Abstract
Imaging, especially contrast-enhanced computed tomography (CECT) for anatomy and positron emission tomography (PET) with labeled 18F fluorodeoxyglucose for physiologic detail, is critical for staging carcinomas of the oropharynx. As the incidence of human papillomavirus (HPV) infection and related carcinomas of the tonsil and base of tongue (BOT) increases, experience with CECT and PET for staging HPV+ tumors is growing. No imaging modality, however, can determine whether the tumor is HPV+. There are some unique challenges posed by HPV+ oropharyngeal squamous cell carcinoma (SCC). In most locations of the head and neck, a malignancy enhances more than surrounding normal structures, which facilitates tumor mapping. Unfortunately, normal lymphoid tissue of the oropharynx, in the BOT and palatine tonsillar fossa, enhances on CECT and gadolinium enhanced magnetic resonance imaging in a manner similar to SCC. The primary tumor may be small or even occult at presentation, and easily over-looked on CECT. PET coupled with CECT has made a true “unknown primary” very rare, as the metabolically active tumor is almost always detectable on PET. The nodal metastases, so common with HPV+ SCC, can be truly cystic; and as such, can be misdiagnosed as a second branchial cleft cyst, a congenital benign lesion. These pitfalls, coupled with the complex anatomy of the upper aerodigestive tract, make staging these tumors difficult. In this monograph we describe the anatomy of the oropharynx and review the imaging modalities available for staging. Figures highlight the points raised in the text.
Keywords: Oropharynx, Squamous cell carcinoma, Human papillomavirus related carcinoma, Computed tomography, Positron emission computed tomography
Introduction
Squamous cell carcinoma (SCC) accounts for more than 90 % of the pathology in neoplasms of the oropharynx (OP). Minor salivary tumors, lymphomas and sarcomas are the chief other etiologies of OP neoplasia [1]. Unfortunately, there is considerable overlap in imaging appearance between SCC and other OP neoplasias. Oropharynx squamous cell carcinoma (OPSCC) is a common head and neck cancer [2]. While incidence rates in the United States population are on the decline for many primary cancer sites including lung, colon, rectum, prostate, stomach, and cervix; rates are on the increase for others including oropharyngeal (human papilloma virus, HPV, related), esophageal (adenocarcinoma), pancreas, liver and intrahepatic bile ducts, thyroid, kidney, and skin (melanoma) [3]. Well-established risk factors for the development of OPSCC include tobacco and alcohol (separately and synergistically), poor oral hygiene, and radiation exposure. In the past decade the increased incidence of OPSCC (base of tongue and tonsil (BTT) subsites), notably in the white male demographic, is linked to HPV infection [4]. It has been estimated that HPV-related tumors of the BTT in the Western countries account for approximately 50 % of all BTT SCC [5]. Despite the increased incidence, population-level survival for this disease has improved and is linked to the different behavior of the HPV-related tumors [6].
Contents and Boundaries of the Oropharynx
The OP is that part of the pharyngeal mucosal space bounded by the soft palate superiorly and the hyoid bone and vallecula inferiorly (Fig. 1). The anterior boundary is the ventral margin of the circumvalatte papilla. As such, the OP communicates with the nasopharynx above, the oral cavity anteriorly, and the larynx and hypopharynx inferiorly. The OP subsites are the ventral soft palate, the posterior and lateral pharyngeal walls, the tonsillar fossa and pillars, and the BOT including the pharyngoepiglottic and glossoepiglottic folds [7].
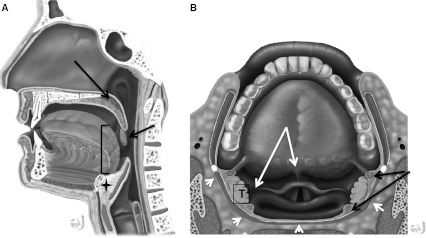
Fig. 1.
Normal anatomy. Sagittal and axial drawings, showing relationship of OP to oral cavity and epiglottis. a Artist rendering of upper aerodigestive tract. Note soft palate (long arrow), and palatine tonsil (short arrow). Brackets denote posterior 1/3 of the tongue. Pre-epiglottic fat is denoted by star. b Upper aerodigestive tract from above. Superior and upper fibers of middle pharyngeal constrictor muscles (white arrowheads). Paired white arrows show pharyngoepiglottic (laterally) and glossoepiglottic (midline) folds. Paired black arrows show palatoglossus (anterior) and palatopharyngeal (posterior) muscles forming the anterior and posterior tonsillar pillars. T palatine tonsil. (Both drawings by Eric Jablonowski, Medical Illustrator, Emory Univeristy.)
The OP is composed of the lymphoid tissue of the palatine and lingual tonsils, and the squamous mucosa, which lines the OP. The superior and middle constrictor muscles and middle layer of deep cervical fascia lie beneath the mucosal surface (Fig. 1). The middle pharyngeal constrictor muscle is contiguous with the buccinator muscle via the pterygomandibular raphe. The pterygomandibular raphe has bony attachments at the mylohyoid line of the mandible and the hamulus of the medial pterygoid plate, and provides a potential route of cancer spread between the OP and oral cavity (OC), the OP and central skull base, and the OP and the masticator space (MS).
Spaces adjacent to the OP include the carotid space (CS), parapharyngeal space (PPS), retropharyngeal space (RPS), and MS. OP is separated from these spaces by at least the middle layer of deep cervical fascia (Fig. 2).
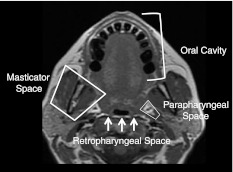
Fig. 2.
