Abstract
The amyloid β-protein (Aβ), which is present predominately as a 40- or 42-residue peptide, is postulated to play a seminal role in the pathogenesis of Alzheimer's disease (AD). Folding of the Aβ21–30 decapeptide region is a critical step in the aggregation of Aβ. We report results of constant temperature all-atom molecular dynamics simulations in explicit water of the dynamics of monomeric Aβ21–30 and its Dutch [Glu22Gln], Arctic [Glu22Gly], and Iowa [Asp23Asn] isoforms that are associated with familial forms of cerebral amyloid angiopathy and AD. The simulations revealed a variety of loop conformers that exhibited a hydrogen bond network involving the Asp23 and Ser26 amino acids. A population of conformers, not part of the loop population, was found to form metastable β-hairpin structures with the highest probability in the Iowa mutant. At least three β-hairpin structures were found that differed in their hydrogen bonding register, average number of backbone hydrogen bonds, and lifetimes. Analysis revealed that the Dutch mutant had the longest β-hairpin lifetime (≥500ns), closely followed by the Iowa mutant (≈500ns). Aβ21–30 and the Arctic mutant had significantly lower lifetimes (≈200ns). Hydrophobic packing of side chains was responsible for enhanced β-hairpin lifetimes in the Dutch and Iowa mutants, whereas lifetimes in Aβ21–30 and its Arctic mutant were influenced by the backbone hydrogen bonding. The data suggest that prolonged β-hairpin lifetimes may impact peptide pathogenicity in vivo.
Keywords: Amyloid β-protein, molecular dynamics, Alzheimer's disease, nucleation, protein folding, lifetimes, aggregation
Introduction
Alzheimer's disease (AD) is a progressive, age-associated, neurological disorder that is rapidly reaching epidemic proportions. The pathognomonic signs of AD are extracellular amyloid plaques and intraneuronal neurofibrillary tangles that are composed of the amyloid β-protein (Aβ) and tau protein, respectively. Progression of AD is associated with profound neuronal loss, the precise cause of which remains enigmatic. Historically, amyloid plaques were thought to cause AD1–4. However, accumulating evidence suggests that the dominant neurotoxins in AD are oligomeric forms of Aβ5–13. If so, then understanding the mechanisms of the toxicity of Aβ oligomers holds therapeutic potential.
Achieving this understanding requires the determination of the structures of the toxins and a correlation of structure with neurotoxicity. Experimental and computational studies have addressed possible mechanisms of Aβ folding and oligomer formation for both full-length alloforms, Aβ1–40 and Aβ1–42 (e.g. Refs.9,14 and references therein). Precise experimental determination of the structure of low-order oligomers, and even the monomer, has proven to be difficult because Aβ belongs to the class of intrinsically disordered proteins15,16. Even partially ordered Aβ conformers are transient and unstable17,18. A zero-length chemical cross-linking approach has enabled the stabilization of Aβ1–40 oligomers, the isolation of pure Aβ1–40 monomers, dimers, trimers, and tetramers, and the determination of order-specific neurotoxicity19. In general, the tendency of Aβ to aggregate makes experimental studies particularly difficult20–23. For this reason, many experimental and computational studies have focused on fragments of Aβ that preserve key properties of the full-length alloforms while aggregating less rapidly24–36. This preservation of structure in fragments, however, is not universal37.
An important fragment is the Aβ21–30 decapeptide. Using limited proteolysis and solution NMR, Lazo et. al.38 have shown that this region within both Aβ1–40 and Aβ1–42 monomers is protease-resistant. Surprisingly, the isolated decapeptide also is protease resistant, suggesting that it functions as an Aβ monomer folding nucleus. Subsequent computational studies revealed the presence of bends or loops stabilized by hydrophobic packing between side chains of Val24 and Lys2839–42. A combined experimental and computational study using solution NMR and replica exchange MD (REMD) simulations concluded that the Aβ21–30 fragment existed mostly (but not entirely) in unstructured conformations, lacking any secondary structure or persistent hydrogen bonding (HB) network, but showing evidence for an Asp23-Lys28 salt bridge (SB) formation that could stabilize the Aβ fibril structure43. Intriguingly, a minority population of conformers contained a β-turn centered at Val24-Gly25.
Further clues to the mechanistic bases of Aβ-induced disease come from families in which mutations in the structural gene for Aβ, the amyloid β-protein precursor gene (APP), cause familial forms of AD (FAD) and cerebral amyloid angiopathy (CAA). In these cases, a number of single amino acid mutations within Aβ at positions 22 and 23 result in more aggressive forms of AD and in vitro aggregate more readily than the wild type (WT) Aβ. Examples include the Arctic [Glu22Gly], Dutch [Glu22Gln], and Iowa [Asp23Asn] mutants44–50. Experimental and computational studies on Aβ21–30 decapeptides containing these amino acid substitutions showed that the turn in the Val24-Lys28 region was destabilized, which resulted in intramolecular interactions between the Aβ21–30 region and the rest of the full-length peptide that may facilitate oligomerization and fibril formation51. A follow-up computational study suggested that mutations at position 23 produced peptide conformers different from those produced by mutations at position 2252. Ion mobility spectrometry coupled with computational studies further found that a HB network involving Asp23 contributed substantially to turn formation and stability, suggesting that mutations at position 23 (Iowa) led to significant structural changes, whereas mutations at position 22 ([Glu22Gln], [Glu22Gly], [Glu22Lys]) led to only subtle variations to the overall folded structure of Aβ21–3053.
In the Aβ system, folding units observed in the Aβ monomer or sub-regions thereof can be maintained in higher-order assemblies. Solid state NMR of fibrils formed by full-length Aβ1–40 showed that the fibrils comprise two β-strand regions (12–24 and 30–40) connected by a bend in the 25–29 region. This bend brought the two β-strands together to form parallel β-sheets through side chain-side chain interaction54,55. Several computational studies of full-length Aβ showed significant structure, such as β-strands, in several regions of the peptide, with some of them spanning the 21–30 decapeptide region56–62. However, other studies did not report any β-structure63,64.
Additional experimental studies supported the importance of the 21–30 peptide turn in controlling aggregation propensity. Aβ1–40 with a lactam (Asp23/Lys28) bridge showed that such a pre-formed bend/turn increased fibrillogenesis by a factor of 1000 relative to the WT peptide65. Also, by forming a disulfide bond between amino acids 21 and 30 (using cysteine substitutions: [Ala21Cys] and [Ala30Cys]), full-length Aβ monomers formed a β-hairpin with strands comprising amino acids 17–23 and 30–36 that resulted in toxic oligomeric species66. Interestingly, this disulfide bond prevented further assembly of these oligomers into amyloid fibrils suggesting that if there was structure in the unmodified 21–30 decapeptide region that initially forms and nucleates the folding of the Aβ, this structure was metastable. If an intermediate, but benign, structure within the 21–30 decapeptide region could be stabilized, this would be a new therapeutic option, as it could destabilize a potentially toxic state67. A recent study has shown that maintenance of a benign conformational state in the transthyretin tetramer may be an effective strategy for treating diseases like familial amyloid polyneuropathy or systemic amyloidosis68.
Here, using constant temperature all-atom MD in explicit water, the dynamics of Aβ21–30 and its Dutch, Arctic, and Iowa mutants was examined. The decapeptide structural landscape comprises a variety of loop structures and at least three types of metastable β-hairpins. Differences in the structure and lifetimes of these metastable β-hairpins were observed among the four different decapeptides. These different β-hairpins could play a critical role in nucleating full-length Aβ folding while the differences in structure and lifetimes reported here could be responsible for distinct folding pathways and aggregation propensity for each of these decapeptides.
Methods
MD Simulations
Long-time constant temperature MD simulations of the decapeptide Aβ21–30 monomer in explicit TIP4P69 water were performed. All atoms were explicitly considered with potential energies given by the OPLS/AA70 force field within the GROMACS 4.0.571,72 package and using the NPT ensemble73. The system was confined in a cubic box with periodic boundary conditions. MD simulations were carried out at a constant temperature of T=283K corresponding to in vitro experiments38. In the simulations, amino acids were numbered sequentially starting from Ala21 through Ala30, corresponding to position 21 through 30 of the full length amyloid β-protein. The WT Aβ21–30 had the primary structure Ala-Glu-Asp-Val-Gly-Ser-Asn-Lys-Gly-Ala, where Glu22 and Asp23 were negatively charged and Lys28 was positively charged. Following Lazo et al38, the Aβ21–30 was blocked with NH3+ and CO2− at the N− and C− termini, respectively. Simulations with the Arctic [Glu22Gly], Dutch [Glu22Gln], and Iowa [Asp23Asn] mutations were also considered. In each case, the peptide was initially solvated by inserting it in the center of a previously-equilibrated cube of water molecules of side 43 Å. This insertion deleted all water molecules overlapping or in close proximity (<2.4Å) to any of the monomer atoms, resulting in a system with an approximate number of water molecules of 2,500. To maintain system neutrality (for WT only), a single Na+ ion was inserted far from the peptide.
