Abstract
The regulator of G protein signaling 9-2 (RGS9-2) is a constituent of G protein-coupled receptor (GPCR) macromolecular complexes with a major role in regulation of GPCR activity in the central nervous system. Previous in situ hybridization and Western blot studies revealed that RGS9-2 is expressed in the superficial dorsal horn of the spinal cord. In the present study, we monitored tail withdrawal latencies to noxious thermal stimuli and performed in vitro whole-cell patch clamp electrophysiological recordings from neurons in lamina II of the spinal dorsal horn to examine the role of RGS9-2 in the dorsal horn of the spinal cord in nociceptive behaviours and opiate mediated modulation of synaptic transmission. Our findings obtained from RGS9 knockout mice indicate that the lack of RGS9-2 protein decreases sensitivity to thermal stimuli and to the analgesic actions of morphine in the tail immersion paradigm. This modulatory role of RGS9-2 on opiate-mediated responses was further supported by electrophysiological studies showing that hyperpolarization of neurons in lamina II of the spinal dorsal horn evoked by application of DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin, a mu opioid receptor agonist) was diminished in RGS9 knockout mice. The results indicate that RGS9-2 enhances the effect of morphine and may play a crucial role in opiate-mediated analgesic mechanisms at the level of the spinal cord.
Keywords: Tail immersion test, Spinal cord, Knockout mice, DAMGO, Dorsal horn
Regulator of G protein signaling 9-2 (RGS9-2) plays a major role in the modulation of G protein-coupled receptor (GPCR) activities in the central nervous system [17]. As a constituent of GPCR macromolecular complexes, RGS9-2 interacts with Gα subunits or several adaptor molecules like R7BP [1,13] to modulate receptor responsiveness and deactivation kinetics as well as the amplitude and duration of postsynaptic potentials [10,12,14]. It has also been demonstrated that activation of RGS9-2 has an inhibitory effect on receptor endocytosis [14,21]. A bulk of recent in vitro and in vivo studies reveal that drug treatment- or genetic mutation-evoked changes in the composition of RGS9 complexes have major consequences on GPCR sensitivity [12,14,8,4,15]. Results obtained from experiments on genetic animal models have demonstrated a potent role of RGS9-2 in psychostimulant, opiate, antipsychotic and antiparkinsonian drug responses [14,18,22,3,9].
In situ hybridization as well as Western blot analysis revealed a substantial expression of RGS9-2 in the nociceptive recipient superficial laminae of the spinal dorsal horn [22]. Although the regulatory role of RGS9-2 in synaptic transmission has been extensively studied in higher brain regions [21,4,15,18] the potential contribution of RGS9-2 to neural mechanisms underlying spinal pain transmission is poorly understood. To fill this gap we initiated a series of studies on the role of RGS9-2 in spinal nociceptive processing.
In the present study we monitored tail withdrawal latencies to noxious thermal stimuli and performed in vitro whole-cell patch clamp electrophysiological recordings from neurons in lamina II of the lumbar dorsal horn to investigate the role of RGS9-2 in the modulation of nociceptive behaviour and opiate-mediated synaptic transmission in the spinal dorsal horn. Findings obtained from RGS9 knockout mice and their wild-type littermates suggest that RGS9-2 enhances the effect of morphine and may play a crucial role in opiate-mediated antinociception at the level of the spinal cord.
Experiments were carried out on male RGS9 knockout (RGS9KO) mice and their wild-type controls (RGS9WT) [5]. Care and handling of animals followed the Helsinki Declaration and European Union regulations and adhered to the guidelines of the Committee for Research and Ethical Issues of IASP. All animal study protocols were approved by the Animal Care and Protection Committees of the University of Debrecen and University of Crete.
2–4 month old RGS9KO mice and their wild-type controls were tested for tail withdrawal latencies (TWL) to noxious thermal stimuli using the warm water tail-immersion assay [2]. Animals were habituated for 7 days in the tail immersion apparatus. On day 7, animals were gently held in a cloth towel and the tip of their tail (the terminal 2–3 cm) was immersed into hot water. The temperature of the water was accurately set at 54 °C. The time elapsing between the immersion and removal of the tail from the warm water was recorded and considered as TWL [2]. If the animals did not remove their tail from the hot water in 15 s (cutoff time) the trial was terminated by the investigator. For morphine analgesia studies, the drug was injected subcutaneously in a dose of either 1 or 3 mg/kg immediately after testing the baseline response latencies. The tail withdrawal latency test was repeated 30 min after morphine injection. Naloxone (NLX, 0.3 mg/kg s.c., Sigma) was administered 30 min after morphine administration and tail withdrawal latencies were monitored 5 min post NLX injection. From the measured values, the maximal possible effect (MPE) was calculated using the following equation: MPE = ([latency-baseline]/[cutoff-latency]). Results indicate mean values ± SEM. Statistical differences between experimental groups were computed using a two way ANOVA followed by the Bonferroni post hoc test.
