Abstract
Morphologic assessment is one of the most basic tools that pathologists use to classify tumors. Human papillomavirus (HPV)-related squamous cell carcinoma of the oropharynx has unique morphologic features that can be readily recognized under the microscope. Yet, these features are not widely recognized or uniformly reported. In our practice, we group oropharyngeal squamous cell carcinomas into ‘nonkeratinizing’, ‘nonkeratinizing with maturation’, and ‘keratinizing’ histologic types. The ‘nonkeratinizing’ type has a very strong association with HPV, while the ‘keratinizing’ type has a weaker association with the virus. ‘Nonkeratinizing with maturation’ is intermediate but much more closely related to the ‘nonkeratinizing’ type. This classification system parallels that of sinonasal and nasopharyngeal squamous cell carcinomas where nonkeratinizing squamous cell carcinomas are widely recognized histologic variants. This review will discuss this classification system and its utility in routine clinical practice.
Keywords: Human papillomavirus, Nonkeratinizing squamous cell carcinoma, Morphology, Oropharynx
Introduction
Routine histologic evaluation of patient specimens is one of the most basic tools that pathologists use to characterize disease processes. Currently, oropharyngeal squamous cell carcinomas (SCCs) are morphologically classified by the World Health Organization (WHO) into well, moderately, and poorly differentiated groups with separation of less common, but distinct, histologic variants such as adenosquamous carcinoma, basaloid squamous cell carcinoma, and verrucous carcinoma, among others, from the larger group [1]. Over the past decade, however, it has become increasingly recognized that the majority of human papillomavirus (HPV)-related SCCs of the oropharynx also have unique histologic features that can be recognized microscopically, although these tumors are not currently classified as a unique subtype of SCC in the current WHO classification of head and neck tumors [2–6]. Furthermore, identification of these morphologic indicators of HPV infection can aid a pathologist in clinical practice in many different ways, for example, in the triaging of cases for further HPV testing, and may be particularly useful in settings where ancillary testing is not available (such as resource-limited practices or intraoperative frozen sections).
Histologic Typing: ‘Keratinizing,’ ‘Nonkeratinizing’ and ‘Nonkeratinizing with Maturation’
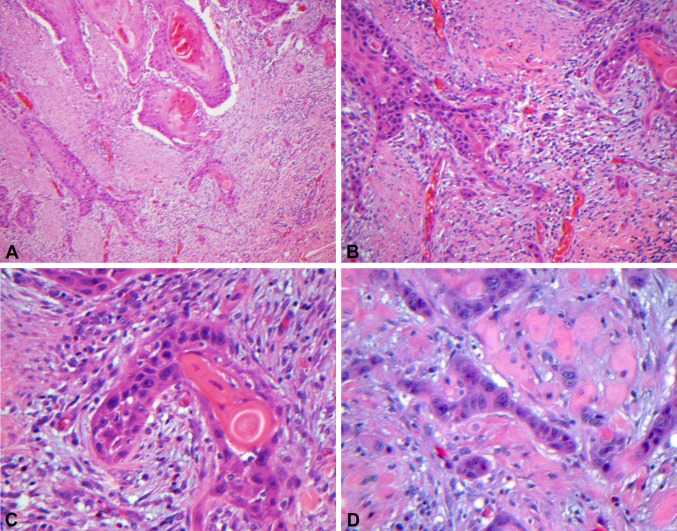
The majority of HPV-related oropharyngeal SCCs have a ‘nonkeratinizing’ appearance, while HPV-unrelated tumors are typically ‘keratinizing.’ Microscopically, HPV-related ‘nonkeratinizing’ tumors tend to form large nests that have pushing borders with little stromal response, frequent mitoses and often central comedo necrosis. The cells are ovoid to spindle-shaped with indistinct cell borders and have hyperchromatic nuclei that lack prominent nucleoli. Squamous maturation is either absent or is limited (Fig. 1a, b, c). In contrast, non-HPV-related ‘keratinizing’ SCCs are typically composed of infiltrative nests with prominent stromal desmoplasia. The tumor cells are polygonal with distinct cell borders and more abundant, eosinophilic cytoplasm. Squamous maturation is diffuse (Fig. 2a, b, c, d).
