Abstract
The multikinase inhibitor sorafenib is the first oral agent to show activity against human hepatocellular carcinoma (HCC). Although the clinical application of sorafenib has shown good tolerability in the studied populations, it also causes multiple human dose-limiting toxicities. Thus, there is a strong need to reduce the overall dose of sorafenib. We have reported that the epidermal growth factor receptor variant III (EGFRvIII) expression can decrease the sensitivity of HCC cells to chemotherapeutic drugs. Therefore, we sought to explore whether EGFRvIII can affect the sensitivity of HCC cells to sorafenib. In this study, we observed that EGFRvIII expression significantly decreased the sensitivity of HCC cells to sorafenib. To enhance the antitumor effect and reduce the overall dose of sorafenib, we evaluated the combined effects of CH12, a monoclonal antibody against EGFRvIII, and sorafenib on the growth of HCC cells expressing EGFRvIII in vitro and in vivo. The results showed that, when CH12 was combined with sorafenib, the tumor growth suppression effect was significantly increased, and the concentration of sorafenib required for growth inhibition was substantially reduced. Mechanistically, the combination could more noticeably downregulate the phosphorylation of constitutively active extracellular signal-regulated kinase (ERK), Akt (Thr308), and signal transducer and activator of transcription 3 (STAT3) than sorafenib alone. Collectively, these findings demonstrate that CH12 interacts additively with sorafenib to strongly inhibit the tumor growth of HCC xenografts expressing EGFRvIII by enhancing the sorafenib-mediated inhibition of the MEK/ERK, phosphoinositide 3-kinase/AKT, and STAT3 pathways.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related death worldwide [1,2]. Over the past decades, the incidence of HCC has increased worldwide, especially in eastern Asia and sub-Saharan Africa [1,3]. HCC is clinically characterized by its invasiveness, poor prognosis, and limited therapeutic opportunities. At present, surgery is the most effective treatment for HCC. However, tumor recurrence after a curative liver resection is high [4]. For advanced stage disease, systemic pharmacotherapy is usually the final and main treatment. Previous clinical investigations have shown that traditional systemic chemotherapy cannot provide survival benefits for patients with HCC [2]. Thus, there is a strong need for new, effective, and well-tolerated treatment strategies for this aggressive disease. Sorafenib was the first successful molecular targeted therapy for advanced HCC, as demonstrated in The Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol trial, and it offers hope for improving the treatment of HCC [5].
Sorafenib (Nexavar), an oral multiple kinase inhibitor, was approved by the Food and Drug Administration to treat advanced HCC and is the first clinically approved targeted drug therapy for HCC [6,7]. Sorafenib shows remarkable inhibition of the serine-threonine kinase Raf, which is part of the Ras/MEK/ERK signaling pathway, and suppresses several receptor tyrosine kinases involved in tumor progression and tumor angiogenesis, including vascular endothelial growth factor receptors 2 and 3, platelet-derived growth factor receptor, FLT3, and c-KIT [7]. Although the clinical application of sorafenib has shown good tolerability in the studied populations, it also causes multiple human dose-limiting toxicities, including anorexia, gastrointestinal bleeding, and hand-foot syndrome, and many patients need to permanently withdraw from treatment because of severe skin toxicity [8]. Therefore, reducing the overall dose of sorafenib is a desirable goal.
Although the epidermal growth factor receptor (EGFR) has been successfully targeted for cancer therapy [9], for example, by the monoclonal antibody cetuximab (C225, Erbitux) [10,11], the presence of EGFR gene mutations may result in a limited clinical response to EGFR-targeting therapies in HCC patients. The most common EGFR variant is EGFRvIII (also called de2-7 EGFR) [12], which can promote tumor cell growth in vitro and in vivo [13]. EGFRvIII has been found in head and neck squamous cell carcinoma, non-small cell lung carcinoma, breast cancer, glioma, ovarian carcinoma, and HCC but has not been detected in normal tissue [13–17]. Recently, we also observed its expression in liver cancer cell lines, such as SMMC-7721 cells [18].
Because EGFRvIII expression can decrease the sensitivity of HCC cell lines to chemotherapeutic drugs, such as 5-fluorouracil [18], it may also account for the limited therapeutic effect of sorafenib. CH12, an anti-EGFRvIII monoclonal antibody developed in our laboratory, can preferentially bind to EGFRvIII and significantly inhibit the growth of Huh-7-EGFRvIII and SMMC-7721 xenografts in vivo, with a growth inhibition ratio much higher than cetuximab [19]. We hypothesized that CH12 may enhance the sorafenib-mediated growth inhibition of HCC xenografts expressing EGFRvIII, and we found that combining sorafenib with CH12 more effectively inhibits HCC cell line growth than either agent alone. These results lay the groundwork for possible future clinical evaluation of this drug combination as an HCC therapy.
Materials and Methods
Cell Culture
The human HCC cell lines Huh-7, Huh-7-EGFRvIII (Huh-7 cells with exogenous EGFRvIII overexpression [18]) and SMMC-7721 (Chinese Academy of Science, Shanghai, China) were used. All of the cell lines were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Drugs
Sorafenib (BAY 43-9006) was purchased from Toronto Research Chemicals, Inc (Canada). For in vitro studies, sorafenib was dissolved in dimethyl sulfoxide (Sigma, St Louis, MO) at various concentrations. For in vivo studies, sorafenib was formulated at a concentration four-fold that of the highest dose in a cremophor EL-ethanol (50:50) solution. This four-fold stock solution was prepared fresh daily. The final dosing solutions were prepared on the day of use by diluting the stock solution to one-fold with endotoxin-free distilled water and vortexing immediately before dosing. The chimeric mAb CH12 (IgG1) was produced in dihydrofolate reductase-deficient CHO DG44 cells as described previously [19]. The chimeric mAb C225 were purchased from Merck (La Jolla, CA).
In Vitro Cell Proliferation Assay
The effect of the test agents on cell viability was assessed with the CCK-8 assay. The cells (2000 per well) were seeded. After 24 hours, the cells were exposed to various concentrations of the test agents in DMEM with 10% fetal bovine serum (FBS) for 48 hours. The controls received the dimethyl sulfoxide vehicle at a concentration equal to that of drug-treated cells. After 48 hours, cell proliferation was measured using a CCK-8 kit (Dojindo Laboratories, Rockville, MD). CCK-8 solution (10 µl) was added to 100 µl of culture media, and the optical density was measured at 450 nm. Three independent experiments were performed.
