Abstract
Cardioembolic cerebral infarction (CI) is the most severe subtype of ischaemic stroke but some clinical aspects of this condition are still unclear. This article provides the reader with an overview and up-date of relevant aspects related to clinical features, specific cardiac disorders and prognosis of CI. CI accounts for 14−30% of ischemic strokes; patients with CI are prone to early and long-term stroke recurrence, although recurrences may be preventable by appropriate treatment during the acute phase and strict control at follow-up. Certain clinical features are suggestive of CI, including sudden onset to maximal deficit, decreased level of consciousness at onset, Wernicke’s aphasia or global aphasia without hemiparesis, a Valsalva manoeuvre at the time of stroke onset, and co-occurrence of cerebral and systemic emboli. Lacunar clinical presentations, a lacunar infarct and especially multiple lacunar infarcts, make cardioembolic origin unlikely. The most common disorders associated with a high risk of cardioembolism include atrial fibrillation, recent myocardial infarction, mechanical prosthetic valve, dilated myocardiopathy and mitral rheumatic stenosis. Patent foramen ovale and complex atheromatosis of the aortic arch are potentially emerging sources of cardioembolic infarction. Mitral annular calcification can be a marker of complex aortic atheroma in stroke patients of unkown etiology. Transthoracic and transesophageal echocardiogram can disclose structural heart diseases. Paroxysmal atrial dysrhyhtmia can be detected by Holter monitoring. Magnetic resonance imaging, transcranial Doppler, and electrophysiological studies are useful to document the source of cardioembolism. In-hospital mortality in cardioembolic stroke (27.3%, in our series) is the highest as compared with other subtypes of cerebral infarction. Secondary prevention with anticoagulants should be started immediately if possible in patients at high risk for recurrent cardioembolic stroke in which contraindications, such as falls, poor compliance, uncontrolled epilepsy or gastrointestinal bleeding are absent. Dabigatran has been shown to be non-inferior to warfarin in the prevention of stroke or systemic embolism. All significant structural defects, such as atrial septal defects, vegetations on valve or severe aortic disease should be treated. Aspirin is recommended in stroke patients with a patent foramen ovale and indications of closure should be individualized. CI is an important topic in the frontier between cardiology and vascular neurology, occurs frequently in daily practice, has a high impact for patients, and health care systems and merits an update review of current clinical issues, advances and controversies.
Keywords: Cardioembolic stroke, recurrent embolization, atrial fibrillation, cardiac source of emboli, outcome, oral anticoagulation, heart failure.
1. THE CLINICAL RELEVANCE OF CARDIOEMBOLIC CEREBRAL INFARCTION
Stroke is the leading cause of disability and the second most common cause of death worldwide [1-3]. Accurate definition of the mechanism of stroke is crucial as this will guide the most effective care and therapy. Cardioembolic cerebral infarction accounts approximately for one quarter of all cerebral infarcts [4-8]. In most cases, recurrence of cardioembolism can be prevented by oral anticoagulants. Therefore, for a patient with a cerebral infarct, early confirmation of a diagnosis of cardioembolic cerebral infarction is extremely important in order to initiate anticoagulation therapy for an adequate secondary prevention [9-13].
In the Sagrat Cor Hospital of Barcelona Stroke Registry, the frequency of cardioembolic stroke is 18% [14], a similar percentage than that in the studies of Bougousslavsky et al. [15] (16%) and Timsit et al. [16] (19.4%), higher than that reported by Vázquez et al. [17] (14%) and de Al-Rajed et al. [18] (14%), but lower than the percentages of Rothrock et al. [19] (22%) and Norrving and Löwenhielm [20] (30.6%). However, the incidence of cardioembolic cerebral infarction increases with age [14]. In the subgroup of patients younger than 65 years of age, cardioembolic cerebral infarction occurred in 14.6% of cases but in very old patients (age ≥ 85 years) cardioembolic stroke reached 36% of cases and is the most frequent ischaemic subtype (Table 1).
Table 1.
Distribution of Cerebral Infarctions According to Age in the Sagrat Cor Hospital of Barcelona Stroke Registry
| Subtype of cerebral infarction (n = 1840) | Years of age | |||
|---|---|---|---|---|
| < 65 (n= 314) | 65–74 (n=501) | 75–84 (n=722) | ≥ 85 (n=303) | |
| Cardioembolic | 46 (14.6) | 100 (20) | 213 (29.5) | 109 (36) |
| Atherothrombotic | 66 (21.0) | 159 (31.7) | 233 (32.3) | 95 (31.4) |
| Lacunar | 93 (29.6) | 159 (31.7) | 173 (24) | 59 (19.5) |
| Unknown cause | 61 (19.4) | 69 (13.8) | 81 (11.2) | 37 (12.2) |
| Unusual cause | 48 (15.3) | 14 (2.8) | 22 (3.0) | 3 (1) |
Percentages in parenthesis.
Embolism from the heart to the brain results from one of three mechanisms: blood stasis and thrombus formation in an enlarged (or affected by another structure alteration) left cardiac chamber (e.g., left ventricular aneurysm); release of material from an abnormal valvular surface (e.g., calcific degeneration); and abnormal passage from the venous to the arterial circulation (paradoxical embolism) [3]. Cardiac emboli can be of any size, but those of arising from the cardiac chambers are often large and hence especially likely to cause severe stroke, disability and death. Cardioembolic cerebral infarction is the most severe ischaemic stroke subtype, with high in-hospital mortality rate (6–27%) and a substantial number of patients with neurological dysfunction at the time of hospital discharge; however, the risk of early embolic recurrence varies between 1 and 10% [3,6,21,22] (Fig. 1).
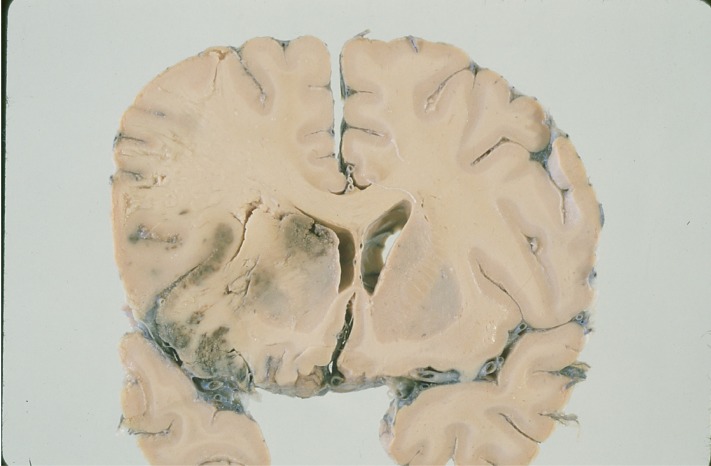
Fig. (1).
Histopathological specimen showing a hemorrhagic cerebral infarction of a cardioembolic origin with signs of ventricular displacement and brain herniation in the territory of the middle cerebral artery.
There is no gold standard for making the diagnosis of cardioembolic cerebral infarction. Neuroimaging findings that support cardioembolic stroke include simultaneous or sequential strokes in different arterial territories. Owing to their large size, cardiac emboli flow to the intracranial vessels in most cases and cause massive, superficial, single large striatocapsular or multiple infarcts in the middle cerebral artery. Therefore, cardioembolic cerebral infarctions predominate in the distribution territories of the carotid and the middle cerebral artery. Bilateral or multilevel posterior infarcts are suggestive of cardioembolism [23]. The presence of a potential major cardiac source of embolism in the absence of significant arterial disease remains the mainstay of clinical diagnosis of cardioembolic cerebral infarction [22]. When cardiac and arterial disease coexist (such as atrial fibrillation and ipsilateral carotid atheroma), determining the etiology of the ischemic stroke becomes more difficult. However, in many patients, history, physical examination, and routine diagnostic tests (electrocardiogram and findings on neuroimaging studies) are sufficient to easily make the diagnosis of most presumed cardiac emboligenic condition (e.g., atrial fibrillation, recent myocardial infarction, heart failure, prior rheumatic disease, splinter hemorrhages) [23]. An important exception is paroxysmal atrial fibrillation, which can be detected by 24−48 hour Holter monitoring immediately after stroke. However, 24-hour Holter may not be sufficient for diagnosing paroxysmal atrial fibrillation and there is evidence supporting the value of prolonged cardiac monitoring. In a recent study of Gaillard et al. [24], transtelephonic ECG monitoring increased detection rate of paroxysmal atrial fibrillation in stroke and TIA patients whose 24-hour Holter monitoring was unrevealing, especially if they had frequent premature atrial ectopic beats, recent anterior circulation infarct on MRI, or both. The implantation of subcutaneous devices for up to 14 months of rhythm monitoring increases the detection of paroxysmal atrial fibrillation [25]. Prolonged cardiac rhythm monitoring increases the detection of paroxysmal atrial fibrillation.
Transthoracic echocardiogram can disclose structural cardiopathies (dilated cardiomyopathies, mitral stenosis and other structural ventricular diseases and intraventricular thrombus, vegetations or tumors) and enables measurement of the left atrial size and left ventricular systolic function [1,2,23]. Transesophageal echocardiogram is able to study the aortic arch and ascending aorta, left atrium and left atrial appendages, intra-arterial septum, pulmonary veins and valve vegetations [1-3,23]. Transesophageal echocardiography is more likely to be helpful in young patients with stroke, stroke of unknown cause and in patients with non-lacunar stroke. Although second harmonic imaging has increased sensitivity of transthoracic echocardiography, contrast transesophageal echocardiography remains the standard echocardiographic technique, particularly in young patients with cryptogenic stroke. Transcranial Doppler (TCD) allows a first-line non-invasive diagnosis of right-to-left shunt caused by a patent foramen ovale by detecting bubble signs in the middle cerebral artery after the injection of agitated saline in the antecubital vein. The most important limitation of contrast TCD is the absence of a temporal bone window in 10% of patients who suffer stroke, a fact which particularly affects the older population. However, TCD does not distinguish intracardiac shunts from extracardiac shunts [22,26].
Cardiac magnetic resonance imaging (MRI) and nuclear cardiology studies (assessment of myocardial perfusion and analysis of ventricular function) may be useful in selected patients [22,23].
2. CHARACTERISTIC CLINICAL FEATURES OF CARDIOEMBOLIC CEREBRAL INFARCTION
There are no absolute criteria for the diagnosis of cardioembolic cerebral infarction, although the following is required: 1) compatible clinical picture, 2) recognition of an emboligenic heart disease and 3) exclusion of carotid and/or cerebral atherosclerosis or other cause for the stroke [1,22].
Clinical features that support the diagnosis of cardioembolic stroke includes sudden onset to maximal deficit (< 5 min), which is present in 47−74% of cases and decreased level of consciousness at onset in 19−31% of cases [27,28]. In the study of Timsit et al. [29], altered consciousness was a predictive factor of cardioembolic cerebral infarction, with an odds ratio (OR) of 3.2 as compared with atherothrombotic infarction. Sudden onset of neurological deficit occurs in 79.7% of cases of cardioembolic cerebral infarction and in 38% of lacunar infarcts and in 46% of thrombotic infarctions (P < 0.01).
