Abstract
Perhaps one of the most important developments in head and neck oncology of the past decade is the demonstration that patients with human papillomavirus (HPV)-mediated oropharyngeal cancers have significantly improved outcomes, compared to HPV-negative counterpart patients. This has become the basis for clinical trials investigating the impact on “treatment deintensification” for patients with HPV-mediated oropharyngeal cancers. Unfortunately, the significance of HPV in non-oropharyngeal head and neck cancers is much less certain. Our goal is to systematically review the published data regarding the role HPV in carcinomas of the oral cavity, larynx, sinonasal tract and nasopharynx with respect to HPV detection frequency, viral activity, and association with outcome. We also present preliminary data on HPV16/18 transcriptional status in oral cavity carcinomas, as well as salivary gland neoplasia, as determined by nested reverse transcription PCR for HPV E6/E7 RNA. The weighted prevalence (WP) of HPV DNA detection in 4,195 oral cavity cancer patients is 20.2 %, (95 % CI 16.0 %, 25.2 %). HPV16 is the most common type detected. Importantly, no data currently demonstrates a significant association between the presence of HPV DNA and improved outcome. The WP of HPV DNA in 1,712 laryngeal cancer patients is 23.6 %, (95 % CI 18.7 %, 29.3 %). Similarly, no association has yet been demonstrated between HPV DNA status and outcome. The WP of HPV DNA detection in 120 sinonasal cancer patients is 29.6 % (95 % CI 17.8 %, 44.9 %), and in 154 nasopharyngeal carcinoma patients is 31.1 %, (95 % CI 20.3 %, 44.5 %). Recent preliminary data also suggests an association between HPV and certain salivary gland neoplasms. The clinical significance of these findings is unclear. The published data strongly support the need for studies on patients with oral and laryngeal carcinomas that will be powered to find any differences in clinical outcome with respect to HR-HPV and p16 overexpression.
Keywords: HPV, Squamous carcinoma, Oral cavity, Laryngeal, Larynx, Mucoepidermoid carcinoma, Salivary
Introduction
Perhaps one of the most important developments in head and neck oncology of the past decade is the demonstration that patients with human papillomavirus (HPV)-mediated oropharyngeal cancers have significantly improved clinical outcomes, compared to patients with HPV-negative oropharyngeal carcinomas. Clinical trials are underway to investigate the impact of “treatment deintensification” for patients with HPV-mediated oropharyngeal cancers. The possibility of offering patients with HPV-mediated cancers less aggressive adjuvant therapy is especially relevant given the potential for devastating radiation toxicities. Can treatment deintensification strategies also be applied to HPV-positive cancers at non-oropharyngeal sites? Does the presence of HPV in non-oropharyngeal carcinomas represent viral-mediated carcinogenesis, or merely “bystander” infection? Lastly, even if HPV promotes carcinogenesis in non-oropharyngeal head and neck cancers, does it impact clinical outcome? Compared to the state of knowledge regarding HPV and oropharyngeal cancers, the significance of HPV in non-oropharyngeal head and neck cancers is unfortunately uncertain. These questions are especially important as laryngeal and oral cavity cancers are more common than oropharyngeal cancers.
Most publications focus on HPV DNA detection frequencies in non-oropharyngeal head and neck cancers, and only a few studies have directly addressed the impact of HPV on clinical outcome. The goal of this article is to systematically review the published data regarding the role of HPV in carcinomas of the oral cavity, larynx, sinonasal tract, and nasopharynx with respect to detection frequency, viral activity, and association with outcome. We also present some emerging data on HPV in salivary neoplasia.
Methods
The Pubmed search engine was used to identify studies published in English published from January 2000 through March 2012 using the MeSH terms “HPV”, “Human Papillomavirus”, “Head and Neck Cancer”, “Oral Cavity”, “Laryngeal”, “Larynx”, “Sinonasal”, “Nasal”, “Nasopharynx”, and “Polymerase Chain Reaction” (PCR). Oral cavity includes tongue (excluding tongue base), gum, floor of mouth, buccal mucosa, and hard palate. Data regarding the hypopharynx was not abstracted, for concern of possible anatomic overlap with oropharynx. The following arbitrary restraints were placed on this review; we excluded non-English manuscripts, manuscripts published before 2000, case reports, and investigations using non-PCR techniques or only in-situ PCR. If data were available using multiple techniques, only the PCR-generated data were extracted [1]. Only data generated from primary squamous cell carcinomas (SCC) were extracted, and we excluded reports if: (1) the detection methods were not well-detailed, and/or (2) HPV data could not be extracted per anatomic site. We took care to avoid tallying overlapping patient cohorts [2–13]. The final extracted data included: county, year of publication, anatomical site, HPV type, detection method of including primers, and summary of findings for cancers, patient controls, benign lesions, potentially premalignant lesions, and premalignant lesions, when available. For consistency, low-risk HPV data were grouped together with “HPV-positive, all types”. The weighted prevalence (WP) with 95 % confidence intervals (CI) was calculated using Comprehensive Meta-Analysis, version 2 (Meta-Analysis.com). Weights are based on the inverse variance from the random effects analysis which includes the “within-studies” variance plus the “between-studies” variance.
Results
One hundred publications fulfilled the above criteria and serve as the basis of this review; [1–100] the great majority of them relied on HPV DNA detection by PCR analysis. Detection of HPV DNA supports the idea that HPV is “associated” with a cancer. However, it does not distinguish whether HPV is transcriptionally active and thus might promote carcinogenesis (e.g., “driver” infection) or transcriptionally inactive (referred to as “passenger” or “bystander” infection). The usual accepted criteria to support HPV-mediated carcinogenesis are: (1) demonstration of high-risk HPV E6/E7 RNA; (2) p16 overexpression; (3) viral integration; and lastly (4) wild type 53 protein. These criteria have important caveats. The p16 gene may be methylated in HPV-mediated cancers. HPV integration is not necessary for promoting carcinogenesis. Lastly, while the HR-HPV+/wild-type p53 profile is the expected genetic phenotype of never-smokers with HPV-mediated oropharyngeal cancers, this polarized relationship is not observed in patients with oral cavity cancer, as will be discussed [101]. Furthermore, nondisruptive p53 mutations can be demonstrated in HPV-mediated head and neck cancers, and are thought to have an additive impact on overall p53 functional loss [102].
HPV and Cancers of the Oral Cavity
Table 1 summarizes 60 publications on 4,195 patients with oral cavity SCC. A total of 705/4,195 oral cavity carcinomas contained HPV DNA, (all types); the WP is 20.2 %, (95 % CI 16.0 %, 25.2 %). No geographical differences were seen. A subgroup of 53 oral verrucous carcinomas were studied; 22/53 contained HPV DNA, with a higher WP, 47.2 % (95 % CI 13.6 %, 83.6 %), as compared to usual oral SCC [34, 37, 79]. The difference in the WP between oral verrucous carcinomas and usual-type SCC is not statistically significant (p = 0.15); this is probably due to the small number of oral verrucous carcinomas studied, and the wide 95 % confidence intervals.
Table 1.
HPV DNA detection frequencies in oral cavity carcinomas
| Author | Year | Country | Method, primers, amplicon detection | Number of HPV positive cancers | Total cancers studied | HPV positive cancers (%) |
|---|---|---|---|---|---|---|
| Badaracco | 2007 | Italy | PCR, MY09/MY11, GP5/GP6 | 8 | 60 | 13.3 |
| Baez | 2004 | Puerto Rico | PCR, HPV16 E6/E7 ORF | 13 | 36 | 36.1 |
| Bagan | 2007 | Spain | PCR, MY09/MY11 | 0 | 6 | 0.0 |
| Balderas - Laenza | 2007 | Mexico | PCR, MY09/MY11, GP5/GP6 | 26 | 62 | 41.9 |
| Barwad | 2011 | India | PCR, MY09/MY11, not nested, agarose gel | 16 | 34 | 47.1 |
| Boscolo-Rizzo | 2009 | Italy | PCR, HPV16 specific primers | 2 | 10 | 20.0 |
| Bouda | 2000 | Greece | PCR | 18 | 19 | 94.7 |
| Boy | 2006 | South Africa | PCR, HPV16/18 specific primers | 7 | 59 | 11.9 |
| Braakhuis | 2004 | Netherlands | PCR, GP5/GP6, typing | 6 | 106 | 5.7 |
| Correnti | 2004 | Venezuela | PCR, MYO9/MY11, not nested, agarose gel, Digene Sharp Signal Assay typing | 8 | 16 | 50.0 |
| Dahlgren | 2004 | Scandinavia | PCR, GP5/GP6, CPI/CPIIG, agarose gel | 2 | 85 | 2.4 |
| Deng | 2011 | Japan | PCR, MYO9/MY11, GP5/GP6, E1 consensus primers | 9 | 25 | 36.0 |
| Dong | 2003 | USA | PCR, HPV16/18 specific primers | 3 | 16 | 18.8 |
| Elango | 2011 | India | PCR, MY09/MY11, GP5/GP6, HPV16 specific primers | 30 | 60 | 50.0 |
| El-Mofty | 2003 | USA | PCR, SPF10, INNO-LiPA line probe | 0 | 15 | 0.0 |
| Feher | 2009 | Hungary | PCR, MY09/MY11, GP5/GP6 | 31 | 65 | 47.7 |
| Fischer | 2003 | Germany | PCR, L1 consensus primers | 0 | 2 | 0.0 |
| Fujita | 2008 | Japan | PCR, SPF10, sequencing | 11 | 23 | 47.8 |
| Furniss | 2007 | USA | PCR, SPF1A, SPF2B, HPV16 E6 specific primers | 38 | 150 | 25.3 |
| Gillison | 2000 | USA | PCR, MY09/MY11, HPV16E7 HPV18E7 Dot blot | 10 | 84 | 11.9 |