Immunoblot Analysis
The cells were seeded and incubated in six-well plates in DMEM with 10% FBS for 24 hours and exposed to various concentrations of CH12, sorafenib, or a combination in 2% FBS-supplemented DMEM for 24 hours. The cell lysates were then collected. The tumor tissues were surgically excised and frozen in liquid nitrogen. Then the tissues were homogenized in tumor lysis buffer, and the lysates were collected. The proteins were quantified using the BCA Kit (Pierce, Rockford, IL). The proteins (20 µg) were separated with 10% SDS-PAGE gels and transferred to nitrocellulose membranes (Millipore Billerica, MA). The membranes were blocked with 5% skim milk and incubated overnight at 4°C with primary antibodies. The following antibodies were used: mAb 12H23, anti-phospho-EGFR (Tyr1068) (Abcam, Cambridge, United Kingdom) and anti-GAPDH (Kang-Chen Bio-tech, Shanghai, China) antibodies. The anti-phosphor-ERK, anti-ERK1, anti-phospho-Akt (Thr308), anti-phospho-Akt (Ser473), anti-Akt, anti-phospho-MEK, anti-MEK, anti-Bcl-xL, and anti-p27 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The other antibodies, including anti-STAT3 (signal transducer and activator of transcription 3) and anti-phospho-STAT3 (p-STAT3; Tyr705), were obtained from Cell Signaling (Cell Signaling Technology, Danvers, MA). The immune complexes were detected through incubation of the membrane with horseradish peroxidase-conjugated goat antimouse antibody or goat antirabbit antibody (Santa Cruz Biotechnology) for 1 hour at room temperature and subsequent exposure of the membrane to enhanced chemiluminescence reagents (Pierce, Thermo Scientific, Rockford, IL).
In Vivo Antitumor Effects
Huh-7-EGFRvIII cells (3 x 106) were subcutaneously injected into 4- to 6-week-old nude mice. When the tumor volumes reached an average of approximately 100 mm3, mice were randomly assigned to one of the following treatment groups (n = 6 for each group): 1) a daily oral dose of vehicle solution and thrice-weekly intraperitoneal injections of phosphate-buffered saline (PBS; control group), 2) a daily oral dose of sorafenib at 10 mg/kg (sorafenib group), 3) intraperitoneal injections of CH12 (25 mg/kg) thrice weekly (CH12 group), and 4) a daily oral dose of sorafenib at 10mg/kg plus an intraperitoneal injection of CH12 at 25 mg/kg thrice weekly (sorafenib-plus-CH12 group). All of the mice were treated for 2 weeks. The tumor volumes were measured every other day in two dimensions with Vernier calipers. The tumor volumes were calculated using the following formula: length x width2 x 0.5. Two weeks after the final treatment, mice were anesthetized and sacrificed through cervical dislocation. The tumors were surgically excised and weighed. Tumor tissues from the in vivo experiments were collected for Western blot analysis and immunohistochemical studies. Mice were manipulated and housed according to protocols approved by the Shanghai Medical Experimental Animal Care Commission.
Immunohistochemical Analysis
To assess angiogenesis and cell proliferation in tumors, formalin-fixed, paraffin-embedded tumor tissues were immunostained using rat antimouse CD34 (Abcam) and anti-Ki-67 (Santa Cruz Biotechnology). After deparaffinization and rehydration, the tissue sections were incubated with 3% hydrogen peroxide in methanol to quench endogenous peroxidase. The sections were blocked for 30 minutes with 1% bovine serum albumin and incubated overnight with the primary antibody at 4°C. Sections were then washed with PBS and incubated with an horseradish peroxidase-conjugated secondary antibody for 45 minutes. Then, the products were visualized using a diaminobenzidine staining kit (Tiangen Biotech, Beijing, China) and counterstained in hematoxylin. The measurement of Ki-67 labeling index and microvessel density was performed as described previously [16].
TUNEL Assay
Tumor tissue sections were first deparaffinized and rehydrated and were then incubated with proteinase K (20 µg/ml) for 20 minutes at 37°C. After several washes with PBS, the sections were incubated with TUNEL assay buffer (Beyotime Biotechnology, Nanjing, China) for 1 hour at 37°C in the dark. Then, the slides were rinsed in PBS three times and visualized under a Zeiss LSM confocal microscope (Carl Zeiss, Jena, Germany). TUNEL-positive cells were counted at x400 magnification. The apoptotic index was calculated as a ratio of apoptotic cell number to the total cell number in each field.
Statistical Analysis
All data are presented as the mean ± SE. Data were examined using an analysis of variance (ANOVA) and the least significant differences method for multisample comparisons. P < .05 was considered statistically significant.
Results
EGFRvIII Expression Decreases the Sensitivity of HCC Cell Lines to Sorafenib
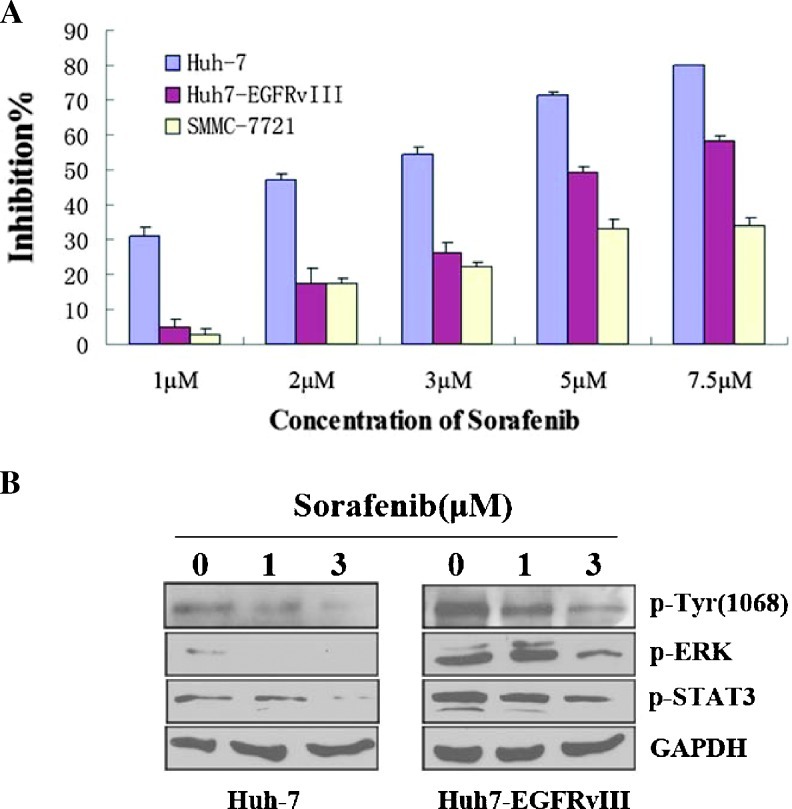
To assess whether EGFRvIII expression affected the sensitivity of HCC cell lines to sorafenib, we first investigated the inhibitory effect of different concentrations of sorafenib (1, 2, 3, 5, and 7.5 µM) on Huh-7, Huh-7-EGFRvIII, and SMMC-7721 cells. As shown in Figure 1A, the Huh-7 cells were more sensitive to sorafenib than the Huh-7-EGFRvIII and SMMC-7721 cells, both of which expressed EGFRvIII [18], especially in the low-concentration groups.
