Abstract
This article systematically reviews the literature on the impact of collaboration between pharmacists and general practitioners and describes its effect on patients' health. A systematic literature search provided 1041 articles. After first review of title and abstract, 152 articles remained. After review of the full text, 83 articles were included. All included articles are presented according to the following variables: (i) reference; (ii) design and setting of the study; (iii) inclusion criteria for patients; (iv) description of the intervention; (v) whether a patient interview was performed to involve patients' experiences with their medicine-taking behaviour; (vi) outcome; (vii) whether healthcare professionals received additional training; and (viii) whether healthcare professionals received financial reimbursement. Many different interventions are described where pharmacists and general practitioners work together to improve patients' health. Only nine studies reported hard outcomes, such as hospital (re)admissions; however, these studies had different results, not all of which were statistically significant. Randomized controlled trials should be able to describe hard outcomes, but large patient groups will be needed to perform such studies. Patient involvement is important for long-term success.
Keywords: family physician, interprofessional relations, patient care, pharmaceutical care, pharmaceutical services, pharmacist
Introduction
To provide best pharmaceutical care practice, it is important that all relevant persons are involved and work together as a healthcare team [1], [2]. As a result, healthcare providers should have a complete patient and medical record. Besides medical information from healthcare providers, a consultation round with the patient is also necessary to determine patients' problems and patients' needs. Active participation of patients during treatment could help to achieve better patient outcomes [3], [4]. In primary care, the triangle of the pharmacist–general practitioner (GP)–patient is important for providing optimal pharmaceutical care. According to the definition of Cipolle et al., pharmaceutical care is ‘a patient-centered practice in which the practitioner assumes responsibility for a patient's drug-related needs and is held accountable for this commitment’[5].
An important tool for pharmaceutical care is regular medication reviews or medication reconciliation next to the assessment of patient needs and the development of a care plan. A medication review is defined as ‘a structured, critical examination of a patient's medicines with the objective of reaching an agreement with the patient about treatment, optimizing the impact of medicines, minimizing the number of medication-related problems and reducing waste’[6]. Medication reconciliation is defined as ‘the process of obtaining and maintaining a complete and accurate list of the current medication use of a patient across healthcare settings’[7]. In 2002, the Medicines Partnership defined four levels of medication review [6] (Figure 1). An ad hoc review (level 0) consists of an isolated question to a patient. A prescription review (level 1) is a review of a patient's medicine by a pharmacist. A treatment review (level 2) requires cooperation between pharmacist and GP (or medical specialist) to review a patient's medicines with the patient's full notes. Finally, a clinical medication review (CMR; level 3) requires face-to-face cooperation between pharmacist and/or GP and the patient in order to review a patient's medicines and conditions. When performing a higher level of medication review, cooperation must increase. In 2008, the four levels were reviewed and redefined to three types in order to focus on the purpose of medication review [8]. One important reason was that medicines use review (MUR), a new development in medication review services, did not fit within the previously defined levels of medication review. A MUR is conducted with the patient (level 3) but without access to the patient's full notes (level 2). In this new classification, prescription review (type 1), concordance and compliance review (type 2) and clinical medication review (type 3) are defined (Table 1) [8]. However, we believe not all different kinds of medication review are covered within these new defined types of medication reviews, e.g. the former level 2, treatment review, where a pharmacist cooperates with a GP to review the patient's medicines with the patient's full notes. Several classifications of medication review activities are being used but none covers all different activities. One similarity is that both the highest level and/or type of medication review requires the patient's presence. We therefore decided to focus on the participation of the patient.
Figure 1.
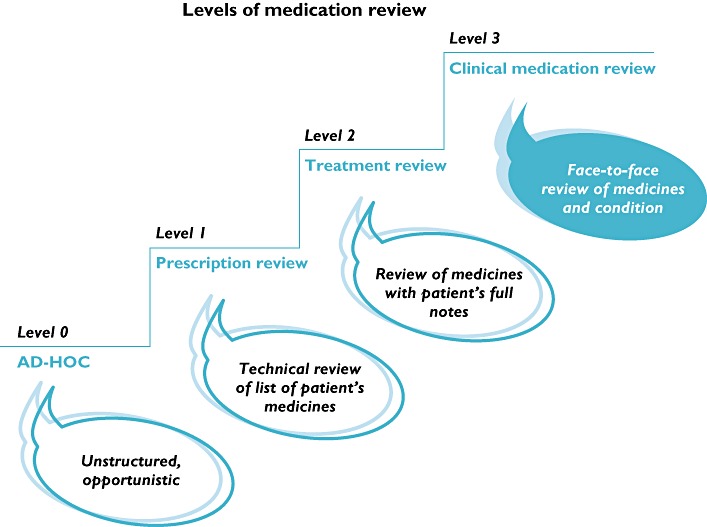
Different levels of medication review (reproduced with the permission of the authors) [6]
Table 1.
Characteristics of types of medication reviews [8]
| Purpose | Patient's presence | Access to patient's notes | All prescription medicines | Prescription, complementary and over-the-counter medicines | Review of medicines and/or condition | |
|---|---|---|---|---|---|---|
| Type 1: prescription review | Address technical issues relating to prescription | No | Possibly | Possibly | No | Medicines |
| Type 2: concordance and compliance review | Address issues relating to patient's medicine-taking behaviour | Usually | Possibly | Yes | Yes | Medicines use |
| Type 3: clinical medication review | Address issues relating to the patient's use of medicines in the context of their clinical condition | Yes | Yes | Yes | Yes | Medicines and condition |
So far, there are no systematic reviews available that compare the different types of cooperation between family doctors and pharmacists and their impact on patient outcomes. This article systematically reviews the literature on the impact of collaboration between pharmacists and GPs and describes their outcomes on patients' health.
Methods
A systematic literature search was performed in the databases PubMed and Embase (period until 16 June 2011) with keywords described in Table 2. Abstracts and articles were reviewed by two authors independently (M.M.E.G and J.J.deG.). Articles were first reviewed based on title and abstract (n = 1041) and second on full text (n = 152). Only English and Dutch written articles were included. Full-text articles were excluded based on language (n = 2), lack of cooperation (n = 11), lack of patient outcomes (n = 41), short communication, e.g. letter, summary or abstract (n = 15), and duplicate articles (n = 7). Finally, seven additional articles were included, five from references from other articles and two that were not available in the databases at time of the literature search. In total, 83 articles were included (Figure 2). The measure of agreement between the two reviewers, defined as Cohen's kappa (κ), was calculated using SPSS 18.0.3.
Table 2.
Keywords used for literature search
| ‘Pharmacists’[Mesh] OR ‘Pharmaceutical Services’[Mesh] OR ‘pharmaceutical care’[All Fields] OR pharmaceutical service* [All Fields] OR ‘pharmacists’[All Fields] OR ‘pharmacist’[All Fields] OR pharmaceutical practice* [All Fields] |
| AND |
| ‘Physicians, Family’[Mesh] OR ‘Family Practice’[Mesh] OR ‘family practice’ OR ‘general practice’ OR general practitioner* OR family practitioner* |
| AND |
| ‘Patient Care’[Mesh] OR ‘Interprofessional Relations’[Mesh] OR cooperation [All Fields] OR ‘referral and consultation’[Mesh Terms] OR ‘consultation’[All Fields] OR ‘patient care’[All Fields] OR review* |
| AND |
| medical record* OR prescription record* OR ‘pharmaceutical care’ OR medication |
used to search multiple options at the same time (singular vs. plural).
Figure 2.
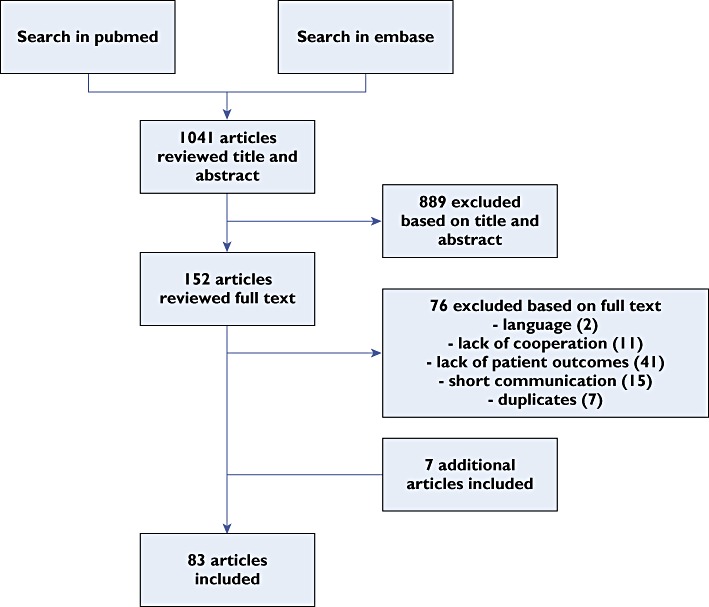
Article selection
Results
The two authors, who reviewed the titles and abstracts, reached strong agreement (κ= 0.766). All included articles after review of full text (n = 83) are presented in Table 3 according to the following variables: (i) reference; (ii) design and setting of the study; (iii) inclusion criteria for patients; (iv) description of the intervention; (v) whether a patient interview was performed to involve patients' experiences with their medicine-taking behaviour; (vi) outcome; (vii) whether healthcare professionals received additional training; and (viii) whether healthcare professionals received financial reimbursement. Articles are arranged in alphabetical order.
Table 3.
