Abstract
Human papillomavirus (HPV) testing is now recommended as part of the work up for patients with oropharyngeal squamous cell carcinoma (OPSCC) and those patients with cervical lymph node metastasis of unknown origin. The laboratory testing strategy should accurately assess the presence or absence of oncogenic HPV infection in routinely collected tumour samples that are subject to standard fixation protocols, alcohol-fixed cytological preparations and formalin-fixed tissue samples. The HPV status should correlate with biologically relevant outcome measures such as overall, disease-specific and disease-free survival. Whilst increased expression of p16 by immunohistochemistry is considered to be a surrogate marker of oncogenic HPV infection and is a validated independent prognostic biomarker, only HPV specific tests provide definitive evidence of the aetiological agent. We provide an overview of HPV testing in OPSCC, justifying the use of HPV specific tests. We examine the analytical accuracy of HPV specific tests against the ‘reference’ test—high risk HPV mRNA in fresh tissue—and contrast this with the performance of p16 immunohistochemistry as a stand alone test. We highlight the added value of HPV specific tests in prognostication, clinical trial design, and population-based disease surveillance. We consider that HPV specific testing is the starting point for developing increasingly informative biomarker panels in the context of ‘stratified medicine’. We briefly frame test information in the context of disclosure of HPV status to patients. We conclude that only a testing strategy that includes HPV specific tests can deliver more effective care for patients with OPSCC. The international head and neck oncology community should work together to clearly define the minimum requirements for assigning a diagnosis of HPV-related OPSCC in order to ensure consistent reporting of this emerging and increasingly prevalent disease.
Keywords: Oropharynx, Squamous cell carcinoma, Human papillomavirus, Molecular diagnosis
Introduction
The head and neck oncology community recognises the utility of human papillomavirus (HPV) testing in routine clinical practice [1]. Evidence-based clinical management guideline documents recommend HPV testing for head and neck squamous cell carcinomas; specifically, for those arising in the oropharynx and metastatic squamous cell carcinoma of unknown origin (National Comprehensive Cancer Network, USA; College of American Pathologists; ENT UK; Royal College of Pathologists, UK). Significantly, the guidelines are not prescriptive about the laboratory tests required to establish HPV status since an ‘international standard’ for HPV testing in head and neck cancer is yet to be defined [2].
Analytical Performance of HPV Tests
Sustained and persistent high-risk HPV E6/E7 viral oncogene expression is essential for the initiation and maintenance of an HPV-driven malignant phenotype [1]. Detection of high-risk HPV E6/E7 mRNA transcripts in oropharyngeal squamous cell carcinoma (OPSCC) correlates with cellular genotoxic damage and gene expression changes that drive the hallmarks of cancer [3]. Consequently, the theoretical analytical ‘reference’ or ‘gold standard’ test for oncogenic HPV infection is the demonstration of transcriptionally active high-risk HPV in fresh tissue; usually by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) amplifying high-risk HPV E6/E7 mRNA transcripts [4, 5]. Whilst this test has not been widely used to evaluate OPSCC, it can be justified as the most appropriate ‘reference’ test because fresh tumour samples contain intact mRNA molecules that can be amplified with high fidelity, in a controlled fashion, against a constitutively expressed endogenous gene, thus allowing precise quantitative assessment of transcript abundance. Quality control can be enhanced by using parallel negative and positive controls, including samples with known HPV genotype (e.g. SiHa, HeLa, CaSki cells) that can be used to set the detection threshold. As with any laboratory analysis, there are caveats that relate to issues of controlling for the amount of starting material (tissue weight, cell number, total amount of RNA/DNA), methodological protocols (primer constructs, amplification conditions, selection of fluorescent reporter systems) and threshold setting. Whilst qRT-PCR for high-risk HPV E6/E7 transcripts is achievable in research laboratories, its utility in clinical practice is limited, not least by the sub-optimal preservation of routine biopsy samples that contain degraded RNA molecules. Therefore, in clinical practice, an HPV testing strategy needs to be effective on alcohol preserved tissue fragments and disaggregated cells derived from fine needle aspiration biopsy material and formalin-fixed paraffin-embedded (FFPE) tissue samples. Notwithstanding the requirement for analytical accuracy against a ‘reference’ assay, the HPV test or combination of tests should have clinical relevance; for OPSCC, the HPV status should correlate with clinical parameters such as overall, disease-specific and disease-free survival [1].