In 4.7−12% of cases, cardioembolic cerebral infarction show a rapid regression of symptoms (the spectacular shrinking deficit syndrome) [30−33]. The recognition of this syndrome is important for a clinical suspicion of the cardioembolic origin of the cerebral infarction [33]. This dramatic improvement of an initially severe neurological deficit may be due to distal migration of the embolus followed by recanalization of the occluded vessel [34,35].
Wernicke’s aphasia or global aphasia without hemiparesis are other common secondary symptoms of cardioembolism [22,34,35]. In the posterior circulation, cardioembolism can produce Wallenberg’s syndrome, cerebellar infarcts, top-of-the basilar syndrome, multilevel infarcts, or posterior-cerebral-artery infarcts. Visual-field abnormalities, neglect, and aphasia are also more common in cardioembolic than in non-cardioembolic stroke [36, 37].
A classic cardioembolic presentation include onset of symptoms after a Valsalva-provoking activity (coughing, bending, etc.) suggesting paradoxical embolism facilitated by a transient rise in right atrial pressure and the co-occurrence of cerebral and systemic emboli [1,36].
On the other hand, other clinical symptoms classically associated with cardioembolic cerebral infarction, such as headache, seizures at onset [30] and onset during activity are not specific for cardioembolic stroke [5,34]. In addition, some signs or syndromes, such as lacunar clinical presentations (e.g., pure motor hemiparesis or ataxic hemiparesis), a lacunar infarct and particularly, multiple lacunar infarcts, make cardioembolic origin unlikely [38]. Cardiac embolism is a very rare cause of lacunar infarction (2.6−5% of cases) [39,40] (Fig. 2).
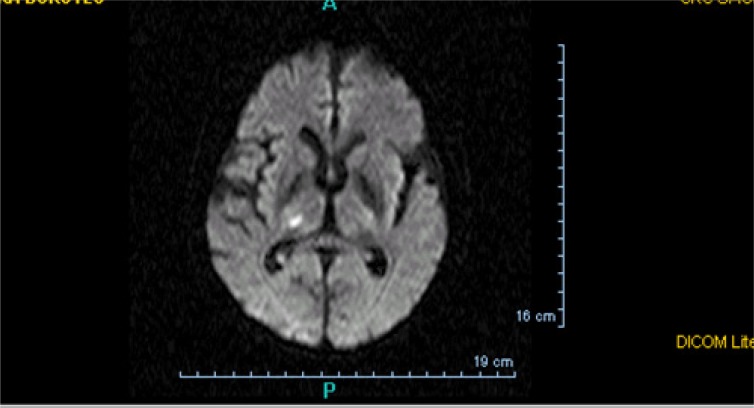
Fig. (2).
Lacunar infarct on brain MRI (diffusion-weighted sequences). Lacunar infarct makes cardioembolic origin unlikely.
Hemorrhagic transformation of an ischemic infarct and early recanalization of an occluded intracranial vessel are suggestive of a cardiac origin of the stroke [1-4]. Hemorrhagic transformation occurs in up to 71% of cardioembolic strokes (Fig. 3). As many as 95% of hemorrhagic infarcts are caused by cardioembolism. There are two types of hemorrhagic transformation: petechial or multifocal, which is normally asymptomatic and secondary hematoma, which has mass effects and clinical deterioration [41,42]. Secondary hematomas are unusual and are found in 0.8% of cases in our stroke registry [14]. A common nomenclature divides haemorrhages into HI1, HI2, PH1, PH2 and remote PH. HI has been defined as a petechial infarction without space-occupying effect and PH was defined as a haemorrhage (coagulum) with mass effect. HIs are of two subtypes: HI1 (small petechiae) and HI2 (more confluent petechiae). Similarly, there are three subtypes of PH: PH1 (≤ 30% of the infarcted area with some mild space-occupying effect), PH2 (>30% of the infarcted area with significant space-occupying effect) and remote PH (clot remote from infarcted area) The traditional explanation for hemorrhagic transformation is that the infarct is caused by blockage of a large artery by the thrombus; this blockage then causes local vascular spasm [1,4]. Release of this local spasm and fragmentation of the thrombus allow the thrombus to migrate distally, exposing ischemic tissues and damaged vessel walls and capillaries to reperfusion. Arterial dissection at the site of impact of the thrombus is an alternative explanation.
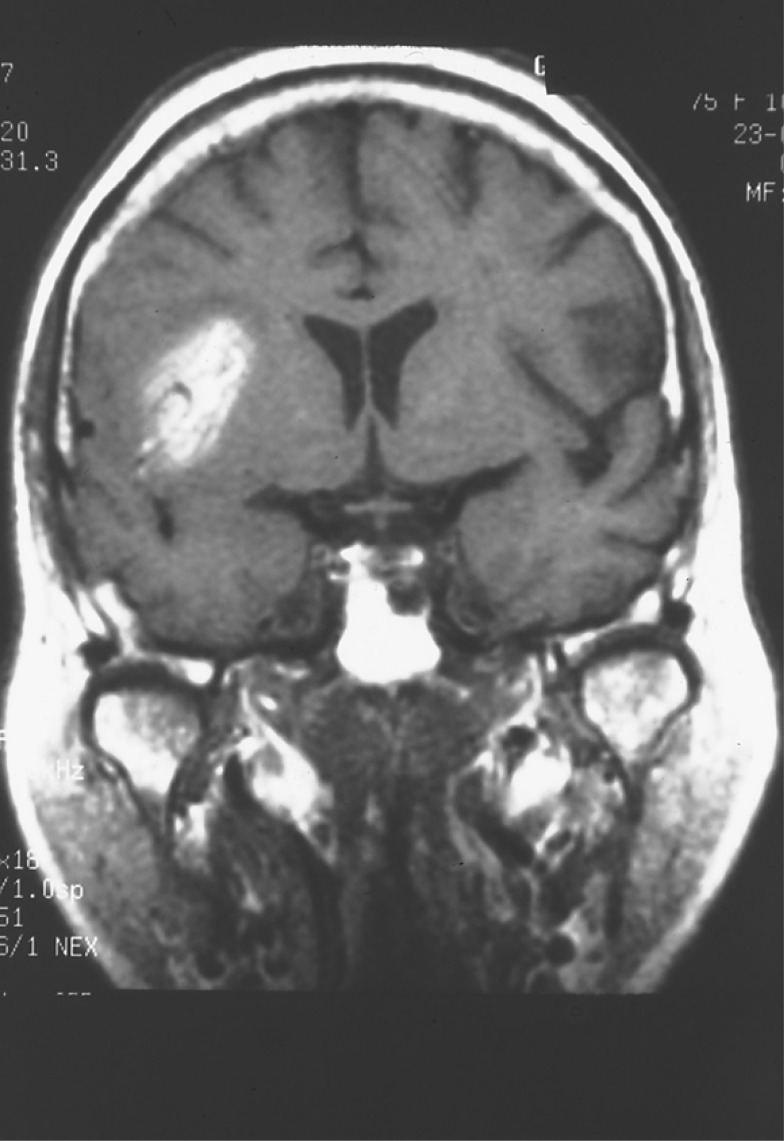
Fig. (3).
Hemorrhagic cardioembolic infarction in a patient with a spectacular shrinking deficit syndrome visualized in the brain MRI study (spin-echo hyperintensity T1-weighted image).
Decreased alertness, total circulation infarcts, severe strokes (NIHSS >14), proximal middle cerebral artery occlusion, hypodensity in more than one third of the middle cerebral artery territory and delayed recanalization (> 6 hours after stroke onset) together with absence of collateral flow predict hemorrhagic transformation in acute cardioembolic cerebral infarction [2,5].
3. CARDIAC DISEASES CAUSING CARDIOEMBOLIC CEREBRAL INFARCTION
A number of cardiac conditions have been proposed as potential sources of embolism. The risk of embolism is heterogeneous. The more common high risk cardioembolic conditions are atrial fibrillation, recent myocardial infarction, mechanical prosthetic valve, dilated myocardiopathy, and mitral rheumatic stenosis. Other major sources of cardioembolism include infective endocarditis, marantic endocarditis, and atrial myxoma. Minor sources of cardioembolism are patent foramen ovale, atrial septal aneurysm, atrial or ventricular septal defects, calcific aortic stenosis, and mitral annular calcification [1,6].
Atrial fibrillation is the most important cause of cardioembolic cerebral infarction [22,27,28]. Atrial fibrillation is the commonest sustained cardiac arrhythmia. Prevalence of atrial fibrillation increases with age, reaching a peak of 5% in people over 65 years of age, and both its incidence and prevalence are increasing. The disorder is associated with valvular heart disease, thyroid disorders, hypertension, and recent heavy drinking of alcohol. In Western populations, most causes of atrial fibrillation are unrelated to mitral valve disease. Instead, atrial fibrillation is now mainly secondary to ischemic or hypertensive heart disease. The attributable risk of stroke due to atrial fibrillation rises from 1.5% at the age of 50 to 24% at the age of 80. The incidence of stroke in people with non-valvular atrial fibrillation is estimated to be 2 to 7 times higher than in people without atrial fibrillation and for those with valvular atrial fibrillation, the risk is 17 times higher than that in age-matched controls. Chronic and recurrent atrial fibrillation appears to carry very similar stroke risk. Atrial fibrillation in the absence of organic heart disease or risk factors (lone atrial fibrillation) appears to carry significantly lower risk especially in younger patients (approximately 1.3% per year). Atrial fibrillation causes stroke because it leads to inadequate contraction of, and leads to stasis that is most marked in the left atrial appendage. Stasis is associated with increased concentrations of fibrinogen, D-dimer, and von Willebrand factor, which are indicative of a prothrombotic state, which in turn predisposes to thrombus formation with consequent increased rate of cerebral embolization [2]. In these patients, left ventricular dysfunction and left atrial size were independent echocardiographic predictors of later thromboembolism. Other factors associated with a particular high embolic risk are spontaneous echo contrast, left atrial thrombus or aortic plaque detected by transesophageal echocardiogram. Heart failure, hypertension, age > 75 years, and diabetes mellitus increase the risk of stroke in a more moderate but additive fashion [4].
The bradycardia-tachycardia (sick sinus) syndrome can be associated with cerebral embolic events.
Approximately 2.5% of patients with acute myocardial infarction experience a stroke within 2 to 4 weeks of the infarction, and 8% of men and 11% of women will have an ischemic stroke within the next 6 years. Factors that enhance the risk of stroke include severe left ventricular dysfunction with low cardiac output, left ventricular aneurysm (Fig. 4) or thrombus, and associated arrhythmias such as atrial fibrillation. Patients with an ejection fraction of less than 28% had a relative risk of stroke of 1.86 compared with patients with an ejection fraction greater than 35%. The incidence of early embolism is high, possibly up to 22% in the presence of a mural thrombus and is most likely when the thrombus is mobile or protrudes into the ventricle [6,7].
