Abstract
Helminths are parasitic organisms that can be broadly described as “worms” due to their elongated body plan, but which otherwise differ in shape, development, migratory routes and the predilection site of the adults and larvae. They are divided into three major groups: trematodes (flukes), which are leaf-shaped, hermaphroditic (except for blood flukes) flatworms with oral and ventral suckers; cestodes (tapeworms), which are segmented, hermaphroditic flatworms that inhabit the intestinal lumen; and nematodes (roundworms), which are dioecious, cylindrical parasites that inhabit intestinal and peripheral tissue sites. Helminths exhibit a sublime co-evolution with the host´s immune system that has enabled them to successfully colonize almost all multicellular species present in every geographical environment, including over two billion humans. In the face of this challenge, the host immune system has evolved to strike a delicate balance between attempts to neutralize the infectious assault versus limitation of damage to host tissues. Among the most important cell types during helminthic invasion are granulocytes: eosinophils, neutrophils and basophils. Depending on the specific context, these leukocytes may have pivotal roles in host protection, immunopathology, or facilitation of helminth establishment. This review provides an overview of the function of granulocytes in helminthic infections.
Keywords: Helminths Eosinophil, neutrophil, basophil, filariasis, TH2 response, reactive oxygen species, reactive nitrogen species, nitric oxide.
INTRODUCTION
Granulocytes are a class of leukocytes characterized by the presence of lobulated nuclei and secretory granules in their cytoplasm. They are generated from hematopoetic stem cells, which can differentiate into common lymphoid progenitor cells or common myeloid progenitor cells. Hematopoetic stem cells, once developed into progenitors, acquire specialized features of a particular cell type while losing lineage differentiation potential [1]. While lymphoid lineage cells include T, B and natural killer cells, differentiation of the myeloid lineage gives rise to mast cells, megakaryocytes, erythrocytes, dendritic cells (DC), macrophages and granulocytes [2]. The use of chemical dyes for selective cell staining enabled Paul Ehrlich in the late 19th century to describe three different types of granulocytes (eosinophilic, neutrophilic and basophilic polymorphonuclear cells) [3]. They are highly conserved, with relatively simple forms found in some invertebrates (such as molluscs), while all vertebrates are endowed with at least one type of granulocyte. Under normal conditions the human blood contains up to 50% neutrophils; whereas eosinophils comprise only 1-5%, and basophils less than 1% of circulating blood leukocytes.
Granulocytes can secrete a wide variety of mediators without de novo protein synthesis; a particularly important characteristic that highlights their key role in innate and adaptive immune functions. The capability of granulocytes, most notably eosinophils, to release toxic cationic proteins has been considered historically as an effector mechanism against extracellular organisms [4], although these molecules have also been implicated in tissue damage. Thus, granulocyte-mediated immunopathology is observed in hyperre-activity during some nematode infections and is also frequently manifested in allergic responses such as asthma [5]. The release of granule proteins can be induced through binding of antigen-IgE complexes to the high affinity IgE receptor (FcεRI) that triggers a tightly controlled phosphorylation cascade [6]. The classical view of granulocyte function has been reconsidered over the last decades, as new data have demonstrated that this cell type has roles other than that of a terminal effector cell [5, 7].
The functional analysis of granulocytes in helminth infections relies on interventional studies and includes suitable animal models in conjunction with immunological or genetic tools to interfere with normal granulocyte development and function. Despite the caveat that laboratory model organisms may not always be the natural host of the parasite, and therefore cannot represent all processes observed in natural infections of livestock or humans, clearly many paradigms translate well between the species and have led not only to greater understanding of parasitic diseases, but in several cases, to successful therapies. This review is not intended to cover the entire field of granulocyte biology but to focus on their functions in relation to a particularly complex foe, the helminth parasites. In particular the role of eosinophils, basophils and neutrophils in host protection, immunopathology or facilitation of helminth establishment will be discussed.
TH2 IMMUNITY TO HELMINTH INFECTIONS
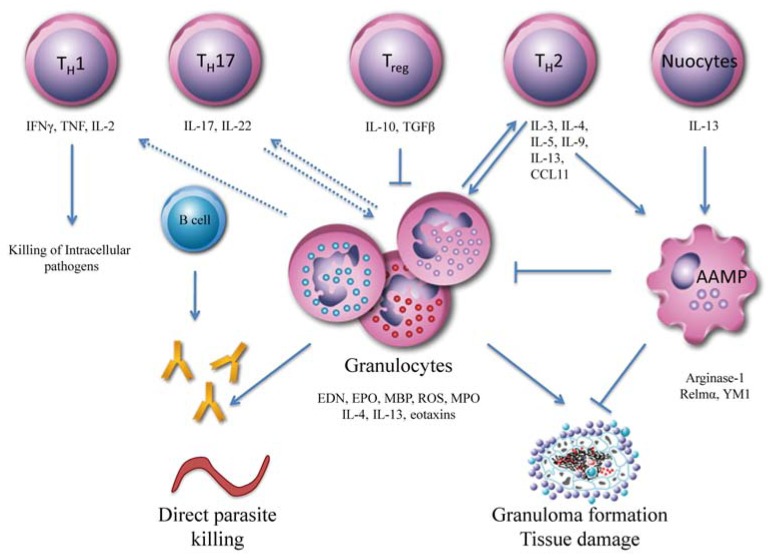
In response to an infection, a variety of cells becomes activated and collaborates in the effort to control and eliminate invading pathogens (see Fig. 1). TH1 cells mainly produce IFNγ, which is important for classical macrophage activation and the clearance of many intracellular microbes. Large extracellular pathogens face immune mechanisms that are of a TH2-type, characterized by an elevation of peripheral blood eosinophilia and accompanied by profound increases in cytokine production Interleukin (IL)-3, IL-4, IL-5, IL-9, IL-13) and granulocyte macrophage colony-stimulating factor (GM-CSF) as well as induction of the antibody isotypes immunoglobulin (Ig) G1, IgG4 and IgE. In mice lacking the key component of TH2-type immunity, i.e. CD4+ T cells, protective immunity to the nematodes Nippostrongylus brasiliensis [8], Litomosoides sigmodontis [9] and other helminths [10] is lost, providing evidence to support the importance of such responses in parasite clearance. Historically, it was hypothesized that TH2 responses are induced by suboptimal antigen presentation and consequently, ineffective stimulation of the TH1 pathway. However, helminth products can drive TH2 immunity even in the presence of TH1 inducers. For example, when stimulated with Schistosoma mansoni soluble egg antigen (SEA), DC are able to induce TH2 responses in the presence of bacterial TH1 stimuli [11].
Fig. (1). T cell mediated effector mechanisms against pathogens.
Innate immune mechanisms are the first to respond to place against infection. They consist of soluble factors, such as complement proteins, together with many cellular components including mast cells, macrophages, dendritic cells, natural killer cells and granulocytes (eosinophils, neutrophils and basophils). Adaptive immune responses develop more slowly, but result in increased antigen specificity and immunological memory, and are orchestrated by CD8+ T cells, CD4+ T cells and B lymphocytes. Among the T-helper (TH) cells, TH1 cells are protective against intracellular pathogens and TH17 cells against extracellular bacteria, whereas TH2 cells play a central role against extracellular pathogens, such as large helminths. The induction and maintainance of a TH2- type environment is strongly supported by recently discovered nuocytes and TH2 responses also play an important role in wound healing by the induction of alternatively activated macrophages (AAMP). Excessive effector T cell responses are controlled by regulatory T cells (Treg) in order to prevent immunopathology, as seen in allergic reactions (overinduction of TH2) or autoimmune diseases (overinduction of TH1 or TH17).
A more complex picture is now emerging, in which IL-25, IL-33 and thymic stromal lymphopoietin (TSLP) [12] have been shown to be strong inducers of TH2-type immunity. Blockade of IL-25 in mice, for example, results in reduced hyper-reactivity and inflammation in a model of ovalbumin-induced airway hypersensitivity [13]. This was associated with reduced eosinophil influx into the lung and suppression of IgE production. The effects induced by IL-25 seem to be very similar to those induced by IL-33, in that administration of both cytokines causes eosinophilia, splenomegaly, IgE secretion, production of other TH2-type cytokines, goblet hyperplasia and mucus production [14, 15]. TSLP is yet another potent TH2-type inducer found particularly in epithelial cells of the lung, skin and gut [16]. It belongs to the IL-7 family, and among other functions sensitizes DC to promote a TH2 developmental pathway. In depth investigation of these molecules led to the identification of lineage-marker negative cells; i.e., nuocytes, natural helper cells, innate helper cells and multi-potent progenitor cells, which are intimately involved in the generation of TH2-type responses via the production of IL-13. The role of these new cell types in immune responses has been extensively discussed in [17]. As this review will focus on only those aspects that are relevant to granulocyte function, one should refer to [10] for a thorough overview of more general features of TH2 immunity.
The host-parasite interface, which is predominantly in the gut or peripheral tissues, is the location where downstream effector mechanisms of TH2-type responses are developed for parasite expulsion or killing. In the gut, IL-13 promotes goblet cell hyperplasia and augments mucus production [18], contributing to worm expulsion. For the targeting of parasites in peripheral tissues, antibody production and recruitment of effector cells that release cytotoxic molecules upon activation (e.g., via cross-linking of Fc-receptors) eventually damage the parasite, although if these processes are not properly controlled, they can also harm the host.
In addition to the well-described effector function against helminthic parasites, TH2 responses are part of adaptive and therefore memory responses, which also result in tissue repair mechanisms. Since tissue destruction is a common manifestation of many helminth infections, limiting parasite-mediated damage is critically important in mitigating disease sequelae. For instance, both Ascaris and Nippostrongylus can lead to damage of the lung tissue during migration through the host. The TH2 cytokines, IL-4 and IL-13 for example, are potent inducers of molecules involved in wound healing processes, such as resistin-like-molecule-α (RELMα ), arginase, matrix metallopeptidase 12 (MMP12) and triggering receptor expressed on myeloid cells 2 (TREM-2) [19]. Along with such TH2 responses comes the alternative activation of macrophages which has been shown for many helminth infections. Indeed, although evolutionary hypotheses can be difficult to test, one intriguing proposal is that TH2 responses may have emerged as a tissue repair mechanism rather than being primarily anti-parasitic [20].
EOSINOPHILS
Eosinophils are mainly associated with allergic responses (such as asthma), viral infections or helminthiases. They are equipped with a variety of receptors (including those for chemokines, cytokines, immunoglobulins, complement and serine proteases) that enable them to be recruited into affected tissue sites and release granule contents [5, 21]. Mainly IL-5, but also IL-3 and GM-CSF promote eosinophil development whereas recruitment is mediated (among others) by chemokine ligand 11 (CCL11) and CCL26 (eotaxins), which in turn are controlled by IL-13. Most notably in helminth infections, ligation of parasite-specific immunoglobulins to Fc receptors is critically important for a process known as antibody-dependent cytotoxicity (ADCC), which results in activation of the eosinophils. However, they can also be activated by a number of other mediators, such as Toll-like receptor (TLR) ligands, chemokines and cytokines. Activation leads to classical exocytosis, in which the complete granule contents are released by fusion with the cellular membrane; and cytolysis, in which the plasma membrane ruptures and granules are deposited extracellularly (extensively reviewed in [5]). A highly regulated process termed “piecemeal degranulation” can occur also in eosinophils. This is characterized by emergence of vesicles from the granules, which traffic through the cytoplasm to the cell membrane for release of transported contents [22]. Although these different pathways to degranulation are likely to be tightly controlled, the exact mechanisms remain unclear.
Eosinophilic granules are primarily composed of the following cytotoxic, cationic proteins: major basic protein (MBP), eosinophil peroxidase (EPO), eosinophilic cationic protein (ECP) and eosinophil-derived neurotoxin (EDN) [5]. They are also equipped with a number of cytokines, chemokines and growth factors for very rapid release, which can alert the immune system to combat pathogenic organisms. Importantly, several of these mediators have additional functions, such as communicating with other cells by signaling interactions, including endothelial cells and mast cells (for more detailed review see [21]). Although the amounts produced are not comparable to the levels released by T cells, the fact that such molecules exist in preformed granules allows eosinophils to act as excellent gatekeepers during parasite entry to rapidly initiate immune responses.
Interventional studies on eosinophils have used a variety of approaches: eosinophil or IL-5 depleting antibodies; genetic modification leading to overexpression of IL-5 (IL-5 transgenic hypereosinophilic mice) or vice-versa IL-5 deficiency; abrogation of the eosinophil lineage (ΔdblGATA), or suicide activation during development induced by diphtheria toxin (TgPHIL) [23]. In addition, deficiency of recruitment factors such as the chemokine CCL11 [24] leads to reduced numbers of eosinophils. Information on the functional relevance of specific eosinophil-related mechanisms can be obtained from granule protein deficiency (e.g. MBP [25], EPO [26]) or by the use of µMT mice [27], which do not produce functional antibodies and thus cannot mediate ADCC.
NEUTROPHILS
Neutrophils are the most numerous type of leukocytes, and with a circulating half-life of only 6–8 hours their lifespan is shorter compared to that of eosinophils. The differentiation factors for neutrophils are G-CSF, IL-6, GM-CSF and IL-3 while multiple inflammatory mediators, including leukotriene B4, complement component C5, IL-8 and TNFα are able to induce neutrophilia [28]. When neutrophils are recruited to an inflamed compartment, apoptosis can be inhibited resulting in extended survival. Neutrophils are classically characterized as phagocytic cells that remove and destroy invading microorganisms; hence neutrophils are a vital component of the innate immune system and play a crucial role in host defence [29]. This is illustrated by the potentially fatal complications that arise in neutropenic individuals, such as patients with WHIM syndrome [30]; or by the morbidity associated with clinical conditions in which genetic polymorphisms give rise to abnormal neutrophil physiology, for instance, as leukocyte adhesion deficiency 1 (LAD1) and chronic granulomatous disease [31].
Neutrophils are the first cell type recruited to the site of an acute inflammatory response and are characterized by their ability to act as phagocytic cells, to release lytic enzymes, and to produce reactive oxygen species with anti-microbial potential [32, 33]. These released products may act in conjunction with cells resident in the affected tissue, such as macrophages and mast cells, to amplify the initial inflammatory response [34] and induce the recruitment of additional neutrophils, other leukocyte populations (including monocytes and lymphocytes), and (in TH2 scenarios) eosinophils and basophils. It has become apparent that neutrophils are important mediators of the TH17 pathway of resistance to pathogens [35]. This arm of the immune system is characterized by a sustained inflammatory milieu that also may give rise to an adaptive immune response. However, in certain circumstances, the excessive activation of such cellular defences may cause inappropriate damage to host tissues. This has been linked to the pathogenesis of a variety of diseases, including adult respiratory distress syndrome [36], chronic obstructive pulmonary disease [37], and sudden-onset fatal asthma [38]. Because of their predominant role in phagocytosis of microbial pathogens, neutrophils have often been overlooked in helminth infections, although this is beginning to be addressed in animal models and hence indications for their involvement in immune responses to helminths will be discussed. Tools for research on neutrophils comprise mainly antibodies against GR-1, Ly6G, PMN or G-CSF; whereas in genetic mouse models, CXCR2- and NE-deficient [39] mice have been used.
BASOPHILS
Since they are comparatively rare, the immunology of basophils has been neglected for many years, but these cells are now recognized for their role in initiating immune responses in addition to their effector function. They share many features with mast cells, including FcεRI expression and the capacity to secrete reactive oxygen and nitrogen species [40], TH2 cytokines, and histamine. However, in contrast to mast cells, which are located in tissues and have the potential to proliferate in response to IL-3, IL-4 and IL-9, basophils circulate as fully matured cells in the blood. IL-3 is the main factor responsible for their optimal activation, population expansion and survival [41], although more recently it has been shown that TSLP acts in concert with IL-3, to propagate these effects [42]. Interestingly, in response to TSLP but independently of IL-3 a different phenotype of basophils was induced, which exhibited reduced degranulation capacity but higher IL-4 and IL-6 production; moreover the gene expression profile in these cells was enriched in cell communication and adhesion.
Basophils are characterized by the presence of basophilic granules and surface expression of high affinity Fcα RI for binding of IgE in addition to cytokine receptors, chemokine receptors and complement receptors [43]. Upon ligation they release chemical mediators, such as leukotriene C4 and histamine, and particularly the TH2 cytokines IL-4 and IL-13, which implicates basophils in immune responses elicited by helminthiases [44]. Basophils display a remarkable potential to contribute to the symptoms of allergic inflammation through the release of histamines and leukotriene, and have been proposed to play a key role by inducing class switching to IgE in B cells [45]. Recent studies suggest that basophils induce IgE-mediated chronic allergic inflammation [46] and IgG1-mediated systemic anaphylactic shock [47]. Depletion of basophils during IgE-mediated dermatitis reduced the number of eosinophils and neutrophils in the skin, suggesting an important role for basophils in the initiation of allergic responses and more importantly, in interactions between granulocytes [48]. Several studies have indicated that basophils may be important in the TH2 polarisation of the immune response [49], mainly by secreting IL-4. This will be discussed in the following section.
Tools for the study of basophils mainly consist of antibody-mediated depletion targeting CD200 receptor 3 (Ba103) or the high affinity Fcα receptor (MAR-1) [49]. Recently, an inducible basophil-deficient mouse was generated (Mcpt8DTR), in which injection of diphtheria toxin led to a transient depletion of basophils [50]. In addition, mice that have the Cre recombinase expressed under the control of the carboxypeptidase A3 promotor have been crossed to mice bearing a floxed allele of Mcl-, also showed a marked deficiency of basophils [51]. Reporter mice, in which either IL-4 [52] or basophil-specific protease expression [53] can be monitored, offer the possibility to track activated basophils in vivo.
RECENTLY RECOGNIZED FUNCTIONS OF GRANULOCYTES
In addition to the classical downstream effector functions of granulocytes, such as ADCC, which direct the release of toxic molecules against pathogens, granulocytes express a particularly rich repertoire of TLRs, the key molecules that mediate innate immune responses. These receptors recognize ligands from infectious agents termed “pathogen-associated molecular patterns” (PAMPs). Binding of PAMPs to TLRs activates cytoplasmic adaptor molecules that trigger a protein kinase cascade, leading to induction or suppression of inflammatory response genes. From the thirteen TLRs identified to date, most are expressed by granulocytes (see Table 1). The majority of characterized TLR ligands are derived from viruses or unicellular pathogens, although more recently, a small number of PAMPs from helminths has been discovered. These include molecules from the trematode Schistosoma mansoni: the lipid lysophosphatidylcholine (lyso-PC) [54] and the glycan lacto-N-fucopentaose III [55], which are ligands for TLR-2 and -4, respectively. While lyso-PC is involved in eosinophil recruitment, the lysophosphatidylserine (lyso-PS) [56], another ligand for TLR-2, is able to skew immune responses towards IL-10 production and T cell hyporesponsivness. Such suppression is an important mechanism during apoptosis by the host´s own phosphatidylserine [57]. This shows that different molecules can stimulate the same TLRs with varying outcomes, whereas a single molecule, irrespective of whether the molecule is derived from the parasite or the host, can induce a similar type of response, i.e. immunosuppression. Co-evolution of the host and parasite has honed many of such immune evasion strategies in order for the parasite to thrive in its host. In nematodes, the excretory-secretory glycoprotein ES-62 from Acanthocheilonema viteae can also bind to TLR-4 [58], while the polysaccharide chitin, which is widespread among nematodes and other non-vertebrate phyla (Table 1), is a ligand for TLR-2. In addition, the unique biology of certain filarial nematodes (e.g., Wuchereria bancrofti and Onchocerca volvulus), which have an obligate relationship with a bacterial symbiont (Wolbachia; see last section), results in the activation of TLRs that recognise bacterial PAMPs during infections with these species [59, 60]. Whereas ligation of ES-62 to TLR-4 leads to deactivation of mast cells and therefore prevents immune-mediated damage, a converse effect has been attributed to Wolbachia recognition by TLRs in filarial infections, which appears to be a strong inducer of pathology in humans [60]. Importantly, despite the apparent ability of TLRs to recognize helminth products, there is little evidence that this process translates into induction of the adaptive TH2 pathway. Indeed, other molecules, such as C-type lectin receptors may be more important than TLRs in this context, but the precise mechanisms remain elusive [10]. For a more comprehensive overview of the innate recognition of helminths by the mammalian immune system, the reader is directed to other recent reviews [61, 62].