Included articles (n = 83)
| Reference | Design/setting | Patients | Intervention | Patient interview | Outcome | Additional training | Financial reimbursement |
|---|---|---|---|---|---|---|---|
| Bell SJ et al., Australia (2006) [27] | Descriptive study, 11 community pharmacists/11 GPs (March–November 2003). | Patients ≥17 years and taking ≥1 psychotropic drug for mental illness (n = 49). | Referral by GP. HMR by pharmacist. Case conference and recommendations to GP. | Yes | High-incidence DRPs identified. Pharmacists identified higher incidence of overall drug use than documented by referring GPs. | One-day mental health training workshop. HMR performed by accredited pharmacist. | No |
| Bereznicki BJ et al., Australia (2008) [28] | Case–control study, 35 community pharmacies (intervention) (May 2006 to April 2007)/28 community pharmacies (follow-up) (May 2007 to April 2008). | Asthma patients receiving ≥3 reliever canisters in preceding 6 months (intervention: n = 1551/follow-up: n = 718). | Educational material and GP referral. | No | Improved self-reported asthma control and asthma-related QoL after 6 months*. Sustained improvement after 12 months. | No | No |
| Bereznicki BJ et al., Australia (2011) [29] | |||||||
| Blenkinsopp A et al., UK (1991) [30] | Pilot study, 6 community pharmacists/15 GPs (18 month study period). | Patients whom the pharmacist thought should seek immediate medical advice (n = 120). | Use of a notification card to be used by community pharmacists when referring patients to their GP. | Yes | Notification card was found helpful and can stimulate and contribute towards developing and fostering collaboration between the two professions. | No | No |
| Bond CM et al., UK (2007) [31] | Randomized controlled trial, 20 community pharmacists/43 GPs (12 month study period). | Patients ≤65 years, receiving repeat medication for angina [n = 706 (340 intervention/366 control)] or hypertension [n = 1308 (656 intervention/652 control)]. | Trained community pharmacists reviewed medical records of eligible patients and made recommendations to the GP. | No | Improved prescribing of antiplatelet drugs*. Majority of outcome measures showed no change. | Pharmacists attended 1 week training course, 1 day workshop after 1 week, and feedback session after 6 months. | No |
| Bonner CJ & Carr B, Australia (2002) [32] | Intervention study, 1 community pharmacy/1 GP (study period unknown). | Patients ≥4 drugs prescribed and a significant medical history (n = 50). | Medical summary card. CP supplied with medical records and conducted consultation. | Yes | Medical summary cards unlikely to solve noncompliance. Collaborative model used was considered worthwhile. | No | No |
| Brulhart MI & Wermeille JP, Switzerland (2011) [33] | Prospective study, 10 nursing homes (January 2007 to December 2009). | All patients from nursing home (n = 329). | Medication review service, pharmaceutical care model integrated into the multidisciplinary team in nursing homes. | No | 3.7 DRPs per patient detected. 93% of interventions led to a rapid change in medication and/or follow-up. Alimentary tract and metabolism were main classes involved in interventions. | No | No |
| Bryant LJM et al., New Zealand (2011) [34] | Randomized controlled trial, 26 community pharmacists/63 GPs (12 month study period). | Patients ≥65 years with ≥5 prescribed medicines (n = 269 intervention/229 control). | Clinical medication review. | Yes | No significant difference in QoL. Improved MAI score after 6 months*. | No | NZ$160 per review (pharmacist). |
| NZ$50 per patient enrolled (GP). | |||||||
| Capoccia KL et al., USA (2004) [35] | Randomized controlled trial, large university family practice clinic (November 1999 to March 2001). | Patients diagnosed with a new episode of depression and started on an antidepressant medicine (n = 41 intervention/33 control). | Follow-up contact and care provided by CP with primary care provider and, if needed, study psychiatrist. | Yes | 440 interventions performed. No difference in overall antidepressant medication adherence, satisfaction with depression care or overall care. Mental health scores improved during follow-up for both groups. | No | No |
| Boudreau DM et al., USA (2002) [36] | |||||||
| Carter BL et al., USA (2009) [37] | Prospective, cluster randomized, controlled clinical trial, 6 community-based family medicine residency programmes (16 August 2005 to 9 April 2008). | Patients >21 years with a diagnosis of essential hypertension taking 0–3 antihypertensive medications (n = 192 intervention/210 control). | Drug therapy recommendations by CP to physicians. BP measurements and 24 h BP monitoring by research nurses. Measurements at 3 and 6 months. | No | Improvement in BP control*. | Team-building exercises for intervention physicians and pharmacists. Two initial 90 min training session to intervention pharmacists. | No |
| Castelino RL et al., Australia (2010) [38] | Retrospective cross-sectional audit, 7 accredited pharmacists (HMRs performed between February 2006 and October 2009). | Patients ≥65 years originally referred for HMR service (n = 270). | HMR. | Yes | Decreased MAI score*. | No (performed by accredited pharmacists). | No |
| Castelino RL et al., Australia (2011) [39] | Retrospective study, 7 accredited pharmacists (HMRs performed between February 2006 and October 2008). | Patients ≥65 years (n = 224). | HMR. | Yes | Pharmacists identified ≥1 DRP in 98% of the patients, mean of 5 recommendations per patient. | No (performed by accredited pharmacists). | No |
| Chambers LW et al., Canada (2005) [40] | Descriptive study, 27 community pharmacies/14 GPs (May–December 2003). | Patients ≥65 years (n = 983). | Cardiovascular health awareness programme. | Yes | Pharmacists noticed increased patient traffic and dialogue with their patients, as well as with GPs. | Training sessions for volunteer peer health educators. | No |
| Chen J & Britten N, UK (2000) [41] | Descriptive study, 3 CPs (3 month study period). | Patients referred by any member of the PHCT to a CP (n = 25). | Pharmacist consultation. | Yes | Referral to CP was slow. CPs have a possibility of providing independent medication advice within primary care. | No | No |
| Coleman DJ et al., UK (2001) [42] | Prospective cohort, single GP surgery located with the pharmacy in a health centre (6 month study period). | Patients ≥65 years with ≥5 repeat medications. | Domiciliary visiting programme of three visits. | Yes | Wide variety of problems identified, including medicines management, health beliefs and concordance and therapeutic and adverse reactions. Patients experienced a positive attitude being visited by the pharmacist. Not possible to calculate cost savings. | No | No |
| Patients <65 years with ≥5 repeat medications and significant disablement. | |||||||
| Patients ≥80 years taking ≥3 specified medications on repeat prescription (n = 100). | |||||||
| Community Pharmacy Medicines Management Project Evaluation Team, UK (2007) [43] | Randomized controlled trial, 9 sites in England (62 pharmacists/164 GPs) (November 2002 to May 2004). | Patients >17 years with coronary heart disease (n = 980 intervention/513 control). | Initial consultation, further consultations according to pharmacist-determined patient need. | Yes | Improvement in single computed satisfaction score for patients' most recent pharmacy visit*. No statistically significant differences found for any of the main outcome measures. | Yes | No |
| Crotty M et al., Australia (2004) [44] | Cluster-randomized controlled trial, 10 nursing homes (study period unknown). | Resident nursing home with difficult behaviour about whom staff would like more advice and information, prescribed ≥5 medications (n = 50 intervention/54 control/50 within-facility control). | Two multidisciplinary case conferences. | No | Higher decrease in MAI score for intervention group*. | No | No |
| Denneboom W et al., The Netherlands (2007) [19] | Randomized controlled trial, 28 pharmacists/77 GPs (March–May 2004). | Patients ≥75 years using ≥5 prescription medicines (n = 387 case conference/351 written feedback). | Treatment review by pharmacist following written feedback or case conference with GP. | No | Feedback in personal contact led to more medication changes compared with written feedback*. Pharmacists having personal contact spent more time*. | Training session for pharmacists. | No |
| Dolovich L et al., Canada (2008) [45] | Large-scale demonstration project, 7 family medical practice sites (24 month study period). | Patients with high level of medication-related risk (n = 1554). | Pharmacists integrated within interdisciplinary healthcare teams consult patients. | Yes | At least one DRP identified in 93.8% patients. Average of 4.4 DRPs identified per patient. | 2 day training workshop (pharmacists). | No |
| Doucette WR et al., USA (2005) [46] | Retrospective observational study, 1 independent community pharmacy (October 2000 to September 2002). | Patients taking ≥4 chronic medications and ≥1 defined disease state (n = 203). | MTM. Drug-related issues identified by pharmacist are recorded and classified. Pharmacist makes recommendations to GP. | Yes | 5.9 DRPs per patient identified for 2 year period under study, almost 3 issues per patient each year. Drug therapy may be improved by comprehensive MTM services. | No | Yes (Iowa Medicaid pharmaceutical case management project). |
| Earle KA et al., UK (2001) [47] | Feasibility study, 6 pharmacies located in catchment area of 1 hospital (16 weeks from October 1999). | Consenting volunteers (n = 263). | Blood pressure measurement (twice, time interval of 5 min). | No | Physician–pharmacist partnership feasible for surveillance and management of hypertension. | Two 3 h sessions on separate days. | No |
| Farris KB et al., Canada (2004) [9] | Single group pre–post design, 6 PHCTs (GP/pharmacist/nurse) (September 1999 to April 2000). | Patients defined by inclusion criteria (n = 199). | Home visits by pharmacists for medication review and discussion of patient care and medication-related issues within PHCTs. | Yes | Improved self-reported compliance after 3 and 6 months*. Decrease in visits to GPs and emergency departments. Decrease in hospital admissions. | Each PHCT received 4.5 h of team development training, and a professional facilitator met with each team every 6–8 weeks. | Yes (paid for travel and time to collaborate). |
| Fejzic JB & Tett SE, Australia (2004) [48] | Descriptive study, 7 GPs (6 month study period). | Patients from former Yugoslavia prescribed ≥1 medication (n = 25). | Medication management review. | Yes | Average of 6 DRPs identified per patient. | No | No |
| Fiss T et al., Germany (2010) [49] | Nonrandomized, prospective cohort, 9 community pharmacists (part 1, November–December 2005; part 2, March–May 2006; part 3, October 2006 to March 2007). | Inclusion based on GP's decision (part 1, n = 18; part 2, n = 23; part 3, n = 37). | To determine feasibility of HMR (part 1), followed by implementation of HMR (parts 2 + 3). HMR performed by practice assistant. | Yes | HMR useful; pharmacists and GPs lack sufficient time. Data transfer sheet supports collaboration and prevents loss of data. | No | No |
