Abstract
Much recent attention has highlighted a subset of head and neck squamous cell carcinomas (HNSCCs) related to the human papillomavirus (HPV) that is characterized by an epidemiologic, demographic, and clinical profile that deviates from the profile of conventional non-HPV-related HNSCC. Lost in the dash to develop and implement diagnostic assays to detect the presence of HPV in HNSCCs is the unpretentious observation that these HPV-HNSCCs are also distinctive with respect to their microscopic appearance, and that an awareness of these characteristic morphologic features can facilitate the diagnosis of HPV-related HNSCC (HPV-HNSCC). This review will delineate the microscopic appearance of HPV-HNSCC, spotlight ways in which the misinterpretation of these microscopic features can lead to diagnostic confusion, and provide recommendations for appropriate terminology when diagnosing HPV-HNSCC.
Keywords: Human papillomavirus, Head and neck cancer, Basaloid, Tumor grade, Tonsils, Oropharynx, Reticulated epithelium, Non-keratinizing squamous cell carcinoma
Introduction
Squamous cell carcinoma of the head and neck (HNSCC) has long been regarded as a monotonous disease entity. Important distinctions between anatomic subsites and natural histories have largely been ignored given the uniformity of histopathology and response to treatment. Recent work suggests considerable differences between some HNSCCs that go beyond variations related to tumor subsite and stage. In particular, a subset of HNSCC is now known to be associated with the human papillomavirus (HPV) [1, 2]. These HPV-related HNSCCs (HPV-HNSCCs) tend to occur more frequently in younger, male patients; tobacco smoking does not appear to be a strong cofactor in the development of these tumors; they most frequently occur in the oropharynx; and they are associated with improved clinical outcomes [3, 4]. In effect, recognition of this HPV association amounts to the identification of a new and distinct disease entity.
Given the importance of recognizing this subset of HNSCCs, investigators continue to debate the method of choice for HPV detection. Sometimes lost in this wrangling is the observation that these HPV-HNSCCs have a distinct and consistent morphologic appearance such that categorization by HPV status can actually begin at the microscope. Admittedly, not every HPV-HNSCC rigidly conforms to any one description, and indeed some HPV-HNSCCs deviate from the prototype in sizable and reliable ways. While others have addressed these HPV variants in another section of this special edition of the journal, this review will detail the microscopic appearance of the more prototypic HPV-HNSCC. An awareness of this appearance can alert the pathologist to the presence of HPV; inform the interpretation of various histologic parameters as they relate to tumor grade and the presence of invasion; enlighten communication with the treating physician; and guide the interpretation of ancillary HPV tests.
The Microanatomy of the Lingual and Palatine Tonsils
Numerous studies have documented a strong predilection of HPV-HNSCC for the oropharynx, specifically the lingual and palatine tonsils [5]. This preferential targeting likely reflects complex biological interactions between HPV and the highly specialized lymphoepithelium lining the tonsillar crypts known as the reticulated epithelium. Although much remains to be learned about the biology of HPV-related tumorigenesis, the reticulated epithelium may provide an immune-privileged site which can inhibit virus-specific T cells and thereby facilitate immune evasion at the time of initial HPV infection and subsequent virus-induced malignant transformation (unpublished observations).
The microscopic features of the reticulated epithelium lining the tonsillar crypts are unique. As the stratified squamous epithelium covering the surface of the tonsils extends into the recesses of the tonsillar crypts, a dense lymphoid infiltrate obscures the junction between the lymphoid and epithelial components and splinters the epithelial sheath into irregular nests and cords. These nests and cords no longer exhibit the polarization and surface maturation that characterize the stratified squamous epithelium lining the surface of the tonsils. At the cytologic level, the epithelial cells take on a basaloid appearance (i.e. high nuclear-to-cytoplasmic ratio, absence of cytoplasmic keratinization) with vesicular nuclei and loss of distinct cytoplasmic borders and intercellular bridges (Fig. 1).
Fig. 1.