Table 1.
Toll-Like Receptors Expressed by Human Granulocytes and their Natural Ligands
| TLR | Ligands | Associated pathogens* | Granulocyte expression# |
|---|---|---|---|
| TLR-1 | Triacylated lipoproteins | Bacteria | Neutrophils, eosinophils [291] |
| TLR-2 | Lipoproteins | Bacteria# | Neutrophils [292], Basophils [293], Eosinophils [291] |
| Zymosan | Fungi | ||
| Ara-lipoarabinomannan | Mycobacteria | ||
| Lysophosphatidylcholine (54) | Schistosomes | ||
| Lipotechoic acid | Gram +ve bacteria | ||
| Chitin (294; 290) | Fungi, protists, nematodes, arthropods | ||
| TLR-3 | dsRNA | Viruses | Eosinophils [295] |
| TLR-4 | Lipopolysaccharide | Gram -ve bacteria | Neutrophils [291], Basophils [293], Eosinophils [296] |
| Heat-shock proteins | Various | ||
| Lacto-N-fucopentaose III (55) | Schistosomes | ||
| Glycoprotein ES-62 (58) | Filarial nematodes | ||
| TLR-5 | Flagellin | Bacteria | Neutrophils [291], Eosinophils [296] |
| TLR-6 | Diacylated lipoproteins | Mollicutes and rickettsiae# | Neutrophils [297], Eosinophils [296] |
| TLR-7 | ssRNA | Viruses | Eosinophils [298], Neutrophils [291] |
| TLR-8 | ssRNA | Viruses | Neutrophils [299] |
| TLR-9 | Unmethylated CpG oligodeoxynucleotides | Bacteria, viruses | Neutrophils [289], Basophils [293], Eosinophils [291] |
| TLR-10 | Unknown | - | Eosinophils [291], Basophils [293], Neutrophils [291] |
Irrespective of our ignorance of the exact pathways involved, several line of evidence suggest that granulocytes are indeed key participants in bridging innate and adaptive immunity. It is now widely accepted that eosinophils [63], neutrophils [64] and basophils [65] not only have the ability to sense and react to PAMPs but also to phagocytose, process and present antigen to naïve T cells. Whereas presentation of helminth-derived antigens by neutrophils remains to be demonstrated, eosinophils from healthy human donors stimulated with Brugia malayi extracts or isolated from rodents after infection with B. malayi, increased their surface MHC class II expression and upregulated costimulatory molecules such as CD40 and CD86 [66, 67]. Furthermore, eosinophils exposed to Strongyloides stercoralis were capable of inducing IL-5 production by T cells in vitro [68] and in vivo after adoptive transfer of antigen-exposed eosinophils [69]. They may also play a role in maintaining TH2 responses by secretion of IL-4 [70].
Recently, a major focus has been on basophils, which have been reported to act as antigen-presenting cells and to be an important source of IL-4 and IL-13, prerequisites for the induction of TH2 responses. For example, depletion of basophils results in impaired protection against several parasites; e.g. the gastrointestinal helminths N. brasiliensis [71] and Trichuris muris [19]. In the latter study, it was also shown that basophils expressed MHC class II and IL-4 and were recruited to the lymph nodes upon challenge of mice with S. mansoni eggs, thereby having the potential to interact with naïve T cells in peripheral lymphoid tissues. A major secreted product of S. mansoni eggs, alpha-1, also known as IL-4-inducing principle of schistosome eggs (IPSE), has been demonstrated to activate mouse [72] and human basophils, causing release of IL-4 in vitro [73, 74], which was dependent on IgE crosslinking [74]. Despite this induction of an immune response, a recent study by Sullivan and colleagues [53] reported that in accordance with [75], basophils may not be implicated in protective responses during primary helminth infection. A similar observation was made in murine infection with the filarial parasite L. sigmodontis [76], since removal of basophils did not alter the outcome of infection, despite a reduction in TH2 immunity. However, basophils may play a major role in type-2-mediated secondary infection in conjunction with CD4+ T cells, as depletion of IL-4 and IL-13 in both basophils and CD4+ T cells was necessary to abrogate protection. It was elegantly shown that IL-4 production in basophils and also eosinophils was restricted to affected tissues; e.g., the lung in primary N. brasiliensis infection [53]. However, other studies have shown that TH2 induction can occur in the absence of basophils. IL-3 deficient mice, for example, show an impaired recruitment of basophils to lymph nodes, but this did not affect elicitation of TH2 responses upon infection with N. brasiliensis [77]. Indeed, it has been suggested that IL-4 produced by basophils may be more important in maintaining TH2 immunity, rather than inducing this response [7]. Hence, the in vivo relevance of granulocytes as antigen-presenting cells or as TH2 inducers during helminth infections requires further investigation.
ROLE OF GRANULOCYTES AGAINST TREMATODES, CESTODES AND NEMATODES
Trematodes
Commonly known as “flukes”, trematodes have a dorsoventrally flattened body with bilateral symmetry, ranging from a few millimeters up to 7-8 cm in size. In general, snails are involved as intermediate hosts and the development includes several larval stages before reaching adulthood. Trematodes of medical importance include Schistosoma spp. (blood flukes), Clonorchis sinensis, Fasciola spp. (liver flukes) and Paragonymus westermani (lung fluke), of which Schistosoma spp. are the best-studied parasites. Five species infect humans, with S. mansoni, S. japonicum, S. intercalatum and S. mekongi inducing intestinal and liver disease, whereas S. haematobium affects the urinary tract. Following the release of parasite eggs into water, a free-living motile larval stage (miracidium) hatches to infect freshwater snails. After transformation and asexual multiplication within the snail, cercariae are liberated, which penetrate human skin and develop into migrating schistosomulae in order to settle within the mesenteric veins or the perivesical venous plexus of the bladder. Females can produce up to 3000 eggs per day that must passage through the gut or bladder wall in order to be transmitted [78]. The severe morbidity that is associated with schistosomiasis is a result of granulomatous reactions to the highly immunogenic eggs that are backwashed, becoming entrapped in the liver. In a minor proportion of infected individuals, these lesions fail to resolve and may progress to hepatosplenomegaly, chronic hepatic fibrosis, portal hypertension, and ultimately, death [79, 80].
Schistosoma: Migratory Stages
The capacity of eosinophils to adhere to Schistosoma spp. was demonstrated decades ago to be dependent on the activation of complement [81]. The in vitro killing of schistosomulae required the cytotoxic activity of activated human eosinophils, but not neutrophils, in combination with heat-inactivated sera from infected patients, a process dependent on IgG1 and IgG3 [82-84]. Also, in vitro incubation of schistosomulae with eosinophils and specific antibodies prior to their injection into rodents led to a marked reduction of parasite viability [85], demonstrating the importance of eosinophils in direct parasite killing. Release of toxic molecules, such as elastase and hydrogen peroxide is an important mechanism that efficiently kills the parasites, as has been demonstrated for Fasciola hepatica [86] and schistosomes, with both molecules being liberated by neutrophils, eosinophils and macrophages [87].
Additional involvement of granulocytes in the priming of immune responses has been investigated over the past decades. It is well known that the infection with Schistosoma spp. begins with a marked TH1 response, shifting towards a strongly TH2 polarized response. The first three hours of transformation from cercariae into skin-stage schistosomulae coincides with the release of pre-formed cercarial gland material into the dermis. This heterologous mixture of highly glycosylated secretions contains several proteases to aid dermal penetration but is also implicated in immunomodulation (reviewed in [88]). In primary experimental mouse infections, neutrophils are rapidly recruited to the skin infection site [89, 90], followed by macrophages, Langerhans cells and DC. Fluorescent labeling of gland contents reveals that all these populations phagocytose the released products and subsequently migrate to the skin-draining lymph nodes, delivering and presenting schistosomal antigen to CD4+ T cells [90, 91] to initiate a predominantly TH1-biased cytokine responses (i.e., IFNα , IL-2). Interestingly however, DC exposed to released products prime a TH2 response in vitro and in vivo [92]. Indeed, after the onset of patent infection and egg deposition in hepatic and intestinal tissues (see below) the initial prevailing TH1 response is modulated in favour of a strongly polarized TH2 response (IL-4, IL-5, IL-13) that coincides with peak granuloma formation when the eggs are produced [94] (reviewed by [95, 96]). Excretory/secretory (E/S) products of invading larvae and those secreted by lung-stage schistosomulae, may also contribute to this second phase induction via a direct TH2 priming effect on DC [92, 97]. Consistent with this, dermal conditioning of antigen-presenting cells by eosinophils during multiple larval infections of the skin further down-regulates the TH1 response in a mechanism requiring a recruitment of large numbers of RELM-α + eosinophils providing an enriched TH2 cytokine (IL-4, IL-13) skin microenvironment that conditions DC and macrophage populations that can subsequently impact negatively on egg granuloma size in the liver [93].
In non-permissive rats (schistosomes are spontaneously eliminated in the third week of infection), parasite elimination coincides with eosinophilia and elevated IgE [98] and ADCC plays a major role [99]. Because mice and humans are permissive to primary and re-infections, this may potentially reflect the fact that mice, rats and humans show profound divergence in the propensity of eosinophils to degranulate [100, 101] and display differences in eosinophil high affinity IgE receptor expression [102-104]. Vaccination of mice with radio-attenuated, but not normal cercariae, leads to a high level (60-70%) of immunoprotection to subsequent challenge (reviewed by [105]). The site of arrestment of attenuated schistosomulae is in the lungs, but migration from the skin to the lungs is also substantially retarded, pointing towards the priming of protective immunity at one or both sites [106, 107]. The location of challenge attrition, however, is exclusively at the lung migration site (reviewed in [108]). IFNγ-producing CD4+ T cells are recruited to this site during challenge infection potentially mediating parasite killing via classical activation of macrophages producing iNOS via an IFNγ and TNFRI-dependent pathway [109, 110]. However, TH2 responses also appear important as IL-4Rα-/- mice are not protected despite strong TH1 responses and cellular infiltration into the lungs [111]. Indeed, in TH1 deficient mice (IFNγR-/-, IL-12p40-/-), a significant level of protection (as much as 40%) is evident and coincides with a marked eosinophil influx and elevation in circulating IgE [105]. The direct role of eosinophil ADCC as a mechanism of parasite attrition remains contentious however, as IL-4 or IL-5 depletion of eosinophils and IgE does not reduce vaccination-mediated challenge protection in mice [112].
Schistosoma: Granuloma Formation
Granuloma formation is initiated by release of eggs that are deposited in the liver, intestinal or bladder tissues. S. mansoni eggs secrete a limited range of molecules [113], which can polarize toward a marked CD4+ T cell programmed inflammation with IL-4 and IL-13 [114]; however, CCL3 [115], an activator of granulocytes, is also produced. In S. mansoni, the two most abundant egg-secreted products both direct the TH2 anti-egg response, but utilize distinct mechanisms. IPSE has been shown to induce basophil release of IL-4 in vivo, [72] providing an early innate source of this polarizing TH2 cytokine at the infection site, and possibly within the draining mesenteric lymph nodes, as basophils can migrate to secondary lymphoid tissue upon activation. A second molecule, Omega-1, mediates TH2 polarization via internalization by DC [116, 117]. The TH2 priming ability of DC exposed to Omega-1 is dependent on the molecule’s ribonuclease activity, and DC that have internalized Omega-1 display altered cytoskeletal architecture, possibly modifying their antigen presentation efficiency [117]. Because these molecules are restricted to S. mansoni [118], distinct TH2 polarizing, but as yet undefined processes, must occur in other schistosome species.
The balance between TH1 and TH2 cytokines determines the extent of granulomatous inflammation and thus larger granulomas are usually associated with strong anti-egg TH2 responses, whereas minimal lesions are observed when TH1 cytokines dominate [119]. In humans, variation in colonic granuloma size is positively associated with peripheral blood mononuclear cell responsiveness to egg antigen. TH2 responses are critical in the survival of murine hosts during acute-stage egg deposition because the absence of IL-4 signaling is lethal shortly following the onset of egg production [45, 120-122].
In the S. mansoni mouse model, egg granuloma formation consists of a dynamic acute phase followed by a chronic phase. The acute phase occurs when anti-egg TH2 reactivity is at a maximum both at the deposition sites and within the mesenteric lymph node and spleen (reviewed in [95, 96]). This phase also coincides with maximal granuloma size in hepatic tissue, and peaks at approximately 8 weeks post-infection (3 weeks after the onset of egg deposition). At this stage, within hepatic tissues, eosinophils constitute the majority of cells (>50%) [123] and tend to be most abundant proximal to the egg surface, reflective of their rapid recruitment. The production and release of reactive oxygen species (ROS) probably has detrimental effects on the parasite and the administration of melatonin, a well-known antioxidant, prevented most of the damage that normally occurs in mice infected with S. mansoni, e.g., eosinophil infiltration [124]. In contrast, neutrophils comprise a very minor population (between <1-2% in the liver and undetectable in the gut) [123, 125] of the cellular infiltrate in egg granulomas. However, it must be noted that in intestinal granulomas, macrophages are the predominant cell type and a significant, yet reduced, proportion of eosinophils (44% in the colon and 21% in the ileum) are recruited [125]. Despite their preeminence within schistosome egg granulomas, the protective function of eosinophils remains elusive. Whereas in the IL-5 knock out system hepatic granulomas are significantly smaller and IL-13-dependent tissue fibrosis reduced (although the authors examined a later time point, at +16 weeks) [123], eosinophil-lineage-negative mice (ΔdblGATA, TgPHIL) show no impaired survival after the onset of egg production up to 11 weeks post-infection. Strikingly, no difference in granuloma size was observed, demonstrating a compensatory recruitment of other leukocyte populations into granulomas in the absence of eosinophils [23]. In addition, gross hepatic damage and fibrosis were not significantly different in these mice.
Cestodes
The Cestoda (cestodes or tapeworms), in common with the Trematoda discussed above, is a class of parasites in the phylum Platyhelminthes. However, unlike the trematodes, the cestodes are universally hermaphroditic. The anatomy of adult cestodes in the major subclass, the Eucestoda, is simple: the rounded scolex features suckers and/or hooks that enable the tapeworm to attach to the wall of the intestine, while the remainder of the body forms the flattened strobila (attaining several metres in length in some species), which is composed of segments or ‘proglottids’. Mature proglottids either release eggs into the gastrointestinal tract, or detach from the strobila and emerge intact from the anus before the eggs are liberated. Once the larvated eggs (oncospheres) or a motile embryonic form are ingested by a suitable intermediate host, the larval metacestode migrates from the intestine into other tissues, where it forms either a spherical or multilobular cyst, or an elongated sparganum. In most species, the lifecycle is completed when this tissue stage is consumed by the definitive host, although some species of tapeworms utilise two intermediate hosts, whereas others have lost the requirement for an intermediate host [78].
In general, adult tapeworms cause little pathology in humans, but the cystic stage can induce life-threatening complications. The species of greatest medical importance are the pig tapeworm (Taenia solium), which causes neurocysticercosis, and two species in the genus Echinococcus that cause either alveolar (E. multilocularis) or cystic (E. granulosus) echinococcosis (the latter is also known as ‘hydatid disease’) [78].
Few studies have investigated the role of neutrophils in cestode infections and these cells are rarely observed in association with metacestodes in tissue [126], although they are well represented in infiltrates around intracranial infections with Mesocestoides corti in mice [127]. However, neutrophil chemokinesis in response to extracts from T. taeniaeformis metacestodes [128] and the killing of cestodes by reactive oxygen species released from neutrophils have been demonstrated in vitro [129]. Moreover, protective immunity stimulated by CpG oligodeoxynucleotides in the murine T. crassiceps model was associated with neutrophil influx into the site of infection [130]. With respect to basophils, human infections with both E. granulosus and E. multilocularis induce specific IgE production in which these antibodies often remain bound to the surface of basophils; in this state, they are responsive to a factor released by mononuclear cells that triggers histamine release [131]. Moreover, basophils secrete both IL-4 and IL-13 when stimulated with extracts from E. multilocularis, and this response is dependent on surface-bound IgE [132]. Thus, specific histamine secretion and degranulation of basophils stimulated with Echinococcus antigens in vitro has been developed as a highly sensitive diagnostic test for infection with these parasites [133].
Metacestodes are particularly potent inducers of eosinophilia. Early studies in rodent models, using species such as M. corti, T. taeniaeformis, T. crassiceps, Hymenolepis nana and H. diminuta, established peripheral eosinophil levels that enabled pioneering functional studies on these cells before the advent of IL-5-overexpressing mice [134]. This eosinophilia usually extended into tissue infiltration around the developing cyst [135]. Secondary challenge of rodents resulted in a rapid eosinophilia that was associated with “eosinophilotactic factors”; these also induced eosinophilia when transferred passively into naive hosts via serum [136]. Moreover, cestodes were found to secrete products that had marked chemotactic activity for eosinophils by direct stimulation [137]. Not only recruitment but also the activity of the eosinophil may contribute to resistance to reinfection, as they have been shown to release extracellular oxygen radicals in the intestine of mice infected with H. nana, which was associated with larval degradation [138].
In pigs naturally infected with cysts of E. granulosus, heavy parasitosis was associated with a massive infiltration of eosinophils around nascent cysts, which led to death and resorption of the parasite [139]. This may reflect concomitant immunity that increases with host age [140]. Additionally, mouse strains naturally resistant to T. taeniaeformis exhibited potent eosinophil responses to metacestodes in the liver, in which degranulation was clearly associated with parasite degeneration [141, 142]; and eosinophils were also implicated in the resistance of ‘rapid responder’ mice to larval stages of H. nana [143] and M. corti [144]. In the case of adult tapeworms in the intestine, eosinophils were associated with expulsion of the strobila of H. diminuta in the rat, and this process was partially dependent on T-cells, as anti-thymocyte serum reduced both eosinophilia and the destrobiliation response [145]. Similar results were obtained when the survival of larval T. taeniaeformis was compared between naturally resistant and severe combined immunodeficiency mouse strains [146]. In mice challenged with protoscoleces from hydatid cysts, a murine strain deficient in the complement component C5 showed higher levels of parasite establishment and larger cysts than did wild-type mice; these differences were associated with reduced levels of eosinophil infiltration in the C5-deficient strain [147]. However, eosinophil degranulation onto the surface of T. solium cysts in skeletal muscle or the diaphragm of naturally infected pigs did not cause any obvious damage to the metacestode tegument [148]; although in more recent studies, eosinophils were associated with disrupted parasite vesicular membranes in late-stage porcine muscle cysts of T. solium [149] and hydatid cysts in cattle [150]. Conversely, in a time-course experiment performed with T. taeniaeformis in the rat, granulocytes closely associated with the surface of the metacestode became pyknotic and degenerated, while cyst development was completely unhindered [151]. In addition, studies using antibody neutralisation or gene knockout of IL-5 in mice infected with M. corti have failed to identify any role for eosinophils in mediating host protection [152, 153].
