Abstract
The large variation in disposition known for most drugs is also true for anabolic androgenic steroids. Genetic factors are probably the single most important cause of this variation. Further, there are reasons to believe that there is a corresponding variation in efficacy of doping agents. Doped individuals employ a large variety of doping strategies in respect of choice of substance, dose, dose interval, duration of treatment and use of other drugs for enforcement of effects or correction of side effects.
Metabolic steps up-stream and down-stream of testosterone are genetically variable and contribute substantially to the variation in disposition of testosterone, the most common doping agent in sports and in society. Large inter- and intra-ethnic variation in testosterone glucuronidation and excretion is described as well as the pit-falls in evaluation of testosterone doping test results. The hydrolysis and bioactivation of testosterone enanthate is also genetically variable yielding a 2–3 fold variation in excretion rate and serum concentration, thereby implicating a substantial variation in ‘efficacy’ of testosterone.
Given this situation it is logical to adopt the new findings in the doping control programme. The population based cut-off level for the testosterone : epitestosterone ratio should be replaced by a Bayesian interpretation of consecutive tests in the same individual. When combined with the above genetic information the sensitivity of the test is considerably improved. The combination of the three approaches should reduce the rate of falsely negative or positive results and the number of expensive follow-up tests, stipulated by the World Anti-Doping Agency.
Keywords: androgens, doping, genetic variation, testosterone, UGT2B17
Introduction
The capacity of testosterone to enhance physical strength has been known since the late nineteenth century [1], but its real physiological role as the male sex hormone was not revealed until the mid 1930s. The characterization and synthesis of testosterone resulted in the 1939 Nobel Prize in chemistry to Butenandt and Ruzicka.
Testosterone and its pharmacological congeners, whether natural or synthesized, are collectively known as anabolic androgenic steroids (AAS) [2]. They have a clear place in the clinical treatment of certain endocrine disorders such as primary or secondary hypogonadism. However, the use of testosterone in ageing men is not an accepted principle everywhere. Because of the capacity of AAS to enhance muscle strength and performance and affect the physical appearance, the abuse and popularity of these agents has increased markedly in the society and in sports [3]–[5].
The effects of androgens are mediated via the androgen receptor (AR) which is present in most organs. Important functions regulated via the ARs include muscle protein metabolism, bone metabolism, sexual and cognitive functions, erythropoiesis and plasma lipid concentrations [6]. These biological effects are specific for each organ and target tissue [7]. In myocytes, testosterone binds to the intracellular AR, initiating an activation cascade with conformational changes and nuclear translocation of the AR-steroid complex. Binding of the complex to androgen responsive elements (ARE) in the DNA results in specific activation or repression of the transcription in target genes. Testosterone enanthate (∼300 mg week−1) has been shown to increase muscular strength and power compared with placebo. This effect may be observed already after 3 weeks [8].
The first reports of athletes using anabolic steroids searching for an increase in weight and power appeared in 1954 [9]. Among all chemical substances prohibited by the World Anti-Doping Agency (WADA) AAS were the most frequently detected doping agents in 2010, accounting for about 60% of positive results in accredited doping laboratories around the world. Among these testosterone, stanozolol and nandrolone were frequently identified (http://www.wada-ama.org/en/).
Many attempts to estimate the misuse of AAS in society have been made. However, such estimates are difficult to make since the misuse of AAS is often concealed. Estimates of misuse suffer from difficulties in carrying out reliable studies of illicit drug use. In the USA, one assumes that between 1 and 3 million people are thought to have misused or are misusing AAS. The corresponding estimates for Sweden, in which the abuse has been criminalized, vary between 10 000 and 100 000, in a population of 9 million [10]. These estimates indicate a misuse rate of about 1% of the respective populations. The frequency of abuse in non-elite sports varies depending on the sport. In the UK AAS abuse among community weight trainers and health clubs is considered to be 15–30%. The majority of users are people in gyms who misuse these drugs primarily for cosmetic purposes rather than to enhance physical performance [11]. Other groups of AAS users include men (and women) with a propensity for drug misuse and criminality, established drug addicts and criminals.
The most spectacular and highlighted misuse is among elite athletes. Even though this group of abusers is limited [12] the publicity may be extensive. Therefore, doping with natural or exogenous androgen derivatives is a severe challenge to the vision, morals and ethics of sports all over the world.
Androgens
Endogenous androgens
Cholesterol is the starting substance for formation of glucocorticoids, mineralocorticoids and sex steroids (Figure 1). Precursors of androgens are formed in the adrenals and biotransformed in the endocrine target organs, the gonads and the prostate gland. Dihydrotestosterone (DHT) is formed from testosterone in the prostate and is a more potent androgen than testosterone itself. Some of the testosterone precursors have weak androgenic effects such as dehydroepiandrosterone (DHEA) and androstenedione. They may be present in dietary products and abused per se.
Figure 1.
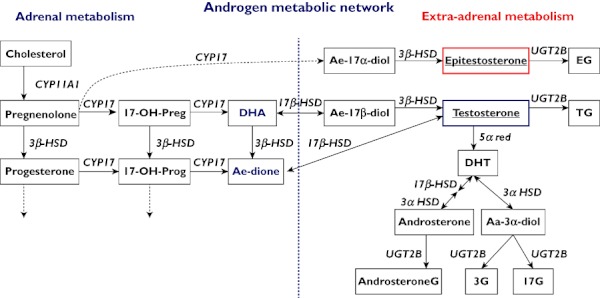
Metabolic network of androgens. Pathways left of the vertical striped line mainly in the adrenals, pathways to the right mainly in the gonads and the prostate. Preg = pregnenolone, Prog = progesterone, DHA = dehydroepiandrosterone, Ae-dione = androstenedione, Ae-17α-diol = Androstene-17α-diol, Ae-17β-diol = Androstene-17β-diol, Aa-3α-diol = Androstane3α-diol, DHT = dihydrotestosterone, G = glucuronide
Most of the end products are eliminated via the kidneys after conjugation with glucuronic acid or sulphate groups by enzymes in the uridine glucuronosyl transferase (UGT) and sulphate transferase (SULT) super families, respectively. As seen in Figure 2 the UGT enzymes have different selectivities for the androgens and androgen metabolites. The specificity, the activity and the genetic variation of the various UGT enzymes is of particular interest in the conjugation and excretion of testosterone and epitestosterone as these steroids and their conjugates are quantified in the urine in conventional testosterone doping tests. As described below, genetic variation in other steroid metabolizing enzymes may also influence the disposition of testosterone and other AAS. The relative contribution of the enzymes to the variation in metabolism and excretion is important to investigate in order to discriminate between the natural and a doping induced urinary excretion profile in the interpretation of results of doping tests.
Figure 2.