Spaces surrounding the oropharynx. Axial T1 MR image at the level of the palatine tonsils shows the fat-filled parapharyngeal space (PPS) lateral to the oropharynx (OP), the oral cavity (OC) and anterior two-thirds of the tongue anterior to the OP, and the retropharyngeal space (fat signal) posterior with respect to the posterior pharyngeal wall
Pattern of Tumor Spread
Tumor spread occurs in a predictable fashion to adjacent structures and draining nodal stations from each of the OP subsites. The tonsil and tonsillar pillars are the most common sites of SCC occurrence (75–80 %) [7]. Tumors that arise here can directly invade through the pharyngeal constrictor muscles to the adjacent PPS (Fig. 3) and from there to the CS and MS. If the tumor follows the anterior tonsillar pillar and palatoglossus muscle, it can involve the soft and hard palate and/or BOT. Posterior tonsillar pillar extension can lead to tumor involving the soft palate, pharyngoepiglottic fold, posterior pharyngeal wall and middle pharyngeal constrictor. Once tumor involves the palate, local extension may affect the tensor and levator palatine muscles and the pterygoid musculature in the MS. Extension along the pterygomandibular raphe can lead to OC (retromolar trigone, buccal mucosa) and skull base involvement. Mucosal or submucosal disease spread to the hypopharynx and nasopharynx also occurs. SCC of the tonsillar complex is commonly (66–76 %) associated with positive lymph nodes at the time of diagnosis: the levels II, III and IV ipsilateral nodes are most commonly affected [7–10].
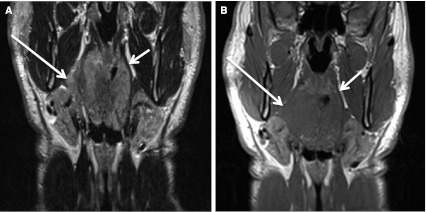
Fig. 3.
Right tonsil cancer with spread through constrictor muscle directly into parapharyngeal fat. a T2 coronal MR shows the tumor (long arrow) is slightly hyperintense to muscle. Notice normal constrictor musculature (short arrow) on left and preservation of fat in the PPS. Right tonsillar SCC is invading right PPS. b T1 coronal MR. The tumor (long arrow) is isointense to muscle, but invasion of PPS is evident. Short arrow points to the normal PPS and constrictor musculature on the left
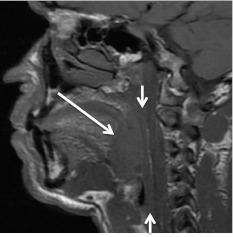
Base of tongue cancers are typically more aggressive and less well differentiated than the cancers arising in the other OP subsites. Tumor can extend anteriorly into the root of tongue and extrinsic tongue musculature. Once in the sublingual space, the tumor can involve the neurovascular bundle. Inferior extension results in tumor in the vallecula and in the pre-epiglottic fat of the supraglottic larynx (Fig. 4). If the tumor extends laterally, the pterygomandibular raphe and anterior tonsillar pillar can be reached; from there the tumor can spread in a predictable fashion along those tissue planes [7–10].
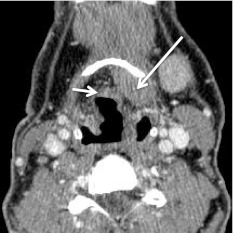
Fig. 4.
Base of tongue cancer invading the pre-epiglottic fat. Axial CECT at level of the hyoid bone shows tumor (long arrow) invading the pre-epiglottic fat. Short arrow denotes normal body of the epiglottis
Cancers here often present at advanced stage with frequent regional nodal (60 % at presentation) and distant metastatic lesions. The richness of the lymphatic drainage for this location helps explain the propensity for bilateral nodal involvement (approximately 30 %) [8]. Nodal levels most commonly affected are II, III, and IV.
Cancers of the soft palate tend to be symptomatic earlier than cancers of the other subsites. Tumor can invade the hard palate (OC), the palatine muscles and the PPS, the tonsillar fossa and pillars with eventual involvement of the nasopharynx and skull base. Proximity of the palatine foramina in the hard palate explains the potential for perineural extension along the palatine nerves and V2. Lymphatic drainage of this subsite is chiefly to levels II and III with retropharyngeal adenopathy sometimes found [7, 11].
Posterior pharyngeal wall tumors are uncommon and can extend in the pharyngeal mucosal space in a cranio-caudal fashion to invade the nasopharynx and hypopharynx and in an anterior direction to affect the tonsil. Lateral extension frequently involves the PPS; posterior extension leads to tumor in the retropharyngeal (RPS) and prevertebral spaces. Lymphatic drainage is to bilateral jugular chain and retropharyngeal nodes [7, 11].
Appreciation of the predictable pattern of tumor spread and nodal involvement can assist in the accurate staging of SCC. Key observations to be made at the time of imaging assessment for OP SCC include carotid encasement, osseous and skull base involvement, prevertebral muscle invasion, and cross midline extension in the tongue.
Staging of Oropharynx Cancer with Imaging (AJCC7)
Imaging is used to gain anatomic information to be able to stage the primary tumor and to detect regional and distant metastatic lesions (T, N, M). HPV status does not affect staging. In assignment of a T designation, tumor size and extension are considered. The local disease extent is best assessed using contrast enhanced computed tomography (CECT) or magnetic resonance imaging (MRI). Positron emission tomography-computed tomography (PET–CT) alone is a powerful modality to determine metabolic activity, but does not provide sufficient anatomic information needed to stage the primary. T1 through T3 lesions are staged based on size. A T1 lesion is less than 2 cm in maximum dimension (Fig. 5a), T2 lesions are 2–4 cm (Fig. 5b), and T3 lesions are greater than 4 cm (Fig. 6). A T4 lesion invades adjacent structures and is further subdivided into T4a (moderately advanced local disease) and T4b (very advanced local disease) (Fig. 7). The T4a lesion may invade the larynx (lingual surface of epiglottis extension by BOT or vallecula lesion does not constitute laryngeal invasion), extrinsic tongue muscles, medial pterygoid muscle, hard palate or mandible. Tumors that encase the internal carotid artery; invade the lateral pterygoid muscle; or extend to the nasopharynx, pterygoid plates and/or skull base are staged as T4b.
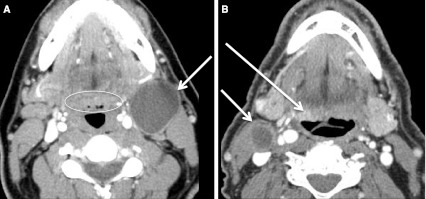
Fig. 5.