Fig. 1.
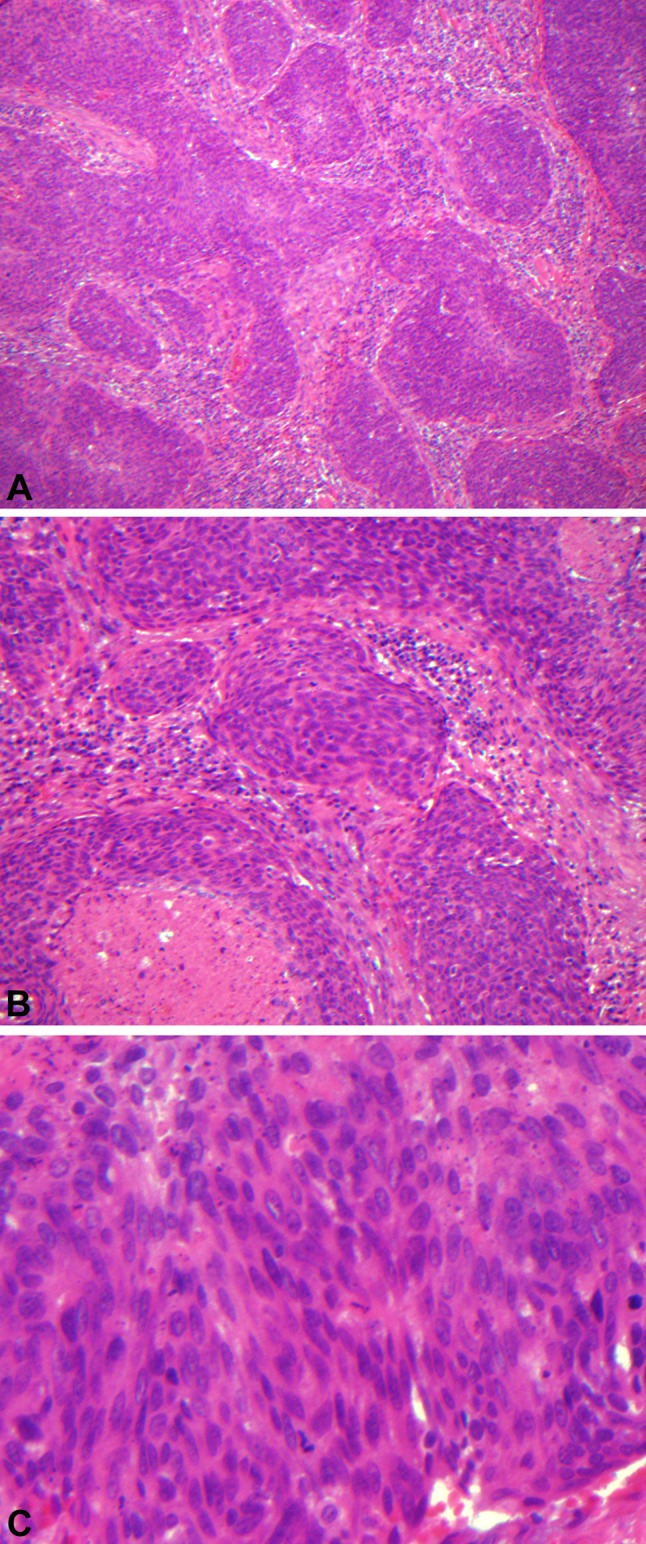
Low (a 100X), medium (b 200X) and high (c 400X) power images of ‘nonkeratinizing’ squamous cell carcinoma. There are large nests of tumor cells that have pushing borders with little stromal response and central comedo necrosis. The cells are ovoid to spindle-shaped with indistinct cells borders and have hyperchromatic nuclei that lack prominent nucleoli. Squamous maturation is minimal
Fig. 2.