The crypts of the tonsils are lined by a specialized squamous epithelium known as the reticulated epithelium. The epithelium is penetrated by numerous interdigitating lymphocytes that obscure the junction between the epithelium and lymphoid stroma, and fragment the epithelium into cords of basaloid cells
The Microscopic Appearance of HPV-HNSCC
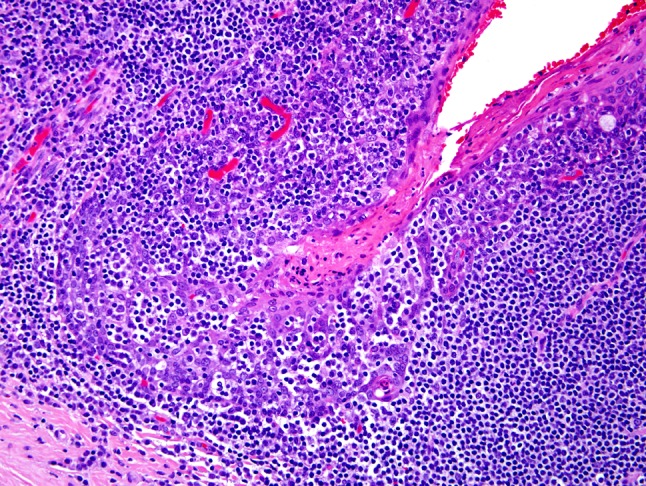
The histologic features of the reticulated epithelium are retained, to varying degrees, in HPV-HNSCC. HPV-HNSCCs consistently arise from tonsillar crypts. Involvement of the tonsillar surface, when it occurs, is generally a secondary phenomenon reflecting colonization of the surface epithelium as the carcinomas spill over from the tonsillar crypts (Fig. 2). This transition between HPV-HNSCCs and the adjacent surface epithelium tends to be abrupt without transitional zones of epithelial precursor lesions. Indeed, the histologic progression through the sequential stages of dysplasia culminating in carcinoma in situ and invasive growth that characterize non-HPV-HNSCCs is not generally evident for HPV-HNSCCs. As these carcinomas infiltrate, they tend to invade as sheets, lobules, or ribbons of cells (Fig. 3). Central necrosis within expanding tumor lobules, sometimes giving rise to cystic degeneration, is a frequent finding. Invasive growth often does not elicit a strong desmoplastic stromal reaction. Instead, the tumor nests are often surrounded by a zone of lymphoid cells. The degree to which these lymphoid cells permeate the tumor lobules as tumor infiltrating lymphocytes (TILS) is highly variable. When the TILS are numerous and disrupt the lobules into cords and individual cells, the HPV-HNSCC can take on a “lymphoepithelial” appearance [6] (Fig. 4). At the cytologic level, the tumor cells display a high nuclear-to-cytoplasmic ratio, syncytial cytoplasm without intercellular bridges, and lack significant cytoplasmic keratinization. These cellular features can impart a distinct basaloid appearance (Fig. 3b).
Fig. 2.
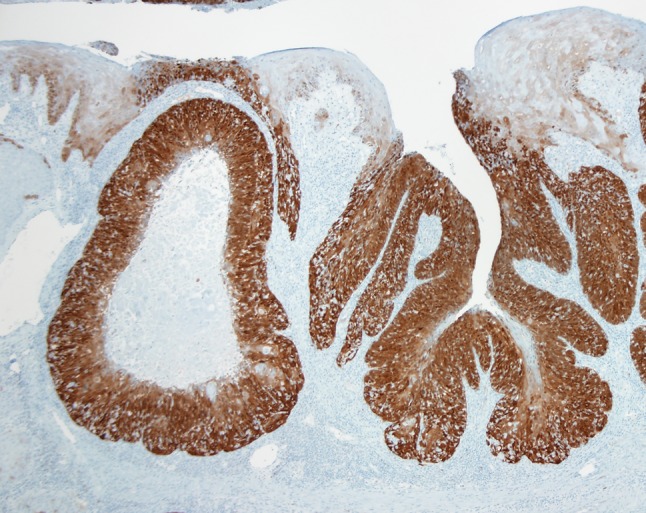
A p16 immunohistochemical stain is used to highlight tumor distribution in the tonsil. HPV-HNSCC (as indicated by strong p16 immunohistochemical staining) tends to target the tonsillar crypts. When the surface epithelium is involved, it is usually by contiguous extension from the tonsillar crypt
Fig. 3.