T1 and T2 OP SCC. a Axial CECT at BOT level shows subtle asymmetrically thickened and enhancing tissue (long arrow) at left BOT and vallecula: the primary T1 SCC (HPV+). This study should be interpreted in conjunction with the physical examination, as normal asymmetric lingual lymphoid tissue may have a similar appearance. There are bilateral (N2c) malignant nodes (short arrows). b Axial CECT, from a different patient, at level of soft palate level shows SCC (HPV+) extending from right tonsil. The tumor measures >2 cm but <4 cm in size
Fig. 6.
T3 primary tumor. Sagittal T1 MR off midline. Tonsil SCC (long arrows) is 5 cm in greatest length (T3) and does not invade adjacent structures. Short arrows denote constrictor musculature. Note the linear high T1 signal posterior to the constrictor muscles of the normal retropharyngeal fat
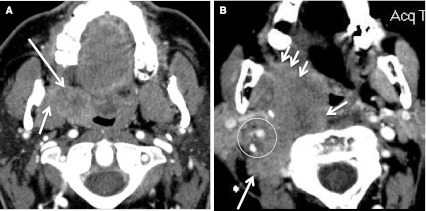
Fig. 7.
T4a and T4b primary SCC. a Axial CECT at level of tonsillar fossa shows HPV+ tonsillar SCC (long arrow) has invaded the right PPS and medial pterygoid muscle (short arrow). This is a T4a lesion. b Axial CECT at the same level, in a different patient. T4b tumor (HPV status unknown) is widely invasive into the prevertebral and paraspinal musculature on the right (short arrows). Tumor surrounds the internal carotid artery and narrows the internal jugular vein (circle), and invades along the pterygomandibular raphe (arrowheads)
Lymph nodes that demonstrate irregular borders (extra-capsular extension of disease), are enlarged by imaging size criteria (greater than 1.5 cm at level I and II; greater than 1.0 cm at levels III-V) as measured in greatest linear dimension in the axial plane, or show evidence of necrotic/cystic change, are considered pathologic by imaging criteria. The N or regional lymph node status designations include N0 (no abnormal nodes), N1 (one <3 cm abnormal node), N2a (single ipsilateral abnormal node >3–6 cm), N2b (multiple ipsilateral abnormal nodes all ≤6 cm), N2c (bilateral or contralateral abnormal lymph nodes), and N3 (6 cm or larger abnormal lymph node) (Fig. 8). Nodal assessment can be achieved with CECT, MRI, ultrasound, or PET–CECT.
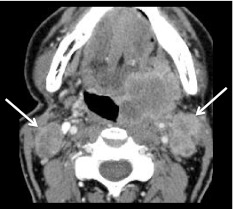
Fig. 8.
Metastatic lymph nodes based on size and central necrosis. Low-density foci in the pathologically enlarged nodes (arrows) bilaterally are imaging criteria for N2c disease. Note the irregular margins of the left IIA nodes likely due to extra-capsular spread (ECS) of tumor into the sternocleidomastoid muscle and fat. The primary tumor is a SCC (HPV+) of left tonsil (shown)
In our opinion, evaluation of the primary tumor, metastatic nodal disease and distant metastatic lesions is best performed with PET coupled with contrast enhanced computed tomography (PET–CECT). This combination has the benefit of a metabolic study and an anatomic examination. PET–CT is especially helpful in the assessment of distant metastases, and there are only two groups in this category: M0 (no distant metastasis) or M1 (distant metastasis) (Fig. 9).
Fig. 9.
Metastatic lesion seen at PET–CT in a patient with primary head and neck SCC. a Fused image with abnormal activity in left level IV and V lymph nodes (circled) as well as a metastatic lesion (arrow) in the left scapula. b CT from the PET–CT at bone window and level settings revealing the lytic bone lesion (arrow)
CECT, MRI, Ultrasound and PET–CT Staging of OP Primary Squamous Cell Carcinoma
While many factors affect overall patient survival, stage at presentation is central to plan treatment, determine prognosis, and predict outcome [12]. Given the rich lymphatic drainage of the OP, OP SCC often present with nodal disease (up to 65 %), most commonly with BOT primary lesions followed in descending order of incidence by tonsil, oropharyngeal wall and soft palate [8]. Patients may present with an identifiable mucosal lesion, a nodal mass, or both. Clinical exam is best for evaluation of the superficial mucosa, but imaging is necessary in the accurate staging of primary lesions. CECT and MRI best determine submucosal and local tumor extension (T stage), while PET–CECT is helpful in the identification of the unknown primary and in evaluation of nodal (N) and other metastatic lesions (M) (Table 1).
Table 1.
Comparison of various imaging modalities used in the assessment of the patient with presumed or proven head and neck malignancy
| Modality | Advantages | Disadvantages | Special considerations |
|---|---|---|---|
| CECT | Excellent T, N assessment Readily available Fast Superior to MRI in evaluation of: Cortical bone |
Artifact from dental amalgam Iodinated contrast: Renal function Allergy |
Re-angled images through the oral cavity can minimize the artifact from amalgam Can be complementary to MRI in T evaluation Can be used for assessment for initial staging, unknown primary workup and treatment follow-up |
| MRI | Excellent T, N assessment Superior to CT in evaluation of: Medullary cavity of bone Perineural disease Cartilage invasion |
Lengthy exam False positives for medullary cavity assessment: Recent dental work Fibrosis Osteonecrosis |
Patient motion decreases study quality Can be complementary to CT in T evaluation Can be used for assessment for initial staging, unknown primary workup and treatment follow-up |
| PET–CT | Excellent for N, M assessment Useful in unknown primary workup and surveillance |
False positives and false negatives (see text for details) Inadequate for T delineation |
Must be cognizant of recent therapy (chemo and XRT) or surgical intervention/biopsy when interpreting study Blood glucose levels can impact study accuracy |
| PET–CECT | Combines the positive qualities of PET–CT with CECT and affords better assessment of T stage over PET–CT alone Useful for initial staging, unknown primary workup and follow up after treatment |
Has the disadvantages of CT and PET–CT as listed above | Must be cognizant of recent therapy (chemo and XRT) or surgical intervention/biopsy when interpreting study Blood glucose levels can impact study accuracy of the PET |
At our institution, patients with a known diagnosis of head and neck SCC undergo an initial staging imaging study, and PET–CECT is our primary initial modality. Non-contrast CT is not accurate in staging, so that patients with iodine allergy usually undergo MRI of the neck at which time multiplanar, multisequence, pre- and post-contrast imaging is performed. Occasionally, if there is extensive oral cavity amalgam artifact or the lesion is poorly demarcated on CECT, MRI of the neck can be performed. Rarely, a patient may have both CECT and MRI examinations, as the two tests may be complementary. Both modalities allow for primary tumor evaluation and local disease extent (T stage).