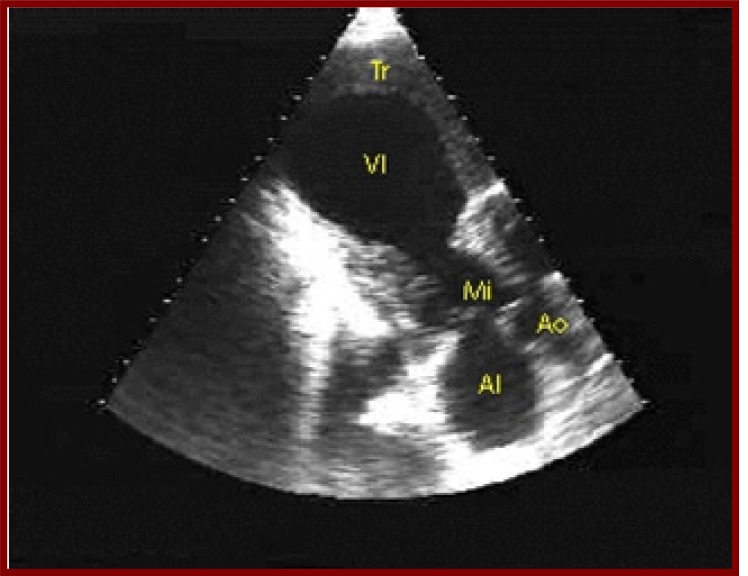
Fig. (4).
Transthoracic echocardiography shows a left ventricular aneurysm (VI) in a patient with history of acute myocardial infarction.
The annual rate of stroke in patients with congestive heart failure is 2%. The risk of stroke correlates with the severity of left ventricular dysfunction. Coexistent disease has a cumulative effect, and the combination of recent congestive heart failure and atrial fibrillation places the patient at particular high risk for cardioembolic stroke [7,43].
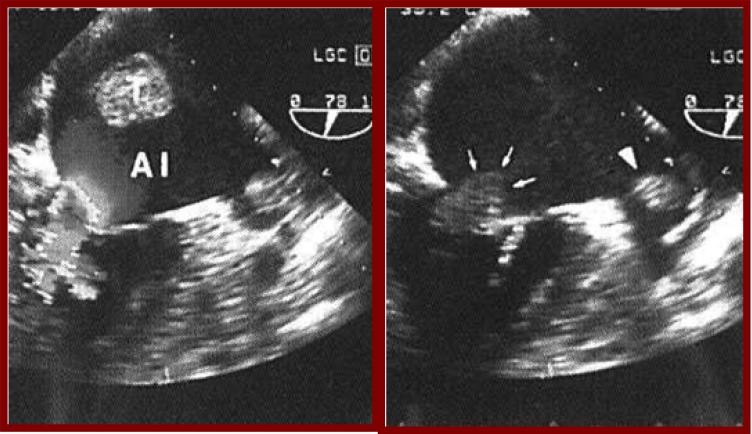
Rheumatic valvular heart disease (Fig. 5) and mechanical prosthetic valves are well recognized risk factors for stroke even in the absence of documented atrial fibrillation. The two most commonly cited rheumatic valve abnormalities are mitral stenosis and calcific aortic stenosis [1,3].
Fig. (5).
Transthoracic echocardiography reveals a thrombus in the left atrium (T) in a patient with double rheumatic mitral valve lesion and atrial fibrillation.
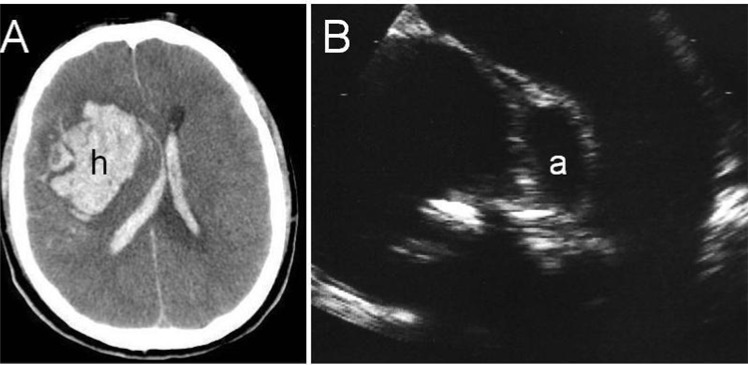
Two types of endocarditis, infective and non-infective, can cause stroke. Non-infective endocarditis can complicate systemic cancer, lupus, and the anti-phospholipid syndrome. Infective endocarditis is complicated by stroke in about 10% of cases. Most stroke happens early (before or during the first 2 weeks of appropriate antimicrobial therapy). Emboli can be multiple especially in the case of infection of prosthetic valves and in infections due to aggressive agents, such as Staphylococcus aureus. Mycotic aneurysm is an uncommon (1−5%) complication of infective endocarditis. They may also enlarge and rupture, which is fatal in many cases (Fig. 6) [44,45].
Fig. (6).
Right lobar hemorrhage (A) secondary to rupture of a mycotic aneurysm in the course of an infective bacterial endocarditis; transthoracic echocardiography (B) shows an abscess in the posterolateral aortic root (a) between the aortic valve leaflets and the mitral valve.
Myxomas account for more than half of primary cardiac tumors and thromboembolism is the most common presenting symptom in patients with myxomas. Other primary cardiac tumors include papillary fibroelastoma [22].
Patent foramen ovale, aortic arch atheroma and mitral annular calcification are emerging cardioembolic sources [26,46].
A patent foramen ovale is present in approximately 25% of the general population, and can be found in up to 40% of younger patients with otherwise cryptogenic stroke [47]. There is a higher risk of stroke with patent foramen ovale, especially when combined with atrial septal aneurysm. In a meta-analysis of case control studies that examined the relative frequency of patent foramen ovale, atrial septal aneurysm, or both, in all patients with ischaemic stroke, cryptogenic stroke and known stroke cause, patent foramen ovale and atrial septal aneurysm were significantly associated with ischaemic stroke in patients younger than 55 years. It was concluded that further studies are needed to establish whether an association exists between patent foramen ovale and ischemic stroke in those older than 55 [48]. There is insufficient evidence to recommend warfarin routinely in patients with cryptogenic stroke and patent foramen ovale. There was no difference of stroke recurrence in cryptogenic stroke between patients with and without massive right-left shunt [49]. The American Heart Association, the American Stroke Association, the American Academy of Neurology [50,51] and the European Stroke Organization [52] recommend antiplatelet agents to prevent recurrent events whilst waiting for the results of ongoing clinical trials regarding closure of patent foramen ovale. In clinical practice, aspirin is the recommended treatment in stroke patients with a patent foramen ovale and indications of closure should be individualized and particularly considered only in young patients with recurrent stroke receiving medical treatment or when anticoagulant treatment is being considered.
Regarding complex aortic arch atheromatosis, in a review of 500 necropsies of patients with neurological diseases, ulcerated aortic plaques were documented in 62 (26%) of 239 patients in whom stroke was the cause of death and only in 13 (5%) of 261 patients who died as a result of other neurological conditions. Likewise, ulcerated aortic plaques were observed in 17 (61%) of 28 patients with cerebral infarction of unknown aetiology as compared with 34 (22%) of 155 patients in whom a cerebral infarction-attributable aetiology was found [53].
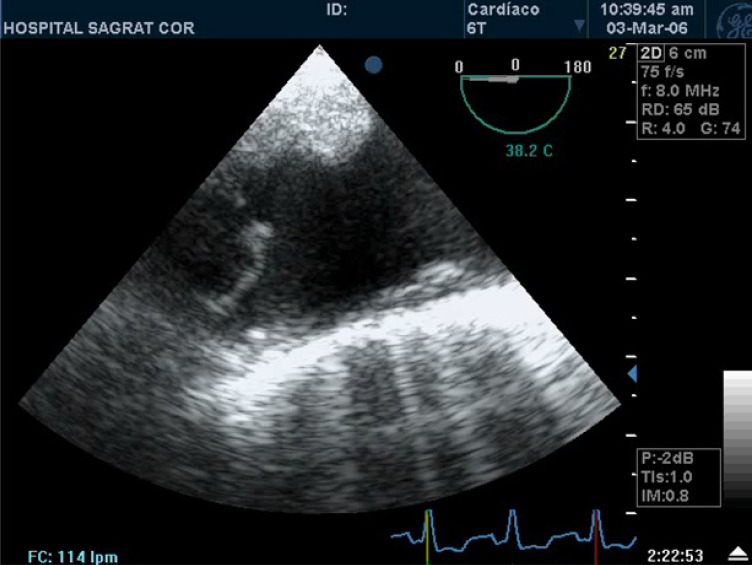
The main emboligenic risk criteria for atheromatous plaques of the aortic arch include plaque thickness ≥ 4 mm and the presence of mobile components [54] (Fig. 7).
Fig. (7).
Complex atheromatosis of the aortic arch on echocardiogram. Main criteria of embolic risk associated with aortic atheromatosis are plaque thickness ≥ 4 mm and the presence of mobile components.
It has been recently shown that complex atheromatous aortic plaques play a causative role in the recurrence of ischaemic stroke in the subgroup of cerebral infarctions of undetermined aetiology [55,56]. The efficacy of anticoagulation versus antiplatelet therapy in the prevention of stroke recurrence in patients with atherothrombosis of the aortic arch and a recent (< 6 months) cerebral or peripheral embolic event is the objective of the ongoing ARCH (Aortic Arch Related Cerebral Hazard Trial) study from France.
The protective effect of statin theraphy on the incidence of stroke and other embolic events in patients with severe thoracic aortic plaque was reported in a matched-paired analysis [57].
Mitral annular calcification is a chronic degenerative process characterized by calcium and lipid deposition in the fibrous support of the mitral valve. Mitral annular calcification has been cited as a possible source of cerebral embolism with a relative risk of stroke of 2.1 in the Framingham Study independent of traditional risk factors for stroke [58]. In a recent study in patients with ischaemic stroke of uncertain etiology, dense mitral annular calcification was an important marker of aortic arch atherosclerosis with high risk of embolism [46].
Spontaneous echo contrast is an independent echocardiographic risk factor for left atrial thrombus and its appendage and cardiac thromboembolic events.
Cardiological substrate and pathophysiological mechanisms presumptively involved in cardioembolic stroke in the Sagrat Cor Hospital of Barcelona Stroke Registry [59] are shown in (Table 2). Atrial dysrhythmia without structural cardiac disease was documented in 89 (22%) patients, with a mean (SD) age of 75 (4) years (range 63–90 years). All these patients had normal electrocardiographic findings and 90% were asymptomatic. The cardiac condition associated with cardiogenic stroke was atrial fibrillation in 88 patients (chronic 67, paroxysmal 18, persistent 3) and atrial flutter in 1. A previous diagnosis of atrial dysrhythmia had been established in the outpatient setting in 51% of patients but none of the patients received anticoagulation.
Table 2.