This HPV-HNSCC infiltrates the lymphoid stroma of the tonsil as expanding tumor lobules with central necrosis. The surface epithelium lacks the dysplastic changes that typify most non-HPV-HNSCCs (a). The absence of keratinization, cell borders, and abundant cytoplasm (i.e. high nuclear-to-cytoplasmic ratio) often imparts a “basaloid” appearance (b)
Fig. 4.
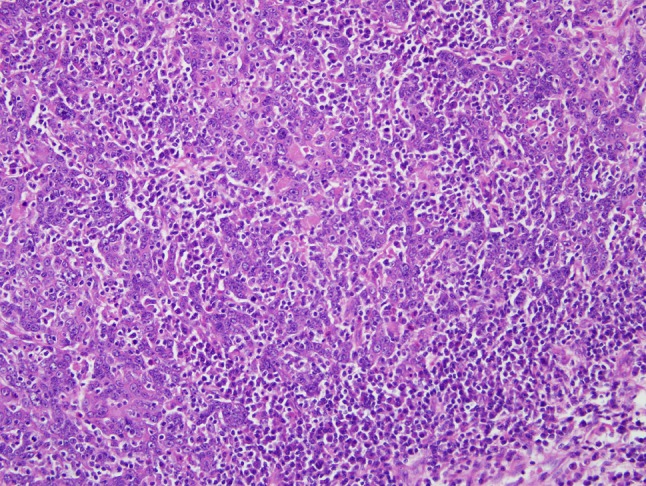
In HPV-HNSCC, the tumor nests are often permeated by lymphocytes. In some cases, a very brisk lymphoplasmacytic infiltrate will disrupt the tumor nests into irregular cords and individual cells in a lymphoepithelial-like fashion
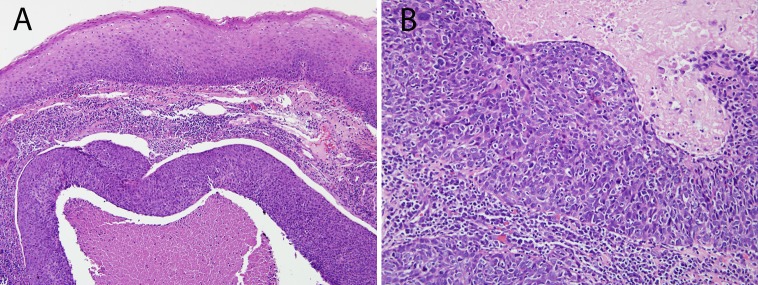
In lymph node metastases, the presence of cystic degeneration is a common finding that sometimes appears to recapitulate the formation of tonsillar crypts (Fig. 5). Its presence should warrant strong consideration of an HPV-related metastasis from the tonsil. These squamous lined cysts of the lateral neck are sometimes clinically and histologically mistaken for branchial cleft cysts.
Fig. 5.
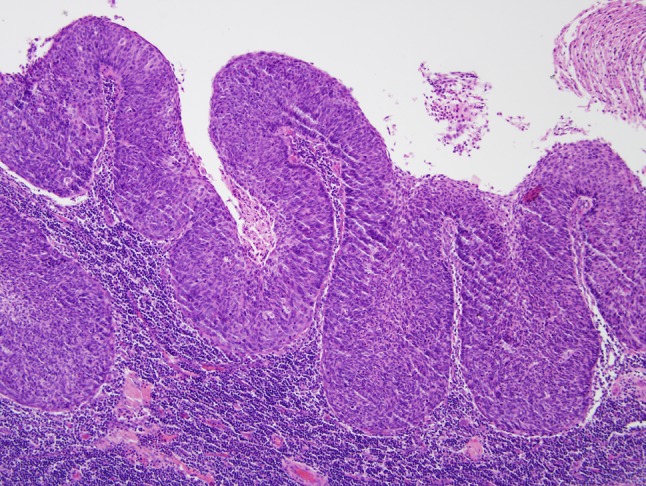
When dealing with metastatic HNSCC to a cervical lymph node, the presence of cystic change and a non-keratinizing morphology should raise the possibility of origin from the base of tongue or palatine tonsil
The morphology of HPV-HNSCC may simply reflect origin from the tonsillar crypts, but several observations suggest that these features may represent an HPV-associated phenomenon. This basaloid phenotype has been noted in HPV-related cancers arising in non-oropharyngeal sites such as the vulva, penis, and anus. As for the head and neck, El-Mofty noted that HPV-positivity is more common in non-keratinizing than in keratinizing forms of squamous cell carcinoma involving the sinonasal tract [7]. The morphology of HPV-HNSCCs arising outside of the oropharynx and nasopharynx, however, remains to be fully characterized.
