Abstract
Human papillomavirus (HPV) infection is emerging as a major prognostic and predictive marker in head and neck squamous cell carcinoma (HNSCC). Researches are focused on the development of HPV detection assays specially designed for HNSCC. The HPV diagnosis in these tumours is relevant toprognosis even in an already-developed tumour, whereas in the cervix, where the HPV is the cause of almost all tumours, this information has less clinical relevance. The better outcome of HPV-associated HNSCC raises the question about the best methodologies to distinguish between HPV and non-HPV-associated SCC. However, no consensus has been reached on the optimal way to identify HPV-associated SCC and ancillary studies have utilised many different methodologies, including HPV polymerase chain reaction testing, HPV in situ hybridization analysis, immunohistochemical staining for p16, and newer techniques that are currently under investigation. The objective of this review is to explain and give examples of various techniques of HPV detection highlighting how they might be used clinically. Although currently insufficiently specific due to the possibility of HPV infection originating at other sites, methodologies utilising serum and plasma to measure HPV infection will also be described, mostly for their potential future development and use. Finally, DNA/RNA microarray platforms will be briefly summarized for their capacity to identify the profile of molecular changes in any particular HPV+/HPV− cancer. In this way, it is expected to be possible to correlate the appropriate transcriptome-based diagnosis to the patients’ specific cancer risk.
Keywords: HNSCC, HPV, DNA/RNA microarray, PCR, RT-PCR, E7 monoclonal, ISH, IHC, Real-time PCR, Serum antibodies, Oropharynx cancer
Introduction
During recent years, evidence of human papillomavirus (HPV) infection emerged as a major prognostic and predictive marker in head and neck squamous cell carcinoma (HNSCC). The HPV involvement was first proposed in 1983 by Syrjanen et al. [1] and then supported by several other authors on the basis of: (1) morphological similarities between genital and oropharyngeal epithelia; (2) the broad epithelial tropism of HPV; (3) the detection of high-risk (HR) HPV genotypes in samples of oral squamous cell carcinoma; and (4) finally, the HPV-induced immortalization of human oral keratinocytes in vitro. The evident similarities between both cervical and head and neck tumours prompted the utilisation of the same HPV diagnostic procedures in working-up the latter. Epidemiological study and early diagnosis as predictive of possible cancer development can be and has been conducted with methodologies valid for both tumours with differences in the typologies of sampling. On the other hand, there is now compelling evidence that specially designed methodologies must be employed in HNSCC because in these tumours the association with HPV is relevant to prognosis, whereas in the cervix this information has less clinical relevance. Thus, distinction between HPV positive and HPV-negative HNSCC is important in relation to clinical outcome. One study reported a three-year survival rate of 82.4% for HPV-positive tumors versus 57.1% for HPV-negative tumors and additional studies confirmed this result [2]. This effect appears unrelated to the particular treatment regimen, as the prognosis was better for patients treated with any therapy. Furthermore, the better outcome of HPV-associated SCC raises the question about the need for aggressive postoperative treatment. Therefore, it is conceivable that in the near future treatment strategies may target specific molecular pathways that differ between HPV and non-HPV-associated SCC, increasing the importance of this distinction. However, no consensus has been reached on the optimal way to identify HPV-associated SCC, and ancillary studies in the distinction between HPV-positive and -negative SCC have utilised many methods. These different methodologies include HPV polymerase chain reaction (PCR) testing, HPV in situ hybridization (ISH) analysis, immunohistochemical (IHC) staining for p16, and newer techniques that are currently under investigation.
The objective of this chapter is to summarize the various techniques of HPV detection highlighting how they might be used clinically. Possible methods are outlined in Table 1.
Table 1.
HPV detection methods
| Methods | Specimens | Advantages | Disadvantages |
|---|---|---|---|
| Southern blotting assay | Fresh/frozen samples | High specificity | Not easily applied to FFPE samples |
| Ability to differentiate between episomal and integrated DNA | Cumbersome practical/clinical utilization | ||
| To detect as little as 0.1 copies of viral DNA per cell | |||
| HPV PCR | Fresh/frozen samples | High sensitivity | Low specificity |
| FFPE | Cost effective | Provides no quantitative measure of viral load | |
| Oral cavity brushing/washing | Several commercially available primer sets | No confirmation of transcriptionally active virus | |
| Any body fluid | Assesses for papillomavirus other than HPV16 | Full spectrum of sequence amplification not published | |
| SPF10 primers capable of amplifying highly degraded DNA samples | No distinction between episomal and integrated DNA | ||
| No distinction between neoplastic HPV+ cells and non-neoplastic HPV+ cells | |||
| Real-time PCR | Fresh/frozen samples FFPE | Sensitivity 92% | False positive and false negative products |
| Oral cavity brushing/washing | Specificity 97% | No direct evidence of viral integration | |
| Any body fluid | Ability to differentiate between episomal and integrated DNA | No direct evidence of oncogene expression | |
| Labour-intensive | |||
| Reverse Transcriptase PCR | Fresh/frozen samples | High sensitivity | Time consuming |
| FFPE | Detection of clinically significant HPV infection within tumour specimens | Technically difficult to be used in routine screening | |
| Evidence of active oncogene transcription | |||
| In situ hybridization high risk HPV | Fresh samples | Specificity 100% | Insufficient clinical sensitivity (83%) to be used in routine screening |
| FFPE | Ability to differentiate between episomal and integrated DNA | Technically difficult to be used in routine screening | |
| Commercially available tests | |||
| p16 immunostaining | Fresh/frozen samples | High sensitivity | Surrogate marker; |
| FFPE | Accessible to most laboratories | Sensitivity less than reported for the existence of HPV positive, non-p16 over-expressing sub-type | |
| Oral cavity brushing/washing | Easily applied to FFPE tissue | Specificity not ideal | |
| Presence of transcriptionally active virus through marker of host cell feedback mechanism | |||
| Signal amplification methods for HPV DNA (HCII and Cervista) | Fresh samples | FDA approved method for HPV detection | False positives products |
| FFPE | Easy to be performed | No typing | |
| Oral cavity brushing/washing | No distinction between episomal and integrated DNA | ||
| No distinction between neoplastic HPV+ cells and non-neoplastic HPV+ cells | |||
| IHC with anti-E6-E7 antibodies | FFPE | Ability to prove that HPV DNA is being expressed | No validated commercial kits |
| Detection of HPV oncoproteins | Few data on practical/clinical utilization | ||
| PCR in situ hybridization (PISH) | Fresh samples | High sensitivity | Very high technical difficult for routine screening |
| FFPE | High specificity | No data on practical/clinical utilization | |
| Antibodies against early/late HPV protein | Serum/plasma | Useful test when applied to epidemiological studies | Poor specificity due to HPV infection at other anatomical sites |
| Does not require biopsy | Not all exposed individuals develop antibody response | ||
| L1 cross-reactivity from low-risk HPV further reducing specificity | |||
| DNA/RNA microarray | Fresh/frozen samples | Identification of molecular portrait of gene expression profiles in HPV-positive and negative HNSCC | No agreement in the preferential use of pure epithelial cell material (micro-dissection) or fresh biopsy material (containing stromal and immune cells) |
| FFPE (mostly for DNA) | Identification of subgroups of HNSCCs with gene expression profiles correlating with different prognosis | Predictive gene expression profiles is still elusive | |
| Oral cavity brushing/washing | High costs | ||
| Saliva |
Although currently poorly specific due to the possibility of HPV infection originating at other sites, methodologies utilising serum and plasma to measure HPV will be also be described in this chapter, mostly for their potential future development.