Figure 1.
EGFRvIII expression decreases the sensitivity of Huh-7-EGFRvIII and SMMC-7721 cell lines to sorafenib. (A) HCC cells were exposed to drugs at different concentrations (1, 2, 3, 5, and 7.5 µM) for 48 hours, and cell viability was analyzed with CCK-8. (B) Huh-7 and Huh-7-EGFRvIII cells were treated with 1 or 3 µM sorafenib in 2% FBS-supplemented DMEM for 24 hours, and the cell lysates were collected. The expression levels of phosphorylated EGFR, ERK, and STAT3 were examined.
Previous studies indicated that sorafenib suppressed tumor growth in the HCC model partly via inhibiting the Raf/MEK/ERK pathway [20] and STAT3 [21]. STAT3 is constitutively elevated in many human solid tumors and hematologic malignancies and has been shown to actively participate in cell growth and survival [22]. To better understand how EGFRvIII induces sorafenib resistance at a molecular level, we examined the effects of sorafenib on the phosphorylation of EGFR (p-EGFR), ERK (p-ERK), and STAT3 (p-STAT3). In the absence of sorafenib treatment, the p-EGFR, p-ERK, and p-STAT3 levels were higher in Huh-7-EGFRvIII cells than in Huh-7 cells. After sorafenib treatment, the p-EGFR, p-ERK, and p-STAT3 levels were more noticeably downregulated in Huh-7 cells than in Huh-7-EGFRvIII cells (Figure 1B). Together, these data indicate that EGFRvIII may confer at least partial resistance to sorafenib in HCC cell lines and that the higher levels of ERK and STAT3 phosphorylation may be one of the mechanisms underlying the lower sensitivity of EGFRvIII-positive HCC cells to sorafenib.
Inhibition of Huh-7, Huh-7-EGFRvIII, and SMMC-7721 Cell Growth by Sorafenib Plus CH12
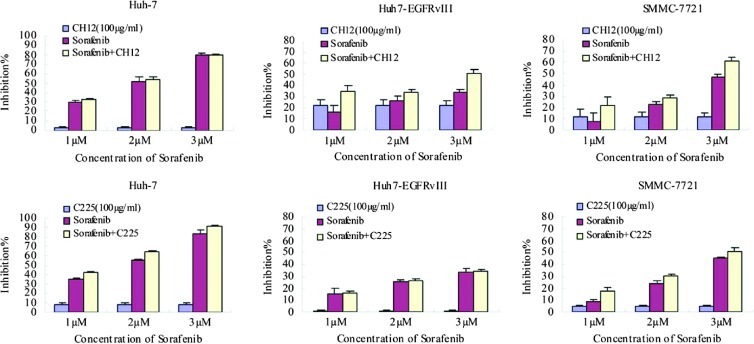
To investigate the combined effects of CH12 and sorafenib on HCC cells, we examined the effects of both drugs on cell viability of Huh-7, Huh-7-EGFRvIII, and SMMC-7721 cells. At the same time, we also evaluated the effects of sorafenib in combination with EGFR inhibitor C225 (cetuximab) on these cells. The cell viability was measured with the CCK-8 assay. Our data illustrated that the combination of CH12 and sorafenib enhanced the antitumor effects of sorafenib in a dose-dependent manner on Huh-7-EGFRvIII and SMMC-7721 cells expressing EGFRvIII. However, there were no obvious additive antitumor effects on Huh-7 cells when sorafenib was combined with CH12 (Figure 2). The possible interpretation is that CH12 barely bind to Huh-7 cells in vitro [19]. The combination of C225 and sorafenib exhibited the enhanced antitumor effects on Huh-7 and SMMC-7721 cells. However, sorafenib in combination with C225 exerted a lower growth inhibitory effect on SMMC-7721 cells than sorafenib in combination with CH12. Moreover, the combined effects of C225 and sorafenib were not observed on Huh-7-EGFRvIII cells (Figure 2).
Figure 2.
The growth inhibition of Huh-7, Huh-7-EGFRvIII, and SMMC-7721 cells by CH12, C225 (100 µg/ml), sorafenib (1, 2, or 3 µM), or the combination. HCC cells were exposed to the drugs for 48 hours and were analyzed with CCK-8. Data are expressed as the percentage inhibition of cell growth. Bars, SD.