Some of the same microscopic features that typify HPV-HNSCC also contribute to certain diagnostic ambiguities. Areas that are particularly prone to diagnostic uncertainty include the distinction between carcinoma in situ and invasive carcinoma, the assignment of tumor grade, and the separation of HPV-HNSCC from the basaloid variant of HNSCC.
Carcinoma In Situ Versus Invasive Carcinoma
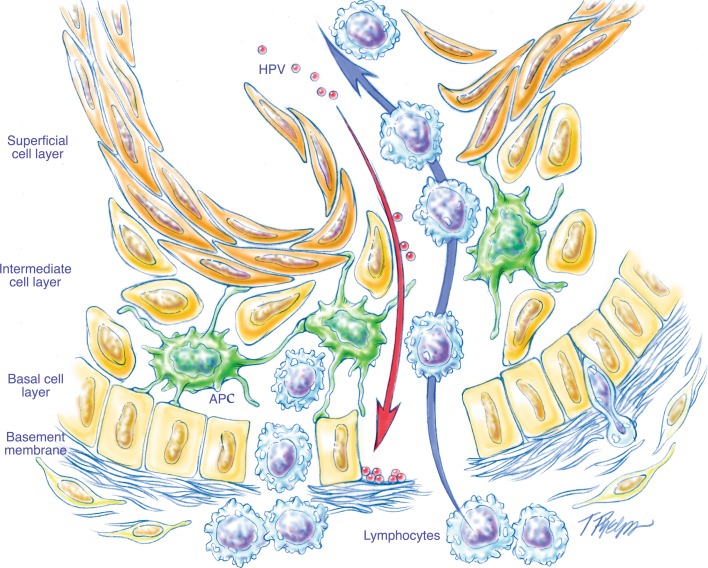
For squamous neoplasia of the head and neck, invasive tumor growth is microscopically recognized as rupture of the basement membrane, irregular extensions of tumor cells into the stroma underlying the surface epithelium, and subsequent incitement of a desmoplastic stromal reaction (e.g. fibrosis, edema, and chronic inflammation). For many of the HPV-HNSCCs, the difficulty in distinguishing carcinoma in situ from invasive carcinoma is heightened by: (1) origin from the tonsillar crypts beneath the surface epithelium; (2) the oft absent tell-tale sign of invasion—stromal desmoplasia; (3) the well-recognized propensity of small, sometimes occult, tonsillar carcinomas to metastasize to regional nodes in the absence of clear-cut stromal invasion (Fig. 6); (4) the blurred junction of the reticulated epithelium and the underlying lymphoid stroma; and (5) the porous nature of this epithelial/lymphoid junction where the basal cell layer is incomplete and its supporting basement membrane is disrupted and non-contiguous (Fig. 7) [8]. In effect, the time-honored microscopic approach to the recognition of tumor invasion may not be valid for those HPV-HNSCCs arising from the tonsillar crypts. Until the histologic progression of HPV-related neoplasia of the tonsils is better characterized and the critical transition marking infiltrative growth is more clearly-demarcated, an aggressive approach that regards all HPV-related neoplasia of the tonsils as potentially malignant, even in the absence of those histologic features that have been traditionally used to diagnose invasion, may be warranted.
Fig. 6.