Direct HPV Tests
Southern Blotting Assay
In a Southern blot, the genomic DNA is extracted from a specimen and digested by restriction enzymes. The product is resolved in agarose gel electrophoresis that separates the DNA based on the size of each fragment. The DNA fragments separated by this method are transferred to a nitrocellulose or nylon membrane and hybridized with cloned HPV genomic probes labelled with isotopic (P32) or non-isotopic (digoxigenin) techniques. The detection of the labelled DNA hybrids indicates HPV is present in a given sample. Southern blotting is an assay that has long been one of the standard techniques for the detection of HPV DNA; it has the ability to differentiate between episomal and integrated DNA, particularly by bi-dimensional agarose gel [3], and can detect as little as 0.1 copies of viral DNA per cell. This method has some technical variability and requires a significant amount of DNA and cannot be applied to formalin-fixed, paraffin-embedded (FFPE) tissue samples because they contain cross-linked, degraded nucleic acid. Southern blot has a theoretically higher specificity but is clearly less sensitive than PCR. Yeudall et al. [4] utilized both type-specific PCR and Southern blot for HPV 16/18 and reported that there was a manifest variation in the two techniques with a higher sensitivity of type-specific PCR.
Nevertheless, the Southern blot assay is still the best method to detect integrated, or episomal, or both forms of viral DNA and can be useful for comparing results of other methods of viral integration detection; however, this method has no practical/clinical utilization.
Polymerase Chain Reaction for HPV Detection
Polymerase chain reaction and reverse transcriptase PCR (RT-PCR) are processes in which a signal sequence of DNA or RNA (properly, the cDNA after reverse transcription) is amplified several orders of magnitude through several rounds of denaturing at high temperature (95°C), annealing of complimentary oligonucleotide primers at a lower temperature (usually below the melting point, i.e. 55°C), and DNA replication at an intermediate temperature (72°C) by a heat-resistant DNA polymerase. PCR represents a highly-sensitive, widely-available, and cost-effective method of HPV detection. In theory, it can be used to detect as little as one copy of a DNA sequence and can be utilised in FFPE tissue or fresh tissue from oral biopsies, although it is more sensitive on fresh frozen tissue compared to FFPE tissue [5]. However, standard PCR techniques have a number of drawbacks in comparison to ISH: (1) they have lower specificity; (2) they do not allow distinction between HPV that is present in the neoplastic cells and HPV that is present in surrounding non neoplastic epithelium or stroma; (3) they cannot distinguish between episomal and integrated HPV DNA; and (4) they are technically cumbersome to perform [5]. These are significant limitations of PCR because it decreases the ability to distinguish clinically relevant HPV infection. Moreover, while primers targeting the conserved L1 region are commonly employed, this region may be deleted during viral integration, potentially reducing the sensitivity. However, the loss of L1 is not seen in a significant number of cases and, thus, likely does not have a major influence on sensitivity of HPV detection [6]. Several PCR amplification techniques are commercially available. These PCR screening assays commonly have primers designed to amplify a region of DNA that is present in multiple HPV types (most commonly within the highly conserved L1 gene) [6]. Since most commercially available PCR kits use consensus sequences from multiple HPV subtypes, specific typing is generally not possible through PCR alone. Among the more commonly commercially available primer sets are PGMY09/11, GP5+/GP6+ (Fig. 1), and SPF10 LiPA [7]. All three target sequences within the L1 gene though they are of varying length (450 base pairs, 140 base pairs, and 65 base pairs, respectively). Targeting shorter stretches of DNA generally results in higher sensitivity on FFPE tissue, as DNA fragmentation often occurs during extraction from the archived tissue [7]. Thus, the GP5+/GP6+ and SPF10 primers are more ideal for use in FFPE tissue from surgical specimens. During the last few years, many novel PCR-based HPV detection assays have been described, including those that target other conserved regions within viral L1 (such as Roche Amplicor, Branchburg, NY, USA) or E1 (such as PapilloCheck, Greiner Bio-one, Frickenhausen, Germany) regions. In addition, modifications on existing broad-spectrum PCR systems were conducted, aiming at better targeting HPV types, which did not react that efficiently with the original assay. Examples of the latter are multiplex variants of GP5+/6+-PCR such as BSGP5+/6+-PCR, the Abbott Real-Time High Risk HPV test (Abbott, IL, USA), and MGP PCR [7]. More recently, multiplex assays have also been developed that use primers targeting different viral regions of different HPV types, rather than a conserved region [8]. Thus, differences in sensitivities between the different primer sets could explain the discrepancy in the final outcome of reports dealing with HPV in clinical samples. Furthermore, few studies have been done to directly compare the sensitivities of these primer sets in detecting HPV in oropharyngeal cancers [9]. Equally important for the final assay outcome is the read-out system used to detect the PCR products. Many read-out systems are based on hybridization of PCR products to oligonucleotide probes targeting internal regions flanked by both primers. In the ‘reverse’ hybridization format, oligonucleotides are immobilized on a solid support, hybridized to (biotin-) labelled PCR products, and ultimately visualized via colorimetric or fluorescent staining procedures. One commonly used read-out system that is useful for screening purposes but is not able to detect single genotypes involves an enzyme immunoassay (EIA) staining procedure. In this assay, after capturing of biotinylated PCR products to streptavidin-coated microplate wells, the immobilized PCR products are hybridized with cocktails of labelled (e.g. with digoxigenin) oligoprobes specific for the HPV types of interest. For genotyping purposes, the reverse hybridization techniques are more suitable with the oligoprobe specific for a single genotype immobilized on many different solid support like strips, filters, microarrays, and microsphere beads. An example of a microarray support developed in our laboratory is reported in Fig. 2.