Reduction of p-EGFR, p-Akt (Thr308), and p-STAT3 Levels and Inhibition of MEK/ERK Signaling by Sorafenib Plus CH12 In Vitro
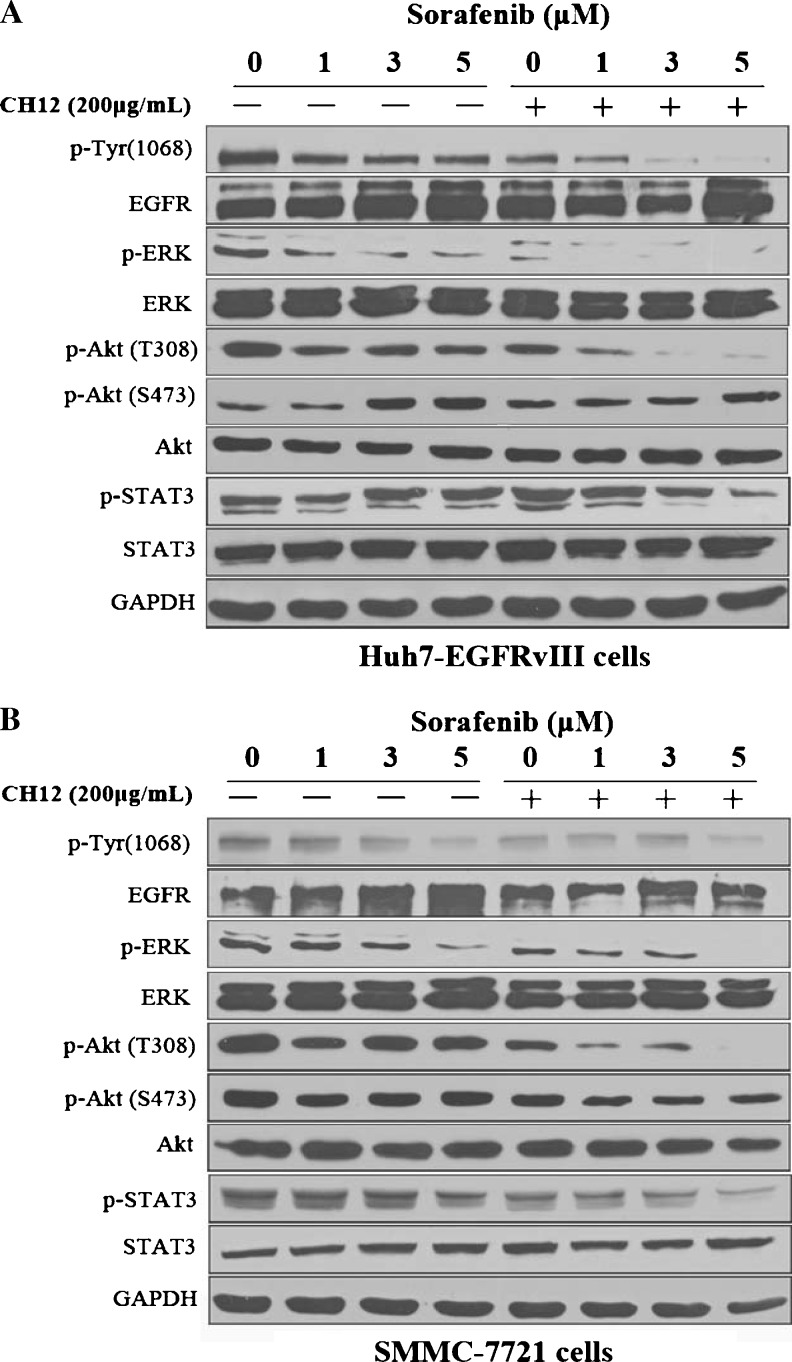
Next, we investigated the mechanisms underlying the reduction in cell proliferation caused by the combination of sorafenib plus CH12. Previous reports have suggested that the constitutively active kinase activity and autophosphorylation of the carboxyl terminus of EGFRvIII can promote tumor cell growth in vitro and in vivo [13,18]. Therefore, we examined the total and phosphorylated EGFR levels in Huh-7-EGFRvIII and SMMC-7721 cells treated with various does of sorafenib, CH12, or the combination through Western blot analysis. In the two cell lines, we observed a dramatic down-regulation of p-EGFR in the combination groups (Figure 3).
Figure 3.
Dose-dependent effects of sorafenib plus CH12 on the activation of EGFR, ERK, Akt, and STAT3 in Huh-7-EGFRvIII cells (A) and SMMC-7721 cells (B). The cells were exposed to the drugs and were then lysed. A total of 20 µg of soluble protein was separated through SDS-PAGE. Protein levels were detected with Western blot analysis. GAPDH was used as the loading control.
Previous studies have indicated that sorafenib and CH12 can both downregulate the phosphorylation of ERK and Akt in HCC xenografts [19,20]. Therefore, we investigated the p-ERK and p-Akt (Thr308) levels in Huh-7-EGFRvIII and SMMC-7721 cells treated with sorafenib and CH12. As shown in Figure 3, although sorafenib dose-dependently inhibited ERK phosphorylation, its combination with CH12 led to a more noticeably reduction in p-ERK levels. The p-Akt (Thr308) levels were also considerably inhibited by the sorafenib-plus-CH12 combination treatment compared with the single-agent treatment. Recent studies indicated that sorafenib, as a single agent, could increase the Akt (Ser473) phosphorylation, and the Akt (Ser473) may be one of the main mediators of sorafenib resistance [23,24]. Here we also detected the levels of p-Akt (Ser473). Intriguingly, in Huh-7-EGFRvIII cells, sorafenib alone caused the upregulated the levels of p-Akt (Ser473), but in SMMC-7721 cells, we did not find an obvious change in p-Akt (Ser473). When sorafenib was combined with CH12, the levels of p-AKT (Ser473) were slightly downregulated in both Huh-7-EGFRvIII and SMMC-7721 cells compared with single-agent sorafenib treatment. Total ERK and Akt levels were unchanged in both cell lines (Figure 3).
To verify whether the therapeutic effect was dependent on the downregulation of p-STAT3 by the combination of sorafenib and CH12, we further assayed the expression of STAT3 in treated HCC cells. As shown in Figure 3, combination treatment could reduce STAT3 phosphorylation in Huh-7-EGFRvIII and SMMC-7721 cell lines, although the phosphorylation levels of STAT3 were downregulated to a greater degree in SMMC-7721 cells than in Huh-7-EGFRvIII cells, whereas there was no obvious decrease when both cell lines were treated with sorafenib or CH12 alone. The total STAT3 protein level was not affected by sorafenib and CH12 treatment.
Additive Effect of Sorafenib Plus CH12 on Huh-7-EGFRvIII Xenograft Tumor Growth In Vivo
To investigate whether the combined effect of sorafenib and CH12 in HCC cell lines expressing EGFRvIII had potential clinical implications, we assessed the in vivo effect of sorafenib plus CH12 on the growth of HCC xenograft tumors. The clinical recommended daily dose of sorafenib is 800 mg, and in a previous report, the dosage of sorafenib used in mice bearing HCC models was generally 30 mg/kg per day [20]. Because CH12 enhanced the antitumor effect and reduced the dosage of sorafenib in the in vitro cytotoxicity assay, we used a lower dosage of sorafenib (10 mg/kg per day) to test the combined effect of sorafenib and CH12 on Huh-7-EGFRvIII xenografts. Tumor-bearing mice were treated with vehicle or 10 mg/kg per day sorafenib orally for 14 days or 25 mg/kg per day CH12 intraperitoneally three times a week for 2 weeks. All of the animals tolerated the treatments well, without observable signs of toxicity, and had stable body weights during the study.