| Gonzalez | 2007 | Argentina | PCR, MY09/MY11, GP5/GP6, | 15 | 25 | 60.0 |
| Gudleviciene | 2009 | Lithuania | PCR, HPV16/18 specific primers, agarose gel | 1 | 13 | 7.7 |
| Ha | 2002 | USA | PCR, HPV16 E6/E7 primers, real time quantitative PCR | 1 | 34 | 2.9 |
| Halimi | 2011 | Iran | PCR, MY09/MY11 then typed, agarose gel | 6 | 30 | 20.0 |
| Hansson | 2005 | Scandinavia | PCR, MY09/MY11, GP5/GP6, agarose gel, sequenced | 15 | 85 | 17.6 |
| Harris | 2011 | USA | PCR, MY09/MY11, GP5/GP6, type specific primers | 2 | 25 | 8.0 |
| Herrero | 2003 | Multiple countries | PCR, GP5/GP6, enzyme immune assay typing | 30 | 766 | 3.9 |
| Ibieta | 2005 | Mexico | PCR, MY09/M11, GP5/GP6, typed | 21 | 50 | 42.0 |
| Jalouli | 2010 | India | PCR, MY09/M11, not nested, agarose gel, typed with HPV16/18 specific primers, and sequenced | 15 | 62 | 24.2 |
| Kaminagakura | 2011 | Brazil | PCR, GP5/GP6, agarose gel | 22 | 114 | 19.3 |
| Kansky | 2006 | Slovenia | PCR, MY09/M11, GP5/GP6, WD72, WD76, agarose gel, typing by restriction fragment length polymorphism | 4 | 44 | 9.1 |
| Klozar | 2008 | Czech | PCR, GP5/GP6, not nested, chemoluminescence detection of hybridized amplicon, sequencing | 2 | 10 | 20.0 |
| Klussmann | 2001 | Germany | PCR, consensus primers, HPV16 specific primers, real time PCR | 4 | 22 | 18.2 |
| Koppikar | 2005 | India | PCR, L1 primers and GP5/GP6 | 28 | 83 | 33.7 |
| Koskinen | 2003 | Scandinavia | PCR, MY09/MY11,GP5/GP6, SPF10, INNO-LiPA typing, FAP 59/64, CP65/70, CP66/69, type specific real time PCR | 7 | 13 | 53.8 |
| Kristoffersen | 2012 | Scandinavia | PCR, MY09/MY1, GP5/GP6 | 8 | 50 | 16.0 |
| Laco | 2011 | Czech Republic | PCR, GP5/GP6 | 3 | 24 | 12.5 |
| Lopes | 2011 | England | PCR, GP5/6 Q-PCR HPV16/18 | 2 | 142 | 1.4 |
| Luo | 2007 | Tapei | PCR, MY09/M11, GP5/GP6, typed by HPV gene chip | 13 | 51 | 25.5 |
| Montaldo | 2010 | Italy | PCR, MY09/M11, agarose gel, sequenced | 21 | 68 | 30.9 |
| Mork | 2001 | Scandinavia | PCR, GP5/GP6 CpI, CpII E1, E6 specific primers for HPV6/11/16/18/33 | 4 | 91 | 4.4 |
| Neme | 2006 | Hungary | PCR, MY09/MY11, type specific, E2 for integration | 33 | 79 | 41.8 |
| Pannone | 2012 | Italy | PCR, MY09/M11, GP5/GP6, 8% polyacrylamide gel | 3 | 6 | 50.0 |
| Popovic | 2010 | Serbia | PCR, consensus primers typing | 6 | 60 | 10.0 |
| Ribeiro | 2011 | Multiple countries | PCR, MY09/MY11, no nesting, HPV16E7 specific primers, agarose gel, typing by restriction fragment length polymorphism | 0 | 483 | 0.0 |
| Ringstrom | 2002 | USA | PCR MY09/MY11, agarose gel, typing by restriction fragment length polymorphism | 2 | 41 | 4.9 |
| Ritchie | 2003 | USA | PCR, MY09/MY11 agarose gel, dot blot, then heminested PCR MY09. GP5 | 10 | 94 | 10.6 |
| Saghravanian | 2011 | Iran | PCR, GP5/GP6 | 3 | 21 | 14.3 |
| Sand | 2000 | Scandinavia | PCR, MY09/MY11, agarose gel | 3 | 24 | 12.5 |
| Schlecht | 2011 | USA | PCR, MY09/11, dot blot | 5 | 38 | 13.2 |
| Seraj | 2011 | Iran | PCR, HPV 16/18 specific primers, agarose gel | 25 | 94 | 26.6 |
| Sethi | 2011 | USA | PCR, SPF10, INNO-LiPA typing | 33 | 120 | 27.5 |
| Slebos | 2006 | USA | PCR, MY09/MY11, sequenced | 0 | 15 | 0.0 |
| Smeets | 2007 | Netherlands | PCR, GP5/GP6 real time quantitative PCR | 9 | 30 | 30.0 |
| Smith | 2008 | USA | PCR,MY09/MY11, GP5/GP6, then typed | 27 | 166 | 16.3 |
| Soderberg | 2008 | USA | PCR, MY09/MY11, GP5/GP6, then sequenced | 1 | 18 | 5.6 |
| Sugiyama | 2007 | Japan | PCR, HPV16 E7 specific primers, agarose gel | 24 | 66 | 36.4 |
| Tachezy | 2005 | Czech Republic | PCR, GP5/GP6, then sequenced | 3 | 12 | 25.0 |
| van Monsjou | 2012 | Netherlands | PCR, INNO-LiPA typing | 2 | 20 | 10.0 |
| Zhang | 2004 | China | PCR, HPV 16/18 E6 specific primers, agarose gel | 54 | 73 | 74.0 |
| Total | 705 | 4,195 | ||||
The most common HPV type detected in oral cancers is HPV16. A notable outlier is a study from South Africa which detected only HPV18, and not HPV16, in patients with oral cancer [23]. HPV18 was detected in a smaller percentage of oral cancers, some oral carcinomas had dual infections with HPV16/18 [51, 70, 96]. Rarer types detected in oral cancers were HPV8, HPV31, HPV38, and HPV66 [56, 68, 93]. Low-risk HPV is rarely detected in oral cancers, and when present might represent a “bystander” infection rather than possibly “driver” infection [37, 42, 64, 68, 80].
HPV RNA and Oral Cavity Cancer
As mentioned, demonstrating viral oncogene transcription suggests, but does not prove, that viral-mediated carcinogenesis is mechanistically possible (“driver infection”). There is a paucity of published data regarding HR-HPV E6/E7 RNA in oral cancers [3, 7, 21, 81, 85]. The common approach of these studies is to perform reverse-transcription PCR on HPV-positive cancers. Only four studies demonstrated that HR-HPV E6/E7 RNA were present in a total of 17/20 (85 %) HPV-positive oral carcinomas tested [3, 7, 21, 85].
Another approach is to perform parallel PCR and reverse-transcription PCR assays for all specimens [81]. In a study of 109 patients with head and neck cancer from multiple anatomic sites, three oral cancers were DNA+/RNA− (reflecting either low-level or no transcription, and possibly “passenger” infections), three oral cancers were DNA+/RNA+ (possibly driver infections), and four other cancers were DNA−/RNA+ [81]. This last set of HPV transcriptionally-active, yet DNA-negative carcinomas is still consistent with the possibility of HPV-mediated carcinogenesis, and speaks to the idea of greater detection sensitivity of reverse-transcription PCR compared with PCR. We have studied a cohort of 89 consecutive patients with oral cavity SCC, and determined the rate of HPV16/18 E6/E7 RNA, by nested reverse transcription PCR on archival tumor samples (unpublished data). We demonstrated that 30 patients (33.7 %) had either HPV16 or HPV18; no double infections were present.
HPV, Oral Cavity Carcinoma, and Outcome
Only three published studies on patients with oral cavity carcinoma specifically examined the impact of HPV on outcome [51, 89, 101]. Kaminagakura studied 114 patients and found a nonsignficant trend towards improved survival for 22 HPV-positive patients [51]. Sugiyama studied 66 patients in total, 62 with outcome data; 23 of these patients were HPV-positive [89]. They demonstrated a nonsignficant trend towards improved overall survival for HPV-positive oral cavity cancer patients [89]. Smith found no association with HPV and outcome for patients with oral carcinoma, based on either serology (116 patients, 13 with HPV positive serology) or tumor HPV detection (100 patients, 12 with tumors positive for HR-HPV DNA) [101]. Interestingly, in our unpublished cohort of 89 patients with oral cavity carcinoma, no significant association was found for patients with either HPV16/18 E6E7 RNA and time to disease progression or disease specific survival.
Oral HPV in Control Populations
Table 2 addresses the issue of HPV oral reservoirs in healthy controls populations: there are 22 studies on 5,095 healthy patients. Most studies examined shed cells and/or saliva, which cannot distinguish between oral cavity and oropharyngeal viral reservoirs; both Klussmann [55] and Anderson [15] examined tonsillectomy specimens. However, all of these studies address the issue of intraoral HPV prevalence in healthy populations. A total of 259/5,095 normal controls had detectable HPV DNA (all types); the WP was 6.9 %, (95 % CI 3.5 %, 13.2 %). Interestingly, Smith and colleagues demonstrated a bimodal age distribution with the two prevalence peaks of 2.5 % for children under 1 year, and 3.3 % for volunteers between ages 16 and 20 [103]. Some of the relatively larger studies from India [30], Scandinavia [60], and China [96] demonstrate significantly greater oral HPV carrier rates as compared to the other studies, suggesting that real geographic differences may be present.
Table 2.
HPV DNA detection frequencies in oral/oropharyngeal controls from healthy patients
| Author | Year | Country | Number of positive normal specimens | Total specimens studied | HPV positive oral/oropharyngeal controls (%) |
|---|---|---|---|---|---|
| Anderson | 2007 | Scotland | 0 | 24 | 0.0 |
| Deng | 2011 | Japan | 1 | 47 | 2.1 |
| D’Souza | 2007 | USA | 11 | 200 | 5.5 |
| Elango | 2011 | India | 31 | 46 | 67.4 |
| Feher | 2009 | Hungary | 3 | 72 | 4.2 |
| Fujita | 2008 | Japan | 7 | 10 | 70.0 |
| Gonzalez | 2007 | Argentina | 0 | 60 | 0.0 |
| Hansson | 2005 | Scandinavia | 15 | 320 | 4.7 |
| Herrero | 2003 | Multiple countries | 91 | 1,527 | 6.0 |
| Jimenez | 2001 | Venezuela | 2 | 20 | 10.0 |
| Kansky | 2006 | Slovenia | 3 | 45 | 6.7 |
| Klussmann | 2001 | Germany | 0 | 14 | 0.0 |
| Koppikar | 2005 | India | 5 | 102 | 4.9 |
| Kristoffersen | 2012 | Scandinavia | 28 | 50 | 56.0 |
| Luo | 2007 | Tapei | 8 | 90 | 8.9 |
| Migaldi | 2012 | Italy | 1 | 81 | 1.2 |
| Montaldo | 2010 | Italy | 0 | 52 | 0.0 |
| Pannone | 2012 | Italy | 0 | 15 | 0.0 |
| Pinto | 2008 | Canada | 6 | 129 | 4.7 |
| Ribeiro | 2011 | Multiple countries | 2 | 898 | 0.2 |
| Saghravanian | 2011 | Iran | 0 | 18 | 0.0 |
| Smith | 2007 | USA | 23 | 1235 | 1.9 |
| Zhang | 2004 | China | 22 | 40 | 55.0 |
| Total | 259 | 5,095 | |||
The WP of intraoral HPV detection is significantly lower than the rate of HPV detection in oral carcinomas (6.9 vs. 20.2 %, respectively, p = 0.0002). The findings suggest that HPV may contribute to oral cancer carcinogenesis. However, this relationship does not establish causality.