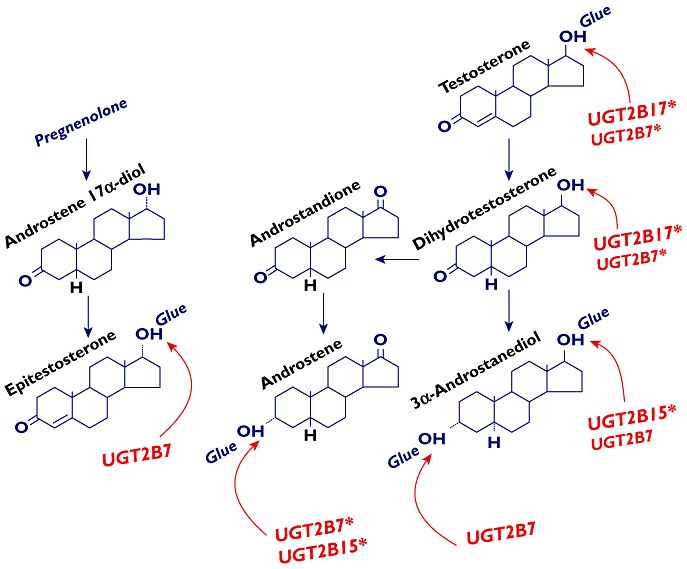
Uridine diphospho glucuronosyl transferase (UGT) enzymes in androgen glucuronidation. UGT2B preference for different substrates is indicated by arrows at the glucuronidation site. Small letters in the enzyme names indicate less quantitative importance. An asterisk indicates effect on androgen disposition by a polymorphism in the enzyme gene
Exogenous androgens
There are a large number of chemical substances with androgenic characteristics and abuse potential. These include legal drugs and illegal chemical entities available as pure compounds or constituents in dietary products. New illicit chemical entities with androgenic effects are also produced challenging the development of analytical methods to detect them. The best matrix to analyze is serum and urine since the results generally reflect current or recent abuse of the agents. Analysis of hair may also serve as a complement. However, the presence of AAS in hair reflects past misuse of these agents [13], [14], the location in the hair being dependent on time of misuse.
WADA releases annual lists of substances that are prohibited in sports. The list contains agents forbidden at all time, i.e. in and out of competition as well as agents forbidden in competition only (Table 1) (http://www.wada-ama.org). The agents are classified according to their mechanism of action, in most cases psychopharmacological or endocrine. In addition, the diuretic drugs are listed because they may mask the detection of certain doping agents.
Table 1.
Classes and subgroups of agents prohibited at all times (or at competition only*) according to the World Anti-Doping Agency
| Substance group | Features and effects | Examples |
|---|---|---|
| Anabolic androgenic steroids (AAS) | Anabolic effects | Endogenous AAS |
| Exogenous AAS | ||
| Peptide hormones, growth factors and related substances | Stimulate growth, erythropoiesis, steroid synthesis, and metabolism | Erythropoietin, hCG, LH, insulins, corticotropin, GH, IGF1, etc |
| β2-adrenoceptor stimulants | Bronchodilating effects | |
| Hormone antagonists and modulators | Conceal side effects of AAS | Aromatase inhibitors, selective oestrogen receptor modulators (e.g. tamoxifen), other anti-oestrogenic substances |
| Diuretics and other masking substances | Pharmacological manipulation and masking effects | Diuretics, epitestosterone, probenicid, 5α-reductase inhibitors (finasteride, plasma expanders) |
| Stimulants* | Promote psychological motivation and physical performance | Amphetamine. cocaine, methamphetamine, strychnine, sibutramine, methylphenidate, selegiline, ephedrine |
| Narcotic analgesic agents* | May make the individual ‘euphoric’ | Morphine, heroin, other opioids |
| Cannabinoids* | May make the individual ‘euphoric’ | Hashish, marijuana |
| Glucocorticoids (cortisone)* | Targeted sedation | |
| Alcohol*† | Reduces pulse rate and trembling | |
| β-adrenoceptor blockers*‡ |
Prohibited in competition.
Forbidden in certain sports (racing).
Forbidden in shooting sports.
When medically justified, the sportsman and sportswoman may get a therapeutic use exemption which allows the doctor to prescribe listed drugs for a named and approved medical indication. The purpose of the WADA list is to communicate the names of drugs known, or suspected, to improve performance in sports. This policy of WADA has been formulated in WADA code chapter 3.4, where criteria for including substances and methods are given (http://www.wada-ama.org). Drugs that are used to counteract the side effects of doping agents are also forbidden and therefore included in the list. Such drugs include oestrogen receptor antagonists that antagonize the oestrogenic side-effects of AAS. Drugs aimed at concealing the doping agents are also prohibited, e.g. if they interfere with the analytical method used in the doping test. There are also a number of prohibited practices in sports that are forbidden. Such manipulative procedures include blood doping (blood transfusion, use of recombinant erythropoietin, etc), gene doping and manipulation of the doping control samples collected. All these procedures are aimed to improve aerobic performance by increasing oxygen flow to the muscles and other peripheral tissues.
However, the abuse of AAS and other agents aimed at changing the physical appearance to the muscular type, enhancing physical performance and reinforcing ‘the self’ is much larger in society than it is in elite sports. Much of the abuse of AAS takes place in the gym environment. For obvious reasons we know only little about the difference in the spectrum of abused substances between these groups.
We have a fair view of the substance array that is preferred among abusers in society from the calls to our Anti-Doping Hotline at the department. Table 2 shows the distribution of substances alleged to be abused by the callers [3], [10]. The statistics are based on calls made between 1996 and 2006. When the first 5 years were compared with the last 5 years we noticed an increased interest, as assessed by the questions, in nandrolone, and an equally large decrease in the interest in methandienone. All other substances were equal in this respect. It is to be noted that the abuse pattern in society is mimicking the abuse pattern in sports, where testosterone, stanozolol and nandrolone are the top rated agents.
Table 2.
Classes and subgroups of agents preferred by people in society calling into the Swedish Anti-Doping Hotline between 1996 and 2006. Numbers refer to reported abuse in % of all callers
| Type of substance | Name and % of all in group | Also known as: |
|---|---|---|
| Anabolic androgenic steroids | Testosterone (27) | Sustanone, Testoviron etc |
| Methanedienone (22) | Dianabol | |
| Nandrolone (19) | Deca-Durabol | |
| Stanozolol (10) | Winstrol | |
| Other substances (22) | ||
| Other hormones and receptor active agents | hCG, tamoxifen (58) | |
| GH, IGF1, insulin (42) | ||
| Other substances | Ephedrine (24) | |
| Clenbuterol (11) | ||
| γ-OH-buturic acid (4) | ||
| Narcotics, unspecified (16) | ||
| Dietary supplements (17) | ||
| Creatine (11) | ||
| Alcohol (1) | ||
| Prescription drugs (16) |
Androgen disposition and genetic variation
Genetic variation in genes involved in the synthesis, breakdown and elimination of androgens may affect the bioavailability of administrated AAS, and hence affect the degree of anabolic and toxic effects of doping. Moreover, genetic variation in the androgen receptor (AR) may modulate the pharmacodynamic effects of AAS. As a corollary, several of these polymorphisms may change the serum concentrations of AAS and excretion of AAS metabolites in the urine. Therefore, genetic variations are an important source of confounders in doping tests. Several functional polymorphisms in these genes known to alter the systemic ‘load’ and excretion rate of androgens are discussed below in relation to doping and doping control.