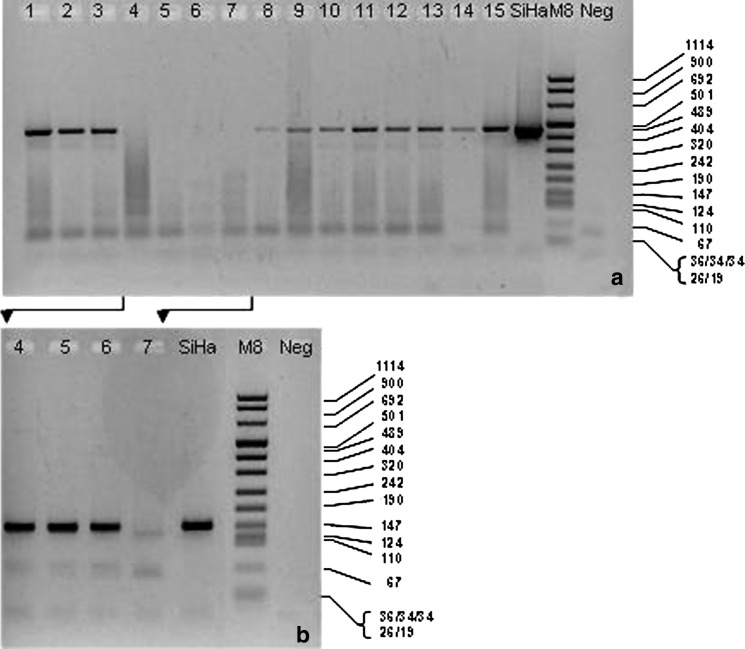
Fig. 1.
PGMY/GP nested PCR. DNA from several oropharynx cancers were subjected to PCR with PGMY primers (a). A second PCR of the previous amplified products with GP primers (nested PCR) was performed for samples 4–7 (b) showing three more positive samples. SiHa is a cell line with integrated HPV-16. M8 = DNA Molecular Weight Marker VIII (Roche); fragment length in bp is indicated. Neg = a control without DNA
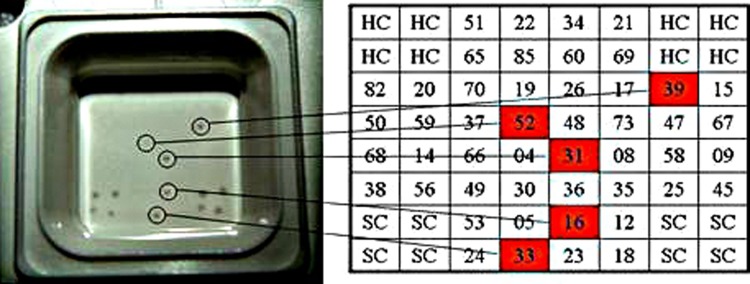
Fig. 2.
Presence of multiple HPV types in oral rinses in a microarray device. Extracted DNA was amplified with a specific set of primers for alpha- and beta-HPV and the amplified products were biotin-labelled and hybridized on a microarray well where the NH2-labelled oligonucleotide probes of the indicated HPV types and the appropriate controls were spotted on plastic devices coated with a proprietary polymer (LifeLineLab s.r.l. Pomezia -Italia). After the hybridization reaction, the hybridized target DNA was detected by enzymatic, colorimetric development. The sample scored positive spots for different HPV. SC = PCR control; HC = hybridization control
The microsphere bead support is utilised in a recently developed flow cytometry-based method. In this system, each type-specific oligonucleotide probe is covalently attached to a specifically coloured microsphere bead set. During analysis, individual microspheres are analysed by two lasers; the first laser allows identification of the microsphere set with the type-specific probe, the second laser allows quantification of the PCR product by exciting a reporter fluorochrome coupled to the hybridized PCR product [10].
As noted previously, the clinical relevance of detecting HPV DNA is particularly important for head and neck tumours. The presence of latent virus leads to false positive results due to the ability of PCR to detect just a few copies of HPV DNA per cell. Attempts have been made to resolve this issue through use of real-time PCR, which provides a quantitative analysis of viral load. Real-time PCR allows for quantification of target DNA via colorimetric markers that accumulate during PCR amplification. This quantitative approach may allow for identification of more clinically relevant high viral loads (Fig. 3). Sensitivity is estimated at 92% and specificity at 97% when using a cut-off viral load of >0.5 copies per cell; therefore, false positives and false negatives still exist [11].
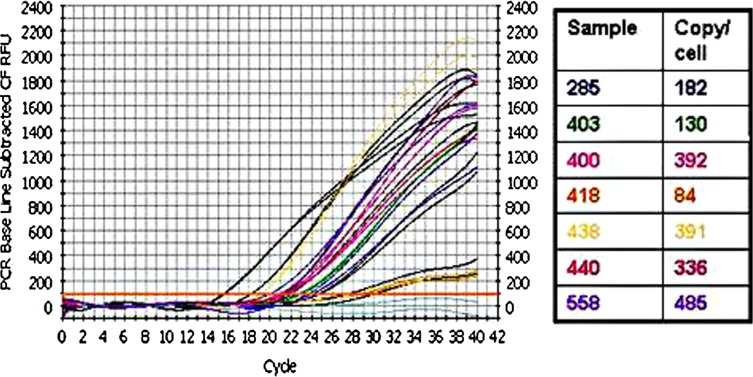
Fig. 3.