| Forstrom MJ et al., USA (1990) [50] | Intervention study using a non-equivalent control group design, 1 study clinic (6 month study period). | Hypertension patients taking oral antihypertensive medication (n = 154 intervention/172 control). | Formal, written consultation by the CP directed to prescribing physician, placed directly into patients' medical records. | No | Decrease cost of antihypertensive drug therapy per patients-day*. Reduction in use of the target drugs. | No | No |
| Gilbert AL et al., Australia (2002) [51] | Participatory action research, 6 divisions of general practice (63 pharmacists/129 GPs) (March 1999 to March 2000). | Patients at risk of having DRPs (n = 1000). | Collaborative service delivery model, involving a preliminary case conference, home visit and a second case conference. | Yes | On average 2.5 DRPs identified per patient. 42% of recommendations were documented as having been implemented. | No | No |
| Goldstein R et al., UK (1998) [52] | Intervention study, 47 GP–pharmacist partnerships from 2 health authorities (study period unknown). | Patients receiving ≥6 medications via repeat prescriptions (n = 1564). | Review patients' notes and repeat prescriptions, meet with GPs to discuss and make suggestions. 3 month review of patients' records and notes. | No | GPs agreed with nearly 60% of suggestions made by pharmacists. They only acted upon 56% of the problems they agreed with. | 1 day training session (pharmacists). | No |
| Graffen M et al., Australia (2004) [10] | Pre-/postintervention randomized controlled trial, 8 GPs (18 month study period). | Patients ≥65 years, ≥5 regular medications (n = 402). | Prescription review and QoL measures. | No | Improvement in 2 of 9 domains of QoL measured*. No difference in hospitalization rates. | No | No |
| Harris IM et al., USA (2009) [53] | Prospective, observational cohort study, family medicine clinic (2000–2001). | Using ≤5 medications, multiple medical conditions (n = 92). | Medication therapy review. | Yes | DRPs found in 90% of patients. Improvement in clinical status in 45% of patients. | No | No |
| Haxby DG et al., USA (1988) [54] | Retrospective study, 5 CPs (9 January to 6 March and 16 July to 4 September 1985). | All patient consultations in which CPs made recommendations (n = 59). | Consultation by CP. | Yes | GPs rated 88% consultations very useful and implemented 98% of recommended actions. 88% of patients were rated as improved clinically. | No | No |
| Hellström LM et al., Sweden (2011) [55] | Prospective controlled study, 3 internal medicine wards at a university hospital (November 2006 to April 2008). | Patients ≥65 years prescribed ≥1 drug for regular use (n = 109 intervention/101 control). | Medication reconciliation upon admission and discharge and medication review and monitoring during hospital stay. | Yes | Decreased number of drugs with ≥1 inappropriate rating*. No significant difference in MAI scores. | No | No |
| Holland R et al., UK (2005) [11] | Randomized controlled trial, after discharge from acute or community hospitals (October 2000 to December 2002). | Patients ≥80 years, admitted to hospital as an emergency, and prescribed ≥2 drugs on discharge (n = 437 intervention/435 control). | Home visit by pharmacist with 1 follow-up visit. | Yes | Increased readmission to hospital*. No significant difference in QoL scores. Difference in scores on visual analogue scale in favour of control group*. | 2 day training course. | Yes |
| Pacini M et al., UK (2007) [56] | |||||||
| Hourihan F et al., Australia (2003) [57] | Descriptive study, 10 community pharmacists (December 1999 to May 2000). | Patients ≥18 years who were not currently treated by a medical practitioner for elevated cholesterol or blood pressure (n = 204). | Innovative pharmacist-delivered health promotion and screening service for cardiovascular risk factors. | Yes | 89% had at least one modifiable risk factor for cardiovascular disease, and 80% received healthy lifestyle advice. | Information evening, two evenings with self-directed learning and skills-based workshops (pharmacists). | No |
| Jameson J et al., USA (1995) [58] | Prospective randomized trial, 1 family health centre (June 1991 to December 1992). | Patients at risk for DRPs (n = 27 intervention/29 control). | Single consultation by CP with high-risk patient and GP. | Yes | Decreased number of drugs, number of doses and 6 month cost*. | No | No |
| Jamieson LH et al., UK (2010) [59] | Randomized, open, cross-over trial, pharmacist-managed clinic with usual care delivered by GP in GP practice (14 month study period). | Hypertensive patients (n = 16 study control/17 control study). | Pharmaceutical care during 4 scheduled visits. | Yes | Reduction in systolic and diastolic blood pressure*. | No | No |
| Kiel PJ & McCord AD, USA (2005) [60] | Retrospective chart review, 22 GPs/2 CPs (June 2003 to April 2004). | Patients 18–90 years, diagnosed with diabetes mellitus, and baseline glycated haemoglobin ≥6.0% (n = 157). | Pharmacist-coordinated diabetes management programme. | No | Improvement of glycated haemoglobin and low-density lipoprotein values*. | No | No |
| King MA & Roberts MS, Australia (2001) [61] | Controlled trial, 3 nursing homes (10 April to 4 December 1996). | Residents of 3 nursing homes (n = 245). | Sessions of 3 case conference reviews. | No | Reduction in the number of medication orders and total cost of medications. | No | Maximum $91.55 per hour (GPs). |
| $40 per hour (CP). | |||||||
| Krska J et al., UK (2001) [62] | Randomized controlled trial, 6 general medical practices (18 month study period, 1997–1998). | Patients ≥65 years, ≥2 chronic disease states taking ≥4 prescribed medicines regularly (n = 168 intervention/164 control). | Development of PCP listing all PCIs, together with desired output, actions and outcomes. | Yes | Patients with a PCP had fewer PCIs*. No reduction in costs, health-related QoL, contacts with health and social services. | No (performed by clinically trained pharmacists). | No |
| Kwint HF et al., The Netherlands (2003) [63] | Intervention study, 1 healthcare centre (27 February to 30 September 2002). | Patients using ≥1 repeat prescriptions (n = 6559 repeat prescriptions). | Monitoring repeat prescriptions by pharmacists in cooperation with GPs. | Yes | DRPs identified in 17% of repeat prescriptions. Monitoring repeat prescriptions by pharmacist jointly with GP results in higher quality of care. | No | No |
| Kwint HF et al., The Netherlands (2011) [64] | Randomized controlled study, 6 community pharmacies (October 2007 to February 2008). | Patients ≥65 years using ≥5 medicines, living at home (n = 63 intervention/55 control). | Review of data from GP and pharmacy by two independent pharmacists. | No | Increased number of drug changes*. Decreased mean number of potential DRPs*. | No (performed by experienced pharmacists). | No |
| Leendertse AJ, The Netherlands (2011) [12] | Randomized, controlled multicentre study, 42 primary healthcare settings (2008–2009). | Patients ≥65 years using ≥5 medicines, ≥1 medicine from ATC class A or B, non-adherence (n = 364 intervention/310 control). | Patient-centred, structured, pharmaceutical care process. | Yes | Decreased medication-related hospital admissions. No statistically significant differences in survival, ADE and QoL. | Yes | No |
| Lenaghan E et al., UK (2007) [13] | Randomized controlled trial, 1 general practice/1 community pharmacist (March 2002 to June 2003). | Patients >80 years, living in their own homes, prescribed ≥4 oral daily medicines (n = 69 intervention/67 control). | HMR. | Yes | Mean difference in change of number of items prescribed*. No differences in hospital admissions and care home admissions. | No (performed by experienced pharmacist). | No |
| Lobas NH et al., USA (1992) [65] | Intervention study, family practice clinic (28 March 1988 to 15 June 1989). | Patients with ≥1 defined prognostic indicator (n = 184). | Patient education and counselling by pharmacist followed by consultation with physician to discuss recommendations. | Yes | Decreased costs and improvement of QoL. | No | No |
| Lowe CJ et al., UK (2000) [66] | Randomized controlled trial, 1 general practice with CP based for duration of the study (study period unknown). | Patients ≥65 years taking ≥3 drugs (n = 77 intervention/84 control). | 3 home visits (1 questionnaire; 2 education; 3 repeat questionnaire). | Yes | Increase in compliance and patient knowledge*. | No | No |
| Makowsky MJ et al., Canada (2009) [14] | Multicentre controlled clinical trial, 2 internal and 2 family medicine teams in 3 teaching hospitals (30 January 2006 to 2 February 2007). | Patients with diagnoses of coronary artery disease, pneumonia, chronic obstructive pulmonary disease, heart failure or type 2 diabetes mellitus (n = 221 intervention/231 control). | Thorough medication history and medication reconciliation by pharmacist (at admission). Medication reconciliation (at discharge) following written summary. | Yes | Team care patients more likely than usual care patients to receive care specified by indicators (all disease states* except for heart failure). Lower rate 3 month hospital readmission*. | No | No |
| Mangiapane S et al., Germany (2005) [67] | Intervention study, 39 community pharmacies/84 GPs (October 2001 to March 2002). | Patients 18–65 years having a physician's diagnosis of asthma (n = 183). | Five meetings between pharmacists and patients scheduled over 12 months. | Yes | Improved peak flow measurements*. Decreased self-reported symptoms and asthma severity*. Improved inhalation technique*. | 13 h to provide asthma services (at least one full-time pharmacist at each participating pharmacy) | Max. €75 (about $100 US) per patient to pharmacists and physicians when data were provided at baseline and after 6 and 12 months. |
| McDermott ME et al., UK (2006) [68] | Descriptive study, 1 general practice (study period unknown). | Patients ≥18 years who received ≥2 prescriptions for chronic pain during a 120 day period (n = 230). | Medical record reviews, patient questionnaires and (for selection of patients) patient interviews. | Yes | Per patient, 1–4 recommendations based on questionnaire and record review. 11 additional recommendations made for 9 individuals based on 23 interviews. | No | No |
| Nazareth I et al., UK (2001) [15] | Randomized controlled trial, 3 acute general and 1 long-stay hospital (June 1995 to March 1997). | Patients ≥75 years taking ≥4 medicines at discharge (n = 181 intervention/181 control). | Development of discharge plan by hospital pharmacist. Home visit by community pharmacist between 7 and 14 days after discharge. | Yes | Mean of 5.5 h for pharmacists to prepare and administer each care plan. No reduction in hospital readmissions at 3 or 6 months. Increased knowledge about medication was only effect revealed by patient interviews. | No | £20 per patient after returning a completed data sheet giving details of the visit (pharmacist) |