Metabolism – phase I enzymes
Aldo-keto reductases (AKR1C)
Aldo-keto reductases are divided into three families of which the AKR1C members AKR1C1, AKR1C2, AKR1C3, AKR1C4 and AKR1D1 have been shown to play an important role in steroid metabolism as reviewed by Bauman et al. [15]. Genetic variations in AKR1C genes may regulate the local concentration of steroid hormones. AKR1C3 is involved in the formation and inactivation of testosterone and a Glu77Gly exchange has been shown to be associated with testosterone serum concentration [16].
Cytochromes P450 (CYPs)
CYPs are the most important phase I enzymes in drug metabolism, accounting for the metabolism of more than 60% of all drugs. Several of the members of the CYP superfamily are also important catalysts in the metabolic network of steroids, e.g. CYP3A4 catalyzing 4-hydroxylation of testosterone. There is a large variation in the CYP3A4 activity between individuals, partly explained by polymorphisms in the CYP3A4 gene and partly by induction or inhibition by exogenous compounds and drugs. These polymorphisms have not been investigated in relation to testosterone concentrations in vivo, but it is likely that the genetic make-up may determine the systemic concentration of testosterone.
CYP17 exerts 17α-hydroxylase activity as well as 17,20-lyase activity, using pregnenolone and progesterone as substrates. Moreover, CYP17 has been described as a putative enzyme involved in the synthesis of epitestosterone [17]. A promoter polymorphism (T > C) has been shown to correlate with the urinary epitestosterone concentrations in healthy men thereby influencing the T : E ratio [18]. Many of the individuals with a natural T : E above 4 have a low excretion of epitestosterone. There is further discussion of the implications of CYP17 genetics for the T : E test below.
CYP19, also known as aromatase, is involved in the formation of oestrogen from testosterone. Abuse of high doses of AAS is often accompanied by aromatase inhibitors (or oestrogen receptor antagonists) in order to prevent gynaecomastia. These drugs are included in the WADA list of prohibited androgens and androgen modulators (Table 1). Several polymorphisms in this gene have been associated with altered serum concentrations of testosterone and oestrogens [19], [20]. It is possible that genetic variation in this gene may determine the risk for AAS abusers to develop gynaecomastia, as well as other side effects induced by the abuse.
Esterases
Many AAS on the market are available as esters in order to achieve a retarded release. These pro-drugs need to be hydrolyzed prior to activation. Recently we showed that PDE7B is involved in the hydrolysis of testosterone enanthate [21] and nandrolone decanoate (unpublished results) with implications for the serum concentrations and bioavailability of testosterone enanthate. It is conceivable that higher serum concentrations of testosterone convey an advantage in terms of strong androgenic influence on the organ receptors, thus being likely to improve the physical achievements. The therapeutic implications of the genetic variation in esterase activity are unclear at the present time, but the findings may have relevance for many therapeutic drugs administered as esters. Interestingly, genetic variation in PDE7B also affects the T : E ratio [21] which is discussed in relation to doping tests below.
Metabolism – phase II enzymes
Uridine glucuronosyl transferases (UGTs)
UGT enzymes are considered to be the main enzymes for inactivation and quantitative metabolic elimination of steroid hormones. UGT2B17 has been shown to be the main enzyme in testosterone glucuronidation activity in vitro and in vivo[22], [23]. A deletion polymorphism in this gene was identified and characterized [24]. We studied the deletion in a large number of healthy individuals and demonstrated that it was associated with low or negligible testosterone excretion in urine in individuals devoid of the enzyme. We were able to demonstrate a large variation in the gene deletion both within, and between ethnic populations with important consequences for the interpretation of the T : E test [23]. This polymorphism and its impact on doping control is further discussed below.
An additional polymorphism was recently identified in the UGT2B17 promoter region (−155G A) affecting the systemic concentrations of 3α-androstanediol glucuronide, a biomarker of testosterone doping [25]. There is a large variation in urinary testosterone concentrations in UGT2B17 carriers, and this SNP may contribute to the variation in excretion within each UGT2B17 deletion polymorphism mode.
A functional genetic variation in the UGT2B7 gene (H268Y) has been shown to correlate with serum testosterone and DHT concentrations [26], indicating that UGT2B7 has some importance for the regulation of the circulatory concentrations of bioactive sex steroids. UGT2B7 has been identified as the main phase II enzyme involved in epitestosterone glucuronidation. However, the H268Y polymorphism did not affect the epitestosterone glucuronidation activity in vitro or in vivo[18], [27]. Whether other SNPs in the UGT2B7 (or in other epitestosterone metabolizing enzyme genes) could explain the inter-individual variation in epitestosterone excretion needs to be investigated.
A UGT2B15 polymorphism (D85Y) has been described [28]. This polymorphism is strongly associated with the serum concentration of 3α-androstanediol-17-glucuronide in men [29]. The role of this SNP may be more important in UGT2B17 del/del individuals since UGT2B15 is supposed to substitute partly for the deficiency in testosterone glucuronidation activity in these individuals. We have shown that UGT2B17 del/del subjects homozygous for the D85 allele had significantly higher urinary concentrations of androsterone glucuronide and 3α-androstanediol glucuronide than those with one or two Y-alleles [18].
Sulphotransferases (SULT)
Even though glucuronides are the main conjugated metabolites of androgens, many steroids are also sulphated to different extent. For some steroids such as DHEA, sulphation is the major metabolic phase II pathway. As a matter of fact DHEA and DHEA sulphate are the most abundant androgen precursors in the circulation. Genetic variation in SULT2A1 has been correlated with the concentrations of DHEA-S. DHEA is a weak androgen and its use is prohibited by WADA. However, sulphated androgens are currently not determined in the standard doping control programme.
Transporters
Lately genetic polymorphisms in many transporter proteins have been shown to affect the outcome of drug treatment. The superfamily of organic anion transporting polypeptides (OATP), encoded by solute carrier organic anion transporter (SLCO) genes, mediates the uptake of various endogenous compounds including hormones. SLCO1B3 is involved in the uptake of several hormones including testosterone and SLCO2B1 mediates the transport of steroid conjugates, such as DHEA sulphate. Both these SLCOB genes are polymorphically expressed and have been shown to be functional, i.e. there is a correlation with the capacity to transport hormones [30]. Whether genetic variation in SLCOB genes influences the concentration of exogenous androgens remains to be studied.
Binding proteins and receptors
Steroid hormone binding globulin (SHBG)
Testosterone and DHT bind with high affinity to SHBG, thereby regulating the concentration of bioactive testosterone. Several genetic polymorphisms have been characterized in the human SHBG gene. The (TAAAA)n repeat polymorphism located at a distance of ∼700 bp from the transcription start site has been associated with SHBG concentrations in several populations [31], [32]. Moreover, a promoter A > G polymorphism, located 8 bp from the transcription start site, has also been associated with SHBG levels [33]. SHBG polymorphisms are associated with serum concentration of DHT and testosterone [34], [35]. Since genetic variation in the SHBG gene affects both the binding (and thereby bioavailability) and the urinary excretion of androgens, polymorphisms in SHBG may modulate both the effects of steroid doping and the analysis of illicit AAS. However this remains to be further elucidated.
