Abstract
When pain is refractory to systemic opioid and non-opioid analgesic therapy and palliative chemoradiation or ablative or stimulant neurosurgical procedures are not possible, palliative treatment becomes limited, particularly if the patient wishes to be at home at the end of life. Intracerebroventricular (ICV) infusion of morphine in the home setting might be presented as an option. The present article reviews the basic and clinical evidence of the efficacy and safety of ICV administration of opioids. Information was gathered from various bibliographic sources, including PubMed and others, and summarized and evaluated to assess the efficacy and safety of ICV opioids for pain relief. Results from ICV infusion of morphine into terminally ill patients refractory to other pain treatments have been reported since the early 1980s. Good efficacy has been achieved for the vast majority of patients, without serious development of analgesic tolerance. There have also been a low incidence of adverse effects, such as constipation and respiratory depression, and a significant retention of alertness associated with this route of administration. Intracerebroventricular infusion of opioid analgesics thus appears to be a safe and effective therapy for the palliative treatment of refractory pain.
Keywords: analgesia, intracerebroventricular, Ommaya, opioids, palliative and hospice care, refractory pain
Introduction
As recently stated in a comprehensive review of treatment of cancer pain, ‘Whether or not primary disease-modifying therapy is possible, a large proportion of patients with pain . . . need symptomatic treatments’[1] and, to an increasing extent, patients who have a terminal illness want to spend their remaining time at home. Therefore, a dominant goal of modern care should involve palliation of pain at the end of life in the home setting.
At the point of patient acceptance of an incurable illness, pain can be refractory to standard treatment options. In a recent case report [2], the oral opioid regimen exceeded 5000 mg oral morphine equivalent per day. The pain remained poorly controlled even after transition to intravenous hydromorphone (>275 mg day−1), methadone by patient-controlled analgesia (continuous 5 mg h−1; button control at 5 mg (15 min) −1) and intravenous ketamine (10–25 mg h−1). The pain became the chief obstacle to the patient's desire to be at home at the time of death. Owing to the specifics of her condition, no palliative chemoradiation or ablative or stimulant neurosurgical options were available, and administration of analgesics spinally was not possible. Good results were ultimately achieved when a device capable of delivering medication into the right lateral cerebral ventricle was implanted. The authors report that the implantation was successful: ‘The patient died comfortably in her own home 46 days after the reservoir placement, at a final morphine ICV [intracerebroventricular] infusion rate of 0.5 mg h−1’[2].
With the concept of palliative pain management becoming more acceptable and widespread in general, the question arises: how invasive, effective and safe is ICV administration of opioid analgesics?
Infusion process
In 1963, Pakistani-born neurosurgeon Ayub Ommaya reported the design of a new device that could circumvent the difficulties associated with repeated introduction of or removal of fluids from the cerebroventricular space [3]. Such a device, with essentially the same design and bearing his name, remains in use for this and similar (e.g. spinal) applications. The device essentially consists of a compressible capsule under the scalp attached to a flexible catheter that terminates in a lateral cerebral ventricle. The capsule, located under the scalp, serves as a reservoir for the drug, which can be refilled by syringe multiple times. Application of mechanical pressure acts as a simple pump and forces drug out of the capsule and into the indwelling catheter, and thereby into the lateral cerebral ventricle (Figure 1). If necessary or no longer needed, the device can be replaced or removed. Ommaya used the device to infuse amphotericin B to treat patients who had cryptococcal meningitis [3] and methotrexate in order to treat inoperable brain tumours [4]. The technique was soon adopted by others (e.g. [5]).
Figure 1.
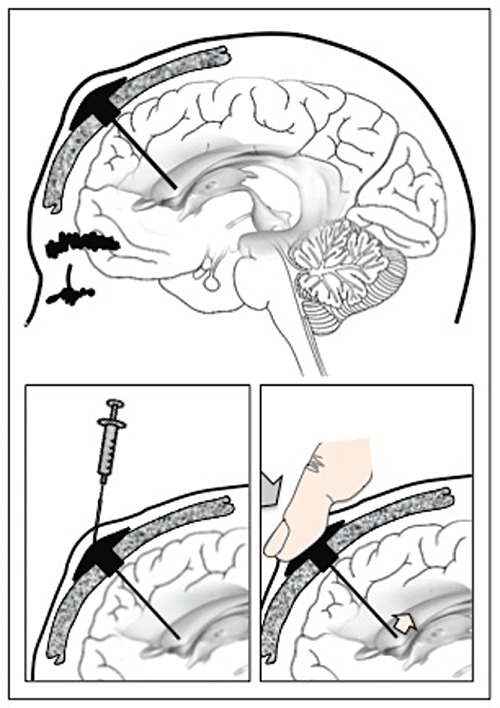
Artistic rendering of an intracerebroventricular infusion of opioids for the relief of refractory pain. Based on the original publication by Ommaya [3] with modification
Clinical results
The earliest studies of ICV opioid use for pain relief in humans, to the best of our knowledge, were published in 1978 by Hosobuchi and Li [6], Catlin et al. [7] and Foley et al. [8]. In all three studies, human β-endorphin was infused. Representative of the three, Hosobuchi and Li [6] reported the naloxone-reversible relief of intractable pain in three patients. In 1979, Foley et al. [8] published a more extensive study of a 43-year-old patient who had cancer pain intractable to oral oxycodone 60 mg daily for 4 weeks, to whom they infused 0.1–7.5 mg β-endorphin via subcutaneous reservoir into the right lateral cerebral ventricle at 1–2 week intervals over a period of 10 weeks. A dose of 7.5 mg, but not lower doses, produced a decrease in pain as measured by visual analogue scale, one of the few studies to do so, and in pupil size, plus an increase in mood. Onset of analgesia was 15 min after infusion and peaked at 3 h. The patient did not request analgesic medication until 24 h after the infusion. The authors also report elevated plasma prolactin levels and lower plasma growth hormone levels starting at doses lower than the analgesic dose.