| Needham DS et al., UK (2002) [69] | Descriptive study, 14 community palliative care teams (10 month study period). | Patients with a life expectancy <12 months (n = 25). | Identification of PCIs and discussing these with patient and/or their carer. | No | 81% of interventions judged to have shown positive benefit to patients. | Training in symptom control and pharmaceutical aspects of palliative care (pharmacists). | No |
| Nishtala PS et al., Australia (2009) [70] | Retrospective study, 10 CPs (1 January to 30 June 2008). | Residents ≥65 years (n = 500). | Residential medication management review. | Yes | Decrease drug burden index score of study population*. Total of 1433 DRPs identified in 96% of residents. GPs acceptance and implementation were 72.5 and 58.1%, respectively. | No (performed by accredited pharmacists). | No |
| Nishtala PS et al., Australia (2011) [71] | |||||||
| Niquille A & Bugnon O, Switzerland (2010) [72] | Cross-sectional study, 11 community pharmacists/61 GPs (July 2007 to June 2008). | Cardiovascular outpatients 56–75 years (n = 85). | Community pharmacist-led medication review. | Yes | Cardiovascular outpatients could benefit from standardized medication review. GPs took 70% of recommendations into account. | 1 day training (pharmacists). | No |
| Patterson SM et al., UK (2010) [73] | Cluster randomized controlled trial, 22 nursing homes (March 2006 to June 2007). | Elderly people residing in nursing homes (n = 173 intervention/161 control). | CMR. | Yes | At 12 months, smaller proportion of residents from intervention group taking inappropriate psychoactive medicines*. Intervention is cost-effective. | Yes | No |
| Patterson SM et al., UK (2011) [74] | |||||||
| Phelan M et al., UK (2008) [75] | Randomized controlled trial, 13 general medical practices (May 2001 to March 2004). | Patients ≥55 years consulting their GP with pain, stiffness or both in one or both knees (n = 106). | Pharmacy review. Consultations with pharmacist (3–6) over a period of 10–12 weeks. | Yes | Improved pain control scores*. Reduction in prescribing costs between initial and final consultations. | No (performed by pharmacist with extensive experience). | No |
| Raynor DK et al., UK (2000) [76] | Intervention study, 6 community pharmacists (May 1997 to May 1998). | Patients ≥65 years, prescribed ≥4 regular medicines and living alone (n = 143). | Home visit by pharmacist and development of action plan followed by second home visit to discuss medication regimen and explain changes. | Yes | Reduction in number of patients with ≥1 problem from 94 to 58%*. Decrease reported non-adherence from 38 to 14%*. | 3 evening workshops (each 3 h) for pharmacists. | Yes (pharmacists for attending training workshops and time spent in providing the service). |
| RESPECT trial team, UK (2010) [77] | Randomized controlled trial, 24 general practices/62 community pharmacies (May 2002 to September 2005). | Patients ≥75 years taking ≥5 drugs on repeat prescription (n = 760). | Pharmacists interviewed patients, developed and implemented PCP together with patients' GPs, monthly medication review. | Yes | No impact on UK-MAI. No significant decrease of QoL measures. | Two sessions for participating pharmacists involving their associated GP prescribers in the second session. | No |
| Roughead EE et al., Australia (2009) [16] | Retrospective cohort study, database from Australian Government's Department of Veterans' Affairs (1 January 2004 to 1 July 2006). | War veterans and war widows with heart failure (n = 273 exposed/5444 unexposed). | HMR. | Yes | 45% reduction in hospitalization rate for heart failure*. | No (performed by accredited pharmacist). | Yes |
| Roughead EE et al., Australia (2011) [17] | Retrospective cohort study, database from Australian Government's Department of Veterans' Affairs (1 January 2004 to 1 July 2006). | War veterans ≥65 years with ≥2 dispensing of warfarin and had an HMR (n = 816 exposed/16 320 non-exposed). | HMR. | Yes | 79% reduction in likelihood of hospitalization due to an episode of bleeding 2–6 months after HMR*. | No (performed by accredited pharmacists). | No |
| Ryan-Woolley BM et al., UK (2000) [78] | Feasibility study, convenience sample 5 community pharmacists/8 general practices (17 GPs) (April 1997 to June 1998). | Patients 45–75 years with stable angina receiving ≤4 prescribed medications for their ischaemic heart disease (n = 208). | PCPs established by pharmacists in collaboration with patients. Interventions and GP referrals were made as necessary. | Yes | Pharmacists and GPs able to work together to deliver a specified programme of secondary prevention for patients with stable ischaemic heart disease. High level of confidence in and acceptance of community pharmacist as partner. | 6 h of training (pharmacists). | No |
| Schmidt I et al., Sweden (1998) [79] | Randomized controlled trial, 33 nursing homes (15 experimental/18 control) (14 month study period). | All residents from 36 selected nursing homes (n = 626 experimental/1228 control). | Assessment number and selectivity of drugs prescribed before and after 12 month intervention. | No | Decreased prescribing of antipsychotics, nonrecommended hypnotics and antidepressants*. | No | No |
| Schneider J & Barber N, UK (1996) [80] | Pilot study, 16 community pharmacists/14 GPs (June–July 1993). | Housebound patients who were having some difficulty with their medication due to a medical, physical or psychological condition (n = 39). | Home visit by community pharmacist. Follow-up visit after 1 month for patients who were given advice and/or for whom interventions were requested. | Yes | Average initial visit was 56 min. Medication records for 29 of 39 patients were inaccurate. GPs did not seem to consider majority of the interventions to be urgent. | One evening training (pharmacists). | No |
| Schulz M et al., Germany (2001) [81] | Controlled intervention study, 48 pharmacies (12 month study period). | Asthma patients identified by means of medication or by patients' self-report (n = 161 intervention/81 control). | Assessment of patients' inhalation techniques in 9 meetings between pharmacists and patients. Pharmacists detected and solved DRPs in cooperation with patients and GPs. | Yes | Increased FEV1 for intervention group patients. Patients in intervention group perceived within-group improvement in asthma severity from 6 to 12 months*. Improved inhalation technique and knowledge in intervention group*. Improved QoL in intervention group*. | Training for intervention pharmacies: medical, pharmaceutical and pharmacological knowledge (5 h), communication skills (6 h), use of study protocol and documentation forms (2 h). | No |
| Sellors J et al., Canada (2001) [82] | Randomized controlled trial, 24 community pharmacists each linked with a GP (study period unknown). | Patients ≥5 medications (n = 387). | Pharmacists make recommendations to GPs to prevent and solve DRPs and simplify medication regimens. | Yes | Average of 3 DRPs per patient. GPs followed 84% of recommendations. After 5 months, GPs implemented 57%. | 1 day training workshop (pharmacists). | No |
| Sellors J et al., Canada (2003) [83] | Randomized controlled trial, 24 community pharmacists/48 GPs (study period unknown). | Patients ≥65 years, taking ≥5 medications (n = 431 intervention/458 control). | Structured medication assessment by pharmacist in physician's office. | Yes | GPs acted on majority of recommendations. No effect on number and cost of medications, healthcare use and cost or QoL. | No | No |
| Shimp LA et al., USA (1986) [84] | Pilot study, 2 board-certified family physicians (study period unknown). | Patients ≥60 years, ≥2 chronic medical problems, ≥6 medications (n = 17). | Medication history in home of patient. Interview by a pharmacist-trained layperson. Systematic review by CP. | Yes | Average of four therapeutic recommendations generated per patient (range 1–9). | No | No |
| Soendergaard B et al., Denmark (2006) [85] | Development project, 1 community pharmacist worked 20 h per week in a GP practice with 3 GPs (June 2002 to November 2003). | Patients prescribed >4 drugs on a regular basis (n = 40). | CMR. | Yes | Average of 2.6 DRPs defined per patient. 83% of pharmacist's recommendations accepted by GP, 77% implemented at end of study. | No | No |
| Sorensen L et al., Australia (2004) [86] | Randomized controlled trial, 3 Australian states, 92 GPs/53 community pharmacists (August 1999 to May 2000). | Polypharmacy patients (1 out of 10 defined inclusion criteria) (n = 177 intervention/223 control). | Medication review report using GP information and home-visit findings. | Yes | Decrease of Duke's Severity of Illness Visual Analogue Scale (DUSOI-A). | 2 educational sessions dealing with prescribing issues for pharmacists and GPs. | AUS$ 199.85 per patient (GP). |
| AUS$ 115 per patient (pharmacist). | |||||||
| Stafford L et al., Australia (2011) [87] | Prospective, controlled cohort study, patients discharged from 8 hospitals (November 2008 to December 2009). | Patients >18 years, discharged from hospital taking warfarin (n = 129 postdischarge cohort/139 usual care cohort). | 2–3 home visits by pharmacist within 8–10 days after discharge hospital, including INR monitoring, education and HMR. | Yes | Reduction in combined major and minor haemorrhagic events to 90 days postdischarge*. Reduction in thrombotic events. Increased persistence with warfarin therapy*. | Training programme to up-skill pharmacists in issues associated with warfarin use (accredited pharmacists). | No |
| Stafford L et al., Australia (2011) [88] | |||||||
| Stergachis A et al., USA (1987) [89] | Case–control study, 2 family practice clusters, 17 GPs (15 month study period). | Patients receiving high-cost NSAIDs (no. of patients unknown). | Pharmacists providing clinical pharmacy services to physicians and patients. | No | No effect on drug costs. | No (performed by experienced CPs) | No |
| Stuijt CCM et al., The Netherlands (2008) [90] | Observational study, 1 residential nursing home (1 April 2003 to 1 April 2004). | Patients from 1 residential nursing home (n = 30). | Pharmacist-led medication review. | Yes | Decreased MAI score after intervention*. | No | No |
| Towle I & Adams J, UK (2006) [91] | Prescribing intervention study, 1 GP practice with 2 GPs (April 2002 to June 2005). | Patients ≤70 years with a repeat benzodiazepine prescription (n = 206). | Development and implementation of benzodiazepine prescribing policy. | Yes | Benzodiazepine prescribing rate decreased by 67%. | No | No |
| Villeneuve J et al., Canada (2010) [92] | Open cluster randomized controlled trial, 15 clusters of physicians (total 77) and pharmacists (total 108) (May 2005 to January 2008). | Patients with dyslipidaemia (n = 108 intervention/117 control). | Collaborative care. | Yes | Collaborative primary care feasible for management of dyslipidaemia. No significant effects on low-density lipoprotein cholesterol and other cardiovascular risk factors. | 1 day training workshop (pharmacists). | $50 per patient (clinicians). |
