Abstract
The metabolic syndrome has been a useful, though controversial construct in clinical practice as well as a valuable model in order to understand the interactions of diverse cardiovascular risk factors. However the increasing importance of the circulatory system in particular the endothelium, in both connecting and controlling organ function has underlined the limitations of the metabolic syndrome definition. The proposed “Circulatory Syndrome” is an attempt to refine the metabolic syndrome concept by the addition of recently documented markers of cardiovascular disease including renal impairment, microalbuminuria, arterial stiffness, ventricular dysfunction and anaemia to more classic factors including hypertension, dyslipidemia and abnormal glucose metabolism; all of which easily measured in clinical practice. These markers interact with each other as well as with other factors such as aging, obesity, physical inactivity, diet and smoking. The final common pathways of inflammation, oxidative stress and hypercoagulability thereby lead to endothelial damage and eventually cardiovascular disease. Nevertheless, the Circulatory (MARC) Syndrome, like its predecessor the metabolic syndrome, is only a small step toward an understanding of these complex and as yet poorly understood markers of disease.
Keywords: Circulatory system, metabolic syndrome, renal function, ventricular function, arterial stiffness, anaemia.
BACKGROUND
In medical science, a syndrome is defined as an “aggregate of symptoms and signs associated with any morbid process and constituted together they produce the picture of the disease” [1]. These components are usually caused by a unifying underlying pathology and their combination confers a risk that is different from the sum of the parts. The main purpose of such a description is to help in the diagnosis, treatment and prognosis of the disease.
The metabolic syndrome was first described by G.M. Reaven in 1988 to describe a cluster of risk factors contributing to the incidence of diabetes, cardiovascular events and also mortality [2]. The definition of this syndrome remains a matter of debate and has been revised on several occasions by different organizations [3-8]. Despite such diversity, obesity, hyperglycemia, dyslipidemia and hypertension have been constant syndrome components and the central concept of such descriptions is the unity of the background pathophysiologic process and the interaction between the elements.
Criticisms Against Metabolic Syndrome
Several epidemiologic studies have illustrated the relationship between the metabolic syndrome, cardiovascular events and mortality [9-16]; however the syndrome was criticised by the American Diabetes Association a few years ago for its modest consistency and limited clinical application [11] and the use of the term metabolic syndrome was discouraged. Although the predictive performance of the syndrome for diabetes incidence has been stressed in several studies and meta-analyses [17], several issues remain unresolved including the presence of potential gender differences in the risk for incident diabetes associated with the metabolic syndrome and whether the metabolic syndrome offers additional prediction beyond its components [18]. Also, its value for both cardiovascular and all-cause mortality is questioned [17]. Hence, it is now important to look back at the issue to ensure about its consistency and usefulness. For instance, the following consideration must be taken for a new definition:
Insulin resistance has been presumed to be the common pathway for all features of the metabolic syndrome [19]; yet insulin related measurements are not standardized and vary widely [20, 21]. Despite the general belief, hyperinsulinemia and insulin resistance are not equivalent terms [11]. Besides, while 78% of individuals with metabolic syndrome have insulin resistance, only 48% of patients with insulin resistance have the metabolic syndrome [22]. Therefore the association of hyperinsulinemia and other elements of metabolic syndrome are not constant and many other factors may also play important roles as underlying mechanisms in clustering the risk factors. In other words, insulin resistance may simply be one of many abnormalities linked to a more fundamental, truly unifying pathophysiology [11].
Metabolic syndrome diagnosis is not always associated with higher cardiovascular risk, for example an increased risk was not observed in elderly diabetics, non-diabetic American Indians and women with suspected CV disease but normal angiography [17, 23-25]. Additionally, application of different definitions of the syndrome causes 15-20% disagreement in patients classification [11] and changes the predictive value of the syndrome diagnosis for CV disease and mortality [10, 15, 26]. Therefore the association of the included syndrome components with CV disease and with each other is uncertain. Even reports supporting the metabolic syndrome state that “detecting the metabolic syndrome is only one part of the overall CV risk assessment and is not an adequate tool to estimate 10-year risk for coronary heart disease”[27]. This is possibly because of many other related factors which have not been included as syndrome criteria. In fact, residual analysis of many longitudinal studies demonstrates a high unexplained variance (as much as 47%) when metabolic syndrome components were considered as independent variables [11].
Although several epidemiologic studies have demonstrated the relationship between metabolic syndrome and microalbuminuria, this factor was only incorporated into the World Health Organisation criteria for the syndrome. Likewise, renal failure is now recognised as an independent CV risk factor, as is anaemia, but they have not been considered as a part of the metabolic syndrome.
Despite a large body of evidence of the strong and independent association of endothelioarterial dysfunction with mortality, CV disease, diabetes and renal function[28-40], its impact has been overlooked in metabolic syndrome. Also, cardiac disease has been considered simply as an outcome of metabolic syndrome while it should be an interacting part of the syndrome.
By and large, the current evidence strongly suggests that the metabolic syndrome definition needs significant amendments, which should include the addition of other factors. Furthermore it must be standardized. Therefore, we introduce the term of “circulatory syndrome” instead as a more refined clinical construct since it is composed of many disease markers including cardiac, arterial and renal (respectively abbreviated as MARC) components as well as the traditional risk factors[41].
Definition of the New Concept
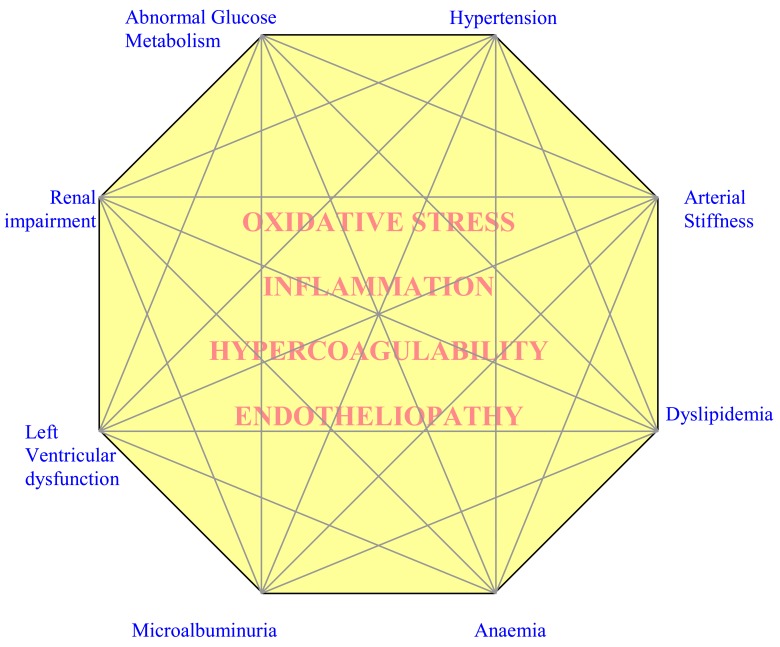
Circulatory (MARC) syndrome is a cluster of risk markers with synergic effects. The proposed syndrome consists of eight major components (Fig. 1):
Fig. (1).
