04 (19) |
| N11—C10A—C6A | 122.29 (19) | O11—C25—C27 | 109.03 (19) |
| N11—C10A—C10 | 119.73 (18) | C26—C25—C27 | 113.4 (2) |
| C6A—C10A—C10 | 117.98 (19) | O11—C25—C28 | 101.29 (18) |
| N11—C11A—C5A | 110.44 (16) | C26—C25—C28 | 110.3 (2) |
| N11—C11A—C12 | 111.89 (16) | C27—C25—C28 | 112.1 (2) |
| C5A—C11A—C12 | 109.07 (16) | C25—C26—H26A | 109.5 |
| N11—C11A—H11A | 108.5 | C25—C26—H26B | 109.5 |
| C5A—C11A—H11A | 108.5 | H26A—C26—H26B | 109.5 |
| C12—C11A—H11A | 108.5 | C25—C26—H26C | 109.5 |
| O12—C12—C12A | 109.25 (16) | H26A—C26—H26C | 109.5 |
| O12—C12—C11A | 111.80 (16) | H26B—C26—H26C | 109.5 |
| C12A—C12—C11A | 112.89 (16) | C25—C27—H27A | 109.5 |
| O12—C12—H12 | 107.6 | C25—C27—H27B | 109.5 |
| C12A—C12—H12 | 107.6 | H27A—C27—H27B | 109.5 |
| C11A—C12—H12 | 107.6 | C25—C27—H27C | 109.5 |
| O1—C12A—C12 | 108.78 (15) | H27A—C27—H27C | 109.5 |
| O1—C12A—C4A | 110.67 (16) | H27B—C27—H27C | 109.5 |
| C12—C12A—C4A | 110.79 (17) | C25—C28—H28A | 109.5 |
| O1—C12A—H12A | 108.9 | C25—C28—H28B | 109.5 |
| C12—C12A—H12A | 108.9 | H28A—C28—H28B | 109.5 |
| C4A—C12A—H12A | 108.9 | C25—C28—H28C | 109.5 |
| O2—C13—H13A | 109.5 | H28A—C28—H28C | 109.5 |
| O2—C13—H13B | 109.5 | H28B—C28—H28C | 109.5 |
| H13A—C13—H13B | 109.5 | H14A—O14—H14B | 117 (5) |
| O2—C13—H13C | 109.5 | C29—O15—H15O | 109.5 |
| H13A—C13—H13C | 109.5 | O15—C29—H29A | 109.5 |
| H13B—C13—H13C | 109.5 | O15—C29—H29B | 109.5 |
| O4—C14—O6 | 127.63 (18) | H29A—C29—H29B | 109.5 |
| O4—C14—N6 | 122.40 (19) | O15—C29—H29C | 109.5 |
| O6—C14—N6 | 109.94 (17) | H29A—C29—H29C | 109.5 |
| O6—C15—C18 | 109.6 (2) | H29B—C29—H29C | 109.5 |
| O13—P2—O1—C12A | 77.02 (16) | C10A—N11—C11A—C12 | −145.40 (18) |
| O2—P2—O1—C12A | −159.94 (14) | O5—C5A—C11A—N11 | −67.4 (2) |
| O3—P2—O1—C12A | −50.11 (15) | N6—C5A—C11A—N11 | 50.7 (2) |
| O13—P2—O2—C13 | 179.4 (2) | O5—C5A—C11A—C12 | 56.0 (2) |
| O3—P2—O2—C13 | −55.6 (2) | N6—C5A—C11A—C12 | 174.10 (16) |
| O1—P2—O2—C13 | 54.1 (2) | N11—C11A—C12—O12 | −50.3 (2) |
| O13—P2—O3—C4 | −78.07 (16) | C5A—C11A—C12—O12 | −172.83 (16) |
| O2—P2—O3—C4 | 159.09 (14) | N11—C11A—C12—C12A | 73.3 (2) |
| O1—P2—O3—C4 | 49.09 (16) | C5A—C11A—C12—C12A | −49.2 (2) |
| P2—O3—C4—C4A | −53.3 (2) | P2—O1—C12A—C12 | 175.96 (12) |
| C5A—O5—C4A—C4 | −174.89 (16) | P2—O1—C12A—C4A | 54.02 (19) |
| C5A—O5—C4A—C12A | 61.5 (2) | O12—C12—C12A—O1 | 51.19 (19) |
| O3—C4—C4A—O5 | −70.5 (2) | C11A—C12—C12A—O1 | −73.9 (2) |
| O3—C4—C4A—C12A | 52.1 (2) | O12—C12—C12A—C4A | 173.05 (15) |