Patients with suspected head and neck cancer should undergo a thorough physical examination prior to imaging, and the exam findings should be available to the radiologist. Mucosal lesions are best and sometimes only detected on physical exam. At CECT and MRI, small tumors of the tonsillar fossa or BOT can be difficult to distinguish from the adjacent enhancing and bulky lymphoid tissue. The lymphoid tissue is often normally asymmetric, which makes it even harder to detect the primary. In fact, the incidence of malignancy in asymmetric tonsillar tissue in an otherwise normal individual is reported at only 5 % [13]. As tumors grow, they invade the adjacent normal tissues and/or grow in an exophytic fashion, and both patterns are more easily detected with CECT or MRI (Fig. 10). In general, non-necrotic SCC tumors show mild to moderate enhancement on CECT. On MRI the tumors are isointense to muscle on T1-weighted images, slightly hyperintense to muscle on T2-weighted images, and enhance following gadolinium administration (Fig. 11). Application of fat saturation techniques on T2 and post-contrast T1 sequences increases tumor conspicuity. The larger tumors often appear heterogeneous in density (CECT) or signal intensity (MRI) due to tumor necrosis. As tumors invade adjacent structures, the fascial boundaries are pushed and ultimately violated. Often the T1 pre-contrast MR images are the most sensitive in demonstrating local extent of disease into the OC and replacement of the normal high T1 signal in the fat of the adjacent PPS and RPS spaces by the intermediate tumor signal (Fig. 3).
Fig. 10.
Appearance of tumor on CECT. a Exophytic growth into the oropharyngeal lumen of a T2 left tonsil SCC (HPV+) is marked with an arrow. b Bulky stage T4b SCC (HPV−) right tonsil primary (circled) is locally invasive, invading the PPS and masticator space. This was the second head and neck primary SCC for this patient. Note bilateral changes of prior selective neck dissection
Fig. 11.
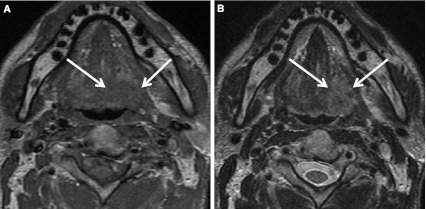
Base of tongue SCC (HPV+). a T1 axial MR shows base of tongue SCC (arrows) is isointense to muscle. b On T2 axial MR image tumor is slightly hyperintense to muscle
Computed tomography viewed at bone window and level settings is especially useful in the assessment of the cortex of the maxilla and mandible; MRI is especially useful in the description of tumor margins, medullary bone, and identification of perineural disease. When tumor invades bone or cartilage, MRI will show replacement of the normal marrow or cartilage signal with signal that is isointense/isoenhancing to tumor. Unfortunately, recent dental extraction, post radiation treatment fibrosis, and osteoradionecrosis can cause false positives for bone involvement on MRI [8].
Both CECT and MRI are useful in nodal evaluation where malignant involvement is suspected in abnormally enlarged, irregularly margined (extra-capsular extent of tumor) and/or cystic/necrotic lymph nodes (N stage). As the single most important prognostic indicator, nodal status needs to be accurately defined. Wide variation in sensitivity and specificity for detection of nodal metastases by CECT and MRI has been reported in the literature. Sensitivity and specificity, respectively, is reported between 54–95 and 39–100 % for CECT, and between 64–92 % and 40–81 % for MRI [14]. Scanning technique and interpreter experience will influence those results. Ultrasound and PET–CECT have also been used to assess for nodal metastases.
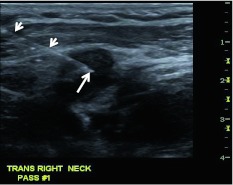
Ultrasound has several drawbacks in staging of the neck, as it is operator dependent, takes relatively longer to scan the whole neck, and is inadequate for assessment of the retropharyngeal nodes. In our practice, ultrasound is the primary tool for imaging guided biopsy, especially for lymph nodes that may be indeterminate on other modalities. Normal cervical nodes have intact fatty hila and are generally elliptical or oval in shape (Fig. 12). Features of a pathologic lymph node at ultrasound include replacement of the hilar fat (loss of normal nodal architecture) and loss of the normal blood flow at the hilum (Fig. 13). Depending upon the solid versus cystic nature of the lymph node, the tumor-replaced node may have increased vascularity while a necrotic or cystic node is devoid of normal blood flow and parenchyma.
Fig. 12.
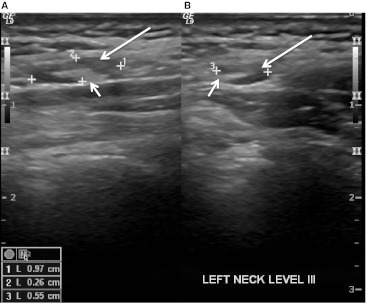
Ultrasound appearance of a normal level III lymph node. Longitudinal (a) and transverse (b) images show the echogenic normal fat-filled hilum (short arrows) and uniform hypoechoic appearance of the cortex (long arrows). Calipers outline the lymph node and node measurement is available on the bottom left side of the figure
Fig. 13.