Androgen receptor (AR)
The androgen receptor (AR) is expressed in many tissues and is activated through binding of testosterone and DHT. Activation of AR interacts with a broad variety of physiological processes and has important physiological effects on bone density, skeletal muscle growth, prostate growth and the mood. Therefore it is likely that genetic variation in AR may correlate with both anabolic effects and adverse side effects of androgen abuse. It has been speculated that these polymorphisms may affect the sensitivity to adverse psychic reactions such as aggression. Several polymorphisms have been described in the AR affecting the receptor affinity. In particular, a trinucleotide repeat (CAG) has been extensively studied and associated with disease risks, total fat free mass in men and acne, a common side effect of AAS abuse [36]. In addition to their binding to AR, the testosterone metabolite 3α-androstanediol can bind to GABAA receptors, known to have an important role in the mediation of aggression [37] and it is possible that genetic variation in this receptor gene may predispose for inter-individual variation observed in behavioural side effects.
Doping tests and genetic confounders
So far, as described above, we know of only genetic variations in three enzyme (UGT2B17, CYP17 and PDE7B) genes with a substantial influence on the T : E ratio. Below follows an in-depth analysis of the implications of genetic polymorphisms for the tests and the interpretation of the test results.
UGT2B17
The conventional testosterone doping test includes measurement of the T : E ratio in urine. The old threshold for suspected testosterone doping was lowered from 6 to 4 in 2004 (http://www.wada-ama.org) because there were occasional reports about individuals with very low natural basal T : E values. Even so, a number of subjects turned out to test falsely negative [38], [39]. As expected the new lower threshold level also captures a larger number of false positive subjects requiring further analysis (see below).
Given this situation we were interested in the normal variation of testosterone excretion, both within, and between ethnic groups. The major part of testosterone is excreted as the glucuronide and we found a 100- or more fold variation in excretion rate, both in a Caucasian and an Asian study group [23]. Unraveling this variation in testosterone glucuronide excretion yielded the first insight into the profound genetic influence on the T : E ratio. There was a conspicuous bipolarity in the excretion profile in both Caucasians and Asians. Interestingly, a majority (75%) of Asian subjects fell within the ‘low excretion’ group compared with 9% among the Caucasians, and the widely different distribution into the two modes indicated a monogenic background of the excretion pattern (Figure 3). Indeed, a monogenic background of the excretion pattern was confirmed when we investigated at that time the newly described deletion polymorphism in the uridine glucuronosyl transferase 2B17 gene (UGT2B17) which encodes the major UGT enzyme catalyzing the glucuronidation of testosterone. There was a near complete concordance between genotype and phenotype [23] in that all subjects devoid of the enzyme (del/del) had minimal or negligible excretion of testosterone and some of its metabolites, in contrast to subjects with the ins/del or ins/ins genotypes.
Figure 3.
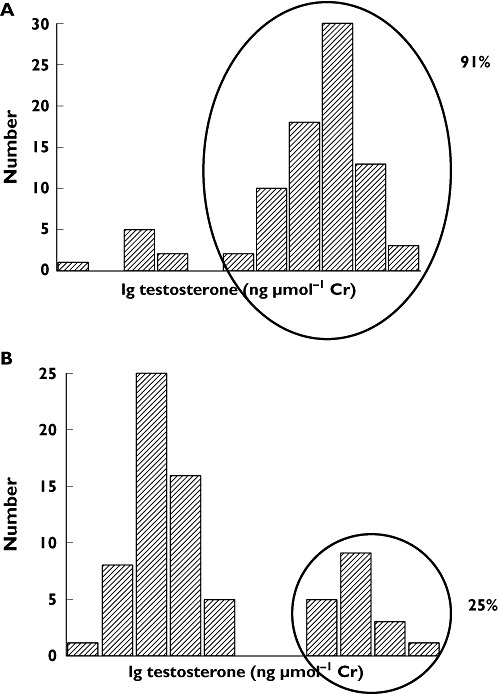
Frequency distribution of total testosterone (unconjugated and conjugated) excretion in urine in a group of Swedish (A) and Korean (B) healthy male volunteers. Excretion expressed as ng µmol–1 creatinine
The conspicuous intra- and interethnic differences in testosterone excretion gave cause for concern about how to interpret the classical urinary T : E screening test as an indicator of exogenous administration of the hormone. First, how would the 100-fold difference in urinary excretion of testosterone between the Caucasian and the Asian groups affect the basal T : E ratio? We assumed, and were able to confirm that the degree of variation in T : E ratio would be similar, as the excretion of epitestosterone which is given in the denominator, was not affected by the UGT2B17 deletion polymorphism. Figure 4 depicts the basal excretion rates of testosterone and epitestosterone glucuronides, and the resulting T : E ratio in different genetic panels of UGT2B17. Second, how would individuals with the different genotypes respond to doping with testosterone? In order to answer this question we challenged healthy male volunteers with 500 mg testosterone enanthate and found interesting and worrying results in respect of the possibilities to interpret T : E test results without genetic information about relevant enzymes [39]. In this work, albeit the number was small, we found that about 15% of the ins/ins individuals already showed T : E ratios above 4 prior to the challenge, and 40% of the del/del subjects never reached the threshold of 4 on the days when the T : E ratio is peaking. This problem would be sizeable in populations with a large number of individuals with the UGT2B17 gene deletion. Given this situation some 11 or 31% of Caucasians and Asians, respectively, will test either falsely positive or falsely negative in the current T : E analysis under the experimental conditions described (Table 3).
Figure 4.
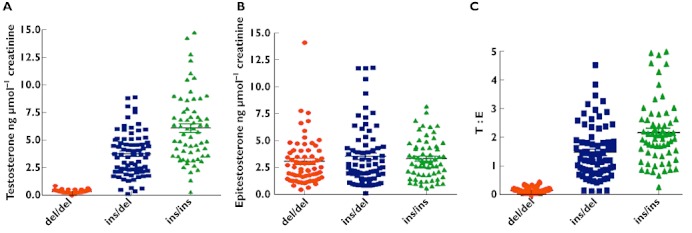
Relation between the UGT2B17 genotype and the urinary excretion of testosterone (A), epitestosterone (B) and the testosterone : epitestosterone (T : E) ratio (C) in Swedish and Korean healthy subjects from Jakobsson et al. [23] and Schulze et al. [18]. The number of indivuduals were 65 del/del, 75 ins/del, and 63 ins/ins where del/del represents subjects devoid of the enzyme
Table 3.