The extent of the ability of eosinophils to control cestode infections may thus be dependent on the innate resistance of the host, the development of concomitant immunity, or effective vaccination. In sheep that have become partially immune to T. hydatigena through natural exposure, larvae from an experimental challenge become encircled by layers of eosinophils that are in turn surrounded by T- and B-cells as the immune response progresses [154], while in primary infection, no such infiltration is observed [155]. Immunotherapeutic vaccination with native antigen in pigs naturally infected with T. solium resulted in a marked ingression of eosinophils around the metacestodes, and these cells destroyed the developing parasites by degranulating on the tegument [156]. Follow-up studies demonstrated that immunisation with native antigen was effective at eliminating cysts whether the pigs acquired infection before or after vaccination, and in both cases eosinophils were instrumental in parasite destruction [157]. Inoculation of T. solium metacestode antigen into mice also induced a pronounced eosinophilia with the production of specific IgE [158]. Interestingly, mice challenged with viable oncospheres of several different species of cestodes showed complete eosinophil-mediated cross-resistance to oncospheres of H. nana [159].
It is intriguing that despite this preponderance of data from natural infection in domestic animals and in laboratory models, IL-5 and eosinophilia are actually associated with an increased risk of recurrent infection with E. granulosus in humans [160]. Moreover, disease severity in human neurocysticercosis is related to eosinophil numbers, among other factors [161], although this is not surprising since the brain is an immunoprivileged site. In contrast, the positive association between TH-2 responses (including the production of IL-5 from PBMC in vitro) and disease severity in humans infected with E. multilocularis did not extend to circulating levels of eosinophils, which were not elevated in any patient group [162].
Indirect evidence for a protective role for granulocytes in cestode infections can be inferred from the immunomodulatory molecules expressed by these parasites that interfere with granulocyte function. For instance, both E. granulosus and E. multilocularis produce “antigen B” (AgB), a large polymeric lipoprotein found in cyst fluid [163]. Components of this complex can inhibit neutrophil chemotaxis and the platelet-activating factor-mediated respiratory burst of these cells in vitro [164]; moreover, AgB drives a potent, non-protective Th-2 response that is positively associated with progressive disease in E. granulosus-infected patients [165]. A tegumental protein from E. granulosus, EgTeg, was also found to inhibit neutrophil chemotaxis and induce IL-4 production from the T-cells of infected patients [166]. These data are consistent with the observed loss of the chemotactic response to parasite antigens in neutrophils recovered from the peritoneal cavity of mice infected with E. multilocularis [167]. Furthermore, an antigen extract from T. solium metacestodes induced DNA fragmentation and inhibition of phagocytosis in neutrophils in vitro [129]. Cestode products that can disrupt eosinophil function have also been identified, such as a cysteine protease secreted by metacestodes of E. multilocularis that digests host eotaxin, preventing the infiltration of eosinophils into the peritoneal cavity of infected mice [168]. In addition, prior administration of a ribonucleopeptide from the metacestode of T. solium abrogated tissue eosinophilia and parasite destruction in mice subcutaneously implanted with metacestodes [169], while an annexin secreted by this parasite was reported to induce eosinophil apoptosis in vitro [170]. This latter finding is also supported by recent data revealing high levels of eosinophil apoptosis in the peritoneum of mice infected with T. crassiceps [171]. Thus, cestodes may have evolved immune evasion strategies to limit eosinophil degranulation, as it has been demonstrated that recombinant human ECP can damage protoscoleces of E. granulosus in vitro. Moreover, in the same study, ECP was detected both on the surface of hydatid cyst walls and in cyst fluid from human-derived parasite material [172].
Nematodes
The phylum Nematoda (roundworms) is characterized by a cylindrical body shape and includes a number of parasites that are pathogenic in humans and/or livestock. Nematodes are equipped with a cuticle, which requires constant molting during development and this is produced by the hypodermis, a thin lining beneath the cuticle that forms two longitudinal cords medial to a dorsal and ventral cord. They are equipped with longitudinal muscle cells, but unlike the Annelida, they lack circular muscles. A remarkable feature of these parasites is their adaptation to very different tissues of the host, ranging from the gut to the dermal tissues, and from the lymphatics to the coelomic cavities. Therefore, they have developed a variety of migration pathways that can be very simple, as for the order Oxyurida (which undertake out their lifecycle entirely within the gastrointestinal system); or more complex, as for Ascaris lumbricoides (which is ingested as an embryonated egg, hatches as an infective larva in the gastrointestinal system, travels through the blood to the lungs, then migrates back into the gastrointestinal system). In contrast with oral uptake, larvae of nematodes such as Strongyloides stercoralis or Necator americanus enter the host via penetration of the skin. Thus the host has had to evolve a range of strategies to combat these diverse parasites [173].
Soil-Transmitted Nematode Infections
Species in the genera Ascaris (giant roundworm), Ancylostoma and Necator (hookworms), Strongyloides (threadworms), and Trichuris (whipworms) are soil-transmitted parasites that together constitute the most prevalent helminth infections of humans. Among these, Strongyloides spp. is the only genus that is able to replicate within the host. Although generally not fatal, high infection rates are associated with chronic morbidity that may include anemia and malnutrition [174]. As for many other helminths, expulsion of gastrointestinal (GI) nematodes is highly dependent on TH2 responses and depletion of CD4+ T cells leads to delayed expulsion of the parasites (extensively reviewed in [175]).
Much of our current knowledge derives from model systems, of which Heligmosomoides polygyrus in mice has a strictly enteric life cycle, invading the muscularis of the jejunum [176] for a short period, after which it migrates back into the lumen. Infection induces a pronounced infiltration of eosinophils and neutrophils, but also mast cells into the mucosa and submucosa [177]. Evidence for the ability of granulocytes to attack H. polygyrus (previously known as Nematospiroides dubius) larvae was provided by in vitro experiments, in which eosinophils and neutrophils from infected animals were able to reduce the infectivity of larvae in the presence of fresh sera [178], suggesting a complement mediated adherence of granulocytes. In vivo, neutrophils accumulate immediately around the parasite, followed by alternatively activated macrophages, DC, CD4+ T cells and eosinophils [179]. However, in secondary but not primary infections, initiated after anti-helminthic treatment of mice, anti-IL-4 or IL-4Rα treatment blocked polyclonal IgE responses and abrogated protective immunity, despite a substantial increase in the number of eosinophils. In contrast, anti-IL-5 treatment prevented H. polygyrus-induced eosinophilia, whereas it did not alter the parasite loads [180]. Therefore, even though granulocytes are associated with memory responses, their importance for parasite expulsion may be minor in this system. This could be due to the fact that once adult, H. polygyrus resides within the lumen and other type-2 dependent mechanisms are more important in controlling worm burden, such as mucus production [181]. Similarly, in T. muris infection (in which larvae migrate to the cecum and proximal colon and reside in tunnels of actin-rich, brush-border epithelium [182]), ablation of IL-5 with blockade of eosinophil accumulation in a resistant mouse strain did not facilitate a patent infection [183]. Furthermore, mice devoid of Fc-γ receptor were able to expel the parasite, arguing against a role for ADCC in Trichuris infection. The same research group recently showed that IL-4-producing eosinophils accumulated in the mesenterial draining lymph nodes, but again, eosinophil-deficient mice efficiently generated CD4+-T cells and TH2 cytokines resulting in worm expulsion [184]. Data regarding inflammatory responses to Trichuris in humans are rare. However, in patients infected with T. trichiura, biopsies of colon tissue revealed an infiltration of eosinophils and neutrophils even in light infections and changes in other cell types (other than a reduction in plasma cells) were not observed [185].
Nippostrongylus brasiliensis is another parasite residing in the GI tract, but in contrast to the abovementioned nematodes, the lifecycle includes host entry via the skin and passage through the lung tissue prior to settlement in the gut. It has been demonstrated that during infection with this species, eosinophilia is due to prolonged survival within tissues, rather than an increased turnover [186]. In this infection, the skin is an important site for immunity against the invading organism. It has been shown in vitro that the relative importance of the three pathways (classical, alternative and lectin) of complement activation differs depending on the species of helminth and the host [187-189]. However, when complement-deficiency of all three pathways was produced on an IL-5 transgenic background in order to facilitate the strong eosinophil-dependent resistance to N. brasiliensis in vivo [190], this resulted in a reduction in eosinophil accumulation and extracellular EPO activity, but only a minor change in larval numbers recovered from the lung. This indicates the importance of other, complement-independent mechanisms. Furthermore, in mice devoid of eosinophilopoesis (ΔdblGATA mice), resistance to infection with N. brasiliensis is abrogated [191]. A more recent study by this group gives weight to the importance of eosinophils explicitly in the skin, as eotaxin- and STAT6-deficient mice on an IL-5 transgenic background showed reduced eosinophil infiltration into the skin and had impaired resistance to N. brasiliensis, but not H. polygyrus, which has no skin phase in its lifecycle [192]. This suggests that migration via the skin is controlled to a much greater degree by granulocyte-mediated defence mechanisms than is colonization of the gut.
The importance of TH2 immunity in infection with N. brasiliensis is indubitable, and T cells play a major role in the development of a TH2-type environment [193]. The cooperation of lineage marker-negative cells in TH2 immunity has been observed in infection with N. brasiliensis. Using IL-4- and IL-13-reporter mice to track these cells, a dominant IL-13-producing, innate cell population (nuocytes) expanded in response to the infection, similar to that seen after injection of IL-25 or IL-33. More importantly, inducing this population by administration of IL-25 was sufficient for worm clearance and restored eosinophilia [194].
In humans, gut-dwelling helminthes, such as Ascaris, Ancylostoma, and Necator may cause of pulmonary eosinophilia (Löffler´s syndrome) during migration through lung tissue. This disease is characterized by pulmonary infiltrates and peripheral blood eosinophilia and the symptoms are usually mild, but can become severe, depending on the number of ingested eggs [195]. Sputum examinations show eosinophils and Charcot-Leyden crystals [196], and larvae covered with eosinophils have been observed, which may reflect immobilization and subsequent destruction of the parasite [197]. The intensity and selectivity of inflammatory recruitment suggests that granulocytes are not simply responding to tissue injury caused by migrating larvae as a repair mechanism, but are actively targeting or being targeted by the parasites, since parasite-derived chemotactic factors have been reported to recruit eosinophils and neutrophils [198]. Neutrophils have been shown to react quickly in response to nematode body fluids by chemotaxis, activation and superoxide anion production, mediated via CXCR1 and CXCR2 (receptors for the neutrophil chemotactic factor IL-8) [199]. Although the role of neutrophils in helminth infections has been understudied compared with that of eosinophils, neutrophils have the ability to release eosinophil chemotactic factors in addition to superoxide anions [5], and thus may contribute to eosinophil recruitment, as has been shown for infection of guinea-pigs with Schistosoma japonicum [200].
In infection with Strongyloides spp., the role of neutrophils has been evaluated in greater detail. The life cycle of this nematode includes a free-living stage, and infective larvae enter the host by penetration through the skin or gut mucosa, followed by migration through the lungs, where they ascend the tracheobronchial tree to be swallowed before colonizing the small bowel. Thus, a clinical manifestation of such infection in humans is the Löffler syndrome. In immunocompromised hosts, e.g., AIDS patients, strongyloidiasis can lead to hyperinfection syndrome, as protective mechanisms are highly dependent on T cells [195]. Abraham and colleges have investigated the importance of granulocytes in murine infection with S. stercoralis. In this infection, eosinophils and/or IL-5 are essential components of the innate protective immune response [201]. In CXCR2-deficient mice that have a severe defect in the recruitment of neutrophils, impaired protection against primary infection with S. stercoralis occurred that was comparable to that seen in eosinophil-depleted mice (anti-CCR3 treatment). Interestingly, only neutrophils, but not eosinophils, seemed to be required for the establishment of adaptive immune responses to larvae after immunization [202]. On a molecular level, MBP has been identified to be involved in eosinophil-mediated larval killing and myeloperoxidase in neutrophil-mediated killing [203].
Food-Borne Nematode Infections: Trichinella
Among helminth species, Trichinella has an important impact on public health and the economy and regulatory controls imposed on all susceptible animal species intended for human consumption have been implemented. Thus, trichinellosis is a major zoonotic disease, which is endemic in less developed countries of Eastern Europe, Asia and Latin America and the global prevalence is estimated in the millions [204], but it occurs also sporadically in developed countries in Europe and in North America, where raw or undercooked pork and wild game may be consumed as delicacies.
Trichinella is an intracellular nematode infecting mainly pigs, small rodents and more broadly all mono-gastric mammals. Digestion of meat of infected animals leads to the release of muscle stage 1 larvae that penetrate inside the intestinal epithelial cells of the small intestine where they moult until becoming adults (males and females). There, they mate and females release newborn stage 1 larvae. These invasive larvae migrate from the intestinal epithelium to the skeletal striated muscle cells, their definitive niche in the host (nurse cells) [205]. In response to infection, the host develops a non-specific immune response in the intestinal mucosa followed by a protective response, which will not prevent the parasite from settling in the muscle cell but will block the migration of newborn larvae in case of reinfection by the same species of Trichinella. During enteric infection, developing worms damage columnar epithelium, depositing their shed cuticula. In vitro systems have allowed analysis of the response of epithelial cells to infection, however in the absence of other host cells and tissues. Despite the destruction of epithelial cells, co-culture of muscle larvae induced up-regulation of transcripts for the proinflammatory mediators IL-1β and chemokines IL-8 and ENA-78 [206]. Infections in humans have been associated with eosinophils and TH2-type responses [205] and re-stimulation of peripheral blood mononuclear cells showed strong IL-5 responses at the mRNA and protein level. Importantly, a strong correlation exists between levels of IgE and blood eosinophilia during the first period of infection. A massive reduction of eosinophils occurs during the acute stage of trichinellosis and this has been shown to predict the severity of the outcome of infection. This phenomenon may be due to a massive peripheral migration [207] and gives weight to the importance of eosinophils in pathology. There is also evidence that eosinophils can kill T. spiralis larvae in vitro [208]. However, a role for eosinophils in host defense against T. spiralis in vivo is not convincing as the evidence is contradictory. Although infection of rodents with T. spiralis stimulated a basophilia as well as an eosinophilia [209], the parasite survival is not or marginally modified during a primary infection in IL-5-deficient mice, IL-5 transgenic mice, or in mice depleted of eosinophils with specific antibodies [210]. In addition, CCR3-deficient mice do not recruit eosinophils to nurse cells, and the number of necrotic nurse cells on histologic sections of tongue decreased [211]. Although intestinal infection with T. spiralis is not altered by eosinophil deficiency, eosinophil deficiency limits parasite destruction in muscle [212]. In this study, two models of eosinophil depletion (Δdbl-GATA and TgPHIL mice) were used to study muscle inflammation during T. spiralis infection. Eosinophils are prominent in infiltrates surrounding infected muscle cells of wild-type mice whereas, in both models infiltrates completely lack eosinophils. These granulocytes are also absent in the blood of infected mice, in contrast to wild-type mice. The recovery rate of T. spiralis muscle larvae was lower in these both strains of mice, by 60-70% in TgPHIL mice and 48% in Δdbl-GATA mice respectively. In addition, the TH1 response was enhanced (increased levels of IFNγ in in vitro lymph node cell culture) and the TH2 response downregulated (decreased IL-4 in in vitro culture). Larval survival improved when mice were treated with inhibitors of inducible NO synthase, implicating the NO pathway in T. spiralis clearance. These results show that muscle larvae are damaged by an immune response driven by TH1 cells, which seem to be downregulated by eosinophils. Thus, eosinophils may play a dual role in T. spiralis infection, with both effector and regulatory function.
Humans as Non-Permissive Hosts
In several other cases of food-borne nematode infections, the parasite is unable to complete its lifecycle in humans. In unnatural hosts, defence mechanisms are usually stronger due to the fact that helminth-induced downregulation of the immune system is part of the evolutionary co-adaptation process. As a corollary to this immune dysregulation, serious complications can result from immune reactions to the migrating larvae. Angiostrongylus cantonensis, a species widespread in South-East Asia and the Pacific, naturally parasitizes the pulmonary artery of rats. First-stage larvae in rat faeces infect the molluscan intermediate host (various species of slugs and snails), and humans can become infected by ingesting raw or undercooked molluscs, paratenic hosts (commonly prawns or crabs), or unwashed vegetables directly contaminated with infective larvae. In rats, the larvae migrate to the brain before establishing patent infections in the pulmonary arteries; however, in humans, infection terminates with the immature adult stage in the central nervous system. Angiostrongyliasis is the leading cause of human eosinophilic meningitis, and animal studies have demonstrated that the pathogenesis of the disease is clearly linked to the non-permissive nature of the human host. For instance, EPO levels in cerebrospinal fluid (CSF) were significantly higher in infected guinea-pigs (non-permissive) compared with rat (permissive) hosts [213]. Moreover, when young adult worms were transferred into the pulmonary arteries of non-permissive hosts, degenerative changes associated with eosinophil infiltration and the deposition of EPO onto the worm cuticle were observed, whereas no such cellular reaction was provoked in rats [214]. In the cerebellum, eosinophil degranulation in non-permissive hosts was apparently responsible for ‘bystander’ damage to host Purkinje cells [214].
More recent studies have demonstrated that the pathophysiology in non-permissive hosts is not only associated with eosinophil degranulation per se, but also with the levels of plasminogen activators and matrix metalloproteinase-9 (MMP-9) [215]. This enzyme has been associated with eosinophils in the mouse model [216] and was specifically localized to the ‘small’ granules [217]. An imbalance between MMP-9 and tissue inhibitors of metalloproteinases in the subarachnoid space of infected mice may be the key host reaction that leads to manifestations of the disease [218]. Analysis of cytokine responses in the CSF of mice detected IL-4 and IL-5 by two to three weeks post-infection, which were expressed by T-cells but not eosinophils [219]; while eotaxin and macrophage inflammatory protein-1 in murine CSF were chemotactic for eosinophils in vitro [220]. In humans, the concentrations of eotaxin-2 [221], IL-5, IL-10 and TNF-α are correlated with eosinophilia [222]. Accordingly, treatment of infected mice with an anti-CCR3 monoclonal antibody decreased eosinophil infiltration into the CSF, and the levels of eotaxin and IL-5, as well as disease severity, were significantly reduced [223]. However, somatic extracts from young adult worms contain at least two proteins that have direct chemotactic activity for guinea pig eosinophils [224].
Early studies using eosinophils in vitro suggested that immunological protection against A. cantonensis was mediated by ADCC. With the advent of the genetic knockout era, the critical role of IL-5 and eosinophilia in controlling worm burden in mice was confirmed. Thus, angiostrongyliasis is a classic case of a helminthic infection in which eosinophils are both the key effector of protective immunity and the primary cause of disease symptoms in non-permissive hosts. Over evolutionary history, the natural host appears to have reached equilibrium with the parasite, since eosinophilia in the CSF could be induced in cerebrally-infected rats by injection of antigens from first-stage larvae or eggs, whereas extracts from young adult worms (i.e., the stage that affects the brain) failed to stimulate this response [225]. Indeed, the severity of disease in mice can be ameliorated by the administration of IL-12, which was shown to successfully reduce meningeal eosinophilia via a switch to a type-1 response, in combination with an anthelminthic (mebendazole). Importantly, IL-12 alone was less effective, as the viable worms were still able to cause mechanical damage to the parenchyma [226]. The efficacy of this combination treatment may result, in part, from its ability to reverse the observed resistance of murine eosinophils to apoptosis in the CSF [227].