There is a paucity of data on the analytical performance of HPV tests against the ‘reference’ test in head and neck cancers. A seminal piece of work by Smeets et al. [6] examined the ability of a portfolio of HPV tests to classify FFPE samples against matched fresh samples characterised by RT-PCR for HPV16 E6/E7 mRNA. This work demonstrated that an RT-PCR assay for HPV16 E6*I mRNA developed specifically for FFPE material was the only test that accurately classified the samples into the correct HPV positive and negative groups; the test had 100% sensitivity and specificity. Other assays, namely PCR for HPV DNA using consensus primers (GP5+/GP6+), HPV16/18 fluorescent in situ hybridisation (ISH) and p16 immunohistochemistry (IHC) had sub-optimal performance when used as stand alone tests (Table 1). The authors acknowledge that one of the factors limiting the clinical utility of their ‘best performing’ test is the technical challenge of retrieving amplifiable mRNA from FFPE material. However, they went on to show that a combination of p16 IHC and GP5+/GP6+ PCR for HPV DNA also provided optimal analytical sensitivity and specificity in their test set and highlighted the potential of this two-tiered approach for translation into clinical diagnostics [6]. More recently, Schache et al. [7] corroborated these findings using a similar comparative testing strategy against the ‘reference’ test; the study demonstrated the limitations of using single tests to accurately classify HPV status, but raised the possibility that a combination of tests on FFPE material can provide a classification approaching the analytical ‘gold standard’ (Table 1).
Table 1.
Performance of HPV tests on formalin-fixed paraffin-embedded biopsy material against the ‘reference’ test
| HPV test | Smeets et al. [6] | Schache et al. [7] | Schlecht et al. [12] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Sensitivity (%) | Specificity (%) | No. | Sensitivity | Specificity | No. | Sensitivity | Specificity | |
| RNA RT-PCR | 48 | 100 | 100 | ND | ND | ND | ND | ND | ND |
| DNA qPCR | 47 | 92 | 97 | ND | ND | ND | ND | ND | ND |
| HPV PCR | 48 | 100 | 89 | ND | ND | ND | ND | ND | ND |
| HPV ISHa | 45 | 83 | 100 | 97 | 88% | 88% | 73 | 67% | 61% |
| p16 IHCb | 44 | 100 | 79 | 97 | 94% | 82% | 80 | 56% | 93% |
| p16 IHC and HPV ISH | 42 | 92 | 100 | 97 | 88% | 90% | ND | ND | ND |
| p16 IHC and HPV PCR | 44 | 100 | 100 | ND | ND | ND | ND | ND | ND |
All studies used RT-PCR on frozen tissue as the ‘reference’ test. HPV16 E6/E7 mRNA [6]. HPV16, 18, 33 E6 mRNA [7]. HPV16 E6 mRNA [12]
aFluorescent HPV16/18 ISH [6]. Chromogenic high-risk HPV ISH (Ventana Medical Systems, USA) [7, 12]
bCINtec Histology (Roche mtm laboratories AG, Germany) [6, 7]. Pharmingen p16 IHC (BD Biosciences, USA) [12]
p16 Immunohistochemistry as a Stand Alone ‘HPV Test’
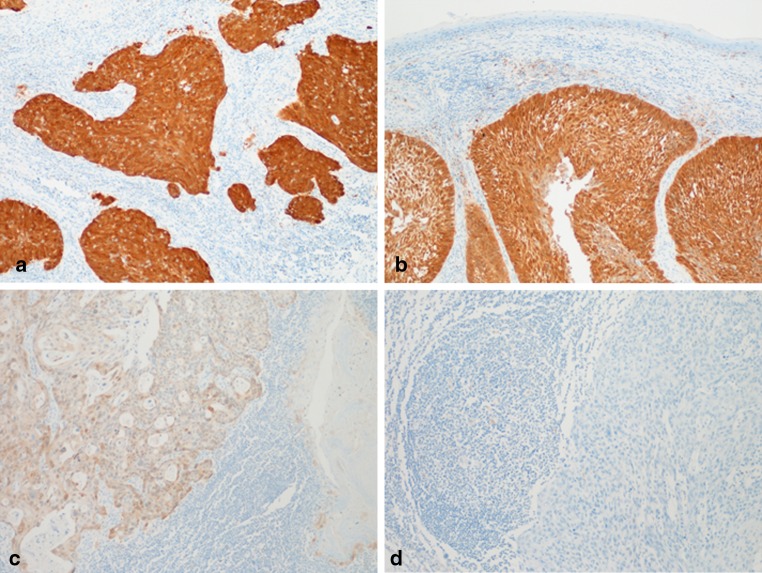