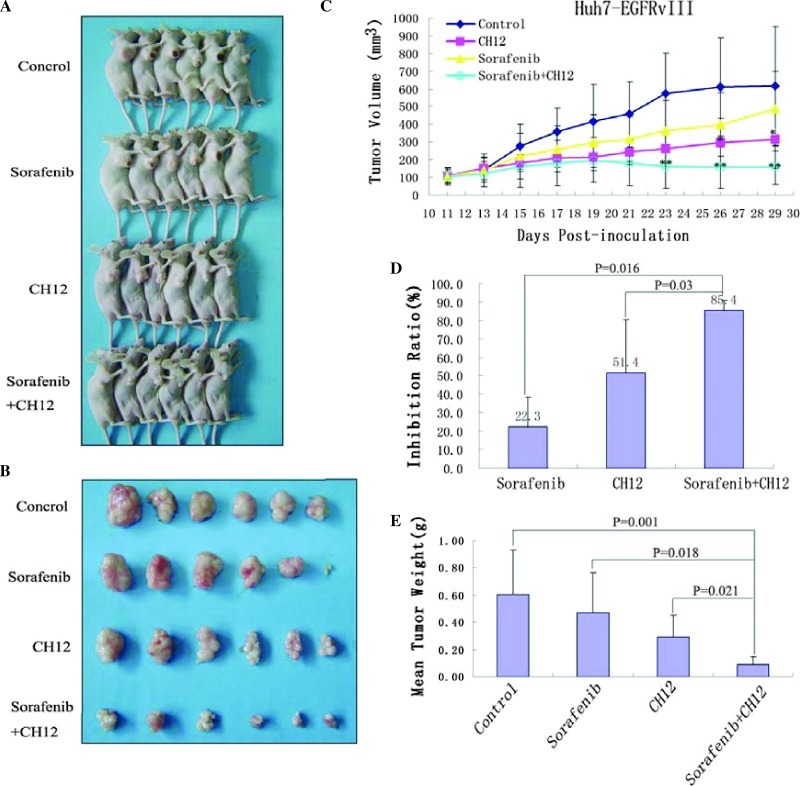
As shown in Figure 4C, tumor growth was considerably inhibited by treatment with sorafenib plus CH12 at day 21 after tumor cell's inoculation (vs sorafenib or CH12 treatment alone, P < .05). The inhibitory ratios of sorafenib, CH12, and the combination on day 30 were 22.3%, 51.4%, and 85.4%, respectively (Figure 4D). Tumor weight was also measured at the end of the study. Our data showed that the cotreatment of CH12 and sorafenib at a low dosage, which was minimally effective when used as a single agent, reduced the tumor weight significantly when given as a combination (vs sorafenib or CH12 treatment alone, P < .05; Figure 4E). Together, these data indicate that the combination of sorafenib and CH12 exhibits better antitumor activity in vivo.
Figure 4.
Antitumor effects of sorafenib plus CH12 on Huh-7-EGFRvIII xenografts in established models. Huh-7-EGFRvIII cells (3 x 106) were subcutaneously injected into 4- to 6-week-old nude mice when the tumors had reached a mean tumor volume of 100 mm3. (A and B) Mice were treated with solvent control, sorafenib, CH12, or a combination of CH12 plus sorafenib. After 29 days, the mice were sacrificed, and whole tumor tissues were isolated as described in Materials and Methods. (C) Data are expressed as mean tumor volumes. (D) Data are expressed as the percentage inhibition of tumor growth. (E) Analysis of tumor weight. Columns, mean (n = 6). *P < .05 versus control. **P < .05 versus sorafenib or CH12 treatment alone.
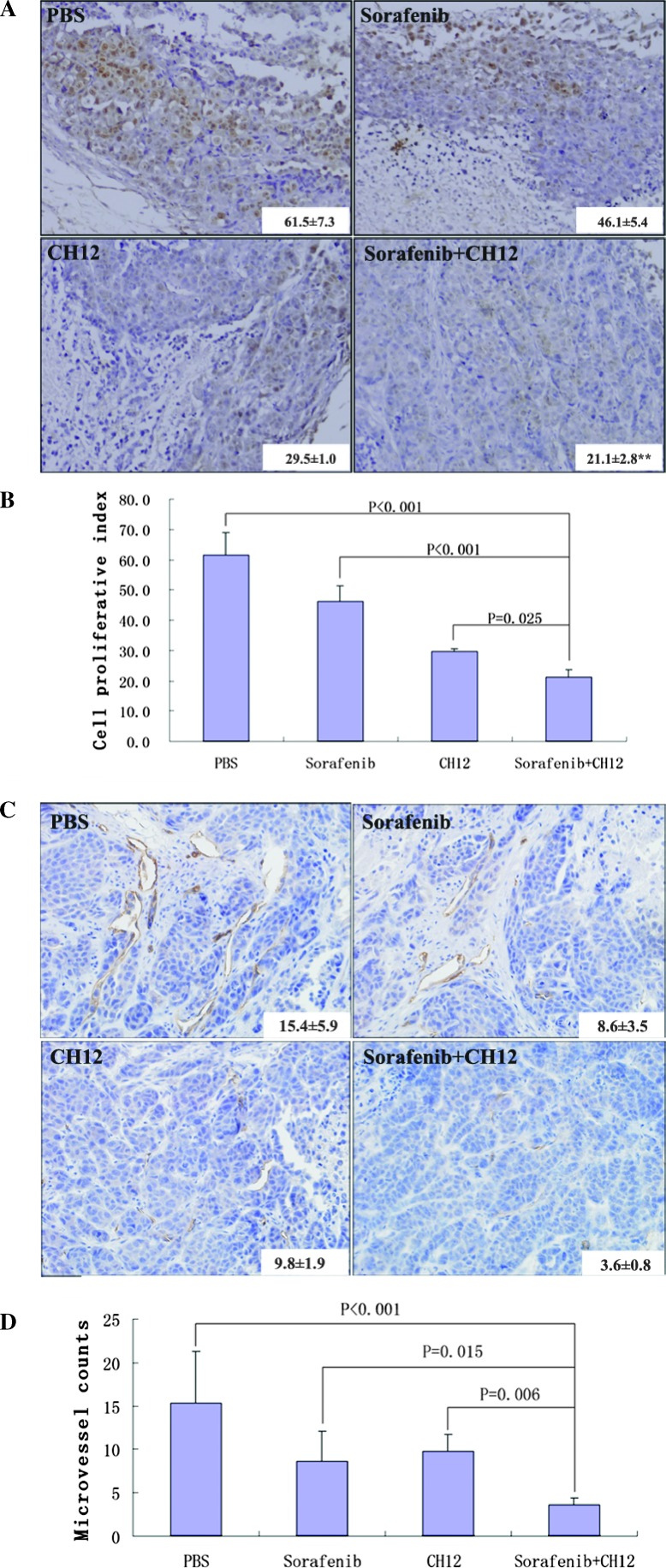
Combined Treatment of Sorafenib and CH12 Reduced the Cell Proliferation and Angiogenesis and Enhanced Apoptosis in Huh-7-EGFRvIII Tumors
To further elucidate the mechanism underlying the in vivo growth suppression caused by CH12 and sorafenib, we determined the proliferative rate and angiogenesis of tumors in the control and treated mice. In xenograft tumors, the proliferative index, measured with Ki-67 staining, was significantly lower for the CH12-plus-sorafenib combination-treated tumors than the vehicle-treated control tumors (P < .01; Figure 5, A and B) and CH12- or sorafenib-treated tumors (P < 05; Figure 5, A and B).
