Abstract
AIM
To investigate trends in spontaneous reporting to the French Pharmacovigilance system of ‘serious’ (SADRs) and ‘non-serious’ (NSADRs) adverse drug reactions over time.
METHODS
Annual SADR : NSADR ratios were calculated for each drug and their evolution tested with linear trend tests.
RESULTS
Among the 39 new active substances commercialized in France in 2000, 16 had sufficient data to perform linear trend tests. An increasing linear relation was found for five widely prescribed drugs, a non-significant increasing trend for eight others, i.e. drugs mostly used in hospitals.
CONCLUSION
ADR reports mainly concern NSADRs during first years of marketing. Reports of SADRs are proportionally more frequent later.
Keywords: ‘serious’ adverse drug reactions, adverse drug reactions, pharmacovigilance, spontaneous reporting
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Several factors are known to influence spontaneous reporting of adverse drug reactions (ADRs). Among them, ‘seriousness’ of the reaction is one of the most important.
However, evolution in the reporting of ‘serious’vs.‘non-serious’ ADRs over time for the same drug remains unknown.
WHAT THIS STUDY ADDS
Spontaneous reports mainly involve ‘non-serious’ ADRs during the first years of marketing and ‘serious’ ADRs later, particularly for drugs with non-hospital use.
Introduction
Despite the development of several pharmacoepidemiological methods [1], [2], spontaneous reporting of adverse drug reactions (ADRs) remains the cornerstone of pharmacovigilance [3], [4]. However, few studies have investigated chronological trends in spontaneous reporting of ADRs over periods of time. Weber described a higher reporting rate in the early years of a product's life followed by a subsequent decline [5] and Haramburu et al. found that unlabelled (‘unexpected’) ADRs were mainly reported during the early years of marketing [6].
The ‘seriousness’ of ADRs contributes to reporting of ADRs [4], [7]. However, the respective trends in reporting of ‘serious’ (SADRs) vs.‘non-serious’ ADRs (NSADRs) remain poorly quantified. Thus, the aim of the present study was to investigate trends in spontaneous reporting of SADRs and NSADRs to the French National PharmacoVigilance Network during the 2000s.
Methods
We performed an observational descriptive study using the French PharmacoVigilance Database (FPVD). This database has been described previously [8], [9]. Briefly, it involves all spontaneous reports registered since 1984 in France. According to French law, every health practitioner must report ‘serious’ or ‘unexpected’ (unlabelled) adverse events to their regional pharmacovigilance centre of which there are 31 in France. ‘Serious’ adverse events are defined as any untoward medical occurrence that at any dose results in death, requires hospital admission or prolongation of existing hospital stay, results in persistent or significant disability/incapacity, is life threatening, results in cancers, congenital anomalies or birth defects, as well as any medical event that would be regarded as serious if they had not responded to acute treatment [10]. Reported adverse events are then assessed in the regional pharmacovigilance centre by a college of specialists, pharmacologists and clinicians. By consensus, the college eventually validates the case as an ADR, classifies it as ‘serious’ or ‘non-serious’ and calculates a causality assessment score. Causality assessment (imputation) is performed according to the French method used by all the Regional Centres of PharmacoVigilance [8], [11]. All suspected ADRs are registered in the FPVD. For each report, information about the patient (age, gender, medical history), drug exposure (suspected and other associated non-suspected drugs), and ADR characteristics (‘serious or ‘non-serious’, ‘expected’ or ‘unexpected’, causality score) is recorded in the FPVD. A detailed summary of clinical description is added at the end of each pharmacovigilance case report [8]. ADRs are coded according to the MedDRA terminology [12].
Among all medicinal products with a new drug approval in France in 2000, we selected the new active substances. For each drug, we counted SADRs and NSADRs recorded from 2000 to 2010 in the FPVD. After excluding years with less than five ADRs reported, the annual SADR : NSADR ratios were calculated for each drug. Lastly, the annual evolution of SADR : NSADR ratios was tested for linear trend for each drug when at least five ADRs per year were reported for at least 3 years. We conducted linear regression and used Student's t-test (threshold for α at 5%). Statistical analyses were performed using SAS9.2TM software (SAS Institute Inc., Cary, North Carolina, USA).