Another food-borne nematode infection, although with a more global distribution, is anisakiasis, caused by members of the genus Anisakis. The natural definitive hosts of Anisakis spp. are marine mammals, but humans can become transiently infected by consumption of raw or undercooked fish (paratenic hosts) containing third-stage larvae. Thus, in common with angiostrongyliasis, humans are non-permissive hosts, although development of the nematode is normally truncated at an earlier stage (i.e., the L4) within the gastrointestinal mucosa. Infection is associated with gastrointestinal symptoms and allergic-type immunological reactions [228]. Crude extracts from Anisakis larvae exhibited potent chemotactic activity for eosinophils in vitro and in vivo [229], and induced an eosinophilic phlegmonous reaction in the ileum of rabbits without prior exposure [230]. However, live L3 implanted into the abdominal cavity of mice generated an intense infiltration of neutrophils, which was not displaced by eosinophils until granulomas matured from 14 days post-infection [231]. Further studies on the granulomas revealed that although eosinophils degranulated onto the cuticle of the parasite, they lacked the ability to kill the larvae; whereas macrophage adherence was associated with gross damage to the parasite surface [232]. Immunopathogenesis in humans was associated with transcript levels for MBP, eotaxin and inducible nitric oxide synthase (iNOS) in eosinophilic infiltrates within the intestinal wall; while ECP and EDN concentrations in the sera of patients were also elevated [233]. Importantly, allergic responses to Anisakis spp. are not dependent on exposure to live larvae, and thus sensitisation to parasite antigens can occur following consumption of fish containing dead parasites, or even via inhalation or skin contact with allergens in fish-processing plants [228, 234]. In a mouse model of allergic inflammation, intranasal administration of E/S material from Anisakis L3 triggered the production of IL-17, IL-6 and the neutrophilic chemokine CXCL1, in conjunction with neutrophil infiltration into the lung. Furthermore, the induction of IL-6 and CXCL1 in mouse embryonic fibroblast cells by Anisakis ES products was dependent on TLR3 (Table 1), although surprisingly, RNase treatment of the E/S did not abrogate this effect [235]. The key Anisakis allergen (Ani s 4) has been identified as a cystatin, and the recombinant protein was capable of activating basophils from allergic patients [236]. Indeed, basophil activation assays have been employed both to diagnose Anisakis allergy and to detect allergens in fish muscle [237].
Beneficial and Detrimental Effects of Granulocytes in Helminth Infections: Lessons from Filariasis
Among the helminths, filarial nematodes constitute the greatest burden of human morbidity worldwide. These parasites are transmitted by haematophagous arthropod vectors, such as mosquitoes and blackflies (Simulium spp.), which become infected by ingesting first-stage larvae (microfilariae) from the blood or skin of the definitive human host. Within the intermediate host, the larvae moult twice and become infective third-stage larvae (L3) that migrate to the head of the vector. The L3 are deposited on the skin of the new definitive host during a blood meal, and migrate via the lymphatics to their specific tissue predilection site, where they moult twice prior to maturation as dioecious adults. Fertilised female worms are ovoviviparous, giving birth to fully formed microfilariae rather than eggs. Filarial worms are responsible for three major neglected tropical diseases: lymphatic filariasis (caused by Wuchereria bancrofti and Brugia spp.), in which the adult worms reside in the lymphatic vessels; onchocerciasis (caused by O. volvulus), in which the adult worms are found in subcutaneous nodules; and loiasis (caused by Loa loa), where adult worms migrate throughout the connective tissues. In lymphatic filariasis, it is the adult worms that are responsible for the symptoms of the disease (lymphoedema and hydrocoele); while in onchocerciasis, the nodules are benign and it is the death of microfilariae in the skin and eyes that triggers severe dermatitis and visual impairment (River Blindness). In loiasis, the migrating adult worms can cause relatively minor pathology (Calabar swellings).
Several rodent models of filariasis have been developed, including those using the human parasites B. malayi and O. volvulus, which undergo only partial development in laboratory mice; alongside the rodent parasites Acanthocheilonema viteae (a natural parasite of the jird, Meriones unguiculatus) and Litomosoides sigmodontis (a natural parasite of the cotton rat, Sigmodon hispidus). The latter is a particularly powerful model species, as it can complete its development in BALB/c mice, exposing the complete filarial lifecycle to the tractable technologies of murine immunology [238]. Several studies using transgenic mice have sought to determine the role of granulocytes in protective immunity against filarial nematodes. For instance, in mice lacking B-cell maturation and antibody production (µMT strain), it has been demonstrated that the vast majority of incoming L3 are killed by an innate immune response dominated by neutrophils [239]. However, a clear consensus has emerged from experiments in several of the above rodent models that the protective immunity induced by irradiated L3 is mediated by eosinophils and antibody within two days of challenge [240-242]. Hence, the potency of this vaccination method was abated both in µMT mice, in which eosinophils were recruited to the site of infection but failed to degranulate [239], and in mice depleted of eosinophils or IgE [243]. In contrast, in primary infections in mice over-expressing IL-5, targeting of the larval stages by eosinophils occurs from day 10 post-infection, and the young adults become embedded in eosinophil-rich granulomas. The difference in the timing of killing relative to irradiated vaccination can be attributed to the delay required to produce specific antibodies in a primary infection [244]. The precise effector mechanism deployed by eosinophils when targeting filariae has not been fully elucidated, although knockout of EPO or MBP led to increased worm burdens of L. sigmodontis in mice, suggesting a role for these granule proteins in defense against the worms. It is not known whether this was primarily due to reduced effector capacity of degranulating eosinophils, or an indirect mechanism involving altered levels of IL-10, IL-5 and/or IL-4, which were affected by knockout of the granule proteins [245].
Several lines of evidence from the L. sigmodontis model indicate that neutrophils also have an important role in control of the adult worm burden. Thus, when the accumulation of neutrophils in granulomas around the adult worms was abrogated by neutralization of G-CSF, parasite killing was inhibited, despite the normal presence of eosinophils in the granulomas [246]. Knockout of IFN-γ greatly reduced the effector function of neutrophils and resulted in an increased worm burden, probably because these mice also exhibited decreased production of the key neutrophil activator, TNF-α [247]. When combined with an additional knockout of IL-5, neutrophil chemotaxis and phagocytosis (assessed in vitro) were even more impaired, worm burdens were higher than in single knockout mice, and TNF-α production was markedly reduced [248]. In contrast, only a single study has attempted to determine the role of basophils in protective immunity against filarial infection. Depletion of these cells had no effect on adult worm numbers in the L. sigmodontis model, despite a significant reduction in IgE levels, eosinophilia and production of IL-4 in treated mice [76].
Mouse models of filariasis are hampered by the lack of immunopathology generated during infection, and thus analysis of an extremely important aspect of the human diseases is largely restricted to clinical studies. However, keratitis (resembling that observed in onchocerciasis) can be induced in mice by direct injection of filarial extracts into the cornea [249]. Studies using this model have shown that neutrophil accumulation occurs early after injection of O. volvulus crude antigen, independently of adaptive immune responses [250]. This can be attributed to a particularity of filarial nematodes, in which many (but not all) species contain endosymbionts of the genus Wolbachia [251]. Consequently, depletion of these bacteria from filarial extracts prevented both innate recruitment of neutrophils and ocular pathology in the mouse model. Granulocytes are also involved in secondary immune responses in the eye, which are induced when injection of the corneal stroma is preceded by subcutaneous immunization of the mice with filarial antigen. In this situation, both neutrophils and eosinophils infiltrate the cornea, although P-selectin-dependent eosinophil influx occurs later than the neutrophil response [252]. Ocular damage was abrogated in CXCR2 knockout mice [253], in which neutrophil recruitment was prevented, but not in P-selectin-deficient mice, which lacked eosinophilic infiltrates [252]. In contrast to neutrophil accumulation, eosinophil influx into the corneal stroma is dependent on adaptive responses; i.e., filarial-specific antibodies [254] and a functional T-cell response [255].
Human onchocerciasis manifests as a disease spectrum between two clinical “poles”: the generalized or hyporesponsive form (which is more common), and the localized or hyperreactive form (if unilaterally predominant also known as “Sowda”). In the hyporesponsive form, the skin contains millions of microfilariae and more than 50 adult worms may be present in a single individual. In contrast, the Sowda form is characterized by extremely low densities of dermal microfilariae, low numbers of adult worms in enlarged inflammatory nodules, and severe lichenification of the skin. The pathogenesis of Sowda can be attributed to a more potent TH2 response than is observed in hyporesponsive onchocerciasis, as revealed by higher levels of parasite-specific IgE [256], elevated numbers of eosinophils and mast cells in nodules [257], increased concentrations of EDN in serum [258], and a significant association with a polymorphism in the IL-13 gene [259], which could lead to enhanced induction of the TH2 pathway. Moreover, eosinophils from Sowda patients exhibited greater chemotactic responses to platelet-activating factor (PAF) in vitro than did eosinophils from patients with the hyporesponsive form; whereas neutrophil chemotaxis to PAF and the synthetic peptide fMLP was diminished in Sowda compared with the migration observed with neutrophils from hyporesponsive individuals [260]. In lymphatic filariasis, a rare clinical manifestation known as “tropical pulmonary eosinophilia” (TPE) represents another form of extreme TH2 polarization induced by hyperreactivity to microfilariae; this syndrome is associated with massive accumulations of eosinophils in the lung, the site where microfilariae are killed as well as peripherally [261]. In addition, IgE antibodies against filarial γ-glutamyl transpeptidase have been implicated in the pathogenesis of TPE, as they react with the host homologue of this molecule in the pulmonary epithelium, leading to autoimmunity [262]. Recently, the processes underlying the clinical responsiveness of TPE to treatment with diethylcarbamazine (DEC) were investigated in a mouse model of airway sensitization, and IL-5-dependent eosinophilopoiesis was found to be suppressed by this drug via a mechanism requiring both iNOS and Fas ligand [263].
An abundance of literature demonstrates that granulocytes are the key players mediating microfilarial killing following anthelminthic treatment. Interestingly, two of the most important compounds currently used in mass control programs for filarial infections, ivermectin and DEC, do not affect the viability of microfilariae in vitro at concentrations that are effective in vivo. This is probably because of a combination of subtle, sub-lethal effects on the microfilarial sheath [264] and/or secretory apparatus [265] together with immunopotentiating activity [266, 267]. Interestingly, for ivermectin, a dose dependent effect on the generation of eosinophilic-derived toxic oxygen intermediates was found, i.e. when applied in low concentrations, the release of these molecules was enhanced, whereas it was reduced at higher doses of the drug [268]. Among many reactive oxygen species, hydrogen peroxide, which can be produced by granulocytes, was the most effective in killing microfilariae of O. cervicalis [269]. The administration of DEC or ivermectin in human patients leads to a rapid (i.e., within 1 – 3 days) accumulation of microfilariae in the lymph nodes, to which eosinophils adhere and degranulate, destroying the target [270, 271]. In onchocerciasis, chemotherapy with DEC induces the “Mazzotti reaction”, a syndrome which includes fever, urticaria and hypotension. This adverse reaction is associated with elevated levels of MBP and EDN in serum and the release of MBP by eosinophils in the skin [272]. Moreover, neutrophils and their granule contents are also abundant in the microabscesses surrounding dermal microfilariae following DEC treatment [273]. Since DEC also kills the microfilariae in the eye, leading to an influx of inflammatory cells that mediate keratitis and other eye damage, it is now contraindicated as chemotherapy for onchocerciasis.
Adverse events following the chemotherapy of filarial infections have also been linked to the rapid release of Wolbachia endosymbionts from dying worms. Treatment with DEC led to significantly elevated levels of Wolbachia DNA in serum, which were correlated with blood neutrophil counts and the concentration of calprotectin [274]. Furthermore, reactions scores, neutrophil counts and serum calgranulin B levels were positively correlated with Wolbachia DNA concentrations after ivermectin chemotherapy. Accordingly, in patients infected with B. malayi, severe adverse reactions associated with DEC and albendazole treatment can be mitigated by prior administration of doxycycline [275], which depletes Wolbachia from filarial adult worms and microfilariae [276]. Since antibiotic chemotherapy of onchocerciasis kills the adult worms slowly via an indirect mechanism (i.e., targeting of the endosymbiont [277, 278]), these regimens do not generate adverse reactions triggered by the sudden release of large quantities of filarial and bacterial antigens. Within the collagenous nodules containing the adult worms, the presence of the endobacteria is associated with extensive neutrophil infiltration without tissue damage, profuse pus formation or necrosis; whereas eosinophils are normally scarce [279, 280]. In the bovine model of onchocerciasis, O. ochengi, oxytetracycline chemotherapy results in the depletion of Wolbachia, a gradual decline in the nodular neutrophil population, and a massive infiltration of eosinophils [280]. These cells were observedby histology to contribute to the killing of the adult worms by degranulating on the cuticle. Importantly, this sequence of events is not seen following administration of an adulticidal drug with a direct mode of action (i.e., an anthelminthic without an antibacterial effect), indicating that the symbiosis between Wolbachia and O. ochengi may involve protection of the worms from eosinophil effector mechanisms through the recruitment of a less effective neutrophil response [281].
CONCLUDING REMARKS
Despite the enormous strides made in our knowledge of the biology of granulocytes and their interactions with helminths over the past decades, new data have not allowed the parasitology community to develop a ‘grand synthesis’ that definitively explains this relationship. Indeed, perhaps such a ‘Holy Grail’ was always an illusory goal considering the great diversity of helminths, which bridge two invertebrate phyla with an estimated evolutionary divergence time of more than one billion years [282]. Moreover, humans are afflicted by a considerable variety of helminth species that often undergo extensive morphological changes within the lifecycle, migrating through different tissues to reach their final predilection site. On the host side of the equation, the granulocytes that were once perceived to be terminally differentiated, ‘kamikaze’ effector cells are now recognized to be considerably more sophisticated, in that they can influence the initial development of acquired immune responses through antigen presentation, and can modulate the response of other leukocytes in processes such as wound healing. Once the intrinsic differences between the immune responses of different mammalian hosts are also considered, the wonder is that any consistent patterns have emerged from analysis of granulocyte-helminth interactions.
The neglected basophil has recently experienced its own period in the limelight in the context of helminth infections [7]. While the potential role of basophils as APCs that can induce TH2 responses is important and intriguing, they do not appear to have an indispensable function in this respect. Moreover, few studies have established an effector function for these cells against helminths, although whether this simply reflects a relative paucity of data relative to eosinophils remains to be determined. However, it is clear that basophils are responsible for the amplification of TH2 responses in helminth infections and are the key player in allergic hypersensitivity reactions to worm antigens.
Consequently, the eosinophil remains essentially unchallenged as the cell at the centrepiece of the granulocyte-helminth relationship. New studies continue to reinforce the paradigm that eosinophils are the key granulocyte mediating protective immunity in the adaptive context in humans and large animals, particularly with regard to species with tissue-migrating stages. Immunity to cestodes in humans may represent an exception to this pattern, but this a field where few clinical studies have been performed, and of the three groups of helminths, our knowledge of the immunology of cestode infections remains the least well developed. Conversely, evidence from mouse models has often failed to demonstrate a convincing role for eosinophils in conferring protection. However, it is important to note a key difference between human and mouse eosinophils: the latter poorly express receptors for IgE (Fc-ε; [283]). Thus, although murine eosinophils can undergo the respiratory burst when Fc-γ receptors are ligated by an opsonised target [283], synergism between these cells and parasite-specific IgE is not a mechanism that is available to the murine host. One explanation for these differences could be that the evolution of effector function between host species has led murine neutrophils to exhibit a role which overlaps with that of human eosinophils, as the former are often associated with adaptive immunity to helminths in mouse models. In contrast, in helminth infections of humans, most studies suggest that neutrophils are only important during the innate response. The problems of interpreting eosinophil responses to helminths in mouse models have been discussed in detail by other researchers [284, 285], and a key factor that may lead to underestimation of the importance of eosinophils in acquired immunity is the potency of innate responses directed against helminths that are not natural parasites of mice. Thus, the evidence from mouse models must always be assessed in relation to the specific host-parasite relationship, and data from natural mouse-helminth systems may be more informative than artificial infection of mice with human parasites that lack a natural rodent reservoir. However, data from non-permissive hosts are necessary to aid interpretation of the human response to incidental infection with worms such as Anisakis spp. and Angiostrongylus.
The evidence for the singular contribution of eosinophils to immunopathology in helminth infections is unequivocal. Indeed, it is well recognized that the mechanisms which eosinophils deploy to destroy a target can lead to damage of host tissue, unless this process is tightly regulated. The degree of immunopathology that a helminth infection induces in the human host is dependent on two principal factors. Firstly, when humans are non-permissive hosts, the risk of immunopathology is increased (on those rare occasions when the parasite overcomes innate defenses), relative to infection with a parasite capable of normal development. The severity of disease manifestations is then determined by the migration route of the worm and the site of transient establishment. Thus, helminths that lodge in immunoprivileged tissues such as the eye or brain are more likely to invoke granulocyte-mediated damage than worms located in the gut. Secondly, host genetics underlying atopy are critical in determining the balance between protection and immunopathology in helminth infections, as exemplified by Sowda in onchocerciasis and allergic responses to Anisakis proteins. Perhaps the most informative example of the fine balance between the beneficial and pathological roles of eosinophils (and other granulocytes) is the chemotherapy of filarial infections, where these cells are activated and effectively clear microfilariae, but simultaneously release products that can drive severe adverse events.
Recent data indicate that the relationship between helminths and eosinophils has evolved to the point where worms ‘perceive’ these cells as a developmental cue to accelerate their progression through the lifecycle in partially immune hosts. Thus, in mice that mount a vigorous eosinophil response during larval entry, surviving adult worms of L. sigmodontis exhibit an accelerated growth [244], reach patency earlier, and produce more microfilariae, relative to worm development in hosts that lack such a response [286]. A related observation was made in mice infected with T. spiralis, in which larval killing was enhanced in eosinophil-deficient mice [287]. Perhaps these studies represent the most pertinent answers to the question, “Who is calling the shots?” that have been performed to date.
Table 2.