Low (a 100X), medium (b 200X) and high (c, d 400X) power images of ‘keratinizing’ squamous cell carcinoma. There are infiltrative nests of tumor cells with prominent stromal desmoplasia. The tumor cells are polygonal with distinct cell borders and more abundant, eosinophilic cytoplasm. Keratin pearls are present. Squamous maturation is diffuse even in poorly differentiated tumors that lack keratinization (D)
In some cases, tumors have histologic features of both ‘keratinizing’ and ‘nonkeratinizing’ SCCs. When tumors have at least some areas with definitive ‘nonkeratinizing’ morphology but also have significant (greater than 10% tumor surface area) squamous maturation (‘keratinizing’ features), we refer to them as ‘hybrid’ or ‘nonkeratinizing SCCs with maturation’ (Fig. 3a, b). ‘Nonkeratinizing SCCs with maturation’ also have a strong association with HPV but the virus is slightly less frequently detected than in purely ‘nonkeratinizing’ tumors [2, 7].
Fig. 3.
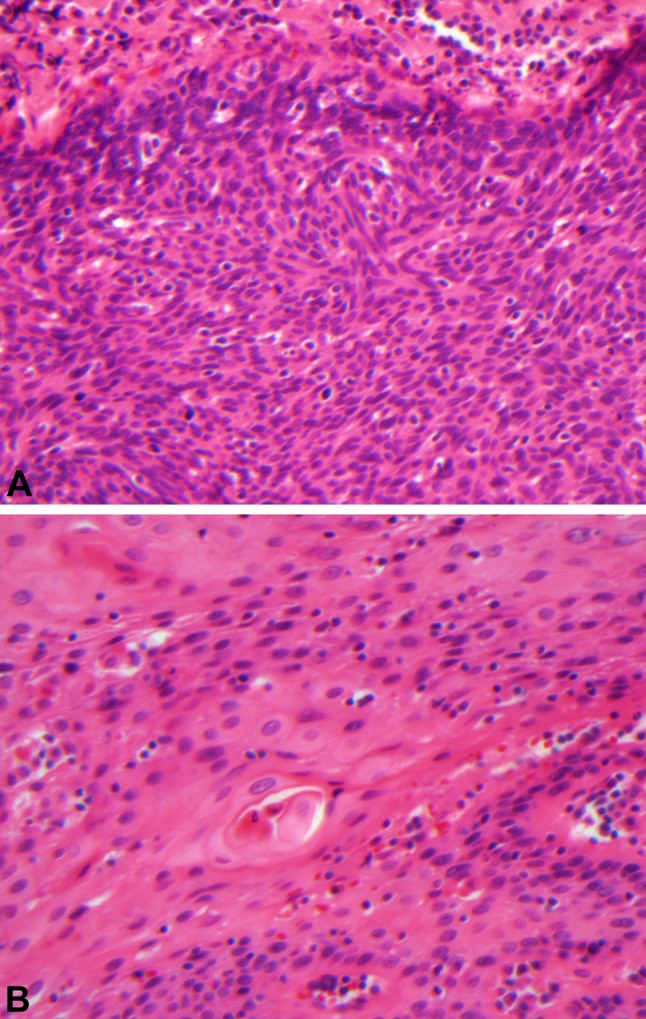
‘Nonkeratinizing squamous cell carcinoma with maturation’ has both areas with features of ‘nonkeratinizing’ squamous cell carcinoma (a 400X) as well as areas of ‘keratinizing’ squamous cell carcinoma (b 400X). The latter comprises greater than 10% of the tumor
In our experience, ‘nonkeratinizing’ is the most common histologic type of oropharyngeal SCC. Approximately 50% of oropharyngeal SCCs are ‘nonkeratinizing,’ while 25% are ‘keratinizing’ and another 25% are ‘nonkeratinizing with maturation’ [2, 7]. However, the frequency of these histologic types may vary in different patient populations.
