Abstract
OBJECTIVES
To determine the activity and tolerability of 100-mg once-daily (QD) dasatinib in patients with metastatic castration-resistance prostate cancer (CRPC). Dasatinib, an oral Src family kinase inhibitor, has demonstrated both preclinical and clinical activity with twice-daily dosing in patients with metastatic CRPC.
METHODS
Chemotherapy-naive men with metastatic CRPC and increasing prostate-specific antigen levels were treated with dasatinib 100 mg QD. The primary measurement was a composite lack of disease progression, according to the Prostate Cancer Working Group 2 criteria, determined every 12 weeks during the study. The other analyses included changes in the prostate-specific antigen level, bone lesions, soft tissue disease, and bone turnover markers (urine N-telopeptide and bone alkaline phosphatase).
RESULTS
The present trial was designed before the publication of the recent Prostate Cancer Working Group 2 criteria; however, the analyses are presented to conform to the updated guidelines. A total of 48 patients received dasatinib. A lack of disease progression was observed in 21 patients (44%) at week 12 and in 8 (17%) at week 24. Urine N-telopeptide was reduced by ≥40% from baseline in 22 (51%) of 43 patients, and bone alkaline phosphatase was decreased in 26 (59%) of 44 patients. Dasatinib was well-tolerated, with only 6 patients (13%) with drug-related grade 3–4 adverse events and 3 (6%) with grade 3 adverse events. The most common treatment-related adverse events (≥20%) were fatigue, nausea, diarrhea, headache, and anorexia.
CONCLUSIONS
Dasatinib 100 mg QD has a favorable safety profile and maintains a similar degree of activity as the previously reported twice-daily dosing schedules. These data support additional study of dasatinib 100 mg QD for metastatic CRPC.
Prostate cancer (CaP) is the second-leading cause of cancer-related deaths in men in the Western world.1 Metastases to the bone are common (~80%) and result in pain, fractures, hypercalcemia, spinal cord compression, and bone marrow insufficiency.2 The current standard for the treatment of metastatic castration-resistant CaP (CRPC) is docetaxel chemotherapy, offering a modest survival benefit.3,4 Bone-targeting agents, such as bisphosphonates and denosumab, a nuclear factor-κB ligand inhibitor, reduce the incidence of skeletal complications in patients with bone-metastatic CRPC.4–10 However, well-tolerated targeted therapies for metastatic CRPC are needed that offer dual antitumor and positive bone effects, delaying the time until chemotherapy initiation.
Dasatinib (Sprycel, Bristol-Myers Squibb, New York, NY) is an oral tyrosine kinase inhibitor with potent activity against the nonreceptor tyrosine kinases, c-Src and other Src family kinases.11 Src and Src family kinase are located within several signaling pathways involved in CaP and promote tumor cell proliferation, survival, migration, and transition to androgen-independent growth.12,13 Dasatinib inhibits cancer cell proliferation, reduces CaP xenograft growth, and inhibits formation of lymph node metastases in androgen-sensitive and androgen-resistant tumors.11,12,14 In a C42B orthotopic nude mouse model of prostate bone tumors, dasatinib decreased the prostate-specific antigen (PSA), increased bone mineral density, decreased serum calcium, and potentiated the activity of docetaxel chemotherapy.15 In bone resorption assays, dasatinib reduced osteoclast proliferation and calcium release.16
A Phase II study (CA180085) was conducted to determine the clinical activity and safety of dasatinib. Yu et al17 reported the safety and activity of dasatinib at 100 mg and 70 mg twice-daily (BID). At these doses, dasatinib demonstrated biologic activity, as evidenced by decreases in bone turnover markers; however, toxicities (≥40% of patients) developed, including pleural effusions, fatigue, nausea, and diarrhea.17 Because of these toxicities and evidence from a dose-optimization study of chronic myelogenous leukemia that once-daily (QD) dasatinib can offer equal efficacy yet less toxicity,18 the trial was amended. In the present expansion cohort of patients, the goal was to determine whether 100 mg QD dosing would improve tolerability while maintaining the biologic (bone markers) and clinical (Prostate Cancer Working Group 2 [PCWG2] criteria19 of a lack of progression) activity.