An illustrative Circulatory Syndrome; A cluster of cardiac, renal, arterial and circulatory markers of disease that are interconnected through the endothelium; the common media (underlying factors) include oxidative stress, inflammation, hypercoagulability state and endotheliopathy which contribute in the main mechanisms of the phenomena; the third dimetion (precipitating factors) include age, obesity, physical inactivity and smoking which accelerate the phenomena
Abnormal glucose metabolism
Hypertension
Renal impairment
Microalbuminuria
Arterial stiffness
Left ventricular dysfunction
Dyslipidemia
Anaemia
The above markers construct a network of associations while the strength of associations creates manifestation nodes. Then, the syndrome may have several facets of presentation and any given individual may exhibit some dominant features.
The above markers can be simply and non-invasively assessed in outpatient clinical settings, although more complex assessments would be necessary for additional workups.
All of these “markers” are expressed on a background of oxidative stress, inflammation, hypercoagulability and endotheliopathy (underlying factors) and can be accelerated by factors such as aging, obesity, smoking and physical inactivity (predisposing factors).
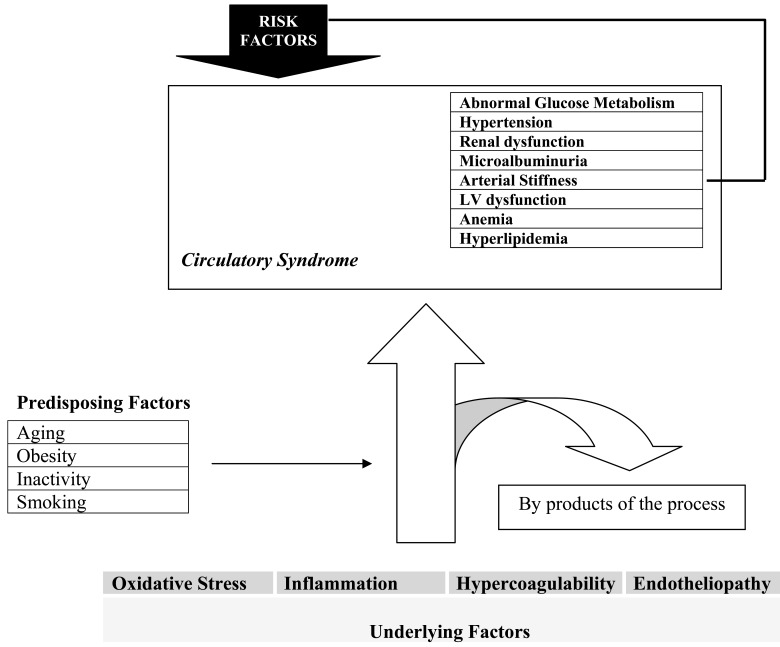
While mechanisms underlying the circulatory (MARC) syndrome are poorly understood, it must be strongly stated that vascular-endothelial pathways link all and all are of pathological significance. Activation of the renin-angiotensin system, insulin resistance and increased sympathetic activation are all by-products of the underlying pathogenic process (Fig. 2). Since these markers represent the extent of the underlying disease process, they could also manifest as risk factors for other components and thereby enhance their development. The final outcome in this model is the general circulatory health level of the individuals or the ability of the circulatory system to respond to body demands including exercise tolerance and ischemia related symptoms and signs.
Fig. (2).
Relationships between the underlying and precipitating factors with the Circulatory Syndrome. The eight major markers of the syndrome can also play a risk factor role for other factors and progression of the syndrome.
RATIONALES FOR THE INCLUSION OF THE COMPONENTS
Circulatory (MARC) syndrome shares some elements with the metabolic syndrome. However it includes additional metabolic and non-metabolic factors (Table 1).
Table 1.
Preliminary Diagnostic Criteria for Circulatory Syndrome
| Abnormal Glucose Metabolism |
| Fasting Plasma Glucose >6.1 mmol/l ; or |
| 2hr post prandial >7.8 mmol/l |
| Hypertension |
| SBP≥130 mmHg; and/or |
| DBP≥ 85 mmHg |
| GFR |
| MDRD eGFR <90 ml/min/1.73 m2 |
| Microalbuminuria |
| Urinary Albumin creatinine ratio (ACR) [two occasions] |
| >2.5 (male) |
| >3.5 (female) |
| Arterial Stiffness |
| Upper quartile for PWV, AI or ambulatory PP in the population |
| Left ventricular dysfunction |
| Any evidence of systolic or diastolic; |
| Imaging techniques or |
| Exercise test (MET <6, impaired systolic BP response) or |
| BNP> 100 pg/ml |
| Previous myocardial infarction |
| Anemia |
| Hb< 12 female |
| HB<13 male |
| Dyslipidemia |
| Triglyceride ≥ 1.7 mmol/l or |
| HDL<1 (male) or <1.3 (female) mmol/l or |
| Elevated Apolipoprotein B |
GFR: Glomerular Filtration Rate, PWV: Pulse Wave Velocity, AI: Augmentation Index, PP: Pulse Pressure, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure, MET: Estimated multiples of resting oxygen uptake, BNP: Brain Natriuretic Peptide, HDL: High Density Lipoprotein
(1). Factors associated with abnormal glucose metabolism:
Diabetes and abnormalities in glucose metabolism are well known risk factors for cardiac, arterial and renal disease as well as anemia [42, 43]. Although insulin resistance and hyperinsulinemia can be attributed to these complications, they may occur with or without insulin resistance because several other mechanisms including advanced glycation end products, autonomic nervous instability, imbalance between the renin-angiotensin system and nitric oxide, hemodynamic changes and endothelial dysfunction with subsequent ADMA accumulation (an inhibitor for nitric oxide synthesis) and adiponectin deficiency also contribute in the process [44-46].
(2). Lipid abnormalities:
Dyslipidemia including increased LDL and TG as well as low HDL is a major risk in patients with chronic renal disease, hypertension and diabetes[14, 47-49]. Genetic variants of lipoprotein lipase correlate with the presence and degree of albuminuria [50]. Dyslipidemia is an independent determinant of progression toward chronic kidney disease and is a known cardiac risk factor [51, 52]. It also contributes to arterial micro-inflammation and atherosclerosis[53]. From different perspective, the correction of lipid abnormalities can reduce albuminuria in subjects with the metabolic syndrome [54], decrease inflammatory markers[55], improve renal function[56], increase arterial compliance[57], improve left ventricular function [55] and prevent CV events[53]. It is noteworthy that obesity was not incorporated into our criteria since there is an opposite relationship between BMI and survival in CKD (reverse epidemiology) [58] and therefore less obese patients with CKD reach to ESRD.
