Abstract
The great majority of HPV-related carcinoma of the oropharynx is nonkeratinizing squamous cell carcinoma. More recently, an increasing number of squamous cell carcinoma variants that are HPV positive are being reported in the oropharynx, as well as in other head and neck sites. As a result, several clinical and pathologic questions have emerged. Importantly, questions raised include whether the virus is biologically active and involved in the pathogenesis of these tumors, and whether there are clinical implications with regard to patient outcome and treatment modality changes that may be needed in HPV-related variants. Examples of HPV-related squamous cell carcinoma variants that will be addressed here include: basaloid squamous cell carcinoma, undifferentiated carcinoma, adenosquamous carcinoma, papillary squamous carcinoma, and small cell carcinoma. Some investigations have suggested a favorable prognosis in some variants, analogous to that of the conventional nonkeratinizing (basaloid) carcinoma, while others showed poorer outcome. So far, the number of studies on this subject is limited and the number of cases evaluated in each investigation is few. Because of this, it is prudent at this stage not to alter management protocols as a result of identification of HPV in these variants and to await additional studies.
Keywords: Human papillomavirus, Squamous cell carcinoma variants, Basaloid squamous cell carcinoma, Undifferentiated carcinoma, Adenosquamous carcinoma, Papillary squamous carcinoma, Small cell carcinoma
Introduction
The greatt majority of HPV-related squamous cell carcinoma of the oropharynx show nonkeratinizing morphology, as described in detail in other parts of this monograph. Rarely, however, HPV-positive squamous cell carcinoma of the head and neck exhibits variant morphologic features. In extraoropharyngeal head and neck anatomic sites, conventional SCC variants are usually unrelated to HPV while in the oropharynx they are more likely to be HPV positive. There is a general conception that HPV positivity is associated with a favorable patient outcome regardless of the morphologic type. Identification of HPV as a causative agent in these variants is, therefore, not merely of academic interest but rather of potential clinical significance in the determination of the patients’ outcome and in the selection of proper treatment modalities. In the case of carcinoma of the uterine cervix, it is uncertain whether the morphologic variant adenosquamous carcinoma (AdSC) has a better or worse survival rate than conventional SCC. Some studies report a poorer prognosis [1–3] while others find the prognosis to be equal [4–6]. Cervical AdSC is HPV-related in up to 95 % of the cases [7], consequently outcome differences between HPV-positive and -negative AdSC are difficult to investigate. In the head and neck however, this type of investigation may be less challenging because HPV-negative variants are more readily available. Nevertheless, studies on the prognostic significance of HPV-positive SCC variants in the head and neck are few, and the number of cases studied is limited. Some of these studies show evidence to suggest that specific HPV-related morphologic variants may have a favorable outcome, similar to HPV-positive conventional nonkeratinizing SCC, which is better than in the case of HPV-unrelated ones [8–10]. Yet, other studies suggest that some variants, such as small cell carcinoma, are associated with a rather poor prognosis.
Examples of SCC variants that were shown to be HPV-related and will be discussed here include: Basaloid squamous cell carcinoma (BSCC) [8, 9], undifferentiated carcinoma (UDCA) [10], AdSC [11], papillary squamous carcinoma (PSCC) and small cell carcinoma. It is of importance to emphasize that the mere detection of HPV DNA in the tumor cells is not in itself evidence of a causative relationship. An etiologic role for HPV in these SCC variants should be established by a distinct molecular profile in the tumor cells caused by transcriptional activation of the virus genome.