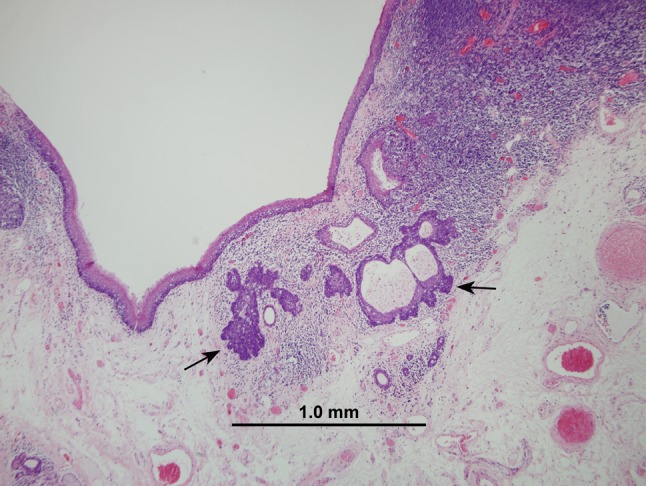
This small focus of HPV-HNSCC was detected in the tonsil of a patient who presented with bulky lymph node metastases. Some HPV-HNSCCs appear to possess the capacity to metastasize even when small in size and seemingly confined to a tonsillar crypt
Fig. 7.
Schematic representation of the reticulated epithelium lining the tonsillar crypts. A porous basement membrane facilitates the migration of lymphocytes and antigen presenting cells (APCs). This lack of structural integrity of the basement membrane confounds the distinction between in situ and invasive disease (figure borrowed from [8])
Histologic Grading of HPV-HNSCC
Tumor grade is a semi-quantitative measurement of differentiation expressed as the degree to which a tumor resembles the normal tissue from which it arises. As a rule of thumb, the more poorly-differentiated a tumor is, the more aggressive its behavior. HPV-HNSCCs are widely perceived as poorly or undifferentiated carcinomas. This perception is largely based on the immature appearance of a tumor cell that widely diverges from the stratified squamous epithelium that lines the surface of the tonsil. Using the surface epithelium as a point of reference for phenotypic divergence, however, may not be appropriate for those HPV-HNSCCs arising from the tonsillar crypts. HPV-HNSCCs often retain the appearance of the reticulated epithelium from which they arise, and thus might best be regarded as highly differentiated tumors based on this more suitable comparison (Fig. 8). Recognition of HPV-HNSCC as well-differentiated, rather than poorly or undifferentiated, is appropriate as it more accurately reflects histogenic derivation and more fittingly associates tumor grade with expected clinical behavior.
Fig. 8.
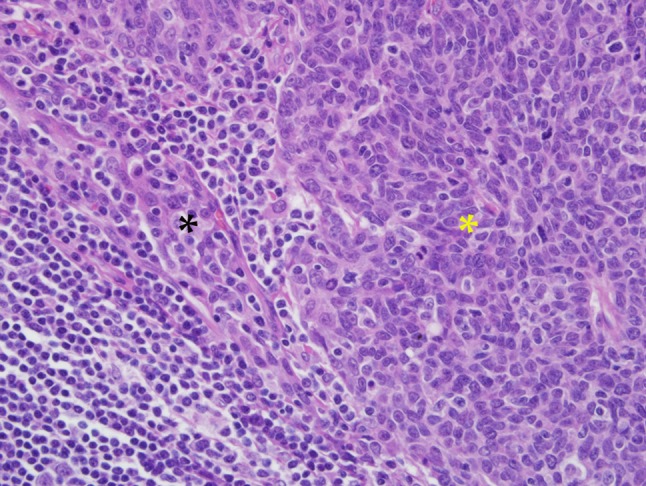
This HPV-HNSCC (yellow asterisk) maintains a close morphologic resemblance to reticulated crypt epithelium (black asterisk) from which it takes origin
Although certain histopathologic features may be helpful in predicting outcomes for patients with oropharyngeal squamous cell carcinoma, the presence of these features (e.g. degree of keratinization) so consistently track with HPV status that their role as prognostic indicators independent of HPV status is probably very limited [9]. Few studies have tried to identify histopathologic parameters that might help identify the subgroup of HPV-HNSCC associated with unfavorable clinical outcomes. Scantlebury et al. [10] made a preliminary observation that the presence of anaplasia and tumor cell multinucleation (Fig. 9) are predictive of poorer clinical outcomes in patients with non-keratinizing p16 positive oropharyngeal carcinomas, but this initial observation has yet to be confirmed by others.