Results
Among 409 new drug approvals, 39 new active substances were identified. Linear trend tests were performed for the 16 which had sufficient ADR reports for analyses (Table 1). A significant increasing linear relation of annual SADR : NSADR ratios was found for celecoxib, esomeprazole, peginterferon alpha 2a, pioglitazone and risedronate. The three most significant increases are illustrated in Figure 1. A non-significant trend for increasing linear relation was found for etanercept, interferon alpha, interferon beta, oxcarbazepine, rosiglitazone, tibolone, trastuzumab and verteporfine. A non-significant decreasing relation was observed for galantamine, levetiracetam and a significant one for oxycodone.
Table 1.
‘Serious’ : ‘non-serious’ adverse drug reaction (ADR) ratios and number of reported ADRs over time for the 16 drugs for which linear trend tests were possible
| ‘Serious’ : ‘Non-serious’ adverse drug reaction ratios (total number of reports) per year | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | P |
| Celecoxib | 0.22 (n = 28) | 0.70 (n = 503) | 0.99 (n = 189) | 1.61 (n = 128) | 1.35 (n = 134) | 2.42 (n = 82) | 2.40 (n = 34) | 1.16 (n = 41) | 1.31 (n = 37) | 1.92 (n = 38) | 2.40 (n = 31) | 0.0165 |
| Esomeprazole | – | – | 0.59 (n = 27) | 0.52 (n = 41) | 0.96 (n = 100) | 1.42 (n = 138) | 1.17 (n = 180) | 1.03 (n = 176) | 1.21 (n = 208) | 1.42 (n = 278) | 1.90 (n = 278) | 0.0033 |
| Etanercept | 2.33 (n = 20) | 1.14 (n = 30) | 2.12 (n = 25) | 1.37 (n = 57) | 1.94 (n = 100) | 1.77 (n = 119) | 3.12 (n = 103) | 1.36 (n = 118) | 1.82 (n = 141) | 2.05 (n = 107) | 2.87 (n = 93) | 0.3312 |
| Galantamine | – | 4.00 (n = 5) | 1.91 (n = 32) | 1.12 (n = 34) | 1.08 (n = 25) | 0.50 (n = 21) | 2.43 (n = 24) | 1.77 (n = 36) | 2.44 (n = 31) | 0.88 (n = 32) | 0.55 (n = 31) | 0.7351 |
| Interferon α | 0.77 (n = 53) | 0.78 (n = 32) | 2.00 (n = 18) | 0.85 (n = 37) | 0.86 (n = 26) | 1.25 (n = 27) | 1.40 (n = 12) | 0.67 (n = 25) | 0.89 (n = 17) | 2.33 (n = 10) | 3.00 (n = 12) | 0.0760 |
| Interferon β | 1.00 (n = 14) | 0.92 (n = 23) | 0.60 (n = 8) | 0.29 (n = 9) | 1.30 (n = 7) | 2.00 (n = 9) | 0.30 (n = 13) | 3.00 (n = 8) | 1.25 (n = 9) | 3.00 (n = 4) | 2.00 (n = 6) | 0.1271 |
| Levetiracetam | – | 2 (n = 6) | 0.67 (n = 10) | 1.00 (n = 12) | 1.33 (n = 21) | 0.94 (n = 35) | 0.69 (n = 27) | 1.03 (n = 73) | 0.95 (n = 127) | 1.07 (n = 116) | 1.26 (n = 86) | 0.4857 |