Figure 5.
Sorafenib plus CH12 treatment leads to the decreased growth of Huh-7-EGFRvIII xenograft tumors. (A) Tumor sections were stained with Ki-67. (B) The cell proliferative index was assessed as the percentage of total cells that were Ki-67-positive in six randomly selected high-power fields (x400) in xenografts from six mice of each group. (C) Tumor sections were immunostained with anti-CD34 antibody. Microvessel density values were analyzed by measuring the number of stained microvessels from six randomly selected fields (x200) in xenografts from six mice of each group. (D) Qualitative analysis of tumor vascularization. Data are means ± SE. All of the data were analyzed with the ANOVA. P < .05 was considered statistically significant.
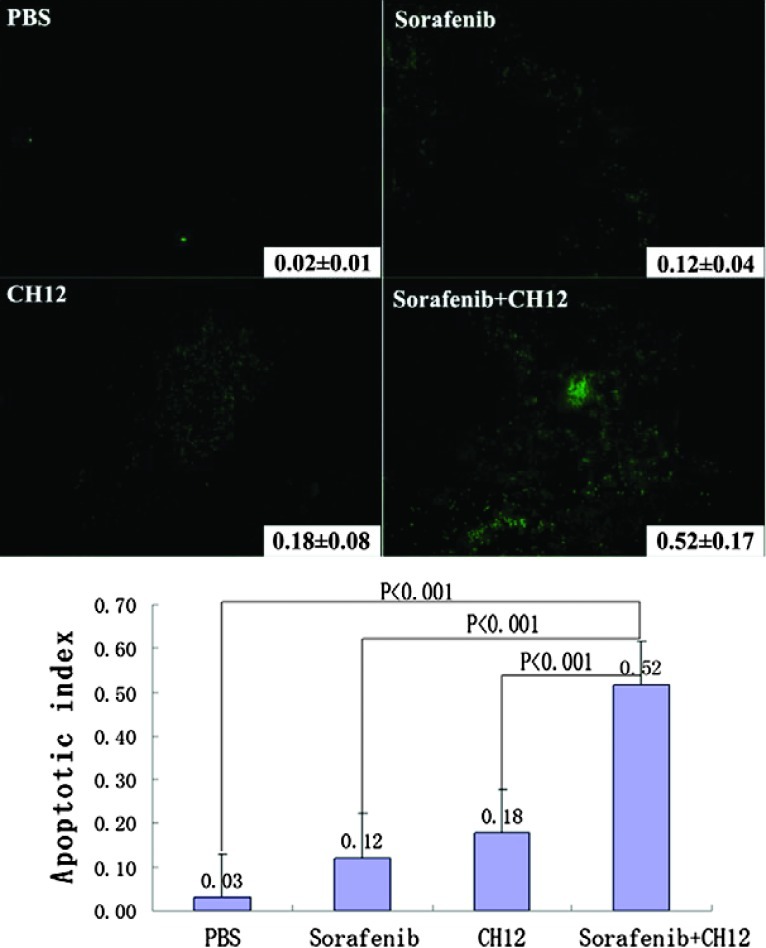
The extent of tumor vascularization was analyzed by immunostaining of tumors from treated and control groups for CD34. Fewer CD34-positive tumor microvessels were observed in the CH12-plussorafenib group than in the other three groups (P < .05; Figure 5, C and D). In addition, analysis of the apoptotic index through TUNEL staining showed a significant increase in the number of apoptotic cells in the group of sorafenib in combination with CH12 as compared with the other three groups (P < .01; Figure 6).
Figure 6.
Sorafenib plus CH12 treatment leads to an increase in apoptosis of Huh-7-EGFRvIII xenograft tumors. Apoptotic cells were detected using the TUNEL assay. The apoptotic index was assessed by the ratio of TUNEL-positive cells to the total number of cells from six randomly selected high-power fields (x400) in xenografts from six mice of each group. Data are mean ± SE. All data were analyzed by ANOVA. P < .05 was considered statistically significant.
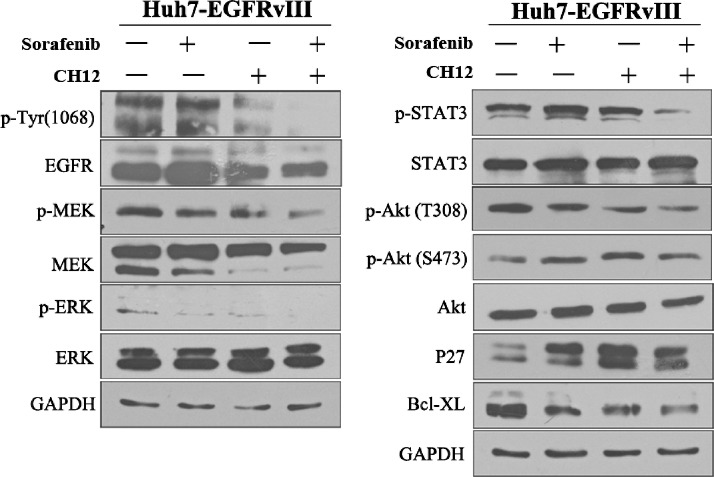
Molecular Mechanisms Underlying the Additive Antitumor Effect of Sorafenib Plus CH12 In Vivo
To understand the molecular events occurring in sorafenib-plus-CH12-treated tumors, some key signaling molecules were examined with Western blot analysis (Figure 7). As Figure 7 shows, compared with the other three groups, the p-EGFR, p-ERK, p-MEK, p-Akt (Thr308), and p-STAT3 levels were dramatically downregulated in the CH12-and-sorafenib combination therapy group, which is consistent with the results in vitro. However, we did not observe the obvious change in p-Akt (Ser473) in the combination treatment of sorafenib with CH12. In our previous study, CH12 treatment enhanced the expression of p27, a negative regulator of the cell cycle, and reduced the expression of Bcl-xL, which can inhibit apoptosis [19]. Therefore, in this study, we also determined the p27 and Bcl-xL status in the CH12-plus-sorafenib-treated tumors. We found that Bcl-xL was more noticeably downregulated, whereas the up-regulation of p27 was not obvious in the combination therapy group compared with the CH12- or sorafenib-treatment-alone groups (Figure 7).