HPV in Benign, Potentially Premalignant, and Premalignant Oral Lesions
Table 3 addresses the issue of HPV detection frequency in a spectrum of oral lesions, summarizing cross-sectional data for DNA detection (all HPV types) in patients with benign lesions, potentially premalignant lesions, (leukoplakia, lichen planus, submucosal fibrosis), and premalignant lesions (high-grade dysplasia). Only two studies addressed benign oral lesions: HPV was detected in 23/48 (47.9 %) of benign biopsies. No conclusions or comparisons can be drawn due to the limited nature of this data.
Table 3.
HPV DNA detection frequencies in benign, potentially premalignant (PPM), and premalignant (PM) oral specimens
| Author | Year | Country | Number HPV positive benign Bx | Total benign Bx studied | HPV positive benign (%) | Number PPM HPV positive Bx | Total PPM Bx | HPV positive PPM (%) | Number PM HPV positive Bx | Total PM Bx studied | HPV positive PM (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bagan | 2007 | Spain | 0 | 4 | 0 | ||||||
| Bouda | 2000 | Greece | 25 | 29 | 86.2 | 5 | 5 | 100 | |||
| Feher | 2009 | Hungary | 57 | 163 | 35.0 | ||||||
| Gonzalez | 2007 | Argentina | 1 | 8 | 12.5 | 15 | 31 | 48.4 | |||
| Ha | 2002 | USA | 0 | 44 | 0 | 1 | 58 | 1.7 | |||
| Jalouli | 2010 | India | 11 | 12 | 91.7 | ||||||
| Jimenez | 2001 | Venezuela | 22 | 40 | 55 | ||||||
| Kristoffersen | 2012 | Scandinavia | 32 | 50 | 64 | ||||||
| Luo | 2007 | Tapei | 14 | 46 | 30.4 | ||||||
| Saghravanian | 2011 | Iran | 0 | 19 | 0 | ||||||
| Sand | 2000 | Scandinavia | 8 | 29 | 27.6 | ||||||
| Total | 23 | 48 | 54 | 144 | 6 | 63 | |||||
Bx Biopsy
Potentially premalignant = Leukoplakias, Lichen Planus, Oral Submucousal Fibrosis [49]
Premalignant = High-grade dysplasias
Ten reports studied a group of lesions classified as “potentially premalignant” oral lesions (leukoplakia, lichen planus, submucous fibrosis); HPV DNA was detected in 54/144 lesions; the WP is 41.4 %, (95 % CI 25.8 %, 58.9 %), which is also significantly higher than the intraoral HPV carrier rate. This also suggests that HPV may also promote potentially premalignant lesions, although it does not establish causality. Finally, only two studies investigated premalignant oral lesions (high-grade dysplasia): HPV was detected in 6/63 (9.5 %) of cases. No conclusions can be made due to the paucity of data.
Interestingly, one small study demonstrated that patients with submucous fibrosis demonstrated the highest HPV prevalence (11/12, or 91.7 %) [49]. Oral submucous fibrosis is a potentially premalignant condition caused by areca nut chewing and histologically characterized by increased submucosal collagen deposition and squamous mucosal atrophy. Access to basal reserve cells is required for establishing HPV mucosal infection. The transitional zone between uterine ectocervix and endocervix is an example of a region particularly vulnerable to HPV infection. It is possible that oral mucosal atrophy due to submucous fibrosis allows for greater exposure of epithelial basal cells, and therefore greater vulnerability to HPV infection. There is a need to follow-up with larger studies investigating the incidence of HPV in patients with betel nut-induced submucous fibrosis.
HPV and Cancers of the Larynx
Disproportionately fewer laryngeal cancers have been studied for HPV as compared to oral cancers (1,712 vs. 4,195, respectively) within the same time period (2000–2012) despite the fact that global incidences for these two cancers are on the same order of magnitude [104]. Table 4 summarizes the 41 publications on 1,712 patients with laryngeal SCC. HPV DNA has been detected in 436/1,712 laryngeal cancers; the WP is 23.6 %, (95 % CI 18.7 %, 29.3 %). No geographical differences were seen. Only three laryngeal verrucous carcinomas were studied, HPV was detected in 2/3 tumors, one was low-risk (LR) and the other was high-risk (HR) [39].
Table 4.
HPV DNA detection frequencies in laryngeal carcinomas
| Author | Year | Country | Method, primers, amplicon detection | Number of cancers HPV+ | Total cancers studied | Cancers HPV+ (%) |
|---|---|---|---|---|---|---|
| Almadori | 2001 | Italy | PCR, MY09/MY11, enzyme immune assay typing | 15 | 42 | 35.7 |
| Anderson | 2007 | Scotland | PCR, GP5/GP6, real time quantitative PCR | 2 | 64 | 3.1 |
| Badaracco | 2007 | Italy | PCR, MY09/MY11, GP5/GP6 | 4 | 30 | 13.3 |
| Baez | 2004 | Puerto Rico | PCR, HPV16E6/E7 ORF | 24 | 52 | 46.2 |
| Baumann | 2009 | USA | PCR, GP5/GP6, enzyme immune assay typing | 6 | 38 | 15.8 |
| Boscolo-Rizzo | 2009 | Italy | PCR, HPV16 specific primers | 1 | 38 | 2.6 |
| Deng | 2011 | Japan | PCR, MY09/MY11, GP5/GP6, E1 consensus primers | 2 | 16 | 12.5 |
| Duray | 2011 | Belgium | PCR, GP5/GP6, type specific primers and real time quantitative PCR | 44 | 59 | 74.6 |
| El-Mofty | 2003 | USA | PCR, SPF10, INNO-LiPA line probe | 2 | 7 | 28.6 |
| Fakhry | 2008 | USA | PCR, MY09/MY11, Roche Molecular systems probe array | 0 | 34 | 0.0 |
| Fischer | 2003 | Germany | PCR, L1 consensus primers | 13 | 34 | 38.2 |
| Furniss | 2007 | USA | PCR, SPF1A, SPF2B, HPV16E6 specific primers | 14 | 45 | 31.1 |
| Gillison | 2000 | USA | PCR, MY09/MY11, HPV16/18E7 specific primers | 16 | 86 | 18.6 |
| Gudleviciene | 2009 | Lithuania | PCR, HPV16/18 specific primers, gel | 6 | 18 | 33.3 |
| Guvenc | 2008 | Turkey | PCR, nested MY09/MY11, GP5/GP6 | 7 | 50 | 14.0 |
| Hassumi | 2012 | Brazil | PCR, GP5/GP6 | 7 | 53 | 13.2 |
| Kleist | 2004 | Germany | PCR, MY09/MY11, types specific primers, polyacrylamide gels, sequencing | 6 | 38 | 15.8 |
| Klussmann | 2001 | Germany | PCR, consensus primers, HPV16 specific primers | 1 | 14 | 7.1 |
| Koppikar | 2005 | India | PCR, probably MY09/MY11 | 0 | 2 | 0.0 |
| Koskinen | 2007 | Scandinavia | PCR, MY09/MY11, GP5/GP6, SPF10, INNO-LiPA line probe | 3 | 69 | 4.3 |
| Liu | 2010 | China | PCR, GP5/6, HPV16/18 specific primers, agarose gel | 29 | 84 | 34.5 |
| Major | 2005 | Hungary | PCR, MY09/MY11, GP5/GP6, HPV 6/11/16 type specific primers, agarose gel | 8 | 16 | 50.0 |
| Manjarrez | 2006 | Mexico | PCR, L1C1/L1C2, typing by restriction fragment length polymorphism | 2 | 16 | 12.5 |
| Mork | 2001 | Scandinavia | PCR, GP5/GP6, CpI, CpII, HPV16 type specific primers | 1 | 32 | 3.1 |
| Morshed | 2010 | Poland | PCR, SPF10, agarose gel, enzyme immune assay typing, INNO-LiPA genotyping | 33 | 93 | 35.5 |
| Oliveira | 2006 | Brazil | PCR, GP5/GP6, HPV type specific primers | 41 | 110 | 37.3 |
| Reidy | 2004 | USA | PCR, HPV type specific primers, agarose gel | 6 | 6 | 100.0 |
| Ringstrom | 2002 | USA | PCR, MY09/MY11, agarose gel, typing by restriction fragment length polymorphism | 1 | 10 | 10.0 |
| Schlecht | 2011 | USA | PCR, MY09/11, dot blot | 8 | 32 | 25.0 |
| Sethi | 2011 | USA | PCR, SPF10, INNO-LiPA line probe | 26 | 111 | 23.4 |
| Slebos | 2006 | USA | PCR, MY09/MY11, sequenced | 1 | 9 | 11.1 |
| Smith | 2008 | USA | PCR, MY09/MY11 | 4 | 40 | 10.0 |
| Smith | 2000 | USA | PCR, MY09/MY11, agarose gel, sequenced | 11 | 44 | 25 |
| Snietura | 2011 | Poland | PCR (Abbott Molecular Real Time High-Risk HPV) | 0 | 65 | 0.0 |
| Stephen | 2012 | USA | PCR, HPVE6 specific primers, real time quantitative PCR | 21 | 77 | 27 |
| Szladek | 2005 | Hungary | PCR, MY09/MY11, GP5/GP6, then typed | 12 | 25 | 48.0 |
| Torrente | 2005 | Chile | PCR, MY09/MY11, E2 for integration, typing by restriction fragment length polymorphism | 10 | 31 | 32.3 |
| Van Houten | 2001 | Netherlands | PCR, GP5/GP6, enzyme immune assay typing | 0 | 5 | 0.0 |
| Van Monsjou | 2012 | Netherlands | PCR, INNO-LiPA line probe | 0 | 2 | 0.0 |
| Venuti | 2000 | Italy | PCR, MY09/MY11, E2 for integration, typing by restriction fragment length polymorphism | 13 | 25 | 52.0 |
| Vlachtsis | 2005 | Greece | PCR, “consensus primers” | 36 | 90 | 40.0 |
| 436 | 1,712 | |||||
The most common HPV type detected in laryngeal cancers is HPV16. Compared to oral carcinomas, a greater diversity of other HPV types has been detected: HPV18, HPV26, HPV31, HPV33, HPV39, HPV36, HPV45, HPV51, HPV52, HPV58, HPV59, HPV66, and HPV69 [8, 9, 14, 20, 26, 29, 39, 65, 71, 90, 94]. Low-risk HPV are uncommonly detected, and might represent incidental “bystander” rather than possibly “driver” infection [29, 39, 65, 75, 90, 94]. Rarely, integrated low-risk HPV has been found; viral integration does suggest viral-mediated carcinogenesis [90, 94].