The two studies described above [6, 7] both highlight the sub-optimal analytical performance of p16 IHC as a surrogate marker for oncogenic HPV infection. When used in isolation, increased p16 expression is highly sensitive (94–100%), but lacks specificity (79–82%). Whilst the ‘reference’ test of transcriptionally active HPV is generally accepted only when performed on well preserved fresh frozen material, two groups have successfully used qRT-PCR to amplify HPV16 E6/E7 mRNA transcripts from FFPE samples [8, 9]. Notwithstanding the methodological problems of extracting satisfactory mRNA from FFPE material, both studies concur that p16 IHC lacks specificity (84–91%) for oncogenic HPV infection [8, 9]. These data suggest that overexpression of p16, without information from HPV specific tests, lacks analytical accuracy for oncogenic HPV infection. Furthermore, empirical and subjective thresholds for determining p16 positivity limit its use as a stand alone biomarker for HPV infection. For example, Smeets et al. [6] broadly defined p16 positivity as any amount of staining regardless of the staining intensity or percentage of tumour cells labelled. By contrast, Schache et al. [7] defined p16 positivity as strong and diffuse nuclear and cytoplasmic staining in greater than 70% of the tumour; in line with much of the work emanating from the USA [10]. Review of cases from the latter cohort [7] identified three false negative cases (qRT-PCR positive/p16 IHC negative). Whilst two of the false negative cases were completely negative, the third case showed weak p16 staining below the pre-determined threshold for the study (Fig. 1). Comparison of the staining pattern of this case with the photomicrographs published by Smeets et al. [6] suggests that they would have most likely scored this case as p16 positive (Fig. 1c). Therefore, whilst the majority of OPSCCs are categorically positive or negative for p16 by immunohistochemistry, equivocal staining patterns are occasionally (less than 5%) encountered in clinical practice, which confounds interpretation (Figs. 1, 2) [11]. This information calls into question the validity of empirically defined cut-offs for HPV-driven p16 overexpression.
Fig. 1.
Increased expression of p16 by immunohistochemistry does not correlate precisely with the presence of high-risk HPV E6/E7 mRNA by qRT-PCR. a and b High-risk HPV E6/E7 mRNA negative. c and d High-risk HPV E6/E7 mRNA positive [7]
Fig. 2.
Although the majority of OPSCCs stained using p16 immunohistochemistry are either categorically positive (see Fig. 1a, b) or negative (see Fig. 1d), equivocal staining patterns are occasionally encountered in clinical practice: a–d Show equivocal p16 staining, highlighting the problem of empirically defined cut-offs for HPV-driven p16 overexpression
Another variable in the assessment of p16 levels is the choice of reagents and immunohistochemistry protocol. Whereas the studies outlined above [6–10] employed the widely used proprietary p16 testing kit from mtm laboratories (CINtec Histology, Roche mtm laboratories AG, Germany), Schlecht et al. [12] used a p16 monoclonal antibody from Pharmingen (BD Biosciences, San Diego, USA). The antibody had a different performance profile to the CINtec Histology kit; when compared to HPV16 E6/E7 mRNA extracted from matched frozen samples it lacked sensitivity (56%), but had a specificity of 93% (Table 1) [12].