Naïve 2–4 month old male RGS9KO and their littermate RGS9WT mice were injected with 30 μl of 4% formalin in the plantar surface of the left hindpaw and nociceptive responses (licking and biting) were recorded for 60 min, in 10 min intervals. Animals were euthanized immediately after the end of the experiment. The first 10 min (phase I) are associated with activation of primary afferent nociceptors from the chemical stimulus, while the remaining time interval (phase II) is related to tissue injury responses [23].
Experiments were performed in 3–10 week old mice (10 per genotype). Animals were decapitated under deep ether anaesthesia and the spinal cord was removed. A block of the spinal cord containing spinal segments L1–L5 was dissected and 300–400 μm thick transverse slices were cut with a Vibratome. Spinal cord slices were then incubated in normal ACSF (NaCl, 126 mM; KCl, 3 mM; CaCl2, 2 mM; MgCl2, 2 mM; NaH2PO4, 1.3 mM; NaHCO3, 26 mM; glucose, 10 mM) at room temperature for at least 1 h prior to recording.
All recordings were performed according to a protocol described by Szûcs et al. [20]. Briefly, in the recording chamber, slices were continuously perfused with ACSF (perfusion rate: 1–3 ml/min) at room temperature. Neurons in lamina II were visually identified under a Zeiss Axioskop FS microscope equipped with a 40× DIC objective and recorded in whole-cell current-clamp configuration with an Axopatch-1D amplifier (Axon Instruments, Union City, CA, USA) by using 7–10 MΩ glass microelectrodes (Harvard Apparatus Ltd., Kent, UK). The intracellular solution contained (in mM) K-gluconate, 126; KCl, 4; ATP-Mg, 4; GTP-Na2, 0.3; Na2-phosphocreatine, 10 and HEPES, 10 at pH 7.2. Resting membrane potential of the cells was measured, input resistance was calculated and firing patterns of the neurons were investigated by applying 800 ms long incrementing current steps ranging from −30 pA to 130 pA. Data were filtered at 5 kHz, recorded in a computer and analyzed off-line after the recording session. After this 2–5 min long recording session, DAMGO (Sigma) was applied in 2–4 μM concentrations into the bath for 2–5 min, and the response of the cell to DAMGO application was recorded. The application of the drug was followed by a 10–15 min wash out period. After the wash out period the slice was discarded; only one cell was recorded from each slice.
Recorded data were analyzed with Clampfit 9.0 analysing software. Numerical data are presented as mean ± SEM. Because of the negative results obtained in the RGS9KO mice, we were not able to make any statistical comparison between sets of data obtained from wild-type and mutant animals.
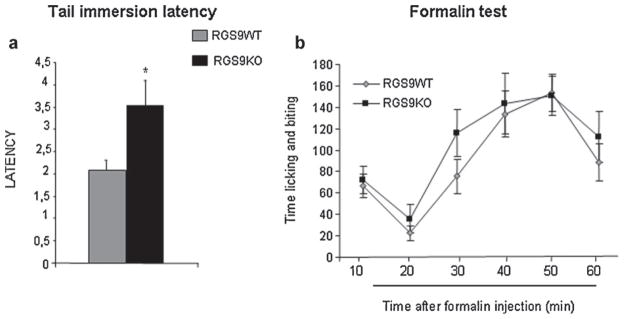
To assess the role of RGS9-2 actions in spinal cord mediated nociceptive responses, we used the 54 °C water tail immersion paradigm. Fig. 1a demonstrates that knockout of the RGS9 gene affects baseline latencies in the tail immersion test, as RGS9KO mice showed a 3.5 ± 0.5 s latency, whereas their wild-type controls showed a lower withdrawal latency (2.08 ± 0.2 s). This difference in tail immersion latencies is reversed by application of the opioid receptor antagonist naloxone (5 mg/kg s.c., WT/NLX latency = 2.4 ± 0.19 and KO/NLX latency = 2.9 ± 0.3).
Fig. 1.