The SETTLE algorithm74 was used to hold constant the covalent bonds of the water molecules. The bonds involving hydrogens were constrained according to the LINCS protocol75. Neighbor lists updated every 10 simulation steps were used for the non-bonded interactions. To calculate the electrostatic interactions, a Particle Mesh Ewald method76 was used with a cut-off distance of 10 Å for non-bonded interactions and a 1.2 Å grid spacing for the Fourier transform in the reciprocal space for all trajectories. A timestep of 2 fs was used in all trajectories. The temperature was controlled by coupling the system to an external bath73,77. The energy of the system was first minimized for 20,000 steps by applying the conjugate gradient algorithm that relaxed all atoms except the Cα atoms. Next, the Cα atoms were released and minimized again for another 20,000 steps. The system was gradually heated in the NVT ensemble from 0 to 283K by harmonically constraining all Cα atoms for 100 ps. Following this step, and still constraining the Cα atoms, another 200 ps were performed in the NPT ensemble at 283 K and 1 atm, followed by a brief 50 ps NPT simulation with no constraints to generate the starting configuration for the production runs. Because of the way that the overlapping water molecules were deleted in the solvation step above, these NPT equilibration steps allowed for water molecules to fill in holes left from the insertion of the decapeptide, thus shrinking the size of the system by about 1 Å in all directions.
To study the dynamics of the WT decapeptide, 34 trajectories were generated with total running times between 100 and 300 ns, resulting in 8.8 µs of total simulation time. The initial conformations for these trajectories were selected from previously-generated stretched chain conformations (conformations with large Rg). Water molecules were given different velocities in each trajectory (based on a Maxwell velocity distribution), making the trajectories independent of each other. The number of trajectories for each of the mutant peptides, which were initially in a stretched chain conformation, was: (i) 7 trajectories for the Arctic mutant, resulting in 710 ns, (ii) 7 trajectories for the Dutch mutant, resulting in 700 ns, and (iii) 8 trajectories for the Iowa mutant, resulting in 900 ns of simulation time. These trajectories had total running times between 100 ns and 200 ns each.
Trajectories to estimate lifetimes of β-hairpin structures (described below) were carried out starting from the pre-formed β-hairpin under consideration. The time at which this β-hairpin would unfold was taken as the breaking time, τi for that trajectory labeled i. A total of 10 trajectories per β-hairpin conformation were performed for each of the four variants (WT, Arctic, Dutch, and Iowa), resulting in 120 additional trajectories. Because these unfolding events were independent of each other, individual values for τi were the result of a Poisson process in which state A (β-hairpin) could change into state B (unstructured) with a constant probability per unit time, with τi belonging to an exponential distribution. Each β-hairpin lifetime was thus given by the characteristic time of an exponential fit to the distribution of all breaking times {τi}. To minimize error, the fitting was performed by finding distributions of breaking times {τi} using 3 to 7 bins. This produced five distributions for which exponential fits yielded five corresponding lifetimes. The lifetime for each β-hairpin was then obtained by averaging these five individual lifetimes and obtaining standard deviations. Because each trajectory would be carried out until its β-hairpin structure unfolded, total trajectory times varied but remained in the range from 100 ns to 400 ns. In less than 6% of the trajectories β-hairpin structures did not unfold at the 400 ns maximum trajectory length. Total accumulated simulation time for this β-hairpin lifetime analysis amounted to 16.5 µs. Additionally, one replica-exchange MD simulation was performed to test the stability of the β28 hairpin conformation (see Supplementary Material (SM) section), amounting to 1.2 µs of total accumulated running time.
In total, this paper presents data derived from more than 28.8 µs in simulated trajectory times when considering all trajectories used to study the extended conformations and β-hairpin simulation trajectories.
Structural Determinants
To quantify average structures of the decapeptides, contact maps were constructed by counting the number of Cα-Cα distances between different pairs of amino acids that would fall below 7Å (creating a contact) and dividing this number by the total number of frames in the trajectories. Similarly, HB contact maps were calculated by counting the number of particular HBs and dividing by the total length of simulations. A HB was defined if the distance between the donor and acceptor was ≤3Å and the angle θ between the donor-hydrogen-acceptor atoms was in the range 160° ≥ θ ≤ 200°. If, in addition, two hydrogen-bonded atoms were of opposite charges, they formed a SB78.
To quantify changes in structure of the decapeptide as a function of time, the radius of gyration Rg and specific distances between atoms were measured and labeled using the notation R(x,y), were R was the distance between amino acid x and amino acid y at a particular time41. The distances that were taken into consideration were: (i) R(4,8) and R(3,6), the distances between the two Cα atoms of Val24 and Lys28, and Asp23 and Ser26, respectively (ii) R(2,8) the distance between Lys28(Nζ) and Glu22(Cδ), and (iii) R(3,8) the distance between Lys28(Nζ) and Asp23(Cδ).
The combined solvent accessible surface area (SASA), S(x,y), between amino acids x and y79 were calculated to monitor for hydrophobic contact formation using the VMD package80. Of particular importance was S(4,8), the combined SASA of the Val24 and Lys28 side chains, the S(3,6), S(3,7), and S(2–4,6–8). The S(2–4,6–8) represented the total SASA of the decapeptide excluding the glycines and the terminal alanines. The secondary structure propensity per amino acid was calculated by using the program STRIDE81 within VMD. The quantity D, used to measure correlations between relative orientations of side chains of two amino acids, was obtained by calculating the normalized scalar product between the vectors defined by the direction of the side chains of Val24 and Lys2841.
To find representative conformations from a trajectory, a cluster algorithm based on the matrix of RMSDs as a measure of distance between two peptide conformations was used82. In this algorithm a representative conformation was found by first measuring all mutual distances (defined by their RMSD distance) between conformations. Given an upper cutoff for this distance, neighborhoods of common conformations were defined and the one with the largest number of conformations selected. Within this selected neighborhood (cluster), the central structure (centroid) was chosen as a representative conformation. This entire neighborhood of conformations was then removed from the pool of conformations and the algorithm was repeated. In the present work, RMSDs were measured only taking into account the backbone Cα atoms of amino acids 22 through 28. A cluster was defined as that in which neighboring conformations had an RMSD equal or less than 1 Å.
Results
We first present distance and HB contact maps for each decapeptide variant, which are used to identify the main (whole-trajectory average) structural elements of the four decapeptides. Spontaneously formed, metastable, β-hairpin structures appearing in the trajectories are then characterized using secondary structure and hydrogen bonding analyses. Finally, the lifetimes of these β-hairpin structures are obtained and discussed in terms of hydrophobicity, electrostatic interactions, and hydrogen bonding.