Possibly the earliest studies in which morphine was infused were published in 1982 by Leavens et al. [9], Roquefeuil et al. [10], [11] and San Emeterio et al. (which is cited as a personal communication without details in [12]). Leavens et al. [9] infused ICV morphine into the frontal region of the right ventricle in four patients with intractable cancer pain. Prior use of oral or parenteral opioids gave unsatisfactory relief of pain. If successful, ICV treatment would continue at home by a trained responsible family member as a form of patient-controlled analgesia by proxy. The infusion of morphine caused transient nausea and vomiting and facial tingling, but pain relief was an excellent 80–100%. The minimal effective dose was 2.5–4.0 mg. None of the patients developed infections. Roquefeuil et al. [10], [11], [13], [14] reported on an increasing series of patients into whom morphine was infused initially into the third ventricle [10] and subsequently into the lateral ventricle. The inclusion criteria included intractable pain of neoplastic origin refractory to oral or subcutaneous morphine and other analgesic drugs, diffuse pain related to multiple metastases, a short life expectancy according to the grade of neoplastic lesion, efficacy of central administration of morphine by peridural route, and necessity of stopping peridural administration because of local problems or mechanical disturbance of the device. In all, good analgesia was obtained, with absence of respiratory depression and low doses (even when the patients were allowed to self-administer the infusion).
Lobato et al. [12] reported the results of ICV infusion of morphine in a series of 17 terminal patients who had intractable pain caused by orofacial, neck and disseminated cancer. The catheter tip was placed in the frontal horn of the right lateral ventricle, and the infusion was performed on an outpatient basis by trained persons. The course of therapy was 0.2–4 months. Pain relief was reported as ‘good’ or ‘excellent’ in all of the patients. The initial dose was 0.75 mg (8 h)−1, 0.25–1 mg (12 h)−1, 0.25–1 mg (24 h)−1, 0.25 mg (48 h)−1 or 0.75 mg (72 h)−1. The final dose was unchanged in nine of the patients and was greater than the initial dose in six of the patients. Side-effects occurred in nine patients, and were generally mild and short lasting. Respiratory depression occurred in only one patient and was said to be easily reversed by naloxone. Interestingly, naloxone did not reverse the analgesia. Also interestingly, constipation was not a side-effect, at least not reported. Experience with a larger number of patients (197) was published 4 years later [15]. The authors reported that their patients had favourable analgesic efficacy without noticeable neurological changes or side-effects severe enough to discontinue therapy. They also noted that tolerance to the ICV analgesic effect was much less marked than with parenteral administration.
Despite some questioning early on of the rigour of ICV analgesic studies and possible confounding factors [16], subsequent studies seem to support the early promising results. A Cochrane review by Ballantyne and Carwood published in 2005 [17] assessed 13 trials [9], [12], [18]–[28] of ICV opioids. The majority of the reviewed studies, like the majority subsequently, did not quantify pain relief using a formal measure (e.g. a pain scale). Qualitative terms, such as ‘unsatisfactory’, ‘good’ or ‘excellent’, were used. With that caveat, the review found that 215 patients (73%) were reported to have ‘excellent’ analgesia. Of the remainder, 56 patients (19%) were reported to have ‘good’ analgesia, leaving only 20 patients (7%) who were reported to have ‘unsatisfactory’ analgesia. The side-effects of persistent nausea, urinary retention and transient pruritis occurred less frequently with ICV catheters compared with spinal catheters (epidural or subarachnoid). Side-effects that occurred more frequently with ICV than spinal administration were respiratory depression (4.3%), sedation (11%) and confusion (13%). Constipation occurred remarkably less frequently by the ICV route than either spinal route (4% vs.‘the majority of patients’). The incidence of major infection was 4.4% for ICV infusion compared with 2.4–8.6% for spinal infusion. Based on the totality of the data from the multiple uncontrolled trials evaluated, the Cochrane review concluded that ‘The available data indicated ICV to be at least as effective against pain as other neuroaxial treatments and ICV may be a successful treatment of patients whose cancer pain is resistant to other treatments’. It also concludes ‘However, more rigorous reporting of efficacy and complications is needed before it will be clear whether or not ICV should be pursued as a first-line neuroaxial treatment’.
The results of other studies are in agreement with those that are covered in the Cochrane review [29]–[36]. Serrié[37], in addition to measuring the analgesic effect, measured the levels of the morphine metabolite morphine-6-glucuronide (M6G) and suggests that the metabolite was responsible for 33–67% of the analgesic effect. Lazorthes et al. [38] admitted to having an initial critical attitude to this technique because of the proximity of the brainstem and diencephalon and potential risks of central side-effects such as vomiting, sedation or respiratory depression. However, their experience over 10 years (82 patients) was very positive. Good pain relief was achieved in all but two patients. Side-effects were absent or only mild and transient. Likewise, Seiwald et al. [39] also reported that ICV morphine resulted in good to excellent analgesia in 95% of patients with somatogenic pain. However, they report that ICV morphine is not at all or is only minimally effective against neurogenic pain.
The use of an implantable ICV pump has also been reported to be effective against intractable cancer pain [40]. The authors suggest that the high cost of a pump is justifiable in patients with a life expectancy greater than 3 months.