MATERIAL AND METHODS
Patients
Male patients with histologically confirmed CaP, metastasis, and ≥2 serial increases in the PSA level with a castrate serum testosterone level of <50 ng/dL, were eligible. The patients with pleural or pericardial effusion were excluded. Other key inclusion/exclusion criteria have been previously described by Yu et al.17 Concomitant luteinizing hormone-releasing hormone agonists were continued for patients without surgical castration. The patients already receiving bisphosphonates were allowed to continue their use throughout the study. However, the treatment could not begin within 3 weeks of study entry or during the study period.
All patients provided written informed consent before enrollment into the trial. The ethics committees of all participating sites approved the study protocol. The clinical trials registry number was NCT00385580.
Study Design
The original study (CA180085) was a Phase II, open-label study conducted at 12 centers in the United States, Italy, and France. The protocol was amended to include the present cohort of patients who received dasatinib 100 mg QD.
Adverse events (AEs) were classified according to the National Cancer Institute Common Toxicity Criteria, version 3.0.20 Dosing interruptions for drug-related toxicity were allowed, and patients could continue dasatinib when the toxicities had recovered to baseline or grade 1 or less. If unacceptable toxicity recurred despite optimal supportive care or if the toxicity was grade 3 or greater, the treatment was discontinued. Unlike the previously reported BID dosing regimen, dose reductions were not allowed for the new cohort.
Evaluation of Clinical Activity
The primary study endpoint was a composite endpoint (referred to as the composite response/stable disease [SD] rate), developed before the PCWG2 recommendations and defined as any of the following: (a) confirmed >50% PSA decline; (b) SD for any duration using the Response Evaluable Criteria in Solid Tumors (RECIST); (c) confirmed complete response or confirmed partial response using RECIST; or (d) confirmed disappearance of the lesions by radionuclide bone scan, at any point during the study period, including week 12. To follow the recent PCWG2 guidelines19 suggesting that Phase II trials should alter the focus from the measurement of response to the lack of progression, the data were reanalyzed and reported. The RECIST response and SD were considered in the definition of the lack of progression. The lack of progression using bone scans was assessed in combination with RECIST at multiple points. PSA changes were not included as a marker of response or progression and have been reported separately.
Tumor progression was defined according to the RECIST or as ≥1 definite new lesion on a bone scan. Patients continued therapy with asymptomatic increases in the PSA level or the appearance of new lesions on a bone scan before 24 weeks, provided they had no other evidence of progression (eg, RECIST findings or clinical symptoms). However, new lesions on a bone scan have been reported as progression, even if before the 24-week assessment.
The secondary endpoints included changes in the bone scan findings, RECIST, PSA level, and bone turnover markers (bone alkaline phosphatase [BAP] and urine N-telopeptide [uNTX]). The PSA levels were measured at the screening visit and every 4 weeks during the study. Serial bone scans were obtained approximately every 12 weeks, and the findings were classified as the disappearance of lesions, SD, or the appearance of new lesions. Investigators performed tumor assessments for the patients with measurable lesions at baseline and every 12 weeks using the modified RECIST.21 An analysis of the tumor measurements and a determination of the lack of response excluded pelvic lymph nodes <2 cm, according to the PCWG2 recommendations.19
The serum BAP and uNTX were measured at screening and every 4 weeks during the study to assess for treatment-related changes in bone metabolism. A 40% decrease in uNTX at 3 months was previously reported to be associated with a significant (17%) reduction in the risk of death, regardless of the baseline level.7 Therefore, patients with a ≥40% decrease in uNTX from baseline are reported. Additionally, because treatment-associated normalization of elevated uNTX has been shown to correspond with a 59% reduction in death and 49% increase in skeletal-related event (SRE)-free survival in men with CRPC,7 the proportion of patients with baseline uNTX levels greater than the upper limit of normal (63 nM/mmol creatinine), with a reduction to normal limits (3–63 nM/mmol creatinine) is also presented.
Statistical Analysis
Analyses of efficacy and safety were performed using all available data from patients treated with dasatinib. A 2-sided 95% exact confidence interval for the composite response/SD rate was calculated. The lack of progression was evaluated at 12 and 24 weeks, depending on the availability of the assessments. Statistical comparisons between the BID and QD groups were not predesignated in the protocol and were exploratory. Selected AE rates were compared using Fisher’s exact test, and P values were calculated for descriptive purposes only. Therefore, no adjustment was made for multiple comparisons.