Fig. 9.
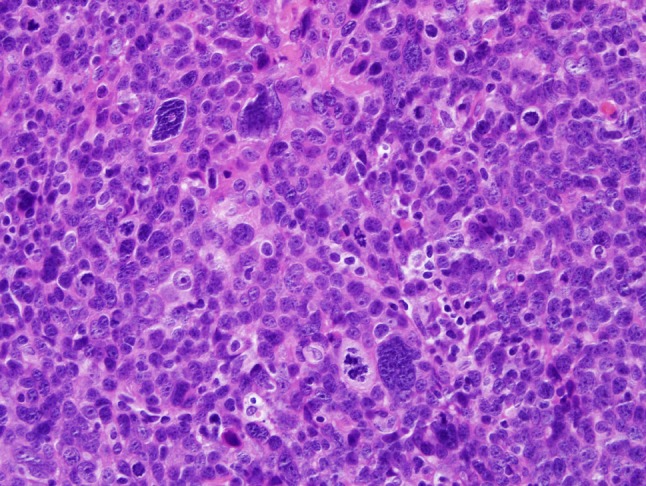
Occasionally, large pleomorphic cells may be encountered in an HPV-HNSCC. The significance of these is not known with certainty, but they may be associated with more aggressive tumor behavior
Separation of HPV-HNSCC from the Basaloid Variant of HNSCC
Basaloid squamous cell carcinoma (BSCC) of the head and neck is set apart as a distinct subtype of squamous cell carcinoma based on its striking basaloid morphology and its aggressive behavior. Its morphologic distinction from HPV-HNSCC can be difficult as both tumors are characterized by the lobular growth of basaloid cells (Fig. 10). Morphologic similarities aside, HPV-HNSCCs do not share the same aggressive clinical behavior that characterizes the basaloid variant of squamous cell carcinoma. When basaloid HNSCCs are separated on the basis of HPV status, HPV-positivity is a favorable prognostic indicator that is useful in identifying those basaloid tumors that depart from the highly aggressive behavior associated with the BSCC variant. In their evaluation of basaloid HNSCCs, Begum et al. found that the presence of HPV was significantly associated with improved overall survival even though patients with HPV-HNSCCs were more likely to present with lymph nodes metastases [11]. In effect, the detection of HPV permits resolution of a less aggressive component within a high grade subtype of head and neck carcinoma.
Fig. 10.
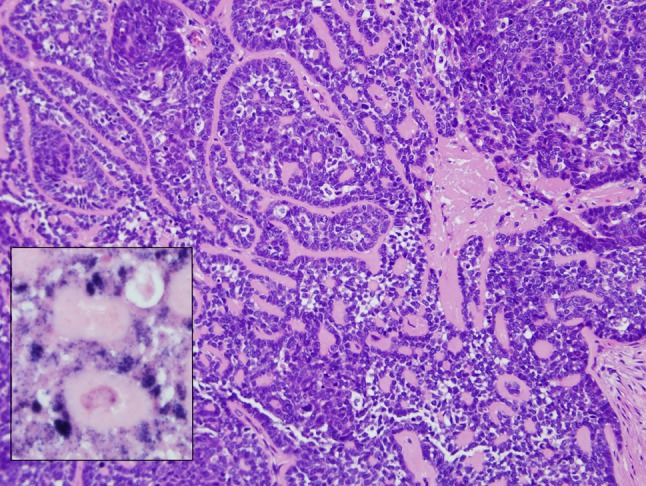
HPV-HNSCC with prominent basaloid features including lobules of hyperchromatic cells separated by hyaline stromal deposits. This tumor can only be separated from the basaloid variant of HNSCC by detection of HPV. In this case, HPV in situ hybridization demonstrates the presence of abundant hybridization signals in the nuclei of the tumor cells (inset)
HPV and the Small Cell Phenotype
The favorable prognosis conferred by HPV positivity consistently trumps traditional prognostic features predicated on morphology, such as tumor grade and histologic subtype. As noted above, the detection of HPV is a favorable prognostic feature even in those variants of HNSCC that traditionally have been regarded as a highly aggressive (e.g. basaloid variant). However, the small cell variant is a most notable exception. Bishop et al. recently described a series of HPV-related oropharyngeal carcinomas with well-developed features of small cell carcinoma including small anaplastic cells with hyperchromatic nuclei, scant cytoplasm, and immunohistochemical evidence of neuroendocrine differentiation [12]. Most of these cases arose in close association with a more conventional HPV-HNSCC, and the presence of HPV was detected in both the small cell and squamous cell components.