Cardiac Disorders and Pathophysiological Mechanisms Presumptively Associated with Cardioembolic Stroke in 402 Patients. Distribution by Cardiac Source Risk Groups. Sagrat Cor Hospital of Barcelona Stroke Registry
| Cardiac source of embolism | Total patients | |
|---|---|---|
| Arrhythmia without structural heart disease | 89 (22.1%) | |
| Atrial fibrillation | 88 | |
| Atrial flutter | 1 | |
| Isolated structural heart disease | 81 (20.1%) | |
| Ischaemic heart disease | 35 | |
| Acute myocardial infarction | 3 (thrombus 2) | |
| Left ventricular aneurysm | 7 (thrombus 3) | |
| Left ventricular ejection fraction < 40% | 12 | |
| Akinesia/dyskinesia ≥ two segments | 13 (thrombus 3) | |
| Dilated cardiomyopathy | 24 (thrombus 5) | |
| Mitral annular calcification | 14* | |
| Cardiac tumour | 4 | |
| Aortic prosthetic valve | 4 | |
| Endocarditis | 2 | |
| Atrial septal aneurysm with patent foramen ovale | 2 | |
| Rheumatic mitral valve disease | 1 | |
| Mitral valve prolapse | 1 | |
| Calcified aortic stenosis | 1 | |
| Moderate mitral valve regurgitation | 1 | |
| Structural heart disease and atrial arrhythmia | 232 (57.7%) | |
| Atrial fibrillation | 230 | |
| Atrial flutter | 2 | |
| Hypertrophic hypertensive cardiac disease | 120 | |
| Rheumatic mitral valve disease | 49 (thrombus 7) | |
| Ischaemic heart disease | 19 | |
| Left ventricular aneurysm | 3 (thrombus 1) | |
| Left ventricular ejection fraction < 40% | 9 | |
| Akinesia/dyskinesia two segments | 7 (thrombus 1) | |
| Mitral annular calcification | 26† | |
| Dilated cardiomyopathy | 13 (thrombus 2) | |
| Mitral valve prolapse | 4 | |
| Mitral prosthetic valve | 3 (thrombus 2) | |
| Lipomatous hypertrophy of the atrial septum | 2 | |
| Hypertrophic cardiomyopathy | 2 | |
| Atrial septal aneurysm and patent foramen ovale | 2 | |
| Severe mitral regurgitation | 2 | |
In 8 patients in association with a structural cardiac source of embolism (dilated cardiomyopathy, n=2; ischaemic heart disease with ventricular ejection fraction < 40%, n=2; acute myocardial infarction, n=1; left ventricular aneurysm, n =1; aortic prosthetic valve, n=1; mitral leaflet calcification with moderate regurgitation, n=1).
In 10 patients in association with a structural cardiac source of embolism (hypertensive left ventricular hypertrophy, n=8; mitral leaflet calcification with severe degenerative type regurgitation, n=2).
Structural cardiac disease with sustained sinus rhythm was diagnosed in 81 (20%) of patients. Left ventricular systolic dysfunction was documented in 59 patients (ischemic heart disease in 35 and dilated cardiomyopathy in 24) associated with intraventricular thrombosis in 13. Other less frequent cardiac disorders included mitral annular calcification, cardiac tumors, aortic prosthetic valve, endocarditis, atrial septal aneurysm with patent foramen ovale, rheumatic mitral valve disease, mitral valve prolapse, calcified aortic stenosis with embolism during catheterization, and moderate mitral valve regurgitation [59].
In the remaining 232 (58%) patients, structural cardiac disorders were associated with atrial fibrillation in 230 cases and atrial flutter in 2. Hypertensive left ventricular hypertrophy was documented in 120 cases followed by rheumatic mitral valve disease in 49 cases and left ventricular dysfunction in 32 cases (ischemic heart disease in 19 and dilated cardiomyopathy in 13). Other less frequent cardiac disorders complicated with atrial fibrillation included mitral valve prolapse, mitral prosthesis, hypertrophic cardiomyopathy, lipomatous hypertrophy of the atrial septum, severe mitral regurgitation, and atrial septal aneurysm with patent foramen ovale [59].
The frequency of the different cardiac disorders in the overall series of 402 patients with cardioembolic stroke is shown in (Table 3). Atrial fibrillation was documented in 79.1% of patients (in association with structural cardiac disease in 72% of cases) followed by hypertensive left ventricular hypertrophy in 29.8% of patients, left ventricular dysfunction in 22.6%, rheumatic mitral valve disease in 12.4%, and mitral annular calcification in 9.9%. Mitral valve prolapse, atrial septal aneurysm with patent foramen ovale and degenerative heart valve disease were observed in only 1% of the patients. In the group of 118 patients with hypertensive left ventricular hypertrophy associated with atrial fibrillation, anteroposterior diameter of the left atrium was significantly larger than in the group of 88 patients with lone atrial fibrillation (45 ± 3 mm vs. 41 ± 3 mm, P < 0.001). On the other hand, 80.6% of these patients were asymptomatic, 50.5% had other vascular risk factor (cigarette smoking, diabetes mellitus, hyperlipidemia) besides hypertensive disease, and although a previous diagnosis of atrial dysrhythmia had been established in the outpatient setting in 43.7% of patients, none of the patients received anticoagulation at the time of stroke onset [59].
Table 3.
Frequency of the Different Cardiological Substrate in 402 Patients with Cardioembolic Stroke in the Sagrat Cor Hospital of Barcelona Stroke Registry
| Cardiac source of embolism | Total patients | |
|---|---|---|
| Atrial fibrillation | 318 (79.1%) | |
| Lone atrial fibrillation | 88 | |
| Associated with structural cardiac disease | 230 | |
| Hypertensive left ventricular hypertrophy | 120 (29.8%) | |
| Associated with atrial fibrillation | 118 | |
| Associated with atrial flutter | 2 | |
| Left ventricular systolic dysfunction | 91 (22.6%) | |
| Sinus rhythm | 59 | |
| Atrial fibrillation | 32 | |
| Rheumatic mitral valve disease | 50 (12.4%) | |
| Mitral annular calcification | 40 (9.9%) | |
| Mitral valve prolapse | 5 (1.2%) | |
| Atrial septal aneurysm with patent foramen ovale | 4 (1%) | |
| Degenerative heart valve disease | 4 (1%) | |
4. CARDIOEMBOLIC CEREBRAL INFARCTION-RELATED MORTALITY
Cardioembolic cerebral infarction are the subtype of ischemic infarcts with the highest in-hospital mortality during the acute phase of stroke [60−62]. In our experience and in agreement with the clinical series of Caplan et al. [60], the in-hospital mortality rate of cardioembolic cerebral infarction was 27.3% as compared with 0.8% for lacunar infarcts and 21.7% for atherothrombotic stroke (P < 0.01). Cardioembolic infarction is also associated with a lower rate of absence of functional limitation at discharge from the hospital, which may be related to the greater size of the lesion of cardioembolic stroke [15,28].
In a recent study carried out by our group in 231 patients with cardioembolic cerebral infarction with an in-hospital mortality rate of 27.3%, causes of death were as follows: a) non-neurological in 54% (n = 34), including pneumonia in 9, heart disease in 7, pulmonary thromboembolism in 7, sepsis in 5, sudden death in 4, and other causes in 2; b) neurological in 39.5% (n = 25), including brain herniation in 17, recurrence of cerebral ischemia in 6, and cerebral hemorrhage in 2; and of unknown cause in 6.5% (n = 4) [63].
Early recurrent embolisms (within the first 7 days of stroke onset) were observed in 9 patients (3.9%) (peripheral embolisms in the extremities in 4, cerebral in 5). Only one patient was receiving therapeutic anticoagulation.
Mortality in patients with early embolic recurrence was 77.7% (7 of 9 cases) as compared with 25% for the remaining patients (P < 0.001). In the 5 patients with recurrent cerebral embolisms, the mortality rate was 100%. Two of the four patients with peripheral embolism died (mortality rate 50%) [63]. (Table 4) shows the relationship between cardiovascular risk factors and in-hospital mortality in patients with cardioembolic cerebral infarction. In another clinical study four clinical variables were significantly associated with in-hospital mortality: age, congestive heart failure, hemiparesis, and decreased level of consciousness. However, when early recurrent embolism was added to the logistic regression model, this variable was associated with the highest risk for death (OR = 33.5).
Table 4.
Predictive value of cardiovascular risk factors for in-hospital death in all brain infarctions and in cardioembolic stroke in the Sagrat Cor Hospital of Barcelona Stroke Registry
| Stroke subtype | Odds ratio (95% confidence interval) | Pvalue |
|---|---|---|
| All brain infarctions | ||
| Atrial fibrillation | 2.33 (1.84 to 2.96) | 0.000 |
| Heart failure β) | 1.96 (1.33 to 2.89) | 0.001 |
| COPD | 1.56 (1.01 to 1.89) | 0.044 |
| Previous cerebral infarction | 1.43 (1.07 to 1.89) | 0.014 |
| Age | 1.05 (1.03 to 1.06) | 0.000 |
| Hyperlipidemia | 0.58 (0.39 to 0.85) | 0.006 |
| Cardioembolic infarction | ||
| Peripheral arterial disease | 2.18 (1.17 to 4.05) | 0.014 |
| Previous cerebral infarction | 1.75 (1.16 to 2.63) | 0.007 |
| Heart failure | 1.71 (1.01 to 2.90) | 0.047 |
| Age | 1.06 (1.04 to 1.08) | 0.000 |
COPD: chronic obstructive pulmonary disease.
Early and late embolic recurrences are not exceptional in cardioembolic cerebral infarction [61,63−66]. Recurrences are more frequent during the first days of stroke [11]. In the study of Sacco et al. [67], in which recurrences within the first 30 days were assessed, mortality was also significantly higher in the group of recurrences (20%) than in the group without recurrences (7.4%); survivors after stroke recurrence also showed a longer hospital stay. In the study of Yasaka et al. [68], mortality was also significantly higher in patients with recurrent embolism (19.6%) as compared with the remaining patients (8.8%).
Taking into account that in our series, only one patient with recurrent embolism was treated with therapeutic anticoagulation, we agree with Chamorro et al. [9,44] in the need of starting early prophylactic anticoagulation with sodium heparin in patients with cardioembolic infarction, with strict control of partial thromboplastin time (between 1.5 and 2) in order to prevent iatrogenic bleeding due to excessive anticoagulation.
Early neurological deterioration (END) is present in 8.3% of cardioembolic stroke patients. Cardioembolic stroke patients with END in comparison with patients without END showed a worse early prognosis with statistically significant differences in absence of neurological deficit at hospital discharge (5% vs. 17.3%), length of hospitalization (30.8 vs. 18.5 days) and in-hospital mortality (47.5% vs. 8.4%). In the multivariate analysis, early seizures, severe headache and hypertension were independent clinical predictors of END. Cardioembolic stroke with END constitutes a subgroup of patients with severe prognosis [69].