There is further information to indicate that p16 expression and high-risk HPV infection are not synonymous. It is well recognised that p16 is expressed by keratinocytes of reticulated epithelium lining the crypts of the oropharyngeal mucosa in the absence of HPV infection. The staining pattern is typically weak and patchy, but incorrect interpretation of this feature could lead to the conclusion that the crypt epithelium shows ‘molecular’ evidence of HPV-driven precancer. In addition, Begum et al. [13] demonstrated p16 expression at non-oropharyngeal sites (9 of 131; 7%) in the absence of HPV DNA by ISH. More recently, Harris et al. [14] detected p16 in a small cohort of young patients with oral tongue SCC (11/25; 44%) that was independent of HPV infection. Whilst the findings of these studies may be confounded by the use of different p16 antibody clones and immunohistochemistry protocols, they support the contention that p16 IHC lacks sufficient accuracy as a surrogate marker of oncogenic HPV infection. They also raise the possibility that in some circumstances elevated p16 expression occurs independently of HPV infection.
Utility of HPV Tests in Prognostication and Clinical Trials
Analytical performance of HPV tests against a laboratory standard should reflect clinical parameters such as overall, disease-specific and disease-free survival. There is compelling evidence that p16 expression is an independent prognostic marker. In their landmark publication, Ang and colleagues [15] provided the strongest evidence to date for the use of p16 as a stand alone prognostic biomarker: individuals with p16 positive OPSCC had similar overall and progression-free survival to those with HPV DNA positive status (3-year overall survival 84 vs. 82% and 3-year progression-free survival 74 vs. 74%). In both instances, outcomes were significantly better than for patients with HPV negative OPSCC (3-year overall survival 57% and 3-year progression-free survival 43%). Similarly, Lewis et al. [16] demonstrated that clinical outcomes for p16 IHC positive/HPV negative cases, defined using both ISH and PCR methods, did not differ significantly from p16 positive/HPV positive cases, with both demonstrating a statistically improved survival by comparison with p16 negative/HPV negative tumours, implying that HPV specific tests are redundant for prognostic purposes. In addition, it is a cost-effective test that is easy to interpret and benefits from excellent inter-observer agreement [11]. This information has been used to devise the first randomised controlled clinical trials for patients with HPV-related OPSCC: RTOG 1016 in the USA and DeESCALaTE-HPV in Europe will register patients for their studies on the basis of p16 positivity alone. Both trials will use CINtec Histology with cut-offs defined as strong nuclear and cytoplasmic staining in greater than 70% of the tumour [10].
Whilst many head and neck oncologists are convinced that p16 is sufficient for patient stratification, others remain skeptical. Perrone et al. [9] suggest that p16 assessment alone produces a heterogeneous group of patients; sub-analysis of a small group (n = 15) of patients with p16 positive/HPV DNA negative tumours, measured by high-risk HPV ISH, had similarly poor overall survival to those patients with HPV negative tumours. A recently published UK study presents interesting data showing that patients with p16 negative/HPV DNA positive OPSCCs, assessed using CINtec Histology and SPF10 PCR primers, are a clinically distinct group that show survival benefits when chemotherapy (cisplatin or carboplatin ± 5-fluorouracil) is added to their radiation regimen; nevertheless, as a group they still had a worse prognosis than individuals with p16 positive tumours [17]. Schache et al. [7] recently demonstrated that prognostication is improved by using HPV specific tests; p16 IHC alone was not considered specific enough to recommend for use in clinical trials, whereas, the combination of p16 IHC and qPCR for HPV-16 DNA was the best discriminator of favourable outcome. These studies suggest that whilst p16 overexpression can be used as a prognostic marker, its utility as a predictive marker in clinical trials may be confounded by a lack of specificity for oncogenic HPV infection. This argument will become particularly pertinent as targeted therapies emerge for HPV positive OPSCC. Whilst molecular targeted agents, such as small interfering RNA (siRNA) molecules, are being developed and tested in pre-clinical models, therapeutic vaccines aimed at clearing existing HPV infections and treating pre-cancer and established cancers are already being evaluated in clinical trials [18]. It is difficult to envisage administering these highly-specific, targeted treatments on the basis of a surrogate marker of oncogenic HPV infection when simple laboratory tests exist to establish the presence of the aetiological agent.