Real time PCR for HPV viral load. DNA for HPV-16 positive oropharyngeal cancers were subjected to PCR in an iCycler BioRad Appartus with specific HPV-16 primers in a SYBR green I containing iQ Supermix (BioRad Lab, Hercules, CA, USA). The graph is the output from the apparatus; black lines are fivefold dilution of a HPV-16 plasmid from 1.94 × 107 to 3.1 × 104 viral copy; grey lines are negative controls without DNA. In the right panel the calculated viral load for each sample is shown
Furthermore, recent studies have demonstrated the ability to distinguish between episomal and integrated HPV DNA by real-time PCR assays. The HPV gene for E2 protein, a regulator of E6 and E7 protein expression, is a common breakpoint prior to viral integration into the host genome and its gene disruption results in upregulation of the oncogenic proteins. When E2 is disrupted, PCR with primers designed to amplify the entire E2 gene will fail [12]. Thus, comparing PCR amplification of the E2 gene with a gene known to rarely be disrupted during integration (such as the E6 gene) can suggest (albeit only indirect evidence) whether the viral DNA is integrated or not, as the amplification ratio of E2–E6 would be lower in integrated HPV compared to episomal HPV [13] (Fig. 4). However, HPV DNA breakpoints are known to be variable, so E2 disruption is not necessarily seen in all integrated cases and episomal E2 may be present even with integrated E2, limiting the sensitivity of this technique. Another criticism of this method is that it still provides no direct evidence of viral integration. However, analysis of tonsillar carcinoma conclusively showed that, similar to preneoplastic cervical lesions and cancers, HPV-16 integration in head and neck carcinoma (HNC) is not a prerequisite for carcinogenesis, since HPV-16 genomes persist and are transcribed as unintegrated plasmids in some tumours [14]. Determining whether the prevalence of unintegrated and integrated HPV-16 genomes in HNC differs from that in cervical lesions will require larger epidemiologic studies. Furthermore, the presence of mRNA from integrated as well as unintegrated HPV DNA indicate that viral oncogene transcripts play a fundamental role in the induction and maintenance of the transformed status [13]. Thus, PCR methods targeted against mRNA were developed in order to provide evidence of active HPV gene transcription. There are a number of commercially available assays for the detection of HPV by RT-PCR. These kits target mRNA of the oncogenic E6 and E7 proteins utilising isothermal mRNA amplification methods such as nucleic acid sequence-based amplification (NASBA) and transcription-mediated amplification (TMA). These assays utilize a reverse transcriptase to generate cDNA first, RNase H to degrade the RNA template (in case of NASBA) and a T7 RNA polymerase to produce multiple RNA copies from the cDNA. Commercially available methods detect E6/E7 mRNA in a real-time format using different molecular beacon probes for five high-risk HPV types (i.e. HPV 16, 18, 31, 33 and 45) in two assay runs to allow genotyping [15]. RT-PCR amplification of viral E6/E7 mRNA is now considered the “gold standard” for the detection of clinically significant HPV infection within tumour specimens as it detects transcriptionally active HPV. The method is reliable not only when applied to fresh frozen specimens, but also when applied to FFPE samples [16]. However, the method has the disadvantage of being time consuming and technically difficult.
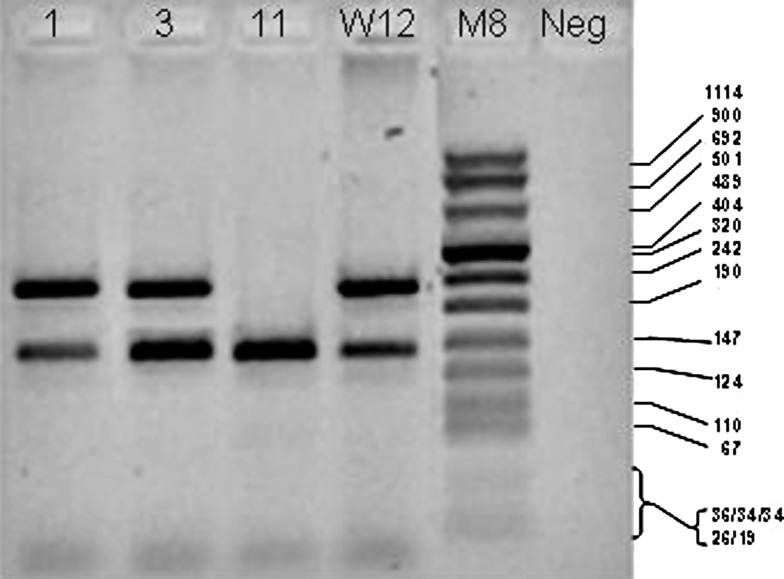
Fig. 4.
PCR for E2 and E6 genes of HPV-16. DNA extracted from oropharynx tumours were amplified with specific primers for the E2 and E6 genes of HPV 16. Note that in lane 11, the E2-amplified band is lacking indication of viral integration; in lane 1, the presence of both E2 and E6 bands indicates the presence of episomal virus like in the W12 control, whereas in line 3 the higher intensity of E6 band suggests (thereafter it was confirmed by E2/E6 ratio) the simultaneous presence of integrated and episomal forms. W12 is a cell line containing episomal HPV-16 DNA. M8 = DNA Molecular Weight Marker VIII (Roche); DNA fragment length in bp is indicated. Neg = a control without DNA
Overall, PCR is a reliable, sensitive marker of HPV DNA and RT-PCR may be a sensitive marker of HPV mRNA. Nonetheless, PCR and RT-PCR cannot localize HPV to the area of neoplasia and other techniques like ISH can provide this information together with a higher clinical specificity and the ability to reliably distinguish episomal from integrated HPV DNA.
In Situ Hybridization for HPV
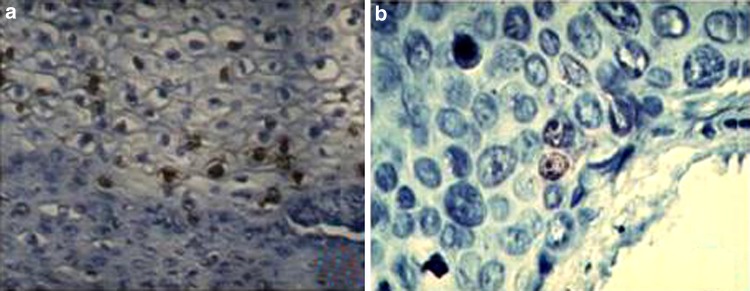
In situ hybridization (ISH) is the only molecular method allowing reliable detection and identification of HPV in topographical relationship to their pathological lesions. Unlike in other molecular methods, in ISH the whole HPV detection procedure occurs within the nuclei of infected cells and not on solid supports or in solutions. The result of the hybridization reaction is evaluated microscopically and the appearance of an appropriate precipitate within the nuclei of epithelial cells is indicative of the presence of HPV in the specimen being tested. In addition, the physical state of the virus can be evaluated by the presence of punctuate signals for integrated virus and diffuse signals for episomal virus (Fig. 5). In this way, ISH may overcome some of the limitations of PCR by detecting only clinically relevant infection. Although the specificity of this method is high (100%), the sensitivity is not ideal (83%) [11]. It has been estimated that around 10 copies of virus per cell must be present in order for ISH to detect HPV, although newer ISH kits with signal enhancement techniques are thought to be more sensitive. Numerous technically validated HPV ISH assays are commercially available, most containing a cocktail of probes targeting multiple types of HPV but probes for individual types can be used if subtyping is clinically relevant, as in oropharyngeal SCC where HPV-16 is by far the most commonly found [12]. Many commercially available tests have demonstrated similar specificity in HPV detection of cervical specimens, but to our knowledge, comparisons of the commercially available tests in HPV detection of oropharyngeal lesions have not been performed. The sensitivity of the assay is increased by signal enhancement techniques. One such technique is tyramide signal amplification, also known as catalysed reporter deposition (CARD), which has been shown to have a 10- to 100-fold increase on sensitivity. In this system, peroxidase-conjugated streptavidin is applied to a DNA–DNA hybridization mixture, followed by incubation with biotinylated tyramide. Peroxidase-conjugated streptavidin is then applied, and lastly, the chromogenic substrate diaminobenzidine is added. Such techniques have increased the sensitivity of ISH to the extent that it can detect one to two copies of DNA per cell. Nevertheless, the sensitivity of ISH is still less than that seen in PCR analysis. However, ISH is more specific for HPV infection than p16 immunohistochemical staining. Although commercially available HPV assays based on ISH have been validated technically, they are insufficiently clinically validated. In addition, current ISH-based assays are considered by many experts in the field to be too laborious and to have insufficient clinical sensitivity to be used in routine screening.