5. RECURRENT STROKE AFTER A CARDIOEMBOLIC CEREBRAL INFARCTION
The risk of early stroke recurrence in cerebral infarctions in general ranges between 1% to 10% according to the different series [64,67]. Some studies have shown that recurrences within the first 3 months are more common in cardioembolic infarction than in atherothrombotic infarcts. The risk of early embolic recurrence in cardioembolic cerebral infarction varies between 1% and 22%. In the Cerebral Embolism Task Force, for example, it was estimated that around 12% of patients with cardioembolic infarctions would develop a second embolism within the first 2 weeks of the onset of symptoms [12]. In our experience, embolism recurrence during hospitalization occurred in 24 of 324 patients with cardioembolic stroke consecutively attended over a 10-year period (6.9% of cases) [70]. Embolic recurrence occurred within the first 7 days of neurological deficit in 12 patients (50%). The mean time of recurrence after stroke onset was 12 days. Recurrence of embolism within the first 30 days was observed in 5 of the 81 patients (6.1%) in the study of Yamanouchi et al. [71] in patients with cardioembolic cerebral infarction and non-valvular atrial fibrillation, in 6% of cerebral infarcts in the study of Sacco et al. [72], in 3.3% of patients from the Stroke Data Bank [67], and in 4.4% of patients included in the Lausanne Stroke Registry [73].
In our study, embolism recurrence was multiple in 3 cases (12.5%), which is consistent with data in the study of Yamanouchi et al. [71] in which 7 of 21 patients with cardioembolic infarctions had two or more stroke recurrences. The maximal risk of recurrence was the immediate period after the cardioembolic cerebral infarction.
Mortality in patients with recurrent embolism was two-fold higher as compared with the remaining patients (70.8% vs 24.4%) [70], in agreement with the study of Sacco et al. [72] (19% vs 8%) in cerebral infarctions in general.
It is important to know factors associated with early embolic recurrence in cardioembolic cerebral infarction because patients in which these risk factors are present constitute a subgroup with the highest risk severity, requiring early treatment and strict medical control. However, risk factors for stroke recurrence are less known than risk factors for first-ever stroke. In our experience, alcohol abuse (OR = 21.8), hypertension with valvular heart disease and atrial fibrillation (OR = 4.3), nausea and vomiting (OR = 3.7), and previous cerebral infarct (OR = 3.2) were clinical predictors of cardioembolic stroke recurrence. In addition to these four variables, cardiac events (tachyarrhythmia, heart failure or acute myocardial infarction that occurred as medical complication during the patient’s hospital stay) were selected in the logistic regression model based on clinical, neuroimaging, and outcome variables (OR = 4.25).
The association of hypertension with valvular heart disease and atrial fibrillation was a predictive variable of stroke recurrence but none of these variables was statistically significant when they were independently analyzed. In another study, valvular heart disease associated with congestive heart failure was the only predictive factor of stroke recurrence [74]. Although the presence of a structural cardiac disorder in a well known risk factor for system embolization [75,76], Lai et al. [77] also showed that patients with hypertension associated with non-valvular atrial fibrillation had a higher risk of embolic recurrence as compared to patients with only hypertension or with non-valvular atrial fibrillation only.
Involvement of cardiac center in the medulla oblongata may predispose to arrhythmias and cardiac arrest during the acute phase of stroke. Therefore, the presence of nausea and vomiting is a symptom usually associated with an infarction in the vertebrobasilar territory or progression compression of the brainstem due to an infarction in the carotid territory with transtentorial herniation, a clinical condition that can cause heart rhythm disturbances by concomitant involvement of the cardiac center and predispose to a potential cardioembolic recurrence [78−83].
In contrast to data observed in our study, the presence of a previous cerebral infarction was not a predictor of recurrence in the study of Sacco et al [72]. However, other authors consider the presence of a cerebral infarction is one of the most powerful predictive factors recurrent embolism [76,77]. In the study of van Latum et al. [84], a previous thromboembolism of any kind was also a significant predictor of stroke recurrence.
Alcohol abuse was an important predictor of recurrent embolism in our experience of cardioembolic infarction [70], which is similar to that observed in the study of Sacco et al. [72]. There is evidence of a strong relationship between stroke and alcohol: a) alcohol intoxication is a risk factor for cerebral infarction [85]; b) a higher frequency of alcohol abuse among stroke patients has been demonstrated [86−89]; c) other studies even claim that continued alcohol abuse is a true risk factor for stroke [90−92]. In Caucasian populations, "J-shaped" relationship has been documented between the protective effect of mild daily alcohol consumption and an increase in the risk of cerebral infarction by increasing daily alcohol consumption [86-88]. Although its effect on cardioembolic stroke is still unclear, there are several pathophysiological mechanisms by which alcohol can cause stroke [86, 93−104].
Any of the mechanisms outlined above may predispose to a new embolism, although the presence of a non-ischemic cardiomyopathy associated with the possibility of cardiac arrhythmia are probably the more common potential mechanisms.
A classification system based on independent risk factors for stroke and used in clinical practice for predicting stroke in patients with non-valvular atrial fibrillation is the CHADS2 index [1,22] (acronym for Congestive heart failure, Hypertension, Age, Diabetes mellitus and stroke). CHADS2 is formed by assigning 1 point each for the presence of congestive heart failure, hypertension, age 75 years or older, and diabetes mellitus, and by assigning 2 points for history of stroke or transient ischemic attack. Those patients with CHADS2 score of 0 or 1 have a low annual risk of stroke (1%), CHADS2 score of 2 identifies patients with moderate risk (annual risk of 2.5%), and patients with a score of 3 or greater are estimated to have a high risk of stroke (annual risk > 5%).
Early embolism in the main independent risk factor for in-hospital mortality in patients with cardioembolic infarction [64]. Non-invasive (anticoagulation) or invasive (devices) therapies for prevention strategies may be needed. Timing of initiation of anticoagulant treatment remains an area of uncertainty, since there is concern regarding exacerbating the risk of hemorrhage into regions of infarction (”hemorrhagic transformation”) after ischemic stroke. Guidelines propose arbitrary deferral of anticoagulation for 2 weeks in patients hospitalized with stroke by extrapolation from acute trials with full-dose heparin, where reduced early recurrent ischemic stroke is balanced by increased hemorrhagic risk. In patients with transient ischemic attack or minor stroke and with exclusion of cerebral hemorrhage, oral anticoagulation can be initiated within 3-5 days. However, we agree with Chamorro et al. [9] that secondary prevention with anticoagulants should be started immediately if possible in high recurrent embolic cardioembolic stroke risk patients without contraindications, such as falls, poor compliance, uncontrolled epilepsy, or gastrointestinal bleeding. Thus, contrary to the recommendation to delay anticoagulation in patients with extensive cardioembolic infarction or marked neurological deficit, immediate anticoagulation may be indicated in this subpopulation of cardioembolic infarction with maximal risk for early cardioembolic recurrence. According to Yasaka et al. [68], early anticoagulation with intravenous sodium heparin reduces the frequency of recurrent events and would reduce mortality, providing that it is initiated as soon as possible and maintaining activated thromboplastin time values below twice the control values. Oral anticoagulation with warfarin would be indicated later.
In summary, not all cardioembolic strokes should be treated with anticoagulation. Cardiac indications for anticoagulation are: atrial fibrillation, mural thrombi, prosthetic valves, marantic endocarditis. Anticoagulation is not indicated for infectious endocarditis. Antiplatelets are recommended in patent foramen ovale, mitral annular calcification, and mitral valve prolapse. Treatment of cardiac tumors needs surgery [105].
Dabigatran is a potent, direct, competitive inhibitor of thrombin that, like ximelagatran, does not require regular monitoring. Dabigatran has been shown to be non-inferior than warfarin in the prevention of stroke or systemic embolism [106].
Left atrial appendage occlusion and ablation procedures for atrial fibrillation are other possible therapeutic cardiac options in selected patients [22].
Cardiac source of clot might probably represent the stroke subtype with more uniform fibrin-rich clots and higher efficacy of thrombolysis. However, results of randomized clinical trials of intravenous thrombolysis have demonstrated no significant difference in final outcome in tPA-treated patients based on confirmed stroke mechanism [107].
6. CLINICAL DIFFERENCES BETWEEN CARDIOEMBOLIC INFARCTION AND ATHEROTHROMBOLIC INFARCTION
Clinical data exclusive for cardioembolic cerebral infarction or atherothrombotic infarctions are lacking [1,108]. However, to establish an early diagnosis of cardioembolic infarction may have a therapeutic interest. A clinical study has shown that atrial fibrillation and sudden onset of symptoms were independently associated with cardioembolic stroke, whereas hypertension, chronic obstructive pulmonary disease (COPD), diabetes mellitus, hyperlipidemia and age were significantly associated with atherothrombotic infarction [109].
On the other hand, clinical data traditionally related to cardioembolic cerebral infarction, such as seizures or headache, were not predictors of cardioembolic stroke, which is consistent with results of the studies of Ramirez-Lassepas et al. [110], Kittner et al. [111,112], and Caplan et al. [27].
Also, considering that the oldest old represents the faster-growing segment of the elderly in developed countries [113], in a clinical study after multivariate analysis atrial fibrillation (OR = 3.77), female gender (OR =2.52), hypertension (OR = 0.35), and diabetes (OR = 0.16) were independent clinical factors for developing lacunar infarction in the very elderly and suggest that the cardioembolic pathogenetic mechanisms may be more frequent than generally established for lacunar infarcts in stroke patients [114].
7. IMPACT OF ATRIAL FIBRILLATION IN CARDIOEMBOLIC AND ATHEROTHROMBOTIC CEREBRAL INFARCTION
Atrial fibrillation is the main cardiac disorder in the different series of cardioembolic cerebral infarction from industrialized countries reported in the literature [1,2,61,115]. However, atrial fibrillation can be also observed in atherothrombotic infarcts, not as an embolic etiology but a marker of other conditions that lead to ischemic stroke, such as atherosclerosis. It may be therefore considered as an epiphenomenon or a clinical manifestation of atherosclerotic disease [75]. In this respect, not all cerebral infarctions in patients with atrial fibrillation are of cardioembolic origin [28]. In our study, atrial fibrillation was diagnosed in 16.5% of patients with thrombotic occlusion or arterial stenosis grater than 70% presumably responsible for the cerebral infarction [116]. In these cases, some clinical or echocardiographic findings related to cardioembolism, such as recent congestive heart failure or increase of the left atrial size, or left ventricular dysfunction were absent [117,118]. Bogousslavsky et al. [28] showed that 76% of patients with cerebral infarcts in the carotid vascular territory with atrial fibrillation, the presumable pathophysiological mechanism of stroke was cardioembolic since a significant arterial vascular disease could not be documented. However, in 11% of the cases, the presumable mechanism was atherosclerosis because severe arterial stenosis or occlusion correlated with clinical features, and in the remaining 13%, the cerebral infarct could be explained by occlusion of small perforating arterial vessels in association with hypertension.