Importantly, the presence of HPV may not convey a favorable prognosis when it comes to this small cell phenotype [12]. HPV-related small cell carcinoma of the oropharynx appears to share the same aggressive clinical features of its counterpart in the uterine cervix and lung where the small cell phenotype is associated with early distant spread and poor overall survival. Consequently, the small cell phenotype should be regarded as a poorly/undifferentiated form of HPV-related oropharyngeal carcinoma where tumor morphology supersedes HPV positivity as a prognostic indicator.
Diagnostic Terminology in the Reporting of HPV-HNSCC
Given the distinctiveness of HPV-HNSCC as a biological and clinical subtype of HNSCC, the reporting of these cancers must make careful use of diagnostic terminology that asserts their relationship with HPV, and yet does not confound the treating clinician [13]. This task is not necessarily straightforward given that the microscopic makeup of these cancers, namely, the absence of cytoplasmic maturation together with the basaloid appearance of the tumor cells, may easily lead to miscommunication regarding expected tumor behavior. The current and widespread practice of assigning these tumors with an advanced histologic grade (e.g. poorly differentiated or undifferentiated) is not only fallacious but inappropriately infers an aggressive clinical course. Similarly, the common use of the term “basaloid” as a diagnostic descriptor, though morphologically correct, invites an erroneous connection with the basaloid variant of squamous cell carcinoma—the subtype of HNSCC notorious for aggressive clinical behavior. Until consensus panels put forward a classification scheme for oropharyngeal carcinomas that underscores their relationship with HPV while avoiding confusion with the aggressive basaloid variant, it is our practice to: (1) suspend the use of inapt descriptors such as “poorly differentiated” and “basaloid”; (2) replace the conventional grading scheme for HNSCC of the oropharynx with a scheme that is in current use for the nasopharynx (e.g. keratinizing and non-keratinizing types); and (3) routinely report on the HPV status of all HNSCCs arising in the oropharynx [14]. For oropharyngeal carcinomas that are found to be HPV positive, we prefer the term “HPV-related squamous cell carcinoma” for its simplicity, clarity, and directness.
The Influence of HPV Morphology on HPV Testing
Awareness of the morphologic profile of HPV-HNSCC can help direct the initiation and interpretation of HPV detection assays. The morphology of HPV-related oropharyngeal carcinoma is consistently retained when these tumors metastasize to regional and distant sites. When encountered in cervical lymph node metastases, particularly when accompanied by cystic change, these features reliably point to the oropharynx as the site of tumor origin—a very helpful insight when dealing with that important group of patients who present with lymph node metastases of unknown origin [15, 16]. Similarly, for the squamous cell carcinoma in the lung of a patient with a prior HPV-related oropharyngeal carcinoma, the non-keratinized basaloid morphology suggests a lung metastasis over a new lung primary [17, 18]. In both of these diagnostic scenarios, an appreciation for the morphologic features of HPV-HNSCC should prompt definitive HPV testing.
HPV morphology may also facilitate the interpretation of HPV testing in those instances where there is a disparity between the morphologic findings and the test results. A high level of p16 expression is commonly used as indirect evidence of HPV infection, but its value as a surrogate marker is limited by suboptimal specificity. P16 staining of an HNSCC that lacks HPV morphology should prompt consideration of additional testing for more direct evidence of HPV [19]. HPV in situ hybridization, on the other hand, is sometimes disparaged for its lack of sensitivity. Absence of hybridization signals in an oropharyngeal carcinoma exhibiting classic HPV-related changes should prompt consideration of repeat testing, the use of a broader spectrum of HPV hybridization probes, or the employment of some other detection strategy.