Predicted rate of false negatives and false positives using the T : E ratio without genetic information
| Ethnic group | UGT2B17 genotype false negatives | UGT2B17 genotype false positives | Sum of false positives and false negatives |
|---|---|---|---|
| Caucasians | 3.7% | 7.7% | 11.4% |
| Asians | 27% | 4.2% | 31.2% |
When a dual gene based cut-off level was applied the sensitivity was considerably improved. Thus, if the cut-off level for the del/del subjects was set to 1 and to 6 for the other genotypes, the T : E test sensitivity increased from 59 and 29% to 100% on days 6 and 11, respectively after challenge with 500 mg testosterone enanthate. The corresponding increase for day 2 was from 6 to 53%.
However, the T : E test, as well as the analysis of several other endogenous steroids and luteinizing hormone (LH), is only a screening test prior to further analysis of the 13C : 12C isotope ratio by isotope ratio mass spectrometry (IRMS) (Technical Document 2004EAAS WADA). This analysis is expensive but it discriminates exogenous from endogenous compounds in that the former contain less 13C than the latter. The future strategy to avoid this procedure is to make a prospective longitudinal study in the individual. This approach is based on the fact that the population-based T : E ratio varies much more than the individual endogenous ratio. The future test programme for testosterone will adopt a Bayesian inference technique for analysis of consecutive T : E samples in the individual [40], as has been applied in the detection of blood doping with the ‘blood pass’[41]. The indivudual cut-off limit adapts itself as a function of previous test results. Thus, we have shown that such a programme returned lower individual cut-off ratios in each del/del subject as compared with the population based cut-off ratio The combination of urine T : E analysis, Bayesian interpretation and genetic information was better than each of the approaches alone and markedly improved the sensitivity of the doping test [42].
CYP17
Even though 66% of the variation in T : E ratio may be ascribed to the UGT2B17 polymorphism, other genetic variation clearly influences this ratio, e.g. the CYP17 enzyme. This enzyme is rate limiting in the synthesis of androgens and the gene harbours a T > C (A1>A2) promoter polymorphism. We found that the T > C polymorphism was associated with the urinary glucuronide concentrations of epitestosterone [18]. The epitestosterone concentrations in the urine were 1.6 times higher in subjects carrying one or two mutant alleles (A1/A2 or A2 A−1/2) compared with individuals homozygous for the A1 allele. As a corollary the T : E ratio was 64% higher in A1/A1 homozygotes. The CYP17 promoter polymorphism may partly explain high natural T : E ratios (>4) that are seen in some individuals. Therefore, it is important to study further this genetic variation in a larger population and particularly in subjects with naturally high T : E ratios in order to establish the impact of this polymorphism in the CYP17 gene.
Phosphodiesterase 7B (PDE7B)
Widely different serum testosterone concentrations were observed in healthy volunteers challenged with a single dose of 500 mg testosterone enanthate [21]. The serum concentration profiles aggregated into either a ‘high-rise’ or a ‘low-rise’ group irrespective of the UGT2B17 genotype and the urinary testosterone excretion which, as expected, varied extensively in these subjects.
The rise in serum concentration of testosterone and several testosterone metabolites, of which some are active, was found to be associated with a polymorphism in the PDE7B gene in a genome wide association study. An intron 1 G > A polymorphism resulted in a 2.5- and a 3.9-fold increase in testosterone serum concentration in homozygous G allele carriers and in carriers of one or two A alleles, respectively [21].
Individuals homozygous for a G-allele with lower serum concentrations of testosterone also had lower T : E ratios than carriers of the PDE7B A-allele for the first 5 days after the testosterone enanthate dose, irrespective of the UGT2B17 genotype. However, on days 13 and 15 this relation was reverted in individuals devoid of the enzyme. The reason for this complex excretion pattern is unclear.
Multi-gene influence on the T : E ratio
When all the enzymes of importance for the disposition of testosterone are considered the situation becomes more complex. The effect of various genes on the increase in T : E ratio after a challenge with testosterone is depicted in Figure 5. The T : E ratio was measured daily for 15 days and the baseline value was deducted from the average of the measured values. Interesting conclusions emerge from the findings. First, the figure shows that UGT2B17 has a marked effect on the increase of the T : E ratio with a gene dose effect irrespective of the other genotypes. Second, PDE7B genetics have an impact on the rise of the ratio, particularly in the UGT2B17 ins/del and del/del groups except in the CYP17 wt/wt–UGT2B17 ins/ins haplotypes.
Figure 5.
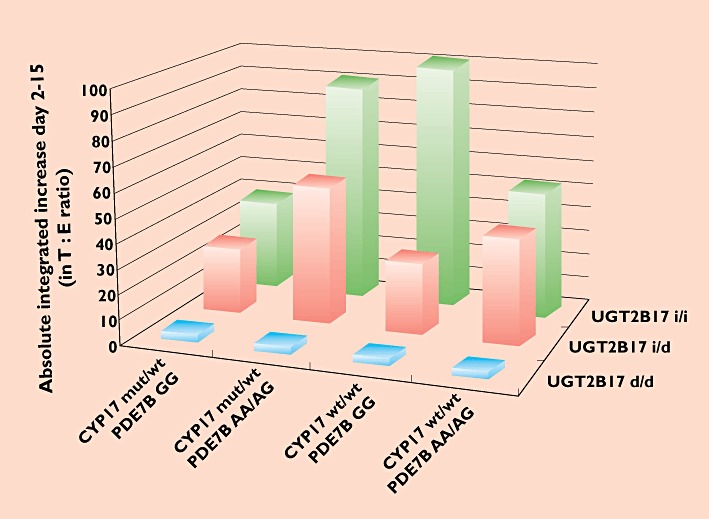
Combined influence of polymorphisms in the UGT2B17, CYP17 and PDE7B genes on the increase in T : E ratio after a single dose of 500 mg testosterone enanthate. The bars represent the absolute average increase on days 2–11 and 13 and 15 as compared with the basal T : E ratio
Third, CYP17 genetics have less influence on the T : E values. The mutant A2 (C) variant is related to an increased excretion of epitestosterone thereby decreasing the ratio. We did, however, not observe any consistent difference in the T : E ratio between the CYP17 genotype panels, probably because the genetic influence on the epitestosterone excretion is marginal compared with the effect of testosterone administration on the T : E ratio. Moreover, the numbers may be too small to demonstrate an effect on the T : E ratio. It is likely that this CYP17 polymorphism plays a more pronounced role in populations with high T : E-ratios [18].
How does the composite effect of the genes affect the T : E ratio over time?
We studied the T : E ratio for 15 days in a UGT2B17 del/del panel with two different genotype combinations of PDE7B (Figure 6). As stated above it was not possible to discern any effect on the T : E ratio of CYP17 genetic variation in this experimental setting.
Figure 6.