Site(s) of action
In order to determine whether the analgesic effect elicited by ICV morphine is produced at structures proximal to the infusion site or at more distal sites (e.g. the spinal cord), Tafani et al. [41] infused 123I-labelled ICV morphine to eight patients suffering from intractable chronic cancer pain. Scans of the radiotracer obtained by γ-scintigraphy showed only slight diffusion beyond the ventricular system up to 1 h after the infusion. The great majority (90%) of the radiotracer remained localized within the brain (Figure 2). No tracer was detectable in the spinal cord up to 30 min postinfusion and only 5% was detected at 1 h, and it did not extend beyond the thoracic region. Diffusion was greatest when the catheter was placed in the occipital horn. In this case, radiotracer accumulated in the lateral ventricle, where the parenchymal exchange surface is largest. Diffusion was lowest when the catheter was placed close to the foramen of Monro or into the third ventricle.
Figure 2.
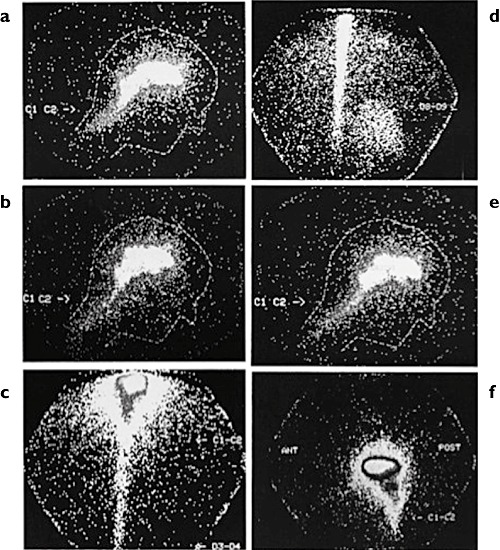
γ-Scintigraphy images of human brain and spinal cord after intracerebroventricular infusion of [123I]iodomorphine into the right lateral cerebral ventricle, as follows: (a) side view 15 min postinjection; (b) side view 30 min postinjection; (c) rear view 60 min postinjection; (d) rear view 60 min postinjection; (e) side view 60 min postinjection; and (f) side view 60 min postinjection. Reprinted from [41] with permission
There thus appears to be only slight diffusion of ICV morphine away from the ventricular system and into surrounding tissue. The walls of the cerebral ventricles and surrounding tissue contain abundant opioid receptors (e.g. [42]–[45]). A direct effect in this region is consistent with a mean analgesic onset latency of about 20 min, a time at which there is no detectible drug in the spinal cord. It should be noted, however, that even small amounts of drug migration to the spinal cord might contribute to the analgesic effect because, at least in animal models, morphine has a synergistic site–site (spinal–supraspinal) analgesic effect [46].
Pharmacokinetics
Sandouk et al. [47] evaluated the pharmacokinetics and pain relief after ICV infusion by Ommaya device of 0.28–0.61 mg morphine into the frontal horn of the lateral ventricle at the level of the foramen of Munro of seven male patients (42–68 years old) with intractable cancer pain. Ventricular and lumbar (L3–L4) cerebrospinal fluid (CSF) was collected. Pain level was assessed by visual analogue scale. Ventricular morphine was approximately 20 000 ng ml−1 on infusion and declined to approximately 10 ng ml−1 at 24 h. Lumbar morphine reached approximately 200 ng ml−1 at about 4 h and declined to about 10 ng ml−1 at 24 h. Thus, the morphine diffused poorly from ventricle (volume of approximately 10 ml [48]) to spine (the ratio of mean area under the curve (AUC)ventriclevs. mean AUCspine was 52.6). Analgesia occurred within a few minutes. The authors suggest that the short latency to onset of analgesic effect corresponds to the high levels of morphine in periventricular tissues and that the duration is enhanced by the morphine at the spinal level.
Metabolites
In order to assess the possible contribution of metabolites to the analgesic effect of ICV morphine, Smith et al. [49] measured CSF and plasma levels of morphine and its two major metabolites, morphine-3-glucuronide (M3G) and M6G. Morphine-6-glucuronide is a potent analgesic [50]; M3G is not analgesic, is thought to produce the excitatory side-effects observed in patients receiving large doses of morphine [51], and attenuates the antinociceptive effects of ICV morphine or M6G when given ICV to rats [52]–[54]. The authors report successful induction of analgesia with ICV morphine in more than 200 patients with pain associated with head and neck cancer, mid-line pain, diffuse pain and neuropathic pain [29], [55]. In this cohort consisting of 23 patients infused with 0.15–0.5 mg ICV morphine, the plasma levels of morphine declined to undetectable levels by 7 days postinfusion; neither M3G nor M6G was detectable in the plasma of most patients throughout the period. By 24 h following infusion, trough CSF morphine concentration rose to approximately 20 µm (which is about 50-fold higher than baseline), a level that would activate all three of the major opioid receptor types (µ, δ and κ) [56]. Morphine-3-glucuronide was present in CSF, presumably formed by metabolism within the brain, and declined to about 0.03–0.3% of the level of morphine over 10 days. Morphine-6-glucuronide was undetectable in the CSF or at baseline throughout the 10 day period.
Sandouk et al. [57] reported the formation of M3G and M6G in ventricular CSF (maximum at 3 h) following ICV infusion of morphine into the frontal horn of the lateral ventricle (level of foramen of Monro) of four male cancer patients with intractable pain. Goudas et al. [58] infused ICV morphine by Ommaya device in three terminal cancer patients suffering from intractable pain. Morphine-6-glucuronide levels remained essentially at baseline in the ventricles and cisternae for 45–70 h, despite the large increases in morphine levels in the same regions during infusion.