| C4A—O5—C5A—N6 | 177.32 (15) | C11A—C12—C12A—C4A | 48.0 (2) |
| C4A—O5—C5A—C11A | −63.7 (2) | O5—C4A—C12A—O1 | 67.9 (2) |
| C14—N6—C5A—O5 | −107.9 (2) | C4—C4A—C12A—O1 | −52.1 (2) |
| C6A—N6—C5A—O5 | 65.7 (2) | O5—C4A—C12A—C12 | −52.8 (2) |
| C14—N6—C5A—C11A | 131.70 (19) | C4—C4A—C12A—C12 | −172.90 (16) |
| C6A—N6—C5A—C11A | −54.7 (2) | C15—O6—C14—O4 | 19.3 (4) |
| C8—N7—C6A—C10A | 0.9 (3) | C15—O6—C14—N6 | −162.6 (2) |
| C8—N7—C6A—N6 | 173.44 (18) | C6A—N6—C14—O4 | −153.2 (2) |
| C14—N6—C6A—N7 | 28.9 (3) | C5A—N6—C14—O4 | 19.8 (3) |
| C5A—N6—C6A—N7 | −144.36 (18) | C6A—N6—C14—O6 | 28.6 (3) |
| C14—N6—C6A—C10A | −158.1 (2) | C5A—N6—C14—O6 | −158.35 (18) |
| C5A—N6—C6A—C10A | 28.6 (3) | C14—O6—C15—C18 | 53.6 (3) |
| C6A—N7—C8—N9 | 1.9 (3) | C14—O6—C15—C16 | −71.1 (3) |
| C6A—N7—C8—N8 | −174.69 (17) | C14—O6—C15—C17 | 171.2 (2) |
| C10—N9—C8—N7 | −1.1 (3) | C20—O8—C19—O7 | 26.9 (3) |
| C10—N9—C8—N8 | 175.53 (18) | C20—O8—C19—N8 | −154.39 (18) |
| C24—N8—C8—N7 | −103.6 (2) | C24—N8—C19—O7 | 8.5 (3) |
| C19—N8—C8—N7 | 55.7 (3) | C8—N8—C19—O7 | −150.5 (2) |
| C24—N8—C8—N9 | 79.5 (2) | C24—N8—C19—O8 | −170.32 (18) |
| C19—N8—C8—N9 | −121.1 (2) | C8—N8—C19—O8 | 30.8 (2) |
| C8—N9—C10—O10 | 178.0 (2) | C19—O8—C20—C22 | 47.2 (3) |
| C8—N9—C10—C10A | −2.3 (3) | C19—O8—C20—C23 | −76.6 (3) |
| C11A—N11—C10A—C6A | −3.5 (3) | C19—O8—C20—C21 | 166.0 (2) |
| C11A—N11—C10A—C10 | 175.77 (18) | C25—O11—C24—O9 | 6.4 (3) |
| N7—C6A—C10A—N11 | 175.01 (19) | C25—O11—C24—N8 | −173.45 (17) |
| N6—C6A—C10A—N11 | 2.5 (3) | C19—N8—C24—O9 | 26.3 (3) |
| N7—C6A—C10A—C10 | −4.3 (3) | C8—N8—C24—O9 | −175.6 (2) |
| N6—C6A—C10A—C10 | −176.77 (18) | C19—N8—C24—O11 | −153.78 (18) |
| O10—C10—C10A—N11 | 5.0 (3) | C8—N8—C24—O11 | 4.4 (2) |
| N9—C10—C10A—N11 | −174.64 (18) | C24—O11—C25—C26 | −61.7 (3) |
| O10—C10—C10A—C6A | −175.7 (2) | C24—O11—C25—C27 | 63.3 (3) |
| N9—C10—C10A—C6A | 4.7 (3) | C24—O11—C25—C28 | −178.3 (2) |
| C10A—N11—C11A—C5A | −23.7 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N9—H9N···O14 | 0.88 | 1.84 | 2.718 (3) | 179 |
| O12—H12O···O7i | 0.84 | 2.40 | 3.090 (2) | 139 |
| O12—H12O···O9i | 0.84 | 2.32 | 2.997 (2) | 138 |
| O14—H14A···O15 | 0.84 (3) | 1.97 (3) | 2.792 (4) | 167 (4) |
| O14—H14B···O13ii | 0.83 (4) | 1.97 (4) | 2.780 (3) | 165 (4) |
| O15—H15O···O10 | 0.84 | 1.96 | 2.775 (3) | 162 |
| C4—H4B···O4iii | 0.99 | 2.31 | 3.142 (3) | 141 |
| C5A—H5A···O13iv | 1.00 | 2.34 | 3.307 (3) | 162 |
| C12A—H12A···O4iii | 1.00 | 2.38 | 3.200 (2) | 138 |
| C16—H16B···O7v | 0.98 | 2.44 | 3.230 (4) | 137 |