The Role of Granulocytes in Helminth Infection in Animals and Humans
| Trematodes | Cestodes | Nematodes | |
|---|---|---|---|
| Host protection | |||
| In vitro damage or killing |
Schistosoma spp. (81-84, 87) Fasciola hepatica (86) |
Heligmosomoides polygyrus (178) Trichinella spirialis (208) |
|
| In vivo damage or killing | Schistosoma mansoni (85) |
Hymenolepis spp. (138, 145, 159) Taenia spp. (141, 142, 154, 156, 157) |
Heligmosomoides polygyrus (180) Litomosoides sigmodontis (239, 245, 246, 248) Nippostrongylus brasiliensis (71, 75, 191,192, 194) Trichuris muris (19) Strongyloides stercoralis (202) Onchocerca ochengi (280, 281) Onchocerca volvulus (243) |
| Antigen presentation |
Brugia
malayi (66, 67) Strongyloides stercoralis (68, 69) |
||
| Granulocyte activation and induction of TH2 responses |
Schistosoma
mansoni, IPSE (72-74) Schistosoma mansoni (90, 91, 93) |
Cysticercus
cellulosae (129) Echinococcus spp. (132) |
Litomosoides sigmodontis (76) Nippostrongylus brasiliensis (53, 75) |
| Granulocyte recruitment | Schistosoma mansoni, skin (89, 90, 93, 123, 124) |
Echinococcus
granulosus (139) Mesocestoides corti (127, 144) Taenia spp. (128, 130, 135, 136, 137, 149) |
Anisakis
simplex (229-231, 236) Heligmosomoides polygyrus (179) Onchocerca volvulus (279) Trichuris trichiura (185) |
| Parasite establishment | |||
| Inhibition of granulocyte recruitment and function | Schistosoma mansoni, lyso-PS (56) |
Echinococcus multilocularis (167, 168) Taenia solium (129, 169) Taenia crassiceps (171) |
Trichinella spiralis (212) Acanthocheilonema viteae, ES-62 (58) |
| Immunopathology | |||
| Association with pathology | Schistosoma mansoni (93, 123, 124) |
Anisakis
simplex (213, 225) Onchocerca volvulus/Wolbachia (252, 253) Onchocerca volvulus (256-258, 272, 288) Brugia malayi (262) |
|
ACKNOWLEDGEMENTS
The authors are supported by the EU [Nr. 242131, “EPIAF” (BLM, CM, SS)], by the Biotechnology & Biological Sciences Research Council (BLM), and Pfizer Animal Health (BLM). CM and SS are supported by the bilateral programm "PROCOPE", which is financed through the German Academic Exchange Service (DAAD, with funds from the BMBF) and the French Embassy. SS is also supported by a grant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine (“A-WOL”). The authors thank Achim Hoerauf, Institute for Medical Microbiology, Immunology and Parasitology, University Hospital Bonn, Germany, for critical reading of the manuscript.
REFERENCES
- 1.Kondo M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol. Rev. 2010;238:37–46. doi: 10.1111/j.1600-065X.2010.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doulatov S, Notta F, Eppert K, Nguyen L T, Ohashi P S, Dick J E. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat. Immunol. 2010;11:585–593. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 3.Himmelweit E. The Collected Papers of Paul Ehrlich Vol. 1- Histology, Biochemistry and Pathology. Vol. 1. London and New York: Pergmon Press; 1956. p. 653. [Google Scholar]
- 4.Sorci G, Faivre B. Inflammation and oxidative stress in vertebrate host-parasite systems. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009;364:71–83. doi: 10.1098/rstb.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shamri R, Xenakis J J, Spencer L A. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343(1 ):57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heneberg P, Draberova L, Bambouskova M, Pompach P, Draber P. Down-regulation of protein-tyrosine phosphatases activates an immune receptor in the absence of its translocation into lipid rafts. J. Biol. Chem. 2010;285:12787–12802. doi: 10.1074/jbc.M109.052555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voehringer D. Basophils in immune responses against helminths. Microbes Infect. 2011;13:881–887. doi: 10.1016/j.micinf.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Katona I M, Urban J F, Jr, Finkelman F D. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J. Immunol. 1988;140:3206–3211. [PubMed] [Google Scholar]
- 9.Al-Qaoud K M, Taubert A, Zahner H, Fleischer B, Hoerauf A. Infection of BALB/c mice with the filarial nematode Litomosoides sigmodontis : role of CD4+ T cells in controlling larval development. Infect. Immun. 1997;65:2457–2461. doi: 10.1128/iai.65.6.2457-2461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen J E, Maizels R M. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 11.Cervi L, MacDonald A S, Kane C, Dzierszinski F, Pearce E J. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation segregate the antigens to distinct intracellular compartments and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J. Immunol. 2004;172:2016–2020. doi: 10.4049/jimmunol.172.4.2016. [DOI] [PubMed] [Google Scholar]
- 12.Saenz S A, Taylor B C, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballantyne S J, Barlow J L, Jolin H E, Nath P, Williams A S, Chung K F, Sturton G, Wong S H, McKenzie A N. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Fort M M, Cheung J, Yen D, Li J, Zurawski S M, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst S D, Zurawski G, Leach M W, Gorman D M, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan T K, Zurawski G, Moshrefi M, Qin J, Li X, Gorman D M, Bazan J F, Kastelein R A. IL-33 an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler S F, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neill D R, McKenzie A N. Nuocytes and beyond: new insights into helminth expulsion. Trends Parasitol. 2011;27:214–221. doi: 10.1016/j.pt.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Hasnain S Z, Evans C M, Roy M, Gallagher A L, Kindrachuk K N, Barron L, Dickey B F, Wilson M S, Wynn T A, Grencis R K, Thornton D J. Muc5ac: a critical component mediating the rejection of enteric nematodes. J. Exp. Med. 2011;208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrigoue J G, Saenz S A, Siracusa M C, Allenspach E J, Taylor B C, Giacomin P R, Nair M G, Du Y, Zaph C, van Rooijen N, Comeau M R, Pearce E J, Laufer T M, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat. Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen J E, Wynn T A. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan S P, Rosenberg H F, Moqbel R, Phipps S, Foster P S, Lacy P, Kay A B, Rothenberg M E. Eosinophils: biological properties and role in health and disease. Clin. Exp. Allergy . 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 22.Melo R C, Weller P F. Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol. Histopathol. 2010;25:1341–1354. doi: 10.14670/hh-25.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swartz J M, Dyer K D, Cheever A W, Ramalingam T, Pesnicak L, Domachowske J B, Lee J J, Lee N A, Foster P S, Wynn T A, Rosenberg H F. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood . 2006;108:2420–2427. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothenberg M E, MacLean J A, Pearlman E, Luster A D, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J. Exp. Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denzler K L, Farmer S C, Crosby J R, Borchers M, Cieslewicz G, Larson K A, Cormier-Regard S, Lee N A, Lee J J. Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J. Immunol. 2000;165:5509–5517. doi: 10.4049/jimmunol.165.10.5509. [DOI] [PubMed] [Google Scholar]
- 26.Denzler K L, Borchers M T, Crosby J R, Cieslewicz G, Hines E M, Justice J P, Cormier S A, Lindenberger K A, Song W, Wu W, Hazen S L, Gleich G J, Lee J J, Lee N A. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J. Immunol. 2001;167:1672–1682. doi: 10.4049/jimmunol.167.3.1672. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 28.Summers C, Rankin S M, Condliffe A M, Singh N, Peters A M, Chilvers E R. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A, Cassatella M A, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 30.Balabanian K, Lagane B, Pablos J L, Laurent L, Planchenault T, Verola O, Lebbe C, Kerob D, Dupuy A, Hermine O, Nicolas J F, Latger-Cannard V, Bensoussan D, Bordigoni P, Baleux F, Le Deist F, Virelizier J L, Arenzana-Seisdedos F, Bachelerie F. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 31.Etzioni A. Loss of endothelial surface expression of E-selectin—a third LAD syndrome. Blood. 1999;94:3956. [PubMed] [Google Scholar]
- 32.Mollinedo F, Borregaard N, Boxer L A. Novel trends in neutrophil structure, function and development. Immunol. Today. 1999;20:535–537. doi: 10.1016/s0167-5699(99)01500-5. [DOI] [PubMed] [Google Scholar]
- 33.Cassatella M A, Gasperini S, Russo M P. Cytokine expression and release by neutrophils. Ann. N. Y. Acad. Sci. 1997;832:233–242. doi: 10.1111/j.1749-6632.1997.tb46251.x. [DOI] [PubMed] [Google Scholar]
- 34.Fialkow L, Wang Y, Downey G P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 35.Cua D J, Tato C M. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 36.Chollet-Martin S, Jourdain B, Gibert C, Elbim C, Chastre J, Gougerot-Pocidalo M A. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS. Am. J. Respir. Crit. Care Med. 1996;154:594–601. doi: 10.1164/ajrccm.154.3.8810592. [DOI] [PubMed] [Google Scholar]
- 37.Williams T J, Jose P J. Neutrophils in chronic obstructive pulmonary disease. Novartis Found Symp. 2001;234:136–141. doi: 10.1002/0470868678.ch9. [DOI] [PubMed] [Google Scholar]
- 38.Sur S, Crotty T B, Kephart G M, Hyma B A, Colby T V, Reed C E, Hunt L W, Gleich G J. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am. Rev. Respir. Dis. 1993;148:713–719. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- 39.Tkalcevic J, Novelli M, Phylactides M, Iredale J P, Segal A W, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–210. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- 40.Heneberg P, Draber P. Regulation of cys-based protein tyrosine phosphatases via reactive oxygen and nitrogen species in mast cells and basophils. Curr. Med. Chem. 2005;12:1859–1871. doi: 10.2174/0929867054546636. [DOI] [PubMed] [Google Scholar]
- 41.Lantz C S, Boesiger J, Song C H, Mach N, Kobayashi T, Mulligan R C, Nawa Y, Dranoff G, Galli S J. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 42.Siracusa M C, Saenz S A, Hill D A, Kim B S, Headley M B, Doering T A, Wherry E J, Jessup H K, Siegel L A, Kambayashi T, Dudek E C, Kubo M, Cianferoni A, Spergel J M, Ziegler S F, Comeau M R, Artis D. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone K D, Prussin C, Metcalfe D D. IgE mast cells basophils and eosinophils. J Allergy Clin. Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan B M, Locksley R M. Basophils: a nonredundant contributor to host immunity. Immunity. 2009;30:12–20. doi: 10.1016/j.immuni.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Fallon P G, Richardson E J, McKenzie G J, McKenzie A N. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 46.Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T, Minegishi Y, Yonekawa H, Karasuyama H. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, Kawano Y, Minegishi Y, Shimizu T, Karasuyama H. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Obata K, Mukai K, Tsujimura Y, Ishiwata K, Kawano Y, Minegishi Y, Watanabe N, Karasuyama H. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 49.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat. Rev. Immunol. 2009;9:9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- 50.Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K, Kawano Y, Minegishi Y, Yokozeki H, Watanabe N, Karasuyama H. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J. Clin. Invest. 2010;120:2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lilla J N, Chen C C, Mukai K, Benbarak M J, Franco C B, Kalesnikoff J, Yu M, Tsai M, Piliponsky A M, Galli S J. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohrs K, Wakil A E, Killeen N, Locksley R M, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan B M, Liang H E, Bando J K, Wu D, Cheng L E, McKerrow J K, Allen C D, Locksley R M. Genetic analysis of basophil function in vivo. Nat. Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magalhaes K G, Almeida P E, Atella G C, Maya-Monteiro C M, Castro-Faria-Neto H C, Pelajo-Machado M, Lenzi H L, Bozza M T, Bozza P T. Schistosomal-derived lysophosphatidylcholine are involved in eosinophil activation and recruitment through Toll-like receptor-2-dependent mechanisms. J. Infect. Dis. 2010;202:1369–1379. doi: 10.1086/656477. [DOI] [PubMed] [Google Scholar]
- 55.Thomas P G, Carter M R, Atochina O, Da'Dara A A, Piskorska D, McGuire E, Harn D A. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J. Immunol. 2003;171:5837–5841. doi: 10.4049/jimmunol.171.11.5837. [DOI] [PubMed] [Google Scholar]
- 56.van der Kleij D, Latz E, Brouwers J F, Kruize Y C, Schmitz M, Kurt-Jones E A, Espevik T, de Jong E C, Kapsenberg M L, Golenbock D T, Tielens A G, Yazdanbakhsh M. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 57.Parker L C, Prestwich E C, Ward J R, Smythe E, Berry A, Triantafilou M, Triantafilou K, Sabroe I. A phosphatidylserine species inhibits a range of TLR- but not IL-1beta-induced inflammatory responses by disruption of membrane microdomains. J. Immunol. 2008;181:5606–5617. doi: 10.4049/jimmunol.181.8.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodridge H S, Marshall F A, Else K J, Houston K M, Egan C, Al-Riyami L, Liew F Y, Harnett W, Harnett M M. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J. Immunol. 2005;174:284–293. doi: 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 59.Brattig N W, Bazzocchi C, Kirschning C J, Reiling N, Büttner D, Ceciliani F, Geisinger F, Hochrein H, Ernst M, Wagner H, Bandi C, Hoerauf A. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J. Immunol. 2004;173:437–445. doi: 10.4049/jimmunol.173.1.437. [DOI] [PubMed] [Google Scholar]
- 60.Turner J D, Langley R S, Johnston K L, Gentil K, Ford L, Wu B, Graham M, Sharpley F, Slatko B, Pearlman E, Taylor M J. Wolbachia lipoprotein stimulates innate and adaptive immunity through Toll-like receptors 2 and 6 to induce disease manifestations of filariasis. J. Biol. Chem. 2009;284:22364–22378. doi: 10.1074/jbc.M901528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perrigoue J G, Marshall F A, Artis D. On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell Microbiol. 2008;10:1757–1764. doi: 10.1111/j.1462-5822.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venugopal P G, Nutman T B, Semnani R T. Activation and regulation of toll-like receptors (TLRs) by helminth parasites. Immunol. Res. 2009;43:252–263. doi: 10.1007/s12026-008-8079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akuthota P, Wang H, Weller P F. Eosinophils as antigen-presenting cells in allergic upper airway disease. Curr. Opin. Allergy Clin. Immunol. 2010;10:14–19. doi: 10.1097/ACI.0b013e328334f693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright H L, Moots R J, Bucknall R C, Edwards S W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010;49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 65.Nakanishi K. Basophils as APC in Th2 response in allergic inflammation and parasite infection. Curr. Opin. Immunol. 2010;22:814–820. doi: 10.1016/j.coi.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Mawhorter S D, Pearlman E, Kazura J W, Boom W H. Class II major histocompatibility complex molecule expression on murine eosinophils activated in vivo by Brugia malayi. Infect. Immun. 1993;61:5410–5412. doi: 10.1128/iai.61.12.5410-5412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mawhorter S D, Kazura J W, Boom W H. Human eosinophils as antigen-presenting cells: relative efficiency for superantigen- and antigen-induced CD4+ T-cell proliferation. Immunology. 1994;81:584–591. [PMC free article] [PubMed] [Google Scholar]
- 68.Padigel U M, Lee J J, Nolan T J, Schad G A, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect. Immun. 2006;74:3232–3238. doi: 10.1128/IAI.02067-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Padigel U M, Hess J A, Lee J J, Lok J B, Nolan T J, Schad G A, Abraham D. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J. Infect. Dis. 2007;196:1844–1851. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinkai K, Mohrs M, Locksley R M. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 71.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–2825. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 72.Schramm G, Mohrs K, Wodrich M, Doenhoff M J, Pearce E J, Haas H, Mohrs M. Cutting edge: IPSE/alpha-1 a glycoprotein from Schistosoma mansoni eggs induces IgE-dependent antigen-independent IL-4 production by murine basophils in vivo. J. Immunol. 2007;178:6023–6027. doi: 10.4049/jimmunol.178.10.6023. [DOI] [PubMed] [Google Scholar]
- 73.Falcone F H, Dahinden C A, Gibbs B F, Noll T, Amon U, Hebestreit H, Abrahamsen O, Klaucke J, Schlaak M, Haas H. Human basophils release interleukin-4 after stimulation with Schistosoma mansoni egg antigen. Eur. J. Immunol. 1996;26:1147–1155. doi: 10.1002/eji.1830260528. [DOI] [PubMed] [Google Scholar]
- 74.Schramm G, Falcone F H, Gronow A, Haisch K, Mamat U, Doenhoff M J, Oliveira G, Galle J, Dahinden C A, Haas H. Molecular characterization of an interleukin-4-inducing factor from Schistosoma mansoni eggs. J. Biol. Chem. 2003;278:18384–18392. doi: 10.1074/jbc.M300497200. [DOI] [PubMed] [Google Scholar]
- 75.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J. Immunol. 2010;184:344–350. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 76.Torrero M N, Hubner M P, Larson D, Karasuyama H, Mitre E. Basophils amplify type 2 immune responses but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J. Immunol. 2010;185:7426–7434. doi: 10.4049/jimmunol.0903864. [DOI] [PubMed] [Google Scholar]
- 77.Kim S, Prout M, Ramshaw H, Lopez A F, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3 but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J. Immunol. 2010;184:1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castro G A. Helminths: Structure, Classification, Growth, and Development. Medical Microbiology. Galveston : The University of Texas Medical Branch ; 1996. [PubMed] [Google Scholar]
- 79.Andrade Z A. Pathology of human schistosomiasis. Mem. Inst. Oswaldo Cruz. 1987;82(Suppl 4 ):17–23. doi: 10.1590/s0074-02761987000800005. [DOI] [PubMed] [Google Scholar]
- 80.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 81.Ramalho-Pinto F J, McLaren D J, Smithers S R. Complement-mediated killing of schistosomula of Schistosoma mansoni by rat eosinophils in vitro. J. Exp. Med. 1978;147:147–156. doi: 10.1084/jem.147.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Capron M, Capron A, Torpier G, Bazin H, Bout D, Joseph M. Eosinophil-dependent cytotoxicity in rat schistosomiasis. Involvement of IgG2a antibody and role of mast cells. Eur. J. Immunol. 1978;8:127–133. doi: 10.1002/eji.1830080211. [DOI] [PubMed] [Google Scholar]