HPV RNA and Laryngeal Cancer
HPV RNA was studied in only four publications and was detected in 8 of 10 HPV positive laryngeal carcinomas tested [2, 3, 21, 81].
HPV, Laryngeal Carcinoma, and Outcome
Only four published studies, Duray [29], Morshed [8, 9], Stephen [88], and Vlachtsis [95] examined the impact of HPV on the outcome of a total of 319 patients; 134 were HPV positive. No association of HPV status with outcome was found.
Laryngeal HPV in Control Populations
Table 5 addresses the issue of latent laryngeal HPV infection in control populations (usually autopsies or laryngeal brushings), and summarizes DNA detection data for five studies, all HPV types, in normal larynges. HPV DNA has been detected in 12/107 normal larynges; the WP is 9.6 %, (95 % CI 2.9 %, 27.2 %). There is a nonsignficant trend comparing the WP for laryngeal “HPV carrier rate” and HPV detection rate in laryngeal carcinomas, p = 0.11. This suggests that HPV might promote laryngeal cancer, but does not establish causality.
Table 5.
HPV DNA detection frequencies in normal larynges
| Author | Year | Country | Number HPV positive normal | Total normal studied | HPV positive laryngeal normal (%) |
|---|---|---|---|---|---|
| Guvenc | 2008 | Turkey | 0 | 50 | 0.0 |
| Kleist | 2004 | Germany | 0 | 5 | 0.0 |
| Smith | 2000 | USA | 2 | 12 | 16.7 |
| Szladek | 2005 | Hungary | 10 | 40 | 25.0 |
| Total | 12 | 107 | |||
HPV in Other Laryngeal Lesions
The association of HPV6/11 with laryngeal papillomas is well-established [105]. On the other hand, very few studies have examined the rate of HPV detection in other benign laryngeal lesions. Morshed [8] did not find any HPV in 22 vocal cord nodules. Duray [29] studied 35 biopsies of vocal nodules (n = 20), chronic laryngitis (n = 13) and papillomas (n = 6) and detected HPV in 27/35 (77 %) of these specimens; however, the HPV detection rate was not subclassified by histology. Smith [10] detected HPV in 3/10 (30 %) laryngeal leukoplakias. Lastly, Morshed [8] detected HPV in laryngeal mucosa adjacent to cancer in 4/49 (8.2 %) cases. No comparisons or conclusions can be drawn from this limited data.
HPV in Sinonasal Cancers
The association of HPV with Schneiderian inverted papillomas (IP) is well-established; the HPV WP is 25.2 % (95 % CI 14.7, 35.6 %), for IP studied by PCR with consensus primers [106]. The HPV WP significantly increases for IP with high-grade dysplasia (WP 55.8 %, 95 %CI 30.5, 81.0 %) and IP with carcinoma (WP 55.1 %, 95 %CI 37.0, 73.2 %), as compared to combined IP without dysplasia plus with mild-dysplasia (WP 22.3 %, 95 %CI 15.9, 28.6 %) (p < 0.02, Wald t test). The published findings support the role of low-risk HPV in the etiology of benign Schneiderian IP, and the idea that high-risk HPV is responsible for malignant progression of IP [106].
The published data specifically regarding sinonasal SCC and HPV are quite sparse and suffers from heterogeneity with respect to association with IP. Table 6 summarizes 9 publications of 120 patients with sinonasal carcinoma: HPV DNA (all types) was detected in 31/120 cancers and the WP is 29.6 % (95 % CI 17.8 %, 44.9 %). HPV16 is most commonly detected. Because of the heterogeneity of this group, no conclusions can be drawn from this WP.
Table 6.
Frequencies of HPV DNA detection in sinonasal carcinomas
| Author | Year | Country | Method, primers, amplicon detection | Number HPV positive cancers | Total cancers studied | HPV positive cancers (%) |
|---|---|---|---|---|---|---|
| Alos | 2009 | Spain | PCR, SPF10, INNO-LiPA typing | 12 | 60 | 20.0 |
| McKay | 2005 | USA | PCR, MY09/MY11, agarose gel | 2 | 3 | 66.7 |
| Kraft | 2001 | Switzerland | PCR, MY09/MY11 | 0 | 4 | 0.0 |
| Hoffman | 2006 | Germany | PCR, MY09/MY11, HPV6/11/16 specific primers | 4 | 20 | 20.0 |
| Mork | 2001 | Scandinavia | PCR, GP5/GP6, CpI, CpII, HPV16 specific primers | 0 | 4 | 0.0 |
| Deng | 2011 | Japan | PCR, MYO9/MY11, GP5/GP6, E1 consensus primers | 3 | 10 | 30.0 |
| Badaracco | 2007 | Italy | PCR, MY09/MY11,GP5/GP6 | 2 | 2 | 100.0 |
| Fischer | 2003 | Germany | PCR, L1 consensus primers | 4 | 4 | 100.0 |
| Sethi | 2011 | USA | PCR, SPF10, INNO-LiPA typing | 4 | 13 | 30.8 |
| Total | 31 | 120 | ||||
HPV RNA and Sinonasal SCC
Only one publication looked at HPV transcriptional activity; two HPV+ SCC arising ex-inverted papillomas also reveal HPV RNA transcription, and evidence of viral integration [66].
HPV, Sinonasal SCC, and Outcome
Only one publication specifically examined clinical outcome for patients with sinonasal carcinomas, with respect to HPV status. Alos and colleagues studied 60 patients with sinonasal SCC, 12 arose ex-IP. Twelve SCC were HPV-positive, including one SCC-ex-IP. Patients with HPV+ sinonasal SCC had significantly improved 5-year progression-free survival rates, 62 % (95 % CI 23 %, 86 %) versus 20 %, (95 % CI 9 %, 34 %, p = 0.004 log rank test), and overall survival rates, 80 % (95 % CI 20 %, 95 %) versus 31 % (95 % CI 14 %, 47 %, p = 0.036 log rank test) [97].
There are limited data regarding sinonasal HPV “carrier” rates. Hoffmann [46] found HPV in 1 of 39 (2.6 %) sinonasal polyps.
HPV in Nasopharyngeal Carcinoma (NPC)
There has been recent interest in the role of HPV in NPC. Table 7 summarizes the 8 PCR-based studies that include 154 patients with NPC. Six of these studies have been published within the last 3 years. HPV 16 was detected. An important caveat to consider is that carcinomas may be of oropharyngeal origin with extension into the contiguous nasopharynx, rather than represent primary NPC. Having said this, three recent studies examined the issue of HPV/Epstein Barr virus (EBV) co-infection by various techniques [98, 99, 107]. These studies suggest a dichotomy of HPV+/EBV− NPC (14/68 cases, 20.5 %) versus HPV−/EBV+ NPC (40/80, 50 %) with no tumors harbouring double HPV/EBV infections [98, 99, 107]. However, NPC with double HPV/EBV infections have been reported [108].
Table 7.
HPV DNA detection frequencies in nasopharyngeal carcinoma
| Author | Year | Country | Method, primers, amplicon detection | Number HPV positive cancers | Total cancers studied | HPV positive cancers (%) |
|---|---|---|---|---|---|---|
| Barwad | 2011 | India | PCR, MY09/MY11, not nested, agarose gel | 1 | 20 | 5.0 |
| Deng | 2011 | Japan | PCR, MY09/MY11, GP5/GP6, E1 primers | 3 | 9 | 33.3 |
| Klussmann | 2001 | Germany | PCR consensus primers, HPV16 specific primers | 1 | 13 | 7.7 |
| Lantri | 2011 | Morrocco | PCR, MY09/MY11, nitrocellulose gel with ISH using type specific probes | 24 | 70 | 34.3 |
| Lo | 2010 | USA | PCR with type-specific E6 primers to HPV16/18, agarose gel | 12 | 28 | 42.8 |
| Maxwell | 2009 | USA | PCR, multiplex competitive PCR with type-specific E6 primers to multiple HR HPV | 4 | 5 | 80 |
| Mork | 2001 | Scandinavia | PCR, GP5/GP6, CpI, CpII E1 consensus primers, HPV16 specific primers | 1 | 7 | 14.3 |
| Schlecht | 2011 | USA | PCR, MY09/11 dot blot | 1 | 2 | 50 |
| Total | 47 | 154 | ||||
HPV RNA in NPC
HPV RNA has been detected in the single HPV+ NPC studied [81].
HPV, NPC, and Outcome
Only one publication specified the outcomes for five patients with NPC, four of whom were HPV+/EBV−, and the remaining patient was HPV−/EBV+ [98]. The limited nature of this data precludes further discussion.
HPV DNA in Salivary Neoplasia
The SEER 9 data demonstrates a trend of increasing incidence for mucoepidermoid carcinoma (MEC) in women, ages 15–34 years [109] reminiscent of the significantly increased incidence of oropharyngeal cancers over the past three decades due to HR-HPV-mediated carcinogenesis. This raises the interesting question as to whether HR-HPV can also be involved in MEC carcinogenesis [109]. The possibility of HPV promoting salivary tumors has been addressed in the literature in a limited manner. Vageli demonstrated HPV16/18 DNA in seven of nine parotid tumors, including an oncocytoma, acinic cell carcinoma, Warthin’s tumor, and a pleomorphic adenoma [110]. While DNA detection does not address the issue of transcriptional activity, and therefore biological relevance, these authors demonstrated relatively high copy number by quantitative real-time PCR for some tumors, which suggests a causative relationship. Recently, Boland and colleagues demonstrated HR-HPV DNA in two of 16 salivary adenoid cystic carcinomas using the Ventana in situ hybridization (ISH) probes [111]. HPV DNA ISH is not the optimum technique for initial exploratory studies. The detection sensitivity of ISH might be very good depending on the context. However, greater tumor sampling is accomplished by PCR on formalin-fixed, paraffin-embedded (FFPE) samples, as compared to ISH, and is therefore the preferred approach for initial exploratory studies.