| $104 per patient (pharmacist). | |||||||
| Vinks THAM et al., The Netherlands (2009) [93] | Pharmacy-based controlled trial, 16 community pharmacies (June 2002 to June 2003). | Patients ≥65 years using ≥6 drugs (n = 98 intervention/98 control). | Medication assessment. | No | Reduction in number of potential DRPs*. | No | No |
| Wermeille J et al., UK (2004) [94] | Prospective pretest-post-test single-group study, 4 community pharmacies (October 1999 to October 2000). | Patients with type 2 diabetes on oral hypoglycaemic therapy (n = 62). | Systematic review by community pharmacist. | Yes | Mean of 2.9 PCIs defined per patient. Decrease in glycated haemoglobin, blood pressure and total cholesterol*. | Support of a research pharmacist. | No |
| Wilcock M & Harding G, UK (2008) [95] | Case–control study, 23 community pharmacies (data collection, end 2006 to April 2007, retrospective). | Patients with coronary heart disease who had received a MUR (n = 294 intervention/360 control). | MUR. | Yes | Over half of pharmacists' recommendations are acted upon. Important to develop pharmacist's role as reviewer and adviser on patients' medications. | No (performed by accredited pharmacists). | No |
| Wong C et al., Canada (1997) [96] | Randomized controlled trial, 2 family practice physicians (4 week study period. | Patients ≥18 years (n = 48 intervention/43 control). | Review of medical records by investigating pharmacist. Survey questionnaire with/without patient interview. | Yes | Almost fourfold increase in identification of DRPs after patient interview*. | No | No |
| Yu K et al., Australia (2007) [97] | Prospective uncontrolled pilot study, 1 teaching hospital (May–September 2005. | Patients admitted with any of the defined risk factors (n = 38). | HMR. | Yes | Reported barriers are time pressure (pharmacists and GPs), lack of preparedness (pharmacists) and financial constraints (pharmacists). | No (performed by accredited pharmacist). | No |
| Zermansky AG et al., UK (2001) [98] | Randomized controlled trial, 4 GP practices (June 1999 to June 2000). | Patients ≥65 years receiving ≥1 drug on repeat prescription (n = 608 intervention/580 control). | CMR. | Yes | More changes in intervention group*. Less rise in numbers of drugs and costs*. | No | No |
| Zermansky AG et al., UK (2006) [99] | Randomized controlled trial, 65 care homes (April 16 to end 2002). | Care home residents ≥65 years taking ≥1 repeat medicine (n = 331 intervention/330 control). | CMR. | Yes | Higher number of medication changes*. Large reduction in number of falls*. | No | No |
Abbreviations: ADE, adverse drug event; ATC, anatomical therapeutic chemical; BP, blood pressure; CMR, clinical medication review; CP, clinical pharmacist; DRP, drug-related problem; FEV1, forced expiratory volume in 1 s; GP, general practitioner; HMR, home medicines review; INR, international normalized ratio; MAI, medication appropriateness index; MTM, medication therapy management; MUR, medicines use review; PCI, pharmaceutical care issue; PCP, pharmaceutical care plan; PHCT, primary healthcare team; QoL, quality of life.
Significant results.
The 83 included articles describe results from 77 studies. Most studies were performed in Europe (n = 40), followed by the USA/Canada (n = 19) and Australia/New Zealand (n = 18). A majority of studies (n = 60) describe patient involvement using a patient interview or consultation (either at home or at the pharmacy/GP practice). About one-third of the studies (26 of 77) provided information on additional training, mostly for participating pharmacists. Only 11 studies reported financial reimbursement for participating healthcare providers. The amounts differed and were not always mentioned.
Studies with a high level of evidence, such as randomized controlled trials, showed more significant results when compared with studies having a lower level of evidence, such as retrospective observational studies. Not all studies were able to conclude with hard outcomes, such as decrease in hospital admissions or costs. Only nine studies reported outcomes on hospital admissions. When we focused more on these studies, we found some differences. Farris et al. [9] showed a nonsignificant decrease in hospital admissions. A single-group pre–post design was used, and 199 patients were included (no power calculation was performed). Each primary healthcare team (PHCT) received training, but team pharmacists were generally not patients' dispensing pharmacists. An average of 3.9 issues were defined per patient, and an average of 59% of issues were resolved. However, these numbers showed large differences between the six PHCTs. Six studies used a randomized controlled trial (RCT) design. Graffen et al. [10] showed no differences in hospitalization rates among 402 included patients (determined by power calculation). No additional training was provided and no patient interview performed. Hospitalizations were determined by asking patients. Holland et al. [11] found a statistically significant increase in hospital admissions. A total of 872 patients were included (determined by power calculation). Pharmacists received a 2 day training course. Leendertse [12] found a nonsignificant decrease in medication-related hospital admissions; however, the intervention did show a statistically significant result for patients with five or more diseases. In this RCT, 674 patients were included (aim 14 200 based on power calculation). Pharmacists received additional training. Lenaghan et al. [13] found no differences in hospital admissions among 136 included patients (aim 164 based on power calculation). Pharmacists did not receive additional training but were experienced in performing home medicines reviews (HMRs). Makowsky et al. [14] found a statistically significant decrease in hospital admissions after 3 but not after 6 months. A total of 452 patients were included (aim 650 based on power calculation). The intervention was performed by experienced pharmacists who did not receive additional training. Nazareth et al. [15] found no differences in hospital admissions among 362 included patients (aim 390 based on power calculation). No additional training was provided for participating healthcare providers. Two cohort studies performed by Roughead et al. [16], [17] showed a statistically significant decrease in hospitalization rates for specific patient groups. Their first study included 273 patients exposed to an HMR and showed a 45% reduction in hospitalization for heart failure patients [16]. Their second study included 816 patients exposed to an HMR and showed 79% reduction in hospitalization for warfarin-associated bleeding [17]. Both studies were performed by experienced pharmacists, and no additional training was provided.
Other significant results found were decreases in number of drug-related problems, improved prescribing of medication, improved quality of life scores, improved medication appropriateness index scores, increased compliance and patient knowledge, and improved clinical values, e.g. cholesterol levels. Most studies described positive outcomes on satisfaction. Healthcare providers and patients were satisfied when they were involved in projects. Studies also showed that when cooperation between healthcare providers and patients occurred, more drug-related problems were defined and solved.
Discussion
A recent Cochrane review focused on health-related outcomes of clinical pharmacy services [18]. Pharmacist interventions resulted in improvement in most clinical outcomes, but these were not always statistically significant. The Cochrane review only describes pharmacist interventions, whereas our review focuses on interventions with cooperation between pharmacists and GPs.
Only nine studies report hard outcomes, such as hospital (re)admissions [9]–[17]. Three studies show a significant decrease in hospital (re)admissions [14], [16], [17], and one study shows an increase in hospital admissions [11]. The intervention described by Holland et al. is performed by a review pharmacist [11]. Also, the intervention by Farris et al. [9] was not performed by the patients' own pharmacist, whereas the other seven studies did include cooperation between the patients' own pharmacist and GP. All studies with no differences in hospital admissions provided no additional training to pharmacists [10], [13], [15]; however, one study did mention that the intervention was performed by experienced pharmacists [13]. Graffen et al. [10] determined hospitalization rates by asking patients, which could result in recall bias. Other studies did provide additional training to pharmacists or mentioned that pharmacists were experienced. This could mean that the pharmacists involved in the selected studies were not able to perform an adequate medication review or medication reconciliation without additional training. Not all studies managed to include sufficient patient numbers necessary according to their power calculation. Two studies did report a decrease in hospital admissions, which could be significant after including higher patient numbers [12], [14] A subanalysis from Leendertse et al. [12] did show a significant decrease in medication-related hospital admissions for patients with ≥5 diseases. In order to retrieve hard outcomes, such as hospital (re)admissions, randomized clinical trials with large numbers of patients are needed. In most studies, numbers are too small to be able to study hard outcomes. Also, the level of cooperation and communication between healthcare providers is important. Denneboom et al. [19] performed a study using level 2 medication review and concluded that feedback in personal contact led to significantly more medication changes when compared with written feedback. When there is personal contact, healthcare providers can motivate their opinion on possible medication changes and, together, might choose a different intervention than they originally had suggested instead of rejecting the proposed intervention outright. When the patient is also involved, the intervention will have a higher chance of long-term success. When patients agree with the proposed intervention, they will be more motivated to change [4], [20]–[22]. The quality of collaboration is also important. Isetts et al. [23] performed a quality assessment of therapeutic determinations made by pharmacists. Decisions made by healthcare providers were found to be clinically credible based on the evaluations and comments of a peer review panel. When pharmaceutical care practitioners collaborate with physicians to provide drug therapy management services, this may help to reduce drug-related morbidity and improve therapeutic outcomes. Several classifications of medication review activities are used, but none of the classification systems is able to cover all different kinds of activities. In order to compare different activities, it is important to develop one classification system that can be used for all different activities performed throughout the whole world.