RESULTS
Patient Characteristics
From October 2006 to July 2008, 48 additional patients were enrolled and received 100 mg dasatinib QD. The median therapy duration was 3.98 months (range 0.10–14.7). At the database lock, February 19, 2009, 7 patients were continuing dasatinib. The last patient finished the study in January 2010. The baseline characteristics and demographic data are listed in Table 1. A flow chart is provided in Figure 1.
Table 1.
Patient baseline demographics and disease characteristics
| Characteristic | Value |
|---|---|
| Total assessable patients (n) | 48 |
| Age (y) | |
| Median | 68 |
| Range | 47–85 |
| Age ≥65 y (n) | 36 (75) |
| Race/ethnicity (n) | |
| White | 46 (96) |
| Black | 1 (2) |
| Other | 1 (2) |
| Interval since diagnosis (mo) | |
| Median | 79 |
| Range | 13–238 |
| ECOG performance status (n) | |
| 0 | 35 (73) |
| 1 | 13 (27) |
| PSA (ng/mL) | n = 48 |
| Median | 43.6 |
| Range | 5.7–471 |
| uNTX (nM/mmol creatinine) | n = 46 |
| Median | 46.0 |
| Range | 8.0–535.0 |
| BAP (U/L) | n = 47 |
| Median | 35.0 |
| Range | 14.0–563.0 |
| Previous therapy (n) | |
| Surgery | 32 (67) |
| Radiotherapy | 37 (77) |
| Bisphosphonates | 12 (25) |
| RECIST-evaluable disease (n) | 20 (42) |
| Bone metastases (n) | 34 (71) |
| Disease sites, target lesions (n) | |
| Lymph node | 18 (38) |
| Pelvis | 1 (2) |
| Prostate mass | 1 (2) |
| Skin/soft tissue | 1 (2) |
| Visceral, lung | 1 (2) |
| Visceral, liver | 1 (2) |
ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen; RECIST, Response Evaluable Criteria in Solid Tumors; uNTX, urine N-telopeptide; BAP, bone alkaline phosphatase.
Data in parentheses are percentages.
Figure 1.
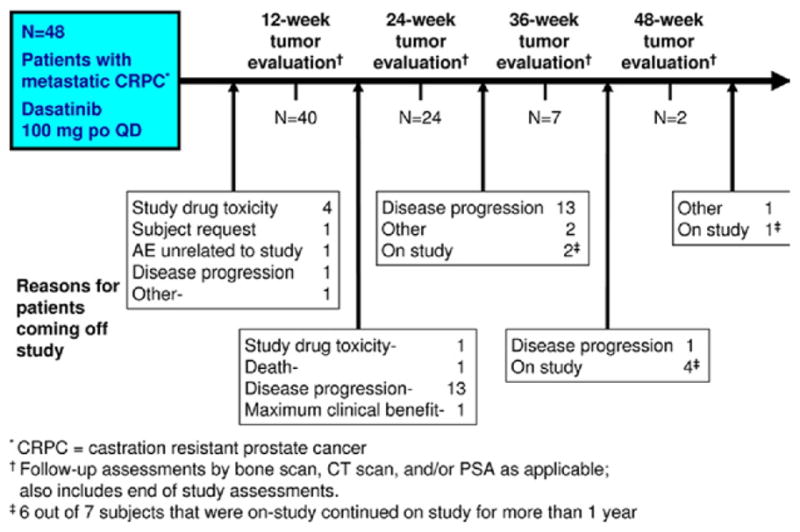
Study schema showing number of patients with 12-week efficacy assessments (including end of study assessment) at which bone scan, computed tomography scans, and/or PSA levels were obtained. Between these 12-week assessments, a list of reasons for patients coming off study is given. Note, 6 of 7 patients still in the study at Clinical Study Report data lock in February 2009 eventually remained with the study with a lack of progressive disease for >1 year. po, oral; CT, computed tomography.