Fig. 5.
ISH for HPV-16. Hybridization with a specific biotinylated probe for E6 HPV-16 on pathological cervical epithelium was carried out by standard procedures. a Diffuse staining indicative of episomal HPV; b punctuate staining for the presence of viral integration
Recently a new chromogenic RNA ISH assay called RNAscope has been utilised to detect E6/E7 mRNA of HPV-16 and other high-risk types on tissue microarrays. RNA ISH seems to be more sensitive than DNA ISH in detecting HPV in OSCC, and it correlates strongly with p16 [17]. However, this new technique is still too laborious to be utilised in routine screening.
Signal Amplification Methods
These techniques are based on an initial hybridization step of nucleic acids in the specimen with target-specific probes after which the signal (i.e. the hybridization event) is amplified and ultimately visualized with one of the various available methodologies. This principle is based not only on the previously described ISH methods but also on the liquid phase hybridization assay. The Hybrid Capture 2 (HCII) HPV DNA Test has been the most important HPV diagnostic assay over the last decade and is still the most frequently used diagnostic HPV test worldwide in cervical cancer screening programs [7]. This is an FDA-approved method for HPV detection in cervical pap smears, and studies have demonstrated its utility in detecting the presence of HPV in lesions of the cervix and oropharynx. It has been used in the majority of key randomized controlled and other clinical trials that have proved the clinical value of HPV testing in general. DNA is extracted from the exfoliated cells, denatured, and converted to single-stranded form. The high-risk HPV HC2 assay uses a mixture of full-length RNA probes representing 13 HPV types (i.e. HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) to hybridize to HPV DNA in heat-alkaline-denatured samples. DNA–RNA hybrids are subsequently captured in microplate wells coated with antibodies that specifically recognize DNA–RNA hybrids. HPV DNA present in the samples is detected by peroxidase-labelled antibodies recognizing the RNA/DNA hybrids, and visualized by chemiluminescence. A more recent liquid-phase assay that is also FDA approved is the Cervista HR HPV test (Hologic, Madison, WI, USA) targeting 14 high-risk HPV types [7]. This system makes use of a Cleavase enzyme that first cleaves a type-specific oligonucleotide probe when it overlaps an invader oligonucleotide at the target DNA recognition site. The resulting 5′-portions of the cleaved probes can subsequently bind to universal hairpin fluorescence resonance energy transfer (FRET) oligonucleotides creating another invasive structure that is recognized as substrate by the Cleavase enzyme. The enzyme then cleaves the FRET oligonucleotides between the fluorophore and quencher molecule, which results in the production of a fluorescence signal. Both HC2 and Cervista assays are specifically designed for HPV detection in cervical scrapings.
Suspicious lesions in the oropharynx can be sampled by brush and the extracted DNA utilised in this liquid-based assay. This test has the advantage of allowing HPV testing without the need for biopsy [18]. However, because the reaction occurs in solution, it does not allow for localization of HPV to a histologic area of interest. In addition, the HR probe cocktail typically used has been shown to detect at least 28 non-targeted HPV types, including many low-risk (LR) HPV types, creating the potential for false positives.
IHC with Anti-E6-E7 Antibodies
While IHC staining against p16 is frequently used as a surrogate marker of HPV, to date, IHC staining against specific HPV proteins has generally not been performed.
Nevertheless, the development of IHC stains against the oncogenic E6 and E7 proteins would have a number of potential advantages over other HPV detection methods. It would have the ability to prove that HPV DNA is being expressed and directly demonstrate that important HPV oncogene proteins are present. Development of reliable antibodies against E6 and E7 protein could be an excellent means for HPV detection in the future.
Many different monoclonal and polyclonal antibodies have been developed and most of these target the HPV-16 E7 protein [19] but, to the best of our knowledge, none has been utilised in HNSCC. Moreover, the procedures for initial fixation of the tissues and the antigen retrieval methods are critical, specifically designed for each antibody, and not well-standardized. Thus, IHC for E7 is far from being a technique for routine analysis. Few of these experimental monoclonal antibodies became recently commercially available for IHC but data on clinical specificity is still lacking.
PCR In Situ Hybridization (PISH)
Over the past two decades, a technique has been developed for combining PCR and ISH, referred to as PCR in situ hybridization (PISH) [5]. In this case, PCR is performed using typical PCR reagents performed on FFPE tissue slides [5]. The slide is then washed, dehydrated in alcohol, and dried. The PCR products present on the slide are then hybridized with specific DNA probes in the same manner that standard ISH is performed (Fig. 6). PISH can be utilized to perform PCR for HPV on intact tissue preparations of SCC followed by in situ hybridization detection, thus combining the sensitivity of PCR with the tissue localization of ISH [8]. Studies looking at HPV detection rates in cervical invasive and in situ SCC have found significantly higher detection rates with PISH compared to ISH alone. However, to date, few studies have looked at the utility of PISH in detecting HPV in oropharyngeal samples [20]. Furthermore, the technique is far from being routinely utilized clinically, as it is a very cumbersome methodology that can be utilised only in very skilled laboratories.
Fig. 6.