While we prefer to classify oropharyngeal SCCs as ‘keratinizing,’ ‘nonkeratinizing’ and ‘nonkeratinizing with maturation,’ the terminology that has been used in the literature is quite variable. HPV-related tumors have alternately been described as ‘basaloid,’ ‘basal-like,’ ‘poorly-differentiated,’ as well as ‘nonkeratinizing’ [4, 5, 8]. The term ‘basaloid’ and ‘basal-like’ have been used primarily because HPV-related tumors typically have a ‘blue cell’ appearance reminiscent of the basal cell layer of squamous epithelium. However, basaloid squamous cell carcinoma is itself a defined, rare histologic variant that is not necessarily a virally-driven tumor, but which may be HPV-related when arising in the oropharynx [4, 9, 10]. Furthermore, the morphology of basaloid squamous cell carcinoma as defined by Wain et al. [11] is distinct from ‘nonkeratinizing’ SCC (see article to follow by El-Mofty SK). Therefore, we believe that the term ‘basaloid’ should be avoided when describing HPV-related ‘nonkeratinizing’ tumors to avoid confusion with that specific variant, especially since HPV-negative basaloid squamous cell carcinoma may be associated with a particularly poor prognosis [9, 10]. Likewise, use of the term ‘basal-like’ may cause confusion with other diagnostic entities with similar names such as cutaneous basal cell carcinoma and basal cell adenocarcinoma of salivary gland origin. ‘Poorly differentiated’ is also a misleading term both because ‘keratinizing’ SCCs may be poorly differentiated as well, and more importantly because ‘poorly differentiated’ implies to the clinicians treating the patients a more primitive and potentially more aggressive tumor type. This is simply not fitting (and actually is paradoxical) for HPV-related tumors, which have consistently been shown to have better patient survival [12, 13]. Therefore, we believe histologic grading of squamous cell carcinoma should be limited to conventional ‘keratinizing’ type SCC.
The variability in terminology that has been applied to HPV-related tumors clearly indicates a need for a consistent classification system for these conventional oropharyngeal SCCs. A uniform classification system would enhance communication both among pathologists and with clinical colleagues taking care of the patients. We feel that the terminology of ‘keratinizing,’ ‘nonkeratinizing,’ and ‘nonkeratinizing with maturation’ best reflects the histologic features of these tumors without implying inaccurate prognostic information or creating confusion with other distinctly different diagnostic entities that may also occur in the oropharynx.
Histologic Types and Human Papillomavirus
The correlation of ‘nonkeratinizing’ morphology in the oropharynx with HPV infection is very strong. In a large study of 239 oropharyngeal SCCs, only 1 out of 125 (< 1%) of the ‘nonkeratinizing’ tumors was both p16 negative (defined as less than 50% of cells with nuclear and cytoplasmic staining) and negative for HPV by DNA ISH [7]. HPV was undetectable (by DNA ISH and PCR) in 13 (10.4%) additional ‘nonkeratinizing’ tumors that were p16 positive [7]. In other words, the HPV DNA detection rate was approximately 88.0% by ISH or PCR, and the p16 positivity rate was approximately 98.4% in ‘nonkeratinizing’ oropharyngeal SCC. In contrast, only 12 of 58 (20.7%) ‘keratinizing’ oropharyngeal tumors were HPV positive and 11 of 58 (19.0%) were p16 positive [7]. ‘Nonkeratinizing with maturation’ tumors were intermediate with an HPV detection rate of 73.5% (36/49) and a p16 positivity rate of 83.7% (41/49) [7]. These results are summarized in Table 1. Similarly, high p16 positivity rates in ‘nonkeratinizing’ and ‘nonkeratinizing with maturation’ tumors were also seen in another study examining the use of histologic typing in routine clinical practice [14]. Biologic activity of the virus found in these tumors has been confirmed not just by p16 immunohistochemistry but also by RNA ISH, which has detected transcriptionally active HPV in approximately 97% of ‘nonkeratinizing’ SCCs [15]. Not surprisingly, ‘nonkeratinizing’ morphology, like HPV and p16 positivity with which it is strongly correlated, is also strongly associated with better patient survival [2]. Whether histologic classification adds any additional prognostic information beyond that of HPV and/or p16 status is uncertain.