Common Folding Structure of Aβ21–30 and Its Mutants
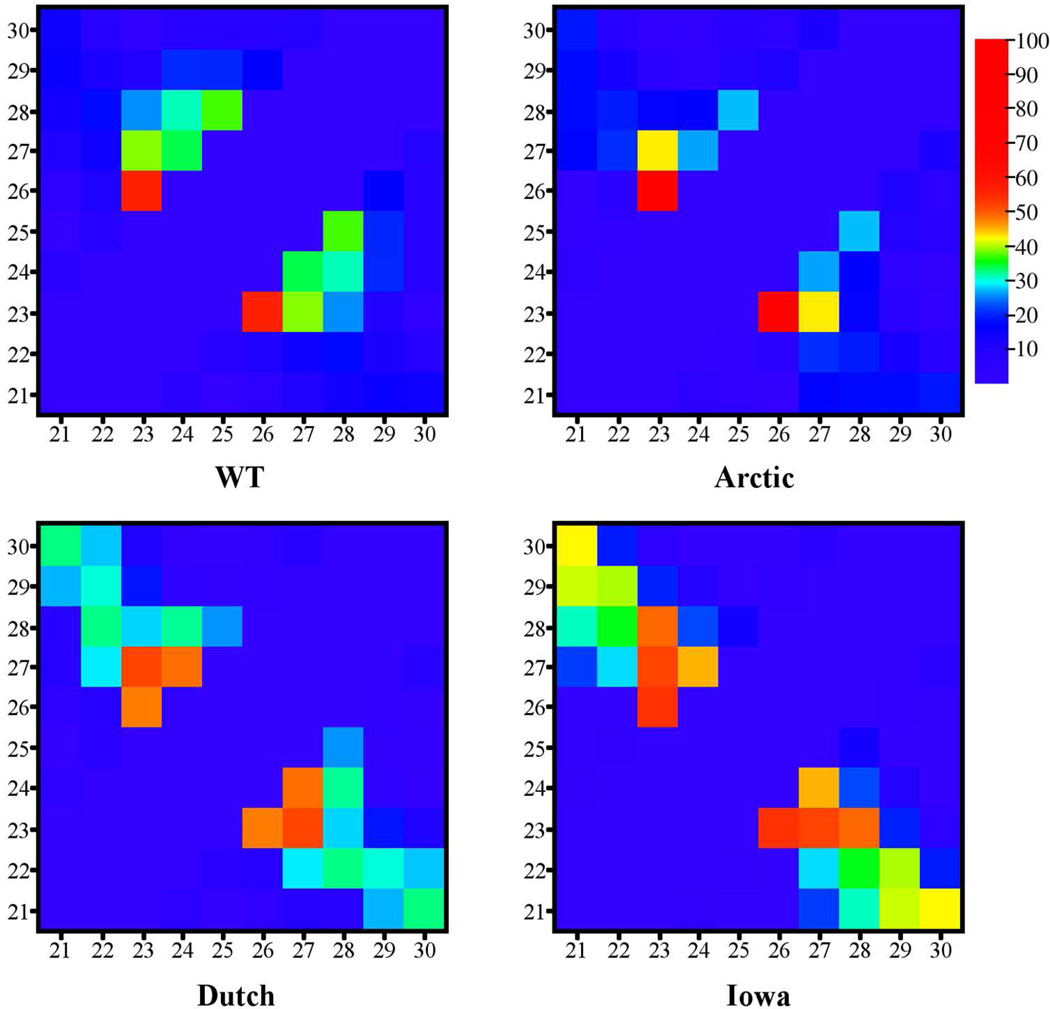
In Figure 1, contact maps between pairs of Cα atoms are shown for the WT and mutant decapeptides. The values of the contacts were calculated as the percentage of the trajectory over which the Cα-Cα distance between amino acids was less than 7 Å. These maps showed that contacts between Asp23 and other inner amino acids (Ser26 and Asn27) were the most prevalent (strongest) for all decapeptide types, suggesting that interactions in the interior of the decapeptides might play a pivotal role in the decapeptide structure. The next four strongest contacts were: Gly25-Lys28, Asp23-Lys28, Val24-Asn27, and Ala21–Ala30 (see Supplementary Material (SM) Table A for specific values). Schematic diagrams in SM Figure I illustrate the location and strength of these contacts. The diagonal feature in the contact map of the Iowa mutant (Figure 1) is indicative of a β-hairpin structure and is discussed below. These data show strong contacts between the inner amino acid Cα atoms that reflected a persistent unstructured turn or loop in the central region in all decapeptide variants.
Figure 1.
Contact maps for the WT (upper left), Arctic (upper right), Dutch (lower left), and Iowa (lower right) mutants where the colors represent the percentage of the trajectory containing each contact. A contact was established whenever the distance between Cα-Cα atoms of different amino acids fell below 7Å. In each map, the lower left corner indicates the Ala21-Ala21 contact while the upper right corner indicates the Ala30-Ala30 contact. The color bar on the right indicates the conversion between the color of the contact and the trajectory percentage for that contact.
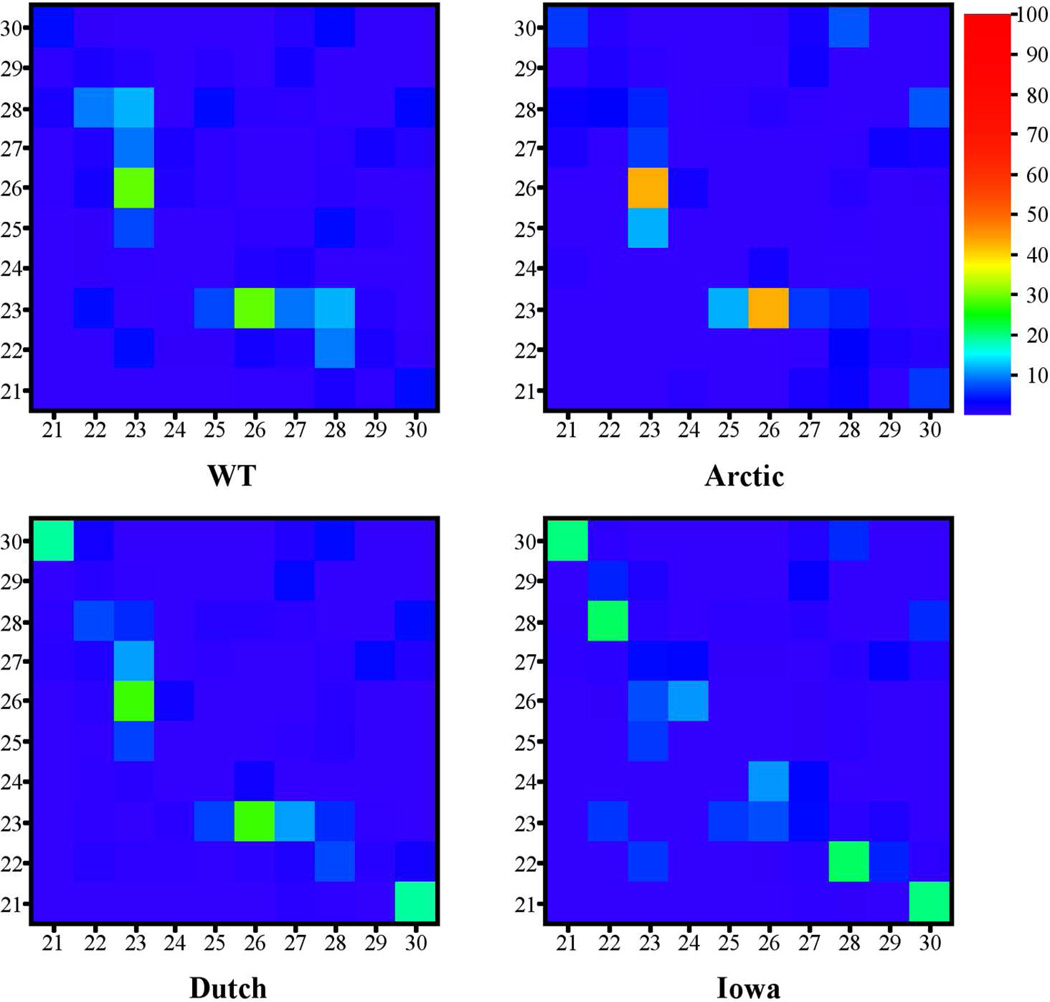
Next, hydrogen bonds between pairs of amino acids were analyzed. Figure 2 shows HB contact maps for all decapeptide types were each contact was calculated as the percentage occurrence of the contacts between pairs of amino acids (strongest HBs are listed in SM Table B). Similarly to Figure 1, the Asp23-Ser26 HB contact was very prominent in the WT, Arctic, and Dutch decapeptides. In the Iowa mutant, however, the dominant HB contact was Glu22-Lys28. Because the Iowa mutant is the only one without a charge at position 23, this result suggested that the absence of charge at position 23 facilitated HB formation. This point was more clearly illustrated when examining specific pairs of atoms forming the HBs (SM Table C). The prevalent HBs for the WT, Arctic, and Dutch decapeptides formed between Asp23(Oδ)-Ser26(H) and Asp23(Oδ)-Ser26(Hγ), unlike in the Iowa mutant. Furthermore, the accumulated percentage of HBs for the WT, Arctic, and Dutch mutants involving the charged Asp23(Oδ) were 54%, 62.7%, and 46.2%, respectively (SM Table C). For the Iowa mutant, the total percentage of HBs formed by the uncharged Asn23(Oδ) was only 9.1%. It is important to note that the absence of charge at position 23 in the Iowa mutant was not compensated by an increase of HB propensity of the charged Glu22(Oε) and formation of the Glu22(Oε)-Lys28(Hζ) SB, but rather by an increased number of backbone HBs. Thus, the absence of charge at position 23 (as exemplified by the Iowa mutant) but not at position 22 (as exemplified by the Arctic and Dutch mutants) changed the HB network and dynamics.