RGS9-2 modulates nociceptive thresholds in the hot water tail immersion assay. The tail withdrawal latency values in the 54 °C tail immersion test were significantly higher in RGS9KO mice compared to their RGS9WT littermates (a), suggesting that RGS9-2 plays an essential role in the modulation of spinal nociceptive transmission. Data are presented as mean ± SEM, n = 8 per group, Student’s t-test, *P < 0.05. (b) Shows responses of RGS9WT and RGS9KO mice in the formalin test. Licking and biting behaviour of animals injected with formalin in the plantar surface of the left hindpaw were recorded for 60 min at 10 min intervals. Data are presented as mean ± SEM, n = 12–13 per group.
As the tail immersion data suggest that loss of RGS9 affects responses to some acute noxious stimuli, we also examined the influence of RGS9 in persistent pain. Therefore, in the next set of studies we monitored responses of RGS9KO mice in the formalin test. In particular, we monitored biting and licking behaviour for 60 min after injection of formalin to the plantar surface of the left hind paw. As shown in Fig. 1b, RGS9KO mice show similar nociceptive behaviour to their wild-type controls, in both phases of the formalin test. No statistically significant differences in nociceptive behaviour were observed between genotypes (two way ANOVA followed by Bonferroni test).
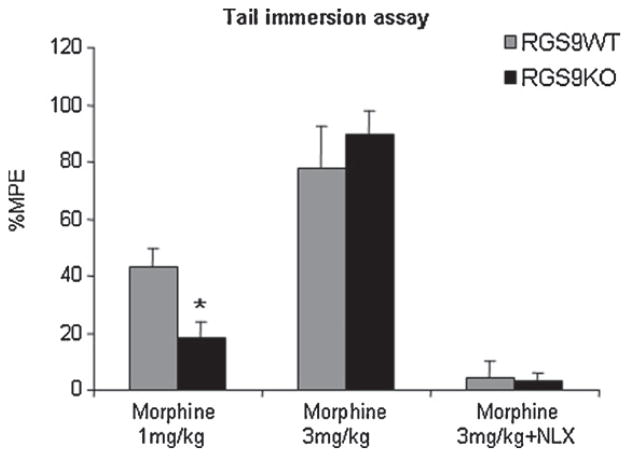
We next examined the antinociceptive effect of opioids in RGS9KO and RGS9WT mice in the tail immersion paradigm. For this reason, we used the mu opioid receptor agonist morphine [6,11]. In this set of studies, tail withdrawal latencies (TWL) were measured at baseline and 30 min following morphine administration (1 or 3 mg/kg s.c.). As shown in Fig. 2, morphine administered at a dose of 1 mg/kg produced an antinociceptive effect in wild-type animals (% MPE = 42.7 ± 7) but had a minor effect in TWL responses in RGS9 knockout mice (18 ± 7). When higher doses of morphine were used, both genotypes showed a robust increase in tail withdrawal latencies (% MPE for RGS9WT = 77.7 ± 15 and for RGS9KO = 89.6 ± 8.6). Morphine response to 3 mg/kg was blocked 5 min after s.c. administration of the opioid receptor antagonist naloxone (NLX) as shown in Fig. 2.
Fig. 2.
RGS9-2 is a positive modulator of morphine analgesia in the tail immersion assay. RGS9KO mice showed decreased sensitivity to morphine in the tail immersion test, at low doses (1 mg/kg s.c.) compared to their RGS9WT controls (n = 11 per genotype). The thermal withdrawal latency is increased in wild-type but not in mutant mice. Both genotypes showed similar analgesic responses (n = 6–8) to morphine at higher doses (3 mg/kg). The analgesic effect of morphine in the tail immersion test is completely blocked following naloxone (NLX) administration (0.3 mg/kg s.c., n = 7–9 per group). Data are expressed as mean MPE ± SEM, *P < 0.05, two way ANOVA followed by Bonferroni post hoc test.