Basaloid Squamous Cell Carcinoma
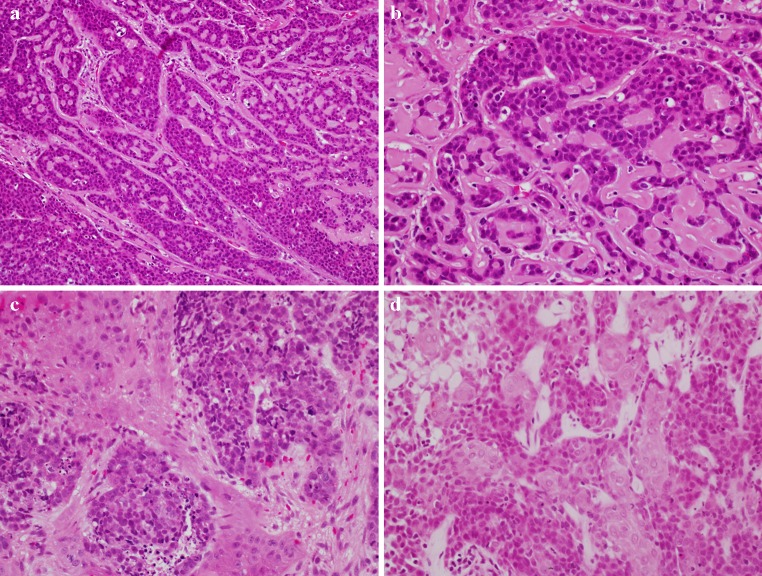
In the upper aerodigestive tract, BSCC is rare variant of conventional SCC. It occurs more commonly in the hypopharynx and larynx and less frequently in the oropharynx. Like SCC, the tumor is typically associated with traditional risk factors like tobacco smoking and alcohol abuse. It is generally considered a high-grade malignant neoplasm with poor prognosis. Microscopically, a biphasic pattern, defined by Wain et al. [12], characterizes BSCC. The tumor shows a basaloid component intimately associated with elements of keratinizing SCC. The basaloid cells are small, crowded with hyperchromatic round nuclei and scant cytoplasm. They form sheets and lobules that produce a “jigsaw puzzle” growth pattern with cystic spaces containing PAS-positive myxoid material. Stromal hyalinization may be present. The squamous component is either surface SCC or severe dysplasia in addition to focal abrupt squamous differentiation within the basal cell areas (Fig. 1). These microscopic features are distinct from, but may be confused with, those of NK SCC as described elsewhere.
Fig. 1.
Basaloid squamous cell carcinoma (BSCC): a Closely packed basaloid cells forming closely opposed sheets and lobules and producing a “jigsaw puzzle” appearance. A microcystic, cribriform-like pattern is also observed. b Higher magnification. Conventional keratinizing squamous cell carcinoma components both separate (c) and intermingled (d) with the basaloid elements
Chernock et al. [8], using in situ hybridization (ISH) for HPV DNA and p16 immunohistochemistry investigated the prevalence of high-risk HPV in 28 cases of BSSC of the head and neck. Twelve cases (43 %) were identified in the oropharynx and 16 (57 %) in the larynx and/or hypopharynx. Nine (75 %) of the oropharyngeal BSCC cases and none of those in the larynx/hypopharynx were HPV-related (p < 0.0001). HPV-positive tumors affected younger patients (p < 0.02). However, no significant statistical difference in patients’ sex, tumor stage, treatment modality or length of follow-up was observed between HPV-positive and -negative tumors. HPV-positive tumors correlated positively with p16 overexpression (Fig. 2) but negatively with p53 expression. Overall survival (OS) was better for patients with HPV-positive tumors (p < 0.05), with 86 % of patients alive at 3 years compared with 53.3 % of patients with HPV-negative BSCC.
Fig. 2.
BSCC: In situ hybridization (ISH) for high-risk HPV DNA showing punctuate blue nuclear staining (a). A p16 immunohistochemical stain showing strong and diffuse reactivity (b)
Similar results have also been reported by Begum and Westra [9] in a study of 53 cases of BSCC of the head and neck. Twenty-one cases (40 %) arose in the oropharynx and 32 (60 %) were from non-oropharyngeal sites. HPV16 was detected in 16 of 21 (76 %) oropharyngeal tumors but only in 2 (6 %) of non-oropharyngeal sites. HPV-positive tumors were also strongly positive for p16 immunostain. OS of patients with HPV-positive tumors was significantly better than in the case of patients with HPV-negative tumors (p < 0.0001).
Undifferentiated Carcinoma
In the head and neck, UDCA is best recognized in the nasopharynx where it is referred to by a variety of names, including lymphoepithelioma, nasopharyngeal type UDCA, and nonkeratinizing UDCA (WHO type III). Microscopically, identical carcinomas are also identified in the parotid gland and in the oropharynx. The salivary gland tumor is known as lymphoepithelial carcinoma. UDCA of the nasopharynx has a strong etiologic relationship to Epstein-Barr virus (EBV) [13]. The tumor is distinctly radiosensitive and is known to have better prognosis than conventional squamous cell carcinoma of that anatomic site (WHO type I) [14, 15]. EBV is also known to play an important role in the etiology of salivary gland lymphoepithelial carcinoma in endemic sites such as Southeast Asia [16] but not in other parts of the world. On the other hand, UDCA of the oropharynx has recently been shown to be predominantly HPV-related [10, 17].