Figure 2.
HB contact maps for the WT (upper left), Arctic (upper right), Dutch (lower left), Iowa (lower right) mutants. The scale on the right indicates the conversion between the color and percentage that each pair of amino acids are connected by HBs.
Analysis of the average secondary structure of each amino acid over the entire trajectories revealed a turn between positions 23 and 27 and unstructured N- and C-termini (SM Figure II), consistent with the contact maps (Figures 1 and 2) that indicated persistent bonding among inner amino acids for all decapeptide variants. In addition, there was a small but statistically significant extended β structure involving amino acids at positions 22/23 and 27/28. The average amount of this extended β structure depended on the decapeptide variant, ranging from ≈5% (Dutch and Arctic) to >25% (Iowa). These extended β structures corresponded to three distinct sporadic types of β-hairpin structures differing by the register of their dominant backbone HBs and labeled β28, β29, and β18. The structure and dynamics of these β-hairpin structures are further examined in the following sections.
Role of Hydrogen Bonding and Hydrophobic Packing in Decapeptide Folding
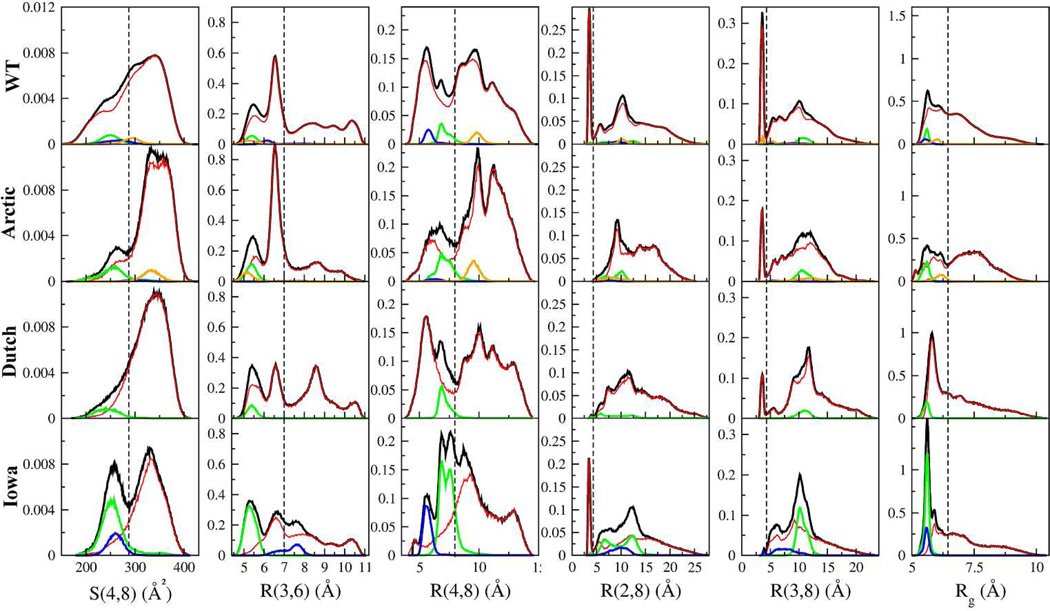
The contact maps of Figures 1 and 2 and secondary structure (SM Figure II) were derived by calculating time-averaged quantities using entire trajectories. These averages, however, do not accurately describe abundance nor frequencies of the different underlying structures since the distributions from which these averages were derived were typically multimodal and the HB contacts did not occur with equal probability throughout each trajectory. To analyze the relative contribution of these structure, histograms of distances between pairs of Cα carbons, radius of gyration (Rg), and the SASA value for S(4,8) for each of the four decapeptide variants were calculated and are shown in Figure 3.
Figure 3.
Normalized histograms of S(4,8), amino acid distances R(x,y), and radius of gyration Rg per mutation. Black lines correspond to each quantity calculated using entire trajectories, while blue, green, and orange lines are the individual contributions to the histograms from the β28, β29, β18 hairpins respectively. The red lines correspond to the histograms calculated excluding the β-hairpins. The dashed vertical lines used to demarcate regions in S(4,8), R(3,6), R(4,8), R(2/3,8), and Rg are at 287 Å2, 7 Å, 8 Å, 4.2 Å, and 6.3 Å, respectively.
For all four peptides, the S(4,8) histograms exhibited a peak at ≈340 Å2 (Figure 3, first column), which corresponded to the loop structure presented in the previous section. The other peaks at lower values of S(4,8) (between 240 and 260 Å2) in the Arctic, Iowa mutants, and to some extent also in the WT peptide, mostly corresponded to β-hairpin structures. The midpoint between the loop and these β-hairpin structures in these histograms was used to draw a dashed line at 287 Å2 as a value that separates these distinct populations. Because Val24 and the n-butyl portion of Lys28 are hydrophobic, the low values of S(4,8) indicated that the hydrophobic side chains were more effectively shielded from the solvent in the β28 and β29 hairpin structures than in the loop structures. However, the β18 hairpin structure in the Arctic had larger values of S(4,8). The uncharacteristic high abundance of β-hairpin structures in the Iowa mutant, first observed in Figure 1, gave rise to a double peaked distribution.
The R(3,6) distance histograms showed a bimodal distribution for R(3,6) < 7 Å in all decapeptides (Figure 3, second column). These two peaks were mostly populated by conformations with a high occurrence of HB pairs Asp23(Oδ)-Ser26(H) and Asp23(Oδ)-Ser26(Hγ) in the WT, Arctic, and Dutch mutants. These HBs were also among the dominant HBs (trajectory-wide) in these decapeptides (SM Table C) and their propensity within and among these peptides corresponded to the relative heights of the R(3,6) peaks. The high percentage of these HBs indicated that the R(3,6) distance was the dominant structural feature in these three decapeptides, also consistent with Figure 1. In contrast, while these two peaks in the Iowa mutant were dominated by structures exhibiting the HB pairs Asn23(Hδ)-Ser26(O) and Asn23(Oδ)-Ser26(H), these two HBs were not dominant when considering the whole trajectory (SM Table C). Instead, the dominant HBs dictating the prevalent structure in the Iowa (Figure 1) came from the large population of β-hairpin conformations found at the R(3,6) ≈ 5.4 Å peak (Figure 3, green line) sustained by the three dominant backbone HBs (listed in SM Table C). A broader conclusion from the data of the four decapeptides is that although HBs involving the Asp23-Ser26 amino acids dominated the R(3,6) structure, the fact that there was a bimodal distribution (R(3,6) ≈ 5.4 Å and 6.5 Å peaks) prevented the decapeptides from locking into a single conformation and allowed the loop to explore different, though related, structures. On the other side of the spectrum, the peak in the Dutch mutant at R(3,6) ≈ 8.5 Å originated from the Asp23(Oδ)-Asn27(H) and Asp23(Oδ)-Asn27(Hδ) HBs. This peak was the most predominant in the Dutch mutant because of the higher combined percentage of these two HBs as compared with the other three decapeptides (SM Table C).
The shapes of the R(4,8) distance distributions (Figure 3, third column, black lines) suggested two regions of low and high R(4,8) populations separated at R(4,8) ≈ 8 Å. This separation was consistent with the compact structure, and thus low R(4,8), of the β28 and β29 hairpins (green and blue lines) that were to the left of this separating line. In previous work41 this separating line was found at a smaller distance of 6.5 Å and was associated with hydrophobic events. However, because the R(4,8) distances did not dominate the structure of the decapeptides (suggested by the lack of Val24-Lys28 contacts in Figure 1), hydrophobicity as indicated by the S(4,8) played a reduced role in the present context. We note that the β18 conformations (orange lines in Figure 3) had increased values of R(4,8), which when combined with their high values of S(4,8) (e.g. in the Arctic, first column) suggested that these β18 hairpin structures might be more open that the β28 and β29 hairpins.