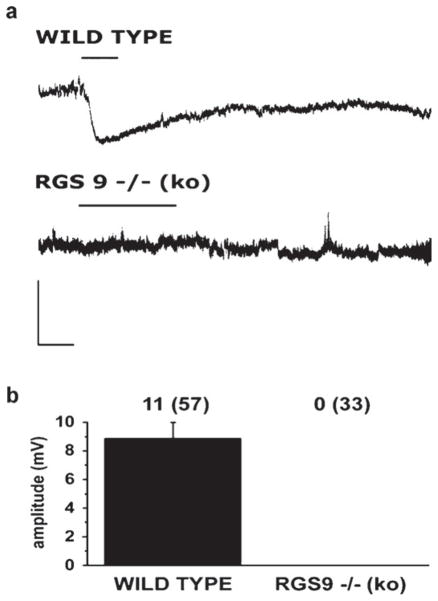
We next tested the effect of the mu opioid receptor agonist DAMGO on 57 and 33 neurons from 41 wild-type and 22 RGS9 knockout animals, respectively. All recorded neurons, regardless whether they were recorded in RGS9WT or RGS9KO animals, presented similar resting membrane potential. The average resting membrane potential of the recorded neurons was −50 ± 1 mV in wild-type and −49 ± 1 mV in RGS9−/− animals. The input resistance of the recorded lamina II neurons proved to be also very similar in the two experimental groups (704 ± 63 MΩ in wild-type and 717 ± 70 MΩ in RGS9 knockout animals).
In RGS9WT animals, 11 of the 57 recorded neurons responded to DAMGO; all of them with a prominent hyperpolarization (Fig. 3a and b). The responses were observed in 20–180 s after the onset of drug application, reached a peak of 8.9 ± 1.1 mV in 1.19 ± 0.13 min after the commencement of the hyperpolarization and lasted for 4.37 ± 0.35 min (Fig. 3a). In RGS9KO animals, the full DAMGO pharmacology protocol was carried out, but none of the 33 recorded neurons responded to the drug (Fig. 3a and b).
Fig. 3.
RGS9-2 is a positive modulator of opiate actions in lamina II neurons. Responses of neurons in lamina II of the spinal dorsal horn to DAMGO are shown in (a). In wild-type animals, 11 of the 57 recorded neurons (from 41 animals) responded to DAMGO; all of them with a prominent hyperpolarization. In RGS9KO animals, none of the 33 recorded neurons (from 22 animals) responded to DAMGO. Scale bars: 20 mV, 1 min. Histogram in (b) shows the amplitude of DAMGO evoked hyperpolarization in RGS9WT and RGS9KO animals. Data are presented as mean ± SEM. The numbers of responding neurons and the total numbers of recorded neurons (in brackets) are indicated above the corresponding column of the histogram.
The present findings demonstrate that deletion of the RGS9 gene decreases sensitivity to hot water in the tail immersion paradigm suggesting that RGS9-2 plays a role in spinal processing of noxious thermal stimuli. On the other hand, deletion of the RGS9 gene does not affect the expression or severity of formalin induced nociceptive behaviour.
Our studies also reveal that functional deletion of the RGS9 gene renders animals less sensitive to morphine in the tail immersion assay. This modulatory role of RGS9-2 on opiate-mediated responses was further supported by electrophysiological studies showing that hyperpolarization of neurons in lamina II of the spinal dorsal horn evoked by application of the mu opioid receptor agonist DAMGO was diminished in mutant mice. These results indicate that enhancement of RGS9-2 activity may reduce the amount of morphine required for attenuation of nociceptive dorsal horn responses, and thus may eliminate side effects and abuse potential associated with morphine application. Experimental data from the present study may be particularly important for the development of treatment strategies targeting pain at the level of the spinal dorsal horn.
In earlier studies we demonstrated that RGS9-2 forms complexes with the mu-opioid receptor [14]. It has also been demonstrated that RGS9-2 interacts with several molecules involved in mu-opioid receptor signaling and desensitization, including β-arrestin-2 and spinophilin [14,4]. The interaction between RGS9-2 and β-arrestin-2 is especially interesting, as it has been shown that beta-arrestin 2 plays a prominent role in morphine analgesia at both spinal and supraspinal sites [16]. Here we provide additional evidence on the active participation of RGS9-2 in opioid receptor mediated nociceptive information processing mechanisms in the spinal dorsal horn.