Ultrasound guided biopsy of an abnormal cervical lymph node. The tip (arrow) of the 23 gauge needle (arrowheads) is in the central portion of the pathologic node. The fat-filled hilum is obliterated and the node is enlarged
Positron emission tomography (PET) imaging is a functional nuclear medicine technique that provides three-dimensional information about glucose utilization by cells in the body. The radionuclide used in PET imaging for SCC patients is fluorodeoxyglucose (FDG), and FDG labeled with 18F is a glucose analog (the 2′ hydroxyl group is replaced by 18F (radioactive isotope) and thus the FDG cannot undergo glycolysis). Normal and malignant cells take up the FDG having recognized the radiotracer as a glucose molecule. The blood glucose level (BGL) of the patient is tested prior to the radiopharmaceutical administration: it is a goal of PET to maximize tracer uptake in malignant tissues. An elevated BGL can yield false negative results. As FDG undergoes natural decay, a pair of photons is produced in an annihilation reaction. The PET scanner detects these photons. The greater the concentration of FDG in an area, the “hotter/brighter” it will appear on PET images. The greater the concentration of FDG, the more metabolically active the tissue is. Primary tumors and metastatic lesions have higher glucose utilization than normal tissues. When PET–CT or PET–CECT is performed, anatomic and functional information are obtained. With PET–CECT, FDG and intravenous (IV) iodinated contrast are both administered. No IV iodinated contrast is administered for a PET–CT. The combination of the two imaging techniques (PET with CT) produces co-registered or fused images where the metabolic activity information from the PET is superimposed upon the anatomic CT images (Fig. 14).
Fig. 14.
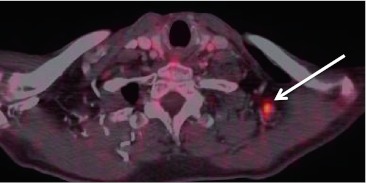
Appearance of cystic HPV+ lymph node on PET–CT. a Non-contrast CT portion of the PET–CT. Right level IIA lymph node (arrow) is largely cystic, with a small and more solid appearing portion (short arrow) of the node. b Fused PET–CT image at the same level as the CT with a relative photopenic region (long arrow) in the cystic portion of the node. The solid component (short arrow) shows avid FDG-uptake. Notice only mildly asymmetric FDG activity in the right tonsillar tissue (circled). Patient presented with the right neck mass and an unknown primary. PET–CT revealed asymmetric activity in the right tonsil. Examination under anesthesia (EUA) with biopsy proved primary SCC (HPV+) tumor in right tonsil
Positron emission tomography-computed tomography is very sensitive in detection of primary and recurrent malignant tumor in the head and neck. PET–CT sensitivity and specificity in the detection of nodal metastases has been reported in the literature as 84–92 and 95–99 % respectively [14]. Head and neck cancer patients are at risk for developing distant metastasis and second primary tumors. PET–CT has been found to have “good performance” in M staging of head and neck cancer, and be more effective than CT of the chest and abdomen alone in detection of metastatic lesions [15]. We routinely perform PET scans with CECT, combining anatomic and functional evaluation into one setting. The Nuclear Medicine section interprets the PET portion of the study and the Head and Neck Radiologist interprets the CECT; and two separate dictations are generated. If there are discordant findings, especially with respect to cervical adenopathy, fine needle aspiration (FNA) with ultrasound guidance is often performed.
All of these techniques have certain drawbacks. Dental filling artifacts degrade CT images and potentially, portions of the OC and OP can be obscured. In an effort to minimize the effects of dental amalgam, re-angled scans are routinely performed from the OC to larynx. The study by Bannas et al. [16], showed that tilted slices avoiding dental work “significantly improve the reader’s sensitivity and are of incremental value for staging of oral and oropharyngeal cancers.” MR images are also affected by dental amalgam artifact, though typically less impacted than CECT images. Each MR sequence can take 2–5 min to complete and patient swallowing or motion can limit diagnostic image quality.
Disadvantages of PET–CT include the decreased spatial resolution offered by this modality (5 mm threshold), chance of false negative results with necrosis (particularly in lymph nodes) and the chance of false positive results in the setting of inflammatory processes, infection, and posttreatment changes. Other causes of false positive or false negative findings in PET are regions of normal physiologic uptake that are misinterpreted. Tissues that are normally metabolically active include salivary tissue, lymphoid tissue, and the thyroid gland. The appearance of lymphoid tissue of Waldeyer’s ring in particular can be confounding. While FDG uptake in these structures is generally symmetric; focal, asymmetric but normal uptake is frequently encountered. Unfortunately, symmetry of uptake in the lymphoid tissue does not preclude malignancy or inflammation and asymmetry of uptake does not always indicate malignancy (false positive) [17, 18]. At our institution, and supported by the literature, areas of concern raised by the PET portion of the PET–CECT are critically assessed by the Head and Neck radiologist on the CECT portion of the exam. If no area of CT concern exists, the more focal area of uptake is interpreted as discordant. We then recommend careful direct clinical evaluation of the site.
Imaging of the Unknown Primary
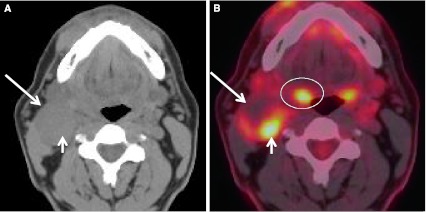
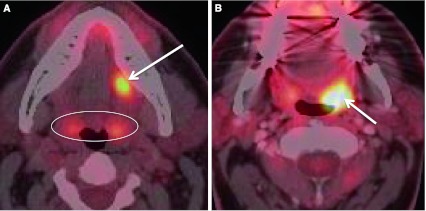
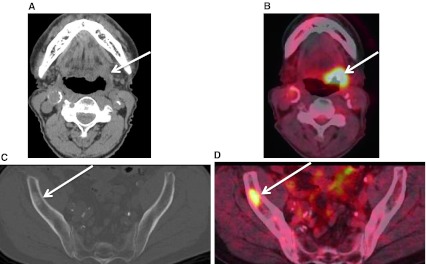
A unique subset of cancer patients is the unknown primary lesion. These patients usually present with a painless neck mass. Greater than 90 % of the primary malignancies are found in Waldeyer’s ring and are SCC [19]. Lesions arising in Waldeyer’s ring, when small, can be obscured by the background enhancement on CECT and MRI, and by FDG uptake of the normal tonsillar tissue. Inherent asymmetry of the lymphoid tissue can “mimic” lesions (Fig. 15). When assessing the patient with the unknown primary, anatomic imaging with CECT and/or MRI is performed. Physiologic assessment with PET–CT or PET–CECT can be performed. Exam under anesthesia with biopsies, targeting areas of concern found with anatomic and physiologic imaging, is pursued (Figs. 14, 16). Addition of FDG-PET to the work up increases detection of the primary cancer site by up to 54 %. PET–CT is also noted to have a high negative predictive value. PET–CT can be “false-positive”, especially in the post biopsy setting related to reactive/inflammatory changes and when normal lymphoid tissue is asymmetric. Interpretation of PET–CT must take into account whether or not the patient has had any recent surgical intervention. Benefits of PET–CT also include the ability to detect unexpected contralateral nodal disease, synchronous malignancies, and distant lymph node involvement [20] (Figs. 17, 18).