(3). Blood pressure abnormalities:
Hypertension is introduced as the leading risk factor of death according to WHO report of global health [59].Hypertension and altered blood pressure circadian rhythm are common co-morbidities with diabetes and pre-diabetic states as well as kidney disease[60]. BP is strongly associated with arterial stiffness and promotes left ventricular dysfunction[61] . In the setting of insulin resistance the vasodilatory effect of insulin can be lost but its renal sodium reabsorption stimulation is preserved. In addition, insulin-induced sympathetic activity increases the prevalence of hypertension in the metabolic syndrome [44]. Furthermore, while salt sensitivity is associated with impaired glucose metabolism, oxidative stress, dyslipidemia and insulin resistance [62, 63] ,it also increases efferent glomerular arteriolar tone and thereby raises glomerular capillary pressure and proteinuria [64]. Moreover, salt sensitivity induces blood pressure abnormalities via renal sodium reabsorption and sympathetic overactivity[65]. Therefore, the interlinking of salt-sensitivity and insulin resistance mechanisms with the clinical outcomes raises the possibility of another underlying mechanism joining these together.
(4). Arterial stiffness:
Decreased arterial compliance is influenced by both atherosclerosis and arteriosclerosis, as well as functional arterial abnormalities [61, 66]. It occurs very early in the process of kidney disease and DM [67, 68], even preceding microalbuminuria [69] and has also been observed in normal individuals with a close family history of DM [68]. Recent studies have illustrated that increased central arterial stiffness in hypercholesterolemia, even in newly diagnosed individuals, is associated with low-grade systemic inflammation [70, 71]. Arterial stiffness in turn increases LV load and leads to ventricular stiffness and diastolic dysfunction [72, 73]. It has also been suggested as the linking factor between renal impairment and CV diseases [74]. Of great importance, decreased arterial compliance predicts mortality in variant patient groups, independently from other risk factors [75-78].
Furthermore, albuminuria, arterial stiffness and intima media thickness increase with the increasing number of metabolic syndrome components even before fulfilling the diagnostic criteria for the syndrome, particularly amongst subjects with type 2 DM [79]. In addition, alterations in BP circadian rhythm and BP profile including non-dipper nocturnal BP is now considered as a manifestation of arterial remodelling and is associated with other manifestation of endothelial dysfunction including mA and arterial stiffness.
(5). Microalbuminuria
is now accepted as a marker of renal, cardiac and arterial damage being predictive for the further development of CV events, renal failure and DM [49, 74]. It is also closely associated with the prevalence of anaemia [43] , hypertension [80] and metabolic syndrome components [79]. Microalbuminuria commonly occurs early in subjects with abnormal glucose metabolism [67, 81] and is correlated with dyslipidemia [82], arterial stiffness [83, 84] and increased coagulability [85] as well as inflammatory markers [86, 87]. Furthermore the presence of microalbuminuria predicts ventricular dysfunction, coronary heart disease and exercise intolerance [88, 89].
(6). Renal impairment:
Kidney function can not be isolated from the health of the heart and arteries and is also associated with the metabolic syndrome components. Alterations in glomerular structure are seen very early in the obesity-mediated metabolic syndrome[90] . Renal hemodynamic reserve is already impaired in patients with asymptomatic left ventricular dysfunction [91]. In addition, the kidney has an important role in insulin and glucose metabolism [92]and insulin resistance has a predictive value for chronic kidney disease[4, 90]. Renal function has been called the Cinderella of CV risk profile [93] and the impact of even minor renal dysfunction on CV function is now well established [74] with endothelial cell dysfunction is likely to be the linking factor between renal and cardiac disease[49, 74, 94]. However endothelial dysfunction in turn is a consequence of inflammation and oxidative stress and is accelerated by these phenomena[95] and is also correlated with a number of the metabolic syndrome components [79]. Decreased arterial compliance increases ventricular wall tension and stiffness and consequently diastolic dysfunction[73]. This in turn may lead to partial renal ischemia, followed by activation of the renin-angiotensin system and tubulointerstitial damage[94]. On the other hand, hyperfiltration which is observed in the early stages of diabetic nephropathy and hypertension [96, 97], leads to increased glomerular pressure and resultant sclerosis which in turn accelerates hypertension[60].
(7). Anemia:
Anemia is a common finding in DM and has multifactorial mechanisms[43]. Early tubulointerstitial occurs which disease decreases EPO production and moreover inflammatory cytokines reduce EPO responsiveness leading to anaemia[98]. It is also associated with the level of albuminuria[43] .Anaemia in turn, increases the progression toward CKD, oxidative stress, tissue ischemia, ventricular stress and mortality[99-101]. Of interest, a recent study demonstrated the contribution of anemia to the frequent diastolic dysfunction in DM, as well as its association with brain natriuretic peptide (BNP) and suggested using this factor to identify diabetic patients at increased risk of cardiac dysfunction [99]. Therefore, accumulating evidence has introduced anemia as an important risk factor for the circulatory system. On the other hand, correction of anemia improves the prognosis in chronic kidney disease, heart failure and DM and its complications as well as decreasing mortality [101-103]
(8). Left Ventricular dysfunction:
In contrast to the metabolic syndrome, ventricular function is proposed as an interactive part of the circulatory syndrome. This idea is supported by reports of a lack of a relationship between the metabolic syndrome and mortality in individuals who have good cardiorespiratory fitness [104]. On the other hand even a mild stage of ventricular failure, as manifested by impaired exercise response is predictive for mortality [105, 106]. Ventricular function determines blood pressure and renal perfusion and in turn is influenced by kidney function, anemia and arterial stiffness and microalbuminuria [73, 107]. Diastolic dysfunction occurs early in DM, is correlated with arterial stiffness and affects exercise response [108]. Furthermore, it has been reported that asymptomatic patients with type 2 DM have subclinical ventricular dysfunction which is related to glycated hemoglubin and LDL cholesterol [109]. Also a recent in vitro study demonstrated that myocyte relaxation and Ca2+ handling are abnormal in early uremia, leading to uremic cardiomyopathy [110].
Additional evidence:
It is of great interest that some hypoglycaemic agents reduce blood pressure via suppression of the renin-angiotensin system and some ACE inhibitors can reduce insulin resistance in addition to reducing microalbuminuria and arterial stiffness, which raises the possibility of the presence of a common pathway for the adverse effects of hyperglycemia and hypertension[111-113]. Likewise, some lipid lowering agents may exhibit mild anticoagulant and hypotensive effects [114] and angiotensin inhibitors have anti-inflammatory actions [115] which also indicate a possible common source of these abnormalities.