HPV Tests in Neck Metastases from Occult OPSCC
A familiar scenario in head and neck clinical practice is the patient that presents with cervical lymph node metastasis from an occult primary head and neck carcinoma. Typically, the first investigation is a fine needle aspiration biopsy and detection of high-risk HPV in the cytological specimen can direct the search for the primary tumour to the oropharynx [19]. The utility of p16 IHC as a single modality test in this scenario is limited since the protein is occasionally overexpressed in non-HPV-related head and neck carcinomas arising in sites other than the oropharynx [13, 14, 20]. Cytologic examination occasionally causes a diagnostic dilemma between sampling of a lymphoepithelial cyst (branchial cyst) and a metastatic squamous cell carcinoma. Potentially, HPV testing could be used to resolve this dichotomy. Two small studies have examined cytologic specimens in this context: one study showed concordance between diffuse, strong p16 staining the presence of HPV-16 DNA, and metastasis from an oropharyngeal primary [21], whereas another study described p16 staining in branchial cyst aspirates in the absence of HPV-16 DNA; however, the ‘cut-off’ for calling a sample p16 positive was not clearly described in the study [22]. Until further data emerges, the results of HPV tests in this specific clinical scenario should be interpreted with caution.
Emerging Histological Subtypes of HPV-Related OPSCC
Although the vast majority of HPV-associated oropharyngeal tumours are conventional non-keratinising squamous cell carcinomas, recent reports have identified the virus in other histological subtypes such as adenosquamous and neuroendocrine carcinomas [23, 24]. These histological subtypes are rare and interpretation of outcome data are limited by the small numbers of cases reported. Nevertheless, future meta-analyses to inform prognosis will be of little value without HPV-specific testing since certain rare types of carcinoma, which are associated with poor outcome, have been found to over-express p16 without any detectable HPV DNA [20].
HPV Tests in Disease Surveillance
Another important aspect of HPV-specific laboratory testing is its use in disease surveillance programs. There is clear evidence of the increasing incidence of HPV-related OPSCC in the USA and parts of Europe and that there are worldwide geographical differences [1]. It is becoming increasingly important for national cancer registries to collect data on HPV-related OPSCC as the information will help to formulate public health programmes, such as public information campaigns and prophylactic vaccination strategies. Contemporary data indicate that the vast majority of HPV-related OPSCC are caused by HPV-16 (95%) with the remainder caused by other high-risk genotypes [1]. With the implementation of vaccination programs for young women, it is likely that OPSCC disease patterns will change over time, albeit with a time lag of decades. In the absence of routine prophylactic vaccination in males, the prediction of the effects of female vaccination on the prevalence of OPSCC in males is difficult. It is also conceivable that the genotype prevalence may change over time as a consequence of vaccination. For practical purposes, ISH has the ability to monitor HPV-16 infection versus other high-risk genotypes, by using a two-tiered testing approach: HPV-16 specific probe followed by high-risk HPV cocktail for HPV-16 negative cases [10]. Whilst this strategy would only give a crude indication of the genotype case mix, any change in the current HPV-16 prevalence could be identified and direct a more focused analysis of changing infection profiles. Currently, this method could not be adopted in Europe as HPV-16 specific probes are not available due to licensing restrictions. It would not be possible to collect such epidemiological data using p16 IHC alone, as it lacks specificity for HPV infection and provides no information about the HPV genotype.
Refining Biomarker Profiles
The concept of ‘personalised medicine’ or ‘stratified medicine’ is underpinned by the use of biomarkers to refine the classification of disease. The detection of HPV infection identifies a group of OPSCC with a common aetiological agent and improved outcomes. However, it is clear from the data produced by Ang et al. [15] that patients with a high nodal category (N2b, N3) who smoke have an intermediate risk of death and disease progression compared with non-smokers who have the lowest risk of death. This raises the possibility that additional biomarkers, particularly those associated with tobacco abuse, for example p53 mutations, may encode additional information that has a significant influence on the biology of HPV-driven OPSCC. It is possible therefore, that those individuals with HPV positive OPSCC that contain wild type p53 have the very best prognosis and would be candidates for dose reduction protocols in a clinical trial setting. Furthermore, genome wide analyses comparing HPV positive and negative OPSCC have demonstrated other candidate genes that could be included as part of the molecular diagnostic criteria [3].