The histograms of the R(2,8) and R(3,8) distances showed the presence (or absence) of SBs between the negatively charged Glu22/Asp23 and positively charged Lys28 (Figure 3, fourth and fifth columns). These SBs were shown as sharp narrow peaks at a distance of ≈4 Å83. According to the charges of the Asp23 and Glu22, both SBs occurred in the WT peptide, whereas in the Arctic and Dutch mutant only the Asp23(Oδ)-Lys28(Hζ) SB formed, and in the Iowa mutant only the Glu22(Oε)-Lys28(Hζ) SB was present. The area below the SB peaks in the R(2,8) and R(3,8) distance histograms was proportional to the number of SBs formed. This in turn reflected a low rate of SB formation, which was consistent with the percentage of SB formation (SM Table C), suggesting a relatively small contribution of SBs to the decapeptide folding dynamics.
The Rg histograms (Figure 3, sixth column) for all of the decapeptide types included a peak at values of Rg<6.5 Å consisting of compact loop conformations as well as all β-hairpin structures. The area A under the curve of each Rg distribution (up to Rg = 6.5 Å) indicated that the level of compactness was variant-specific. The Arctic mutant was, on average, the least compact (A=0.39), followed by the WT peptide (A=0.49), the Dutch mutant (A=0.52), and the Iowa mutant (A=0.58). The second peak in the Arctic mutant for Rg > 6.5 Å arose from structures that also had a large value of S(4,8) (>287 Å2), further examined below.
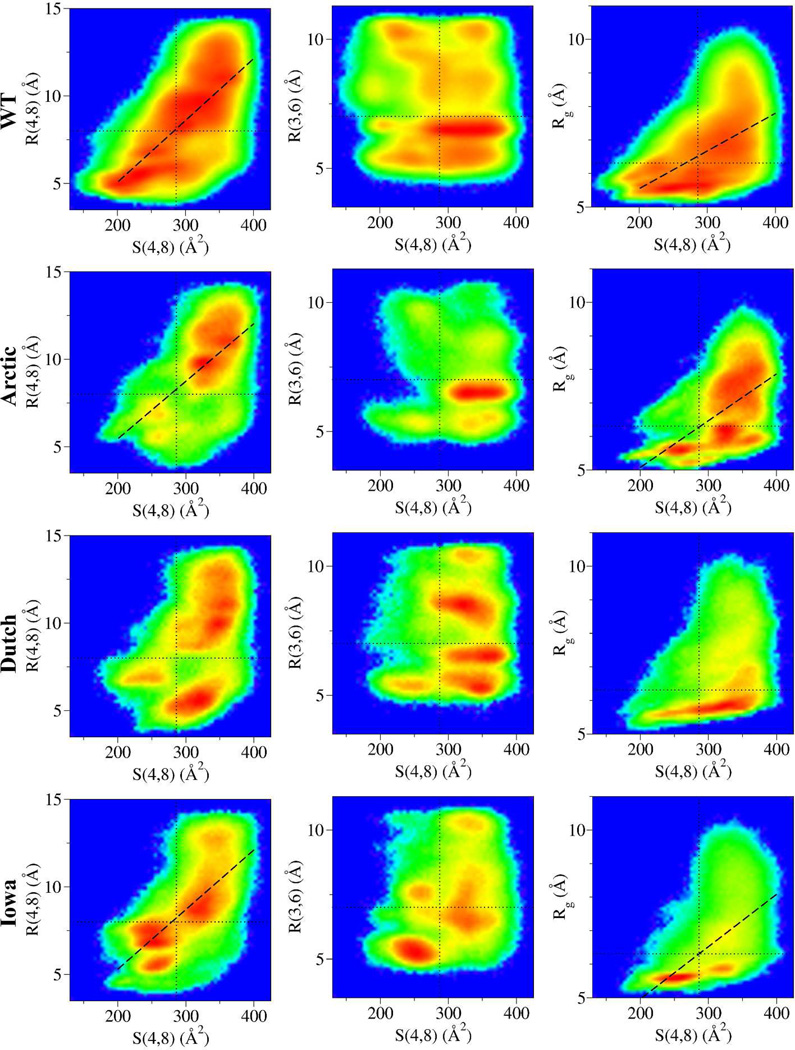
Next, possible correlations between key distances R (R(4,8), R(3,6), and Rg) from Figure 3 and the hydrophobic packing of Val24 and Lys28 side chains S(4,8) were examined. Two-dimensional correlation maps were constructed in which for a given pair of values [S(4,8), R] they: (i) counted the number of conformations simultaneously satisfying those two numbers; and (ii) plotted G[S(4,8), R], the normalized logarithm of this histogram (Figure 4). Data for G[S(4,8), R(4,8)] shown in the first column of Figure 4 showed that R(4,8) and S(4,8) were positively correlated in the WT, Arctic, and Iowa variants as indicated by their Pearson correlation coefficient r = 0.71, 0.59, and 0.68, respectively. The Dutch mutant, however, only showed a weak correlation with r = 0.45 where conformations with low R(4,8) and high values for S(4,8) (lower-right region) weakened the correlation. These results were preserved even when β-hairpin were excluded from the analysis (SM Figure III). The regions with large S(4,8) and R(4,8) values (upper-right) were the most populated in all peptides except in the Iowa mutant, which was characterized by a large population of β-hairpin structures with low S(4,8) and R(4,8) (lower-left region). This behavior was confirmed by calculating G[S(4,8), R(4,8)] excluding all β-hairpin structures (SM Figure III). In the second column of Figure 4, G[S(4,8), R(3,6)] was examined to see whether the dominant Asp23-Ser26 HBs were correlated with hydrophobic packing events. No significant correlation was found, demonstrating that regardless of the Asp23-Ser26 HB, most conformations had high S(4,8) values (also in SM Figure III). The exception was the Iowa mutant with the β-hairpin structures populating the low S(4,8)/low R(3,6) value region (compared to SM Figure III). An important feature is that the peaks in the distribution of R(3,6) (Figure 3) were here segregated into unconnected “islands” with respect to S(4,8) indicating distinct conformational subgroups differing not only by S(4,8) but also by their predominant HBs. In the third column, G[S(4,8), Rg] demonstrated a correlation between the compactness of the decapeptide structure and hydrophobic packing in the WT, Arctic, and Iowa variants with r = 0.57, 0.55, and 63, respectively. Again, in the Dutch mutant this quantity showed only a weak correlation with r = 0.35. However, when excluding in the analysis all of the β-hairpin structures, only the WT preserved a significant correlation with r = 0.54 (SM Figure III). This is in part because when excluding the β-hairpin structures, the remaining conformations mostly corresponded to high values of S(4,8) (first column of Figure 3, red curves) irrespective of compactness. Interestingly, the Dutch mutant with one of the most compact structures, even when excluding β-hairpin structures (Rg in Figure 3 black and red curves), had the weakest correlation between S(4,8) and Rg, indicating that even when considering the most compact conformations, as exemplified in the Dutch variant, they would not necessarily result in hydrophobic packing. Overall, these maps suggested that the WT decapeptide sampled a larger R and S(4,8) phase space than the mutants which were characterized by a more discrete set of preferred conformations in the form of highly populated phase space islands.
Figure 4.
Two-dimensional maps corresponding to the normalized logarithm of the number of configurations G[S,R] plotted according to their R(4,8), R(3,6), and Rg vs S(4,8) values using the data from Figure 3. The rainbow colormap corresponds to the highest number of conformations (red) to zero conformations (blue). Thus, a point in a map with value G[S,R] that is red indicates that that particular pair of R(x,y) and S(4,8) is populated by the maximum number of conformations. The dashed lines are linear regression fits to the data for only those data exhibiting significant correlations. For the WT G[S(4,8),R(4,8)] the Pearson correlation coefficient, r, is r = 0.71 and for the G[S(4,8), Rg] it is r = 0.57. For the Arctic G[S(4,8), R(4,8)] the correlation coefficient is r = 0.59 and for the G[S(4,8), Rg] it is r = 0.55. For the Iowa G[S(4,8), R(4,8)] the correlation coefficient is r = 0.68 and for the G[S(4,8), Rg] it is r = 0.63. The thin dotted vertical lines in the S(4,8) axis are centered at 287 Å2, while the horizontal lines at the R(4,8), R(3,6), and Rg axes are at 8, 7, and 6.3 Å, respectively.