- 83.Butterworth A E, Sturrock R F, Houba V, Mahmoud A A, Sher A, Rees P H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975;256:727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- 84.Vadas M A, David J R, Butterworth A E, Houba V, Sturrock R F, David L, Herson R, Siongok T A, Kimani R. Functional studies on purified eosinophils and neutrophils from patients with Schistosoma mansoni infections. Clin. Exp. Immunol. 1980;39:683–694. [PMC free article] [PubMed] [Google Scholar]
- 85.Dessein A, Butterworth A E, Vadas M A, David J R. Maturation in vivo of Schistosoma mansoni schistosomula after culture in vitro with granulocytes and antibody. Infect. Immun. 1983;39:225–232. doi: 10.1128/iai.39.1.225-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serradell M C, Guasconi L, Masih D T. Involvement of a mitochondrial pathway and key role of hydrogen peroxide during eosinophil apoptosis induced by excretory-secretory products from Fasciola hepatica. Mol. Biochem. Parasitol. 2009;163:95–106. doi: 10.1016/j.molbiopara.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 87.Freudenstein-Dan A, Gold D, Fishelson Z. Killing of schistosomes by elastase and hydrogen peroxide: implications for leukocyte-mediated schistosome killing. J. Parasitol. 2003;89:1129–1135. doi: 10.1645/GE-96R. [DOI] [PubMed] [Google Scholar]
- 88.Jenkins S J, Hewitson J P, Jenkins G R, Mountford A P. Modulation of the host's immune response by schistosome larvae. Parasite Immunol. 2005;27:385–393. doi: 10.1111/j.1365-3024.2005.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Incani R N, McLaren D J. Histopathological and ultrastructural studies of cutaneous reactions elicited in naive and chronically infected mice by invading schistosomula of Schistosoma mansoni. Int. J. Parasitol. 1984;14:259–276. doi: 10.1016/0020-7519(84)90077-8. [DOI] [PubMed] [Google Scholar]
- 90.Paveley R A, Aynsley S A, Cook P C, Turner J D, Mountford A P. Fluorescent imaging of antigen released by a skin-invading helminth reveals differential uptake and activation profiles by antigen presenting cells. PLoS Negl. Trop. Dis. 2009;3:e528. doi: 10.1371/journal.pntd.0000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hogg K G, Kumkate S, Anderson S, Mountford A P. Interleukin-12 p40 secretion by cutaneous CD11c+ and F4/80+ cells is a major feature of the innate immune response in mice that develop Th1-mediated protective immunity to Schistosoma mansoni. Infect. Immun. 2003;71:3563–3571. doi: 10.1128/IAI.71.6.3563-3571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jenkins S J, Mountford A P. Dendritic cells activated with products released by schistosome larvae drive Th2-type immune responses, which can be inhibited by manipulation of CD40 costimulation. Infect. Immun. 2005;73:395–402. doi: 10.1128/IAI.73.1.395-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cook P C, Aynsley S A, Turner J D, Jenkins G R, Van Rooijen N, Leeto M, Brombacher F, Mountford A P. Multiple helminth infection of the skin causes lymphocyte hypo-responsiveness mediated by Th2 conditioning of dermal myeloid cells. (e1001323).PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pearce E J, Caspar P, Grzych J M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J. Exp. Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson M S, Mentink-Kane M M, Pesce J T, Ramalingam T R, Thompson R, Wynn T A. Immunopathology of schistosomiasis. Immunol. Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearce E J, MacDonald A S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 97.Anthony R M, Rutitzky L I, Urban J F, Jr, Stadecker M J, Gause W C. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cutts L, Wilson R A. Elimination of a primary schistosome infection from rats coincides with elevated IgE titres and mast cell degranulation. Parasite Immunol. 1997;19:91–102. doi: 10.1046/j.1365-3024.1997.d01-184.x. [DOI] [PubMed] [Google Scholar]
- 99.Capron M, Capron A. Immunoglobulin E and effector cells in schistosomiasis. Science. 1994;264:1876–1877. doi: 10.1126/science.8009216. [DOI] [PubMed] [Google Scholar]
- 100.Lee J J, Lee N A. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin. Exp. Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 101.Clark K, Simson L, Newcombe N, Koskinen A M, Mattes J, Lee N A, Lee J J, Dent L A, Matthaei K I, Foster P S. Eosinophil degranulation in the allergic lung of mice primarily occurs in the airway lumen. J. Leukoc. Biol. 2004;75:1001–1009. doi: 10.1189/jlb.0803391. [DOI] [PubMed] [Google Scholar]
- 102.Capron M, Jouault T, Prin L, Joseph M, Ameisen J C, Butterworth A E, Papin J P, Kusnierz J P, Capron A. Functional study of a monoclonal antibody to IgE Fc receptor (Fc epsilon R2) of eosinophils, platelets, and macrophages. J. Exp. Med. 1986;164:72–89. doi: 10.1084/jem.164.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dombrowicz D, Quatannens B, Papin J P, Capron A, Capron M. Expression of a functional Fc epsilon RI on rat eosinophils and macrophages. J. Immunol. 2000;165:1266–1271. doi: 10.4049/jimmunol.165.3.1266. [DOI] [PubMed] [Google Scholar]
- 104.Kayaba H, Dombrowicz D, Woerly G, Papin J P, Loiseau S, Capron M. Human eosinophils and human high affinity IgE receptor transgenic mouse eosinophils express low levels of high affinity IgE receptor, but release IL-10 upon receptor activation. J. Immunol. 2001;167:995–1003. doi: 10.4049/jimmunol.167.2.995. [DOI] [PubMed] [Google Scholar]
- 105.Hewitson J P, Hamblin P A, Mountford A P. Immunity induced by the radiation-attenuated schistosome vaccine. Parasite Immunol. 2005;27:271–280. doi: 10.1111/j.1365-3024.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- 106.Mastin A J, Bickle Q D, Wilson R A. Schistosoma mansoni: migration and attrition of irradiated and challenge schistosomula in the mouse. Parasitology. 1983;87:87–102. doi: 10.1017/s0031182000052446. [DOI] [PubMed] [Google Scholar]
- 107.Mountford A P, Coulson P S, Saunders N, Wilson R A. Characteristics of protective immunity in mice induced by drug-attenuated larvae of Schistosoma mansoni. Antigen localization and antibody responses. J. Immunol. 1989;143:989–995. [PubMed] [Google Scholar]
- 108.Coulson P S. The radiation-attenuated vaccine against schistosomes in animal models: paradigm for a human vaccine? Adv. Parasitol. 1997;39:271–336. doi: 10.1016/s0065-308x(08)60048-2. [DOI] [PubMed] [Google Scholar]
- 109.Wynn T A, Oswald I P, Eltoum I A, Caspar P, Lowenstein C J, Lewis F A, James S L, Sher A. Elevated expression of Th1 cytokines and nitric oxide synthase in the lungs of vaccinated mice after challenge infection with Schistosoma mansoni. J. Immunol. 1994;153:5200–5209. [PubMed] [Google Scholar]
- 110.Street M, Coulson P S, Sadler C, Warnock L J, McLaughlin D, Bluethmann H, Wilson R A. TNF is essential for the cell-mediated protective immunity induced by the radiation-attenuated schistosome vaccine. J. Immunol. 1999;163:4489–4494. [PubMed] [Google Scholar]
- 111.Mountford A P, Hogg K G, Coulson P S, Brombacher F. Signaling via interleukin-4 receptor alpha chain is required for successful vaccination against schistosomiasis in BALB/c mice. Infect. Immun. 2001;69:228–236. doi: 10.1128/IAI.69.1.228-236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sher A, Coffman R L, Hieny S, Cheever A W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J. Immunol. 1990;145:3911–3916. [PubMed] [Google Scholar]
- 113.Ashton P D, Harrop R, Shah B, Wilson R A. The schistosome egg: development and secretions. Parasitology. 2001;122:329–338. doi: 10.1017/s0031182001007351. [DOI] [PubMed] [Google Scholar]
- 114.Chiaramonte M G, Schopf L R, Neben T Y, Cheever A W, Donaldson D D, Wynn T A. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J. Immunol. 1999;162:920–930. [PubMed] [Google Scholar]
- 115.Souza P R, Souza A L, Negrao-Correa D, Teixeira A L, Teixeira M M. The role of chemokines in controlling granulomatous inflammation in Schistosoma mansoni infection. Acta Trop. 2008;108:135–138. doi: 10.1016/j.actatropica.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 116.Everts B, Perona-Wright G, Smits H H, Hokke C H, van der Ham A J, Fitzsimmons C M, Doenhoff M J, van der Bosch J, Mohrs K, Haas H, Mohrs M, Yazdanbakhsh M, Schramm G. Omega-1 a glycoprotein secreted by Schistosoma mansoni eggs drives Th2 responses. J. Exp. Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steinfelder S, Andersen J F, Cannons J L, Feng C G, Joshi M, Dwyer D, Caspar P, Schwartzberg P L, Sher A, Jankovic D. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J. Ex. Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McLaren M L, Lillywhite J E, Dunne D W, Doenhoff M J. Serodiagnosis of human Schistosoma mansoni infections: enhanced sensitivity and specificity in ELISA using a fraction containing S. mansoni egg antigens omega 1 and alpha 1. Trans. R. Soc. Trop. Med. Hyg. 1981;75:72–79. doi: 10.1016/0035-9203(81)90014-6. [DOI] [PubMed] [Google Scholar]
- 119.Wynn T A, Cheever A W. Cytokine regulation of granuloma formation in schistosomiasis. Curr. Opin. Immunol. 1995;7:505–511. doi: 10.1016/0952-7915(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 120.Fallon P G, Dunne D W. Tolerization of mice to Schistosoma mansoni egg antigens causes elevated type 1 and diminished type 2 cytokine responses and increased mortality in acute infection. J. Immunol. 1999;162:4122–4132. [PubMed] [Google Scholar]
- 121.Herbert D R, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Forster I, Brombacher F. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 122.Hoffmann K F, Cheever A W, Wynn T A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 123.Reiman R M, Thompson R W, Feng C G, Hari D, Knight R, Cheever A W, Rosenberg H F, Wynn T A. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect. Immun. 2006;74:1471–1479. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.El-Sokkary G H, Omar H M, Hassanein A F, Cuzzocrea S, Reiter R J. Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni. Free Radic. Biol. Med. 2002;32:319–332. doi: 10.1016/s0891-5849(01)00753-5. [DOI] [PubMed] [Google Scholar]
- 125.Weinstock J V, Boros D L. Organ-dependent differences in composition and function observed in hepatic and intestinal granulomas isolated from mice with Schistosoma mansoni. J. Immunol. 1983;130:418–422. [PubMed] [Google Scholar]
- 126.Londono B, Carmona J, Blair S. Comparison between OptiMAL and the thick smear tests for malaria diagnosis in an endemic area during a non-epidemic period. Biomedica. 2002;22:466–475. [PubMed] [Google Scholar]
- 127.Cardona A E, Restrepo B I, Jaramillo J M, Teale J M. Development of an animal model for neurocysticercosis: immune response in the central nervous system is characterized by a predominance of gamma delta T cells. J. Immunol. 1999;162:995–1002. [PubMed] [Google Scholar]
- 128.Camp C J, Leid R W. Chemokinetic factors obtained from the larval stage of the cestode, Taenia taeniaeformis. Parasite Immunol. 1982;4:373–381. doi: 10.1111/j.1365-3024.1982.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 129.Chaible L M, Alba-Loureiro T C, Maia A A, Pugine S M, Valle C R, Pithon-Curi T C, Curi R, De Melo M P. Effect of Cysticercus cellulosae on neutrophil function and death. Vet. Parasitol. 2005;127:121–129. doi: 10.1016/j.vetpar.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 130.Aldridge J R, Johnson E C, Kuhn R E. CpG stimulates protective immunity in Balb/cJ mice infected with larval Taenia crassiceps. J. Parasitol. 2010;96:920–928. doi: 10.1645/GE-2483.1. [DOI] [PubMed] [Google Scholar]
- 131.Aceti A, Celestino D, Teggi A, Caferro M. Spontaneous in vitro generation of histamine releasing factor from mononuclear cells of patients with hydatidosis. Int. Arch. Allergy Immunol. 1992;98:247–251. doi: 10.1159/000236192. [DOI] [PubMed] [Google Scholar]
- 132.Aumuller E, Schramm G, Gronow A, Brehm K, Gibbs B F, Doenhoff M J, Haas H. Echinococcus multilocularis metacestode extract triggers human basophils to release interleukin-4. Parasite Immunol. 2004;26:387–395. doi: 10.1111/j.0141-9838.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- 133.Bresson-Hadni S, Beurton I, Bartholomot B, Vuitton D A, Kern P, Mantion G, Miguet J P. Alveolar echinococcosis. Hepatology. 1998;27:1453–1456. doi: 10.1002/hep.510270543. [DOI] [PubMed] [Google Scholar]
- 134.Strath M, Sanderson C J. Detection of eosinophil differentiation factor and its relationship to eosinophilia in Mesocestoides corti-infected mice. Exp. Hematol. 1986;14:16–20. [PubMed] [Google Scholar]
- 135.Ansari A, Williams J F. The eosinophilic response of the rat to infection with Taenia taeniaeformis. J. Parasitol. 1976;62:728–736. [PubMed] [Google Scholar]
- 136.Leid R W. Immunity to the metacestode of Taenia taeniaeformis in the laboratory rat. Am. J. Trop. Med. Hyg. 1977;26:54–60. doi: 10.4269/ajtmh.1977.26.54. [DOI] [PubMed] [Google Scholar]
- 137.Potter K A, Leid R W. Isolation and partial characterization of an eosinophil chemotacticfactor from metacestodes of Taenia taeniaeformis (ECF-Tt) J. Immunol. 1986;136:1712–1717. [PubMed] [Google Scholar]
- 138.Niwa A, Miyazato T. Reactive oxygen intermediates from eosinophils in mice infected with Hymenolepis nana. Parasite Immunol. 1996;18:285–295. doi: 10.1046/j.1365-3024.1996.d01-102.x. [DOI] [PubMed] [Google Scholar]
- 139.Slais J, Vanek M. Tissue reaction to spherical and lobular hydatid cysts of Echinococcus granulosus (Batsch, 1786) Folia Parasitol. (Praha): 1980;27:135–143. [PubMed] [Google Scholar]
- 140.de Aluja A, Vargas G. The histopathology of porcine cysticercosis. Vet. Parasitol. 1988;28:65–77. doi: 10.1016/0304-4017(88)90019-2. [DOI] [PubMed] [Google Scholar]
- 141.Bortoletti G, Conchedda M, Ferretti G. Damage and early destruction of Taenia taeniaeformis larvae in resistant hosts and anomalous development in susceptible hosts: a light microscopic and ultrastructural study. Int. J. Parasitol. 1985;15:377–384. doi: 10.1016/0020-7519(85)90022-0. [DOI] [PubMed] [Google Scholar]
- 142.Letonja T, Hammerberg C. Taenia taeniaeformis: early inflammatory response around developing metacestodes in the liver of resistant and susceptible mice I. Identification of leukocyte response with monoclonal antibodies. J. Parasitol. 1987;73:962–970. [PubMed] [Google Scholar]
- 143.Bortoletti G, Gabriele F, Palmas C. Kinetics of mast cells, eosinophils and phospholipase B activity in the spontaneous-cure response of two strains of mice (rapid and slow responder) to the cestode Hymenolepis nana. Parasitol. Res. 1989;75:465–469. doi: 10.1007/BF00930974. [DOI] [PubMed] [Google Scholar]
- 144.Lammas D A, Mitchell L A, Wakelin D. Genetic influences upon eosinophilia and resistance in mice infected with Mesocestoides corti. Parasitology. 1990;101:291–299. doi: 10.1017/s0031182000063356. [DOI] [PubMed] [Google Scholar]
- 145.Hindsbo O, Andreassen J, Ruitenberg J. Immunological and histopathological reactions of the rat against the tapeworm Hymenolepis diminuta and the effects of anti-thymocyte serum. Parasite Immunol. 1982;4:59–76. doi: 10.1111/j.1365-3024.1982.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 146.Ishiwata K, Oku Y, Ito M, Kamiya M. Responses to larval Taenia taeniaeformis in mice with severe combined immunodeficiency (scid) Parasitology. 1992;104:363–369. doi: 10.1017/s0031182000061825. [DOI] [PubMed] [Google Scholar]
- 147.Ferreira A M, Breijo M, Sim R B, Nieto A. Contribution of C5-mediated mechanisms to host defence against Echinococcus granulosus hydatid infection. Parasite Immunol. 2000;22:445–453. doi: 10.1046/j.1365-3024.2000.00323.x. [DOI] [PubMed] [Google Scholar]
- 148.Willms K, Merchant M T. The inflammatory reaction surrounding Taenia solium larvae in pig muscle: ultrastructural and light microscopic observations. Parasite Immunol. 1980;2:261–275. doi: 10.1111/j.1365-3024.1980.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 149.Londono D P, Alvarez J I, Trujillo J, Jaramillo M M, Restrepo B I. The inflammatory cell infiltrates in porcine cysticercosis: immunohistochemical analysis during various stages of infection. Vet. Parasitol. 2002;109:249–259. doi: 10.1016/s0304-4017(02)00290-x. [DOI] [PubMed] [Google Scholar]
- 150.Sakamoto T, Cabrera P A. Immunohistochemical observations on cellular response in unilocular hydatid lesions and lymph nodes of cattle. Acta Trop. 2003;85:271–279. doi: 10.1016/s0001-706x(02)00226-7. [DOI] [PubMed] [Google Scholar]
- 151.Engelkirk P G, Williams J F. Taenia taeniaeformis (Cestoda) in the rat: ultrastructure of the host-parasite interface on days 8 to 22 postinfection. J. Parasitol. 1983;69:828–837. [PubMed] [Google Scholar]
- 152.Ovington K S, Behm C A. The enigmatic eosinophil: investigation of the biological role of eosinophils in parasitic helminth infection. Mem. Inst. Oswaldo Cruz. 1997;92:93–104. doi: 10.1590/s0074-02761997000800013. [DOI] [PubMed] [Google Scholar]
- 153.Rawat J, Dixon J B, Macintyre A R, McGarry H F, Taylor M J. IL-4 dependent resistance to the tapeworm Mesocestoides corti (Cestoda) in mice. Parasite Immunol. 2003;25:553–557. doi: 10.1111/j.0141-9838.2004.00666.x. [DOI] [PubMed] [Google Scholar]
- 154.Meeusen E, Gorrell M D, Rickard M D, Brandon M R. Lymphocyte subpopulations of sheep in protective immunity to Taenia hydatigena. Parasite Immunol. 1989;11:169–181. doi: 10.1111/j.1365-3024.1989.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 155.Meeusen E, Barcham G J, Gorrell M D, Rickard M D, Brandon M R. Cysticercosis: cellular immune responses during primary and secondary infection. Parasite Immunol. 1990;12:403–418. doi: 10.1111/j.1365-3024.1990.tb00977.x. [DOI] [PubMed] [Google Scholar]
- 156.Molinari J L, Meza R, Tato P. Taenia solium: cell reactions to the larva (Cysticercus cellulosae) in naturally parasitized, immunized hogs. Exp. Parasitol. 1983;56:327–338. doi: 10.1016/0014-4894(83)90078-4. [DOI] [PubMed] [Google Scholar]
- 157.Molinari J L, Soto R, Tato P, Rodriguez D, Retana A, Sepulveda J, Palet A. Immunization against porcine cysticercosis in an endemic area in Mexico: a field and laboratory study. Am. J. Trop. Med. Hyg. 1993;49:502–512. doi: 10.4269/ajtmh.1993.49.502. [DOI] [PubMed] [Google Scholar]
- 158.Cortes I, Molinari J L, Solano S, Hernandez-Mendoza L, Ramirez A, Tato P. Taenia solium metacestode antigens which are protective for pigs induce Th1/Th2 mixed responses in mice. Parasitol. Res. 2003;90:273–279. doi: 10.1007/s00436-003-0855-0. [DOI] [PubMed] [Google Scholar]