Most studies performed focused on elderly patients with multiple morbidities using multiple medicines (polypharmacy). These patients are at higher risk of complications and would benefit most from a periodic CMR [24], [25]. In many countries, clinical pharmacologists are also involved in CMRs; these studies were not included in this review, because the focus is on pharmacists and GPs as healthcare providers, with whom patients have a relationship.
Besides study design and patient outcomes, we looked at additional training for healthcare providers and financial reimbursement. Performing services such as medication review or medication reconciliation takes time. When performed at a higher level, time investment will increase. We question whether all healthcare providers have sufficient skill and experience to perform these services, knowing it involves complex patients with multiple morbidities and polypharmacy. In about one-third of the included studies, additional training was provided to participating healthcare providers, usually for pharmacists. This training usually concerned education about specific diseases and communication skills. Twelve studies mention that they did not provide additional training, but the studies were performed by accredited and experienced (clinical) pharmacists. Of these studies, six were performed in Australia, where the service called home medicines review (HMR) can only be performed by accredited pharmacists who are reimbursed for their services. Australia is well known for the development and implementation of HMRs.
Some studies focused on cost-effectiveness of medication review services, but none could show significant results. A recent study by Perez et al. [26] showed economic evaluations of clinical pharmacy services from 2001 to 2005. A median benefit-to-cost ratio of 4.81:1 was found, meaning that for every $1 spent a $4.81 reduction in costs or other economic benefits was achieved. When other healthcare providers besides pharmacists, e.g. GPs, are involved, costs of the intervention will increase without knowing what this means for the achieved benefits. More research on cost-effectiveness of multidisciplinary interventions is necessary.
The strength of this article is its use of a systematic literature search. However, we did find five relevant articles outside the performed search, which may mean we missed other articles. One reason for this could be that certain articles do not have well-defined keywords or that cooperation is not explicitly mentioned in the title or abstract. We excluded two articles based on their language (Norwegian and French). We do not believe this influenced our results.
We recommend that future research should include a large randomized clinical trial with high patient numbers focused on hard outcomes, e.g. hospital (re)admissions and cost-effectiveness. With such a study, healthcare providers would be able to show their professional skills and how they can provide benefit to patients at high risk. This will make it easier to determine the necessary additional skills and proper reimbursement for time spent.
Conclusions
Many different interventions are described where pharmacists and GPs work together to improve patients' health. Besides results on patient satisfaction, drug-related problems, quality of life and clinical values, fewer studies report hard outcomes, and results are not all comparable. Randomized controlled trials should be able to describe hard outcomes, but large patient groups will be needed to perform such studies. Patient involvement is important for motivation to change and for a long-term effect of the proposed intervention.
Acknowledgments
We thank Truus van Ittersum, library services of Research Institute SHARE, for her help with the systematic literature search. We thank Timothy Broesamle for editing this article.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.FIP Working Group on Collaborative Practice. FIP Reference Paper Collaborative Practice. 2009. Available at http://www.fip.org/www/uploads/database_file.php?id=319&table_id= (last accessed 12 October 2011)
- 2.Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169:894–900. doi: 10.1001/archinternmed.2009.71. [DOI] [PubMed] [Google Scholar]
- 3.Michie S, Miles J, Weinman J. Patient-centredness in chronic illness: what is it and does it matter? Patient Educ Couns. 2003;51:197–206. doi: 10.1016/s0738-3991(02)00194-5. [DOI] [PubMed] [Google Scholar]
- 4.Holman H, Lorig K. Patients as partners in managing chronic disease. Partnership is a prerequisite for effective and efficient health care. BMJ. 2000;320:526–7. doi: 10.1136/bmj.320.7234.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cipolle RJ, Strand LM, Morley PC. In: Pharmaceutical Care Practice The Clinician's Guide. 2nd edn. New York, NY: The McGraw-Hill Companies, Inc; 2004. [Google Scholar]
- 6.Task Force on Medicines Partnership and The National Collaborative Medicines Management, Services Programme. Room for Review: A guide to medication review: the agenda for patients, practitioners and managers. 2002. Available at http://www.npc.nhs.uk/review_medicines/intro/resources/room_for_review.pdf (last accessed 12 October 2011)
- 7.Karapinar-Carkit F, Borgsteede SD, Zoer J, Smit HJ, Egberts AC, van den Bemt PM. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43:1001–10. doi: 10.1345/aph.1L597. [DOI] [PubMed] [Google Scholar]
- 8.Medicines PP. A Guide to Medication Review 2008. 2008. Available at http://www.npc.co.uk/review_medicines/intro/resources/agtmr_web1.pdf (last 12 accessed October 2011)
- 9.Farris KB, Cote I, Feeny D, Johnson JA, Tsuyuki RT, Brilliant S, et al. Enhancing primary care for complex patients. Demonstration project using multidisciplinary teams. Can Fam Physician. 2004;50:998–1003. [PMC free article] [PubMed] [Google Scholar]
- 10.Graffen M, Kennedy D, Simpson M. Quality use of medicines in the rural ambulant elderly: a pilot study. Rural Remote Health. 2004;4:184. [PubMed] [Google Scholar]
- 11.Holland R, Lenaghan E, Harvey I, Smith R, Shepstone L, Lipp A, et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ. 2005;330:293. doi: 10.1136/bmj.38338.674583.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leendertse AJ. Hospital Admissions Related to Medication. Prevalence, Provocation and Prevention. Utrecht: Utrecht University; 2010. [Google Scholar]
- 13.Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care – the POLYMED randomised controlled trial. Age Ageing. 2007;36:292–7. doi: 10.1093/ageing/afm036. [DOI] [PubMed] [Google Scholar]
- 14.Makowsky MJ, Koshman SL, Midodzi WK, Tsuyuki RT. Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study [ NCT00351676] Med Care. 2009;47:642–50. doi: 10.1097/MLR.0b013e3181926032. [DOI] [PubMed] [Google Scholar]
- 15.Nazareth I, Burton A, Shulman S, Smith P, Haines A, Timberal H. A pharmacy discharge plan for hospitalized elderly patients – a randomized controlled trial. Age Ageing. 2001;30:33–40. doi: 10.1093/ageing/30.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Roughead EE, Barratt JD, Ramsay E, Pratt N, Ryan P, Peck R, et al. The effectiveness of collaborative medicine reviews in delaying time to next hospitalization for patients with heart failure in the practice setting: results of a cohort study. Circ Heart Fail. 2009;2:424–8. doi: 10.1161/CIRCHEARTFAILURE.109.861013. [DOI] [PubMed] [Google Scholar]
- 17.Roughead EE, Barratt JD, Ramsay E, Pratt N, Ryan P, Peck R, et al. Collaborative home medicines review delays time to next hospitalization for warfarin associated bleeding in Australian war veterans. J Clin Pharm Ther. 2011;36:27–32. doi: 10.1111/j.1365-2710.2009.01149.x. [DOI] [PubMed] [Google Scholar]
- 18.Nkansah N, Mostovetsky O, Yu C, Chheng T, Beney J, Bond CM, et al. Effect of outpatient pharmacists' non-dispensing roles on patient outcomes and prescribing patterns. Cochrane Database Syst Rev. 2010;(7) doi: 10.1002/14651858.CD000336.pub2. CD000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denneboom W, Dautzenberg MG, Grol R, De Smet PA. Treatment reviews of older people on polypharmacy in primary care: cluster controlled trial comparing two approaches. Br J Gen Pract. 2007;57:723–31. [PMC free article] [PubMed] [Google Scholar]
- 20.Charles C, Whelan T, Gafni A. What do we mean by partnership in making decisions about treatment? BMJ. 1999;319:780–2. doi: 10.1136/bmj.319.7212.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan JL, Ellis SJ, Chambers R. Defining shared decision making and concordance: are they one and the same? Postgrad Med J. 2002;78:383–4. doi: 10.1136/pmj.78.921.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 23.Isetts BJ, Brown LM, Schondelmeyer SW, Lenarz LA. Quality assessment of a collaborative approach for decreasing drug-related morbidity and achieving therapeutic goals. Arch Intern Med. 2003;163:1813–20. doi: 10.1001/archinte.163.15.1813. [DOI] [PubMed] [Google Scholar]
- 24.Warlé-van Herwaarden M, Kramers C, Sturkenboom M, Van den Bemt PMLA, De Smet PAGM. Targeting outpatient drug safety: recommendations of the Dutch HARM-Wrestling task force. 2011. Available at http://www.knmp.nl/downloads/medicijnen-zorgverlening/medicatieveiligheid/harmwrestlingEnglishcopyrightKNMP.pdf (last accessed 12 October 2011) [DOI] [PubMed]