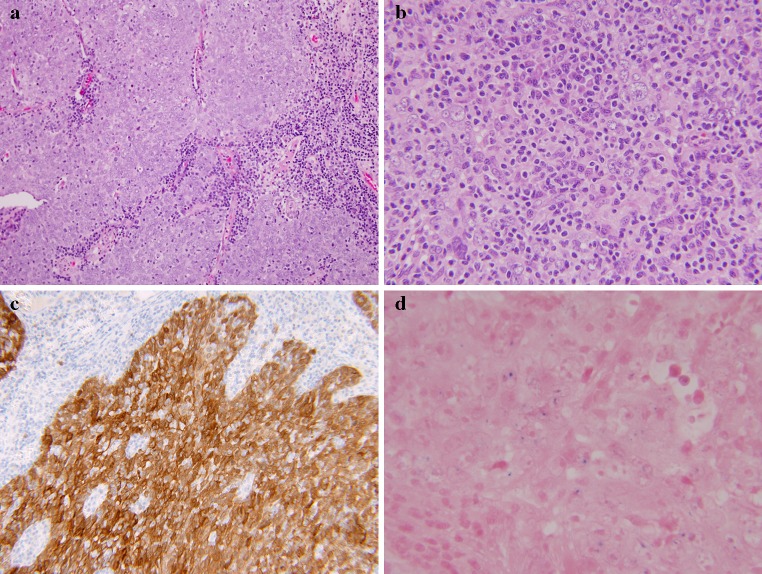
The microscopic features of oropharyngeal UDCA are indistinguishable from those of the nasopharyngeal type, as defined by the WHO [18]. The tumors are composed of solid sheets, trabeculae, nests and single neoplastic cells intimately intermingled with lymphocytes and plasma cells. The epithelial tumor cells are large with indistinct cell borders forming a syncytium (Fig. 3). The nuclei are round to oval and vesicular with large central nucleoli. HPV DNA was identified in these tumors by PCR and ISH. Evidence for biologic activity of the virus was demonstrated by p16 immunohistochemical over-expression (Fig. 3) [10, 17].
Fig. 3.
Undifferentiated (lymphoepithelial) carcinoma of the oropharynx. a Low-power view showing undifferentiated epithelial cells forming a syncytium and intermingled with lymphocytes and plasma cells. b Higher magnification showing tumor cells with large vesicular nuclei and prominent nucleoli. c Strong and diffuse p16 immunoreactivity. d Positive ISH for high-risk HPV DNA
Of 38 published cases of oropharyngeal UDCAs, 25 were in the tonsils, 5 in the base of tongue, 1 in the soft palate, and 7 in the oropharynx NOS. The patient’s ages ranged from 37 to 85 years with an average of 54 and 59 years, between the two studies. The majority (73–80 %) of the patients were male and 81–86 % of the patients had N2–N3 nodal disease at presentation [10, 17] (Table 1).
Table 1.
HPV-related undifferentiated carcinoma of the oropharynx
| References | N | M:F | Average age Y | N+ (%) | p16+ (%) | HPV+ (%) | DSS (%) |
|---|---|---|---|---|---|---|---|
| Singhi et al. [17] | 22 | 8:3 | 54 | 86 | 100 | 86 | 100 |
| Carpenter et al. [10] | 16 | 7:1 | 59 | 81 | 88 | 88 | 100 |
N+ lymph node metastasis, DSS disease specific survival
Overexpression of p16 by immunohistochemistry was demonstrated in 100 % of the cases in one study [17] and by 88 % in the other [10], while HPV DNA was detected in 86 and 88 % of the cases, respectively. EBV was not identified in any of the cases in either study [10, 17]. The 3-year OS was 55 %, while the disease-specific survival (DSS) was 100 % [10], and no tumor recurrence was observed during a median follow-up period of 23 months [17] (Table 1). The patient’s outcome in HPV-positive UDCA of the oropharynx is generally favorable and comparable to that of the conventional HPV-related squamous cell carcinoma.
Adenosquamous Carcinoma
Adenosquamous carcinoma (AdSC) is a rare variant of SCC. In the head and neck, it occurs most commonly in the larynx and, in descending frequency, the oral cavity, sinonasal tract, oropharynx and hypopharynx [11]. As defined by the WHO, it is an aggressive neoplasm originating from the surface epithelium and is characterized by biphasic pattern of differentiation comprised of SCC and true adenocarcinoma [18]. In a review of 93 cases of AdSC of the head and neck, Masand et al. [11] reported an age range of 22–87 years and a male-to-female ratio of 6:1. The local recurrence rate in these cases was 36 %. Regional metastasis was 47 % and distant metastasis 25 %, with the most common sites of metastasis being the lung, skin, liver, kidney and bone in descending order.