HPV RNA in Salivary Neoplasia
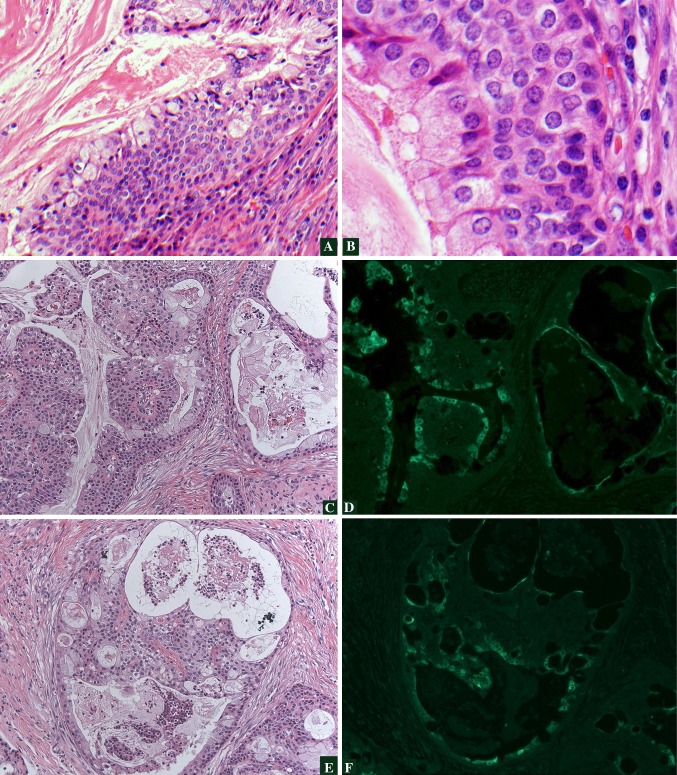
We studied a cohort of 89 patients with MEC for high risk HPV E6/E7 RNA by nested reverse transcription PCR (unpublished data). A total of 42 patients (47.2 %) had either HPV 16 or 18, and seven (7.1 %) had both 16 and 18. Interestingly, there was no predilection of HPV positivity in minor salivary MEC as compared to MEC of the major glands. Eighty four cases were studied by IF with the monoclonal C1P5 antibody which detects E6 protein of both HPV16/18. Eighteen tumors displayed nuclear and or cytoplasmic tumor staining, and the protein was detected in both mucinous and squamoid elements (Fig. 1). All cases positive by IF were HPV16/18 positive by RT-PCR. Fourteen additional MEC were negative by IF and positive by RT-PCR. This preliminary data demonstrates that transcriptionally active HPV16/18 is common in MEC. Thus, HPV may possibly promote the carcinogenesis of these tumors.
Fig. 1.
HPV and Histologya, b demonstrate low- and high-power magnification, respectively, of an HPV-positive, cystic, low-grade MEC with proliferation of basaloid type cells. Immunofluorescence (IF) for HPV16/18 E6 protein in MECc, e demonstrate hematoxylin and eosin stained areas of a MEC which is HPV-positive. d, f represent the corresponding regions demonstrating positive IF staining using an antibody to HPV16/18 E6 protein. Tumor nuclear and cytoplasmic staining is seen (bright green) which correlates with both the glandular and squamoid elements
The significance of the HPV DNA and RNA in salivary neoplasia is presently unknown.
Discussion
Technical Considerations
Despite limiting this systematic literature review to PCR-based studies, the heterogeneity of published data begs the issue of technical considerations, and should be briefly addressed. The gold standard for HPV assay sensitivity is the detection of HPV RNA in frozen tissues [81, 85]. HPV RNA can be present in excess in HPV DNA, and snap frozen tissue does not undergo the extensive nucleic acid/protein cross-linking and continuous DNA/RNA degradation found in FFPE samples. Most, but not all cited studies used FFPE samples. Tables 1 and 4 demonstrate that nested PCR using the MY09/MY11 consensus primers, and nested GP5+/GP6+ consensus primers, is a very common approach. These consensus primers detect a broad spectrum of low-risk and high-risk mucosal HPV types [112]. However, there are two major limitations with this approach: (1) size of the region to be amplified; and (2) potential loss of the L1 gene.
The MY09/MY11 primers amplify a region 450 base pairs long, which is too long for amplifying FFPE samples. The GP5+/GP6+ consensus primers detect a much smaller region, on the order of 150 bp. In general, a nested approach increases amplicon concentration, allowing for better amplicon visualization. However, nested PCR does not abrogate the initial sensitivity bottleneck of using the MY09/MY11 consensus primers. We recommend a nested approach using the GP5 +/GP6 + consensus primers for both rounds of PCR. The SPF10 (Short PCR Fragment) primers amplifies a region in the L1 gene only 65 bp long, so may be even more sensitive than the GP5+/GP6+ primers; however, we have not had direct experience with these primers.
The second limitation is that these primers detect sequences in the L1 region which can be lost upon HPV integration. If HPV is present in both integrated and episomal forms, one would expect positive results. However, if HPV is entirely integrated, PCR using these consensus primers may result in a false negative reaction. This was nicely demonstrated by Duray [29] who detected HPV in an additional 36 % of laryngeal SCC when assayed using type-specific E6/E7 primers. These laryngeal carcinomas harbored HPV that was entirely integrated and resulted in false negative PCR with GP5+/GP6+ primers, but positive PCR with E6/E7 type-specific primers. One can then use the CPIIG/CPI primer pair, which amplifies a 188-bp fragment in the highly conserved E1 ORF region of various skin and mucosal HPV, for specimens negative by PCR with GP5+/GP6+ primers.
General Conclusions
This systematic literature review highlights the present state of our knowledge with respect to three fundamental questions: (1) Is HPV associated with cancers of oral cavity, larynx, sinonasal tract, nasopharynx, and salivary glands? (2) Is there a causative relationship? (3) If HPV mediates carcinogenesis in these sites, does this impart an improved survival akin to HPV-mediated oropharyngeal SCC? As mentioned, this is important as an improved survival could be exploited to develop new treatment strategies. Each non-oropharyngeal site will be summarized with respect to these questions.
HPV and Oral Cavity Cancer
Our systematic review, plus other published reviews support the idea that HPV is significantly present in a subgroup of oral cavity SCC, as compared to control populations; thus, HPV may possibly contribute to oral carcinogenesis. We find that the WP for HPV DNA detection in 60 studies on 4,195 patients is 23.3 %, (95 % CI 18.1 %, 28.5 %). Kreimer reviewed 35 PCR-based studies on 2,642 patients with oral cavity SCC and determined the cumulative pooled prevalence for HPV DNA and oral SCC to be 23.5 %, (95 % CI 21.9, 25.1) [113]. Termine reviewed 47 PCR studies on 4852 patients with oral cavity SCC and determined the cumulative pooled prevalence of HPV DNA to be 39.9 %, (95 % CI 30.2, 49.8) [114]. A limitation of cumulative pooled prevalence is that it assumes homogeneity among the pooled samples. The WP adjusts for standard error per study, and between studies, minimizing the variability of pooled estimates.
Another approach is to compare the odds ratio (OR) for the association of HPV in controls versus cancer patients. Syrjänen reviewed 39 studies of 1,885 patients with oral SCC and 2,248 controls, and demonstrated an OR of 3.98 (95 % CI 2.6, 6.0) for HPV (all types), and OR of 3.86 (95 % CI 2.2, 6.9) for HPV16 [115].
There is limited published data to support causation in this context. Only four studies demonstrated HR-HPV E6/E7 RNA to be present in a total of 17/20 (85 %) HPV positive oral carcinomas tested [3, 7, 21, 85]. We also mentioned our unpublished findings of HPV16/18 E6/E7 RNA in 33.7 % of 89 oral cavity SCC studied by nested reverse transcription PCR. Future studies should address the issue of HPV RNA in oral cavity SCC.
P16 expression status should also be studied specifically in this context of oral cavity SCC, with the important caveat that lack of p16 overexpression does not exclude the possibility of HPV-potentiated carcinogenesis. Most publications correlating p16 expression with HPV status study mixed groups of cancers from different anatomic sites [61, 81, 93]. These studies are not powered to determine the sensitivity and specificity of p16 overexpression as a surrogate biomarker for HPV-mediated oral cavity carcinogenesis. Importantly, future studies should utilize whole tissue section staining rather than tissue microarrays, and specifically document the intensity and distribution of p16 overexpression in oral cavity SCC to access test performance at various “cut-points”.
With respect to etiology, the interaction between HPV, cigarettes, and alcohol exposure is more complex in the oral cavity as compared to the oropharynx. Smith recently reported that among heavy tobacco users, the risk of oropharyngeal carcinoma is greater in HPV-seronegative patients (adjusted OR = 11.0) compared with HPV-seropositive patients (adjusted OR = 4.7); among heavy alcohol users the risk is also greater in HPV-seronegative patients (adjusted OR = 24.3) compared to HPV-seropositive patients (adjusted OR = 8.5) [101]. This is consistent with the concept of mutually exclusive pathways of HPV-mediated carcinogenesis versus cigarette/alcohol-mediated carcinogenesis for oropharyngeal cancers. However, Smith found a different relationship between HPV, tobacco and alcohol for oral cavity cancers. The oral cavity cancer risk among heavy tobacco users was greater in HPV-seropositive patients (adjusted OR = 3.5) compared to HPV-seronegative patients (adjusted OR = 1.4); among heavy alcohol users the risk is also greater for HPV-seropositive patients (adjusted OR = 9.8) compared with HPV-seronegative patients (adjusted OR = 3.1) [101]. This interaction also suggests that profiling tumor suppressor gene mutational status in oral cancers will not result in the predicted dichotomy anticipated in oropharyngeal cancers (HPV+/wild type p53/wild type Rb vs. HPV−/mutated p53/mutated Rb).
Importantly, no data currently supports the idea that HPV is significantly associated with improved outcome for patients with oral cancer. The studies by Kaminagakura [51] and Sugiyama [89] do reveal nonsignficant trends, towards improved survival for patients with HPV-positive cancers. Therefore, future studies on oral cavity SCC should be powered to address the important clinical issue of HPV status (as determined by PCR).
HPV and Laryngeal Cancer
Fewer studies have addressed the association of HPV in laryngeal cancer [113, 116, 117]. Kreimer reviewed 35 PCR-based studies on 1,435 patients with laryngeal SCC and determined the cumulative pooled prevalence for HPV to be 24.0 %, (95 % CI 21.8, 26.3) [113]. We have found the WP of HPV is 23.9 %, (range 0–100 %, 95 % CI 17.1 %, 30.9 %) in laryngeal cancers. Hobbs determined the OR for laryngeal cancer is 2.0 (95 % CI 1.2, 3.4) as compared to controls. The most common HPV type detected in laryngeal cancers is HPV16; HPV18 is the second most common HPV type. However, compared to oral carcinomas, a greater diversity of other HPV types has been detected in laryngeal cancers. Low-risk HPV are uncommonly detected, but cannot be summarily dismissed as “bystander” infections as integrated low-risk HPV has been found in laryngeal cancers.
Very few studies have addressed the issue of causation regarding HPV and laryngeal cancer. HPV transcriptional activity was addressed in a total of nine cancers from three studies [2, 21, 81]. Duray demonstrated that the HPV16 viral load in laryngeal cancers (median 504 copies) was significantly higher than in benign lesions (median 37 copies). This supports the idea of active HPV “driver” infection, and suggested viral-mediated carcinogenesis. Future studies should address the issue of HPV RNA in laryngeal SCC.