- 159.Ito A, Onitake K, Sasaki J, Takami T. Hymenolepis nana: immunity against oncosphere challenge in mice previously given viable or non-viable oncospheres of H. nana, H. diminuta, H. microstoma and Taenia taeniaeformis. Int. J. Parasitol. 1991;21:241–245. doi: 10.1016/0020-7519(91)90015-y. [DOI] [PubMed] [Google Scholar]
- 160.Hernandez-Pomi A, Borras-Salvador R, Mir-Gisbert A. Analysis of cytokine and specific antibody profiles in hydatid patients with primary infection and relapse of disease. Parasite Immunol. 1997;19:553–561. doi: 10.1046/j.1365-3024.1997.d01-173.x. [DOI] [PubMed] [Google Scholar]
- 161.Chavarria A, Fleury A, Garcia E, Marquez C, Fragoso G, Sciutto E. Relationship between the clinical heterogeneity of neurocysticercosis and the immune-inflammatory profiles. Clin. Immunol. 2005;116:271–278. doi: 10.1016/j.clim.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 162.Jenne L, Kilwinski J, Scheffold W, Kern P. IL-5 expressed by CD4+ lymphocytes from Echinococcus multilocularis-infected patients. Clin. Exp. Immunol. 1997;109:90–97. doi: 10.1046/j.1365-2249.1997.4031299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Siracusano A, Margutti P, Delunardo F, Profumo E, Rigano R, Buttari B, Teggi A, Ortona E. Molecular cross-talk in host-parasite relationships: the intriguing immunomodulatory role of Echinococcus antigen B in cystic echinococcosis. Int. J. Parasitol. 2008;38:1371–1376. doi: 10.1016/j.ijpara.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 164.Virginio V G, Taroco L, Ramos A L, Ferreira A M, Zaha A, Ferreira H B, Hernandez A. Effects of protoscoleces and AgB from Echinococcus granulosus on human neutrophils: possible implications on the parasite's immune evasion mechanisms. Parasitol. Res. 2007;100:935–942. doi: 10.1007/s00436-006-0366-x. [DOI] [PubMed] [Google Scholar]
- 165.Rigano R, Profumo E, Bruschi F, Carulli G, Azzara A, Ioppolo S, Buttari B, Ortona E, Margutti P, Teggi A, Siracusano A. Modulation of human immune response by Echinococcus granulosus antigen B and its possible role in evading host defenses. Infect. Immun. 2001;69:288–296. doi: 10.1128/IAI.69.1.288-296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ortona E, Margutti P, Delunardo F, Nobili V, Profumo E, Rigano R, Buttari B, Carulli G, Azzara A, Teggi A, Bruschi F, Siracusano A. Screening of an Echinococcus granulosus cDNA library with IgG4 from patients with cystic echinococcosis identifies a new tegumental protein involved in the immune escape. Clin. Exp. Immunol. 2005;142:528–538. doi: 10.1111/j.1365-2249.2005.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Alkarmi T, Behbehani K. Echinococcus multilocularis: inhibition of murine neutrophil and macrophage chemotaxis. Exp. Parasitol. 1989;69:16–22. doi: 10.1016/0014-4894(89)90166-5. [DOI] [PubMed] [Google Scholar]
- 168.Mejri N, Gottstein B. Echinococcus multilocularis metacestode metabolites contain a cysteine protease that digests eotaxin, a CC pro-inflammatory chemokine. Parasitol Res. 2009;105:1253–1260. doi: 10.1007/s00436-009-1549-z. [DOI] [PubMed] [Google Scholar]
- 169.Tato P, White A C, Jr, Willms K, Rodriguez D, Solano S, Sepulveda J, Molinari J L. Immunosuppression and inhibition of inflammation in mice induced by a small Taenia solium RNA-peptide to implanted T. solium metacestodes. Parasitol. Res. 1996;82:590–597. doi: 10.1007/s004360050170. [DOI] [PubMed] [Google Scholar]
- 170.Yan H L, Xue G, Mei Q, Ding F X, Wang Y Z, Sun S H. Calcium-dependent proapoptotic effect of Taenia solium metacestodes annexin B1 on human eosinophils: a novel strategy to prevent host immune response. Int. J. Biochem. Cell Biol. 2008;40:2151–2163. doi: 10.1016/j.biocel.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 171.Zepeda N, Solano S, Copitin N, Fernandez A M, Hernandez L, Tato P, Molinari J L. Decrease of peritoneal inflammatory CD4+, CD8+, CD19+ lymphocytes and apoptosis of eosinophils in a murine Taenia crassiceps infection. Parasitol. Res. 2010;107:1129–1135. doi: 10.1007/s00436-010-1980-1. [DOI] [PubMed] [Google Scholar]
- 172.Ramos A L, Discipio R G, Ferreira A M. Eosinophil cationic protein damages protoscoleces in vitro and is present in the hydatid cyst. Parasite Immunol. 2006;28:347–355. doi: 10.1111/j.1365-3024.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 173.Anderson R C. Nematode Parasites of Vertebrates. Their Development and Transmission. Oxon, UK : CABI Publishing ; 2000. p. 650. [Google Scholar]
- 174.Hotez P J, Brindley P J, Bethony J M, King C H, Pearce E J, Jacobson J. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jenkins S J, Allen J E. Similarity and diversity in macrophage activation by nematodes trematodes and cestodes. J. Biomed. Biotechnol. 2010. (2010, 262609). [DOI] [PMC free article] [PubMed]
- 176.Anthony R M, Urban J F, Jr, Alem F, Hamed H A, Rozo C T, Boucher J L, Van Rooijen N, Gause W C. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu S K, Cypess R H, van Zandt P. Gastritis caused by multiple Nematospiroides dubius infections. J. Parasitol. 1974;60:790–793. [PubMed] [Google Scholar]
- 178.Penttila I A, Ey P L, Jenkin C R. Reduced infectivity of Nematospiroides dubius larvae after incubation in vitro with neutrophils or eosinophils from infected mice and a lack of effect by neutrophils from normal mice. Parasite Immunol. 1984;6:295–308. doi: 10.1111/j.1365-3024.1984.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 179.Morimoto M, Whitmire J, Xiao S, Anthony R M, Mirakami H, Star R A, Urban J F, Jr, Gause W C. Peripheral CD4 T cells rapidly accumulate at the host: parasite interface during an inflammatory Th2 memory response. J. Immunol. 2004;172:2424–2430. doi: 10.4049/jimmunol.172.4.2424. [DOI] [PubMed] [Google Scholar]
- 180.Urban J F, Jr, Katona I M, Paul W E, Finkelman F D. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc. Natl. Acad. Sci. USA. 1991;88:5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Madden K B, Yeung K A, Zhao A, Gause W C, Finkelman F D, Katona I M, Urban J F, Jr, Shea-Donohue T. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J. Immunol. 2004;172:5616–5621. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 182.Tilney L G, Connelly P S, Guild G M, Vranich K A, Artis D. Adaptation of a nematode parasite to living within the mammalian epithelium. J. Exp. Zool. A Comp. Exp. Biol. 2005;303:927–945. doi: 10.1002/jez.a.214. [DOI] [PubMed] [Google Scholar]
- 183.Betts C J, Else K J. Mast cells eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris. Parasite Immunol. 1999;21:45–52. doi: 10.1046/j.1365-3024.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- 184.Svensson M, Bell L, Little M C, DeSchoolmeester M, Locksley R M, Else K J. Accumulation of eosinophils in intestine-draining mesenteric lymph nodes occurs after Trichuris muris infection. Parasite Immunol. 2011;33:1–11. doi: 10.1111/j.1365-3024.2010.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kaur G, Raj S M, Naing N N. Trichuriasis: localized inflammatory responses in the colon. Southeast Asian J. Trop. Med. Public Health. 2002;33:224–228. [PubMed] [Google Scholar]
- 186.Ohnmacht C, Pullner A, van Rooijen N, Voehringer D. Analysis of eosinophil turnover in vivo reveals their active recruitment to and prolonged survival in the peritoneal cavity. J. Immunol. 2007;179:4766–4774. doi: 10.4049/jimmunol.179.7.4766. [DOI] [PubMed] [Google Scholar]
- 187.Giacomin P R, Wang H, Gordon D L, Botto M, Dent L A. Loss of complement activation and leukocyte adherence as Nippostrongylus brasiliensis develops within the murine host. Infect. Immun. 2005;73:7442–7449. doi: 10.1128/IAI.73.11.7442-7449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yates J A, Higashi G I, Lowichik A, Orihel T C, Lowrie R C, Jr, Eberhard M L. Activation of the alternative complement pathway in normal human serum by Loa loa and Brugia malayi infective stage larvae. Acta Trop. 1985;42:157–163. [PubMed] [Google Scholar]
- 189.Gruden-Movsesijan A, Petrovic M, Sofronic-Milosavljevic L. Interaction of mannan-binding lectin with Trichinella spiralis glycoproteins a possible innate immune mechanism. Parasite Immunol. 2003;25:545–552. doi: 10.1111/j.0141-9838.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 190.Giacomin P R, Gordon D L, Botto M, Daha M R, Sanderson S D, Taylor S M, Dent L A. The role of complement in innate, adaptive and eosinophil-dependent immunity to the nematode Nippostrongylus brasiliensis. Mol. Immunol. 2008;45:446–455. doi: 10.1016/j.molimm.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 191.Knott M L, Matthaei K I, Giacomin P R, Wang H, Foster P S, Dent L A. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int. J. Parasitol. 2007;37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 192.Knott M L, Matthaei K I, Foster P S, Dent L A. The roles of eotaxin and the STAT6 signalling pathway in eosinophil recruitment and host resistance to the nematodes Nippostrongylus brasiliensis and Heligmosomoides bakeri. Mol. Immunol. 2009;46:2714–2722. doi: 10.1016/j.molimm.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 193.Mearns H, Horsnell W G, Hoving J C, Dewals B, Cutler A J, Kirstein F, Myburgh E, Arendse B, Brombacher F. Interleukin-4-promoted T helper 2 responses enhance Nippostrongylus brasiliensis-induced pulmonary pathology. Infect. Immun. 2008;76:5535–5542. doi: 10.1128/IAI.00210-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Price A E, Liang H E, Sullivan B M, Reinhardt R L, Eisley C J, Erle D J, Locksley R M. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chitkara R K, Krishna G. Parasitic pulmonary eosinophilia. Semin. Respir. Crit. Care Med. 2006;27:171–184. doi: 10.1055/s-2006-939520. [DOI] [PubMed] [Google Scholar]
- 196.Chitkara R K, Sarinas P S. Dirofilaria visceral larva migrans and tropical pulmonary eosinophilia. Semin. Respir. Infect. 1997;12:138–148. [PubMed] [Google Scholar]
- 197.Weller P F. Eosinophils: structure and functions. Curr. Opin. Immunol. 1994;6:85–90. doi: 10.1016/0952-7915(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 198.Horii Y, Owhashi M, Fujita K, Nakanishi H, Ishii A. A comparative study on eosinophil and neutrophil chemotactic activities of various helminth parasites. Parasitol. Res. 1988;75:76–78. doi: 10.1007/BF00931196. [DOI] [PubMed] [Google Scholar]
- 199.Falcone F H, Rossi A G, Sharkey R, Brown A P, Pritchard D I, Maizels R M. Ascaris suum-derived products induce human neutrophil activation via a G protein-coupled receptor that interacts with the interleukin-8 receptor pathway. Infect. Immun. 2001;69:4007–4018. doi: 10.1128/IAI.69.6.4007-4018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Owhashi M, Kirai N, Horii Y. Eosinophil chemotactic factor release from neutrophils induced by stimulation with Schistosoma japonicum eggs. Parasitol. Res. 1997;83:42–46. doi: 10.1007/s004360050205. [DOI] [PubMed] [Google Scholar]
- 201.Herbert D R, Lee J J, Lee N A, Nolan T J, Schad G A, Abraham D. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 2000;165:4544–4551. doi: 10.4049/jimmunol.165.8.4544. [DOI] [PubMed] [Google Scholar]
- 202.Galioto A M, Hess J A, Nolan T J, Schad G A, Lee J J, Abraham D. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval strongyloides stercoralis in mice. Infect. Immun. 2006;74:5730–5738. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.O'Connell A E, Hess J A, Santiago G A, Nolan T J, Lok J B, Lee J J, Abraham D. Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect. Immun. 2011;79:2770–2778. doi: 10.1128/IAI.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Alban L, Pozio E, Boes J, Boireau P, Boue F, Claes M, Cook A J, Dorny P, Enemark H L, van der Giessen J, Hunt K R, Howell M, Kirjusina M, Nockler K, Rossi P, Smith G C, Snow L, Taylor M A, Theodoropoulos G, Vallee I, Viera-Pinto M M, Zimmer I A. Towards a standardised surveillance for Trichinella in the European Union. Prev. Vet. Med. 2011;99:148–160. doi: 10.1016/j.prevetmed.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 205.Capo V, Despommier D D. Clinical aspects of infection with Trichinella spp. Clin. Microbiol. Rev. 1996;9:47–54. doi: 10.1128/cmr.9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li C K, Seth R, Gray T, Bayston R, Mahida Y R, Wakelin D. Production of proinflammatory cytokines and inflammatory mediators in human intestinal epithelial cells after invasion by Trichinella spiralis. Infect. Immun. 1998;66:2200–2206. doi: 10.1128/iai.66.5.2200-2206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bruschi F, Korenaga M, Watanabe N. Eosinophils and Trichinella infection: toxic for the parasite and the host? Trends Parasitol. 2008;24:462–467. doi: 10.1016/j.pt.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 208.Kazura J W, Aikawa M. Host defense mechanisms against Trichinella spiralis infection in the mouse: eosinophil-mediated destruction of newborn larvae in vitro. J. Immunol. 1980;124:355–361. [PubMed] [Google Scholar]
- 209.Ogilvie B M, Askenase P W, Rose M E. Basophils and eosinophils in three strains of rats and in athymic (nude) rats following infection with the nematodes Nippostrongylus brasiliensis or Trichinella spiralis. Immunology. 1980;39:385–389. [PMC free article] [PubMed] [Google Scholar]
- 210.Vallance B A, Matthaei K I, Sanovic S, Young I G, Collins S M. Interleukin-5 deficient mice exhibit impaired host defence against challenge Trichinella spiralis infections. Parasite Immunol. 2000;22:487–492. doi: 10.1046/j.1365-3024.2000.00328.x. [DOI] [PubMed] [Google Scholar]
- 211.Gurish M F, Humbles A, Tao H, Finkelstein S, Boyce J A, Gerard C, Friend D S, Austen K F. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. J. Immunol. 2002;168:5730–5736. doi: 10.4049/jimmunol.168.11.5730. [DOI] [PubMed] [Google Scholar]
- 212.Fabre M V, Beiting D P, Bliss S K, Appleton J A. Immunity to Trichinella spiralis muscle infection. Vet. Parasitol. 2009;159:245–248. doi: 10.1016/j.vetpar.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Perez O, Capron M, Lastre M, Venge P, Khalife J, Capron A. Angiostrongylus cantonensis: role of eosinophils in the neurotoxic syndrome (Gordon-like phenomenon) Exp. Parasitol. 1989;68:403–413. doi: 10.1016/0014-4894(89)90125-2. [DOI] [PubMed] [Google Scholar]
- 214.Yoshimura K, Uchida K, Sato K, Oya H. Ultrastructural evidence for eosinophil-mediated destruction of Angiostrongylus cantonensis transferred into the pulmonary artery of non-permissive hosts. Parasite Immunol. 1984;6:105–118. doi: 10.1111/j.1365-3024.1984.tb00785.x. [DOI] [PubMed] [Google Scholar]
- 215.Lan K P, Lai S C. Differences of proteolytic enzymes and pathological changes in permissive and nonpermissive animal hosts for Angiostrongylus cantonensis infection. Vet. Parasitol. 2009;165:265–272. doi: 10.1016/j.vetpar.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 216.Lee H H, Chou H L, Chen K M, Lai S C. Association of matrix metalloproteinase-9 in eosinophilic meningitis of BALB/c mice caused by Angiostrongylus cantonensis. Parasitol. Res. 2004;94:321–328. doi: 10.1007/s00436-004-1196-3. [DOI] [PubMed] [Google Scholar]
- 217.Tseng Y K, Tu W C, Lee H H, Chen K M, Chou H L, Lai S C. Ultrastructural localization of matrix metalloproteinase-9 in eosinophils from the cerebrospinal fluid of mice with eosinophilic meningitis caused by Angiostrongylus cantonensis. Ann. Trop. Med. Parasitol. 2004;98:831–841. doi: 10.1179/000349804X3199. [DOI] [PubMed] [Google Scholar]
- 218.Chen K M, Lee H H, Chou H L, Liu J Y, Tsai B C, Lai S C. Upregulation of MMP-9/TIMP-1 enzymatic system in eosinophilic meningitis caused by Angiostrongylus cantonensis. Int. J. Exp. Pathol. 2005;86:81–89. doi: 10.1111/j.0959-9673.2005.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sugaya H, Aoki M, Yoshida T, Takatsu K, Yoshimura K. Eosinophilia and intracranial worm recovery in interleukin-5 transgenic and interleukin-5 receptor alpha chain-knockout mice infected with Angiostrongylus cantonensis. Parasitol. Res. 1997;83:583–590. doi: 10.1007/s004360050302. [DOI] [PubMed] [Google Scholar]
- 220.Chang E E, Yen C M. Eosinophil chemoattracted by eotaxin from cerebrospinal fluid of mice infected with Angiostrongylus cantonensis assayed in a microchamber. Kaohsiung J. Med. Sci. 2004;20:209–215. doi: 10.1016/s1607-551x(09)70108-1. [DOI] [PubMed] [Google Scholar]
- 221.Intapan P M, Niwattayakul K, Sawanyawisuth K, Chotmongkol V, Maleewong W. Cerebrospinal fluid eotaxin and eotaxin-2 levels in human eosinophilic meningitis associated with angiostrongyliasis. Cytokine. 2007;39:138–141. doi: 10.1016/j.cyto.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 222.Intapan P M, Kittimongkolma S, Niwattayakul K, Sawanyawisuth K, Maleewong W. Cerebrospinal fluid cytokine responses in human eosinophilic meningitis associated with angiostrongyliasis. J. Neurol. Sci. 2008;267:17–21. doi: 10.1016/j.jns.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 223.Chuang C C, Su K E, Chen C W, Fan C K, Lin F K, Chen Y S, Du W Y. Anti-CCR3 monoclonal antibody inhibits eosinophil infiltration in Angiostrongylus cantonensis-infected ICR mice. Acta Trop. 2010;113:209–213. doi: 10.1016/j.actatropica.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 224.Ishida K, Yoshimura K. Characterization of monoclonal antibodies against eosinophil chemotactic factors from young adult worms of Angiostrongylus cantonensis. Parasite Immunol. 1992;14:633–644. doi: 10.1111/j.1365-3024.1992.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 225.Sugaya H, Ishida K, Yoshimura K. Induction of cerebrospinal fluid eosinophilia in rats by the intraventricular injection of Angiostrongylus cantonensis antigen. Int. J. Parasitol. 1997;27:113–117. doi: 10.1016/s0020-7519(96)00165-8. [DOI] [PubMed] [Google Scholar]
- 226.Du W Y, Liao J W, Fan C K, Su K E. Combined treatment with interleukin-12 and mebendazole lessens the severity of experimental eosinophilic meningitis caused by Angiostrongylus cantonensis in ICR mice. Infect. Immun. 2003;71:3947–3953. doi: 10.1128/IAI.71.7.3947-3953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sugaya H, Abe T, Yoshimura K. Eosinophils in the cerebrospinal fluid of mice infected with Angiostrongylus cantonensis are resistant to apoptosis. Int. J. Parasitol. 2001;31:1649–1658. doi: 10.1016/s0020-7519(01)00291-0. [DOI] [PubMed] [Google Scholar]
- 228.Audicana M T, Kennedy M W. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 2008;21:360–379. doi: 10.1128/CMR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tanaka J, Torisu M. Anisakis and eosinophil. I. Detection of a soluble factor selectively chemotactic for eosinophils in the extract from Anisakis larvae. J. Immunol. 1978;120:745–749. [PubMed] [Google Scholar]