Opioid side-effect profile
Analgesic tolerance
The occurrence of analgesic tolerance to ICV morphine is generally reported to be relatively limited in the literature surveyed. Some degree of tolerance to the analgesic effect of morphine occurred in most patients. Several authors comment that analgesic tolerance to ICV morphine was notably more marked in patients who had received large amounts of systemic opioids prior to initiation of ICV infusion (e.g. [12], [38]) and particularly those who had received higher oral doses of slow-release morphine ([38]). The assessment of tolerance development is particularly difficult to quantify because of the relatively short lifespan of this terminally ill patient population and the likely increase in pain as their disease progresses. Another possibility might be opioid-induced hyperalgesia [59].
Respiratory depression
Lobato et al. [12] reported that respiratory depression occurred in only one of their series of 17 patients and that it was easily reversed by naloxone (the details were not given). Nurchi [26] also reports one case of respiratory depression, which was reversed by naloxone. Langlade et al. [60] report a case of respiratory depression following accidental infusion of a dose of morphine 10-fold larger than intended. The patient was a 52-year-old woman who had breast cancer with bone metastases. Following successful pain relief with 0.3 mg ICV morphine daily via Ommaya device, she was discharged from hospital and treated at home, where she was accidentally infused with 3 mg of morphine. Respiratory rate decreased suddenly 12 h later (the ventricular CSF morphine concentration was 6827 ng ml−1 at this time). Naloxone (0.4 mg intravenous plus 0.2 mg intramuscular) reversed the respiratory depressant effect, and it was continued as a continuous infusion 1 h later for a period of 8 h (total dose = 12.8 mg) due to persistent somnolence, miosis and recurrence of hypoventilation. The patient recovered fully from the event and did not complain of pain during naloxone treatment.
Smith et al. [49] also note the lack of respiratory depression in their study of 23 patients. They hypothesize that the lack of respiratory depression might be due to the absence of M6G or the continued presence of M3G in the brain, because ICV M3G attenuates ICV morphine-induced respiratory depression in rats and dogs [61], [62].
Alertness
Given the level of pain experienced by these patients, the amount of mental clouding, sedation or somnolence reported in the studies surveyed is notably low. Smith et al. [49] summarize their representative experience succinctly: ‘A significant feature was the mental alertness of these patients’.
Constipation
In this population, opioid analgesics are considered the mainstay of treatment, but opioid analgesics almost always lead to bowel dysfunction and/or constipation. It is of considerable interest, therefore, that constipation was rarely reported as a side-effect in the ICV studies surveyed. One likely reason is the absence of a contribution of effect on opioid receptors in the gastrointestinal tract. Smith et al. [49] postulate that another reason might be due to the low levels of M6G, because M6G is a more potent inhibitor of gastrointestinal transit than is morphine in mice [63]. Another possibility is that administration of morphine directly to the brain circumvents a possible synergistic spinal/supraspinal constipating effect [64].
Temperature and endocrine effects
The infusion of ICV morphine (0.3–1.0 mg) to eight terminal cancer patients with intractable pain using Ommaya catheters inserted into the right lateral ventricle [65] produced hypothermia (secondary to cutaneous vasodilatation and increased sweating), hyperglycaemia, and an increase in prolactin and growth hormone levels. The hypothermia started about 20 min after infusion, reached a maximum of −0.4°C at 60–90 min, returned to the control level by 120 min, and was observed from the first to fourth week of therapy. The hyperglycaemic effect also started about 20 min after infusion, lasted about 150 min, and disappeared as the treatment continued. Serum prolaction and growth hormone levels rose almost immediately after ICV infusion, peaked at 30 min, and returned to baseline levels at 3 h (growth hormone) or 6 h (prolactin). The elevations were observed during the first to fourth weeks, but disappeared as treatment continued. Thus, the effects were significant (e.g. about sixfold increase for prolactin and more than 10-fold increase for growth hormone), but did not persist beyond 4 weeks of therapy. No intervention was needed.
Seizures
Opioids can decrease seizure thresholds. Intravenous, extradural and intrathecal administration of opioids in both animals and humans can induce epileptic seizures or myoclonic episodes. Kronenburg et al. [66] reported two cases of seizures associated with the use of ICV bolus administration of morphine (5 mg (20 min)−1) in a 71-year-old patient with no known personal or family history of seizure disorders who was being treated for intractable pain secondary to an invasive inoperable epidermoid carcinoma at the left temple. The patient was able to tolerate increased stepwise dosage via continuous infusion mode to treat increasing pain due to tumour progression. The authors recommended that the initiation of ICV morphine therapy and bolus application should be performed carefully and only when constant monitoring is provided for at least 12 h.
Glutathione levels
Consistent with results in rats [67], ICV morphine (0.3 mg) infused by an Ommaya device into the frontal horn of the right lateral ventricle produces an acute decrease in CSF levels of the major endogenous intracellular antioxidant, glutathione [58]. The study did not examine long-term ICV treatment. If the effect persists, and anticipated life expectancy is long enough, coadministration of free radical scavengers might be desirable to prevent possible central nervous system vulnerability to damage from oxidative stress.