Fig. 15.
Appearance of the palatine tonsil on fused images from PET–CT. a The patient’s primary malignancy is in the oral cavity (long arrow) in left lateral floor of mouth. The palatine tonsils (circled) are mildly asymmetric in activity, which can be a normal finding, but must always be confirmed clinically. b This asymmetric metabolic activity on PET–CECT in the left tonsillar fossa (arrow) was a true positive. Biopsy of this site yielded SCC
Fig. 16.
PET–CECT and the unknown primary. Patient presented with palpable neck masses. a CECT neck performed in conjunction with PET shows bilateral enlarged and necrotic lymph nodes (arrows) at levels IIA. b Fused image from the PET–CECT shows subtle but definite asymmetric activity (arrow) at left BOT from a biopsy proven SCC (HPV+) primary. The malignant adenopathy is circled. Note normal salivary tissue activity in the submandibular glands located anterior to the malignant nodes
Fig. 17.
Distant lymph node involvement found at PET–CECT. Fused image from a PET–CECT performed for staging in a patient with BOT primary SCC (HPV+). An otherwise normal appearing left level V lymph node (by CT criteria) was discovered to have metabolic activity consistent with malignancy
Fig. 18.
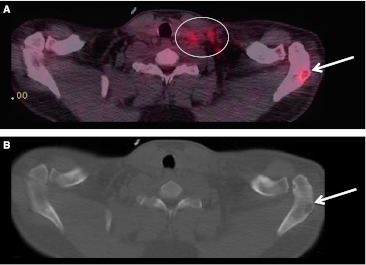
PET–CT detection of an unexpected second primary cancer. Patient was being staged for a known left BOT SCC (HPV−) tumor. a Non-contrast CT image obtained during PET–CT shows the primary lesion (arrow) at the left BOT and caudal left tonsil. b PET–CT fused image demonstrates the metabolically active lesion (arrow). c Non-contrast CT image at level of the bony pelvis, note the sclerotic lesion (arrow) in the right ilium. d On the fused PET–CT image, the sclerotic lesion shows metabolic activity. This osseous metastatic lesion was from an undiagnosed prostate primary
Human Papilloma Virus Associated Squamous Cell Carcinomas: Are There Any Distinguishing Imaging Features?
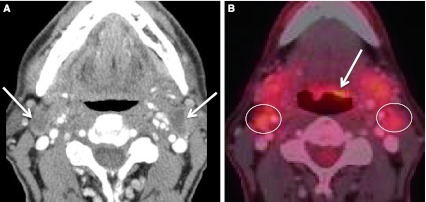
Early evidence suggests HPV associated SCC of the OP has better outcomes compared to HPV-negative (HPV−) SCC [21]. Therefore, it would be useful if imaging could predict HPV status. Unfortunately, there are no unique imaging findings on any modality to determine HPV status (Figs. 19, 20, 21). While HPV− OP SCC can arise in any subsite of the OP, HPV-positive (HPV+) disease is most common in the tonsillar subsite followed by BOT subsite [22]. In our experience, HPV+ tumors often present with smaller primary lesions than the HPV− cancers. It has been reported that HPV+ SCC nodal disease is often cystic [23, 24] or “cystic or necrotic” [25] (Figs. 20, 21). In the editorial by Hudgins and Gillison, two important points were made: often the primary SCC lesion is small, arising in the BOT or tonsil; and that a new neck mass (unless midline or a goiter) in the adult patient must be considered malignant SCC adenopathy until proven otherwise. They cautioned against the assumption that a cystic mass behind the submandibular gland represents a second branchial cleft cyst (Figs. 22, 23). With cross-sectional post contrast imaging, cystic lymph nodes have been defined as “round or ovoid masses with a thin (<2 mm) enhancing capsule, homogeneous fluid content, and no internal complex, irregular, or solid area.” This same reference described a necrotic node as “thicker solid walls and irregular, complex central low attenuation” [23] (Fig. 24). Ultrasound of a cystic node shows an echolucent area in contrast to a necrotic node demonstrating an echogenic area [26]. It has been reported that there is overlap in the HPV+ and HPV− adenopathy, but the majority of truly cystic nodes are from HPV+ tonsillar cancers [23]. In the abstract by Morani, “intranodal cystic changes (defined as areas of central low density) on pretreatment CECT of the neck in patients with OP SCC show favorable association with HPV status.” The authors reported 100 % specificity for HPV positive status in patients with intranodal cystic foci in their study population of 41 patients [24]. In our experience, HPV+ adenopathy is variable in appearance, and can be solid, solid with necrotic foci, or cystic.
Fig. 19.
Imaging appearance of HPV+ and HPV− SCC primary tumors can overlap. a Post-contrast T1 axial image of HPV+ BOT tumor (long arrow) on right. The lesion is mildly heterogeneous in its enhancement pattern. The submandibular gland (short arrow) is adjacent and enhancing to a slightly greater degree than the tumor. b CECT of HPV− BOT tumor with extension into the sublingual space (long arrow) on left. This lesion is also mildly heterogeneous in its enhancement pattern. Note malignant level IIA adenopathy (short arrow)
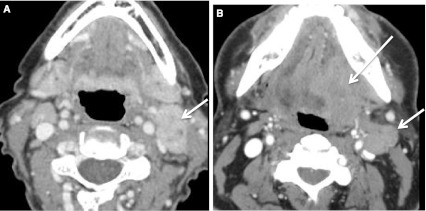
Fig. 20.
Imaging appearance of HPV+ and HPV− lymph nodes can also overlap. a HPV+ T1 SCC tonsil primary (not shown) with ipsilateral lymphadenopathy. The left level IIA node (short arrow) is heterogeneous with low density necrosis on this CECT. b The primary HPV− BOT cancer (long arrow) is invading the extrinsic tongue muscles (T4a). Malignant left level IIA nodes (short arrow) are heterogeneous with low density necrosis on the CECT image
Fig. 21.