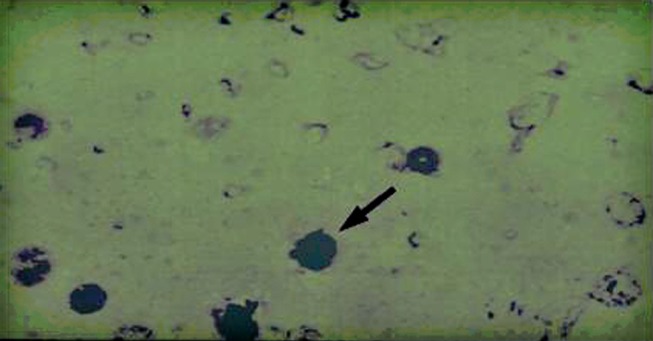
PISH for HPV 16. Cyto-centrifuged cells from an oral rinse of HPV+ OSCC patients were subject to PISH essentially as described by Nuovo [5]. The primers for amplification of the HPV-16 E6 gene were forward-AAGGGCGTAACCGAAATCGGT and reverse-GTTTGCAGCTCTGTGCATA. The PCR products were hybridized with a biotin-labelled HPV-16 DNA probe 5′-CATTTTATGCACCAAAAGAGAACTGCAATG-3′ as in standard ISH procedure. The detection system employed a streptavidin–alkaline phosphatase conjugate and the chromagen blue tetrazolium in the presence of 5-bromo-4-chloro-3-indolynitrolphosphate. The slide was counterstained with methyl green. Dark blush/purple precipitate at the site of hybridization in nuclei (arrow) denotes the presence of HPV-16 DNA
Indirect Tests Correlating with HPV
Immunohistochemical Staining for p16
The E7 protein of HR HPV binds to Rb and in turn causes increased expression of nuclear p16 regulator. As a result, IHC staining for p16 has a sensitivity approaching 100% for the detection of HPV-associated SCC and, consequently, p16 detection is often used as a surrogate marker of HPV infection. It has the advantage of being easy to perform on FFPE tissue, and monoclonal antibodies against p16 are commercially available. However, p16 is overexpressed in a subset of tumors apparently lacking evidence for the presence of HPV DNA. In a series of 239 cases of oropharyngeal SCC, Lewis et al. [21] reported that 78% (n = 187) were positive for the p16 immunohistochemical stain, of which 13.9% were negative for HPV (by ISH and SPF10-PCR) with no difference in outcome. In contrast, a recent study by Thavaraj et al. [22] using a different set of PCR primers (GP5+/GP6+) found that only 2 out of 142 (1.4%) p16 positive tonsillar SCC were negative for HPV by both PCR and ISH.
The difference in the percentage of these p16-positive, HPV-negative tumors between the two studies may represent differences in sensitivities between HPV tests used or may reflect true differences in HPV prevalence in different populations. It is possible that there is a subset of non-HPV-associated tumors with histologic phenotype, molecular characteristics, and prognosis similar to HPV-associated SCC. Indeed, Harris et al. [23] reported an association of p16 overexpression without evidence of HPV in young patients suffering of SCC of the oral tongue. This subtype demonstrated an improved clinical outcome suggesting that p16 positivity is a sensitive marker for non-keratinizing, poorly-differentiated, yet prognostically favourable, SCCs. While p16 may not be a specific marker of HPV infection, it can provide important prognostic information and future therapies aimed at targeting this pathway may be effective in treating p16-positive, HPV-negative SCC.
Gene Expression: DNA/RNA Microarray
A DNA microarray is a collection of microscopic DNA spots attached to a solid surface by a covalent chemical matrix. Each DNA spot contains picomoles (10−12 mol) of a specific DNA sequence, known as probes. Each spot has a unique sequence different from the others in the array and will hybridize only to its complimentary strand. These can be a short section of a gene or other DNA element that are used to hybridize a cDNA or cRNA under high-stringency conditions. Probe-target hybridization is usually detected and quantified by detection of fluorophore-, silver-, or chemiluminescence-labeled targets to determine relative abundance of nucleic acid sequences in the target. DNA microarrays have been successfully used to identify global patterns of gene expression in different human neoplasia, including head and neck cancers. Many investigators have used microarrays to analyze gene expression changes in HNSCC in tissues and cell lines, but little is known about the gene expression changes in HPV-associated HNSCC. The identification of molecular portraits of gene expression profiles in HPV-positive and -negative HNSCC, including their differences, could result in a better understanding of critical events during carcinogenesis.
Gene expression microarrays bind labelled nucleic acid, allowing inference of the level of expression by measuring the extent of binding. Such studies have identified subgroups of HNSCCs with gene expression profiles that correlate with different aspects of prognosis, including recurrence, risk of lymph node metastasis, and overall survival. However, few have attempted to validate their predictive gene expression signature in an independent data set. Martinez et al. [24] compared the cellular gene expression profiles of HPV-positive and negative tissues in normal epithelium and oropharyngeal carcinomas (OPC) with the help of Affymetrix Human U133A Gene Chip® and suggested the specific expression of gene patterns in HPV-positive and HPV-negative oropharyngeal SCC that may serve as potential biomarkers for the development of HNSCC. DNA microarray technology is an exciting, rapidly expanding biotechnological methodology and will hopefully enable both a greater understanding of the biology of head and neck cancer and help in the clinical management of this disease. One barrier to extrapolation of these gene expression signatures into a clinically useful test is the lack of agreement between these prognostic expression signatures, both in terms of content and size. These differences have been attributed mostly to variables such as site within the upper aero-digestive tract, sample preparation, and the platform used for analysis. Besides the concern about the extreme sensitivity of the assay, there is also concern about the use of fresh biopsy material (which introduces heterogeneity into the samples due to the content of stromal and immune cells) or pure epithelial cell material (obtained by laser capture microdissection). Both approaches seem to be reasonable because in other models gene expression changes have been detected in both tumour stroma and epithelial compartments of epithelial cancers. However, the ability to consistently map a particular gene expression signal to clinical outcome or response to therapy is still elusive. In addition, prospective studies, which test the clinical predictive power of these gene expression profiles, are still lacking and it is uncertain if any of these expression profiles will complete the translation into the clinic, considering also the high costs of these assays.