P16 expression status should also be studied in the context of laryngeal cancer, with the same recommendations as above (whole tissue sections, documenting intensity and distribution). Baumann studied a subgroup of 10 laryngeal SCC including 5 HPV+ SCC and demonstrated good correlation [20]. Likewise, Laco also demonstrated good correlation between p16 overexpression and HPV status in 24 laryngeal SCC, 14 of which were HPV+ by chromogenic in situ hybridization (CISH) [118].
Importantly, only four studies [8, 9, 29, 88, 95] examined the impact of HPV on the outcome of a total of 319 patients; 134 of which were HPVpositive. No association of HPV status with outcome was found. Therefore, future studies on laryngeal SCC should be powered to address the important clinical issue of HPV status (as determined by PCR) and association with clinical outcome.
HPV and Sinonasal Cancer
The relationship between HR-HPV promoting malignant progression of inverted papillomas is well established. Future studies should focus on sinonasal SCC specifically unrelated to IP. Good correlation between HPV+ status, detected by either PCR or ISH, and diffuse p16 overexpression, has been reported in sinonasal SCC, albeit in small numbers [97, 119–121]. Strong diffuse p16 expression cannot be accepted as a surrogate HPV biomarker in any untested context, as this pattern of overexpression has also been detected in HPV-negative sinonasal undifferentiated carcinoma [122]. The improved outcome associated with HPV+ sinonasal SCC reported by Alos and colleagues [97] necessitates validation by other groups.
HPV and Nasopharyngeal Cancer
While recent studies suggest a exclusionary dichotomy between HPV-mediated NPC and EBV-mediated NPC [98–100, 102] NPC with double EBV/HPV infections have been reported [108]. An important caveat to future studies is the necessity for stringent exclusion of “NPC” which may have arisen from the oropharynx. With respect to HPV as a driver infection for NPC, three studies have demonstrated good correlation between HPV+ NPC and p16 expression, albeit on a small number of patients [98, 99, 107].
Nowhere is the possibility of “treatment de-escalation” more important that in the situation of skull base radiation, where the treatment related toxicities of optic nerve damage and osteoradionecrosis are most fearsome. Adequately powered, stringent studies comparing outcomes for patients with HPV-mediated NPC, EBV-mediated NPC, double-infected NPC, and “null” viral NPC may be challenging due to the relative rarity of NPC in western populations. However, these studies could have tremendous clinical impact.
HPV and Salivary Tumors
The emerging data demonstrate that HPV is detected in some benign and malignant salivary tumors. We have also mentioned our preliminary data on HPV16/18 E6/E7 RNA in MEC. Correlation of any biomarker or grading schema, with outcome for patients with salivary malignancies is extremely challenging given the overall rarity of even “common” salivary malignancies and the need for even larger sample sizes on multivariate analysis to account for a greater number of possible anatomic tumor sites.
In conclusion, high-risk HPV DNA is present in a significant proportion of oral and laryngeal cancers. There is limited published data on HPV-positive oral and laryngeal carcinomas regarding RNA expression, physical state (episomal vs. integrated), and correlation with tumor suppressor gene mutational status. Therefore, a causative relationship between HPV and these nonpharyngeal cancers has not yet been firmly established. Importantly, only few studies have attempted to correlate HPV status with clinical outcome. This review justifies the need for additional, appropriately powered, well-designed studies to examine the relationship of HPV status and clinical outcome for patients with oral and laryngeal cancer. High risk HPV DNA is also present in a significant proportion of sinonasal, nasopharyngeal, and salivary gland cancers, but the clinical significance of these findings in these malignancies has yet to be clearly defined.
References
- 1.Manjarrez ME, Ocadiz R, Valle L, et al. Detection of human papillomavirus and relevant tumor suppressors and oncoproteins in laryngeal tumors. Clin Cancer Res. 2006;12:6946–6951. doi: 10.1158/1078-0432.CCR-06-1214. [DOI] [PubMed] [Google Scholar]
- 2.Venuti A, Badaracco G, Rizzo C, et al. Presence of HPV in head and neck tumours: high prevalence in tonsillar localization. J Exp Clin Cancer Res. 2004;23:561–566. [PubMed] [Google Scholar]
- 3.Badaracco G, Rizzo C, Mafera B, et al. Molecular analyses and prognostic relevance of HPV in head and neck tumours. Oncol Rep. 2007;17:931–939. [PubMed] [Google Scholar]
- 4.Szarka K, Tar I, Fehér E, et al. Progressive increase of human papillomavirus carriage rates in potentially malignant and malignant oral disorders with increasing malignant potential. Oral Microbiol Immunol. 2009;24:314–318. doi: 10.1111/j.1399-302X.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 5.Fehér E, Gáll T, Murvai M, et al. Investigation of the occurrence of torque tenovirus in malignant and potentially malignant disorders associated with human papillomavirus. J Med Virol. 2009;81:1975–1981. doi: 10.1002/jmv.21627. [DOI] [PubMed] [Google Scholar]
- 6.Houten VM, Snijders PJ, Brekel MW, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer. 2001;93:232–235. doi: 10.1002/ijc.1313. [DOI] [PubMed] [Google Scholar]
- 7.Braakhuis BJ, Snijders PJ, Keune WJ, et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst. 2004;96:998–1006. doi: 10.1093/jnci/djh183. [DOI] [PubMed] [Google Scholar]
- 8.Morshed K, Polz-Dacewicz M, Szymański M, Polz D. Short-fragment PCR assay for highly sensitive broad-spectrum detection of human papillomaviruses in laryngeal squamous cell carcinoma and normal mucosa: clinico-pathological evaluation. Eur Arch Otorhinolaryngol. 2008;265:S89–S96. doi: 10.1007/s00405-007-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morshed K. Association between human papillomavirus infection and laryngeal squamous cell carcinoma. J Med Virol. 2010;82:1017–1023. doi: 10.1002/jmv.21749. [DOI] [PubMed] [Google Scholar]
- 10.Smith EM, Summersgill KF, Allen J, et al. Human papillomavirus and risk of laryngeal cancer. Ann Otol Rhinol Laryngol. 2000;109:1069–1076. doi: 10.1177/000348940010901114. [DOI] [PubMed] [Google Scholar]
- 11.Smith EM, Rubenstein LM, Ritchie JM, et al. Does pretreatment seropositivity to human papillomavirus have prognostic significance for head and neck cancers? Cancer Epidemiol Biomarkers Prev. 2008;17:2087–2096. doi: 10.1158/1055-9965.EPI-08-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith EM, Wang D, Rubenstein LM, et al. Association between p53 and human papillomavirus in head and neck cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17:421–427. doi: 10.1158/1055-9965.EPI-07-2597. [DOI] [PubMed] [Google Scholar]
- 13.Smith EM, Rubenstein LM, Hoffman H, et al. Human papillomavirus, p16 and p53 expression associated with survival of head and neck cancer. Infect Agent Cancer. 2010;5:4. [DOI] [PMC free article] [PubMed]
- 14.Almadori G, Cadoni G, Cattani P, et al. Human papillomavirus infection and epidermal growth factor receptor expression in primary laryngeal squamous cell carcinoma. Clin Cancer Res. 2001;7:3988–3993. [PubMed] [Google Scholar]
- 15.Anderson CE, McLaren KM, Rae F, et al. Human papillomavirus in squamous carcinoma of the head and neck: a study of cases in south east Scotland. J Clin Pathol. 2007;60:439–441. doi: 10.1136/jcp.2005.033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Báez A, Almodóvar JI, Cantor A, et al. High frequency of HPV16-associated head and neck squamous cell carcinoma in the Puerto Rican population. Head Neck. 2004;26:778–784. doi: 10.1002/hed.20046. [DOI] [PubMed] [Google Scholar]
- 17.Bagan JV, Jimenez Y, Murillo J, et al. Lack of association between proliferative verrucous leukoplakia and human papillomavirus infection. J Oral Maxillofac Surg. 2007;65:46–49. doi: 10.1016/j.joms.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 18.Balderas-Loaeza A, Anaya-Saavedra G, Ramirez-Amador VA, et al. Human papillomavirus-16 DNA methylation patterns support a causal association of the virus with oral squamous cell carcinomas. Int J Cancer. 2007;120:2165–2169. doi: 10.1002/ijc.22563. [DOI] [PubMed] [Google Scholar]
- 19.Barwad A, Sood S, Gupta N, et al. Human papilloma virus associated head and neck cancer: a PCR based study. Diagn Cytopathol. 6 Apr 2011. doi:10.1002/dc.21667. [DOI] [PubMed]
- 20.Baumann JL, Cohen S, Evjen AN, et al. Human papillomavirus in early laryngeal carcinoma. Laryngoscope. 2009;119:1531–1537. doi: 10.1002/lary.20509. [DOI] [PubMed] [Google Scholar]
- 21.Boscolo-Rizzo P, Da Mosto MC, Fuson R, et al. HPV-16 E6 L83 V variant in squamous cell carcinomas of the upper aerodigestive tract. J Cancer Res Clin Oncol. 2009;135:559–566. doi: 10.1007/s00432-008-0490-3. [DOI] [PubMed] [Google Scholar]
- 22.Bouda M, Gorgoulis VG, Kastrinakis NG, et al. “High risk” HPV types are frequently detected in potentially malignant and malignant oral lesions, but not in normal oral mucosa. Mod Pathol. 2000;13:644–653. doi: 10.1038/modpathol.3880113. [DOI] [PubMed] [Google Scholar]
- 23.Boy S, Rensburg EJ, Engelbrecht S, et al. HPV detection in primary intra-oral squamous cell carcinomas–commensal, aetiological agent or contamination? J Oral Pathol Med. 2006;35:86–90. doi: 10.1111/j.1600-0714.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 24.Correnti M, Rivera H, Cavazza ME. Detection of human papillomaviruses of high oncogenic potential in oral squamous cell carcinoma in a Venezuelan population. Oral Dis. 2004;10:163–166. doi: 10.1046/j.1601-0825.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 25.Dahlgren L, Dahlstrand HM, Lindquist D, et al. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int J Cancer. 2004;112:1015–1019. doi: 10.1002/ijc.20490. [DOI] [PubMed] [Google Scholar]
- 26.Deng Z, Hasegawa M, Matayoshi S, et al. Prevalence and clinical features of human papillomavirus in head and neck squamous cell carcinoma in Okinawa, southern Japan. Eur Arch Otorhinolaryngol. 2011;268:1625–1631. doi: 10.1007/s00405-011-1515-0. [DOI] [PubMed] [Google Scholar]