- 25.De Smet PA. Hospital admissions related to medications and implementing guidelines. Arch Intern Med. 2009;169:810–1. doi: 10.1001/archinternmed.2009.81. [DOI] [PubMed] [Google Scholar]
- 26.Perez A, Doloresco F, Hoffman JM, Meek PD, Touchette DR, Vermeulen LC, et al. ACCP: economic evaluations of clinical pharmacy services: 2001–2005. Pharmacotherapy. 2008;28:285e–323e. doi: 10.1592/phco.29.1.128. [DOI] [PubMed] [Google Scholar]
- 27.Bell JS, Whitehead P, Aslani P, McLachlan AJ, Chen TF. Drug-related problems in the community setting: pharmacists' findings and recommendations for people with mental illnesses. Clin Drug Investig. 2006;26:415–25. doi: 10.2165/00044011-200626070-00003. [DOI] [PubMed] [Google Scholar]
- 28.Bereznicki BJ, Peterson GM, Jackson SL, Walters H, Fitzmaurice K, Gee P. Pharmacist-initiated general practitioner referral of patients with suboptimal asthma management. Pharm World Sci. 2008;30:869–75. doi: 10.1007/s11096-008-9242-3. [DOI] [PubMed] [Google Scholar]
- 29.Bereznicki B, Peterson G, Jackson S, Walters EH, Gee P. The sustainability of a community pharmacy intervention to improve the quality use of asthma medication. J Clin Pharm Ther. 2011;36:144–51. doi: 10.1111/j.1365-2710.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- 30.Blenkinsopp A, Jepson M, Drury M. Using a notification card to improve communication between community pharmacists and general practitioners. Br J Gen Pract. 1991;41:116–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Bond CM, Fish A, Porteous TH, Reid JP, Scott A, Antonazzo E. A randomised controlled trial of the effects of note-based medication review by community pharmacists on prescribing of cardiovascular drugs in general practice. Int J Pharm Pract. 2007;15:39–46. [Google Scholar]
- 32.Bonner CJ, Carr B. Medication compliance problems in general practice: detection and intervention by pharmacists and doctors. Aust J Rural Health. 2002;10:33–8. doi: 10.1046/j.1440-1584.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 33.Brulhart MI, Wermeille JP. Multidisciplinary medication review: evaluation of a pharmaceutical care model for nursing homes. Int J Clin Pharm. 2011;33:549–57. doi: 10.1007/s11096-011-9506-1. [DOI] [PubMed] [Google Scholar]
- 34.Bryant LJM, Coster G, Gamble GD, McCormick RN. The General Practitioner–Pharmacist Collaboration (GPPC) study: a randomised controlled trial of clinical medication reviews in community pharmacy. Int J Pharm Pract. 2011;19:94–105. doi: 10.1111/j.2042-7174.2010.00079.x. [DOI] [PubMed] [Google Scholar]
- 35.Capoccia KL, Boudreau DM, Blough DK, Ellsworth AJ, Clark DR, Stevens NG, et al. Randomized trial of pharmacist interventions to improve depression care and outcomes in primary care. Am J Health Syst Pharm. 2004;61:364–72. doi: 10.1093/ajhp/61.4.364. [DOI] [PubMed] [Google Scholar]
- 36.Boudreau DM, Capoccia KL, Sullivan SD, Blough DK, Ellsworth AJ, Clark DL, et al. Collaborative care model to improve outcomes in major depression. Ann Pharmacother. 2002;36:585–91. doi: 10.1345/aph.1A259. [DOI] [PubMed] [Google Scholar]
- 37.Carter BL, Ardery G, Dawson JD, James PA, Bergus GR, Doucette WR, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996–2002. doi: 10.1001/archinternmed.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castelino RL, Bajorek BV, Chen TF. Retrospective evaluation of home medicines review by pharmacists in older Australian patients using the medication appropriateness index. Ann Pharmacother. 2010;44:1922–9. doi: 10.1345/aph.1P373. [DOI] [PubMed] [Google Scholar]
- 39.Castelino RL, Bajorek BV, Chen TF. Are interventions recommended by pharmacists during Home Medicines Review evidence-based? J Eval Clin Pract. 2011;17:104–10. doi: 10.1111/j.1365-2753.2010.01375.x. [DOI] [PubMed] [Google Scholar]
- 40.Chambers LW, Kaczorowski J, Dolovich L, Karwalajtys T, Hall HL, McDonough B, et al. A community-based program for cardiovascular health awareness. Can J Public Health. 2005;96:294–8. doi: 10.1007/BF03405169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Britten N. ‘Strong medicine’: an analysis of pharmacist consultations in primary care. Fam Pract. 2000;17:480–3. doi: 10.1093/fampra/17.6.480. [DOI] [PubMed] [Google Scholar]
- 42.Coleman DJ, Portlock J, Brown D. Delivering domiciliary pharmaceutical care from a health centre pharmacy. Int J Pharm Pract. 2001;9:127–37. [Google Scholar]
- 43.Community Pharmacy Medicines Management Project Evaluation Team. The MEDMAN study: a randomized controlled trial of community pharmacy-led medicines management for patients with coronary heart disease. Fam Pract. 2007;24:189–200. doi: 10.1093/fampra/cml075. [DOI] [PubMed] [Google Scholar]
- 44.Crotty M, Halbert J, Rowett D, Giles L, Birks R, Williams H, et al. An outreach geriatric medication advisory service in residential aged care: a randomised controlled trial of case conferencing. Age Ageing. 2004;33:612–7. doi: 10.1093/ageing/afh213. [DOI] [PubMed] [Google Scholar]
- 45.Dolovich L, Pottie K, Kaczorowski J, Farrell B, Austin Z, Rodriguez C, et al. Integrating family medicine and pharmacy to advance primary care therapeutics. Clin Pharmacol Ther. 2008;83:913–7. doi: 10.1038/clpt.2008.29. [DOI] [PubMed] [Google Scholar]
- 46.Doucette WR, McDonough RP, Klepser D, McCarthy R. Comprehensive medication therapy management: identifying and resolving drug-related issues in a community pharmacy. Clin Ther. 2005;27:1104–11. doi: 10.1016/s0149-2918(05)00146-3. [DOI] [PubMed] [Google Scholar]
- 47.Earle KA, Taylor P, Wyatt S, Burnett S, Ray J. A physician-pharmacist model for the surveillance of blood pressure in the community: a feasibility study. J Hum Hypertens. 2001;15:529–33. doi: 10.1038/sj.jhh.1001220. [DOI] [PubMed] [Google Scholar]
- 48.Fejzic JB, Tett SE. Medication management reviews for people from the former Yugoslavia now resident in Australia. Pharm World Sci. 2004;26:271–6. doi: 10.1023/b:phar.0000042879.89551.70. [DOI] [PubMed] [Google Scholar]
- 49.Fiss T, Ritter CA, Alte D, van den Berg N, Hoffmann W. Detection of drug related problems in an interdisciplinary health care model for rural areas in Germany. Pharm World Sci. 2010;32:566–74. doi: 10.1007/s11096-010-9409-6. [DOI] [PubMed] [Google Scholar]
- 50.Forstrom MJ, Ried LD, Stergachis AS, Corliss DA. Effect of a clinical pharmacist program on the cost of hypertension treatment in an HMO family practice clinic. DICP. 1990;24:304–9. doi: 10.1177/106002809002400318. [DOI] [PubMed] [Google Scholar]
- 51.Gilbert AL, Roughead EE, Beilby J, Mott K, Barratt JD. Collaborative medication management services: improving patient care. Med J Aust. 2002;177:189–92. doi: 10.5694/j.1326-5377.2002.tb04730.x. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein R, Hulme H, Willits J. Reviewing repeat prescribing – general practitioners and community pharmacists working together. Int J Pharm Pract. 1998;6:60–6. [Google Scholar]
- 53.Harris IM, Westberg SM, Frakes MJ, Van Vooren JS. Outcomes of medication therapy review in a family medicine clinic. J Am Pharm Assoc. 2009;49:623–7. doi: 10.1331/JAPhA.2009.08069. [DOI] [PubMed] [Google Scholar]
- 54.Haxby DG, Weart CW, Goodman BW., Jr Family practice physicians' perceptions of the usefulness of drug therapy recommendations from clinical pharmacists. Am J Hosp Pharm. 1988;45:824–7. [PubMed] [Google Scholar]
- 55.Hellstrom LM, Bondesson A, Hoglund P, Midlov P, Holmdahl L, Rickhag E, et al. Impact of the Lund Integrated Medicines Management (LIMM) model on medication appropriateness and drug-related hospital revisits. Eur J Clin Pharmacol. 2011;67:741–52. doi: 10.1007/s00228-010-0982-3. [DOI] [PubMed] [Google Scholar]
- 56.Pacini M, Smith RD, Wilson EC, Holland R. Home-based medication review in older people: is it cost effective? Pharmacoeconomics. 2007;25:171–80. doi: 10.2165/00019053-200725020-00008. [DOI] [PubMed] [Google Scholar]
- 57.Hourihan F, Krass I, Chen T. Rural community pharmacy: a feasible site for a health promotion and screening service for cardiovascular risk factors. Aust J Rural Health. 2003;11:28–35. doi: 10.1046/j.1440-1584.2003.00468.x. [DOI] [PubMed] [Google Scholar]
- 58.Jameson J, VanNoord G, Vanderwoud K. The impact of a pharmacotherapy consultation on the cost and outcome of medical therapy. J Fam Pract. 1995;41:469–72. [PubMed] [Google Scholar]
- 59.Jamieson LH, Scally AJ, Chrystyn H. A randomised comparison of practice pharmacist-managed hypertension providing Level 3 Medication Review versus usual care in general practice. J Appl Ther Res. 2010;7:77–86. [Google Scholar]
- 60.Kiel PJ, McCord AD. Pharmacist impact on clinical outcomes in a diabetes disease management program via collaborative practice. Ann Pharmacother. 2005;39:1828–32. doi: 10.1345/aph.1G356. [DOI] [PubMed] [Google Scholar]
- 61.King MA, Roberts MS. Multidisciplinary case conference reviews: improving outcomes for nursing home residents, carers and health professionals. Pharm World Sci. 2001;23:41–5. doi: 10.1023/a:1011215008000. [DOI] [PubMed] [Google Scholar]