Nevertheless, more data is expected to come with new technologies based on direct RNA sequencing that will be able to depict the complete scenario of the RNA within the tumours, including non-coding sequences. Deep sequencing of entire mRNA populations using so called “Next Generation Sequencing” platforms (i.e. Illumina platform) has emerged as an alternative approach to the use of array-based technologies, particularly in biomarker discovery. These methods have the advantage of allowing expression profiling of all potential transcripts, not only those represented on available array platforms. Additionally, both expression analyses and characterization/quantification of alternative splicing patterns can be performed using the same experimental data. The feasibility of the method for viral transcript is further strengthened by our preliminary experiments with the Illumina platform that enabled the identification of two almost identical transcript alignment patterns to the HPV16 genome in cervical cancer cell lines (CaSki and SiHa) and the absence of transcripts aligning to the viral poly-A site, suggesting that viral transcripts use cellular poly-A sites following the integration, at least in cancer cells (Paolini et al. manuscript in prep.). When and if these technologies will be available to clinical practice remains uncertain and currently they represent a new field of study rather than a diagnostic/prognostic tool.
Proxy Measure of HPV Infection
Serum Antibodies Against HPV Antigen
The same HR HPVs involved in HNC also infect/involve the anogenital tract. Since a serologic assay is not site-specific, it could be argued that infections outside the head and neck might influence the specificity of the serum tests. However, in a study on a large Nordic cohort in which serum samples were collected from almost 900,000 individuals, the risk associated with seropositivity in the ELISA test against baculovirus-expressed capsids containing both the L1 and the L2 proteins (major oncogenic HPV types 16, 18, and 33) was largely attributable to infection at the site of the tumour, because the odds ratio was significantly higher for tumors that were positive for HPV-16 DNA (37.5) than for those that were negative (2.1) [25]. This association was also reported in an IARC multicenter study utilising a similar ELISA assay. Different findings between the serologic assays may be expected since the presence of viral-like particles (VLP) reflects changes that occur earlier in the infection process and over a longer time period than does the presence of anti-E6/E7 oncoproteins, which are markers of invasion and later events in the disease process. Indeed, more convincing data are reported for the presence of anti E6-E7 antibodies in the sera of HNC patients suggesting that E6/E7 proteins are markers of HPV-transformed tumour cells inducing an anti-E6/E7 antibody response, whereas among healthy patients without tumors, E6/E7 expression is an uncommon occurrence. A strong correlation exists between detection of HPV-16 tumour DNA and antibodies to HPV-16 E6 or E7 but not to HPV-16 VLP. Recently, antibodies to the entire HPV-16 proteome were quantified by using a new promising multiplexed bead assay, using C-terminal GST-fusion proteins captured onto Luminex beads, as already described in the PCR paragraph. HPV-16 E1, E2, and E7 antibody levels were significantly elevated compared with healthy control samples (p = 0.02) and partners of OPC patients (p = 0.01) suggesting that these antibodies are potential biomarkers for HPV-associated OPC [26]. Moreover, anti-E6/E7 serum levels seem to be potential prognostic markers of survival in patients with HNC as shown in the following post-treatment study on cervical cancer [27]. Decreased levels of E6 and E7 antibodies between pre-and post-treatment are associated with continuous remission [28].
HPV DNA in Plasma
Blood is the only fluid that is in direct contact with all organs and therefore offers an attractive non-invasive method of cancer surveillance. Since the first evidence showing that tumour associated DNA can be detected in the serum of cancer patients, several studies have evaluated different types of tumour DNA as biomarkers for cancer surveillance. The presence of viral DNA in viral-related tumors offers a distinct marker for detection in blood. For example, the presence of Epstein-Barr virus (EBV) DNA in the plasma of nasopharyngeal carcinoma (NPC) patients has been shown to be a sensitive and reliable prognostic marker in NPC. Several large studies have demonstrated that the post-treatment EBV DNA level is highly indicative of persistent tumour or early relapse [29]. In addition, circulating EBV DNA can be considered a test for surveillance as its increase precedes by months the clinical signs of recurrence [29]. In contrast to NPC, the potential clinical application of circulating HPV DNA in OPC cancer has not been investigated. Only one study has evaluated circulating HPV DNA in HNSCC, using a combination of conventional PCR, Southern blot hybridization, and quantitative PCR (qPCR) [30]. In that study, Capone et al. [30] detected E6/E7 HPV DNA in 46% of 13 patients with HPV-16 positive cancer. Since then, no other study on the role of plasma HPV DNA in HNSCC has been published and data on the best performing technology for such an assay (very high sensitivity versus very high specificity) are completely lacking.
HPV DNA in Saliva
In the head and neck area, there is another body fluid that could be suitable for such studies: saliva. Preliminary data are encouraging for the utilisation of saliva (rinses) as a valid specimen to assess the presence of HR HPV, as well as of other prognostic markers. However, the sensitivity of the saliva test is low and the origin of the HPV+ cells can affect the specificity of the test. Indeed, these cells can originate from: HPV-positive tumour cells, any associated HR-HPV infection that led to the development of oropharyngeal cancer, or an independent HR HPV infection. In addition, their number and HPV positivity tend to correlate with cancer burden, with lower detection rates in minimal or early disease.
The development of a Pap test equivalent for the oral cavity has been already utilised but with unencouraging results, likely due to limitations in sampling the relevant tonsillar crypt epithelium, where persistent HPV infection can lead to ASCUS or dysplasia [31]. It is expected in the near future that HPV detection together with high-throughput technologies for saliva transcriptome will help to identify the profile of molecular changes in any particular HPV+/HPV− cancer. In this way, it will be possible to correlate the appropriate salivary transcriptome-based diagnosis to the patients’ specific cancer risk.
Conclusion
All of the aforementioned methods are able to give information about the presence of HR HPV in biological samples, either directly within the tumour or otherwise in the patient. Each test possesses its own strength and weakness and, at the present time, IHC staining for p16 and PCR for HPV appear to be the most sensitive markers of HPV, while ISH confers the greatest specificity. For most clinical laboratories, the combination of a sensitive test (e.g., p16 IHC) and a specific test (e.g., ISH) allows for the best potential to accurately establish the presence or absence of HPV in a given case of SCC. This information is crucial for the prognostic assessment of these patients but it is ineffective for the individuation of people at risk of tumour after HPV infection. Thus, there is a need for improving these HPV diagnostic tools by the detection not just of the virus, but also simultaneous detection of other biological markers like alterations in tumour suppressor gene pathways, modification of gene expression profiles, and microsatellite markers.
In summary, at the present time, many different HPV tests exist and much more information about their specific technical, analytical, and clinical properties are or will be available. Therefore, the choice for an HPV test has to be driven by practical considerations, as well as by the intention for its use. Epidemiological/screening studies require different methods from those needed for the detection of clinically relevant HPV infections. In particular, for the clinically relevant HPV infections it is of utmost importance to use a clinically validated HPV detection assay.
Acknowledgments
Work partially supported by a grant from the Italian Ministry of Health.