Disclosure of HPV Status
HPV testing for OPSCC raises the issue of disclosure of HPV status to the patients who have a positive test result. Discussion of HPV as a possible aetiological factor is currently recommended for all patients with OPSCC [25]. Individual patient circumstance and physician choice may guide the discussion content. For example, the young non-smoking patient may welcome the identification of a possible causative agent, as well as receiving information about improved prognosis. For other patient groups, the information may be less relevant. Once the p16 status is known then the clinician has an ethical obligation to share information about probable HPV status with the patient. It has been argued that the patients’ right to know overrides any consideration regarding a possible difficult discussion in the clinic. A further issue is that HPV is detectable in a significant proportion of p16 negative OPSCC. It follows that only testing for HPV directly can provide the information needed to build an accurate clinical picture to inform the clinical discussion. Increasingly, patients are using the Internet and other sources to find out more about their diseases; however, not all of the available information is validated. This may lead to questions about such matters as the possible value of HPV vaccination as therapy and even whether HIV testing is indicated. In the absence of a laboratory test result that detects HPV directly, meaningful discussion of these issues is necessarily limited. The widespread introduction of HPV testing into cervical screening programmes, albeit for a different purpose, raises similar ethics issues and it is likely that the same principles of disclosure and counselling will apply when formulating guidelines for HPV-related OPSCC.
Cost Effectiveness of HPV Testing
The introduction of routine HPV testing by PCR into UK pathology laboratories as part of the cervical screening programme is likely to drive down test costs due to economies of scale. In any event, the costs of p16 IHC and HPV testing are only a tiny fraction of the costs of the whole oncology regimen. As yet, no evidence base exists to measure cost-effectiveness of HPV testing for OPSCC. Experience from other more common cancers suggests that a predictive marker of response, for example KRAS testing and cetuximab for metastatic colorectal cancer, is both cost-effective and avoids inappropriate therapy. Refinement of the translational tests employed in oncology is typical as clinical experience accumulates, and it is likely that p16 IHC alone will prove insufficient for stratification of patients as the international evidence builds.
Conclusion
The pathologist has an important role in the development of biologically relevant grading and staging systems, whether based on morphologic parameters or more sophisticated biomarker profiles. We think it would be premature to restrict biomarker profiles for OPSCC as there are potential advances that will only be appreciated following accumulation of data in well-characterised retrospective and prospective patient cohorts. When designing clinical trials for patients with OPSCC, it is essential that patient risk factors and HPV-specific biomarkers are considered as part of the registration and randomisation protocols. Collection of tissue from patients with OPSCC that are recruited into clinical trials will, in time, facilitate biomarker discovery and validation studies.
In clinical practice, we think that HPV-related OPSCC should, at the very least, be defined by the aetiological agent using HPV specific tests, and further, that classification should not be restricted to the use of a single surrogate biomarker of HPV infection. Currently, there is insufficient evidence to recommend a particular testing algorithm; however, the combination of p16 IHC along with the detection of HPV DNA by target amplification (PCR) and/or signal amplification (ISH) have been proposed [6–10]. These techniques are feasible in well- equipped pathology laboratories and have the potential to deliver quality assured HPV diagnostics for OPSCC as part of a routine pathology service [11]. In our clinical practice, we define ‘HPV-related OPSCC’ as p16 positive/HPV positive (CINtec Histology, Roche mtm laboratories AG, Germany and Inform HPV III CISH, Ventana Medical Systems Inc, USA on a Ventana Benchmark Autostainer. p16 is defined as positive if there is strong and diffuse nuclear and cytoplasmic staining present in greater than 70% of the tumour specimen. The HPV test is positive if there is evidence of staining that co-localises with the nuclei of malignant cells). For p16 positive/HPV negative cases we inform the clinical team that p16 positivity can be used as an indicator of favourable prognosis, but that there is no evidence of HPV infection per se. Currently, as there is not enough evidence to predict the outcome for p16 negative/HPV positive cases, we group these patients with those classified as p16 negative/HPV negative. All things considered, it is becoming increasingly important for the international head and neck oncology community to agree on the minimum requirements for assigning a diagnosis of ‘HPV-related’ OPSCC, in order to ensure consistent reporting of this emerging and increasingly prevalent disease.