Efficacy
The original predefined primary endpoint of the composite response/SD rate, which included assessments at 12 weeks, was achieved in 12 (25%) of 48 patients (95% confidence interval 13.6%–39.6%). The protocol pre-specified that if the composite response/SD rate was ≤10% that additional study with dasatinib for this patient population would be futile. The PCWG2-defined endpoint of a lack of progression using both the RECIST and bone scan findings was met by 21 (44%) of 48 patients at week 12 and 8 (17%) of 48 patients at week 24. However, 6 patients (13%) continued with the study for >1 year without progression. The median interval to disease progression was 4.7 months (95% confidence interval 2.8–5.5).
All 48 patients had undergone a bone scan at baseline. Of the 40 patients evaluated by bone scan at week 12, 25 (52%) were reported to have no new lesions. At week 24, 21 patients were evaluated by bone scan, and 12 (25%) were reported to have no new lesions.
Of the 48 patients, 20 had RECIST-evaluable lesions at baseline. The disease control rate with confirmed measurable lesions by RECIST (complete response plus partial response, plus SD) was 40% (8 of 20) after 12 weeks. All 8 patients had SD. Of the remaining 12 patients, 8 (40%) had progression, and 4 (20%) were not evaluable. The 8 patients with SD at week 12 were evaluated again at week 24, for a disease control rate by RECIST of 15% (3 of 20, 1 with a confirmed partial response and with 2 SD); 3 (15%) others had progression, and 2 (10%) were not evaluable. The maximal percentage of change from baseline in tumor size is presented for the 16 men with measurable disease at baseline and ≥1 tumor assessment during the study period (Fig. 2A).
Figure 2.
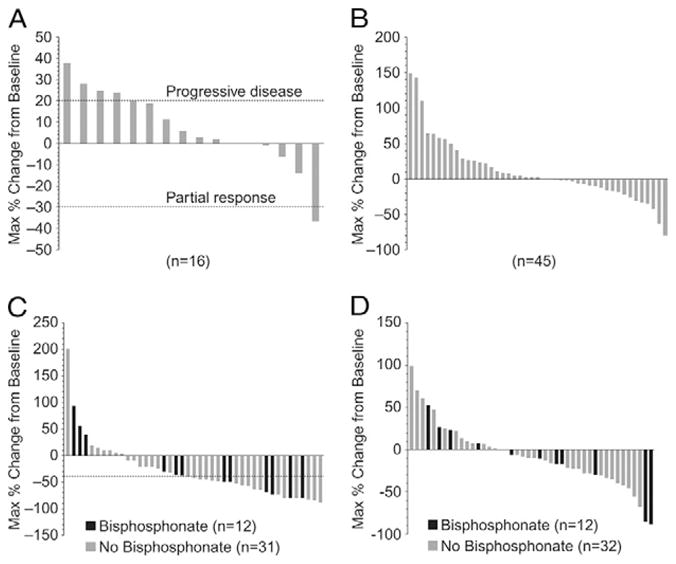
(A) Maximal percentage of change in tumor size from baseline. Dashed line at 20% represents RECIST progression; dashed line at −30% represents RECIST partial response. RECIST could only be reported for 16 (80%) of 20 patients with baseline measurable disease, because 4 patients did not remain with the study long enough to undergo 12-week computed tomography imaging assessment. (B) Maximal PSA change from baseline. PSA level in 2 patients (4%) declined by >50%, and 1 had confirmation of >50% decline. (C) Maximal percentage of change in uNTX levels from baseline. Twelve patients were receiving bisphosphonates. Dashed line represents 40% reduction from baseline. (D) Maximal percentage of change in BAP from baseline. Twelve patients were receiving bisphosphonates.
A confirmed PSA decline of ≥50% from baseline was observed in 1 patient (2%) with no other signs of clinical or radiographic progression. The maximal PSA change from baseline in the individual patients is shown in Figure 2B.
The patients receiving dasatinib exhibited decreased uNTX, independent of bisphosphonate use (Fig. 2C). The number of patients who achieved any decline in uNTX during the study was 33 (77%) of 43 patients. The number of patients who achieved a ≥40% reduction in uNTX during study was 22 (51%) of 43 patients. Of these 22 patients, 6 (50%) of 12 had received bisphosphonates and 16 (52%) of 31 had not. Seventeen patients had uNTx levels greater than the upper limit of normal at baseline. Of these 17 patients, the only patient who was taking bisphosphonates and 9 (56%) of 16 who were not had a decline of uNTX into the normal range. All but 1 patient receiving bisphosphonates had started bisphosphonate therapy (zoledronate in 9 [75%] and aledronate in 3 [25%] of 12) >4 months before the initiation of dasatinib.