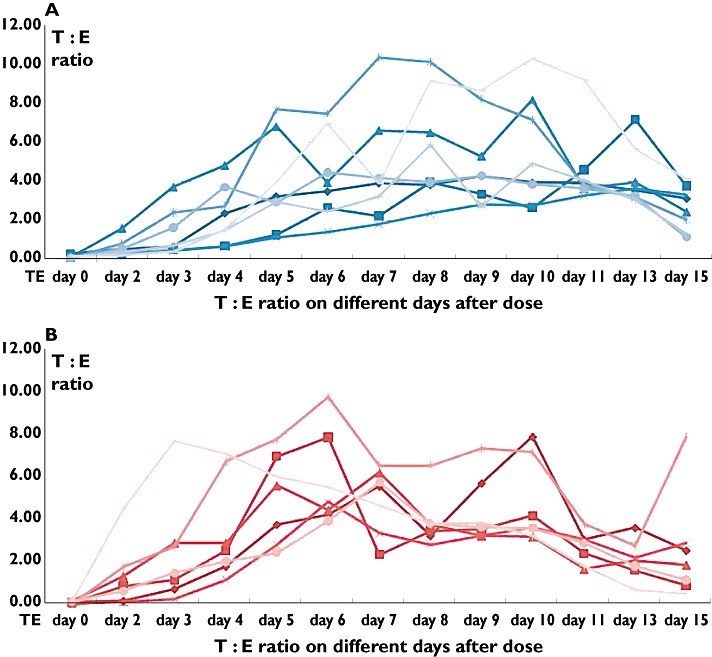
Increase in T : E ratio after a 500 mg testosterone enanthate dose in 15 UGT2B17 del/del individuals harboring either the PDE7B GG (A) or the PDE7B AG/AA genotype (B)
The figure shows that the UGT2B17 del/del individuals with the A allele reached the cut-off level earlier than carriers of the G allele. All carriers of the PDE7B A allele (Figure 6A) reached the cutoff level of 4 in contrast to subjects homozygous for the G allele (Figure 6B). It is to be noted that the profiles vary a lot in respect of time reaching, leaving or remaining in the area above the cut-off line.
As a corollary, a single sample at random time gives a limited possibility to detect true doping, particularly in individuals with the UGT2B17 del/del and PDE7B GG allelic combination. In addition, the doping habits include a large variety of doses and dosing schedules. These increases further emphasize the difficulties in pin-pointing the best time for detection. The situation for other UGT2B17 genotypes is different in that they have an extensive urinary excretion of testosterone well above the threshold (data not shown).
How frequent is the problem with low sensitivity of the T : E doping test?
Assuming an allele frequency of 44% for the mutant CYP17 A2 (C) allele in Caucasians [18], [43], [44] the proportion of the population harbouring one or two A2 alleles is 0.69. For the PDE7B gene, the proportion harbouring at least one A allele that promotes a higher bioavailability of testosterone enanthate would be 0.64 [21]. The UGT2B17 del/del, ins/del and ins/ins genotype frequencies are 0.09, 0.39 and 0.51 in Caucasians whereas the corresponding proportions in Koreans are 0.67, 0.23 and 0.10 [23]. With these numbers the distribution of the gene combinations in a Caucasian and an Asian population sample are very different. This is depicted in Figures 7A and B in which the blue bars in the front line represent the UGT2B17 del/del genotype in various combinations with the other genes. Thus, the blue bars represent the fraction of the two populations with the least, sometimes minimal, effect on the T : E ratio after challenge with one dose of testosterone enanthate. It is conceivable that the sensitivity of the T : E screening test is unsatisfactory in detecting intake of testosterone in populations with high prevalence of the UGT2B17 del allele.
Figure 7.
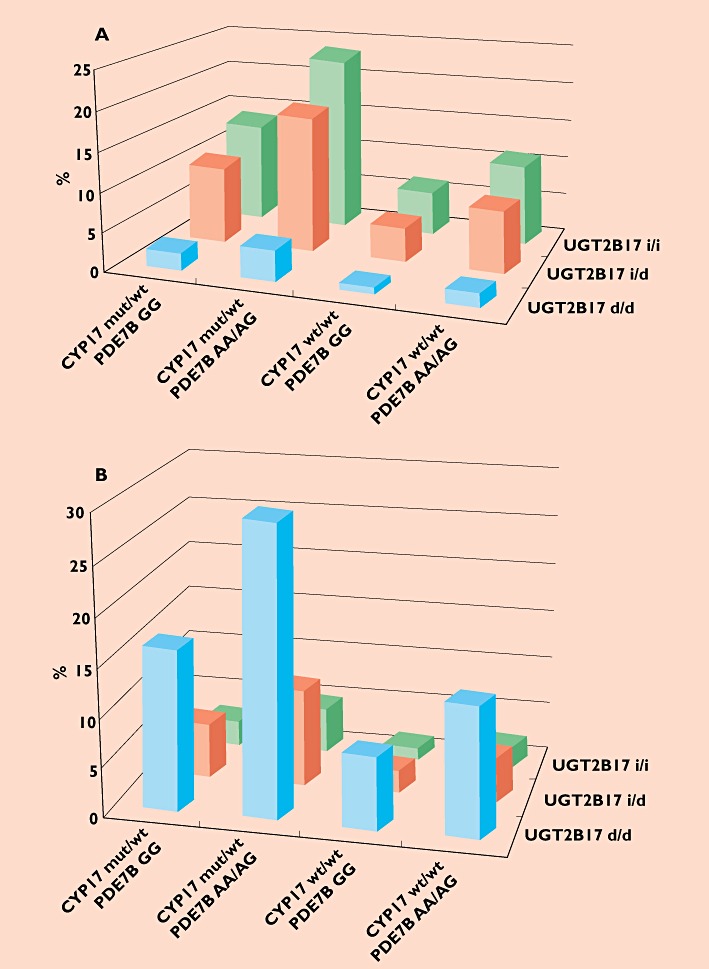
Distribution of different genetic panels in a Caucasian (A) and Korean (B) population. Shown are the distribution of combinations of one polymorphism each in three genes of relevance for the T : E ratio, namely UGT2B17, CYP17 and PDE7B
Conclusion
In summary, large genetic variation in the disposition and effects of anabolic androgenic steroids is a reality that must be considered in the doping control strategy in sports as well as in society. Polymorphisms in three crucial enzyme genes have hitherto been shown to have an impact on the disposition of testosterone and the T : E ratio. Further genetic confounders in other genes cannot be excluded at present time. False negative and false positive results with the current principles for interpretation of the T : E screening test as described will lead to unnecessary and expensive follow-up analyses of many samples. However, when combining the urinary measurement of T : E ratio with allelic determinations in relevant genes and a Bayesian interpretation of the test results, the sensitivity and specificity is considerably improved.
The large variation between individuals in the abuse pattern, androgen doses, androgen genetics and last dose to sample time will further increase the risk to miss detection of true doping in sports and in the society.