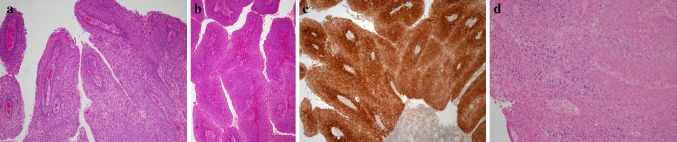
Microscopically, the main feature is true adenocarcinoma and conventional SCC occurring in close proximity but not intermingled. The SCC can either be in situ or invasive. The adenocarcinoma occurs in the deeper parts of the tumor. It consists of tubular, ductal, and glandular structures. Intra-luminal and intracellular mucin may or may not be identified (Fig. 4).
Fig. 4.
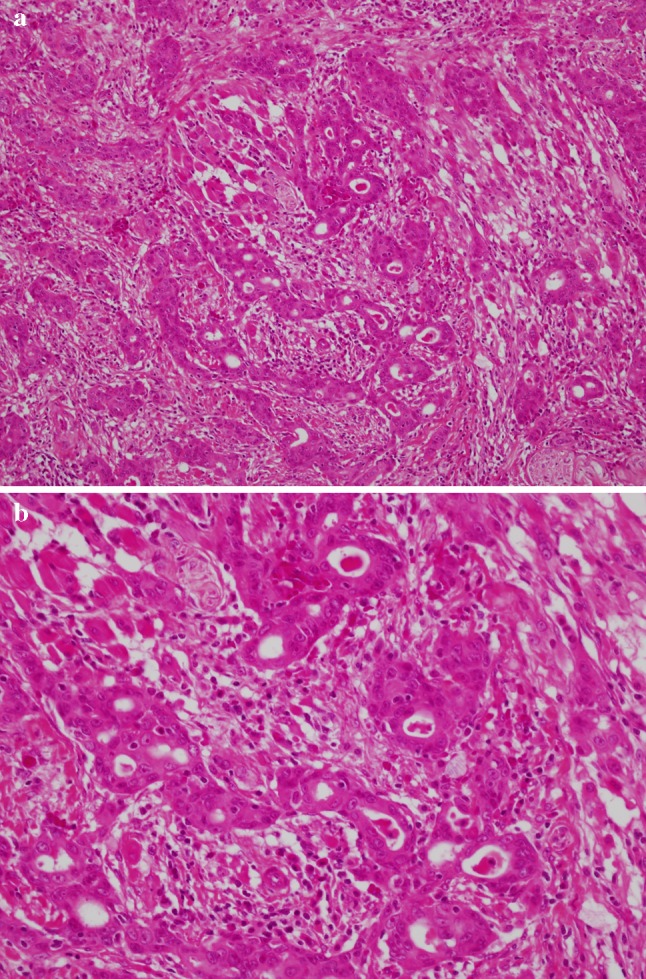
a Morphologic features of adenosquamous carcinoma illustrating biphasic pattern: a squamous cell carcinoma in the upper part of the figure and adenocarcinoma inferiorly. b Higher magnification showing glandular structures of adenocarcinoma
The relationship with HPV was investigated in 18 cases of AdSC of the head and neck by ISH for high-risk HPV DNA and E6 and E7 RNA, as well as by p16 immunohistochemistry [11]. Of the 18 cases, 8 were from the larynx and hypopharynx, 4 from the oral cavity, and 3 each from the oropharynx and nasal cavity. Two oropharyngeal and one nasal AdSCs were HPV-related, as documented by p16 overexpression and positive E6 and E7 RNA ISH. Two additional cases from the larynx/hypopharynx were positive for HPV DNA ISH. They were, however, negative for p16 and E6/E7 RNA ISH, and thus not considered to be etiologically related to a biologically active virus. Both of the HPV-related oropharyngeal AdSC were alive and disease-free at 103 and 24 months, respectively. However, the patient with HPV positive nasal AdSC died with disease after 4.5 months. Among patients with HPV-negative tumors, 43 % died of disease.
Similarly, a recent review of AdSC of the head and neck showed that patients’ outcome was generally poor. Of 93 patients, 36.5 % died of their disease, while 35.9 % had local recurrence, 47.4 % regional metastasis, and 24.7 % distant metastasis [11]. Based on the very limited number of HPV-related AdSC cases studied, there is a suggestion that HPV-positive tumors, particularly in the oropharynx, may have a more favorable prognosis.