Figure 7.
Mechanisms of antitumor activity after treatment with sorafenib plus CH12. Established Huh-7-EGFRvIII xenografts treated with PBS, sorafenib, CH12, or the combination were excised and prepared through homogenization in cell lysis buffer. Tumor lysates (20 µg) were then subjected to SDS-PAGE and immunoblotted for total EGFR, p-EGFR (Tyr1068), totalMEK, p-MEK, total ERK, p-ERK, total Akt, p-Akt (Thr308), p-Akt (Ser473), total STAT3, p-STAT3, P27, and Bcl-XL, as indicated.
Discussion
HCC is a heterogeneous malignancy with complex carcinogenesis [20]. Patients who have advanced HCC are not amenable to treatment with surgery, local ablation, or regional therapy. The chemotherapy for advanced HCC is still unsatisfactory. These issues have led to a search for novel approaches for HCC therapy, including the targeting of the EGFR (e.g., with erlotinib and cetuximab), vascular endothelial growth factor (bevacizumab), or the Raf/MEK/ERK pathway (sorafenib). The multikinase inhibitor sorafenib is the first oral agent to show clinical activity against human HCC. Although the clinical application of sorafenib has shown good tolerability in the studied populations, it also causes multiple toxicities, and a large percentage of patients have to be treated with a reduced dose or stop taking the drug for this reason [25]. This toxicity was the stimulus for us to search for agents that could act additively with sorafenib to enhance its HCC growth inhibition, which could reduce the dosage of sorafenib and thus decrease its adverse effects.
Recently, some studies reported that sorafenib in combination with EGFR inhibitors had synergistic antitumor activity in human non-small cell lung cancer, colorectal cancer, and HCC cells [26,27]. The possible mechanisms were that sorafenib could inhibit the activation of vascular endothelial growth factor-dependent signaling, which is responsible for the intrinsic resistance to anti-EGFR therapies [26], or EGFR inhibitors could restrict the activation of p-ERK and p-AKT, which leads to sorafenib resistance [27]. As demonstrated in our previous report, EGFRvIII expression can decrease the sensitivity of HCC cell lines to a chemotherapeutic drug [18]. In this study, we also observed that EGFRvIII expression reduced the sensitivity of HCC cells to sorafenib. Recently, we developed a mouse-human chimeric monoclonal IgG1 antibody CH12, which is a promising biotherapeutic agent for cells expressing EGFRvIII. CH12, which inhibits the phosphorylation of constitutively active EGFRvIII and its downstream signaling, may be combined with sorafenib to enhance its antitumor effects. The present study shows for the first time that the combination of sorafenib and the monoclonal antibody CH12 can additively inhibit the growth of HCC cells expressing EGFRvIII both in vitro and in vivo. Furthermore, we elucidated the possible mechanisms responsible for this additive interaction.
A previous study reported that the combination of sorafenib (30 mg/kg) plus clodrolip or zoledronic acid could significantly induce HCC xenograft tumor growth inhibition [28]. Here, our study showed that the treatment of Huh-7-EGFRvIII xenografts with CH12 plus a low concentration of sorafenib (10 mg/kg) resulted in significant tumor growth inhibition of approximately 85.4%, which was more effective than that of the single agents.
Many studies have shown that sorafenib inhibits Raf kinase and thus blocks MEK/ERK signaling in HCC cells [29,30]. In this study, we also found that p-ERK was inhibited by both sorafenib and CH12 alone in Huh-7-EGFRvIII and SMMC-7721 cells, but CH12 seemed to promote sorafenib inhibition of the MEK/ERK pathway. Akt plays a critical role in cell survival and acts as a mediator of phosphoinositide 3-kinase (PI3K) signaling [31]. Recently, it has been proposed that Akt may be one of the main mediators of sorafenib resistance, although several studies have produced controversial results. Fujimaki et al. [24] revealed that sorafenib significantly decreased the levels of p-Akt (Thr308) and increased the levels of p-Akt (Ser473) in both HepG2 and PLC/PRF/5 cells. In the present study, our data illustrate that sorafenib alone or in combination with CH12 can downregulate the levels of p-Akt (Thr308) both in Huh-7-EGFRvIII and in SMMC-7721 cells. But sorafenib alone can obviously increase the levels of p-Akt (Ser473) in Huh-7-EGFRvIII cells, although we did not find the same trend in SMMC-7721 cells. We consider that the status of p-Akt activation might not be the same in different types of HCC cells. In addition, Thr308 is phosphorylated by PDK1, a key component of PI3K signaling [32,33], whereas Ser473 is phosphorylated by the elusive PDK2, which was proposed to be mTOR complex 2 including rictor and Sin1 [34]. Thus, we surmise that CH12 enhances the antitumor effect of sorafenib at least partially through the PI3K/AKT (Thr308) pathway. STAT3 has been implicated in the transcription of genes that are associated with cell proliferation and survival, and its constitutive activation has been associated with a number of human epithelial cancers [35]. Recent reports have also indicated that STAT3 activation can be inhibited by sorafenib in glioblastoma and pancreatic cells [21,36]. Here, we investigated the activation status of STAT3 in Huh-7-EGFRvIII and SMMC-7721 cells or xenografts treated with sorafenib and CH12 either singly or in combination. Intriguingly, when sorafenib and CH12 were combined, the phosphorylation of STAT3 was noticeably decreased. However, there was no obvious down-regulation of p-STAT3 when both cell lines were treated with sorafenib or CH12 alone.
Our previous research found that CH12 had the same epitope as ch806 [37], which bound specifically to overexpressed and mutant EGFR. A phase 1 clinical trial indicated that ch806 showed excellent targeting to tumor sites in all patients, with no evidence of uptake by normal tissues and no significant toxicity [38]. In conclusion, we suggest that the combination of sorafenib plus CH12 is a candidate for clinical applications, either to permit the use of a lower dosage or to enhance clinical responses to sorafenib.
Abbreviations
- EGFR
epidermal growth factor receptor
- EGFRvIII
epidermal growth factor receptor variant III
- ERK
extracellular signal-regulated kinase
- HCC
hepatocellular carcinoma
- STAT3
signal transducer and activator of transcription 3
Footnotes
This study is supported by the “Twelfth Five-year Plan” for Science & Technology Research of China (grant no. 2012ZX10002015-007), the National Natural Science Foundation (grant no. 30901820), the Key Program Project of the Shanghai Science and Technology Committee (grant no. 10431903700), and the research fund of the State Key Laboratory of Oncogenes and Related Genes (grant no. 91-10-06).