The maximal percentage of change in BAP for individual patients is presented in Figure 2D. The number of patients who achieved any decline of BAP during the study was 26 (59%) of 44. Reductions in BAP were achieved in 7 (58%) of the 12 patients taking bisphosphonates and 19 (59%) of the 32 patients who did not. Sixteen patients had levels greater than the upper limit of normal of BAP at baseline. Of these 16 patients, 2 (67%) of the 3 taking bisphosphonates had declines in BAP into the normal range, and 2 (15%) of the 13 who were not taking bisphosphonates had declines into the normal range.
Safety and Tolerability
The 100 mg QD dose of dasatinib was generally well-tolerated, and most AEs were grade 1 or 2, reversible, and clinically manageable. Grade 3–4 treatment-related serious AEs were reported in 3 men (6%): 1 (2%) grade 3 pleural effusion, 1 (2%) grade 4 anemia, and 1 (2%) grade 3 ischemia. Grade 1–2 hypocalcemia was experienced by 19 patients (40%); 1 patient (2%) developed transient grade 4 hypocalcemia. Other grade 3 laboratory AEs included hypokalemia in 2 (4%), hyponatremia in 1 (2%), and hypophosphatemia in 2 (4%). All laboratory-related AEs resolved without treatment or discontinuation of dasatinib. Two deaths (4%) unrelated to dasatinib (1 [2%] from septicemia/infection and 1 [2%] reported as unknown) were recorded within 30 days of the last dose of dasatinib.
COMMENT
In the present study, dasatinib, administered at a dose of 100 mg QD to patients with chemotherapy-naive metastatic CRPC, produced signals of clinical activity. The PCWG2-defined endpoint of a lack of progression at 12 and 24 weeks was observed in 21 (44%) of 48 and 8 (17%) of 48 patients with QD dosing. The previously reported results were 20 (43%) of 47 and 9 (19%) of 47 patients with BID dosing.17
The present trial was designed before the publication of the PCWG2 criteria19 and did not require confirmation of bone scan progression. More specifically, only 1 new lesion was considered progression before the use of the PCWG2 criteria, which has recommended ≥2 new lesions, generally confirmed ≥6 weeks later with an additional ≥2 new lesions. Thus, dasatinib’s effect in preventing tumor progression might have been underestimated compared with contemporary clinical trials using the PCWG2 criteria. The median duration of therapy was 3.98 months for the 100 mg QD dose and 2.78 and 2.68 months for the previously reported 70 mg and 100 mg BID doses, respectively.
Given the high incidence of bone morbidity in patients with CaP, we studied the biologic activity of dasatinib by evaluating the bone turnover markers. The reduction of SREs and bone pain associated with bone metastases are important goals to improve the quality of life and potentially extend the survival of patients with CRPC.4,7,17,22–26 Increased levels of the bone resorption marker, uNTX, has been correlated with the presence and extent of bone metastases.7 Moreover, normalization of uNTX in response to treatment has been associated with significant palliative responses, delays in bone disease progression, a trend toward fewer fractures, and a trend toward improved overall survival.4,7,27,28 The uNTX results with QD dasatinib (51% of patients achieved a ≥40% decrease in uNTX at 12 weeks) was exactly the same with the previously reported BID dosing.17
Osteoblastic activity was also measured using the prognostic marker, BAP, whose levels have been correlated with the risk of SREs29 and interval to disease progression30 in patients with CaP. In the present analysis, 59% of patients demonstrated a reduction in BAP. This result also agrees with the previously published BID results showing 63% of patients with a reduction in BAP levels.
In a post hoc analysis of these 2 separate cohorts, the safety profile (any drug-related AEs) of the 100 mg QD dose was generally more tolerable than the pooled 70 and 100 mg BID doses (P = .06; Tables 2 and 3). By reducing dasatinib from BID to QD, the number of patients experiencing drug-related grade 3 or 4 AEs decreased from 32% to 13% (P = .03). The occurrence of pleural effusion decreased from 51% to 19% (P = .001) for the pooled 70 and 100 mg BID and 100 mg QD dosing, respectively. Although the differences between the QD and BID dosing are apparent in Table 2, the present study was not designed to compare safety across the different doses and the P values are for descriptive purposes only.