Clustering Analysis
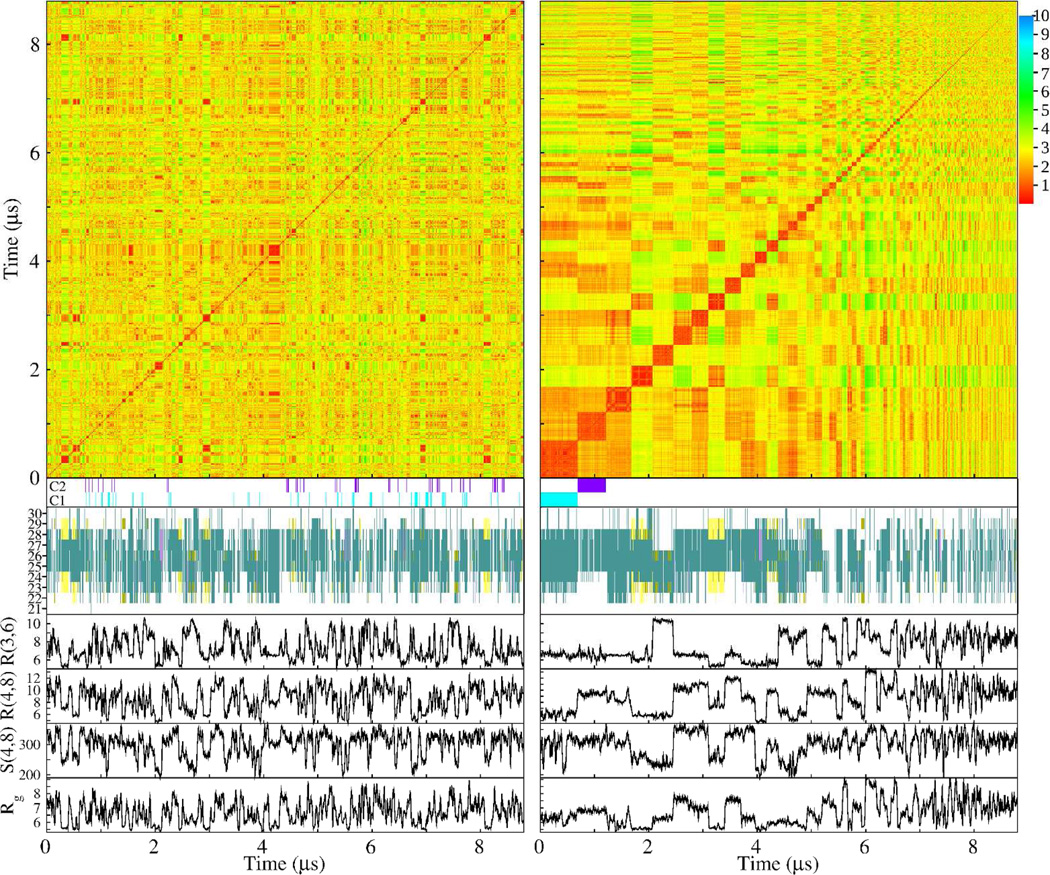
To test whether decapeptide structures could be classified in terms of representative conformations regardless of their R(4,8), R(3,6), Rg, or S(4,8) values, a clustering algorithm (described in the Methods section) was used. In Figure 5, the RMSD matrix of all concatenated trajectories of the WT is shown as a function of simulation time (upper left matrix). This matrix indicated, by using a colormap representation, the RMSD distance between any frame i (x-axis) and frame j (y-axis). Overall, this map indicated large variability of conformations with RMSD distances ranging from less than 1 Å to about 6 Å with small but significant clustering regions. To the right of this map the same data was now sorted according to the cluster to which each timeframe belonged (defined in Methods). The most populated cluster started at the (0,0) position followed by clusters of decreasing population size for increasing values of the x- and y-axes. The most populated cluster, denoted C1, comprised 7.7% of all conformations, followed by C2 and C3, with 5.9% and 5.4%, respectively. The significance of these percentage values as well as the definition of clusters and the correspondence of time frames to clusters is small, however, since all of these quantities were found to be strongly depended on the choice of RMSD cutoff and not on intrinsic properties of the structures (SM Figures IV and V). Nonetheless, under the current definition (RMSD cutoff = 1Å) clusters were determined at every frame of the trajectories, identifying each frame as belonging to either C1 or C2 by using a cyan or purple bar below both RMSD matrices of Figure 5. The scattered distribution of frames in the unsorted matrix on the left belonging to C1 and C2 indicated that conformations belonging to C1 and C2 occurred throughout the length of the trajectories. Below these C1 and C2 colored bars, secondary structures as a function of simulation frame are presented. While in the unsorted trajectories secondary structure elements were uniformly distributed throughout the trajectories, in the sorted trajectories there was clear indication of clustering corresponding to C1, C2, C3, etc. The sorted trajectories indicated that for structures belonging to C1, C2, and C3, amino acids in the central part of the decapeptide exhibited similar turn structures. In contrast, cluster C4 corresponded to β-hairpin conformations. In the four lower graphs in Figure 5 the quantities R(3,6), R(4,8), S(4,8), and Rg again assumed a wide range of values in the unsorted trajectories unlike in the sorted frame sequences where they exhibited regions of constant values that corresponded to each cluster. Importantly, whereas R(3,6) showed a relatively constant value for clusters C1, C2, and C3 in the sorted trajectories (at about 6.5 Å), the other quantities varied radically from cluster to cluster. This result indicated that while there was a commonality in the values of R(3,6) among the predominant clusters, these clusters were nonetheless distinct from each other in other structural aspects, thus indicating the absence of a dominating single representative conformation.
Figure 5.
Clustering analysis for the WT trajectories. The analysis is presented in two ways: by concatenating all trajectories and analyzing them as a function of simulation time (left column) and by sorting the frames of all of the trajectories according to the cluster that each frame belongs to (right column). In each column, the top color matrix is the RMSD matrix indicating the RMSD distances between all pairs of trajectory frames. The cyan and purple horizontal bars (indicated by C1 and C2, respectively) mark those frames belonging to the most populated cluster (C1, cyan) and the second most populated (C2, purple). Below these bars, the secondary structure per amino acid for each frame is shown (colors correspond to turn – green, gold – bridge, yellow – extended β, and magenta – α helix). The four graphs below, trace values for R(3,6), R(4,8), Rg (in Å), and S(4,8) (in Å2) for each trajectory frame.
Structural Characteristics of the β-Hairpin Conformations
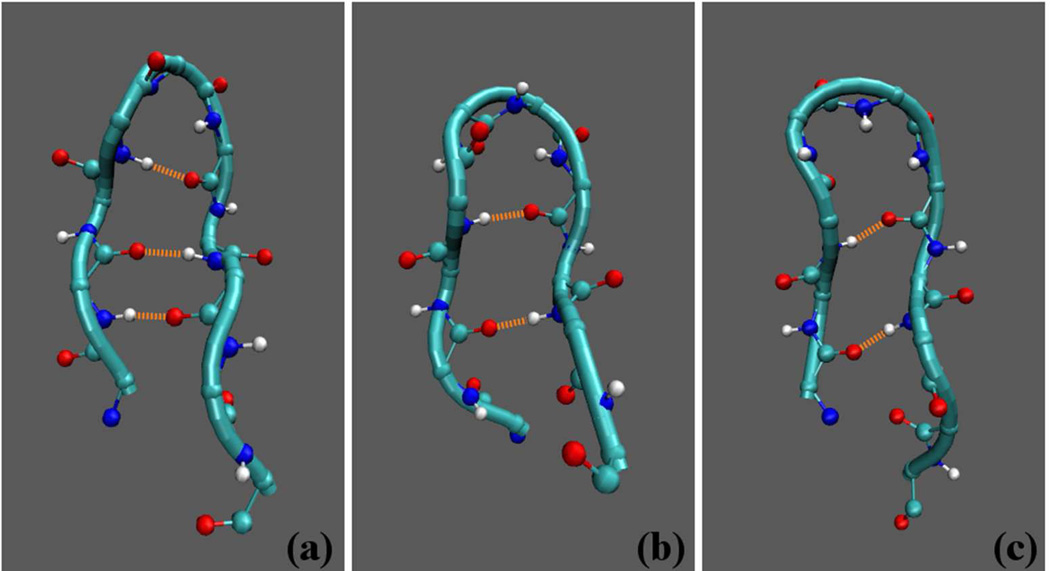
To study in more detail the extended β structures found in the average secondary structure analysis (SM Figure II), the time evolution of the secondary structure was examined. At least three distinct sporadic types of β-hairpin structures were identified (SM Figure VI), the β28, β29, and β18 discussed above, that differed by the register in which pairs of amino acids formed hydrogen bonds. The different registers are shown in Figure 6. For the β28 type, the dominant backbone HBs were between Glu22-Lys28, Ala21–Ala30, and Val24-Ser26 (see Table A). For the β29 type, the dominant backbone HBs were between Glu22-Gly29 and Val24-Asn27. For the β18 type the dominant HB was between Ala21-Lys28. A complete list of the HB propensities within the three β-hairpin conformations is given in Table A. Comparison between HB pair propensities (SM Table C) and HB propensities for particular β-hairpin conformations (Table A) showed that the loop and β-hairpin conformations fundamentally differed. Whereas the β-hairpin structures were associated with the backbone-backbone HB formation, the loop conformations did not have any appreciable backbone-backbone HBs but rather formed HBs between the charged side chain group of Asp23(Oδ) and either backbone atoms or other side chain groups.