| Oxcarbazepine | – | 0.71 (n = 48) | 0.82 (n = 62) | 0.71 (n = 77) | 0.90 (n = 80) | 1.00 (n = 58) | 1.22 (n = 60) | 1.37 (n = 45) | 0.83 (n = 42) | 1.05 (n = 43) | 1.13 (n = 32) | 0.0515 |
| Oxycodone | – | – | – | – (n = 1) | – (n = 2) | 4.00 (n = 5) | 4.00 (n = 15) | 4.67 (n = 17) | 3.75 (n = 19) | 2.25 (n = 26) | 1.29 (n = 39) | 0.0394 |
| Peginterferon-α2a | – | 0.75 (n = 7) | 0.77 (n = 85) | 0.79 (n = 77) | 0.94 (n = 68) | 1.05 (n = 39) | 1.42 (n = 29) | 1.54 (n = 28) | 2.14 (n = 22) | 2.50 (n = 28) | 4.00 (n = 10) | 0.0006 |
| Pioglitazone | – | – | – (n = 3) | 0.22 (n = 11) | 0.30 (n = 13) | 0.50 (n = 15) | 0.73 (n = 19) | 1.07 (n = 29) | 1.00 (n = 24) | 3.00 (n = 24) | 2.50 (n = 7) | 0.0036 |
| Risedronate | – | 0.25 (n = 15) | 0.56 (n = 36) | 0.64 (n = 36) | 0.38 (n = 29) | 1.50 (n = 35) | 0.80 (n = 45) | 1.00 (n = 32) | 0.68 (n = 32) | 1.29 (n = 32) | 3.00 (n = 24) | 0.0197 |
| Rosiglitazone | – | – | – (n = 1) | 0.62 (n = 13) | 0.75 (n = 14) | 1.50 (n = 20) | 1.00 (n = 12) | 1.10 (n = 19) | 1.80 (n = 14) | 5.00 (n = 6) | 2.00 (n = 3) | 0.0503 |
| Tibolone | – | 0 (n = 9) | 0.10 (n = 11) | 0.12 (n = 9) | 0.33 (n = 12) | – (n = 3) | – (n = 2) | 0.25 (n = 5) | – (n = 2) | – (n = 2) | – (n = 0) | 0.1367 |
| Trastuzumab | – (n = 2) | 4 (n = 5) | 3 (n = 12) | 0.62 (n = 13) | 1.25 (n = 18) | 2.14 (n = 22) | 0.86 (n = 52) | 0.88 (n = 68) | 2.69 (n = 59) | 2.28 (n = 59) | 2.12 (n = 53) | 0.6811 |
| Verteporfin | – | 0.87 (n = 15) | 0.17 (n = 40) | 0.24 (n = 31) | – (n = 3) | 0.5 (n = 6) | 1.33 (n = 7) | 0.5 (n = 6) | – (n = 1) | – (n = 2) | – (n = 0) | 0.5882 |
Figure 1.
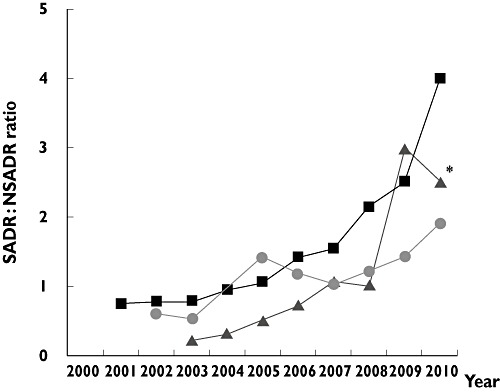
‘Serious’ : ‘non-serious’ adverse drug reaction (SADR : NSADR) ratios over time for esomeprazole, peginterferon-α2a, and pioglitazone (Student's t-test for linear trend, P < 0.01). *Only seven ADR reports for the 2010 pioglitazone ratio. esomeprazole ( ); peginterferon-α2a (
); peginterferon-α2a ( ); pioglitazone (
); pioglitazone ( )
)
Discussion
Our study allows for the discussion of some interesting points. It shows that, during the first years of marketing, reports concern all ADRs, both SADRs and NSADRs. Fortunately, drugs more frequently induce NSADRs than SADRs and the SADR : NSADR ratio was less than 1. Later, practitioners know the main ADRs of the drug and prefer to report only SADRs.