Table 2.
Safety summary for subjects receiving either 100 mg QD dasatinib or pooled 70 mg and 100 mg BID dasatinib doses
| Variable | QD (100 mg; n = 48) | Pooled BID (70 mg and 100 mg; n = 47) |
|---|---|---|
| All deaths | 2 (4.2) | 2 (4.3) |
| Subjects with ≥1 dose interruption (n) | 15 (31.3) | 30 (63.8) |
| Dose interruptions (n) | ||
| 1 | 12 (25.0) | 19 (40.4) |
| 2 | 2 (4.2) | 4 (8.5) |
| 3 | 0 | 4 (8.5) |
| >3 | 1 (2.1) | 3 (6.4) |
| Interval to first dose interruption for toxicity (d) | ||
| Median | 50 | 39 |
| Range | 4–172 | 2–127 |
| Drug-related serious AEs | 3 (6.3) | 6 (12.8) |
| Drug-related AEs leading to discontinuation | 6 (12.5) | 7 (14.9) |
| All AEs | 48 (100.0) | 47 (100.0) |
| Drug-related AEs | 43 (89.6) | 47 (100.0) |
| Drug-related grade 3 or 4 AEs | 6 (12.5) | 15 (31.9) |
QD, once daily; BID, twice daily; AEs, adverse events.
Data in parentheses are percentages.
Table 3.
Adverse events of special interest*
| Adverse Event | QD (100 mg; n = 48)
|
Pooled BID (70 mg and 100 mg; n = 47)
|
||
|---|---|---|---|---|
| Grade 3–4 (n)† | All Grades (n)† | Grade 3–4 (n) | All Grades (n) | |
| Diarrhea | 0 | 13 (27.1) | 2 (4.2) | 29 (61.7) |
| Pleural effusion | 1 (2.1) | 9 (18.8) | 1 (2.1) | 24 (51.1) |
| Nausea | 0 | 13 (27.1) | 1 (2.1) | 22 (46.8) |
| Anorexia | 0 | 10 (20.8) | 0 | 17 (36.2) |
| Fatigue | 0 | 21 (43.8) | 5 (10.6) | 21 (44.7) |
| Dyspnea | 1 (2.1) | 11 (22.9) | 4 (8.5) | 19 (40.4) |
| Rash | 0 | 8 (16.7) | 1 (2.1) | 19 (40.4) |
| Headache | 0 | 13 (27.1) | 0 | 18 (38.3) |
| Asthenia | 1 (2.1) | 9 (18.8) | 2 (4.2) | 13 (27.6) |
| Superficial edema | 0 | 5 (10.4) | 0 | 12 (25.5) |
| Pericardial effusion | 0 | 1 (2.1) | 0 | 11 (23.4) |
| Flushing | 0 | 3 (6.3) | 0 | 10 (21.3) |
| Musculoskeletal pain | 0 | 10 (20.8) | 0 | 9 (19.1) |
Abbreviations as in Table 2.
Data in parentheses are percentages.
AEs of special interest (drug-related AEs) as defined by medical monitor summarized by grouped preferred term (MedDRA version 11.1; AEs of special interest listed defined as AEs reported in ≥20% of population for either the QD or the BID doses.
No grade 4 or 5 AEs observed in ≥10% of subjects for 100 mg QD dose.
CONCLUSIONS
The final results from the present study have demonstrated the biologic activity and tolerability of dasatinib at a dose of 100 mg QD. The bone and potential anti-tumor effects of dasatinib support the clinical and pre-clinical results that dasatinib is a potent inhibitor of Src and other Src family kinases.12,14,15 Additionally, the 100 mg QD dose demonstrated activity with a more acceptable toxicity profile than the previously pooled BID doses. Future clinical trials with dasatinib evaluating survival, bone biomarkers, and SREs are proceeding with 100 mg QD dosing.
Acknowledgments
The present study was funded by Bristol-Myers Squibb.
To Heather Ward, an employee of Bristol-Myers Squibb, who provided professional medical writing assistance.