Summary and perspective
The prevalence of Americans suffering from advanced illness is estimated at greater than 1.5 million [68], and many of these patients rely on opioid analgesics to relieve the associated pain [69]. If the illness progresses to the point that it is terminal, many of these patients wish to spend their remaining time at home. An impediment to the fulfilment of this desire is intractable pain refractory to systemic analgesics and other interventions. The infusion of an opioid into human cerebral ventricles for the relief of intractable pain was published more than 40 years ago, and the overwhelming consensus is that it is safe and effective. Thus, pain that is refractory to systemic analgesic therapy responds well to ICV administration of opioid medication (almost always morphine to date). The pain is relieved without interference with the other sensory modalities. Onset of relief generally occurs within 20–40 min and lasts 12–16 h (depending on the dose). A possible exception is neurogenic pain, but this needs to be confirmed. Almost all of the studies note the relative lack of sedation and respiratory depression in patients who are infused with ICV morphine. Very few even mention constipation. This is remarkable given that the patients are administered palliative doses for pain so severe that it is refractory to all other pharmacological, radiological, surgical or systemic analgesic therapy.
The commonly used infusion device is small, simple to install and use, and non-intrusive enough that patients can be transitioned to the home setting and for hospice care. In addition to the relief of pain, such an intervention contributes to a better quality of remaining life. It also interrupts the otherwise inevitable trajectory toward ever-increasing opioid side-effects (such as constipation) and the therapy needed to treat them. There seems to be no question about its value as a positive psychological as well as medical intervention.
To our knowledge, with the exception of the early studies with β-endorphin, morphine (as the sulfate or hydrochloride salt or as dimorphine) has been the only drug used for ICV infusion. Given the success with this drug, there does not appear to be a compelling reason to test others, but this possibility might be considered.
The reported observation by more than one group that naloxone can reverse the respiratory depression induced by excess ICV morphine, but not the morphine-induced analgesia, raises fundamental basic science questions and also the possible consideration of concomitant ICV dosing of morphine with low-dose naloxone [70]. However, much more investigation is needed.
The issue of the possible misuse, abuse or diversion of home supplies of ICV opioids was not brought up in any of the studies that we found. It is also not difficult to imagine that the sensitive and complex issues of right-to-die, euthanasia, assisted suicide, mercy killing, etc. could become involved.
In summary, the administration of ICV opioid for palliative care in the home setting is associated with opportunities and challenges. The objective benefits of ICV administration of opioid pain relievers appear to include good efficacy, relative ease of insertion and infusion, modest dose escalation, and relatively low incidence and severity of opioid side-effects, particularly constipation and mental clouding. Perhaps the more important subjective benefits are best summarized by the case in Cramond and Stuart [29] relayed by the friend of a patient (they had been prisoners of war together):
‘When I engineered his transfer to your care, he was a pitiful, narcotised, constipated struggler-to-survive . . . When he came back . . . , he was a quite different man to the one that left – relaxed and philosophical, adjusted to his inevitable fate (no more of the “Why me!” stuff); mobile to a limited extent, which he had not been at all before; virtually pain-free and had not a conscious worry at all; and no constipation. We don't realize the full extent of worry and concern this latter causes to those who have not normally suffered from it. Saw him the day before he died when he was still his old, lucid self . . .’.
Acknowledgments
The authors thank Robert Taylor Jr for valuable assistance with the preparation of this paper.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Portenoy RK. Treatment of cancer pain. Lancet. 2011;377:2236–47. doi: 10.1016/S0140-6736(11)60236-5. [DOI] [PubMed] [Google Scholar]
- 2.Adolph MD, Stretanski MF, McGregor JM, Rawn BL, Ross PM, Benedetti C. Intracerebroventricular morphine for refractory cancer pain: transitioning to the home setting. Am J Hosp Palliat Care. 2010;27:326–32. doi: 10.1177/1049909109355150. [DOI] [PubMed] [Google Scholar]
- 3.Ommaya AK. Subcutaneous reservoir and pump for sterile access to ventricular cerebrospinal fluid. Lancet. 1963;282:983–4. doi: 10.1016/s0140-6736(63)90681-0. [DOI] [PubMed] [Google Scholar]