This point was demonstrated for most of the drugs investigated in the study. However, some drugs do not follow this pattern. The non-linear trend in the increasing of the SADR : NSADR ratio concerning drugs restricted to hospital use or prescribed by hospital staff (trastuzumab, verteporfine, etanercept) could be explained by the fact that drug-induced ADRs occur mainly in hospitalized patients and, thus, often prolong the duration of hospitalization, reclassifying the ADR as an SADR. Moreover, the location and the diligent work of French Regional Centres of PharmacoVigilance in hospitals could induce more reporting from hospital practitioners than from others. The decreasing trend observed with levetiracetam, galantamine and oxycodone remains difficult to explain.
Several studies have found that different factors can affect the reporting of ADRs. Among them, the Weber effect is the best known. First described in 1984 with seven non-steroidal anti-inflammatory drugs (NSAIDs), it consists in a reporting rate adjusted to the number of prescriptions which increases in the first years of marketing, and then decreases over time. Typically, the reporting rate peak is observed at the end of the second year post-marketing [5]. In the United States Food and Drug Administration (FDA) PharmacoVigilance databases (the FDA Spontaneous Reporting System and the Adverse Event Reporting System), five of these seven NSAIDs also demonstrated a highest reporting peak at the second year post-marketing [13]. The Weber effect has also been demonstrated with anti-infective, endocrine, pulmonary, and cardiovascular drugs in a pharmaceutical company PharmacoVigilance system [14]. In the FPVD, unlabelled ADR reporting rates of 10 drugs belonging to various pharmacologic classes followed a similar trend (with a peak at the end of the first year) [6]. The introduction of a new omeprazole formulation led to an early Weber effect [15]. Nevertheless, the Weber effect has not been demonstrated for all drugs. For instance, it was not found with vaccines [14]. Similarly, a study in the FDA PharmacoVigilance databases on four angiotensin II receptor blockers found an ADR reporting rate adjusted to the number of prescriptions to be highest in the first year of marketing, followed by a gradual decline [16]. Neither may be the Weber curve a class effect: another descriptive survey in the FDA Adverse Event Reporting System failed in showing a highest reporting peak the second year post-marketing for NSAIDs other than those initially studied by Weber [17]. A similar study in the same database found an increased number of reports until the end of the second year for two selective serotonin re-uptake inhibitors among five [18]. Furthermore, the Weber effect is controversial. Indeed, in the study conducted by Wallenstein et al., the ADR reporting rate adjusted to the number of prescriptions was not consistent with the Weber curve for four NSAIDs. Among them, two were originally studied by Weber [17]. Nonetheless, we should highlight the fact that the UK PharmacoVigilance database, as well as the French one, registers reports from health practitioners. The Weber effect describes their reporting habits. This is not the case of the FDA databases, in which patients report adverse events. For every patient who receives a drug, the drug is new, whatever the delay from the first marketing. As a result, it is not surprizing that the Weber effect does not apply to such databases.
Nevertheless, the Weber effect as well as the one we describe here are not consistent for all drugs. Indeed, many other factors may interact with reporting and perhaps with temporal trends in reporting: the unexpected nature of the ADR and its familiarity to physicians, the length of time the drug has been on the market, the religion of the area, the main characteristics of drug regulation in the country and the seriousness of the ADR [4].
Some limitations of our study should be discussed. The threshold of at least five reported ADRs for at least 3 years is arbitrary. When the annual numbers of reported ADRs are very sparse, the trends observed could be due to chance. However, except for oxycodone, the significant trends that we observed involved drugs with high annual numbers of reported ADRs, and available for at least 8 years. When performing the linear trend tests, we did not include any correction for multiple tests. As a result, some of our results could be statistically significant by chance. Only the lowest P value may be truly significant (the drugs shown in Figure 1). However, we performed 16 tests at the α threshold of 5%. As a result, only one test (exactly 0.8 test) could be significant by chance.