Accordingly, in a patient with cerebral infarction and atrial fibrillation it is important to make an early and precise diagnosis of the subtype of cerebral infarct, although the differential diagnosis between cardioembolic and athero-thrombotic stroke with atrial fibrillation may be difficult to establish at the onset of neurological deficit. In recent classifications of stroke subtypes, this distinction is not made and these patients are included in the subgroup of cerebral infarctions of undetermined cause due to the simultaneous presence of two potential etiologies. However, it should be noted that using the results of appropriate neurological and cardiological studies carried out in a delayed during hospitalization, in most of the cases, it is possible to establish the correct classification of stroke in the definite nosological entity [27].
In our experience based on 2000 patients with acute cerebrovascular disease [116], 1712 (85.6%) had a cerebral infarction. A total of 347 (17.4%) were classified as cardioembolic infarction, 452 (22.6%) as atherothrombotic infarction. Patients with cardioembolic infarction and atrial fibrillation accounted for 76.6% of the cases (n = 226), and patients with atherothrombotic infarction and atrial fibrillation for 16.5% (n = 75).
It should be noted that atrial fibrillation had a negative effect on outcome, both in cardioembolic and atherothrombotic infarction. It has been hypothesized that the worse outcome associated with atrial fibrillation may be explained by a higher prevalence of heart failure and ischemic heart disease. This hypothesis coincides in part with our results, given that a higher occurrence of heart failure in patients with cardioembolic stroke and a higher frequency of ischemic heart disease in patients with atherothrombotic stroke were observed. This may contribute to a decrease in cerebral blood flow as cerebral autoregulatory mechanisms in the ischemic area are impaired [119]. Other authors suggest that chronic atrial fibrillation may cause a significant reduction of regional blood flow [120], which may normalize when sinus rhythm is attained after successful cardioversion [121]. Other studies indicate that an increase in mortality may be explained by the more advanced age of the patients, a higher volume of the lesion, or a higher initial intensity of focal neurological deficit in patients with atrial fibrillation [122,123]. In summary, cerebrovascular disease in ischemic cardioembolic or atherothrombotic infarct is more severe in the presence of atrial fibrillation as compared to patients with normal sinus rhythm.
DISCLOSURE OF CONFLICT OF INTEREST
No conflict of interest.
DISCLOSURE OF SOURCES OF FUNDING
This study was supported by a grant from Fondo de Investigación Sanitaria (FIS PI/081514), Instituto de Investigación Carlos III, Madrid, Spain.
ACKNOWLEDGEMENTS
We thank A. Cartanyà, MD, M. Lowak, MD, and A. Saßmannshausen MD, and N. Amorós MD for their assistance in this study and Marta Pulido, MD, for editing the manuscript and editorial assistance.
Footnotes
This manuscript is an updated version of Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Current Cardiol. Rev. 2010; 6: 150–161.
REFERENCES
- 1.Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Current Cardiol Rev. 2010;6:150–61. doi: 10.2174/157340310791658730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weir NU. An update on cardioembolic stroke. Postgrad Med J. 2008;84:133–42. doi: 10.1136/pgmj.2007.066563. [DOI] [PubMed] [Google Scholar]
- 3.Ferro JM. Brain embolism. Answers to practical questions. J Neurol. 2003;250:139–47. doi: 10.1007/s00415-003-1017-0. [DOI] [PubMed] [Google Scholar]
- 4.Murtagh B, Smalling RW. Cardioembolic stroke. Curr Atherosclr Rep. 2006;8:310–6. doi: 10.1007/s11883-006-0009-9. [DOI] [PubMed] [Google Scholar]
- 5.Ferro JM. Cardioembolic stroke: an update. Lancet Neurol. 2003;2:177–88. doi: 10.1016/s1474-4422(03)00324-7. [DOI] [PubMed] [Google Scholar]
- 6.Di Tullio MR, Homma S. Mechanisms of cardioembolic stroke. Curr Cardiol Rep. 2002;4:141–8. doi: 10.1007/s11886-002-0027-3. [DOI] [PubMed] [Google Scholar]
- 7.MacDougall NJJ, Amarasinghe S, Muir KW. Secondary prevention of stroke. Expert Rev Neurother. 2009;7:1103–5. doi: 10.1586/erc.09.77. [DOI] [PubMed] [Google Scholar]
- 8.Khoo CW, Lip GYH. Clinical outcomes of acute stroke patients with atrial fibrillation. Expert Rev Neurother. 2009;7:371–4. doi: 10.1586/erc.09.11. [DOI] [PubMed] [Google Scholar]
- 9.Chamorro A, Vila N, Saiz A, Alday M, Tolosa E. Early anticoagulation after large cerebral embolic infarction: a safety study. Neurology. 1995;45:861–5. doi: 10.1212/wnl.45.5.861. [DOI] [PubMed] [Google Scholar]
- 10.Cerebral Embolism Study Group. Immediate anticoagulation of embolic stroke: brain hemorrhage and management opinions. Stroke. 1984;15:779–89. doi: 10.1161/01.str.15.5.779. [DOI] [PubMed] [Google Scholar]
- 11.Cerebral Embolism Study Group. Immediate anticoagulation of embolic stroke: a randomized trial. Stroke. 1983;14:668–76. doi: 10.1161/01.str.14.5.668. [DOI] [PubMed] [Google Scholar]
- 12.Cerebral Embolism Task Force. Cardiogenic brain embolism. Arch Neurol. 1986;43:71–84. [PubMed] [Google Scholar]
- 13.Cerebral Embolism Task Force. Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol. 1989;46:727–43. [PubMed] [Google Scholar]
- 14.Arboix A, Vericat MC, Pujades R, Massons J, García-Eroles L, Oliveres M. Cardioembolic infarction in The Sagrat Cor-Alianza Hospital of Barcelona Stroke Registry. Acta Neurol Scand. 1997;96:407–12. doi: 10.1111/j.1600-0404.1997.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 15.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Registry: analysis of 1.000 consecutive patients with first stroke. Stroke. 1988;19:1083–92. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 16.Timsit SG, Sacco RL, Mohr JP, et al. Brain infarction severity differs according to cardiac or arterial embolic source. Neurology. 1993;43:728–33. doi: 10.1212/wnl.43.4.728. [DOI] [PubMed] [Google Scholar]
- 17.Vázquez J, Gendre J, Martí-Vilalta JL. Manifestaciones clínicas del infarto cerebral embólico de origen cardíaco. In: Matías-Guiu J, Martínez-Vila E, Martí-Vilalta JL, editors. Isquemia cerebral. Matas-G. Barcelona, MCR SA: 1990. pp. 185–202. [Google Scholar]
- 18.Al-Rajeh S, Larbi E, Bademosi O, et al. Stroke in a tertiary hospital in Saudi Arabia: a study of 372 cases. Eur Neurol. 1991;31:251–256. doi: 10.1159/000116685. [DOI] [PubMed] [Google Scholar]
- 19.Rothrock JF, Lyden PD, Brody ML, et al. An analysis of ischemic stroke in an urban southern california population. The University of California, San Diego, Stroke Data Bank. Arch Intern Med. 1993;153:619–24. [PubMed] [Google Scholar]
- 20.Norrving B, Löwenhielm P. Epidemiology of first stroke in Lund-Orup, Sweden, 1983-1985. Incidence of first stroke and aged-related changes in subtypes. Acta Neurol Scand. 1988;78:408–13. doi: 10.1111/j.1600-0404.1988.tb03677.x. [DOI] [PubMed] [Google Scholar]
- 21.Arboix A, Cendrós V, Besa M, et al. Trends in risk factors, stroke subtypes and outcome. Nineteen-year data from the Sagrat Cor Hospital of Barcelona Stroke Registry. Cerebrovasc Dis. 2008;26:509–16. doi: 10.1159/000155989. [DOI] [PubMed] [Google Scholar]
- 22.Arboix A, Alió J. Acute cardioembolic stroke: an update. Expert Rev Cardiovasc Ther. 2011;9:367–9. doi: 10.1586/erc.10.192. [DOI] [PubMed] [Google Scholar]
- 23.Ustrell X, Pellisé A. Cardiac workup of ischemic stroke. Current Cardiol Rev. 2010;6:175–183. doi: 10.2174/157340310791658721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaillard N, Deltour S, Vilotijevic B, et al. Detection of paroxysmal atrial fibrillation with transtelephonic EKG in TIA or stroke patients. Neurology. 2010;74:1666–70. doi: 10.1212/WNL.0b013e3181e0427e. [DOI] [PubMed] [Google Scholar]
- 25.Morris JG, Duffis EJ, Fisher M. Cardiac workup of ischemic stroke. Can we improve our diagnostic yield? Stroke. 2009;40:2893–8. doi: 10.1161/STROKEAHA.109.551226. [DOI] [PubMed] [Google Scholar]
- 26.Serena J, Jiménez-Nieto M, Silva Y, Castellanos M. Patent foramen ovale in cerebral infarction. Current Cardiol Rev. 2010;6:162–74. doi: 10.2174/157340310791658794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caplan LR. Brain embolism, revisited. Neurology. 1993;43:1281–7. doi: 10.1212/wnl.43.7.1281. [DOI] [PubMed] [Google Scholar]
- 28.Bogousslavsky J, Cachin C, Regli F, Despland PA, Van Melle G, Kappenberger L. Cardiac sources of embolism and cerebral infarction-clinical consequences and vascular concomitants: The Lausanne Stroke Registry. Neurology. 1991;41:855–9. doi: 10.1212/wnl.41.6.855. [DOI] [PubMed] [Google Scholar]
- 29.Timsit SG, Sacco MS, Mohr JP, et al. Early clinical differentation of cerebral infarction from severe atherosclerotic stenosis and cardioembolism. Stroke. 1992;23:486–91. doi: 10.1161/01.str.23.4.486. [DOI] [PubMed] [Google Scholar]
- 30.Mohr JP, Gautier JC, Hier DB. Middle cerebral artery disease. In: Barnett HJM, Stein BM, Mohr JP, Yatsu FM, editors. Stroke: Pathophysiology, diagnosis and management. New York. Churchill Livingstone: 1992. pp. 361–417. [Google Scholar]
- 31.Minematsu K, Yamaguchi T, Omae T. "Spectacular shrinking deficit": rapid recovery from a major hemispheric syndrome by migration of an embolus. Neurology. 1992;42:157–62. doi: 10.1212/wnl.42.1.157. [DOI] [PubMed] [Google Scholar]