| C18—H18B···O10vi | 0.98 | 2.38 | 3.339 (4) | 166 |
Symmetry codes: (i) −x+1, y+1/2, −z+3/2; (ii) −x+3/2, −y+1, z+1/2; (iii) x+1/2, −y+3/2, −z+1; (iv) x−1/2, −y+3/2, −z+1; (v) −x, y+1/2, −z+3/2; (vi) x−1, y, z.
Ring parameters (Å,°)a
| Ring No.b | Source | Q | θ | φ | Conformation |
| 1 | here | 0.518 (2) | 0.0 (2) | 120 (11) | Chair |
| 2 | here | 0.554 (2) | 173.2 (2) | 159.7 (18) | Chair |
| 2 | PUVCIS | 0.505 (3) | 173.9 (3) | 237 (3) | Chair |
| 2 | ZENSAM | 0.504 (5) | 173.5 (6) | 174 (5) | Chair |
| 2 | GODXEC | 0.755 (3) | 89.8 (2) | 359.6 (3) | Boat |
| 3 | here | 0.460 (2) | 56.7 (2) | 59.1 (3) | Envelope |
| 3 | PUVCIS | 0.478 (3) | 54.7 (4) | 55.7 (4) | Envelope |
| 3 | ZENSAM | 0.481 (4) | 54.4 (5) | 256.6 (6) | Envelope |
| 3 | GODXEC | 0.538 (3) | 86.9 (3) | 0.5 (4) | Boat |
| 4 | here | na | na | na | (Planar) |
| 4 | PUVCIS | 0.090 (3) | 70.9 (19) | 149.6 (19) | Flat-Screw-boat |
| 4 | ZENSAM | 0.093 (5) | 103 (3) | 324 (3) | Flat-Twist-boat |
a. For puckering parameters, see: Cremer & Pople (1975). b. Ring definitions given in text
Angles between ring planes (°)a
| Source | Planes 1,2 | Planes 2,3 | Planes 1,3 |
| here | 63.83 (9) | 13.74 (10) | 72.85 (10) |
| PUVCIS | 70.52 (13) | 16.62 (13) | 85.44 (13) |
| ZENSAM | 70.3 (2) | 17.0 (2) | 85.7 (2) |
| GODXEC | 47.72 (17) | 29.33 (15) | 76.55 (17) |
a. Ring definitions given in text
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2437).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Low, J. N., Cadoret, E., Ferguson, G., López, M. D., Quijano, M. L., Sánchez, A. & Nogueras, M. (1995). Acta Cryst. C51, 2141–2143.
- Low, J. N., Ferguson, G., Dolores, L. M., Luisa, Q. M., Sánchez, A., Nogueras, M. & Cobo, J. (1998). Acta Cryst. C54, IUC9800060.
- Low, J. N., López, M. D., Quijano, M. L., Sánchez, A. & Nogueras, M. (1999). Acta Cryst. C55, 452–454.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Oxford Diffraction (2007). CrysAlis PRO Oxford Diffraction Ltd, Abingdon, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Veldman, A., Santamaria-Araujo, J. A., Sollazzo, S., Pitt, J., Gianello, R., Yaplito-Lee, J., Wong, F., Ramsden, C. A., Reiss, J., Cook, I., Fairweather, J. & Schwarz, G. (2010). Pediatrics, 125, e1249–e1254. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S160053681202867X/bh2437sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681202867X/bh2437Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681202867X/bh2437Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report