- 27.Dong SM, Sun DI, Benoit NE, et al. Epigenetic inactivation of RASSF1A in head and neck cancer. Clin Cancer Res. 2003;9:3635–3640. [PubMed] [Google Scholar]
- 28.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 29.Duray A, Descamps G, Arafa M, et al. High incidence of high-risk HPV in benign and malignant lesions of the larynx. Int J Oncol. 2011;39:51–59. doi: 10.3892/ijo.2011.1031. [DOI] [PubMed] [Google Scholar]
- 30.Elango KJ, Suresh A, Erode EM, et al. Role of human papilloma virus in oral tongue squamous cell carcinoma. Asian Pac J Cancer Prev. 2011;12:889–896. [PubMed] [Google Scholar]
- 31.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27:1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 33.Fischer M, Winterfeld F. Evaluation and application of a broad-spectrum polymerase chain reaction assay for human papillomaviruses in the screening of squamous cell tumours of the head and neck. Acta Otolaryngol. 2003;123:752–758. doi: 10.1080/00016480310001420. [DOI] [PubMed] [Google Scholar]
- 34.Fujita S, Senba M, Kumatori A, et al. Human papillomavirus infection in oral verrucous carcinoma: genotyping analysis and inverse correlation with p53 expression. Pathobiology. 2008;75:257–264. doi: 10.1159/000132387. [DOI] [PubMed] [Google Scholar]
- 35.Furniss CS, McClean MD, Smith JF, et al. Human papillomavirus 16 and head and neck squamous cell carcinoma. Int J Cancer. 2007;120:2386–2392. doi: 10.1002/ijc.22633. [DOI] [PubMed] [Google Scholar]
- 36.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 37.González JV, Gutiérrez RA, Keszler A, et al. Human papillomavirus in oral lesions. Medicina (B Aires). 2007;67(4):363–368. [PubMed] [Google Scholar]
- 38.Gudleviciene Z, Smailyte G, Mickonas A, Pikelis A. Prevalence of human papillomavirus and other risk factors in Lithuanian patients with head and neck cancer. Oncology. 2009;76:205–208. doi: 10.1159/000201573. [DOI] [PubMed] [Google Scholar]
- 39.Güvenç MG, Midilli K, Ozdoğan A, et al. Detection of HHV-8 and HPV in laryngeal carcinoma. Auris Nasus Larynx. 2008;35:357–362. doi: 10.1016/j.anl.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Ha PK, Pai SI, Westra WH, et al. Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res. 2002;8:1203–1209. [PubMed] [Google Scholar]
- 41.Halimi M, Morshedi Asl S. Human papillomavirus infection in lung vs. oral squamous cell carcinomas: a polymerase chain reaction study. Pak J Biol Sci. 2011;14:641–646. doi: 10.3923/pjbs.2011.641.646. [DOI] [PubMed] [Google Scholar]
- 42.Hansson BG, Rosenquist K, Antonsson A, et al. Strong association between infection with human papillomavirus and oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1337–1344. doi: 10.1080/00016480510043945. [DOI] [PubMed] [Google Scholar]
- 43.Harris SL, Thorne LB, Seaman WT, et al. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck. 2011;33:1622–1627. doi: 10.1002/hed.21650. [DOI] [PubMed] [Google Scholar]
- 44.Hassumi-Fukasawa MK, Miranda-Camargo FA, Guimarães MC, et al. Possible implication of Mdm2 as a prognostic marker in invasive laryngeal carcinoma. Eur Arch Otorhinolaryngol. 5 Feb 2012. [Epub ahead of print] PubMed PMID: 22310835. [DOI] [PubMed]
- 45.Herrero R, Castellsagué X, Pawlita M, et al. IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann M, Kahn T, Goeroegh T, et al. Tracing human papillomavirus DNA in nasal polyps by polymerase chain reaction. Acta Otolaryngol. 2000;120:872–875. doi: 10.1080/000164800750061750. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann M, Klose N, Gottschlich S, et al. Detection of human papillomavirus DNA in benign and malignant sinonasal neoplasms. Cancer Lett. 2006;239:64–70. doi: 10.1016/j.canlet.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Ibieta BR, Lizano M, Fras-Mendivil M, et al. Human papilloma virus in oral squamous cell carcinoma in a Mexican population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:311–315. doi: 10.1016/j.tripleo.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Jalouli J, Ibrahim SO, Mehrotra R, et al. Prevalence of viral (HPV, EBV, HSV) infections in oral submucous fibrosis and oral cancer from India. Acta Otolaryngol. 2010;130:1306–1311. doi: 10.3109/00016481003782041. [DOI] [PubMed] [Google Scholar]
- 50.Jimenez C, Correnti M, Salma N, et al. Detection of human papillomavirus DNA in benign oral squamous epithelial lesions in Venezuela. J Oral Pathol Med. 2001;30:385–388. doi: 10.1034/j.1600-0714.2001.300701.x. [DOI] [PubMed] [Google Scholar]
- 51.Kaminagakura E, Villa LL, Andreoli MA, et al. High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int J Cancer. 2012;130:1726–1732. doi: 10.1002/ijc.26185. [DOI] [PubMed] [Google Scholar]
- 52.Kansky AA, Seme K, Maver PJ, et al. Human papillomaviruses (HPV) in tissue specimens of oral squamous cell papillomas and normal oral mucosa. Anticancer Res. 2006;26:3197–3201. [PubMed] [Google Scholar]
- 53.Kleist B, Bankau A, Lorenz G, et al. Different risk factors in basaloid and common squamous head and neck cancer. Laryngoscope. 2004;114:1063–1068. doi: 10.1097/00005537-200406000-00020. [DOI] [PubMed] [Google Scholar]
- 54.Klozar J, Kratochvil V, Salakova M, et al. HPV status and regional metastasis in the prognosis of oral and oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2008;265:S75–S82. doi: 10.1007/s00405-007-0557-9. [DOI] [PubMed] [Google Scholar]
- 55.Klussmann JP, Weissenborn SJ, Wieland U, et al. Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer. 2001;92:2875–2884. doi: 10.1002/1097-0142(20011201)92:11<2875::aid-cncr10130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 56.Koppikar P, deVilliers EM, Mulherkar R. Identification of human papillomaviruses in tumors of the oral cavity in an Indian community. Int J Cancer. 2005;113:946–950. doi: 10.1002/ijc.20664. [DOI] [PubMed] [Google Scholar]
- 57.Koskinen WJ, Chen RW, Leivo I, et al. Prevalence and physical status of human papillomavirus in squamous cell carcinomas of the head and neck. Int J Cancer. 2003;107:401–406. doi: 10.1002/ijc.11381. [DOI] [PubMed] [Google Scholar]
- 58.Koskinen WJ, Brøndbo K, Mellin Dahlstrand H, et al. Alcohol, smoking and human papillomavirus in laryngeal carcinoma: a Nordic prospective multicenter study. J Cancer Res Clin Oncol. 2007;133:673–678. doi: 10.1007/s00432-007-0219-8. [DOI] [PubMed] [Google Scholar]
- 59.Kraft M, Simmen D, Casas R, Pfaltz M. Significance of human papillomavirus in sinonasal papillomas. J Laryngol Otol. 2001;115:709–714. doi: 10.1258/0022215011908955. [DOI] [PubMed] [Google Scholar]
- 60.Kristoffersen AK, Enersen M, Kverndokk E, et al. Human papillomavirus subtypes in oral lesions compared to healthy oral mucosa. J Clin Virol. 2012;53:364–366. doi: 10.1016/j.jcv.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 61.Laco J, Vosmikova H, Novakova V, et al. The role of high-risk human papillomavirus infection in oral and oropharyngeal squamous cell carcinoma in non-smoking and non-drinking patients: a clinicopathological and molecular study of 46 cases. Virchows Arch. 2011;458:179–187. doi: 10.1007/s00428-010-1037-y. [DOI] [PubMed] [Google Scholar]
- 62.Liu B, Lu Z, Wang P, et al. Prevalence of high-risk human papillomavirus types (HPV-16, HPV-18) and their physical status in primary laryngeal squamous cell carcinoma. Neoplasma. 2010;57:594–600. doi: 10.4149/neo_2010_06_594. [DOI] [PubMed] [Google Scholar]
- 63.Lopes V, Murray P, Williams H, et al. Squamous cell carcinoma of the oral cavity rarely harbours oncogenic human papillomavirus. Oral Oncol. 2011;47:698–701. doi: 10.1016/j.oraloncology.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 64.Luo CW, Roan CH, Liu CJ. Human papillomaviruses in oral squamous cell carcinoma and pre-cancerous lesions detected by PCR-based gene-chip array. Int J Oral Maxillofac Surg. 2007;36:153–158. doi: 10.1016/j.ijom.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Major T, Szarka K, Sziklai I, et al. The characteristics of human papillomavirus DNA in head and neck cancers and papillomas. J Clin Pathol. 2005;58:51–55. doi: 10.1136/jcp.2004.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKay SP, Grégoire L, Lonardo F, et al. Human papillomavirus (HPV) transcripts in malignant inverted papilloma are from integrated HPV DNA. Laryngoscope. 2005;115:1428–1431. doi: 10.1097/01.mlg.0000168091.50584.b4. [DOI] [PubMed] [Google Scholar]
- 67.Migaldi M, Pecorari M, Forbicini G, et al. Low prevalence of human Papillomavirus infection in the healthy oral mucosa of a Northern Italian population. J Oral Pathol Med. 2012;41:16–20. doi: 10.1111/j.1600-0714.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 68.Montaldo C, Mastinu A, Zorco S, et al. Distribution of human papillomavirus genotypes in Sardinian patients with oral squamous cell carcinoma. Open Virol J. 2010;4:163–168. doi: 10.2174/1874357901004010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 70.Nemes JA, Deli L, Nemes Z, Márton IJ. Expression of p16(INK4A), p53, and Rb proteins are independent from the presence of human papillomavirus genes in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:344–352. doi: 10.1016/j.tripleo.2005.10.069. [DOI] [PubMed] [Google Scholar]
- 71.Oliveira DE, Bacchi MM, Macarenco RS, et al. Human papillomavirus and Epstein-Barr virus infection, p53 expression, and cellular proliferation in laryngeal carcinoma. Am J Clin Pathol. 2006;126:284–293. doi: 10.1309/UU2J-ADUE-HDWA-TVM9. [DOI] [PubMed] [Google Scholar]
- 72.Pannone G, Rodolico V, Santoro A, et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 immunohistochemistry, consensus PCR HPV-DNA, and in situ hybridization. Infect Agent Cancer. 2012;7:4. doi:10.1186/1750-9378-7-4. [DOI] [PMC free article] [PubMed]
- 73.Pintos J, Black MJ, Sadeghi N, et al. Human papillomavirus infection and oral cancer: a case-control study in Montreal, Canada. Oral Oncol. 2008;44:242–250. doi: 10.1016/j.oraloncology.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Popović B, Jekić B, Novaković I, et al. Cancer genes alterations and HPV infection in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2010;39:909–915. doi: 10.1016/j.ijom.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 75.Reidy PM, Dedo HH, Rabah R, et al. Integration of human papillomavirus type 11 in recurrent respiratory papilloma-associated cancer. Laryngoscope. 2004;114:1906–1909. doi: 10.1097/01.mlg.0000147918.81733.49. [DOI] [PubMed] [Google Scholar]