Table 1.
Relationship between morphology and HPV DNA and p16 status adapted from a large study of oropharyngeal squamous cell carcinomas [7]
| Biomarker | ‘Nonkeratinizing’a 125 (53.9%) |
‘Nonkeratinizing with maturation’a 49 (21.1%) |
‘Keratinizing’ 58 (25.0%) |
|---|---|---|---|
| p16 | |||
| Positive | 123 (98.4%) | 41 (83.7%) | 11 (19.0%) |
| Negative | 2 (1.6%) | 8 (16.3%) | 47 (81.0%) |
| HPV statusb | |||
| Positive | 110 (88.0%) | 36 (73.5%) | 12 (20.7%) |
| Negative | 13 (10.4%) | 13 (26.5%) | 46 (79.3%) |
ap < 0.001 for both p16 and HPV status compared ‘keratinizing’ type
bby DNA ISH or PCR
The reason that the majority of HPV-related oropharyngeal SCCs display ‘nonkeratinizing’ morphology is not known. It is possible that virally-induced molecular genetic changes account for this phenotype. It has been suggested that the interaction of HPV oncoproteins with cell cycle mediators results in the uncoupling of cell proliferation from cell maturation. Another, unproven theory is that these tumors mimic the morphology of normal tonsillar crypt epithelium from which they are derived [16]. HPV-related oropharyngeal SCCs have a predilection for the palatine and lingual tonsillar crypt epithelium. Normal tonsillar crypt epithelium shows less squamous maturation than surface squamous mucosa and often is heavily infiltrated by lymphocytes (Fig. 4a). Like normal tonsillar crypt epithelium, ‘nonkeratinizing’ SCC also lacks significant squamous maturation and often shows a pronounced host lymphocytic response (Fig. 4b). Therefore, it may be that ‘nonkeratinizing’ SCC is an indicator of tonsillar crypt origin, which, in turn, reflects preferential HPV infection at this site. This theory could also explain why not all HPV infected tumors have ‘nonkeratinizing’ morphology—it may be that HPV infection can occur, albeit less frequently, in surface epithelium and contribute to carcinogenesis of a subset of ‘keratinizing’ SCCs as well.
Fig. 4.
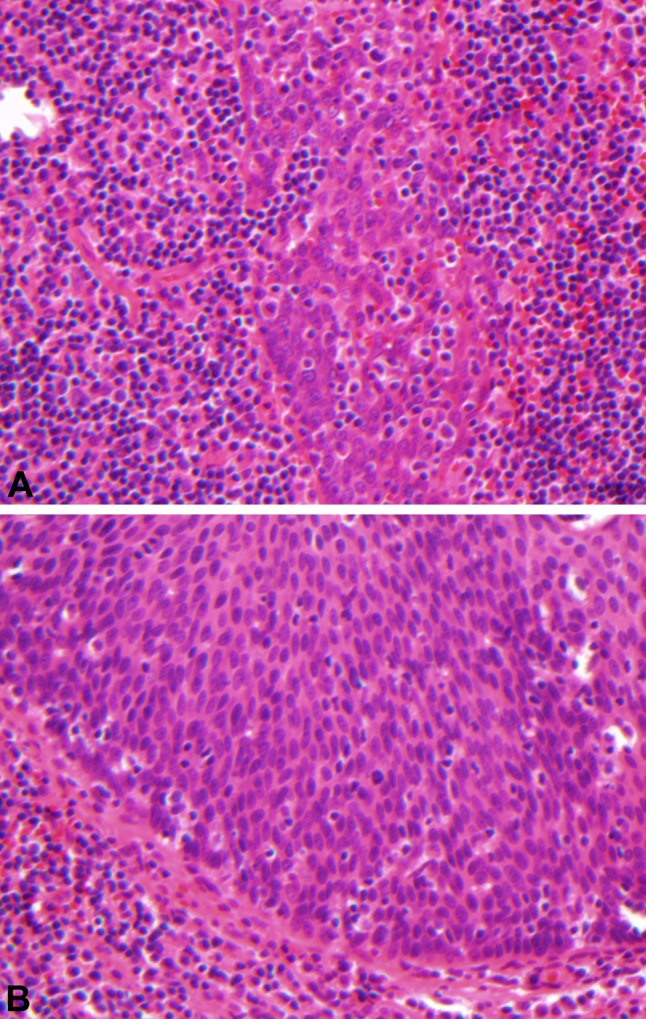
Normal tonsillar crypt epithelium (a 400X) and ‘nonkeratinizing’ squamous cell carcinoma (b 400X) both show little squamous maturation and infiltration by lymphocytes
‘Nonkeratinizing’ Squamous Cell Carcinoma of Non-Oropharyngeal Head and Neck Subsites