- 4.Ommaya AK, Rubin RC, Henderson ES, Rall DP, Gieseke FG, Bering EA, Jr, Bagan M. A new approach to the treatment of inoperable brain tumors. Med Ann Dist Columbia. 1965;34:455–8. [PubMed] [Google Scholar]
- 5.Witorsch P, Williams TW, Jr, Ommaya AK, Utz JP. Intraventricular administration of amphotericin B. Use of subcutaneous reservoir in four patients with mycotic meningitis. JAMA. 1965;194:699–702. doi: 10.1001/jama.194.7.699. [DOI] [PubMed] [Google Scholar]
- 6.Hosobuchi Y, Li CH. The analgesic activity of human beta-endorphin in man (1,2,3) Commun Psychopharmacol. 1978;2:33–7. [PubMed] [Google Scholar]
- 7.Catlin DH, Hui KK, Loh HH, Li CH. Advances in Biochemical Psychopharmacology. New York: Raven Press; 1978. [PubMed] [Google Scholar]
- 8.Foley KM, Inturrisi CE, Kourides IA, Kaiko RF, Posner JB, Houde RW, Li CH. Characteristics and Function of Opioids. Amsterdam, The Netherlands: Elsevier/North-Holland; 1978. [Google Scholar]
- 9.Leavens ME, Hill CS, Jr, Cech DA, Weyland JB, Weston JS. Intrathecal and intraventricular morphine for pain in cancer patients: initial study. J Neurosurg. 1982;56:241–5. doi: 10.3171/jns.1982.56.2.0241. [DOI] [PubMed] [Google Scholar]
- 10.Roquefeuil B, Benezech J, Batier C, Marchal J. Analgesia by intraventricular injection of morphine. Agressologie. 1982;23:119–22. [PubMed] [Google Scholar]
- 11.Roquefeuil B, Blanchet P, Batier C, Benezech J. Intraventricular morphine analgesia. Apropos of 4 cases, 1 with self-administration. Ann Fr Anesth Reanim. 1982;1:649–54. doi: 10.1016/s0750-7658(82)80109-3. [DOI] [PubMed] [Google Scholar]
- 12.Lobato RD, Madrid JL, Fatela LV, Rivas JJ, Reig E, Lamas E. Intraventricular morphine for control of pain in terminal cancer patients. J Neurosurg. 1983;59:627–33. doi: 10.3171/jns.1983.59.4.0627. [DOI] [PubMed] [Google Scholar]
- 13.Roquefeuil B, Benezech J, Batier C, Blanchet P, Gros C, Mathieu-Daude JC. Value of intraventricular morphine analgesia in intractable neoplasm pain. Apropos of 8 cases with self-administration in 4. Neurochirurgie. 1983;29:135–41. [PubMed] [Google Scholar]
- 14.Roquefeuil B, Benezech J, Blanchet P, Batier C, Frerebeau P, Gros C. Intraventricular administration of morphine in patients with neoplastic intractable pain. Surg Neurol. 1984;21:155–8. doi: 10.1016/0090-3019(84)90334-3. [DOI] [PubMed] [Google Scholar]
- 15.Lobato RD, Madrid JL, Fatela LV, Sarabia R, Rivas JJ, Gozalo A. Intraventricular morphine for intractable cancer pain: rationale, methods, clinical results. Acta Anaesthesiol Scand Suppl. 1987;85:68–74. doi: 10.1111/j.1399-6576.1987.tb02672.x. [DOI] [PubMed] [Google Scholar]
- 16.Pawl RP. Intraventricular morphine. Neurosurgery. 1986;18:250–1. doi: 10.1097/00006123-198602000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Ballantyne JC, Carwood CM. Comparative efficacy of epidural, subarachnoid, and intracerebroventricular opioids in patients with pain due to cancer. Cochrane Database Syst Rev. 2005;(1) doi: 10.1002/14651858.CD005178. CD005178. [DOI] [PubMed] [Google Scholar]
- 18.Blond S, Dubar M, Meynadier J, Combelles-Pruvot M, Vitrac P. Cerebral Intraventricular Administration of Morphine in Cancer Patients with Intractable Pain. Utrecht, The Netherlands: VNU Science Press; 1985. [Google Scholar]
- 19.Blond S, Meynadier J, Brichard C, Guieu JD, Willer JC, Le Bars D. Intra-cerebro-ventricular mophinotherapy (ICVM) (n = 79) and study of the supraspinal action of morphine (M) Pain. 1987;30(Suppl. 1):S391. [Google Scholar]
- 20.Dennis GC, DeWitty RL. Long-term intraventricular infusion of morphine for intractable pain in cancer of the head and neck. Neurosurgery. 1990;26:404–7. discussion 407–8. [PubMed] [Google Scholar]
- 21.Esposito S, Delitala A. Transoval administration of opiates into trigeminal cistern for cancer pain. Preliminary report. Neurochirurgia (Stuttg) 1991;34:116–8. doi: 10.1055/s-2008-1052068. [DOI] [PubMed] [Google Scholar]
- 22.Houdek M, Opavsky J, Ostrizek J. Intracerebroventricular application of morphine in the treatment of intractable malignant pain. Acta Univ Palacki Olomuc Fac Med. 1990;128:101–6. [PubMed] [Google Scholar]
- 23.Karavelis A, Foroglou G, Selviaridis P, Fountzilas G. Intraventricular administration of morphine for control of intractable cancer pain in 90 patients. Neurosurgery. 1996;39:57–61. doi: 10.1097/00006123-199607000-00012. discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 24.Lenzi A, Galli G, Gandolfini M, Marini G. Intraventricular morphine in paraneoplastic painful syndrome of the cervicofacial region: experience in thirty-eight cases. Neurosurgery. 1985;17:6–11. doi: 10.1227/00006123-198507000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Lobato RD, Madrid JL, Fatela LV, Gozalo A, Rivas JJ, Sarabia R. Analgesia elicited by low dose intraventricular morphine in terminal cancer patients. Adv Pain Res Ther. 1985;9:673–81. [Google Scholar]
- 26.Nurchi G. Use of intraventricular and intrathecal morphine in intractable pain associated with cancer. Neurosurgery. 1984;15:801–3. [PubMed] [Google Scholar]