- 76.Ribeiro KB, Levi JE, Pawlita M, et al. Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int J Epidemiol. 2011;40:489–502. doi: 10.1093/ije/dyq249. [DOI] [PubMed] [Google Scholar]
- 77.Ringström E, Peters E, Hasegawa M, et al. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res. 2002;8:3187–3192. [PubMed] [Google Scholar]
- 78.Ritchie JM, Smith EM, Summersgill KF, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104:336–344. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 79.Saghravanian N, Ghazvini K, Babakoohi S, et al. Low prevalence of high risk genotypes of human papilloma virus in normal oral mucosa, oral leukoplakia and verrucous carcinoma. Acta Odontol Scand. 2011;69:406–409. doi: 10.3109/00016357.2011.572560. [DOI] [PubMed] [Google Scholar]
- 80.Sand L, Jalouli J, Larsson PA, Hirsch JM. Human papilloma viruses in oral lesions. Anticancer Res. 2000;20(2B):1183–1188. [PubMed]
- 81.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24:1295–1305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seraj JM, Yazdani N, Ashtiani ZO, et al. TP53 gene expression in HPV-positive oral tongue SCC and its correlation with nodal metastasis. Pathol Res Pract. 2011;207:758–761. doi: 10.1016/j.prp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 83.Sethi S, Ali-Fehmi R, Franceschi S, et al. Characteristics and survival of head and neck cancer by HPV status: a cancer registry-based study. Int J Cancer. 23 Oct 2011. doi:10.1002/ijc.26500. [DOI] [PMC free article] [PubMed]
- 84.Slebos RJ, Yi Y, Ely K, et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:701–709. doi: 10.1158/1078-0432.CCR-05-2017. [DOI] [PubMed] [Google Scholar]
- 85.Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 86.Snietura M, Piglowski W, Jaworska M, et al. Impact of HPV infection on the clinical outcome of p-CAIR trial in head and neck cancer. Eur Arch Otorhinolaryngol. 2011;268:721–726. doi: 10.1007/s00405-010-1396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soderberg C, Perez DS, Ukpo OC, et al. Differential loss of expression of common fragile site genes between oral tongue and oropharyngeal squamous cell carcinomas. Cytogenet Genome Res. 2008;121:201–210. doi: 10.1159/000138886. [DOI] [PubMed] [Google Scholar]
- 88.Stephen JK, Chen KM, Shah V, et al. Human papillomavirus outcomes in an access-to-care laryngeal cancer cohort. Otolaryngol Head Neck Surg. 20 Jan 2012. [Epub ahead of print] PubMed PMID: 22267491. [DOI] [PMC free article] [PubMed]
- 89.Sugiyama M, Bhawal UK, Kawamura M, et al. Human papillomavirus-16 in oral squamous cell carcinoma: clinical correlates and 5-year survival. Br J Oral Maxillofac Surg. 2007;45:116–122. doi: 10.1016/j.bjoms.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 90.Szládek G, Juhász A, Kardos G, et al. High co-prevalence of genogroup 1 TT virus and human papillomavirus is associated with poor clinical outcome of laryngeal carcinoma. J Clin Pathol. 2005;58:402–405. doi: 10.1136/jcp.2004.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tachezy R, Klozar J, Saláková M, et al. HPV and other risk factors of oral cavity/oropharyngeal cancer in the Czech Republic. Oral Dis. 2005;11:181–185. doi: 10.1111/j.1601-0825.2005.01112.x. [DOI] [PubMed] [Google Scholar]
- 92.Torrente MC, Ampuero S, Abud M, Ojeda JM. Molecular detection and typing of human papillomavirus in laryngeal carcinoma specimens. Acta Otolaryngol. 2005;125:888–893. doi: 10.1080/00016480510038220. [DOI] [PubMed] [Google Scholar]
- 93.Monsjou HS, Velthuysen ML, Brekel MW, et al. Human papillomavirus status in young patients with head and neck squamous cell carcinoma. Int J Cancer. 2012;130:1806–1812. doi: 10.1002/ijc.26195. [DOI] [PubMed] [Google Scholar]
- 94.Venuti A, Manni V, Morello R, et al. Physical state and expression of human papillomavirus in laryngeal carcinoma and surrounding normal mucosa. J Med Virol. 2000;60:396–402. doi: 10.1002/(sici)1096-9071(200004)60:4<396::aid-jmv6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 95.Vlachtsis K, Nikolaou A, Markou K, et al. Clinical and molecular prognostic factors in operable laryngeal cancer. Eur Arch Otorhinolaryngol. 2005;262:890–898. doi: 10.1007/s00405-005-0916-3. [DOI] [PubMed] [Google Scholar]
- 96.Zhang ZY, Sdek P, Cao J, Chen WT. Human papillomavirus type 16 and 18 DNA in oral squamous cell carcinoma and normal mucosa. Int J Oral Maxillofac Surg. 2004;33:71–74. doi: 10.1054/ijom.2002.0443. [DOI] [PubMed] [Google Scholar]
- 97.Alos L, Moyano S, Nadal A, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115:2701–2709. doi: 10.1002/cncr.24309. [DOI] [PubMed] [Google Scholar]
- 98.Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32:562–567. doi: 10.1002/hed.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lo EJ, Bell D, Woo JS, et al. Human papillomavirus and WHO type I nasopharyngeal carcinoma. Laryngoscope. 2010;120:1990–1997. doi: 10.1002/lary.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laantri N, Attaleb M, Kandil M, et al. Human papillomavirus detection in Moroccan patients with nasopharyngeal carcinoma. Infect Agent Cancer. 2011;6:3. [DOI] [PMC free article] [PubMed]
- 101.Smith EM, Rubenstein LM, Haugen TH, et al. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: a case for multifactor disease. J Oncol. 2012;2012:571862. [DOI] [PMC free article] [PubMed]
- 102.Westra WH, Taube JM, Poeta ML, et al. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14:366–369. doi: 10.1158/1078-0432.CCR-07-1402. [DOI] [PubMed] [Google Scholar]
- 103.Smith EM, Swarnavel S, Ritchie JM, et al. Prevalence of human papillomavirus in the oral cavity/oropharynx in a large population of children and adolescents. Pediatr Infect Dis J. 2007;26:836–840. doi: 10.1097/INF.0b013e318124a4ae. [DOI] [PubMed] [Google Scholar]
- 104.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 105.Bonagura VR, Hatam LJ, Rosenthal DW, et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS. 2010;118:455–470. doi: 10.1111/j.1600-0463.2010.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lawson W, Schlecht NF, Brandwein-Gensler M. The role of the human papillomavirus in the pathogenesis of schneiderian inverted papillomas: a critical review of the evidence. Head Neck Pathol. 2008;2:49–59. doi: 10.1007/s12105-008-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singhi AD, Califano J, Westra WH. High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck. 2012;34:213–218. doi: 10.1002/hed.21714. [DOI] [PubMed] [Google Scholar]
- 108.Punwaney R, Brandwein MS, Zhang DY, et al. Human papillomavirus may be common within nasopharyngeal carcinoma of Caucasian Americans: investigation of Epstein-Barr virus and human papillomavirus in eastern and western nasopharyngeal carcinoma using ligation-dependent polymerase chain reaction. Head Neck. 1999;21:21–29. doi: 10.1002/(sici)1097-0347(199901)21:1<21::aid-hed3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 109.Bleyer A. Cancer of the oral cavity and pharynx in young females: increasing incidence, role of human papilloma virus, and lack of survival improvement. Semin Oncol. 2009;36:451–459. doi: 10.1053/j.seminoncol.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 110.Vageli D, Sourvinos G, Ioannou M, et al. High-risk human papillomavirus (HPV) in parotid lesions. Int J Biol Markers. 2007;22:239–244. doi: 10.1177/172460080702200401. [DOI] [PubMed] [Google Scholar]
- 111.Boland JM, McPhail ED, García JJ, et al. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Mod Pathol. 2012;25:529–536. doi: 10.1038/modpathol.2011.186. [DOI] [PubMed] [Google Scholar]
- 112.Zehbe I, Wilander E. Two consensus primer systems and nested polymerase chain reaction for human papillomavirus detection in cervical biopsies: a study of sensitivity. Hum Pathol. 1996;27:812–815. doi: 10.1016/s0046-8177(96)90454-2. [DOI] [PubMed] [Google Scholar]
- 113.Kreimer AR, Cifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 114.Termine N, Panzarella V, Falaschini S, et al. HPV in oral squamous cell carcinoma vs. head and neck squamous cell carcinoma biopsies: a meta-analysis (1988–2007) Ann Oncol. 2008;19:1681–1690. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 115.Syrjänen S, Lodi G, Bültzingslöwen I, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011;17(Suppl 1):58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 116.Torrente MC, Rodrigo JP, Haigentz M, Jr, et al. Human papillomavirus infections in laryngeal cancer. Head Neck. 2011;33:581–586. doi: 10.1002/hed.21421. [DOI] [PubMed] [Google Scholar]
- 117.Hobbs CG, Sterne JA, Bailey M, et al. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31:259–266. doi: 10.1111/j.1749-4486.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 118.Laco J, Slaninka I, Jirásek M, et al. High-risk human papillomavirus infection and p16INK4a protein expression in laryngeal lesions. Pathol Res Pract. 2008;204:545–552. doi: 10.1016/j.prp.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 119.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 120.Jo VY, Mills SE, Stoler MH, Stelow EB. Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol. 2009;33:1720–1724. doi: 10.1097/PAS.0b013e3181b6d8e6. [DOI] [PubMed] [Google Scholar]
- 121.Stasikowska-Kanicka O, Wągrowska-Danilewicz M, Danilewicz M. Effect of human papillomavirus on cell cycle-related proteins p16INK4A, p21waf1/cip1, p53 and cyclin D1 in sinonasal inverted papilloma and laryngeal carcinoma. An in situ hybridization study. Folia Histochem Cytobiol. 2011;49:34–40. doi: 10.5603/fhc.2011.0006. [DOI] [PubMed] [Google Scholar]
- 122.Wadsworth B, Bumpous JM, Martin AW, et al. Expression of p16 in sinonasal undifferentiated carcinoma (SNUC) without associated human papillomavirus (HPV) Head Neck Pathol. 2011;5:349–354. doi: 10.1007/s12105-011-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]