- 32.Arboix A, Bechich S. Recurrencia precoz de la regresión espectacular del déficit hemisférico neurológico como forma de presentación de un infarto cardioembólico. Rev Neurol. 1998;27:601–3. [PubMed] [Google Scholar]
- 33.Bechich J, Arboix A. Regresión espectacular del déficit hemisférico neurológico. Neurología. 1997;12:45–6. [PubMed] [Google Scholar]
- 34.Martin R, Bogousslavsky J. Embolic versus nonembolic causes of ischemic stroke. Cerebrovasc Dis. 1995;5:70–4. [Google Scholar]
- 35.Hart RG. Cardiogenic embolism to the brain. Lancet. 1992;339:589–94. doi: 10.1016/0140-6736(92)90873-2. [DOI] [PubMed] [Google Scholar]
- 36.Caplan LR. Clinical diagnosis of brain embolism. Cerebrovasc Dis. 1995;5:79–88. [Google Scholar]
- 37.Arboix A, Arbe G, García-Eroles L, Oliveres M, Parra O, Massons J. Infarctions in the vascular territory of the posterior cerebral artery: clinical features in 232 patients. BMC Res Notes. 2011;4:329. doi: 10.1186/1756-0500-4-329. doi: 10.1186/1756-0500-4-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arboix A, Martí-Vilalta JL. Lacunar stroke. Expert Rev Neurother. 2009;9:179–96. doi: 10.1586/14737175.9.2.179. [DOI] [PubMed] [Google Scholar]
- 39.Cacciatore A, Russo LS., Jr Lacunar infarction as an embolic complication of cardiac and arch angiography. Stroke. 1991;22:1603–5. doi: 10.1161/01.str.22.12.1603. [DOI] [PubMed] [Google Scholar]
- 40.Lodder J, Bamford JM, Sandercock PAG, Jones LN, Warlow CP. Are hypertension or cardiac embolism likely causes of lacunar infarction? Stroke. 1990;21:375–81. doi: 10.1161/01.str.21.3.375. [DOI] [PubMed] [Google Scholar]
- 41.Fieschi C, Sette G, Fiorelli M, et al. Clinical presentation and frequency of potential sources of embolism in acute ischemic stroke patients: the experience of the Rome Acute Stroke Registry. Cerebrovasc Dis. 1995;5:75–8. [Google Scholar]
- 42.Bogousslavsky J, Regli J, Uské A, Maeder Ph. Early spontaneous hematoma in cerebral infarct: is primary cerebral hemorrhage overdiagnosed? Neurology. 1991;41:837–40. doi: 10.1212/wnl.41.6.837. [DOI] [PubMed] [Google Scholar]
- 43.Cuadrado-Godia E, Ois A, Roquer J. Heart failure in acute ischemic stroke. Current Cardiol Rev. 2010;6:202–13. doi: 10.2174/157340310791658776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Font MA, Krupinski J, Arboix A. Antithrombotic medication for cardioembolic stroke prevention. Stroke Res Treat. 2011. 607852. doi: 10.4061/2011/607852. [DOI] [PMC free article] [PubMed]
- 45.Arboix A, García-Eroles L, Massons J, Oliveres M, Targa C. Hemorrhagic lacunar stroke. Cerebrovasc Dis. 2000;10:229–234. doi: 10.1159/000016061. [DOI] [PubMed] [Google Scholar]
- 46.Pujadas R, Arboix A, Anguera N, Rafel J, Sagués F, Casañas R. Mitral annular calcification as a marker of complex aortic atheroma in patients with stroke of uncertain etiology. Echocardiography. 2008;25:124–31. doi: 10.1111/j.1540-8175.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 47.Kent DM, Trikalinos TA, Thaler DE. Patent foramen ovale and cryptogenic stroke. N Engl J Med. 2008;358:1519–20. [PubMed] [Google Scholar]
- 48.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. 2000;55:1172–9. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 49.Serena J, Marti-Fàbregas J, Santamarina E, et al. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke. 2008;39:3131–6. doi: 10.1161/STROKEAHA.108.521427. [DOI] [PubMed] [Google Scholar]
- 50.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 51.Messé SR, Silverman IE, Kizer JR, et al. Practice parameter: recurrent stroke with patent foramen ovale and atrial septal aneurysm: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;62:1042–50. doi: 10.1212/01.wnl.0000119173.15878.f3. [DOI] [PubMed] [Google Scholar]
- 52.The European Stroke Organization (ESO) Executive Committee and the ESO Writing Committee. ESO Guidelines for management of ischaemic stroke 2008. Cerebrovasc. Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 53.Amarenco P, Duyckaerts C, Tzourio C, Henin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:21–5. doi: 10.1056/NEJM199201233260402. [DOI] [PubMed] [Google Scholar]
- 54.Meissner I, Khandheria BK, Sheps SG, et al. Atherosclerosis of the aorta: Risk factor, risk marker, or innocent bystander? A prospective population-based transesophageal echocardiographic study. J Am Coll Cardiol. 2004;44:1018–24. doi: 10.1016/j.jacc.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 55.Pujadas R, Arboix A, Anguera N, Oliveres M, Massons J, Comes E. Papel de las placas complejas de ateroma aórtico en la recurrencia del infarto cerebral de etiología incierta. Rev Esp Cardiol. 2005;58:34–40. [PubMed] [Google Scholar]
- 56.Capmany RP, Ibañez MO, Pesquer XJ. Complex atheromatosis of the aortic arch in cerebral infarction. Current Cardiol Rev. 2010;6:184–93. doi: 10.2174/157340310791658712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tunick PA, Nayar AC, Goodkin GM, et al. Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque. Am J Cardiol. 2002;90:1333–5. doi: 10.1016/s0002-9149(02)02870-9. [DOI] [PubMed] [Google Scholar]
- 58.Benjamin EJ, Plehn JF, D'Agostino RB, et al. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327:374–9. doi: 10.1056/NEJM199208063270602. [DOI] [PubMed] [Google Scholar]
- 59.Pujadas RC, Arboix A, Casañas-Muñoz R, Anguera-Ferrando N. Specific cardiac disorders in 402 consecutive patients with ischaemic cardioembolic stroke. Int J Cardiol. 2004;95:129–34. doi: 10.1016/j.ijcard.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Caplan LR, Hier DB, D'Cruz I. Cerebral embolism in the Michael Reese Stroke Registry. Stroke. 1983;14:530–6. doi: 10.1161/01.str.14.4.530. [DOI] [PubMed] [Google Scholar]
- 61.Hornig CR, Brainin M, Mast H. Cardioembolic stroke: results from three current stroke data banks. Neuroepidemiology. 1994;13:318–23. doi: 10.1159/000110398. [DOI] [PubMed] [Google Scholar]
- 62.Lodder J, Krijne-Kubat B, Broekman J. Cerebral hemorrhagic infarction at autopsy: cardiac embolic cause and the relationship to the cause of death. Stroke. 1986;17:626–9. doi: 10.1161/01.str.17.4.626. [DOI] [PubMed] [Google Scholar]
- 63.Arboix A, García-Eroles L, Massons J, Oliveres M. Predictive clinical factors of in-hospital mortality in 231 consecutive patients with cardioembolic cerebral infarction. Cerebrovasc Dis. 1998;8:8–13. doi: 10.1159/000015809. [DOI] [PubMed] [Google Scholar]
- 64.Hart RG, Coull BM, Hart D. Early recurrent embolism associated with nonvalvular atrial fibrillation: a retrospective study. Stroke. 1983;14:688–93. doi: 10.1161/01.str.14.5.688. [DOI] [PubMed] [Google Scholar]
- 65.Broderick JP, Phillips SJ, O'Fallon M, Whisnant JP. Heart diseases as a potential cause of stroke. (Abstract) Stroke. 1990;21:173. [Google Scholar]
- 66.Arboix A, Massons J, Garcia-Eroles L, Comes E, Balcells M, Oliveres M. Recurrent ischemic stroke: study of 605 patients. Med Clin (Barc) 2011;137:541–5. doi: 10.1016/j.medcli.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 67.Sacco RL, Foulkes MA, Mohr JP, Wolf PA, Hier DB, Price TR. Determinants of early recurrence of cerebral infarction. The Stroke Data Bank. Stroke. 1989;20:983–9. doi: 10.1161/01.str.20.8.983. [DOI] [PubMed] [Google Scholar]
- 68.Yasaka M, Yamaguchi T, Oita J, Sawada T, Shichiri M, Omae T. Clinical features of recurrent embolization in acute cardioembolic stroke. Stroke. 1993;24:1681–5. doi: 10.1161/01.str.24.11.1681. [DOI] [PubMed] [Google Scholar]
- 69.Arboix A, Vicens A, Vives JM, García-Eroles L, Massons J. Spontaneous neurological deterioration in acute cardioembolic stroke: a subgroup of patients with early severe prognosis. J Neurol Res. 2011;1:133–8. [Google Scholar]
- 70.Arboix A, García Eroles L, Oliveres M, Massons JB, Targa C. Clinical predictors of early embolic recurrence in presumed cardioembolic infarction. Cerebrovasc Dis. 1998;8:345–53. doi: 10.1159/000015878. [DOI] [PubMed] [Google Scholar]
- 71.Yamanouchi H, Shimada H, Tomonaga M, Matsushita S. Recurrence of embolic stroke in non-valvular atrial fibrillation (NVAF). An autopsy study. Acta Neurol Scand. 1989;80:123–9. doi: 10.1111/j.1600-0404.1989.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 72.Sacco RL, Shi T, Zamanillo MC, Kargman DE. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: The Northern Manhattan Stroke Study. Neurology. 1994;44:626–34. doi: 10.1212/wnl.44.4.626. [DOI] [PubMed] [Google Scholar]
- 73.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke registry: analysis of 1.000 consecutive patients with first stroke. Stroke. 1988;19:1083–92. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 74.Broderick JP, Phillips SJ, O'Fallon M, Frye RL, Whisnant JP. Relationship of cardiac disease to stroke occurrence, recurrence and mortality. Stroke. 1992;23:1250–6. doi: 10.1161/01.str.23.9.1250. [DOI] [PubMed] [Google Scholar]
- 75.Sandercock P, Bamford J, Dennis M, et al. Atrial fibrillation and stroke: prevalence in different types of stroke and influence on early and long term prognosis (Oxforsdshire community stroke project) BMJ. 1992;305:1460–5. doi: 10.1136/bmj.305.6867.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cabin HS, Clubb KS, Hall C, Perlmutter RA, Feinstein AR. Risk for systemic embolization of atrial fibrillation without mitral stenosis. Am J Cardiol. 1990;65:1112–6. doi: 10.1016/0002-9149(90)90323-s. [DOI] [PubMed] [Google Scholar]