Footnotes
G. C. Trudel is an employee of, and owns stocks in, Bristol-Myers Squibb; and P. Paliwal is an employee of Bristol-Myers Squibb.
E. Y. Yu, M. A. Carducci, E. M. Posadas, and G. Wilding are members of the Department of Defense-Sponsored Prostate Cancer Clinical Trials Consortium.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5:21–28. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 3.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 4.Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 5.Fizazi K, Beuzeboc P, Lumbroso J, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2009;27:2429–2435. doi: 10.1200/JCO.2008.18.9811. [DOI] [PubMed] [Google Scholar]
- 6.Fizazi K, Bosserman L, Gao G, et al. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telo-peptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. J Urol. 2009;182:509–515. doi: 10.1016/j.juro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Lipton A, Cook R, Saad F, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 8.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 9.Saad F, Chen YM, Gleason DM, et al. Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clin Genitourin Cancer. 2007;5:390–396. doi: 10.3816/CGC.2007.n.022. [DOI] [PubMed] [Google Scholar]
- 10.Fizazi K, Carducci MA, Smith MR, et al. A randomized phase III trial of denosumab versus zoledronic acid in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2010;28(18 suppl):LBA4507. [Google Scholar]
- 11.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazine-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 12.Nam S, Kim D, Cheng JQ, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 13.Recchia I, Rucci N, Festuccia C, et al. Pyrrolopyrimidine c-Src inhibitors reduce growth, adhesion, motility and invasion of prostate cancer cells in vitro. Eur J Cancer. 2003;39:1927–1935. doi: 10.1016/s0959-8049(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 14.Park SI, Zhang J, Phillips KA, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 15.Koreckij T, Nguyen H, Brown LG, et al. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer. 2009;101:263–268. doi: 10.1038/sj.bjc.6605178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo FR, Barrett YC, Yang Z, et al. Identification and validation of phospho-SRC, a novel and potential pharmacodynamic biomarker for dasatinib (SPRYCEL), a multi-targeted kinase inhibitor. Cancer Chemother Pharmacol. 2008;62:1065–1074. doi: 10.1007/s00280-008-0699-5. [DOI] [PubMed] [Google Scholar]
- 17.Yu EY, Wilding G, Posadas E, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–7428. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 19.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute. [Accessed July 14, 2010];Common Terminology Criteria for Adverse Events version 3.0 (CTCAE) Available from: www.ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf8-9-2006.
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. for the European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.DePuy V, Anstrom KJ, Castel LD, et al. Effects of skeletal morbidities on longitudinal patient-reported outcomes and survival in patients with metastatic prostate cancer. Support Care Cancer. 2007;15:869–876. doi: 10.1007/s00520-006-0203-x. [DOI] [PubMed] [Google Scholar]
- 23.Lipton A, Demers L, Curley E, et al. Markers of bone resorption in patients treated with pamidronate. Eur J Cancer. 1998;34:2021–2026. doi: 10.1016/s0959-8049(98)00277-9. [DOI] [PubMed] [Google Scholar]
- 24.Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 25.Oefelein MG, Ricchiuti V, Conrad W, et al. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168:1005–1007. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 26.Rajpar S, Massard C, Laplanche A, et al. Urinary N-telopeptide (uNTx) is an independent prognostic factor for overall survival in patients with bone metastases from castration-resistant prostate cancer. Ann Oncol. 2010;21:1864–1869. doi: 10.1093/annonc/mdq037. [DOI] [PubMed] [Google Scholar]
- 27.Pectasides D, Nikolaou M, Farmakis D, et al. Clinical value of bone remodelling markers in patients with bone metastases treated with zoledronic acid. Anticancer Res. 2005;25:1457–1463. [PubMed] [Google Scholar]
- 28.Vinholes JJ, Purohit OP, Abbey ME, et al. Relationships between biochemical and symptomatic response in a double-blind randomised trial of pamidronate for metastatic bone disease. Ann Oncol. 1997;8:1243–1250. doi: 10.1023/a:1008238422151. [DOI] [PubMed] [Google Scholar]
- 29.Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong AJ, Creel P, Turnbull J, et al. A phase I-II study of docetaxel and atrasentan in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2008;14:6270–6276. doi: 10.1158/1078-0432.CCR-08-1085. [DOI] [PubMed] [Google Scholar]