- 27.Obbens EA, Hill CS, Leavens ME, Ruthenbeck SS, Otis F. Intraventricular morphine administration for control of chronic cancer pain. Pain. 1987;28:61–8. doi: 10.1016/0304-3959(87)91060-8. [DOI] [PubMed] [Google Scholar]
- 28.Reeve WG, Todd JG. Intraventricular diamorphine via an Ommaya shunt for intractable cancer pain. Br J Anaesth. 1990;65:544–7. doi: 10.1093/bja/65.4.544. [DOI] [PubMed] [Google Scholar]
- 29.Cramond T, Stuart G. Intraventricular morphine for intractable pain of advanced cancer. J Pain Symptom Manage. 1993;8:465–73. doi: 10.1016/0885-3924(93)90189-3. [DOI] [PubMed] [Google Scholar]
- 30.Smith KA, Frank E. Stereotaxic placement of a ventricular catheter and reservoir for the administration of morphine sulfate. Axone. 1991;13:12–5. [PubMed] [Google Scholar]
- 31.Opavsky J, Houdek M. Administration of morphine into the cerebral ventricles in chronic intractable pain. Cesk Neurol Neurochir. 1990;53:264–8. (English Abstract) [PubMed] [Google Scholar]
- 32.Lee TL, Kumar A, Baratham G. Intraventricular morphine for intractable craniofacial pain. Singapore Med J. 1990;31:273–6. [PubMed] [Google Scholar]
- 33.Carlisle DW, Smith KA, Frank E, Meyers FJ. Intraventricular morphine administered by hospice nurses to a patient with intractable pain. Am J Hosp Care. 1989;6:36–9. doi: 10.1177/104990918900600402. [DOI] [PubMed] [Google Scholar]
- 34.Caputi CA, Busca G, Fogliardi A, Rychlicki F, Basili P. Epidural and intraventricular morphine therapy in the treatment of cancer pain. Minerva Anestesiol. 1986;52:351–5. [PubMed] [Google Scholar]
- 35.Ballantyne JC, Carr DB, Berkey CS, Chalmers TC, Mosteller F. Comparative efficacy of epidural, subarachnoid, and intracerebroventricular opioids in patients with pain due to cancer. Reg Anesth. 1996;21:542–56. [PubMed] [Google Scholar]
- 36.Vojkovic SJ, Kristjanson LJ. Case study report of two palliative care patients receiving intracerebroventricular (ICV) analgesia. J Palliat Care. 2003;19:280–3. [PubMed] [Google Scholar]
- 37.Serrié A. Analgesic effect of morphine and its metabolites administered by an intracerebroventricular route. Bulletin. 1995;179:1237–52. discussion 1252–3. [PubMed] [Google Scholar]
- 38.Lazorthes YR, Sallerin BA, Verdie JC. Intracerebroventricular administration of morphine for control of irreducible cancer pain. Neurosurgery. 1995;37:422–8. doi: 10.1227/00006123-199509000-00009. discussion 428–9. [DOI] [PubMed] [Google Scholar]
- 39.Seiwald M, Alesch F, Kofler A. Intraventricular morphine administration as a treatment possibility for patients with intractable pain. Wien Klin Wochenschr. 1996;108:5–8. [PubMed] [Google Scholar]
- 40.Weigl K, Mundinger F, Chrubasik J. Continuous intraventricular morphine- or peptide-infusion for intractable cancer pain. Acta Neurochir Suppl (Wien) 1987;39:163–5. doi: 10.1007/978-3-7091-8909-2_43. [DOI] [PubMed] [Google Scholar]
- 41.Tafani JA, Lazorthes Y, Danet B, Verdie JC, Esquerre JP, Simon J, Guiraud R. Human brain and spinal cord scan after intracerebroventricular administration of iodine-123 morphine. Int J Rad Appl Instrum B. 1989;16:505–9. doi: 10.1016/0883-2897(89)90064-0. [DOI] [PubMed] [Google Scholar]
- 42.Akaike A, Shibata T, Satoh M, Takagi H. Analgesia induced by microinjection of morphine into, and electrical stimulation of, the nucleus reticularis paragigantocellularis of rat medulla oblongata. Neuropharmacology. 1978;17:775–8. doi: 10.1016/0028-3908(78)90093-x. [DOI] [PubMed] [Google Scholar]
- 43.Dickenson AH, Oliveras JL, Besson JM. Role of the nucleus raphe magnus in opiate analgesia as studied by the microinjection technique in the rat. Brain Res. 1979;170:95–111. doi: 10.1016/0006-8993(79)90943-0. [DOI] [PubMed] [Google Scholar]
- 44.Tsou K, Jang CS. Studies on the Site of Analgesic Action of Morphine by Intracerebral Micro-Injection. Sci Sin. 1964;13:1099–109. [PubMed] [Google Scholar]
- 45.Yaksh TL, Rudy TA. Narcotic analgestics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978;4:299–359. doi: 10.1016/0304-3959(77)90145-2. [DOI] [PubMed] [Google Scholar]
- 46.Yeung JC, Rudy TA. Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of antinociceptive action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. J Pharmacol Exp Ther. 1980;215:633–42. [PubMed] [Google Scholar]
- 47.Sandouk P, Serrie A, Urtizberea M, Debray M, Got P, Scherrmann JM. Morphine pharmacokinetics and pain assessment after intracerebroventricular administration in patients with terminal cancer. Clin Pharmacol Ther. 1991;49:442–8. doi: 10.1038/clpt.1991.52. [DOI] [PubMed] [Google Scholar]
- 48.Bull JWD. The Robert Wartenberg Memorial Lecture: the volume of the cerebral ventricles. Neurology. 1961;11:1–9. [Google Scholar]
- 49.Smith MT, Wright AW, Williams BE, Stuart G, Cramond T. Cerebrospinal fluid and plasma concentrations of morphine, morphine-3-glucuronide, and morphine-6-glucuronide in patients before and after initiation of intracerebroventricular morphine for cancer pain management. Anesth Analg. 1999;88:109–16. [PubMed] [Google Scholar]