‘Nonkeratinizing’ morphology is not exclusively seen in the oropharynx but is rare at most other head and neck sites. We have not observed ‘nonkeratinizing’ tumors in the oral cavity, larynx or hypopharynx (except by direct extension from an oropharyngeal primary). Nonkeratinizing squamous cell carcinoma does occur in the nasopharynx (referred to as nonkeratinizing differentiated carcinoma) where its association with Epstein-Barr virus is well documented. More recently, HPV-positive squamous cell carcinomas with nonkeratinizing morphology have also been reported in the nasopharynx [17, 18]. However, it appears that many of these tumors may actually represent oropharyngeal primary tumors masquerading as nasopharyngeal carcinomas. Singhi et al. (2012) found that 4 of 45 nonkeratinizing nasopharyngeal carcinomas (9%) were HPV-positive/EBV-negative [17]. On retrospective review, 3 of these 4 cases involved the oropharynx and the 4th case did not have available staging information [17]. Thus, it seems most likely that these HPV-positive tumors actually arose in the oropharynx with extension to the nasopharynx. Maxwell et al. (2009), also reported 4 HPV-positive/EBV-negative nonkeratinizing SCCs in the nasopharynx but did not comment on presence or absence of oropharyngeal extension [18]. While the possibility of rare HPV-positive tumors truly arising in the nasopharynx cannot be entirely excluded, it seems that given its close proximity to the oropharynx, most may represent extension from the latter. Nonkeratinizing squamous cell carcinoma also occurs in the sinonasal tract (sometimes referred to as cylindrical cell, transitional or Schneiderian carcinoma) where it is thought to arise from the transitional or Schneiderian mucosa of the sinonasal tract. A significant proportion of these do in fact appear to be HPV-related [19, 20]. Like HPV-related oropharyngeal SCC, HPV-positive nonkeratinizing sinonasal SCCs also appear to have better patient outcomes [20]. Both nonkeratinizing SCC of the sinonasal tract and nasopharynx are recognized as distinct histologic subtypes by the World Health Organization [21].
Clinical Utility of Histologic Typing in Oropharyngeal SCC
Morphologic separation of oropharyngeal SCC into ‘keratinizing,’ ‘nonkeratinizing’ and ‘nonkeratinizing with maturation’ types has potential diagnostic utility. p16 is often used as a surrogate marker or initial screening test for HPV-related oropharyngeal tumors. Since ‘nonkeratinizing’ morphology is virtually synonymous with p16 positivity, one may reasonably conclude that p16 testing is unnecessary in this histologic group. p16 negative ‘nonkeratinizing’ SCC of the oropharynx is quite rare and at this point its significance is uncertain. Furthermore, in an interobserver agreement study, no tumor that was classified as ‘nonkeratinizing’ by any of the six pathologists was p16 negative, suggesting that the designation of a tumor as ‘nonkeratinizing’ is a reliable indicator of p16 expression, at least among head and neck pathologists [14].