Several studies in recent years indicate that pre- and postsynaptic opioid receptor mechanisms play a substantial role in spinal pain processing. Our results further support this notion, and suggest that RGS9-2 contributes to the fine-tuning of signaling mechanisms related to opioid receptor-associated molecular complexes. Deletion of the RGS9 gene increases thermal tail withdrawal latencies and decreases sensitivity of lamina II dorsal horn neurons to the MOR agonist DAMGO. Thus, RGS9-2 in the spinal cord enhances MOR responsiveness, and in the absence of the protein higher doses of the drug are required to produce an antinociceptive effect. There are several mechanisms that may contribute to this phenotype. Our hypothesis is that RGS9-2 forms complexes with Gα subunits and other signaling/scaffolding molecules to facilitate mu opioid receptor responsiveness. Deletion of the RGS9 gene affects these protein/protein interactions, decreases the sensitivity of the receptor to morphine and therefore higher doses of the drug are required to produce an antinociceptive response. It is also possible that high morphine doses elicit an antinociceptive response by activating other opioid receptor subtypes (delta, kappa) or by activating opioid receptor heterodimers [19] which may function via mechanisms that do not involve RGS9-2 actions. The differences in tail immersion responses to morphine between genotypes may also derive from diminished nociceptive control from supraspinal sites where RGS9-2 plays a negative modulatory role in MOR responsiveness. As cannabinoid receptors are also involved in spinal analgesic responses, RGS9-2 may affect TRPV channel function via modulation of CB1 cannabinoid receptors [7]. Although our preliminary studies show no role of RGS9-2 in CB1 responses in reward paradigms, future studies will address this possibility.
Comparing our present and earlier findings, it appears that RGS9-2 exerts a different influence on opioid receptor mechanisms at spinal versus supraspinal levels. We have previously reported that deletion of the RGS9 gene increases sensitivity to the rewarding actions of morphine in the place preference paradigm and to the analgesic actions of the drug in the hot plate test, a paradigm assessing mostly supraspinal antinociceptive responses [22]. In contrast, here we demonstrate that in spinal cord mediated behaviours, mutant mice show decreased sensitivity to hot water and morphine. We also show that hyperpolarization of spinal neurons evoked by the application of a mu opioid receptor agonist is abolished in RGS9 knockout mice. Thus, while RGS9-2 acts as a negative modulator of morphine actions in supraspinally mediated behaviours, it acts as a positive modulator of morphine actions in spinal cord mediated responses. As the function of signal transduction molecules depends on local protein/protein interactions, it is possible that this difference in the modulatory effect of RGS9-2 on morphine action at spinal versus supraspinal levels may be related to the composition of signal transduction complexes modulating mu opioid receptor function at different CNS regions. For example, while it is clear that RGS9-2 forms complexes with Gβ5, R7BP and with Gα subunits in the striatum [13,4,15], it remains unknown if these complexes are also present in the spinal cord. In fact, studies using knockout mice reveal that signal transduction molecules involved in mu opioid receptor signaling and desensitization, like beta arrestin-2, differentially modulate morphine responses in spinal versus supraspinal sites [2]. Future investigation of the molecular composition of opioid receptor-associated signaling complexes will assess the function of complexes modulating mu opioid receptor responsiveness in the spinal cord and other brain regions expressing mu opioid receptors.
In summary, our study provides novel information about signal transduction molecules mediating sensitivity to noxious thermal stimuli, and responses to morphine in the spinal cord. As RGS9-2 is a key molecule for analgesia and addiction, understanding the tissue specific actions of this protein is essential for the development of more efficient treatment strategies and novel drug targets.
References