It could be expected that genetic predisposition including nephron underdosing, ACE gene polymorphism, congenital tubular defects and also some other factors such as aging, obesity and smoking produce organ damage susceptibility [48, 116-118].
The above evidence suggests that a genetic profile or a common pathologic process induces a network of metabolic (including alterations in glucose, salt, insulin and lipid metabolism) and hemodynamic abnormalities ( due to renin-angiotensin system stimulation, sympathetic overactivity and decreased nitric oxide bioavailability) which are followed by anaemia, hypercoagulability, tissue ischemia, arterial stiffness, hypertension, renal and cardiac dysfunction, the other features of the circulatory syndrome (Fig. 2).
UNDERLYING PATHOLOGY
It seems that inflammation is the fuel that “burns” the circulatory syndrome. The association between inflammatory markers and both diabetes and hypertension is so strong that these diseases has recently been redefined as inflammatory diseases, as has atheroma[119-122]. Likewise, insulin resistance has a strong link with inflammation, although additional mechanisms such as genetic factors may interfere in this relationship[123]. Additionally, inflammation is known to be a modifier of the relationship between microalbuminuria and hypertension [86, 124]. Hence, CRP has been frequently promoted as a part of the metabolic syndrome [11, 27, 125].
Advanced glycation end products (AGEs) which accumulate in diabetes activate inflammatory cells[119]. Moreover, inflammatory markers such as CRP are now considered to be independent predictors of diabetes [119] and its complications including left ventricular hypertrophy, endothelial dysfunction, albuminuria and renal failure [74, 95, 126, 127].
In addition, high LDL cholesterol induces oxidative stress and increases inflammation [128]. On the other hand HDL and Apolipoprotein A1 have anti-inflammatory and anti-oxidant properties [129].
There is a close relationship between inflammation and hypercoagulability [87, 130]. Furthermore, hypercoagulability is also linked to the metabolic syndrome, dyslipidemia, anaemia and even the hemodynamic response to exercise [129, 131-133]. It is associated with a poorer outcome in coronary artery disease, heart failure and is correlated with the severity of target-organ damage including renal impairment [134-136]. Moreover, diabetic and metabolic syndrome patients are at high risk for thrombotic events [137-139] and have an increased level of clotting factors including tissue plasminogen activator (tPA) and von Willenbrand Factor (vWF) and D-dimer when compared to the controls [140]. Additionally insulin and lipids may have direct effects on inhibition of coagulation and platelet function through the nitric oxide pathway, which is impaired in patients with diabetes [141].
By and large, this interlinking mesh of inflammatory mediators, oxidative stress, endotheliopathy and hypercoagulability makes a common soil for development of the circulatory syndrome.
CLINICAL APPLICATIONS
The above description of the “circulatory (MARC) syndrome” clearly has clinical applications. Identification of commonly evaluated markers such as blood pressure, glucose and lipids in a patient should prompt a search for other circulatory syndrome markers. Suspicion about the presence of the circulatory syndrome should facilitate decision making for diagnostic procedures in asymptomatic but high risk patients. Also treatment of each syndrome component should be accompanied by management of the other components. Furthermore, any difficulty in treating one circulatory syndrome marker should probably leads to a more aggressive treatment program for other components as is currently proposed in patients with renal disease, diabetes and associated hypertension. Hence, management of circulatory syndrome would need an interdisciplinary approach with the collaboration of different medical subspecialties.
PERSPECTIVE
Although circulatory syndrome is theoretically logical, and is supported by evidence from separate studies for each component, integrated epidemiologic studies are required to examine its consistency, validity and predictive value for adverse outcomes such as disability and mortality. Also it is important to investigate whether other markers can be added to the cluster and improve its performance.
CONCLUSION
Circulatory (MARC) syndrome is a cluster of interactive risk markers, combining renal, arterial, cardiac and metabolic elements into a coherent whole. These components develop on a background of inflammation, oxidative stress, hypercoagulability and endotheliopathy and while accelerated by one another, other factors including aging, obesity, physical inactivity and smoking contribute to the syndrome pathology. It’s consistency, diagnostic criteria and clinical utility still need to be investigated.
ACKNOWLEDGEMENT
None declared.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Stedman's Medical Dictionary. 27th edition ed. Baltimore: Lippincott, Williams and Wilkins; 2000. p. 1746. [Google Scholar]
- 2.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Ormezzano O, JP Baguet, P Francois, et al. Is there any real target organ damage associated with white-coat normotension? Clin Auton Res. 2004;14(3):160–6. doi: 10.1007/s10286-004-0174-2. [DOI] [PubMed] [Google Scholar]
- 4.Balkau B, MA Charles. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16(5):442–3. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 5.Einhorn D, GM Reaven, RH Cobin, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9(3):237–52. [PubMed] [Google Scholar]
- 6.Grundy SM, HB Brewer, Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 7.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 9.Alexander CM, PB Landsman, SM Teutsch, et al. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52(5):1210–4. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 10.Athyros VG, ES Ganotakis, MS Elisaf, et al. Prevalence of vascular disease in metabolic syndrome using three proposed definitions. Int J Cardiol. 2006. [DOI] [PubMed]