There are other scenarios where histologic classification may be useful. In resource-limited practices there may be limited access to techniques such as immunohistochemistry, in situ hybridization, or PCR, which are commonly used to perform HPV specific testing. In this situation, morphologic classification may serve as a surrogate indicator of an HPV-related tumor. However, ancillary testing may not be available in some situations even at institutions where ancillary testing is routinely performed or is accessible, making histologic classification beneficial here as well. For example, small biopsy material may have insufficient tissue remaining in the paraffin block for additional studies and ancillary studies are not typically available at the time of intraoperative frozen section evaluation. We have encountered cases where recognition of ‘nonkeratinizing’ morphology on intraoperative frozen section was clinically useful. For example, patients with HPV-related oropharyngeal SCC often present with lymph node metastases to the neck and the primary tumor may be occult. In the setting of an occult primary SCC, it is customary at our institution to perform endoscopic examination of the upper aerodigestive tract mucosa with biopsy and intraoperative frozen section of any suspicious areas with particular attention to the oropharyngeal tonsillar tissue. In one such case, a patient was known to have a p16 positive ‘nonkeratinizing’ SCC metastatic to a neck lymph node. Intraoperatively, a suspicious lesion on the epiglottis was biopsied and submitted for frozen section which showed a ‘keratinizing’ SCC. Because of the histologic discrepancy between this tumor and the lymph node metastasis, the search was continued and a second separate tumor, this time with ‘nonkeratinizing’ morphology, was eventually identified and considered to be the more likely origin of the histologically similar nodal metastasis. The patient had two separate primary SCCs. Without histologic characterization, the first tumor could have been easily considered the primary and the second tumor overlooked.
Similarly, recognition of ‘nonkeratinizing’ morphology may be quite useful when faced with a metastasis outside of the head and neck that is of uncertain primary site. It can be the first clue that the metastasis might be of oropharyngeal origin and this knowledge can be used to effectively guide a panel of immunostains, if needed. Furthermore, in patients who have a known history of oropharyngeal ‘nonkeratinizing’ SCC and subsequently present with a solitary pulmonary SCC, the question often arises whether the SCC in the lung represents a new primary or a metastasis from the prior head and neck SCC. Morphologic evaluation can be very useful since, in our experience, ‘nonkeratinizing’ SCC is rare in lung primary tumors, and, thus would most likely represent a metastasis.
The question remains—can ‘nonkeratinizing’ oropharyngeal SCC be recognized by pathologists in routine clinical practice? Since this is not currently a widely recognized or specific WHO recognized histologic subtype of oropharyngeal SCC, the presence or absence of ‘nonkeratinizing’ features are not routinely described by pathologists. Nevertheless, it seems likely that the distinction between ‘keratinizing’ and ‘nonkeratinizing’ can be made in clinical practice, particularly since this parallels in many ways the accepted WHO classification of sinonasal or nasopharyngeal carcinomas. The previously mentioned interobserver study among six head and neck pathologists did find good agreement in the diagnosis of ‘keratinizing’ and ‘nonkeratinizing’ oropharyngeal SCC, although agreement was poor for ‘nonkeratinizing with maturation’ tumors [14]. This suggests that further education/awareness is needed to achieve more consistent classification.
Summary
We believe that recognition of ‘nonkeratinizing’ morphologic features, a strong indicator of HPV infection in oropharyngeal SCC, is important and, thus, a need for consistent terminology in describing these tumors exists. Morphologic classification is a useful adjunct that does not necessarily replace ancillary HPV testing but may reduce the need to perform p16 immunohistochemistry on a significant subset of cases (‘nonkeratinizing’ tumors). Histologic classification may also provide valuable information in certain settings, particularly when HPV testing is not immediately available. For these reasons, we hope that classification of oropharyngeal SCCs as ‘nonkeratinizing,’ ‘nonkeratinizing with maturation,’ and ‘keratinizing’ histologic types is adapted into clinical practice and becomes recognized by the WHO as representing distinct tumor subtypes.