Papillary Squamous Cell Carcinoma
Papillary squamous cell carcinoma (PSCC) is a poorly understood variant of SCC of the upper aerodigestive tract that is often confused with other exophytic mucosal malignancies, such as verrucous carcinoma (VC) and squamous cell carcinoma with verrucous features (SCCVF). The latter two entities are characterized by excessive keratinization and are broad based. VC does not exhibit cellular atypia while SCCVF does. As defined by the WHO, PSCC is characterized by a predominant papillary growth pattern with thin fibrovascular cores covered by immature basaloid cells or dysplastic cells with minimal or no keratinization [18]. The relationship of HPV to PSCC of the upper aerodigestive tract has, so far, not been well studied.
Carcinomas with papillary growth pattern are known to occur in the uterine cervix and other genital sites, including the penis, vagina, and vulva [19–22]. Several morphologic patterns are described, including PSCC, warty (condylomatous) SCC, and VC. High-risk HPV, particularly type 16, is most commonly associated with the PSCC of the cervix and with warty carcinomas of the penis, vagina, and vulva. Warty carcinoma is described as a papillary tumor with acanthosis, hyperkeratosis and parakeratosis, and common keratin plugging. The most conspicuous feature is the presence of zones of koilocytic atypia with large nuclei and perinuclear halo as well as frequent binucleation, multinucleation, mitosis, and apoptosis [20, 22, 23]. Interestingly, HPV+ warty/condylomatous type papillary carcinoma has not been reported in the upper aerodigestive tract. A very limited number of studies so far have investigated the prevalence and significance of HPV in PSCC of the head and neck [24–26]. Using ISH and PCR, Suarez et al. [24] found HPV in 4 and 5 of 14 total cases, respectively, while Jo et al. [25] found that 15 of 31 cases of PSCC of the head and neck were both p16 and HPV ISH positive. The majority of those (11 cases) were oropharyngeal. In neither of these two studies was patient outcome, between HPV-positive and -negative tumors, adequately investigated.
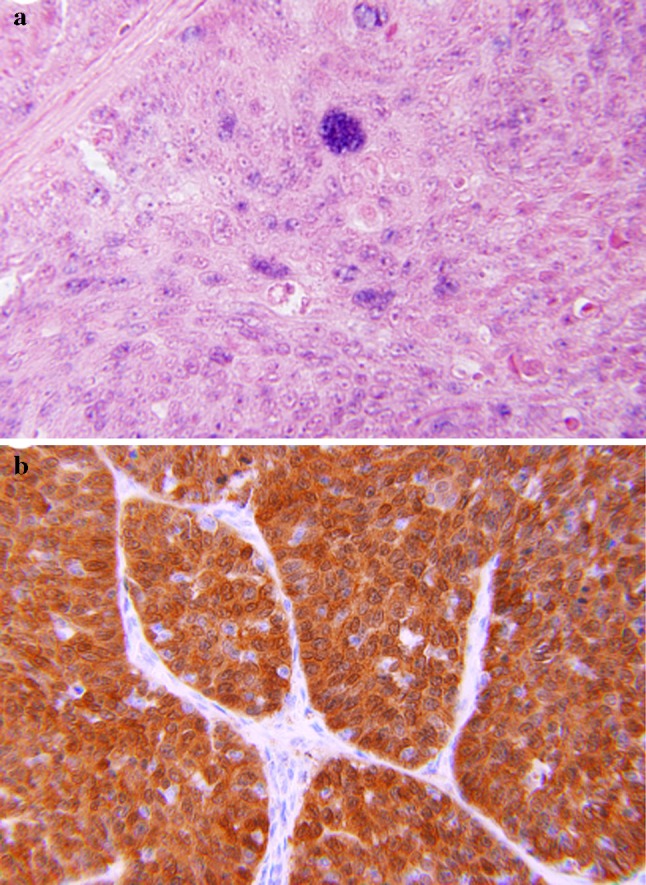
In a recent study [26], we reviewed 48 cases of PSCC of the head and neck, including 7 in the oral cavity, 19 in the oropharynx, and 22 in the larynx. Two morphologic types were identified: (1) a keratinizing (K) type, in which the dysplastic epithelium showed maturation trend with minimal surface parakeratin; and (2) a nonkeratinizing (NK) type, in which the papillae were covered with immature basaloid cells (Fig. 5). An HPV relationship was identified by p16 immunoreactivity, HPV ISH, and E6 and E7 mRNA ISH in a number of tumors (Fig. 5). The majority of these were found in younger patients, occurred more commonly in the oropharynx, had NK morphology, and were less likely to be p53 positive (Tables 2, 3). None of the HPV-related tumors showed warty/condylomatous morphology.
Fig. 5.