- 11.Kahn R, J Buse, E Ferrannini, et al. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28(9):2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 12.Malik S, ND Wong, SS Franklin, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 13.Girman CJ, T Rhodes, M Mercuri, et al. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) Am J Cardiol. 2004;93(2):136–41. doi: 10.1016/j.amjcard.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173(2):309–14. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Hunt KJ, RG Resendez, K Williams, et al. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110(10):1251–7. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 16.Scuteri A, SS Najjar, CH Morrell, et al. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care. 2005;28(4):882–7. doi: 10.2337/diacare.28.4.882. [DOI] [PubMed] [Google Scholar]
- 17.Cameron A. The metabolic syndrome: Validity and utility of clinical definitions for cardiovascular disease and diabetes risk prediction. Maturitas. 2010;65:117–21. doi: 10.1016/j.maturitas.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Ford ES, MB Schulze, T Pischon, et al. Metabolic syndrome and risk of incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam Study. Cardiovascular Diabetology. 2008;7(35) doi: 10.1186/1475-2840-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am. 2004;33(2):283–303. doi: 10.1016/j.ecl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Robbins DC, L Andersen, R Bowsher, et al. Report of the American Diabetes Association's Task Force on standardization of the insulin assay. Diabetes. 1996;45(2):242–56. doi: 10.2337/diab.45.2.242. [DOI] [PubMed] [Google Scholar]
- 21.Wallace TM, JC Levy, DR Matthews. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 22.Liao Y, S Kwon, S Shaughnessy, et al. Critical evaluation of adult treatment panel III criteria in identifying insulin resistance with dyslipidemia. Diabetes Care. 2004;27(4):978–83. doi: 10.2337/diacare.27.4.978. [DOI] [PubMed] [Google Scholar]
- 23.Bruno G, F Merletti, A Biggeri, et al. Metabolic syndrome as a predictor of all-cause and cardiovascular mortality in type 2 diabetes: the Casale Monferrato Study. Diabetes Care. 2004;27(11):2689–94. doi: 10.2337/diacare.27.11.2689. [DOI] [PubMed] [Google Scholar]
- 24.Resnick HE, K Jones, G Ruotolo, et al. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic american indians: the Strong Heart Study. Diabetes Care. 2003;26(3):861–7. doi: 10.2337/diacare.26.3.861. [DOI] [PubMed] [Google Scholar]
- 25.Marroquin OC, KE Kip, DE Kelley, et al. Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women: a report from the Women's Ischemia Syndrome Evaluation. Circulation. 2004;109(6):714–21. doi: 10.1161/01.CIR.0000115517.26897.A7. [DOI] [PubMed] [Google Scholar]
- 26.Lakka HM, DE Laaksonen, TA Lakka, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288(21):2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM, JI Cleeman, SR Daniels, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 28.Amar J, JB Ruidavets, B Chamontin, et al. Arterial stiffness and cardiovascular risk factors in a population-based study. J Hypertens. 2001;19(3):381–7. doi: 10.1097/00004872-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Asmar R, A Rudnichi, J Blacher, et al. Pulse pressure and aortic pulse wave are markers of cardiovascular risk in hypertensive population. Am J Hypertension. 2001;14:91–7. doi: 10.1016/s0895-7061(00)01232-2. [DOI] [PubMed] [Google Scholar]
- 30.Blacher J, ME Safar, AP Guerin, et al. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney international. 2003;63:1852–60. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 31.Boutouyrie P, Tropeano AI, R Asmar, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 32.London GM, JN Cohn. Prognostic application of arterial stiffness: task forces. Am J Hypertens. 2002;15(8):754–8. doi: 10.1016/s0895-7061(02)02966-7. [DOI] [PubMed] [Google Scholar]
- 33.Laurent S, J Cockcroft, L Van Bortel, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 34.Mourad A, A Khoshdel, S Carney, et al. Haemodialysis-unresponsive blood pressure: cardiovascular mortality predictor? Nephrology (Carlton) 2005;10(5):438–41. doi: 10.1111/j.1440-1797.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 35.Khoshdel AR, SL Carney, P Trevillian, et al. Evaluation of arterial stiffness and pulse wave reflection for cardiovascular risk assessment in diabetic and nondiabetic kidney transplant recipients. Iran J Kidney Dis. 2010;4(3):237–43. [PubMed] [Google Scholar]
- 36.Khoshdel AR, SL Carney. HEMODYNAMIC RESPONSE TO EXERCISE PREDICTS THE DEVELOPMENT OF SEVERE RENAL FAILURE. American Heart Association; in 14th World Congress on Heart Disease; July 2008; Toronto, CANADA. 2008. [Google Scholar]
- 37.Khoshdel AR, SL Carney, S White. Disturbed hemodynamic cardiac exercise stress test response in non-smoking, normolipidemic, normotensive, diabetic subjects. Diabetes Res Clin Pract. 2007;75(2):193–9. doi: 10.1016/j.diabres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Khoshdel AR, SL Carney, BR Nair, et al. Better management of cardiovascular diseases by pulse wave velocity: combining clinical practice with clinical research using evidence-based medicine. Clin Med Res. 2007;5(1):45–52. doi: 10.3121/cmr.2007.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khoshdel AR, A Thakkinstian, SL Carney, et al. Estimation of an age-specific reference interval for pulse wave velocity: a meta-analysis. J Hypertens. 2006;24(7):1231–7. doi: 10.1097/01.hjh.0000234098.85497.31. [DOI] [PubMed] [Google Scholar]
- 40.Khoshdel AR, SL Carney, A Gillies, et al. The impact of diabetes mellitus on arterial stiffness in end-stage renal disease (Abstract) Nephrology. 2005. p. A21.
- 41.Khoshdel AR. Metabolic syndrome: Erasing the problem or constructing a better answer. BMJ rapid response at www.bmj.com . 2008. Mar 27th,
- 42.Knudsen ST, PL Poulsen, KW Hansen, et al. Pulse pressure and diurnal blood pressure variation: association with micro- and macrovascular complications in type 2 diabetes. Am J Hypertens. 2002;15(3):244–50. doi: 10.1016/s0895-7061(01)02281-6. [DOI] [PubMed] [Google Scholar]
- 43.Thomas MC, RJ MacIsaac, C Tsalamandris, et al. Anemia in patients with type 1 diabetes. J Clin Endocrinol Metab. 2004;89(9):4359–63. doi: 10.1210/jc.2004-0678. [DOI] [PubMed] [Google Scholar]
- 44.Eckel RH, SM Grundy, PZ Zimmet. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 45.Bongartz LG, MJ Cramer, PA Doevendans, et al. The severe cardiorenal syndrome: 'Guyton revisited'. Eur Heart J. 2005;26(1):11–7. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 46.Becker B, F Kronenberg, JT Kielstein, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16(4):1091–8. doi: 10.1681/ASN.2004090742. [DOI] [PubMed] [Google Scholar]