- 230.Iwasaki K, Torisu M. Anisakis and eosinophil. II. Eosinophilic phlegmon experimentally induced in normal rabbits by parasite-derived eosinophil chemotactic factor (ECF-P) Clin. Immunol. Immunopathol. 1982;23:593–605. doi: 10.1016/0090-1229(82)90322-1. [DOI] [PubMed] [Google Scholar]
- 231.Jones R E, Deardorff T L, Kayes S G. Anisakis simplex: histopathological changes in experimentally infected CBA/J mice. Exp. Parasitol. 1990;70:305–313. doi: 10.1016/0014-4894(90)90112-p. [DOI] [PubMed] [Google Scholar]
- 232.Deardorff T L, Kayes S G, Fukumura T. Human anisakiasis transmitted by marine food products. Hawaii Med. J. 1991;50:9–16. [PubMed] [Google Scholar]
- 233.del Pozo V, Arrieta I, Tunon T, Cortegano I, Gomez B, Cardaba B, Gallardo S, Rojo M, Renedo G, Palomino P, Tabar A I, Lahoz C. Immunopathogenesis of human gastrointestinal infection by Anisakis simplex. J. Allergy Clin. Immunol. 1999;104:637–643. doi: 10.1016/s0091-6749(99)70336-2. [DOI] [PubMed] [Google Scholar]
- 234.Anadon A M, Rodriguez E, Garate M T, Cuellar C, Romaris F, Chivato T, Rodero M, Gonzalez-Diaz H, Ubeira F M. Diagnosing human anisakiasis: recombinant Ani s 1 and Ani s 7 allergens versus the UniCAP 100 fluorescence enzyme immunoassay. Clin. Vaccine Immunol. 2010;17:496–502. doi: 10.1128/CVI.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cho M K, Ahn S C, Kim D H, Yu H S. Parasite excretory-secretory proteins elicit TRIF dependent CXCL1 and IL-6 mediated allergic inflammation. Parasite Immunol. 2010;32:354–360. doi: 10.1111/j.1365-3024.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- 236.Rodriguez-Mahillo A I, Gonzalez-Munoz M, Gomez-Aguado F, Rodriguez-Perez R, Corcuera M T, Caballero M L, Moneo I. Cloning and characterisation of the Anisakis simplex allergen Ani s 4 as a cysteine-protease inhibitor. Int. J. Parasitol. 2007;37:907–917. doi: 10.1016/j.ijpara.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 237.Rodriguez-Mahillo A I, Gonzalez-Munoz M, de las Heras C, Tejada M, Moneo I. Quantification of Anisakis simplex allergens in fresh long-term frozen and cooked fish muscle. Foodborne Pathog. Dis. 2010;7:967–973. doi: 10.1089/fpd.2009.0517. [DOI] [PubMed] [Google Scholar]
- 238.Petit G, Diagne M, Marechal P, Owen D, Taylor D, Bain O. Maturation of the filaria Litomosoides sigmodontis in BALB/c mice comparative susceptibility of nine other inbred strains. Ann. Parasitol. Hum. Comp. 1992;67:144–150. doi: 10.1051/parasite/1992675144. [DOI] [PubMed] [Google Scholar]
- 239.Martin C, Saeftel M, Vuong P N, Babayan S, Fischer K, Bain O, Hoerauf A. B-cell deficiency suppresses vaccine-induced protection against murine filariasis but does not increase the recovery rate for primary infection. Infect. Immun. 2001;69:7067–7073. doi: 10.1128/IAI.69.11.7067-7073.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lange A M, Yutanawiboonchai W, Scott P, Abraham D. IL-4 and IL-5-dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J. Immunol. 1994;153:205–211. [PubMed] [Google Scholar]
- 241.Le Goff L, Loke P, Ali H F, Taylor D W, Allen J E. Interleukin-5 is essential for vaccine-mediated immunity but not innate resistance to a filarial parasite. Infect. Immun. 2000;68:2513–2517. doi: 10.1128/iai.68.5.2513-2517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bleiss W, Oberlander U, Hartmann S, Adam R, Marko A, Schonemeyer A, Lucius R. Protective immunity induced by irradiated third-stage larvae of the filaria Acanthocheilonema viteae is directed against challenge third-stage larvae before molting. J. Parasitol. 2002;88:264–270. doi: 10.1645/0022-3395(2002)088[0264:PIIBIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 243.Abraham D, Leon O, Schnyder-Candrian S, Wang C C, Galioto A M, Kerepesi L A, Lee J J, Lustigman S. Immunoglobulin E and eosinophil-dependent protective immunity to larval Onchocerca volvulus in mice immunized with irradiated larvae. Infect. Immun. 2004;72:810–817. doi: 10.1128/IAI.72.2.810-817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Martin C, Le Goff L, Ungeheuer M N, Vuong P N, Bain O. Drastic reduction of a filarial infection in eosinophilic interleukin-5 transgenic mice. Infect. Immun. 2000;68:3651–3656. doi: 10.1128/iai.68.6.3651-3656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Specht S, Saeftel M, Arndt M, Endl E, Dubben B, Lee N A, Lee J J, Hoerauf A. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect. Immun. 2006;74:5236–5243. doi: 10.1128/IAI.00329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Al-Qaoud K M, Pearlman E, Hartung T, Klukowski J, Fleischer B, Hoerauf A. A new mechanism for IL-5-dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int. Immunol. 2000;12:899–908. doi: 10.1093/intimm/12.6.899. [DOI] [PubMed] [Google Scholar]
- 247.Saeftel M, Volkmann L, Korten S, Brattig N, Al-Qaoud K, Fleischer B, Hoerauf A. Lack of interferon-gamma confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect. 2001;3:203–213. doi: 10.1016/s1286-4579(01)01372-7. [DOI] [PubMed] [Google Scholar]
- 248.Saeftel M, Arndt M, Specht S, Volkmann L, Hoerauf A. Synergism of gamma interferon and interleukin-5 in the control of murine filariasis. Infect. Immun. 2003;71:6978–6985. doi: 10.1128/IAI.71.12.6978-6985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hall L R, Pearlman E. Pathogenesis of onchocercal keratitis (River blindness) Clin. Microbiol. Rev. 1999;12:445–453. doi: 10.1128/cmr.12.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.St. André A, Blackwell N M, Hall L R, Hoerauf A, Brattig N, Taylor M, Ford L, Hise A, Volkmann L, Lass J H, Diaconu E, Pearlman E. A critical role for endosymbiotic Wolbachia bacteria and TLR4 signaling in the pathogenesis of river blindness. Science. 2002;295:1892–1895. doi: 10.1126/science.1068732. [DOI] [PubMed] [Google Scholar]
- 251.Slatko B E, Taylor M J, Foster J M. The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis. 2010;51:55–65. doi: 10.1007/s13199-010-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kaifi J T, Hall L R, Diaz C, Sypek J, Diaconu E, Lass J H, Pearlman E. Impaired eosinophil recruitment to the cornea in P-selectin-deficient mice in Onchocerca volvulus keratitis (River blindness) Invest. Ophthalmol. Vis. Sci. 2000;41:3856–3861. [PubMed] [Google Scholar]
- 253.Hall L R, Diaconu E, Patel R, Pearlman E. CXC chemokine receptor 2 but not C-C chemokine receptor 1 expression is essential for neutrophil recruitment to the cornea in helminth-mediated keratitis (river blindness) J. Immunol. 2001;166:4035–4041. doi: 10.4049/jimmunol.166.6.4035. [DOI] [PubMed] [Google Scholar]
- 254.Hall L R, Diaconu E, Pearlman E. A dominant role for Fc gamma receptors in antibody-dependent corneal inflammation. J. Immunol. 2001;167:919–925. doi: 10.4049/jimmunol.167.2.919. [DOI] [PubMed] [Google Scholar]
- 255.Hall L R, Kaifi J T, Diaconu E, Pearlman E. CD4(+) depletion selectively inhibits eosinophil recruitment to the cornea and abrogates Onchocerca volvulus keratitis (River blindness) Infect. Immun. 2000;68:5459–5461. doi: 10.1128/iai.68.9.5459-5461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mpagi J L, Rickert R, Steinhart H, Brattig N W. Hyperreactive onchocerciasis exhibits reduced arachidonate and linoleate levels in serum triglycerides. Am. J. Trop. Med. Hyg. 2000;62:705–710. doi: 10.4269/ajtmh.2000.62.705. [DOI] [PubMed] [Google Scholar]
- 257.Korten S, Wildenburg G, Darge K, Buttner D W. Mast cells in onchocercomas from patients with hyperreactive onchocerciasis (sowda) Acta Trop. 1998;70:217–231. doi: 10.1016/s0001-706x(98)00029-1. [DOI] [PubMed] [Google Scholar]
- 258.Tischendorf F W, Brattig N W, Buttner D W, Pieper A, Lintzel M. Serum levels of eosinophil cationic protein, eosinophil-derived neurotoxin and myeloperoxidase in infections with filariae and schistosomes. Acta Trop. 1996;62:171–182. doi: 10.1016/s0001-706x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- 259.Hoerauf A, Kruse S, Brattig N W, Heinzmann A, Mueller-Myhsok B, Deichmann K A. The variant Arg110Gln of human IL-13 is associated with an immunologically hyper-reactive form of onchocerciasis (sowda) Microbes Infect. 2002;4:37–42. doi: 10.1016/s1286-4579(01)01507-6. [DOI] [PubMed] [Google Scholar]
- 260.Rubio de Krömer M T, Krömer M, Luersen K, Brattig N W. Detection of a chemotactic factor for neutrophils in extracts of female Onchocerca volvulus. Acta Trop. 1998;71:45–56. doi: 10.1016/s0001-706x(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 261.Vijayan V K. Tropical pulmonary eosinophilia: pathogenesis diagnosis and management. Curr. Opin. Pulm. Med. 2007;13:428–433. doi: 10.1097/MCP.0b013e3281eb8ec9. [DOI] [PubMed] [Google Scholar]
- 262.Lobos E, Nutman T B, Hothersall J S, Moncada S. Elevated immunoglobulin E against recombinant Brugia malayi gamma-glutamyl transpeptidase in patients with bancroftian filariasis: association with tropical pulmonary eosinophilia or putative immunity. Infect. Immun. 2003;71:747–753. doi: 10.1128/IAI.71.2.747-753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Queto T, Xavier-Elsas P, Gardel M A, de Luca B, Barradas M, Masid D, PM E S, Peixoto C A, Vasconcelos Z M, Dias E P, Gaspar-Elsas M I. Inducible nitric oxide synthase/CD95L-dependent suppression of pulmonary and bone marrow eosinophilia by diethylcarbamazine. Am. J. Respir. Crit. Care Med. 2010;181:429–437. doi: 10.1164/rccm.200905-0800OC. [DOI] [PubMed] [Google Scholar]
- 264.Peixoto C A, Rocha A, Aguiar-Santos A, Florencio M S. The effects of diethylcarbamazine on the ultrastructure of microfilariae of Wuchereria bancrofti in vivo and in vitro. Parasitol. Res. 2004;92:513–517. doi: 10.1007/s00436-004-1081-0. [DOI] [PubMed] [Google Scholar]
- 265.Moreno Y, Nabhan J F, Solomon J, Mackenzie C D, Geary T G. Ivermectin disrupts the function of the excretory-secretory apparatus in microfilariae of Brugia malayi. Proc. Natl. Acad. Sci. USA. 2010;107:20120–20125. doi: 10.1073/pnas.1011983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Soboslay P T, Dreweck C M, Hoffmann W H, Lüder C G, Heuschkel C, Gorgen H, Banla M, Schulz-Key H. Ivermectin-facilitated immunity in onchocerciasis. Reversal of lymphocytopenia, cellular anergy and deficient cytokine production after single treatment. Clin. Exp. Immunol. 1992;89:407–413. doi: 10.1111/j.1365-2249.1992.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mukhopadhyay S, Ravindran B. Antibodies to diethylcarbamazine potentiate the antifilarial activity of the drug. Parasite Immunol. 1997;19:191–195. doi: 10.1046/j.1365-3024.1997.d01-192.x. [DOI] [PubMed] [Google Scholar]
- 268.Tischendorf F W, Brattig N W, Hoyer A, Medina-De la Garza C E, Geisinger F. Modulatory effects of antifilarial drugs ivermectin, CGP 6140 and CGP 20376 on the oxidative burst of eosinophilic granulocytes. Acta Trop. 1993;53:27–37. doi: 10.1016/0001-706x(93)90003-t. [DOI] [PubMed] [Google Scholar]
- 269.Callahan H L, Crouch R K, James E R. Hydrogen peroxide is the most toxic oxygen species for Onchocerca cervicalis microfilariae. Parasitology. 1990;100:407–415. doi: 10.1017/s0031182000078690. [DOI] [PubMed] [Google Scholar]
- 270.Racz P, Tenner-Racz K, Luther B, Büttner D W, Albiez E J. Immunopathologic aspects in human onchocercal lymphadenitis. Bull. Soc. Pathol. Exot. 1983;76:676–680. [PubMed] [Google Scholar]
- 271.Wildenburg G, Darge K, Knab J, Tischendorf F W, Bonow I, Buttner D W. Lymph nodes of onchocerciasis patients after treatment with ivermectin: reaction of eosinophil granulocytes and their cationic granule proteins. Trop. Med. Parasitol. 1994;45:87–96. [PubMed] [Google Scholar]
- 272.Ackerman S J, Kephart G M, Francis H, Awadzi K, Gleich G J, Ottesen E A. Eosinophil degranulation. An immunologic determinant in the pathogenesis of the Mazzotti reaction in human onchocerciasis. J. Immunol. 1990;144:3961–3969. [PubMed] [Google Scholar]
- 273.Gutierrez-Pena E J, Knab J, Büttner D W. Neutrophil granule proteins: evidence for the participation in the host reaction to skin microfilariae of Onchocerca volvulus after diethylcarbamazine administration. Parasitology. 1996;113:403–414. doi: 10.1017/s0031182000066543. [DOI] [PubMed] [Google Scholar]
- 274.Keiser P B, Reynolds S M, Awadzi K, Ottesen E A, Taylor M J, Nutman T B. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J. Infect. Dis. 2002;185:805–811. doi: 10.1086/339344. [DOI] [PubMed] [Google Scholar]
- 275.Supali T, Djuardi Y, Pfarr K M, Wibowo H, Taylor M J, Hoerauf A, Houwing-Duistermaat J J, Yazdanbakhsh M, Sartono E. Doxycycline treatment of Brugia malayi-infected persons reduces microfilaremia and adverse reactions after diethylcarbamazine and albendazole treatment. Clin. Infect. Dis. 2008;46:1385–1393. doi: 10.1086/586753. [DOI] [PubMed] [Google Scholar]
- 276.Hoerauf A, Nissen-Pähle K, Schmetz C, Henkle-Dührsen K, Blaxter M L, Büttner D W, Gallin M Y, Al-Qaoud K M, Lucius R, Fleischer B. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Invest. 1999;103:11–18. doi: 10.1172/JCI4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Langworthy S, Renz A, Mackenstedt U, Henkle-Dührsen K, Bronsvoort M, Tanya V, Donnelly M, Trees A. Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: elimination of Wolbachia preceeds worm death and suggests a dependent relationship. Proc. Royal Soc. London Ser. B. 2000;267:1063–1069. doi: 10.1098/rspb.2000.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hoerauf A, Specht S, Buttner M, Pfarr K, Mand S, Fimmers R, Marfo-Debrekyei Y, Konadu P, Debrah A Y, Bandi C, Brattig N, Albers A, Larbi J, Batsa L, Taylor M J, Adjei O, Buttner D W. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: a randomized placebo-controlled study. Med. Microbiol. Immunol. 2008;197:295–311. doi: 10.1007/s00430-007-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Brattig N W, Buttner D W, Hoerauf A. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 2001;3:439–446. doi: 10.1016/s1286-4579(01)01399-5. [DOI] [PubMed] [Google Scholar]
- 280.Nfon C K, Makepeace B L, Njongmeta L M, Tanya V N, Bain O, Trees A J. Eosinophils contribute to killing of adult Onchocerca ochengi within onchocercomata following elimination of Wolbachia. Microbes Infect. 2006;8:2698–2705. doi: 10.1016/j.micinf.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 281.Hansen R D, Trees A J, Bah G S, Hetzel U, Martin C, Bain O, Tanya V N, Makepeace B L. A worm's best friend: recruitment of neutrophils by Wolbachia confounds eosinophil degranulation against the filarial nematode Onchocerca ochengi. Proc. Biol. Sci. 2011;278:2293–2302. doi: 10.1098/rspb.2010.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hausdorf B. Early evolution of the bilateria. Syst. Biol. 2000;49:130–142. doi: 10.1080/10635150050207438. [DOI] [PubMed] [Google Scholar]
- 283.de Andres B, Rakasz E, Hagen M, McCormik M L, Mueller A L, Elliot D, Metwali A, Sandor M, Britigan B E, Weinstock J V, Lynch R G. Lack of Fc-epsilon receptors on murine eosinophils: implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood. 1997;89:3826–3836. [PubMed] [Google Scholar]
- 284.Meeusen E N, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitol. Today. 2000;16:95–101. doi: 10.1016/s0169-4758(99)01607-5. [DOI] [PubMed] [Google Scholar]
- 285.Klion A D, Nutman T B. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 286.Babayan S A, Read A F, Lawrence R A, Bain O, Allen J E. Filarial parasites develop faster and reproduce earlier in response to host immune effectors that determine filarial life expectancy. (e1000525).PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Fabre V, Beiting D P, Bliss S K, Gebreselassie N G, Gagliardo L F, Lee N A, Lee J J, Appleton J A. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J. Immunol. 2009;182:1577–1583. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Brattig NW, Tenner-Racz K, Korten S, Hoerauf A, Büttner D W. Lee. Immunohisology of ectopic secondary lymph follicels in subcutaneous nodules from patients with hyperreactive onchocerciasis (sowda) Parasitol. Res. 2010;107(3 ):657–66. doi: 10.1007/s00436-010-1912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.O'Mahony D S, Pham U, Iyer R, Hawn T R, Liles W C. Differential constitutive and cytokine-modulated expression of human Toll-like receptors in promary neutrophils monocytes and macrophages. Int. J. Med. Sci. 2008;5(1 ):1–8. doi: 10.7150/ijms.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kogiso M, Nishiyama A, Shinohara T, Nakamura M, Mizoguchi E, Misawa Y, Guinet E, Nouri-Shirazi M, Dorey C K, Henriksen R A, Shibata Y. Chitin particles induce size-dependent but carbohydrate-independent innate eosinophilia. J. Leukoc. Biol. 2011;90(1 ):167–176. doi: 10.1189/jlb.1110624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J. Immunol. 2003;1718(8 ):3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 292.Kurt-Jones E A, Mandell L, Whitney C, Padgett A, Gosselin K, Newburger P E, Finberg R W. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100(5 ):1860–1868. [PubMed] [Google Scholar]
- 293.Komiya A, Nagase H, Okugawa S, Ota Y, Suzukawa M, Kawakami A, Sekiya T, Matsushima K, Ohta K, Hirai K, Yamamoto K, Yamaguchi M. Expression and function of toll-like receptors in human basophils. Int. Arch. Allergy Immunol. 2006;140(Suppl 1 ):23–27. doi: 10.1159/000092707. [DOI] [PubMed] [Google Scholar]
- 294.Da Silva C A, Hartl D, Liu W, Lee C G, Elias J A. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J. Immunol. 2008;181(6 ):4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mansson A, Fransson M, Adner M, Benson M, Uddman R, Bjornsson S, Cardell L O. TLR3 in human eosinophils: functional effects and decreased expression during allergic rhinitis. Int. Arch. Allergy Immunol. 2010;151(2 ):118–128. doi: 10.1159/000236001. [DOI] [PubMed] [Google Scholar]
- 296.Wong C K, Cheung P F Y, Ip W K, Lam C W K. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am. J. Respir. Cell Mol. Biol. 2007;37(1 ):85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 297.Nakao Y, Funami K, Kikkawa S, Taniguchi M, Nishiguchi M, Fukumori Y, Seya T, Matsumoto M. Surface-expressed TLR6 participates in the recognition of diacylated lipopeptide and peptidoglycan in human cells. J. Immunol. 2005;174(3 ):1566–1573. doi: 10.4049/jimmunol.174.3.1566. [DOI] [PubMed] [Google Scholar]
- 298.Phipps S, Lam C E, Mahalingam S, Newhouse M, Ramirez R, Rosenberg H F, Foster P S, Matthaei K I. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110(5 ):1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 299.Yanagisawa S, Koarai A, Sugiura H, Ichikawa T, Kanda M, Tanaka R, Akamatsu K, Hirano T, Matsunaga K, Minakata Y, Ichinose M. Oxidative stress augments toll-like receptor 8 mediated neutrophilic responses in healthy subjects. Respir. Res. 2009;10:50. doi: 10.1186/1465-9921-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]