Papillary squamous cell carcinoma: a Keratinizing type, the dysplastic cells show maturation with minimal parakeratin formation. b Nonkeratinizing type with immature basaloid cells. c Strong and diffuse p16 immunoreactivity. d Positive ISH for high-risk HPV DNA (blue nuclear staining)
Table 2.
Papillary squamous cell carcinoma, distribution by site, morphologic features and HPV relationship
| Site (n) | NK (%) | P16+ (%) | P53+ (%) | RNA ISH+ (%) |
|---|---|---|---|---|
| OP (19) | 68.4 | 68.4 | 21.1 | 77.7 |
| Oral (7) | 14.3 | 14.3 (p = 0.012) | 71.4 (p = 0.028) | 25 (p = 0.040) |
| Larynx (22) | 9.0 | 13.6 (p = 0.0005) | 68.2 (p = 0.004) | 21.4 (p = 0.020) |
OP oropharynx, NK nonkeratinizing morphology
Table 3.
P16 positive and negative papillary squamous cell carcinoma; distribution by age, microscopic features, p53 reactivity and patient outcome
| Number (%) | Age Y M |
K (%) | NK (%) | P53+ (%) | P53− (%) | 5 year OS (%) | 5 year DSS (%) | 5 year DFS (%) | |
|---|---|---|---|---|---|---|---|---|---|
| P16+ | 17 (34) | 57 | 4 (23) p < 0.0001 |
13 (77) p < 0.0001 |
2 (12) p < 0.0002 |
15(88) p < 0.0002 |
52 (NS) |
80 (NS) |
58 (NS) |
| P16− | 31 (66) | 63 | 28 (90) | 3 (10) | 22(71) | 9(29) | 54 | 73 | 48 |
K keratinizing squamous cell carcinoma, NK nonkeratinizing squamous cell carcinoma
DSS was favorable in all cases. The 5-year DSS for p16-positive and p16-negative cases was 80 and 70 %, respectively. No statistically significant difference in OS, DSS or disease-free survival (DFS) was found regarding tumor site, morphologic type, or HPV relationship (Table 3). However, a trend towards better DFS was seen in patients with p16 positive/HPV positive tumors.
Small Cell Carcinoma
An association between HPV and neuroendocrine carcinoma of the oropharynx has been shown in two recent studies [27, 28]. Bishop and Westra [27] found that 5 of 9 cases of oropharyngeal small cell carcinoma (poorly differentiated neuroendocrine carcinoma) were HPV-related. In 4 of these cases, the tumors were associated with conventional HPV-related SCC. All 5 tumors were p16-positive by immunohostochemistry and HPV-positive by ISH. All cases showed a characteristic neuroendocrine immunophenotype, including reactivity to synaptophysin and/or chromogranin and CAM 5.2 and were negative for CK 5/6. Three of the 5 patients died within 15 months of diagnosis (mean 10 months) with widely disseminated disease. Such outcome is in sharp contrast to the typical HPV-related nonkeratinizing SCC which is associated with a 3-year survival rate of up to 80 % [29]. Kraft et al. [28] reported 8 oropharyngeal neuroendocrine carcinomas that were HPV-related. Disease recurrence occurred in 5 of 6 patients with available clinical follow-up, with 3 developing metastasis to bone, lung, pleura, adrenal gland, and pancreas.
HPV-related small cell carcinoma of the oropharynx shares common features with small cell neuroendocrine carcinoma of the uterine cervix. Both are associated with high-risk HPV, commonly coexist with non-small cell squamous cell carcinoma, and share the same aggressive clinical behavior with early distant metastasis and poor OS [27, 28, 30, 31].
Discussion
The majority of HPV-related SCC of the oropharynx exhibits a characteristic microscopic appearance, typically a non-keratinizing squamous cell morphology [31–33]. The neoplastic cells show an immature basaloid appearance and are mitotically active. That phenotype is not limited to oropharyngeal sites but it is also observed in other HPV-related tumors in the sinonasal tract, larynx, cervix and other anogenital sites [34–36]. The exact molecular mechanisms underlying the expression of this specific histopathologic phenotype are not clear. It is possible that interactions between HPV oncoproteins and cell cycle mediators may play a role. It is known that high-risk HPV early proteins, particularly E6 and E7, through their interference with Rb and p53 functions, lead to cell cycle progression, cell immortalization, prevention of apoptosis, and essentially uncoupling of proliferation and maturation Chromosomal instability is another property of HPV oncogenesis. It is likely that additional mutations may be responsible for the development of those rare microscopic variants.