- 47.Saydah SH, J Fradkin, CC Cowie. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. Jama. 2004;291(3):335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 48.Fox CS, MG Larson, EP Leip, et al. Predictors of new-onset kidney disease in a community-based population. Jama. 2004;291(7):844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 49.Amann K, C Wanner, E Ritz. Cross-Talk between the Kidney and the Cardiovascular System. J Am Soc Nephrol. 2006;17(8):2112–9. doi: 10.1681/ASN.2006030204. [DOI] [PubMed] [Google Scholar]
- 50.Mattu RK, J Trevelyan, EW Needham, et al. Lipoprotein lipase gene variants relate to presence and degree of microalbuminuria in Type II diabetes. Diabetologia. 2002;45(6):905–13. doi: 10.1007/s00125-002-0824-7. [DOI] [PubMed] [Google Scholar]
- 51.Zoccali C. Cardiorenal risk as a new frontier of nephrology: research needs and areas for intervention. Nephrol Dial Transplant. 2002;17( Suppl 11):50–4. doi: 10.1093/ndt/17.suppl_11.50. [DOI] [PubMed] [Google Scholar]
- 52.Locatelli F, J Bommer, GM London, et al. Cardiovascular disease determinants in chronic renal failure: clinical approach and treatment. Nephrol Dial Transplant. 2001;16(3):459–68. doi: 10.1093/ndt/16.3.459. [DOI] [PubMed] [Google Scholar]
- 53.Colhoun HM, DJ Betteridge, PN Durrington, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 54.Geluk CA, FW Asselbergs, HL Hillege, et al. Impact of statins in microalbuminuric subjects with the metabolic syndrome: a substudy of the PREVEND Intervention Trial. Eur Heart J. 2005;26(13):1314–20. doi: 10.1093/eurheartj/ehi253. [DOI] [PubMed] [Google Scholar]
- 55.Sola S, MQ Mir, S Lerakis, et al. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol. 2006;47(2):332–7. doi: 10.1016/j.jacc.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 56.Elisaf M, DP Mikhailidis. Statins and renal function. Angiology. 2002;53(5):493–502. doi: 10.1177/000331970205300501. [DOI] [PubMed] [Google Scholar]
- 57.Dogra GK, GF Watts, DC Chan, et al. Statin therapy improves brachial artery vasodilator function in patients with Type 1 diabetes and microalbuminuria. Diabet Med. 2005;22(3):239–42. doi: 10.1111/j.1464-5491.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 58.Bakker SJ, RT Gansevoort, D de Zeeuw. Metabolic syndrome: a fata morgana? Nephrol Dial Transplant. 2007;22(1):15–20. doi: 10.1093/ndt/gfl581. [DOI] [PubMed] [Google Scholar]
- 59.Global Health Risks: Morbidity and burden of disease attributable to selected major risks. WHO Global Reports. 2009;70 [Google Scholar]
- 60.Zandi-Nejad K, VA Luyckx, BM Brenner. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47(3):502–8. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 61.Vlachopoulos C, M O'Rourke. Genesis of the normal and abnormal arterial pulse. Curr Probl Cardiol. 2000;25(5):303–67. doi: 10.1067/mcd.2000.104057. [DOI] [PubMed] [Google Scholar]
- 62.Fuenmayor N, E Moreira, LX Cubeddu. Salt sensitivity is associated with insulin resistance in essential hypertension. Am J Hypertens. 1998;11(4 Pt 1):397–402. doi: 10.1016/s0895-7061(97)00490-1. [DOI] [PubMed] [Google Scholar]
- 63.Sharma AM, U Schorr, A Distler. Insulin resistance in young salt-sensitive normotensive subjects. Hypertension. 1993;21(3):273–9. doi: 10.1161/01.hyp.21.3.273. [DOI] [PubMed] [Google Scholar]
- 64.Weir MR. Impact of salt intake on blood pressure and proteinuria in diabetes: importance of the renin-angiotensin system. Miner Electrolyte Metab. 1998;24(6):438–45. doi: 10.1159/000057405. [DOI] [PubMed] [Google Scholar]
- 65.Resnick LM. Ionic basis of hypertension, insulin resistance, vascular disease, and related disorders. The mechanism of "syndrome X". Am J Hypertens. 1993;6(4):123S–134S. doi: 10.1093/ajh/6.4s.123s. [DOI] [PubMed] [Google Scholar]
- 66.Cohn JN, AA Quyyumi, NK Hollenberg, et al. Surrogate markers for cardiovascular disease: functional markers. Circulation. 2004;109(25 ) Suppl 1:IV31–46. doi: 10.1161/01.CIR.0000133442.99186.39. [DOI] [PubMed] [Google Scholar]
- 67.Kimoto E, T Shoji, K Shinohara, et al. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes. 2003;52(2):448–52. doi: 10.2337/diabetes.52.2.448. [DOI] [PubMed] [Google Scholar]
- 68.Hopkins KD, ED Lehmann, RL Jones, et al. A family history of NIDDM is associated with decreased aortic distensibility in normal healthy young adult subjects. Diabetes Care. 1996;19(5):501–3. doi: 10.2337/diacare.19.5.501. [DOI] [PubMed] [Google Scholar]
- 69.Ratto E, G Leoncini, F Viazzi, et al. Ambulatory arterial stiffness index and renal abnormalities in primary hypertension. J Hypertens. 2006;24(10):2033–8. doi: 10.1097/01.hjh.0000244953.62362.41. [DOI] [PubMed] [Google Scholar]
- 70.Pirro M, G Schillaci, G Savarese, et al. Low-grade systemic inflammation impairs arterial stiffness in newly diagnosed hypercholesterolaemia. Eur J Clin Invest. 2004;34(5):335–41. doi: 10.1111/j.1365-2362.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- 71.Wilkinson I, and JR Cockcroft. Cholesterol, lipids and arterial stiffness. Adv Cardiol. 2007;44:261–77. doi: 10.1159/000096747. [DOI] [PubMed] [Google Scholar]
- 72.Mottram PM, BA Haluska, R Leano, et al. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91(12):1551–6. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gates PE, H Tanaka, J Graves, et al. Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur Heart J. 2003;24(24):2213–20. doi: 10.1016/j.ehj.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 74.Ritz E. Heart and kidney: fatal twins? Am J Med. 2006;119(5 ) Suppl 1:S31–9. doi: 10.1016/j.amjmed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Cruickshank K, L Riste, SG Anderson, et al. Aortic Pulse-Wave Velocity and its relationship to Mortality in Diabetes and Glucose intolerance. Circulation. 2002;106:2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 76.Laurent S, P Boutouyrie, R Asmar, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 77.London GM, J Blacher, B Pannier, et al. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38(3):434–8. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 78.Meaume S, A Rudnichi, A Lynch, et al. Aortic pulse wave velocity as a marker of cardiovascular disease in subjects over 70 years old. J Hypertens. 2001;19(5):871–7. doi: 10.1097/00004872-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 79.Yokoyama H, M Kuramitsu, S Kanno, et al. Relationship between metabolic syndrome components and vascular properties in Japanese type 2 diabetic patients without cardiovascular disease or nephropathy. Diabetes Res Clin Pract. 2006. [DOI] [PubMed]