References
- 1.Syrjänen KJ, Pyrhönen S, Syrjänen SM, et al. Immunohistochemical demonstration of human papilloma virus (HPV) antigens in oral squamous cell lesions. Br J Oral Surg. 1983;21:147–153. doi: 10.1016/0007-117X(83)90060-4. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venuti A, Manni V, Morello R, et al. Physical state and expression of human papillomavirus in laryngeal carcinoma and surrounding normal mucosa. J Med Virol. 2000;60:396–402. doi: 10.1002/(SICI)1096-9071(200004)60:4<396::AID-JMV6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Yeudall WA, Campo MS. Human papillomavirus DNA in biopsies of oral tissues. J Gen Virol. 1991;72:173–176. doi: 10.1099/0022-1317-72-1-173. [DOI] [PubMed] [Google Scholar]
- 5.Nuovo GJ. In situ detection of human papillomavirus DNA after PCR-amplification. Methods Mol Biol. 2011;688:35–46. doi: 10.1007/978-1-60761-947-5_4. [DOI] [PubMed] [Google Scholar]
- 6.Agoston ES, Robinson SJ, Mehra KK, et al. Polymerase chain reaction detection of HPV in squamous carcinoma of the oropharynx. Am J Clin Pathol. 2010;134:36–41. doi: 10.1309/AJCP1AAWXE5JJCLZ. [DOI] [PubMed] [Google Scholar]
- 7.Snijders PJ, Heideman DA, Meijer CJ. Methods for HPV detection in exfoliated cell and tissue specimens. APMIS 2010;118:520–8 (Review). [DOI] [PubMed]
- 8.Gheit T, Landi S, Gemignani F, et al. Development of a sensitive and specific assay combining multiplex PCR and DNA microarray primer extension to detect high-risk mucosal human papillomavirus types. J Clin Microbiol. 2006;44:2025–2031. doi: 10.1128/JCM.02305-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remmerbach TW, Brinckmann UG, Hemprich A, et al. PCR detection of human papillomavirus of the mucosa: comparison between MY09/11 and GP5+/6+ primer sets. J Clin Virol. 2004;30:302–308. doi: 10.1016/j.jcv.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt M, Bravo IG, Snijders PJ, et al. Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol. 2006;44:504–512. doi: 10.1128/JCM.44.2.504-512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 12.Badaracco G, Venuti A, Morello R, et al. Human papillomavirus in head and neck carcinomas: prevalence, physical status and relationship with clinical/pathological parameters. Anticancer Res. 2000;20:1301–1305. [PubMed] [Google Scholar]
- 13.Badaracco G, Rizzo C, Mafera B, et al. Molecular analyses and prognostic relevance of HPV in head and neck tumours. Oncol Rep. 2007;17:931–939. [PubMed] [Google Scholar]
- 14.Mellin H, Dahlgren L, Munck-Wikland E, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102:152–158. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 15.Molden T, Kraus I, Skomedal H, et al. PreTect HPV-Proofer: real-time detection and typing of E6/E7 mRNA from carcinogenic human papillomaviruses. J Virol Methods. 2007;142:204–212. doi: 10.1016/j.jviromet.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ukpo OC, Flanagan JJ, Ma XJ, et al. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 18.Bishop JA, Maleki Z, Valsamakis A, et al. Application of the hybrid capture 2 assay to squamous cell carcinomas of the head and neck: a convenient liquid-phase approach for the reliable determination of human papillomavirus status. Cancer Cytopathol. 2011 Jul 12. Epub ahead of print]). [DOI] [PMC free article] [PubMed]
- 19.Lidqvist M, Nilsson O, Holmgren J, et al. Detection of human papillomavirus oncoprotein E7 in Liquid-based cytology using monoclonal antibodies. J Gen Virol. 2011; Oct 19 [Epub ahead of print]. [DOI] [PubMed]
- 20.Chen PC, Pan CC, Kuo C, et al. Risk of oral nonmalignant lesions associated with human papillomavirus infection, betel quid chewing, and cigarette smoking in Taiwan: an integrated molecular and epidemiologic study. Arch Pathol Lab Med. 2006;130:57–61. doi: 10.5858/2006-130-57-ROONLA. [DOI] [PubMed] [Google Scholar]
- 21.Lewis S, Jr, Thorstad WL, Chernock RD, et al. P16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thavaraj S, Stokes A, Guerra E, et al. Evaluation of human papillomavirus testing for squamous cell carcinoma of the tonsil in clinical practice. J Clin Pathol. 2011;64:308–312. doi: 10.1136/jcp.2010.088450. [DOI] [PubMed] [Google Scholar]
- 23.Harris SL, Thorne LB, Seaman WT, et al. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck. 2010; Dec 28 [Epub ahead of print]. [DOI] [PubMed]
- 24.Martinez I, Wang J, Hobson KF, et al. Identification of differentially expressed genes in HPV-positive and HPV-negative oropharyngeal squamous cell carcinomas. Eur J Cancer. 2007;43:415–432. doi: 10.1016/j.ejca.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 26.Anderson KS, Wong J, D’Souza G, et al. Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. Br J Cancer. 2011;104:1896–1905. doi: 10.1038/bjc.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonardo A, Marcante ML, Poggiali F, et al. HPV 16 E7 antibody levels in cervical cancer patients: before and after treatment. J Med Virol. 1998;54:192–195. doi: 10.1002/(SICI)1096-9071(199803)54:3<192::AID-JMV9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Rubenstein LM, Smith EM, Pawlita M, et al. Human papillomavirus serologic follow-up response and relationship to survival in head and neck cancer: a case-comparison study. Infect Agent Cancer. 2011;6:9. doi: 10.1186/1750-9378-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le QT, Jones CD, Yau TK, et al. A comparison study of different PCR assays in measuring circulating plasma Epstein-Barr virus DNA levels in patients with nasopharyngeal carcinoma. Clin Cancer Res. 2005;11:5700–5707. doi: 10.1158/1078-0432.CCR-05-0648. [DOI] [PubMed] [Google Scholar]
- 30.Capone RB, Pai SI, Koch WM, et al. Detection and quantitation of human papillomavirus (HPV) DNA in the sera of patients with HPVassociated head and neck squamous cell carcinoma. Clin Cancer Res. 2000;6:4171–4175. [PubMed] [Google Scholar]
- 31.Fakhry C, Rosenthal BT, Clark DP, et al. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “pap-test equivalent” in high-risk populations. Cancer Prev Res (Phila). 2011;4:1378–1384. doi: 10.1158/1940-6207.CAPR-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]