Acknowledgments
This work was supported by the World Anti-Doping Agency (WADA) and the Swedish National Centre for Research in Sports. We thank Drs Jenny Schulze, Jonas Lundmark and Mats Garle for constructive support in various parts of the work.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Brown-Sequard C. Note on the effects produced on man by subcutaneous injections of a liquid obtained from the testis of animals. Lancet. 1889;134:105–7. [Google Scholar]
- 2.Saudan C, Baume N, Robinson N, Avois L, Mangin P, Saugy M. Testosterone and doping control. Br J Sports Med. 2006;40(Suppl. 1):i21–4. doi: 10.1136/bjsm.2006.027482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjoqvist F, Garle M, Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet. 2008;371:1872–82. doi: 10.1016/S0140-6736(08)60801-6. [DOI] [PubMed] [Google Scholar]
- 4.Barroso O, Mazzoni I, Rabin O. Hormone abuse in sports: the antidoping perspective. Asian J Androl. 2008;10:391–402. doi: 10.1111/j.1745-7262.2008.00402.x. [DOI] [PubMed] [Google Scholar]
- 5.Handelsman DJ, Heather A. Androgen abuse in sports. Asian J Androl. 2008;10:403–15. doi: 10.1111/j.1745-7262.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson JD, Griffin JE. The use and misuse of androgens. Metabolism. 1980;29:1278–95. doi: 10.1016/0026-0495(80)90159-6. [DOI] [PubMed] [Google Scholar]
- 7.Janne OA, Palvimo JJ, Kallio P, Mehto M. Androgen receptor and mechanism of androgen action. Ann Med. 1993;25:83–9. doi: 10.3109/07853899309147863. [DOI] [PubMed] [Google Scholar]
- 8.Rogerson S, Weatherby RP, Deakin GB, Meir RA, Coutts RA, Zhou S, Marshall-Gradisnik SM. The effect of short-term use of testosterone enanthate on muscular strength and power in healthy young men. J Strength Cond Res. 2007;21:354–61. doi: 10.1519/R-18385.1. [DOI] [PubMed] [Google Scholar]
- 9.Wade NA. Anabolic steroids: doctors denounce them, but athletes aren't listening. Science. 1972;176:1399–403. doi: 10.1126/science.176.4042.1399. [DOI] [PubMed] [Google Scholar]
- 10.Eklof AC, Thurelius AM, Garle M, Rane A, Sjoqvist F. The anti-doping hot-line, a means to capture the abuse of doping agents in the Swedish society and a new service function in clinical pharmacology. Eur J Clin Pharmacol. 2003;59:571–7. doi: 10.1007/s00228-003-0633-z. [DOI] [PubMed] [Google Scholar]
- 11.Evans NA. Gym and tonic: a profile of 100 male steroid users. Br J Sports Med. 1997;31:54–8. doi: 10.1136/bjsm.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze JJ. Genetics of Androgen Disposition – Implications for Doping Tests. Stockholm: Karolinska Institutet; 2007. [Google Scholar]
- 13.Kintz P, Cirimele V, Sachs H, Jeanneau T, Ludes B. Testing for anabolic steroids in hair from two bodybuilders. Forensic Sci Int. 1999;101:209–16. doi: 10.1016/s0379-0738(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 14.Deshmukh N, Hussain I, Barker J, Petroczi A, Naughton DP. Analysis of anabolic steroids in human hair using LC-MS/MS. Steroids. 2010;75:710–4. doi: 10.1016/j.steroids.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Bauman DR, Steckelbroeck S, Penning TM. The roles of aldo-keto reductases in steroid hormone action. Drug News Perspect. 2004;17:563–78. doi: 10.1358/dnp.2004.17.9.872570. [DOI] [PubMed] [Google Scholar]
- 16.Jakobsson J, Palonek E, Lorentzon M, Ohlsson C, Rane AEkstrom L. A novel polymorphism in the 17beta-hydroxysteroid dehydrogenase type 5 (aldo-keto reductase 1C3) gene is associated with lower serum testosterone levels in Caucasian men. Pharmacogenomics J. 2006;7:282–9. doi: 10.1038/sj.tpj.6500419. [DOI] [PubMed] [Google Scholar]
- 17.Weusten JJ, Legemaat G, van der Wouw MP, Smals AG, Kloppenborg PW, Benraad T. The mechanism of the synthesis of 16-androstenes in human testicular homogenates. J Steroid Biochem. 1989;32:689–94. doi: 10.1016/0022-4731(89)90513-x. [DOI] [PubMed] [Google Scholar]
- 18.Schulze JJ, Lorentzon M, Ohlsson C, Lundmark J, Roh HK, Rane AEkstrom L. Genetic aspects of epitestosterone formation and androgen disposition: influence of polymorphisms in CYP17 and UGT2B enzymes. Pharmacogenet Genomics. 2008;18:477–85. doi: 10.1097/FPC.0b013e3282fad38a. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J, Tang NL, Ohlsson C, Eriksson AL, Vandenput L, Chan FW, Ching JK, Kwok A, Orwoll E, Kwok TC, Woo J, Leung PC. Association of genetic variations in aromatase gene with serum estrogen and estrogen/testosterone ratio in Chinese elderly men. Clin Chim Acta. 2010;411:53–8. doi: 10.1016/j.cca.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 20.Sowers MR, Wilson AL, Kardia SR, Chu J, Ferrell R. Aromatase gene (CYP 19) polymorphisms and endogenous androgen concentrations in a multiracial/multiethnic, multisite study of women at midlife. Am J Med. 2006;119:S23–30. doi: 10.1016/j.amjmed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Ekstrom L, Schulze JJ, Guillemette C, Belanger ARane A. Bioavailability of testosterone enanthate dependent on genetic variation in the phosphodiesterase 7B but not on the uridine 5′-diphospho-glucuronosyltransferase (UGT2B17) gene. Pharmacogenet Genomics. 2011;21:325–32. doi: 10.1097/FPC.0b013e328344c5c6. [DOI] [PubMed] [Google Scholar]
- 22.Sten T, Bichlmaier I, Kuuranne T, Leinonen A, Yli-Kauhaluoma J, Finel M. UDP-glucuronosyltransferases (UGTs) 2B7 and UGT2B17 display converse specificity in testosterone and epitestosterone glucuronidation, whereas UGT2A1 conjugates both androgens similarly. Drug Metab Dispos. 2009;37:417–23. doi: 10.1124/dmd.108.024844. [DOI] [PubMed] [Google Scholar]
- 23.Jakobsson J, Ekstrom L, Inotsume N, Garle M, Lorentzon M, Ohlsson C, Roh HK, Carlstrom K, Rane A. Large differences in testosterone excretion in Korean and Swedish men are strongly associated with a UDP-glucuronosyl transferase 2B17 polymorphism. J Clin Endocrinol Metab. 2006;91:687–93. doi: 10.1210/jc.2005-1643. [DOI] [PubMed] [Google Scholar]