- 80.Cerasola G, S Cottone, G Mule, et al. Microalbuminuria, renal dysfunction and cardiovascular complication in essential hypertension. J Hypertens. 1996;14(7):915–20. doi: 10.1097/00004872-199607000-00016. [DOI] [PubMed] [Google Scholar]
- 81.Cruickshank K, L Riste, SG Anderson, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106(16):2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 82.Niskanen L, M Uusitupa, H Sarlund, et al. Microalbuminuria predicts the development of serum lipoprotein abnormalities favouring atherogenesis in newly diagnosed type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1990;33(4):237–43. doi: 10.1007/BF00404802. [DOI] [PubMed] [Google Scholar]
- 83.Smith A, J Karalliedde, L De Angelis, et al. Aortic pulse wave velocity and albuminuria in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(4):1069–75. doi: 10.1681/ASN.2004090769. [DOI] [PubMed] [Google Scholar]
- 84.Kohara K, Y Tabara, R Tachibana, et al. Microalbuminuria and arterial stiffness in a general population: the Shimanami Health Promoting Program (J-SHIPP) study. Hypertens Res. 2004;27(7):471–7. doi: 10.1291/hypres.27.471. [DOI] [PubMed] [Google Scholar]
- 85.Peppa-Patrikiou M, M Dracopoulou, and C Dacou-Voutetakis. Urinary endothelin in adolescents and young adults with insulin-dependent diabetes mellitus: relation to urinary albumin, blood pressure, and other factors. Metabolism. 1998;47(11):1408–12. doi: 10.1016/s0026-0495(98)90314-6. [DOI] [PubMed] [Google Scholar]
- 86.Pedrinelli R, G Dell'Omo, V Di Bello, et al. Low-grade inflammation and microalbuminuria in hypertension. Arterioscler Thromb Vasc Biol. 2004;24(12):2414–9. doi: 10.1161/01.ATV.0000147415.40692.7f. [DOI] [PubMed] [Google Scholar]
- 87.Aso Y, N Yoshida, K Okumura, et al. Coagulation and inflammation in overt diabetic nephropathy: association with hyperhomocysteinemia. Clin Chim Acta. 2004;348(1-2):139–45. doi: 10.1016/j.cccn.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Kelbaek H, T Jensen, B Feldt-Rasmussen, et al. Impaired left-ventricular function in insulin-dependent diabetic patients with increased urinary albumin excretion. Scand J Clin Lab Invest. 1991;51(5):467–73. doi: 10.3109/00365519109091641. [DOI] [PubMed] [Google Scholar]
- 89.Estacio RO, JG Regensteiner, EE Wolfel, et al. The association between diabetic complications and exercise capacity in NIDDM patients. Diabetes Care. 1998;21(2):291–5. doi: 10.2337/diacare.21.2.291. [DOI] [PubMed] [Google Scholar]
- 90.Peralta CA, M Kurella, JC Lo, et al. The metabolic syndrome and chronic kidney disease. Curr Opin Nephrol Hypertens. 2006;15(4):361–5. doi: 10.1097/01.mnh.0000232875.27846.7e. [DOI] [PubMed] [Google Scholar]
- 91.Magri P, MA Rao, S Cangianiello, et al. Early impairment of renal hemodynamic reserve in patients with asymptomatic heart failure is restored by angiotensin II antagonism. Circulation. 1998;98(25):2849–54. doi: 10.1161/01.cir.98.25.2849. [DOI] [PubMed] [Google Scholar]
- 92.Sarafidis PA, LM Ruilope. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol. 2006;26(3):232–44. doi: 10.1159/000093632. [DOI] [PubMed] [Google Scholar]
- 93.Ruilope LM, DJ van Veldhuisen, E Ritz, et al. Renal function: the Cinderella of cardiovascular risk profile. J Am Coll Cardiol. 2001;38(7):1782–7. doi: 10.1016/s0735-1097(01)01627-8. [DOI] [PubMed] [Google Scholar]
- 94.Safar ME, GM London, GE Plante. Arterial stiffness and kidney function. Hypertension. 2004;43(2):163–8. doi: 10.1161/01.HYP.0000114571.75762.b0. [DOI] [PubMed] [Google Scholar]
- 95.Zoccali C, R Maio, G Tripepi, et al. Inflammation as a mediator of the link between mild to moderate renal insufficiency and endothelial dysfunction in essential hypertension. J Am Soc Nephrol. 2006;17(4 ) Suppl 2:S64–8. doi: 10.1681/ASN.2005121345. [DOI] [PubMed] [Google Scholar]
- 96.Levine DZ. Hyperfiltration, nitric oxide, and diabetic nephropathy. Curr Hypertens Rep. 2006;8(2):153–7. doi: 10.1007/s11906-006-0012-0. [DOI] [PubMed] [Google Scholar]
- 97.Palatini P, P Mormino, F Dorigatti, et al. Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int. 2006;70(3):578–84. doi: 10.1038/sj.ki.5001603. [DOI] [PubMed] [Google Scholar]
- 98.Weiss G, LT Goodnough. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 99.Srivastava PM, MC Thomas, P Calafiore, et al. Diastolic dysfunction is associated with anaemia in patients with Type II diabetes. Clin Sci (Lond) 2006;110(1):109–16. doi: 10.1042/CS20050184. [DOI] [PubMed] [Google Scholar]
- 100.Smith KJ, AJ Bleyer, WC Little, et al. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59(3):538–48. doi: 10.1016/s0008-6363(03)00468-1. [DOI] [PubMed] [Google Scholar]
- 101.Ritz E. Managing anaemia and diabetes: a future challenge for nephrologists. Nephrol Dial Transplant. 2005;20 (Suppl 6):vi21–5. doi: 10.1093/ndt/gfh1093. [DOI] [PubMed] [Google Scholar]
- 102.Kovesdy CP, BK Trivedi, K Kalantar-Zadeh, et al. Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int. 2006;69(3):560–4. doi: 10.1038/sj.ki.5000105. [DOI] [PubMed] [Google Scholar]
- 103.Streeter RP, Mancini D. Treatment of anemia in the patient with heart failure. Curr Treat Options Cardiovasc Med. 2005;7(4):327–32. doi: 10.1007/s11936-005-0043-x. [DOI] [PubMed] [Google Scholar]
- 104.Katzmarzyk PT, TS Church, SN Blair. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med. 2004;164(10):1092–7. doi: 10.1001/archinte.164.10.1092. [DOI] [PubMed] [Google Scholar]
- 105.Jouven X, JP Empana, PJ Schwartz, et al. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–8. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 106.Williams SG, M Jackson, LL Ng, et al. Exercise duration and peak systolic blood pressure are predictive of mortality in ambulatory patients with mild-moderate chronic heart failure. Cardiology. 2005;104(4):221–6. doi: 10.1159/000088257. [DOI] [PubMed] [Google Scholar]
- 107.Bonapace S, A Rossi, M Cicoira, et al. Aortic distensibility independently affects exercise tolerance in patients with dilated cardiomyopathy. Circulation. 2003;107(12):1603–8. doi: 10.1161/01.CIR.0000051458.39176.43. [DOI] [PubMed] [Google Scholar]
- 108.Boyer JK, S Thanigaraj, KB Schechtman, et al. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93(7):870–5. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 109.Vinereanu D, E Nicolaides, AC Tweddel, et al. Subclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci (Lond) 2003;105(5):591–9. doi: 10.1042/CS20030168. [DOI] [PubMed] [Google Scholar]