Similar imaging features of HPV+ and HPV− primary SCC and the respective malignant lymphadenopathy. a CECT from a patient with a T4b (HPV+) primary (long arrow) of the left tonsil. The carotid artery was encircled at other levels. Note bilateral level IIA lymph nodes (short arrows) with necrosis. An enlarged and solid appearing retropharyngeal lymph node was also abnormal (arrow head). The tumor (long arrow) was invasive and partly necrotic. b CECT shows an invasive T4b (HPV−) SCC tumor (long arrow) invading the parapharyngeal space, medial pterygoid muscle, and carotid space. Pathologic lymph nodes (short arrow) almost entirely necrotic are present at right level II
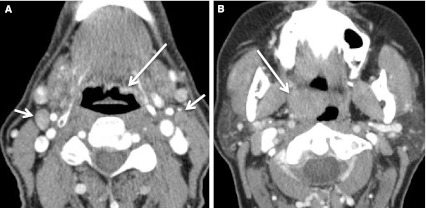
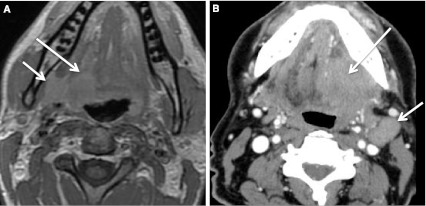
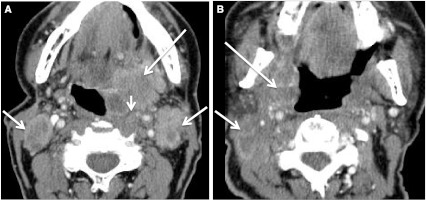
Fig. 22.
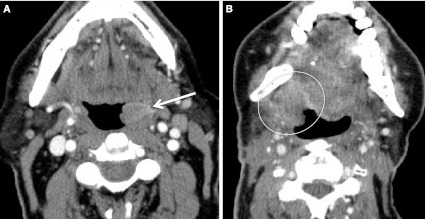
Second branchial cleft cyst and a “mimic”. a CECT obtained in a patient with a palpable left neck mass (arrow). Cystic mass was a biopsy proven branchial cleft cyst. Prominent lymphoid tissue at BOT (circled) related to reflux. b CECT obtained in a patient with a palpable right neck mass (short arrow). This cystic mass is a biopsy proven metastatic right level IIA lymph node in a patient with an “unknown primary;” however, note enhancing mass at the BOT (long arrow), missed on initial scan interpretation. Biopsy of right BOT proved SCC (HPV+)
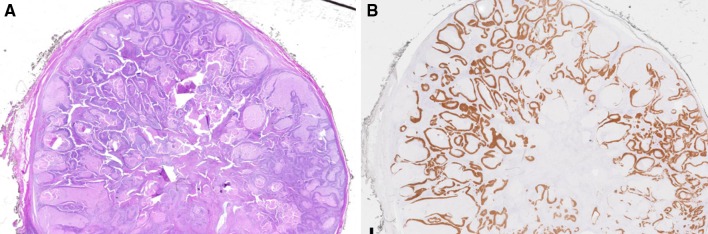
Fig. 23.
Cystic lymph node pathology correlate. a Whole slide scan of a cystic SCC metastasis to a cervical lymph node from a tonsil primary showing a well-circumscribed lymph node almost entirely replaced by tumor forming numerous cystic spaces containing serous fluid and cellular debris. b p16 immunohistochemistry highlights the tumor cells within the lymph node. (Images courtesy of Susan Muller, DMD)
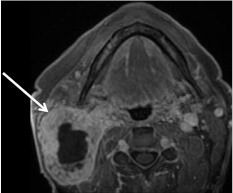
Fig. 24.
Necrotic lymph node. Axial T1 post contrast fat-saturated image of an N3 (greater than 6 cm) right level II lymph node (arrow). Note the thick and irregularly enhancing wall with the complex appearance to the central necrotic non-enhancing area. Patient was diagnosed with an HPV− right tonsil SCC found at biopsy during work up for unknown primary
Imaging During and Following Definitive Treatment
Following definitive treatment, both clinical examination and imaging are used to assess for treatment response. Many factors complicate accurately interpreting the post treatment scan. After neck dissection and radiation and/or reconstruction, normal fascial relationships and symmetry are lost. Scar tissue, inflammatory changes and sterile/treated disease also complicate the image interpretation. Over time, the appearance of a muscle flap will change. The baseline post-treatment exam is often the most challenging to interpret as the patient is now at a “new baseline” appearance with treatment related edematous and inflammatory changes superimposed. These acute changes can lead to false positive CECT, MR and/or PET–CECT results and can potentially obscure residual disease (false negative). In patients having received chemotherapy and radiation therapy, false negative and false positive results can be found on PET–CECT when imaged within 8 weeks following treatment. Therefore, it is recommended that post-treatment anatomic and functional imaging be performed at least 10–12 weeks following completion of radiation therapy. False negative results in PET–CECT can also be seen in necrotic or cystic lymph nodes, and small residual lesions may be beneath the size threshold for detection. False positive results in PET–CECT can result from fasciculation in the flaps; aspiration or fungal pneumonia; fibrosis; and misinterpretation of normal activity in Waldeyer’s ring, muscle, mucosa and salivary tissue. Symmetry is not only lost with respect to architecture of the neck in the post-treatment patient, but also lost with respect to metabolic activity; tissue damage by radiation reduces background physiologic activity on the affected side [27].
Summary
When staging a head and neck tumor, or unknown primary presenting as a neck mass, accurate imaging interpretation is facilitated by close communication between the examining physician and the radiologist. CECT, MRI, PET–CECT, or a combination of these imaging studies is essential to accurately stage the malignancy. Primary cancers of squamous epithelium, lymphatic tissue and salivary glands overlap in imaging appearance and, while there may be distinctive patterns of disease, biopsy and histologic examination are always necessary prior to treatment. HPV+ and HPV− primary SCC lesions are impossible to routinely distinguish from one another on imaging. Both sub-types of SCC arise in the tonsil and BOT, and both can have abnormal appearing (low density) malignant nodes. When the lymph node appears completely cystic, however, there is a greater likelihood that the HPV status of the cancer will be positive.