- 1.Berman DM, Gilman AG. Mammalian RGS proteins: Barbarians at the gates. J Biol Chem. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- 2.Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in beta-arrestin knockout mice. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrera-Vera TM, Hernandez S, Earls LR, Medkova M, Sundgren-Andersson AK, Surmeier JD, Hamm HE. RGS9-2 modulates D2 dopamine receptor-mediated Ca2 -channel inhibition in rat striatal cholinergic interneurons. Proc Natl Acad Sci. 2004;101:16339–16344. doi: 10.1073/pnas.0407416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charlton JJ, Allen BP, Psifogeorgou K, Chakravarty S, Gomes I, Neve RL, Devi LA, Greengard P, Nestler EJ, Zachariou V. Multiple actions of spinophilin modulate mu opioid receptor function. Neuron. 2008;58:238–247. doi: 10.1016/j.neuron.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CK, Eversole-Cire P, Zhang H, Mancino V, Chen YJ, He W, Wensel TG, Simon MI. Instability of GGL domain containing RGS proteins in mice lacking the G protein beta subunit Gbeta5. Proc Natl Acad Sci. 2003;100:6604–6609. doi: 10.1073/pnas.0631825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Fioravanti B, De Felice M, Stucky CL, Medler KA, Luo MC, Gardell LR, Ibrahim M, Malan TP, Jr, Yamamura HI, Ossipov MH, King T, Lai J, Porreca F, Vanderah TW. Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: antinociceptive actions of CB1 inverse agonists. J Neurosci. 2008;28:11593–11602. doi: 10.1523/JNEUROSCI.3322-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold SJ, Hoang CV, Potts BW, Porras G, Pioli E, Kim KW, Nadjar A, Qin C, LaHoste GJ, Li Q, Bioulac BH, Waugh JL, Gurevich E, Neve RL, Bezard E. RGS9-2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson’s disease. J Neurosci. 2007;27:14338–14348. doi: 10.1523/JNEUROSCI.4223-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooks SB, Waldo GL, Corbitt ET, Krumins AM, Harden TK. RGS6, RGS7, RGS9 and RGS11 stimulate GTPase activity of Gi family of G proteins with differential selectivity and maximal activity. J Biol Chem. 2003;278:10087–10093. doi: 10.1074/jbc.M211382200. [DOI] [PubMed] [Google Scholar]
- 11.Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. 2002;18(4 Suppl):S3–S13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- 12.Kovoor A, Seyffarth P, Ebert J, Barghshoon S, Chen CK, Schwarz S, Axelrod JD, Cheyette BN, Simon MI, Lester HA, Schwarz J. D2 dopamine receptors colocalize regulator of G protein signalling 9-2 via the RGS9 DEp domain, and RGS9 knockout mice develop dyskinesias associated with dopamine pathways. J Neurosci. 2005;280:5133–5136. doi: 10.1523/JNEUROSCI.2840-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martemyanov KA, Yoo PJ, Skiba NP, Arshavsky VY. R7BP, a novel neuronal protein interacting with RGS proteins and the R7 subfamily. J Biol Chem. 2005;280:5133–5136. doi: 10.1074/jbc.C400596200. [DOI] [PubMed] [Google Scholar]
- 14.Psifogeorgou K, Papakosta P, Russo SJ, Kardassis D, Gold SJ, Zachariou V. RGS9-2 is a negative modulator of mu opioid receptor function. J Neurochem. 2007;103:617–625. doi: 10.1111/j.1471-4159.2007.04812.x. [DOI] [PubMed] [Google Scholar]
- 15.Psifogeorgou K, Terzi D, Papachatzaki MM, Varidaki A, Ferguson D, Gold SJ, Zachariou V. A unique role of RGS9-2 as a positive or negative modulator of opiate actions in the striatum. J Neurosci. 2011;31:5617–5624. doi: 10.1523/JNEUROSCI.4146-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. AAPS J. 2005:E587–E591. doi: 10.1208/aapsj070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman Z, Gold SJ, Potenza MN, Cowan CW, Ni YG, He W, Wensel TG, Nestler EJ. Cloning and characterization of RGS9-2, a striatal enriched alternatively spliced product of the RGS9 gene. J Neurosci. 1999;19:2016–2026. doi: 10.1523/JNEUROSCI.19-06-02016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman Z, Schwarz J, Gold SJ, Zachariou V, Wein MN, Choi KH, Kovoor A, Chen CK, DiLeone RJ, Schwarz SC, Selley DE, Sim-Selley LJ, Barrot M, Luedtke RR, Self D, Neve RL, Lester HA, Simon MI, Nestler E. J RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;38:941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- 19.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signalling: beta arrestin-2 mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szûcs P, Odeh F, Szokol K, Antal M. Neurons with distinctive firing patterns, morphology and distribution in laminae V–VII of the neonatal rat lumbar spinal cord. Eur J Neurosci. 2003;17:537–544. doi: 10.1046/j.1460-9568.2003.02484.x. [DOI] [PubMed] [Google Scholar]
- 21.Terzi D, Stergiou E, King SL, Zachariou V. RGS proteins in neuropsychiatric disorders. Prog Mol Biol Transl Sci. 2009;86C:299–333. doi: 10.1016/S1877-1173(09)86010-9. [DOI] [PubMed] [Google Scholar]
- 22.Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, Berton O, Neve RL, Sim-Selley LJ, Selley DE, Gold SJ, Nestler EJ. Essential role for RGS9 in opiate action. Proc Natl Acad Sci. 2003;100:13656–13661. doi: 10.1073/pnas.2232594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeitz KP, Giese KP, Silva AJ, Basbaum AI. The contribution of autophosphorylated alpha-calcium-calmodulin kinase II to injury-induced persistent pain. Neuroscience. 2004;128:889–898. doi: 10.1016/j.neuroscience.2004.07.029. [DOI] [PubMed] [Google Scholar]