Summary Points
Most of the HPV-related carcinomas of the head and neck target the oropharynx, particularly the specialized reticulated epithelium lining the tonsillar crypts. Accordingly, HPV-HNSCCs often morphologically resemble this unique epithelium.
Like the reticulated epithelium, these HPV-HNSCCs tend to demonstrate a non-keratinizing or only partially keratinizing morphology. Secondary morphologic features may include the presence of central necrosis with cystic change, tumor infiltrating lymphocytes, and basaloid features; notably absent are surface dysplasia and prominent stromal desmoplasia.
In an oropharyngeal carcinoma, the presence of HPV generally is a favorable prognostic feature that supersedes the basaloid phenotype.
In an oropharyngeal carcinoma, the presence of the small cell phenotype is an unfavorable prognostic feature that supersedes HPV positivity.
When diagnosing an HPV-positive oropharyngeal carcinoma, one should consider suspending the use of inapt labels such as “poorly differentiated”, “basaloid” and other descriptors that would imply an aggressive tumor type. We prefer the term “HPV-related squamous cell carcinoma” for its simplicity, clarity and directness.
A strong appreciation for the morphologic appearance of HPV-HNSCC can help direct the initiation of HPV testing (e.g. clarify the site of tumor origin in patients who develop lymph node or lung metastases) and guide the interpretation of HPV detection assays (e.g. p16 immunohistochemical staining).
References
- 1.Gillison ML, Koch WM, Spafford M, et al. Causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, D.C. Head Neck. 2009;31:1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 5.Begum S, Cao D, Gillison ML, et al. Tissue distribution of HPV 16 DNA integration in patients with tonsillar carcinoma as visualized by HPV 16 in situ hybridization and p16 immunohistochemistry. Clin Caner Res. 2005;11:5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 6.Singhi AD, Stelow EB, Mills SE, Westra WH. Lymphoepithelial-like carcinoma of the oropharynx: a morphologic variant of HPV-related head and neck carcinoma. Am J Surg Pathol. 2010;34:800–805. doi: 10.1097/PAS.0b013e3181d9ba21. [DOI] [PubMed] [Google Scholar]
- 7.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 8.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis and treatment of patients with head and neck squamous cell carcinoma. Annual Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chernock RD, El-Mofty SK, Thorstad WL, et al. HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3:186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scantlebury JB, Zhang Q, Thorstad WL, et al. Anaplastic nuclei and tumor cell multinucleation are strong predictors of poorer survival in nonkeratinizing oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;282–3A.
- 11.Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32:1044–1050. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 12.Bishop JA, Westra WH. Human papillomas virus-related small cell carcinoma of the oropharynx. Am J Surg Pathol. 2011;35:1679–1684. doi: 10.1097/PAS.0b013e3182299cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenig BM, Barnes L, Carlson DL et al. Protocol for the examination of specimens from patients with carcinomas of the pharynx. Based on AJCC/UICCTNM. 7th ed. College of American Pathologists. Available from URL: http://www.cap.org.
- 15.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;15:6469–6475. [PubMed] [Google Scholar]
- 16.Goldenberg D, Khan Z, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-related phenomenon. Head Neck. 2008;39:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 17.Bishop JA, Illei PB, Gabrielson E, Westra WH. HPV analysis in distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2012;36:142–148. doi: 10.1097/PAS.0b013e3182395c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weichert W, Schewe C, Denkert C, et al. Molecular HPV typing as a diagnostic tool to discriminate primary from metastatic squamous cell carcinoma of the lung. Am J Surg Pathol. 2009;33:513–520. doi: 10.1097/PAS.0b013e3181938319. [DOI] [PubMed] [Google Scholar]
- 19.El Naggar A, Westra WH. Immunohistochemical detection P16 expression as a surrogate marker for HPV related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2011. doi:10.1002/hed.21974. [DOI] [PubMed]