Figure 6.
Snapshots of β-hairpin conformations for the WT showing some of the dominant backbone HBs (orange dashed lines) that define each different register. These HBs are (from bottom to top): (a) β28 between Glu22(H)-Lys28(O), Glu22(O)-Lys28(H), and Val24(H)-Ser26(O); (b) β29 between Glu22(O)-Gly29(H) and Val24(H)-Asn27(O); and (c) β18 between Ala21(H)-Lys28(O) and Ala21(O)-Lys28(H). Conformations show the N termini on the left end of the decapeptide. Only backbone atoms are displayed.
Table A.
Table of HB percentage for all β-hairpin types and decapeptide variants. This table was compiled using data gathered from the pre-formed β-hairpin trajectories. Only HBs with percentages greater than 1% are listed. The three most prevalent HB bonds are indicated in bold.
| Hydrogen Bond (%) | β28 | β29 | β18 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | Arctic | Dutch | Iowa | WT | Arctic | Dutch | Iowa | WT | Arctic | Dutch | Iowa | |
| SB | ||||||||||||
| Glu22(Oε)-Lys28(Hζ) | ||||||||||||
| Asp23(Oδ)-Lys28(Hζ) | 27.9 | 23.9 | 22.9 | 12.7 | 2.9 | 16.1 | ||||||
| Charged-SD | ||||||||||||
| Glu22(Oε)-Asp23(H) | 3.9 | 4.1 | 4.3 | 9.0 | ||||||||
| Glu22(Oε)-Asn27(Hδ) | 14.9 | 2.8 | 17.1 | |||||||||
| Asp23(Oδ)-Val24(H) | 1.4 | 1.5 | ||||||||||
| Asp23(Oδ)-Gly25(H) | 9.5 | 12.6 | 8.8 | 5.0 | 2.8 | 3.2 | 2.8 | 2.2 | 4.8 | 9.9 | 1.5 | |
| Asp23(Oδ)-Ser26(H) | 3.3 | 6.3 | 5.0 | 2.2 | 1.1 | |||||||
| Asp23(Oδ)-Ser26(Hγ) | 5.7 | 5.9 | 1.8 | |||||||||
| Gly25(O)-Lys28(Hζ) | 1.0 | |||||||||||
| Ser26(Oγ)-Lys28(Hζ) | 1.7 | |||||||||||
| Ala30(O)-Lys28(Hζ) | 5.1 | 1.2 | 2.5 | |||||||||
| Backbone | ||||||||||||
| Ala21(O)-Lys28(H) | 21.6 | 22.9 | 24.1 | 17.3 | ||||||||
| Glu22(O)-Lys28(H) | 19.3 | 20.9 | 21.2 | 19.4 | ||||||||
| Glu22(O)-Gly29(H) | 29.5 | 24.1 | 28.2 | 32.9 | ||||||||
| Asp23(O)-Ser26(H) | 5.2 | |||||||||||
| Ser26(O)-Asp23(H) | 2.6 | 9.3 | 19.4 | 2.5 | ||||||||
| Ser26(O)-Val24(H) | 20.3 | 20.6 | 19.9 | 24.8 | ||||||||
| Asn27(O)-Val24(H) | 13.9 | 20.2 | 18.1 | 29.8 | ||||||||
| Lys28(O)-Ala21(H) | 3.0 | 23.4 | 9.8 | 19.5 | 27.5 | |||||||
| Lys28(O)-Glu22(H) | 16.5 | 13.4 | 19.2 | 15.3 | ||||||||
| Gly29(O)-Ala21(H) | 1.5 | 8.7 | 3.4 | |||||||||
| Gly29(O)-Glu22(H) | 9.9 | 8.8 | 11.6 | 9.1 | ||||||||
| Ala30(O)-Ala21(H) | 24.8 | 20.2 | 25.9 | 27.8 | 2.5 | 9.4 | 1.8 | 2.9 | 2.0 | 8.8 | 1.5 | 10.1 |
| Ala30(O)-Glu22(H) | 2.3 | 1.3 | 2.7 | |||||||||
| SD-Backbone | ||||||||||||
| Gly25(O)-Asn23(Hδ) | 2.8 | |||||||||||
| Gly25(O)-Ser26(Hγ) | 1.2 | |||||||||||
| Ser26(O)-Asn23(Hδ) | 10.8 | |||||||||||
| Ser26(Oγ)-Asn23(Hδ) | 1.0 | |||||||||||
| Asn27(Oδ)-Ala21(H) | 3.3 | 1.7 | 2.1 | |||||||||
| Asn27(Oδ)-Gly29(H) | 3.6 | 1.1 | ||||||||||
| Lys28(O)-Asn27(Hδ) | 3.7 | 1.5 | 4.8 | |||||||||
| Ala30(O)-Gln22(Hε) | 3.2 | |||||||||||
Because of the specific register of the three β-hairpin types, the Val24 and Lys28 side chains were found to be located at fixed positions relative to each other, yet not necessarily in proximity. Specifically, in the β28 type, the Val24 and Lys28 side chains were on the same side of the hairpin and adjacent to each other (SM Figure VII, top). However, in the β29 type, they occupied opposite sides of the hairpin (SM Figure VII, middle). In the β18 type, they were on the same side, but the side chain of Val24 was within the turn region pointing away from the Lys28 side chain (SM Figure VII, bottom). These data indicated that the quantity S(4,8) would not be an appropriate metric for hydrophobic events in the β-hairpins as low values would not necessarily indicate proximity and hydrophobic contact between Val24 and Lys28 as had been the case for the loop. An alternate measure, the SASA of the whole decapeptide, is discussed below.
Different β-hairpin type structures occurred at different proportions for each decapeptide variant (SM Table D). The particularly high percentages of β28 and β29 in the Iowa mutant were already evident in the strong diagonal contacts in Figure 1, a clear signature of β-hairpin structures. Taken as a whole, these results demonstrated that the absence of charge at position 22 decreased the β29 occurrence, whereas the absence of charge at position 23 increased β28 and β29 occurrences. The β18 occurrence percentage increased for the Arctic mutant. However, of the three β-hairpins the β18 was the most insensitive to mutations.
Degree of Stability of the β-Hairpin Conformations
A simple observation of the time evolution of the β-hairpin structures revealed that these structures would form and unfold, although in a few cases they would remain folded for the duration of the trajectory. Numerous studies38–43,51–53,63,84–86 (also SM text and SM Figure VIII), however, have shown that this decapeptide does not have stable conformations. Thus, the behavior observed here can only be attributed to metastable β-hairpin structures with low occurrence probabilities, manifested in our current dynamical trajectories as short-lived conformations.
Examination of the time evolution of the secondary structure suggested that the β-hairpins had characteristic formation times and lifetimes (the typical time that a β-hairpin structure existed before unfolding or converting into another β-hairpin type) that may be sensitive to mutations at positions 22 and 23. Here, only the lifetime of each β-hairpin structure was examined as a measure of degree of stability. Visual inspection of the fluctuations in the extended structure (SM Figure VI) initially suggested that the β28 type was more stable than the β29 type, followed by the least stable β18 type, consistent with the number of dominant backbone HBs of each β hairpin type, namely 3, 2, and 1 (see Table A).