References
- 1.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braakhuis BJ, Brakenhoff RH, Meijer CJ, Snijders PJ, Leemans CR. Human papilloma virus in head and neck cancer: the need for a standardised assay to assess the full clinical importance. Eur J Cancer. 2009;45(17):2935–2939. doi: 10.1016/j.ejca.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 4.Houten VM, Snijders PJ, Brekel MW, Kummer JA, Meijer CJ, Leeuwen B, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer. 2001;93(2):232–235. doi: 10.1002/ijc.1313. [DOI] [PubMed] [Google Scholar]
- 5.Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 2002;21(10):1510–1517. doi: 10.1038/sj.onc.1205214. [DOI] [PubMed] [Google Scholar]
- 6.Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121(11):2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 7.Schache AG, Liloglou T, Risk JM, Filia A, Jones TM, Sheard J, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17(19):6262–6271. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi W, Kato H, Perez-Ordonez B, Pintilie M, Huang S, Hui A, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27(36):6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 9.Perrone F, Gloghini A, Cortelazzi B, Bossi P, Licitra L, Pilotti S. Isolating p16-positive/HPV-negative oropharyngeal cancer: an effort worth making. Am J Surg Pathol. 2011;35(5):774–777; author reply 777–778. [DOI] [PubMed]
- 10.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 11.Thavaraj S, Stokes A, Guerra E, Bible J, Halligan E, Long A, et al. Evaluation of human papillomavirus testing for squamous cell carcinoma of the tonsil in clinical practice. J Clin Pathol. 2011;64(4):308–312. doi: 10.1136/jcp.2010.088450. [DOI] [PubMed] [Google Scholar]
- 12.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24(10):1295–1305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11(16):5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 14.Harris SL, Thorne LB, Seaman WT, Hayes DN, Couch ME, Kimple RJ. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck. 2011;33(11):1622–1627. doi: 10.1002/hed.21650. [DOI] [PubMed] [Google Scholar]
- 15.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis JS, Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, et al. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34(8):1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junor E, Kerr G, Oniscu A, Campbell S, Kouzeli I, Gourley C et al. Benefit of chemotherapy as part of treatment for HPV DNA-positive but p16-negative squamous cell carcinoma of the oropharynx. Br J Cancer. 2011;106(2):358–65. [DOI] [PMC free article] [PubMed]
- 18.Gillison ML. Human papillomavirus and prevention and therapy of head and neck cancer. In: Harrison LB, Sessions RB, Hong WK, editors. Head and neck cancer a multidisciplinary approach. Philadelphia: Lippincott Williams and Wilkins; 2009. pp. 905–917. [Google Scholar]
- 19.Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13(4):1186–1191. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- 20.Wadsworth B, Bumpous JM, Martin AW, Nowacki MR, Jenson AB, Farghaly H. Expression of p16 in sinonasal undifferentiated carcinoma (SNUC) without associated human papillomavirus (HPV) Head Neck Pathol. 2011;5(4):349–354. doi: 10.1007/s12105-011-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai RK, Erikson J, Pourmand N, et al. p16(INK4a) immunohistochemical staining may be helpful in distinguishing branchial cleft cysts from cystic squamous cell carcinomas originating in the oropharynx. Cancer. 2009;117:108–119. doi: 10.1002/cncy.20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao D, Begum S, Ali SZ, Westra WH. Expression of p16 in benign and malignant cystic squamous lesions of the neck. Hum Pathol. 2010;41(4):535–539. doi: 10.1016/j.humpath.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Masand RP, El-Mofty SK, Ma XJ, Luo Y, Flanagan JJ, Lewis JS., Jr Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of the literature. Head Neck Pathol. 2011;5(2):108–116. doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J Surg Pathol. 2011;35(11):1679–1684. doi: 10.1097/PAS.0b013e3182299cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuman AG, Wolf GT. Human papillomavirus status in head and neck cancer: the ethics of disclosure. Cancer. 2010;116(18):4221–4226. doi: 10.1002/cncr.25210. [DOI] [PubMed] [Google Scholar]