- 24.Wilson W, 3rd, Pardo-Manuel de Villena F, Lyn-Cook BD, Chatterjee PK, Bell TA, Detwiler DA, Gilmore RC, Valladeras IC, Wright CC, Threadgill DW, Grant DJ. Characterization of a common deletion polymorphism of the UGT2B17 gene linked to UGT2B15. Genomics. 2004;84:707–14. doi: 10.1016/j.ygeno.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Hu DG, Gardner-Stephen D, Severi G, Gregory PA, Treloar J, Giles GG, English DR, Hopper JL, Tilley WD, Mackenzie PI. A novel polymorphism in a forkhead box A1 (FOXA1) binding site of the human UDP glucuronosyltransferase 2B17 gene modulates promoter activity and is associated with altered levels of circulating androstane-3alpha,17beta-diol glucuronide. Mol Pharmacol. 2010;78:714–22. doi: 10.1124/mol.110.065953. [DOI] [PubMed] [Google Scholar]
- 26.Swanson C, Lorentzon M, Vandenput L, Labrie F, Rane A, Jakobsson J, Chouinard S, Belanger A, Ohlsson C. Sex steroid levels and cortical bone size in young men are associated with a uridine diphosphate glucuronosyltransferase 2B7 polymorphism (H268Y) J Clin Endocrinol Metab. 2007;92:3697–704. doi: 10.1210/jc.2007-0530. [DOI] [PubMed] [Google Scholar]
- 27.Coffman BL, King CD, Rios GR, Tephly TR. The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268) Drug Metab Dispos. 1998;26:73–7. [PubMed] [Google Scholar]
- 28.Levesque E, Beaulieu M, Green MD, Tephly TR, Belanger A, Hum DW. Isolation and characterization of UGT2B15(Y85): a UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics. 1997;7:317–25. doi: 10.1097/00008571-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Swanson C, et al. The uridine diphosphate glucuronosyltransferase 2B15 D85Y and 2B17 deletion polymorphisms predict the glucuronidation pattern of androgens and fat mass in men. J Clin Endocrinol Metab. 2007;92:4878–82. doi: 10.1210/jc.2007-0359. [DOI] [PubMed] [Google Scholar]
- 30.Hamada A, Sissung T, Price DK, Danesi R, Chau CH, Sharifi N, Venzon D, Maeda K, Nagao K, Sparreboom A, Mitsuya H, Dahut WL, Figg WD. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14:3312–8. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferk P, Teran N, Gersak K. The (TAAAA)n microsatellite polymorphism in the SHBG gene influences serum SHBG levels in women with polycystic ovary syndrome. Hum Reprod. 2007;22:1031–6. doi: 10.1093/humrep/del457. [DOI] [PubMed] [Google Scholar]
- 32.Haiman CA, Riley SE, Freedman ML, Setiawan VW, Conti DV, Le Marchand L. Common genetic variation in the sex steroid hormone-binding globulin (SHBG) gene and circulating shbg levels among postmenopausal women: the Multiethnic Cohort. J Clin Endocrinol Metab. 2005;90:2198–204. doi: 10.1210/jc.2004-1417. [DOI] [PubMed] [Google Scholar]
- 33.Dunning AM, Dowsett M, Healey CS, Tee L, Luben RN, Folkerd E, Novik KL, Kelemen L, Ogata S, Pharoah PD, Easton DF, Day NE, Ponder BA. Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J Natl Cancer Inst. 2004;96:936–45. doi: 10.1093/jnci/djh167. [DOI] [PubMed] [Google Scholar]
- 34.Eriksson AL, Lorentzon M, Mellström D, Vandenput L, Swanson C, Andersson N, Hammond GL, Jakobsson J, Rane A, Orwoll ES, Ljunggren O, Johnell O, Labrie F, Windahl SH, Ohlsson C. SHBG gene promoter polymorphisms in men are associated with serum sex hormone-binding globulin, androgen and androgen metabolite levels, and hip bone mineral density. J Clin Endocrinol Metab. 2006;91:5029–37. doi: 10.1210/jc.2006-0679. [DOI] [PubMed] [Google Scholar]
- 35.Ohlsson C, Wallaschofski H, Lunetta KL, Stolk L, Perry JR, Koster A, Petersen AK, Eriksson J, Lehtimäki T, Huhtaniemi IT, Hammond GL, Maggio M, Coviello AD, EMAS Study Group. Ferrucci L, Heier M, Hofman A, Holliday KL, Jansson JO, Kähönen M, Karasik D, Karlsson MK, Kiel DP, Liu Y, Ljunggren O, Lorentzon M, Lyytikäinen LP, Meitinger T, Mellström D, Melzer D, Miljkovic I, Nauck M, Nilsson M, Penninx B, Pye SR, Vasan RS, Reincke M, Rivadeneira F, Tajar A, Teumer A, Uitterlinden AG, Ulloor J, Viikari J, Völker U, Völzke H, Wichmann HE, Wu TS, Zhuang WV, Ziv E, Wu FC, Raitakari O, Eriksson A, Bidlingmaier M, Harris TB, Murray A, de Jong FH, Murabito JM, Bhasin S, Vandenput L, Haring R. Genetic determinants of serum testosterone concentrations in men. PLoS Genet. 2011;7:e1002313. doi: 10.1371/journal.pgen.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9–15. doi: 10.1177/120347549800300103. [DOI] [PubMed] [Google Scholar]
- 37.Andreen L, Nyberg S, Turkmen S, van Wingen G, Fernandez G, Backstrom T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology. 2009;34:1121–32. doi: 10.1016/j.psyneuen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Garevik N, Strahm E, Garle M, Lundmark J, Stahle L, Ekstrom L, Rane A. Long term perturbation of endocrine parameters and cholesterol metabolism after discontinued abuse of anabolic androgenic steroids. J Steroid Biochem Mol Biol. 2011;127:295–300. doi: 10.1016/j.jsbmb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Schulze JJ, Lundmark J, Garle M, Skilving I, Ekstrom L, Rane A. Doping test results dependent on genotype of uridine diphospho-glucuronosyl transferase 2B17, the major enzyme for testosterone glucuronidation. J Clin Endocrinol Metab. 2008;93:2500–6. doi: 10.1210/jc.2008-0218. [DOI] [PubMed] [Google Scholar]
- 40.Sottas PE, Baume N, Saudan C, Schweizer C, Kamber M, Saugy M. Bayesian detection of abnormal values in longitudinal biomarkers with an application to T/E ratio. Biostatistics. 2007;8:285–96. doi: 10.1093/biostatistics/kxl009. [DOI] [PubMed] [Google Scholar]
- 41.Robinson N, Sottas PE, Mangin P, Saugy M. Bayesian detection of abnormal hematological values to introduce a no-start rule for heterogeneous populations of athletes. Haematologica. 2007;92:1143–4. doi: 10.3324/haematol.11182. [DOI] [PubMed] [Google Scholar]
- 42.Schulze JJ, Lundmark J, Garle M, Ekstrom L, Sottas PE, Rane A. Substantial advantage of a combined Bayesian and genotyping approach in testosterone doping tests. Steroids. 2009;74:365–8. doi: 10.1016/j.steroids.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Wadelius M, Andersson AO, Johansson JE, Wadelius C, Rane E. Prostate cancer associated with CYP17 genotype. Pharmacogenetics. 1999;9:635–9. [PubMed] [Google Scholar]
- 44.Carey AH, Waterworth D, Patel K, White D, Little J, Novelli P, Franks S, Williamson R. Polycystic ovaries and premature male pattern baldness are associated with one allele of the steroid metabolism gene CYP17. Hum Mol Genet. 1994;3:1873–6. doi: 10.1093/hmg/3.10.1873. [DOI] [PubMed] [Google Scholar]