- 77.Lai SM, Alter M, Friday G, Sobel E. A multifactorial analysis of risk factors for recurrence of ischemic stroke. Stroke. 1994;25:958–62. doi: 10.1161/01.str.25.5.958. [DOI] [PubMed] [Google Scholar]
- 78.Canhao P, Melo TP, Salgado AV, et al. Nausea and vomiting in acute ischemic stroke. Cerebrovasc Dis. 1997;7:220–5. [Google Scholar]
- 79.Fisher CM. Vomiting out of proportion to dizziness in ischemic brainstem strokes. Neurology. 1996;46:267. doi: 10.1212/wnl.46.1.267. [DOI] [PubMed] [Google Scholar]
- 80.Jaster JH, Porterfield LM, Bertorini TE, Dohan TC, Jr, Becske T. Stroke and cardiac arrest. Neurology. 1996;47:1357. doi: 10.1212/wnl.47.5.1357. [DOI] [PubMed] [Google Scholar]
- 81.Stober T, Sen S, Anstätt Th, Bette L. Correlation of cardiac arrhythmias with brainstem compression in patients with intracerebral hemorrhage. Stroke. 1988;19:688–92-. doi: 10.1161/01.str.19.6.688. [DOI] [PubMed] [Google Scholar]
- 82.Furlan AJ, Cavalier SJ, Hobbs RE, Weinstein MA, Modic MT. Hemorrhage and anticoagulation after nonseptic embolic brain infarction. Neurology. 1982;32:280–2. doi: 10.1212/wnl.32.3.280. [DOI] [PubMed] [Google Scholar]
- 83.Easton JD. Epidemiology of stroke recurrence. Cerebrovasc Dis. 1997;7(Suppl 1):2–4. [Google Scholar]
- 84.van Latum JC, Koudstaal PJ, Venables GS, van Gijn J, Kappelle LJ, Algra A. for the European Atrial Fibrillation Trial (EAFT) Study Group. Predictors of major vascular events in patients with a transient ischemic attack or minor ischemic stroke and with nonrheumatic atrial fibrillation. Stroke. 1995;26:801–6. doi: 10.1161/01.str.26.5.801. [DOI] [PubMed] [Google Scholar]
- 85.Hillbom M, Kaste M. Does ethanol intoxication promote brain infarction in young adults? Lancet. 1978. pp. 1181–3. [DOI] [PubMed]
- 86.Gill JS, Zezulka AV, Shipley MJ, Gill SK, Beevers DG. Stroke and alcohol consumption. N Engl J Med. 1986;315:1041–6. doi: 10.1056/NEJM198610233151701. [DOI] [PubMed] [Google Scholar]
- 87.Gill JS, Shipley MJ, Tsemenzis SA, et al. Alcohol consumption. A risk factor for hemorrhagic and non-hemorrhagic stroke. Am J Med. 1991;90:489–97. [PubMed] [Google Scholar]
- 88.Camargo CA., Jr Moderate alcohol consumption and stroke. The epidemiologic evidence. Stroke. 1989;20:1611–26. doi: 10.1161/01.str.20.12.1611. [DOI] [PubMed] [Google Scholar]
- 89.Palomäki H, Kaste M. Regular light-to-moderate intake of alcohol and the risk of ischemic stroke. Is there a beneficial effect? Stroke. 1993;24:1828–32. doi: 10.1161/01.str.24.12.1828. [DOI] [PubMed] [Google Scholar]
- 90.Iso H, Kitamura A, Shimamoto T, et al. Alcohol intake and the risk of cardiovascular disease in middle-aged Japanese men. Stroke. 1995;26:767–73. doi: 10.1161/01.str.26.5.767. [DOI] [PubMed] [Google Scholar]
- 91.Beghi E, Bogliun G, Cosso P, et al. Stroke and alcohol intake in a hospital population. A case-control study. Stroke. 1995;26:1691–6. doi: 10.1161/01.str.26.9.1691. [DOI] [PubMed] [Google Scholar]
- 92.Wannamethee SG, Shaper AG. Patterns of alcohol intake and risk of stroke in middle-aged British men. Stroke. 1996;27:1033–9. doi: 10.1161/01.str.27.6.1033. [DOI] [PubMed] [Google Scholar]
- 93.Gorelick PhB. The status of alcohol as a risk factor for stroke. Stroke. 1989;20:1607–10. doi: 10.1161/01.str.20.12.1607. [DOI] [PubMed] [Google Scholar]
- 94.Kaplan NM. Alcohol and hypertension. Lancet. 1995;345:1588–9. doi: 10.1016/s0140-6736(95)90110-8. [DOI] [PubMed] [Google Scholar]
- 95.Puddey IB, Beilin LJ, Vandongen R. Regular alcohol use raises blood pressure in treated hypertensive subjects. A randomised controlled trial. Lancet. 1987. pp. 647–51. [DOI] [PubMed]
- 96.Brigden W, Robinson J. Alcoholic heart disease. BMJ. 1964;2:1283–9. doi: 10.1136/bmj.2.5420.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anonymous. Alcohol and atrial fibrillation. Lancet. 1985;2:1374. [PubMed] [Google Scholar]
- 98.Estruch R. Efectos cardiovasculares del alcohol. Med Clin (Barc) 1995;105:628–35. [PubMed] [Google Scholar]
- 99.Miralles R, Molina L. Fibrilación auricular paroxística e intoxicación alcohólica aguda. Med Clin (Barc) 1985;85:814. [PubMed] [Google Scholar]
- 100.Ettinger PO, Wu CF, De La Cruz C, Jr, Weise AB, Ahmed SS, Regan TJ. Arrhythmias and the "Holiday Heart": alcohol associated cardiac rhythm disorders. Am Heart J. 1978;95:555–62. doi: 10.1016/0002-8703(78)90296-x. [DOI] [PubMed] [Google Scholar]
- 101.MacMahon SW, Norton RN. Alcohol and hypertension: implications for prevention and treatment. Ann Intern Med. 1986;105:124–6. doi: 10.7326/0003-4819-105-1-124. [DOI] [PubMed] [Google Scholar]
- 102.Marmot M, Brunner E. Alcohol and cardiovascular disease: the status of the U shaped curve. BMJ. 1991;303:565–8. doi: 10.1136/bmj.303.6802.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bonita R. Epidemiology of stroke. Lancet. 1992;339:342–7. doi: 10.1016/0140-6736(92)91658-u. [DOI] [PubMed] [Google Scholar]
- 104.Hansagi H, Romelsjö A, de Verdier MG, Andréasson S, Leifman A. Alcohol consumption and stroke mortality. 20-year follow-up of 15077 men and women. Stroke. 1995;26:1768–73. doi: 10.1161/01.str.26.10.1768. [DOI] [PubMed] [Google Scholar]
- 105.Official Guidelines for the diagnosis and treatment of cerebrovascular diseases (2 edition). Catalan Society of Neurology. Cel·lula (ed) Barcelona. 2011. pp. 159–240.
- 106.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 107.Millán M, Dorado L, Dávalos A. Fibrinolytic therapy in acute stroke. Current Cardiol Rev. 2010;6:218–26. doi: 10.2174/157340310791658758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.González EB, Román AR, González-Juanatey JR. Cardioembolic stroke: call for a multidisciplinary approach. Cerebrovasc Dis. 2009;27(Suppl 1):82–7. doi: 10.1159/000200444. [DOI] [PubMed] [Google Scholar]
- 109.Arboix A, Oliveres M, Massons J, Pujades R, García-Eroles L. Early differentiation of cardioembolic from atherothrombotic cerebral infarction: a multivariate analysis. Eur J Neurol. 1999;6:677–83. doi: 10.1046/j.1468-1331.1999.660677.x. [DOI] [PubMed] [Google Scholar]
- 110.Ramirez-Lassepas M, Cipolle RJ, Bjork RJ, et al. Can embolic stroke be diagnosed on the basis of neurologic clinical criteria? Arch Neurol. 1987;44:87–9. doi: 10.1001/archneur.1987.00520130067019. [DOI] [PubMed] [Google Scholar]
- 111.Kittner SJ, Sharkness CM, Sloan MA, et al. Infarcts with a cardiac source of embolism in the NINDS Stroke Data Bank: neurological examination. Neurology. 1992;42:299–302. doi: 10.1212/wnl.42.2.299. [DOI] [PubMed] [Google Scholar]
- 112.Kittner SJ, Sharkness CM, Price TR, et al. Infarcts with a cardiac source of embolism in the NINSDS Stroke Data Bank: historical features. Neurology. 1990;40:281–4. doi: 10.1212/wnl.40.2.281. [DOI] [PubMed] [Google Scholar]
- 113.Arboix A, Miguel M, Císcar E, Garcia-Eroles L, Massons J, Balcells M. Cardiovascular risk factors in patients aged 85 years or older with ischemic stroke. Clin Neurol Neurosurg. 2006;108:638–43. doi: 10.1016/j.clineuro.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 114.Arboix A, Garcia-Eroles L, Massons J, Oliveres M, Targa C. Lacunar infarcts in patients aged 85 years and older. Acta Neurol Scand. 2000;101:25–9. doi: 10.1034/j.1600-0404.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 115.Bogousslavsky J, Van Melle G, Regli F, Kappenberger L. Pathogenesis of anterior circulation stroke in patients with nonvalvular atrial fibrillation: The Lausanne Stroke Registry. Neurology. 1990;40:1046–50. doi: 10.1212/wnl.40.7.1046. [DOI] [PubMed] [Google Scholar]
- 116.Arboix A, García Eroles L, Massons JB, Oliveres M, Pujades R, Targa C. Atrial fibrillation and stroke: clinical presentation of cardioembolic versus atherothrombotic infarction. Int J Cardiol. 2000;73:33–42. doi: 10.1016/s0167-5273(99)00214-4. [DOI] [PubMed] [Google Scholar]
- 117.D'Olhaberriague L, Hernández-Vidal A, Molina L, et al. A prospective study of atrial fibrillation and stroke. Stroke. 1989;20:1648–52. doi: 10.1161/01.str.20.12.1648. [DOI] [PubMed] [Google Scholar]
- 118.Weinberger J, Rothlauf E, Materese E, Halperin J. Noninvasive evaluation of the extracranial carotid arteries in patients with cerebrovascular events and atrial fibrillations. Arch Intern Med. 1988;148:1785–8. [PubMed] [Google Scholar]
- 119.Keller TS, McGillicuddy JE, LaBond VA, Kindt GW. Volume expansion in focal cerebral ischemia: the effect of cardiac output on local cerebral blood flow. Clin Neurosurg. 1982;29:40–50. doi: 10.1093/neurosurgery/29.cn_suppl_1.40. [DOI] [PubMed] [Google Scholar]
- 120.Lavy S, Stern S, Melamed E, Cooper G, Keren A, Levy P. Effect of chronic atrial fibrillation on regional cerebral blood flow. Stroke. 1980;11:35–8. doi: 10.1161/01.str.11.1.35. [DOI] [PubMed] [Google Scholar]
- 121.Petersen P, Kastrup J, Videbaek R, Boysen G. Cerebral blood flow before and after cardioversion of atrial fibrillation. J Cereb Blood Flow Metab. 1989;9:422–5. doi: 10.1038/jcbfm.1989.62. [DOI] [PubMed] [Google Scholar]
- 122.Friedman PJ. Atrial fibrillation after stroke in the elderly. Stroke. 1991;22:209–214. doi: 10.1161/01.str.22.2.209. [DOI] [PubMed] [Google Scholar]
- 123.Censori B, Camerlingo M, Casto L, Ferraro B, Gazzaniga GC, Cesana B. Prognostic factors in first-ever stroke in the carotid territory seen within 6 hours after onset. Stroke. 1993;24:532–5. doi: 10.1161/01.str.24.4.532. [DOI] [PubMed] [Google Scholar]