As the numbers of reported HPV-positive head and neck SCC variants are increasing, particularly in the oropharynx, it is of importance to establish a causal relationship between the virus and the tumors and to show that the virus is actually a “driver” rather than “passenger”. Currently, the number of cases of SCC variants with established HPV relationship is still limited. Based on a limited number of studies, there is some evidence to suggest that variants such as BSCC, UDCA, and PSCC may have similar favorable prognosis as the conventional NKSCC. On the other hand, HPV-related small cell carcinoma has been shown to be associated with early distant metastasis and poor OS. It is thus too early to reach a definite conclusion regarding the biologic behavior of HPV-related SCC morphologic variants. The detection of HPV in these tumors should not be used, at this time, as a justification for considering less intensive multimodality therapy.
References
- 1.Liu FS, Chen CJ, Kan YY, et al. Adenosquamous carcinoma of the cervix-A clinical analysis of 23 cases. Chin Med J. 1990;45:261–265. [PubMed] [Google Scholar]
- 2.Gallup DG, Harper RH, Stock RJ. Poor prognosis in patients with adenosquamous cell carcinoma of the cervix. Obestet Gynecol. 1985;65:416–422. [PubMed] [Google Scholar]
- 3.Wheeless CR, Graham JB. Prognosis and treatment of adenoepidermoid carcinoma of the cervix. Obestet Gynecol. 1970;35:928–932. [PubMed] [Google Scholar]
- 4.Harrison TA, Sevin BU, Koechli O, et al. Adenosquamous carcinoma of the cervix: prognosis in early stage disease treated by radical hysterectomy. Gyncol Oncol. 1993;50:310–315. doi: 10.1006/gyno.1993.1217. [DOI] [PubMed] [Google Scholar]
- 5.Yazigi R, Sandstad J, Munoz AK, et al. adenosquamous carcinoma of the cervix: prognosis in stage IB. Obestet Gynicol. 1990;75:1012–1015. [PubMed] [Google Scholar]
- 6.Angel C, DeBeshter B, Lin JY. Clinical presentation and management of stage I cervical adenocarcinoma: a 25 year experience. Gynico Oncol. 1992;44:71–78. doi: 10.1016/0090-8258(92)90015-B. [DOI] [PubMed] [Google Scholar]
- 7.Ogura K, Ishi K, Matsumoto T, et al. Human papilloma virus localization in cervical adenocarcinoma and adenosquamous carcinoma using in situ polymerase chain reaction: review of the literature of human papillomavirus detection in these carcinomas. Pathol Int. 2006;56:301–308. doi: 10.1111/j.1440-1827.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- 8.Chernock RD, Lewis JS, Jr, Zhang Q, El-Mofty SK. Human papillomavirus positive basaloid squamous cell carcinoma of the upper aerodigestive tract: a distinct clinicopathologic and molecular subtype of basaloid squamous cell carcinoma. Human Pathol. 2010;41:1016–1023. doi: 10.1016/j.humpath.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begun S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32:1044–1050. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter D, El-Mofty SK, Lewis JS., Jr Undifferentiated carcinoma of the oropharynx: a human papillomavirus-associated tumor with favorable prognosis. Mod Pathol. 2011;24:1306–1312. doi: 10.1038/modpathol.2011.87. [DOI] [PubMed] [Google Scholar]
- 11.Masand RP, El-Mofty SK, Ma XJ, et al. Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of literature. Head Neck Pathol. 2011;5:108–116. doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wain SL, Kier R, Vollmer RT, et al. Basaloid-squamous carcinoma of the tongue, hypopharynx and larynx: report of 10 cases. Hum Pathol. 1986;17:1158–1166. doi: 10.1016/S0046-8177(86)80422-1. [DOI] [PubMed] [Google Scholar]
- 13.Nicholls M, Agathanggelou A, Fung K, et al. The association of squamous cell carcinoma of the nasopharynx with Epstein-Barr virus shows geographical variations reminiscent of Burkitt’s lymphoma. J Pathol. 1997;183:164–168. doi: 10.1002/(SICI)1096-9896(199710)183:2<164::AID-PATH919>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Chan JKC, Bray J, McCarron P, et al. Nasopharyngeal carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, et al., editors. World Health Organization classification of tumors—pathology and genetics of head and neck tumors. Geneva: IARC Press; 2005. pp. 85–97. [Google Scholar]
- 15.Lee AW, Sze WM, Au JS, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 16.Chan JK, Yip TT, Tsang WY, et al. Specific association of Epstein-Barr virus with lymphoepithelial carcinoma among tumors and tumor-like lesions of salivary glands. Arch Pathol Lab Med. 1994;118:994–997. [PubMed] [Google Scholar]