We describe here, for the first time, a chronological trend in notification of SADRs (vs. NSADRs). There is a marked increase after 2 to 3 years of marketing. Since post-marketing surveillance of drugs is based on spontaneous reports of ADRs worldwide, the present results should be borne in mind when trying to detect new drug safety alerts. It could also suggest difficulties in detecting delayed or rare NSADRs.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Wilson AM, Thabane L, Holbrook A. Application of data mining techniques in pharmacovigilance. Br J Clin Pharmacol. 2004;57:127–34. doi: 10.1046/j.1365-2125.2003.01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bate A, Lindquist M, Edwards IR. The application of knowledge discovery in databases to post-marketing drug safety: example of the WHO database. Fundam Clin Pharmacol. 2008;22:127–40. doi: 10.1111/j.1472-8206.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 3.Royer RJ. Mechanism of action of adverse drug reactions: an overview. Pharmacoepidemiol Drug Saf. 1997;6(Suppl. 3):S43–50. doi: 10.1002/(sici)1099-1557(199710)6:3+<s43::aid-pds308>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Stephen M. Introduction. In: Talbot J, Walter P, editors. Stephens' Detection of Adverse Drug Reactions. 6th edn. Chichester: John Wiley & Sons, Ltd; 2004. p. 14. [Google Scholar]
- 5.Weber J. Epidemiology of adverse reactions to nonsteroidal antiinflammatory drugs. In: Rainsford KD, Velo GP, editors. Advances in Inflammatory Research. New York: Raven Press; 1984. pp. 1–7. [Google Scholar]
- 6.Haramburu F, Bégaud B, Moride Y. Temporal trends in spontaneous reporting of unlabelled adverse drug reactions. Br J Clin Pharmacol. 1997;44:299–301. doi: 10.1046/j.1365-2125.1997.t01-1-00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasford J, Goettler M, Munter K-H, Müller-Oerlinghausen B. Physicians' knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol. 2002;55:945–50. doi: 10.1016/s0895-4356(02)00450-x. [DOI] [PubMed] [Google Scholar]
- 8.Montastruc J-L, Sommet A, Lacroix I, Olivier P, Durrieu G, Damase-Michel C, Lapeyre-Mestre M, Bagheri H. Pharmacovigilance for evaluating adverse drug reactions: value, organization, and methods. Joint Bone Spine. 2006;73:629–32. doi: 10.1016/j.jbspin.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Moore N, Noblet C, Kreft-Jais C, Lagier G, Ollagnier M, Imbs JL. French pharmacovigilance database system: examples of utilisation. Therapie. 1995;50:557–62. [PubMed] [Google Scholar]
- 10.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 11.Bégaud B, Evreux JC, Jouglard J, Lagier G. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie. 1985;40:111–8. [PubMed] [Google Scholar]
- 12.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Saf. 1999;20:109–17. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 13.Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy. 2004;24:743–9. doi: 10.1592/phco.24.8.743.36068. [DOI] [PubMed] [Google Scholar]
- 14.Brodovicz K, Sharrar R, Hostelley L. The Weber effect – is it real? Pharmacoepidemiol Drug Saf. 2001;10:S138. [Google Scholar]
- 15.de Graaf L, Fabius MA, Diemont WL, van Puijenbroek EP. The Weber-curve pitfall: effects of a forced introduction on reporting rates and reported adverse reaction profiles. Pharm World Sci. 2003;25:260–3. doi: 10.1023/b:phar.0000006518.22231.ea. [DOI] [PubMed] [Google Scholar]
- 16.McAdams MA, Governale LA, Swartz L, Hammad TA, Dal Pan GJ. Identifying patterns of adverse event reporting for four members of the angiotensin II receptor blockers class of drugs: revisiting the Weber effect. Pharmacoepidemiol Drug Saf. 2008;17:882–9. doi: 10.1002/pds.1633. [DOI] [PubMed] [Google Scholar]
- 17.Wallenstein EJ, Fife D. Temporal patterns of NSAID spontaneous adverse event reports: the Weber effect revisited. Drug Saf. 2001;24:233–7. doi: 10.2165/00002018-200124030-00006. [DOI] [PubMed] [Google Scholar]
- 18.Hartnell NR, Wilson JP, Patel NC, Crismon ML. Adverse event reporting with selective serotonin-reuptake inhibitors. Ann Pharmacother. 2003;37:1387–91. doi: 10.1345/aph.1C522. [DOI] [PubMed] [Google Scholar]