The addition of PET–CECT to the imaging armamentarium represents a significant development, as the fusion of anatomic detail to metabolic activity has improved lesion detection for both small primary tumors and metastatic nodal and distant disease. Imaging plays a vital role in the accurate staging of patients, but should not stand-alone. The most value added scan interpretations include correlation between the clinical exam results, history of intervention/biopsy or treatment, and the imaging findings. Patients are best served when a Tumor Board is composed of surgical, medical, pathology and radiologic specialties.
References
- 1.Osborne RG, Brown JJ. Carcinoma of the oral pharynx: an analysis of subsite treatment heterogeneity. Surg Oncol Clin N Am. 2004;13:71–80. doi: 10.1016/S1055-3207(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 2.Filion E, Le Q-T. Oropharynx: epidemiology and treatment outcome. In: Harari PM, Connor NP, Grau C, editors. Functional preservation and quality of life in head and neck radiotherapy. Berlin: Springer; 2009. [Google Scholar]
- 3.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999–2008. CA Cancer J Clin. 2012 (epub ahead of print). doi:10.3322/caac.20141. [DOI] [PubMed]
- 4.Saba NF, Goodman M, Ward K, et al. Gender and ethnic disparities in incidence and survival of squamous cell carcinoma of the oral tongue, base of tongue and tonsils: a surveillance, epidemiology and end results program-based analysis. Oncology. 2011;81:12–20. doi: 10.1159/000330807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Souza F, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 6.Chaturverdi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin DT, Cohen SM, Coppit GL, et al. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clinc N Am. 2005;38:59–74. doi: 10.1016/j.otc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Trotta BM, Pease CS, Rasamny JJ, et al. Oral cavity and oropharyngeal squamous cell cancer: key imaging findings for staging and treatment planning. Radiographics. 2011;31:339–354. doi: 10.1148/rg.312105107. [DOI] [PubMed] [Google Scholar]
- 9.Cohan DM, Popat S, Kaplan SE, et al. Oropharyngeal cancer: current understanding and management. Curr Opin Otolaryngol Head Neck Surg. 2009;17:88–94. doi: 10.1097/MOO.0b013e32832984c0. [DOI] [PubMed] [Google Scholar]
- 10.Stambuk HE, Karimi S, Lee N, et al. Oral cavity and oropharynx tumors. Radiol Clin N Am. 2007;45:1–20. doi: 10.1016/j.rcl.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Wesolowski JR, Mukherji S. Pathology of the pharynx. In: Som PM, Curtin HD, editors. Head and neck imaging. 5. Amsterdam: Elsevier; 2011. [Google Scholar]
- 12.Edge S, Byrd D, Compton C, et al. AJCC7 cancer staging manual. New York: Springer; 2010. [Google Scholar]
- 13.Syms MJ, Birkmire-Peters DP, Holtel MR. Incidence of carcinoma in incidental tonsil asymmetry. Laryngoscope. 2000;11:1807–1810. doi: 10.1097/00005537-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Yoon DY, Hwang HS, Chang SK, et al. CT, MR, US, 18F-FDG PET/CT, and their combined use for the assessment of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Eur Radiol. 2009;19:634–643. doi: 10.1007/s00330-008-1192-6. [DOI] [PubMed] [Google Scholar]
- 15.Xu G-Z, Zhu X-D, Li M-Y. Accuracy of whole-body PET and PET-CT in initial M staging of head and neck cancer: a meta-analysis. Head Neck. 2011;33:87–94. doi: 10.1002/hed.21400. [DOI] [PubMed] [Google Scholar]
- 16.Bannas P, Habermann CR, Jung C, et al. Diagnostic accuracy of state-of-the-art MDCT scanners without gantry tilt in patient with oral and oropharyngeal cancer. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Bhargava P, Rahman S, Wendt J. Atlas of confounding factors in head and neck PET/CT imaging. Clin Nucl Med. 2011;36:e20–e29. doi: 10.1097/RLU.0b013e318212c872. [DOI] [PubMed] [Google Scholar]
- 18.Blodgett TM, Fukui MB, Snyderman CH, et al. Combined PET-CT in the head and neck. Part 1. Physiologic, altered physiologic and artifactual FDG uptake. Radiographics. 2005;25:897–912. doi: 10.1148/rg.254035156. [DOI] [PubMed] [Google Scholar]
- 19.Schmalbach CE, Miller FR. Occult primary head and neck cancer. Curr Oncol Rep. 2007;9:139–146. doi: 10.1007/s11912-007-0012-5. [DOI] [PubMed] [Google Scholar]
- 20.Wong WL, Sonoda LI, Gharpurhy, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the assessment of occult primary head and neck cancers—an audit and review of published studies. Clin Oncol. Available on-line 17 Dec 2011. [DOI] [PubMed]
- 21.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 22.Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldenberg D, Begu S, Westra WH, et al. Cystic lymph node metastatsis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30(7):898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 24.Morani A, Shah G, Eisbruch A, et al. Intranodal cystic changes: a potential radiological signature/biomarker to assess the human papilloma virus status of patients with oropharyngeal carcinoma. AJR 2011;196:A47. Abstract presented in scientific session 14—neuroradiology: head and neck on 4 May 2011 at the ARRS meeting.
- 25.Hudgins PA, Gillison M. Editorial: second branchial cleft cyst: NOT!! AJNR. 2009;30:1628–1629. doi: 10.3174/ajnr.A1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahuga AT, Ying M. Sonographic evaluation of cervical lymph nodes. AJR. 2005;184:1691–1699. doi: 10.2214/ajr.184.5.01841691. [DOI] [PubMed] [Google Scholar]
- 27.King KG, Kositwattanarerk A, Genden E, et al. Cancers of the oral cavity and oropharynx: FDG-PET with contrast enhanced CT in the posttreatment setting. Radiographics. 2011;31:355–373. doi: 10.1148/rg.312095765. [DOI] [PubMed] [Google Scholar]