- 50.Milne RW, Nation RL, Somogyi AA. The disposition of morphine and its 3- and 6-glucuronide metabolites in humans and animals, and the importance of the metabolites to the pharmacological effects of morphine. Drug Metab Rev. 1996;28:345–472. doi: 10.3109/03602539608994011. [DOI] [PubMed] [Google Scholar]
- 51.Sjogren P, Jonsson T, Jensen NH, Drenck NE, Jensen TS. Hyperalgesia and myoclonus in terminal cancer patients treated with continuous intravenous morphine. Pain. 1993;55:93–7. doi: 10.1016/0304-3959(93)90188-U. [DOI] [PubMed] [Google Scholar]
- 52.Smith MT, Watt JA, Cramond T. Morphine-3-glucuronide – a potent antagonist of morphine analgesia. Life Sci. 1990;47:579–85. doi: 10.1016/0024-3205(90)90619-3. [DOI] [PubMed] [Google Scholar]
- 53.Gong QL, Hedner J, Bjorkman R, Hedner T. Morphine-3-glucuronide may functionally antagonize morphine-6-glucuronide induced antinociception and ventilatory depression in the rat. Pain. 1992;48:249–55. doi: 10.1016/0304-3959(92)90065-J. [DOI] [PubMed] [Google Scholar]
- 54.Faura CC, Olaso MJ, Garcia Cabanes C, Horga JF. Lack of morphine-6-glucuronide antinociception after morphine treatment. Is morphine-3-glucuronide involved? Pain. 1996;65:25–30. doi: 10.1016/0304-3959(95)00198-0. [DOI] [PubMed] [Google Scholar]
- 55.Cramond T. Invasive Techniques for Neuropathic Pain. New York: Oxford University Press; 1998. [Google Scholar]
- 56.Sutters KA, Miaskowski C, Taiwo YO, Levine JD. Analgesic synergy and improved motor function produced by combinations of mu-delta- and mu-kappa-opioids. Brain Res. 1990;530:290–4. doi: 10.1016/0006-8993(90)91297-t. [DOI] [PubMed] [Google Scholar]
- 57.Sandouk P, Serrie A, Scherrmann JM, Langlade A, Bourre JM. Presence of morphine metabolites in human cerebrospinal fluid after intracerebroventricular administration of morphine. Eur J Drug Metab Pharmacokinet. 1991;3:166–71. [PubMed] [Google Scholar]
- 58.Goudas LC, Langlade A, Serrie A, Matson W, Milbury P, Thurel C, Sandouk P, Carr DB. Acute decreases in cerebrospinal fluid glutathione levels after intracerebroventricular morphine for cancer pain. Anesth Analg. 1999;89:1209–15. [PubMed] [Google Scholar]
- 59.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–61. [PubMed] [Google Scholar]
- 60.Langlade A, Serrie A, Sandouk P, Thurel C, Cunin G. Levels of morphine and metabolites in CSF during respiratory depression after intraventricular morphine injection. Pain. 1991;44:175–8. doi: 10.1016/0304-3959(91)90134-J. [DOI] [PubMed] [Google Scholar]
- 61.Gong QL, Hedner T, Hedner J, Bjorkman R, Nordberg G. Antinociceptive and ventilatory effects of the morphine metabolites: morphine-6-glucuronide and morphine-3-glucuronide. Eur J Pharmacol. 1991;193:47–56. doi: 10.1016/0014-2999(91)90199-z. [DOI] [PubMed] [Google Scholar]
- 62.Pelligrino DA, Riegler FX, Albrecht RF. Ventilatory effects of fourth cerebroventricular infusions of morphine-6- or morphine-3-glucuronide in the awake dog. Anesthesiology. 1989;71:936–40. doi: 10.1097/00000542-198912000-00018. [DOI] [PubMed] [Google Scholar]
- 63.Paul D, Standifer KM, Inturrisi CE, Pasternak GW. Pharmacological characterization of morphine-6 beta-glucuronide, a very potent morphine metabolite. J Pharmacol Exp Ther. 1989;251:477–83. [PubMed] [Google Scholar]
- 64.Tallarida RJ, Porreca F, Cowan A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989;45:947–61. doi: 10.1016/0024-3205(89)90148-3. [DOI] [PubMed] [Google Scholar]
- 65.Su CF, Liu MY, Lin MT. Intraventricular morphine produces pain relief, hypothermia, hyperglycaemia and increased prolactin and growth hormone levels in patients with cancer pain. J Neurol. 1987;235:105–8. doi: 10.1007/BF00718020. [DOI] [PubMed] [Google Scholar]
- 66.Kronenberg MF, Laimer I, Rifici C, Saltuari L, Bramanti P, Moriggl U, Norer B, Kofler A. Epileptic seizure associated with intracerebroventricular and intrathecal morphine bolus. Pain. 1998;75:383–7. doi: 10.1016/s0304-3959(97)00173-5. [DOI] [PubMed] [Google Scholar]
- 67.Goudas LC, Carr DB, Maszczynska I, Marchand JE, Wurm WH, Greenblatt DJ, Kream RM. Differential effect of central versus parenteral administration of morphine sulfate on regional concentrations of reduced glutathione in rat brain. Pharmacology. 1997;54:92–7. doi: 10.1159/000139474. [DOI] [PubMed] [Google Scholar]
- 68.National Hospice and Palliative Care Organization. NHPCO Facts and Figures: Hospice Care in America. 2010 edn. Alexandria, VA: National Hospital and Palliative Care Organization; 2010. [Google Scholar]
- 69.Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138:507–13. doi: 10.1016/j.pain.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 70.Gan TJ, Ginsberg B, Glass PS, Fortney J, Jhaveri R, Perno R. Opioid-sparing effects of a low-dose infusion of naloxone in patient-administered morphine sulfate. Anesthesiology. 1997;87:1075–81. doi: 10.1097/00000542-199711000-00011. [DOI] [PubMed] [Google Scholar]