Acknowledgments
I would like to thank Walter Clermont for his assistance in preparation of the figures and James S. Lewis Jr. MD for critical review of the manuscript.
Conflict of interest
The author has no conflicts of interest or funding to disclose.
References:
- 1.Johnson N, Franceschi S, Ferlay J, Ramadas K, Schmid S, MacDonald DG, Bouquot JE, Slootweg PJ. Squamous cell carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics: head and neck tumours. Lyon: IARC Press; 2005. pp. 168–175. [Google Scholar]
- 2.Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS. HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic featurtes in predicting patient outcome. Head Neck Pathol. 2009;3:186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:339–345. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity in young patients: a distinct cllinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27(11):1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn AH, Lai CK, Shintaku IP, Elashoff DA, Dubinett SM, Abemayor E, St. John MA. Histopathologic findings of HPV and p16 positive HNSCC. Laryngoscope. 2010;120:1788–1794. doi: 10.1002/lary.21044. [DOI] [PubMed] [Google Scholar]
- 6.Wilczynski SP, Lin BT, Xie Y, Paz IB. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am J Pathol. 1998;152(1):145–156. [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis JS, Jr, Thorstad W, Chernock RD, Haughey BH, Yip JH, Zhang Q, El-Mofty SK. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34(8):1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHugh JB. Association of cystic neck metastases and human papillomavirus-positive oropharyngeal squamous cell carcinoma. Arch Pathol Lab Med. 2009;133:1798–1803. doi: 10.5858/133.11.1798. [DOI] [PubMed] [Google Scholar]
- 9.Chernock RD, Lewis JS, Jr, Zhang Q, El-Mofty SK. Human papillomavirus-positive basaloid squamous cell carcinomas of the upper aerodigestive tract: a distinct clinicopathologic and molecular subtype of basaloid squamous cell carcinoma. Hum Pathol. 2010;41:1016–1023. doi: 10.1016/j.humpath.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Begum S, Westra W. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32(7):1044–1050. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 11.Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: report of 10 cases. Hum Pathol. 1986;17(11):1158–1166. doi: 10.1016/S0046-8177(86)80422-1. [DOI] [PubMed] [Google Scholar]
- 12.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP. Human papillomavirus and survival of patients wtih oropharyngeal cancer. N Eng J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, Sasaki C, Joe J, Camp RL, Rimm DL, Psyrri A. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JS, Khan RA, Masand RP, Chernock RD, Zhang Q, Al-Naief NS, Muller S, McHugh JB, Prasad M, Brandwein-Gensler M, Perez-Ordonez B, El-Mofty SK. Recognition of nonkeratinizing morphology in oropharyngeal squamous cell carcinoma-a prospective cohort and interobserver variability study. Histopathol. 2012;60(3):427–436. doi: 10.1111/j.1365-2559.2011.04092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis JS Jr, Ukpo O, Ma XJ, Flanagan JJ, Luo Y, Thorstad WL, Chernock RD. Transcriptionally-active high-risk human papillomavirus is rare in oral cavity and laryngeal/hypopharyngeal squamous cell carcinomas-a tissue microarray study utilizing E6/E7 mRNA in situ hybridization. Histopathol. [Epub ahead of print]. [DOI] [PubMed]
- 16.Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singhi AD, Califano J, Westra WH. High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck. 2012;34:213–218. doi: 10.1002/hed.21714. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell JH, Kumar B, Feng FY, McHugh JB, Cordell KG, Eisbruch A, Worden FP, Wolf GT, Prince ME, Moyer JS, Teknos TN, Chepeha DB, Stoeker J, Walline H, Carey TE, Bradford CR. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2009;32(5):562–567. doi: 10.1002/hed.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29(10):1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 20.Alos L, Moyano S, Nadal A, Alobid I, Blanch JL, Ayala E, Lloveras B, Quint W, Cardesa A, Ordi J. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115(12):2701–2709. doi: 10.1002/cncr.24309. [DOI] [PubMed] [Google Scholar]
- 21.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]