- 110.McMahon AC, RU Naqvi, MJ Hurst, et al. Diastolic dysfunction and abnormality of the Na+/Ca2+ exchanger in single uremic cardiac myocytes. Kidney Int. 2006;69(5):846–51. doi: 10.1038/sj.ki.5000193. [DOI] [PubMed] [Google Scholar]
- 111.Ando K, T Fujita. Anti-diabetic effect of blockade of the renin-angiotensin system. Diabetes Obes Metab. 2006;8(4):396–403. doi: 10.1111/j.1463-1326.2005.00526.x. [DOI] [PubMed] [Google Scholar]
- 112.Derosa G, AF Cicero, A Dangelo, et al. Thiazolidinedione effects on blood pressure in diabetic patients with metabolic syndrome treated with glimepiride. Hypertens Res. 2005;28(11):917–24. doi: 10.1291/hypres.28.917. [DOI] [PubMed] [Google Scholar]
- 113.Raji A, J Plutzky. Insulin resistance, diabetes, and atherosclerosis: thiazolidinediones as therapeutic interventions. Curr Cardiol Rep. 2002;4(6):514–21. doi: 10.1007/s11886-002-0116-3. [DOI] [PubMed] [Google Scholar]
- 114.Undas A, KE Brummel-Ziedins, KG Mann. Statins and blood coagulation. Arterioscler Thromb Vasc Biol. 2005;25(2):287–94. doi: 10.1161/01.ATV.0000151647.14923.ec. [DOI] [PubMed] [Google Scholar]
- 115.Fliser D, K Buchholz, H Haller. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110(9):1103–7. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- 116.Gross ML, K Amann, E Ritz. Nephron number and renal risk in hypertension and diabetes. J Am Soc Nephrol. 2005;16 (Suppl 1):S27–9. doi: 10.1681/asn.2004110967. [DOI] [PubMed] [Google Scholar]
- 117.Hobson A, PA Kalra, PR Kalra. Cardiology and nephrology: time for a more integrated approach to patient care? Eur Heart J. 2005;26(16):1576–8. doi: 10.1093/eurheartj/ehi375. [DOI] [PubMed] [Google Scholar]
- 118.Lee YJ, JC Tsai. ACE gene insertion/deletion polymorphism associated with 1998 World Health Organization definition of metabolic syndrome in Chinese type 2 diabetic patients. Diabetes Care. 2002;25(6):1002–8. doi: 10.2337/diacare.25.6.1002. [DOI] [PubMed] [Google Scholar]
- 119.Zozulinska D, B Wierusz-Wysocka. Type 2 diabetes mellitus as inflammatory disease. Diabetes Res Clin Pract. 2006.
- 120.Li JJ, CH Fang, RT Hui. Is hypertension an inflammatory disease? Med Hypotheses. 2005;64(2):236–40. doi: 10.1016/j.mehy.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 121.Li JJ, JL Chen. Inflammation may be a bridge connecting hypertension and atherosclerosis. Med Hypotheses. 2005;64(5):925–9. doi: 10.1016/j.mehy.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 122.Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. 2003;169(2):203–214. doi: 10.1016/s0021-9150(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 123.Shoelson SE, J Lee, AB Goldfine. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stuveling EM, SJ Bakker, H Hillege, et al. C-reactive protein modifies the relationship between blood pressure and microalbuminuria. Hypertension. 2004;43(4):791–6. doi: 10.1161/01.HYP.0000120125.08867.42. [DOI] [PubMed] [Google Scholar]
- 125.Ridker PM, PW Wilson, SM Grundy. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109(23):2818–25. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 126.Stehouwer CD, MA Gall, JW Twisk, et al. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157–65. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 127.Palmieri V, RP Tracy, MJ Roman, et al. Relation of left ventricular hypertrophy to inflammation and albuminuria in adults with type 2 diabetes: the strong heart study. Diabetes Care. 2003;26(10):2764–9. doi: 10.2337/diacare.26.10.2764. [DOI] [PubMed] [Google Scholar]
- 128.Ridker PM, N Rifai, NR Cook, et al. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. Jama. 2005;294(3):326–33. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 129.Sagastagoitia JD, Y Saez, M Vacas, et al. Association between inflammation, lipid and hemostatic factors in patients with stable angina. Thromb Res. 2006. [DOI] [PubMed]
- 130.Devaraj S, RS Rosenson, I Jialal. Metabolic syndrome: an appraisal of the pro-inflammatory and procoagulant status. Endocrinol Metab Clin North Am. 2004;33(2):431–53. doi: 10.1016/j.ecl.2004.03.008. table of contents. [DOI] [PubMed] [Google Scholar]
- 131.Matteucci E, J Rosada, M Pinelli, et al. Systolic blood pressure response to exercise in type 1 diabetes families compared with healthy control individuals. J Hypertens. 2006;24(9):1745–51. doi: 10.1097/01.hjh.0000242398.60838.5d. [DOI] [PubMed] [Google Scholar]
- 132.Keung YK, and J Owen. Iron deficiency and thrombosis: literature review. Clin Appl Thromb Hemost. 2004;10(4):387–91. doi: 10.1177/107602960401000412. [DOI] [PubMed] [Google Scholar]
- 133.Nieuwdorp M, ES Stroes, JC Meijers, et al. Hypercoagulability in the metabolic syndrome. Curr Opin Pharmacol. 2005;5(2):155–9. doi: 10.1016/j.coph.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 134.Sechi LA, L Zingaro, C Catena, et al. Relationship of fibrinogen levels and hemostatic abnormalities with organ damage in hypertension. Hypertension. 2000;36(6):978–85. doi: 10.1161/01.hyp.36.6.978. [DOI] [PubMed] [Google Scholar]
- 135.Danesh J, S Lewington, SG Thompson, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. Jama. 2005;294(14):1799–809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 136.Marcucci R, AM Gori, F Giannotti, et al. Markers of hypercoagulability and inflammation predict mortality in patients with heart failure. J Thromb Haemost. 2006;4(5):1017–22. doi: 10.1111/j.1538-7836.2006.01916.x. [DOI] [PubMed] [Google Scholar]
- 137.Vicari AM, MV Taglietti, F Pellegatta, et al. Deranged platelet calcium homeostasis in diabetic patients with end-stage renal failure. A possible link to increased cardiovascular mortality? Diabetes Care. 1996;19(10):1062–6. doi: 10.2337/diacare.19.10.1062. [DOI] [PubMed] [Google Scholar]
- 138.Haffner SM, L Mykkanen, A Festa, et al. Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation. 2000;101(9):975–80. doi: 10.1161/01.cir.101.9.975. [DOI] [PubMed] [Google Scholar]
- 139.Isomaa B, P Almgren, T Tuomi, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 140.Bloomgarden ZT. Third Annual World Congress on the Insulin Resistance Syndrome: Atherothrombotic disease. Diabetes Care. 2006;29(8):1973–80. doi: 10.2337/dc06-zb08. [DOI] [PubMed] [Google Scholar]
- 141.Juhan-Vague I, MC Alessi, A Mavri, et al. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost. 2003;1(7):1575–9. doi: 10.1046/j.1538-7836.2003.00279.x. [DOI] [PubMed] [Google Scholar]