- 62.Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing. 2001;30:205–11. doi: 10.1093/ageing/30.3.205. [DOI] [PubMed] [Google Scholar]
- 63.Kwint HF, Luchtman T, Krijger-Dijkema JM, Eekhof J, De Kanter J. Physician and pharmacist monitor together. Monitoring of repeat prescriptions for drug-related problems. Pharm Weekbl. 2003;138:1732–7. [Google Scholar]
- 64.Kwint HF, Faber A, Gussekloo J, Bouvy ML. Effects of medication review on drug-related problems in patients using automated drug-dispensing systems: a pragmatic randomized controlled study. Drugs Aging. 2011;28:305–14. doi: 10.2165/11586850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 65.Lobas NH, Lepinski PW, Abramowitz PW. Effects of pharmaceutical care on medication cost and quality of patient care in an ambulatory-care clinic. Am J Hosp Pharm. 1992;49:1681–8. [PubMed] [Google Scholar]
- 66.Lowe CJ, Raynor DK, Purvis J, Farrin A, Hudson J. Effects of a medicine review and education programme for older people in general practice. Br J Clin Pharmacol. 2000;50:172–5. doi: 10.1046/j.1365-2125.2000.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mangiapane S, Schulz M, Muhlig S, Ihle P, Schubert I, Waldmann HC. Community pharmacy-based pharmaceutical care for asthma patients. Ann Pharmacother. 2005;39:1817–22. doi: 10.1345/aph.1G180. [DOI] [PubMed] [Google Scholar]
- 68.McDermott ME, Smith BH, Elliott AM, Bond CM, Hannaford PC, Chambers WA. The use of medication for chronic pain in primary care, and the potential for intervention by a practice-based pharmacist. Fam Pract. 2006;23:46–52. doi: 10.1093/fampra/cmi068. [DOI] [PubMed] [Google Scholar]
- 69.Needham DS, Wong IC, Campion PD Hull and East Riding Pharmacy Developmnet Group. Evaluation of the effectiveness of UK community pharmacists' interventions in community palliative care. Palliat Med. 2002;16:219–25. doi: 10.1191/0269216302pm533oa. [DOI] [PubMed] [Google Scholar]
- 70.Nishtala PS, Hilmer SN, McLachlan AJ, Hannan PJ, Chen TF. Impact of residential medication management reviews on drug burden index in aged-care homes: a retrospective analysis. Drugs Aging. 2009;26:677–86. doi: 10.2165/11316440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 71.Nishtala PS, McLachlan AJ, Bell JS, Chen TF. A retrospective study of drug-related problems in Australian aged care homes: medication reviews involving pharmacists and general practitioners. J Eval Clin Pract. 2011;17:97–103. doi: 10.1111/j.1365-2753.2010.01374.x. [DOI] [PubMed] [Google Scholar]
- 72.Niquille A, Bugnon O. Relationship between drug-related problems and health outcomes: a cross-sectional study among cardiovascular patients. Pharm World Sci. 2010;32:512–9. doi: 10.1007/s11096-010-9401-1. [DOI] [PubMed] [Google Scholar]
- 73.Patterson SM, Hughes CM, Crealey G, Cardwell C, Lapane KL. An evaluation of an adapted U.S. model of pharmaceutical care to improve psychoactive prescribing for nursing home residents in northern ireland (fleetwood northern ireland study) J Am Geriatr Soc. 2010;58:44–53. doi: 10.1111/j.1532-5415.2009.02617.x. [DOI] [PubMed] [Google Scholar]
- 74.Patterson SM, Hughes CM, Cardwell C, Lapane KL, Murray AM, Crealey GE. A cluster randomized controlled trial of an adapted U.S. model of pharmaceutical care for nursing home residents in Northern Ireland (Fleetwood Northern Ireland study): a cost-effectiveness analysis. J Am Geriatr Soc. 2011;59:586–93. doi: 10.1111/j.1532-5415.2011.03354.x. [DOI] [PubMed] [Google Scholar]
- 75.Phelan M, Blenkinsopp A, Foster NE, Thomas E, Hay EM. Pharmacist-led medication review for knee pain in older adults: Content, process and outcomes. Int J Pharm Pract. 2008;16:347–55. [Google Scholar]
- 76.Raynor DK, Nicolson M, Nunney J, Petty D, Vail A, Davies L. The development and evaluation of an extended adherence support programme by community pharmacists for elderly patients at home. Int J Pharm Pract. 2000;8:157–64. [Google Scholar]
- 77.RESPECT trial team. Effectiveness of shared pharmaceutical care for older patients: RESPECT trial findings. Br J Gen Pract. 2010;60:e10–9. doi: 10.3399/bjgp09X473295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryan-Woolley BM, Cantrill JA. Professional perspective on a feasibility study of GP-pharmacist collaboration in the management of angina. Int J Pharm Pract. 2000;8:275–84. [Google Scholar]
- 79.Schmidt I, Claesson CB, Westerholm B, Nilsson LG, Svarstad BL. The impact of regular multidisciplinary team interventions on psychotropic prescribing in Swedish nursing homes. J Am Geriatr Soc. 1998;46:77–82. doi: 10.1111/j.1532-5415.1998.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 80.Schneider J, Barber N. Provision of a domiciliary service by community pharmacists. Int J Pharm Pract. 1996;4:19–24. [Google Scholar]
- 81.Schulz M, Verheyen F, Muhlig S, Muller JM, Muhlbauer K, Knop-Schneickert E, et al. Pharmaceutical care services for asthma patients: a controlled intervention study. J Clin Pharmacol. 2001;41:668–76. doi: 10.1177/00912700122010438. [DOI] [PubMed] [Google Scholar]
- 82.Sellors J, Sellors C, Woodward C, Dolovich L, Poston J, Trim K, et al. Expanded role pharmacists: Consulting in family physicians' offices – a highly acceptable program model. Can Pharm J. 2001;134:27–31. [Google Scholar]
- 83.Sellors J, Kaczorowski J, Sellors C, Dolovich L, Woodward C, Willan A, et al. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. CMAJ. 2003;169:17–22. [PMC free article] [PubMed] [Google Scholar]
- 84.Shimp LA, Glazer HM, Reinke CM, Ascione FJ, Peggs JF, Reinhardt RW. A systematic approach to identifying and reducing medication-related problems in family practice patients. Fam Pract Res J. 1986;5:247–54. [PubMed] [Google Scholar]
- 85.Soendergaard B, Kirkeby B, Dinsen C, Herborg H, Kjellberg J, Staehr P. Drug-related problems in general practice: results from a development project in Denmark. Pharm World Sci. 2006;28:61–4. doi: 10.1007/s11096-006-9008-8. [DOI] [PubMed] [Google Scholar]
- 86.Sorensen L, Stokes JA, Purdie DM, Woodward M, Elliott R, Roberts MS. Medication reviews in the community: results of a randomized, controlled effectiveness trial. Br J Clin Pharmacol. 2004;58:648–64. doi: 10.1111/j.1365-2125.2004.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stafford L, Stafford A, Hughes J, Angley M, Bereznicki L, Peterson G. Drug-related problems identified in post-discharge medication reviews for patients taking warfarin. Int J Clin Pharm. 2011;33:621–6. doi: 10.1007/s11096-011-9515-0. [DOI] [PubMed] [Google Scholar]
- 88.Stafford L, Peterson GM, Bereznicki LR, Jackson SL, van Tienen EC, Angley MT, et al. Clinical outcomes of a collaborative, home-based postdischarge warfarin management service. Ann Pharmacother. 2011;45:325–34. doi: 10.1345/aph.1P617. [DOI] [PubMed] [Google Scholar]
- 89.Stergachis A, Fors M, Wagner EH, Sims DD, Penna P. Effect of clinical pharmacists on drug prescribing in a primary-care clinic. Am J Hosp Pharm. 1987;44:525–9. [PubMed] [Google Scholar]
- 90.Stuijt CCM, Franssen EJF, Egberts ACG, Hudson SA. Appropriateness of prescribing among elderly patients in a Dutch residential home: observational study of outcomes after a pharmacist-led medication review. Drugs Aging. 2008;25:947–54. doi: 10.2165/0002512-200825110-00005. [DOI] [PubMed] [Google Scholar]
- 91.Towle I, Adams J. A novel, pharmacist-led strategy to reduce the prescribing of benzodiazepines in Paisley. Pharm J. 2006;276:136–8. [Google Scholar]
- 92.Villeneuve J, Genest J, Blais L, Vanier MC, Lamarre D, Fredette M, et al. A cluster randomized controlled Trial to Evaluate an Ambulatory primary care Management program for patients with dyslipidemia: the TEAM study. CMAJ. 2010;182:447–55. doi: 10.1503/cmaj.090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vinks THAM, Egberts TCG, de Lange TM, de Koning FHP. Pharmacist-based medication review reduces potential drug-related problems in the elderly: the SMOG controlled trial. Drugs Aging. 2009;26:123–33. doi: 10.2165/0002512-200926020-00004. [DOI] [PubMed] [Google Scholar]
- 94.Wermeille J, Bennie M, Brown I, McKnight J. Pharmaceutical care model for patients with type 2 diabetes: integration of the community pharmacist into the diabetes team – a pilot study. Pharm World Sci. 2004;26:18–25. doi: 10.1023/b:phar.0000013465.24857.a8. [DOI] [PubMed] [Google Scholar]
- 95.Wilcock M, Harding G. What do pharmacists think of MURs and do they change prescribed medication? Pharm J. 2008;281:163–7. [Google Scholar]
- 96.Wong C, Cave A, Jarman R. Pharmaceutical care in a family medicine centre. Can Pharm J. 1997;130:35–43. [Google Scholar]
- 97.Yu K, Nguyen A, Shakib S, Doecke CJ, Boyce M, March G, et al. Enhancing continuity of care in therapeutics: Development of a post-discharge home medicines review model. J Pharm Pract Res. 2007;37:22–6. [Google Scholar]
- 98.Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ. 2001;323:1340–3. doi: 10.1136/bmj.323.7325.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes – randomised controlled trial. Age Ageing. 2006;35:586–91. doi: 10.1093/ageing/afl075. [DOI] [PubMed] [Google Scholar]