References
- 1.Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44(suppl 19):96–101. doi: 10.1007/s00535-008-2258-6. [DOI] [PubMed] [Google Scholar]
- 2.Verslype C, Van Cutsem E, Dicato M, Arber N, Berlin JD, Cunningham D, De Gramont A, Diaz-Rubio E, Ducreux M, Gruenberger T, et al. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 10th World Congress on Gastrointestinal Cancer, Barcelona, 2008. Ann Oncol. 2009;20(suppl 7):vii1–vii6. doi: 10.1093/annonc/mdp281. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 4.Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? Oncologist. 2006;11:790–800. doi: 10.1634/theoncologist.11-7-790. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 8.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, et al. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 9.Gainet M, Guardiola E, Dufresne A, Pivot X. Epidermal growth factor receptors (EGFR): a new target for anticancer therapy [in French] Cancer Radiother. 2003;7:195–199. doi: 10.1016/s1278-3218(03)00019-2. [DOI] [PubMed] [Google Scholar]
- 10.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 11.Nygren P, Sorbye H, Osterlund P, Pfeiffer P. Targeted drugs in metastatic colorectal cancer with special emphasis on guidelines for the use of bevacizumab and cetuximab: an Acta Oncologica expert report. Acta Oncol. 2005;44:203–217. doi: 10.1080/02841860510029798. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen MW, Meltorn M, Damstrup L, Poulsen HS. The type III epidermal growth factor receptor mutation. Biological significance and potential target for anti-cancer therapy. Ann Oncol. 2001;12:745–760. doi: 10.1023/a:1011177318162. [DOI] [PubMed] [Google Scholar]
- 13.Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, Freilino ML, Graner MW, Wikstrand CJ, Bigner DD, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 14.Wikstrand CJ, Hale LP, Batra SK, Hill ML, Humphrey PA, Kurpad SN, McLendon RE, Moscatello D, Pegram CN, Reist CJ, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55:3140–3148. [PubMed] [Google Scholar]
- 15.Ou C, Wu FX, Luo Y, Cao J, Zhao YN, Yuan WP, Li Y, Su JJ. Expression and significance of epidermal growth factor receptor variant type III in hepatocellular carcinoma [in Chinese] Ai Zheng. 2005;24:166–169. [PubMed] [Google Scholar]
- 16.Zhou M, Gong B, Gu J, Li Z. EGFRvIII mRNA detection in the serum of patients with hepatocellular carcinoma. Liver Int. 2010;30:925–927. doi: 10.1111/j.1478-3231.2010.02233.x. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Zhang CQ, Feng KT, Li JQ. Clinical significance of EGFR and EGFRvIII expression in tumor and liver tissue of HCC [in Chinese] J SUN Yat-sen Univ (Med Sci) 2006;27:467–471. [Google Scholar]
- 18.Wang H, Jiang H, Zhou M, Xu Z, Liu S, Shi B, Yao X, Yao M, Gu J, Li Z. Epidermal growth factor receptor vIII enhances tumorigenicity and resistance to 5-fluorouracil in human hepatocellular carcinoma. Cancer Lett. 2009;279:30–38. doi: 10.1016/j.canlet.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Jiang H, Wang H, Tan Z, Hu S, Wang H, Shi B, Yang L, Li P, Gu J, Wang H, et al. Growth suppression of human hepatocellular carcinoma xenografts by a monoclonal antibody CH12 directed to epidermal growth factor receptor variant III. J Biol Chem. 2011;286:5913–5920. doi: 10.1074/jbc.M110.192252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 21.Huang S, Sinicrope FA. Sorafenib inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in human pancreatic cancer cells. Mol Cancer Ther. 2010;9:742–750. doi: 10.1158/1535-7163.MCT-09-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 23.Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A, Evers BM. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:4951–4958. [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimaki S, Matsuda Y, Wakai T, Sanpei A, Kubota M, Takamura M, Yamagiwa S, Yano M, Ohkoshi S, Aoyagi Y. Blockade of ataxia telangiectasia mutated sensitizes hepatoma cell lines to sorafenib by interfering with Akt signaling. Cancer Lett. 2012;319:98–108. doi: 10.1016/j.canlet.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors—a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009;10:470–481. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 26.Martinelli E, Troiani T, Morgillo F, Rodolico G, Vitagliano D, Morelli MP, Tuccillo C, Vecchione L, Capasso A, Orditura M, et al. Synergistic antitumor activity of sorafenib in combination with epidermal growth factor receptor inhibitors in colorectal and lung cancer cells. Clin Cancer Res. 2010;16:4990–5001. doi: 10.1158/1078-0432.CCR-10-0923. [DOI] [PubMed] [Google Scholar]
- 27.Blivet-Van Eggelpoel MJ, Chettouh H, Fartoux L, Aoudjehane L, Barbu V, Rey C, Priam S, Housset C, Rosmorduc O, Desbois-Mouthon C. Epidermal growth factor receptor and HER-3 restrict cell response to sorafenib in hepatocellular carcinoma cells. J Hepatol. 2012 doi: 10.1016/j.jhep.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by anti-metastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 30.Wei G, Wang M, Hyslop T, Wang Z, Carr BI. Vitamin K enhancement of sorafenib-mediated HCC cell growth inhibition in vitro and in vivo. Int J Cancer. 2010;127:2949–2958. doi: 10.1002/ijc.25498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YL, Law PY, Loh HH. Inhibition of PI3K/Akt signaling: an emerging paradigm for targeted cancer therapy. Curr Med Chem. 2005;5:575–589. doi: 10.2174/156801105774574649. [DOI] [PubMed] [Google Scholar]
- 32.Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 33.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 34.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 35.Kim DJ, Chan KS, Sano S, Digiovanni J. Signal transducer and activator of transcription 3 (STAT3) in epithelial carcinogenesis. Mol Carcinog. 2007;46:725–731. doi: 10.1002/mc.20342. [DOI] [PubMed] [Google Scholar]
- 36.Yang F, Brown C, Buettner R, Hedvat M, Starr R, Scuto A, Schroeder A, Jensen M, Jove R. Sorafenib induces growth arrest and apoptosis of human glioblastoma cells through the dephosphorylation of signal transducers and activators of transcription 3. Mol Cancer Ther. 2010;9:953–962. doi: 10.1158/1535-7163.MCT-09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Jiang H, Shi B, Wang H, Li J, Wang H, Yao M, Li Z. Identification and characterization of Ch806 mimotopes. Cancer Immunol Immunother. 2010;59:1481–1487. doi: 10.1007/s00262-010-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, Skrinos E, Murone C, Saunder TH, Chappell B, et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci USA. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]