- 17.Singhi AD, Stelow EB, Mills SE, Westra WH. Lymphoepithelial-like carcinoma of the oropharynx; A morphologic variant of HPV-related head and neck carcinoma. Am J Surg Pathol. 2010;34:800–805. doi: 10.1097/PAS.0b013e3181d9ba21. [DOI] [PubMed] [Google Scholar]
- 18.Barnes EL, Eveson JW, Reichart P, Sideransky D. Pathology and genetics of head and neck tumors. In: Kleihues P, Sobin LH, editors. World health Organization Classification of Tumors. Lyon: IARC Press; 2005. [Google Scholar]
- 19.Brinck U, Jacob C, Bau O, Fuzesi L. Papillary squamous cell carcinoma of the uterine cervix: report of three cases and review of their classification. Int J Gynecol Pathol. 2000;19:231–235. doi: 10.1097/00004347-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Cubilla AL, Velazques EF, Reuter VE, Oliva E, et al. Warty (condylomatous) squamous cell carcinoma of the penis; A report of 11 cases and proposed classification of “verruciform” penile tumors. Am J Surg Pathol. 2000;24:505–512. doi: 10.1097/00000478-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Fuste V, Pino M, Perez A, Garcia A, et al. Primary squamous cell carcinoma of the vagina: human papillomavirus detection, p16 INK4A overexpression and clinicopathologic correlation. Histopathology. 2010;57:907–916. doi: 10.1111/j.1365-2559.2010.03727.x. [DOI] [PubMed] [Google Scholar]
- 22.Santos M, Landolfi S, Olivella A, Lloveras B, et al. p16 overexpression identifies HPV-positive vulvar squamous cell carcinomas. Am J Surg Pathol. 2006;30:1347–1356. doi: 10.1097/01.pas.0000213251.82940.bf. [DOI] [PubMed] [Google Scholar]
- 23.Kurman RJ, Toki T, Schiffman MH. Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomavirus. Am J Surg Pathol. 1993;17:133–145. doi: 10.1097/00000478-199305000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Suarez PA, Adler-storthz K, Luna MA, et al. Papillary squamous cell carcinoma of upper aerodigestive tract: a clinicopathologic and molecular study. Head Neck. 2000;22:360–368. doi: 10.1002/1097-0347(200007)22:4<360::AID-HED8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 25.Jo VY, Mills SE, Stoler MH, et al. Papillary squamous cell carcinoma of the head and neck. Frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol. 2009;33:1720–1724. doi: 10.1097/PAS.0b013e3181b6d8e6. [DOI] [PubMed] [Google Scholar]
- 26.Mehrad M, Carpenter DH, Chernock RD, et al. Characterization of the head and neck papillary squamous cell carcinoma variants: clinicopathologic and molecular study with special reference to human papillomavirus (HPV) association. Mod Pathol. 2012;25S:313A. doi: 10.1097/PAS.0b013e318290427d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J Surg Pathol. 2011;35:1679–1684. doi: 10.1097/PAS.0b013e3182299cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraft S, Faquin WC, Krane JF. HPV-associated neuroendocrine carcinoma of the oropharynx: a rare new entity with potentially aggressive clinical behavior. Am J Surg Pathol. 2012;36:321–330. doi: 10.1097/PAS.0b013e31823f2f17. [DOI] [PubMed] [Google Scholar]
- 29.Ang KK, Harris J, Wheeler R, et al. Human Papilloma and survival in oropharyngeal cancer. N Eng J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abeler VM, Holm R, Nesland JM, et al. Small cell carcinoma of the uterine cervix: a clinicopathologic study of 26 patients. Cancer. 1994;73:672–677. doi: 10.1002/1097-0142(19940201)73:3<672::AID-CNCR2820730328>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 31.Wang KL, Yang YC, Wang TY, et al. Neuroendocrine carcinoma of the uterine cervix: a retrospective study of 31 cases with prognostic implications. J Chemother. 2006;18:209–216. doi: 10.1179/joc.2006.18.2.209. [DOI] [PubMed] [Google Scholar]
- 32.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27:1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 33.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):339–345. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS., Jr HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3:186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Mofty SK, Lu DW. Prevalence of high risk human papilloma virus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 36.Lu DW, El-Mofty SK, Wang H. Expression of p16, Rb and p53 proteins in squamous cell carcinoma of the anorectal region harboring human papillomavirus DNA. Modern Pathol. 2003;16:692–699. doi: 10.1097/01.MP.0000077417.08371.CE. [DOI] [PubMed] [Google Scholar]