To measure lifetimes, independent trajectories labeled i were started from pre-formed β-hairpins and their breaking times τi were measured. Lifetimes were then calculated by an exponential fit to the set {τi}, as described in the Methods section. Specifically, τi were obtained by examining the secondary structure as a function of simulation time. The times at which the extended β conformations disappeared were then recorded as τi. A secondary criterion for τi was obtained by examining, in a similar way, the RMSD between the starting conformation (β-hairpin at t=0) and all conformations at later times. The times at which the RMSD rapidly increased were recorded as τi. The respective τi obtained through these two methods typically coincided (SM Figure IX).
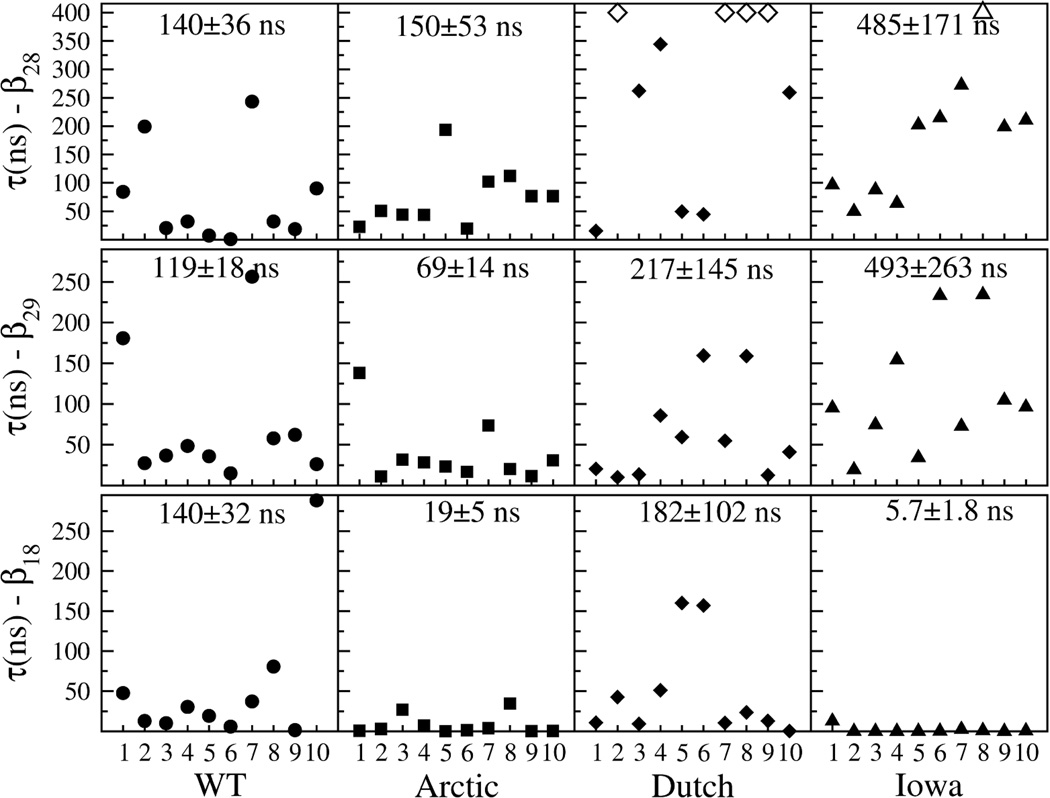
In Figure 7, results for all {τi} from 10 independent trajectories per decapeptide variant for each of the β-hairpin types are plotted. The number at the top of each graph is the calculated lifetime for that particular β-hairpin structure (see Methods section for details). Open symbols indicate a trajectory in which the β-hairpin did not break within the maximal observation time of 400 ns. For the β28 in the Dutch mutant, a lifetime could not be calculated due to the high number of unbroken β-hairpins, but considered to have a lower bound of no less than 500 ns when compared with the β28 and β29 in the Iowa. Overall, the breaking times were typically higher for β28 and lower for β29 and β18 in all four decapeptide variants. In the WT peptide, one β18 trajectory may have biased the β18 lifetime to a value greater than the one for the β29 trajectories. Based on the lifetimes of the dominant β28 and β29 hairpins, these results separated the four decapeptide variants into two groups: (i) the WT and Arctic mutant, with lifetimes below 200 ns; and (ii) the Dutch and Iowa mutants, with lifetimes above 200 ns and as high as 500 ns. For comparison, a similar analysis performed using the C1 and C2 clusters in the WT trajectories (using the data from the cyan and purple bars of Figure 5) yielded a loop lifetime of 15.88±0.11 ns and 13.60±0.10 ns for the C1 and C2 loop conformations, respectively (SM Figure X). These loop lifetimes were an order of magnitude smaller than any of the WT β-hairpin structures.
Figure 7.
Breaking times τi obtained for each of ten trajectories per β-hairpin and decapeptide type. The number at the top of each graph is the average lifetime and standard deviation obtained by fitting an exponential distribution to the data (see Methods section).
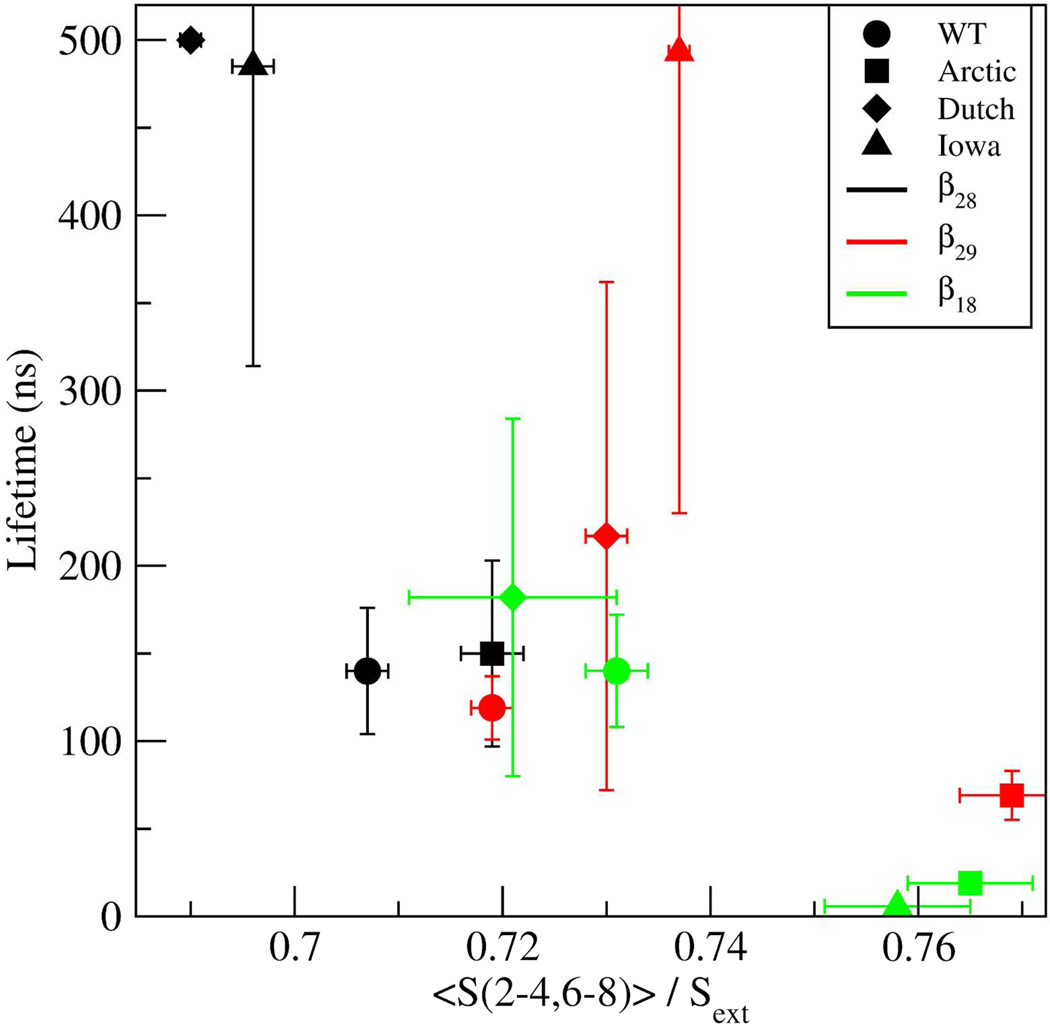
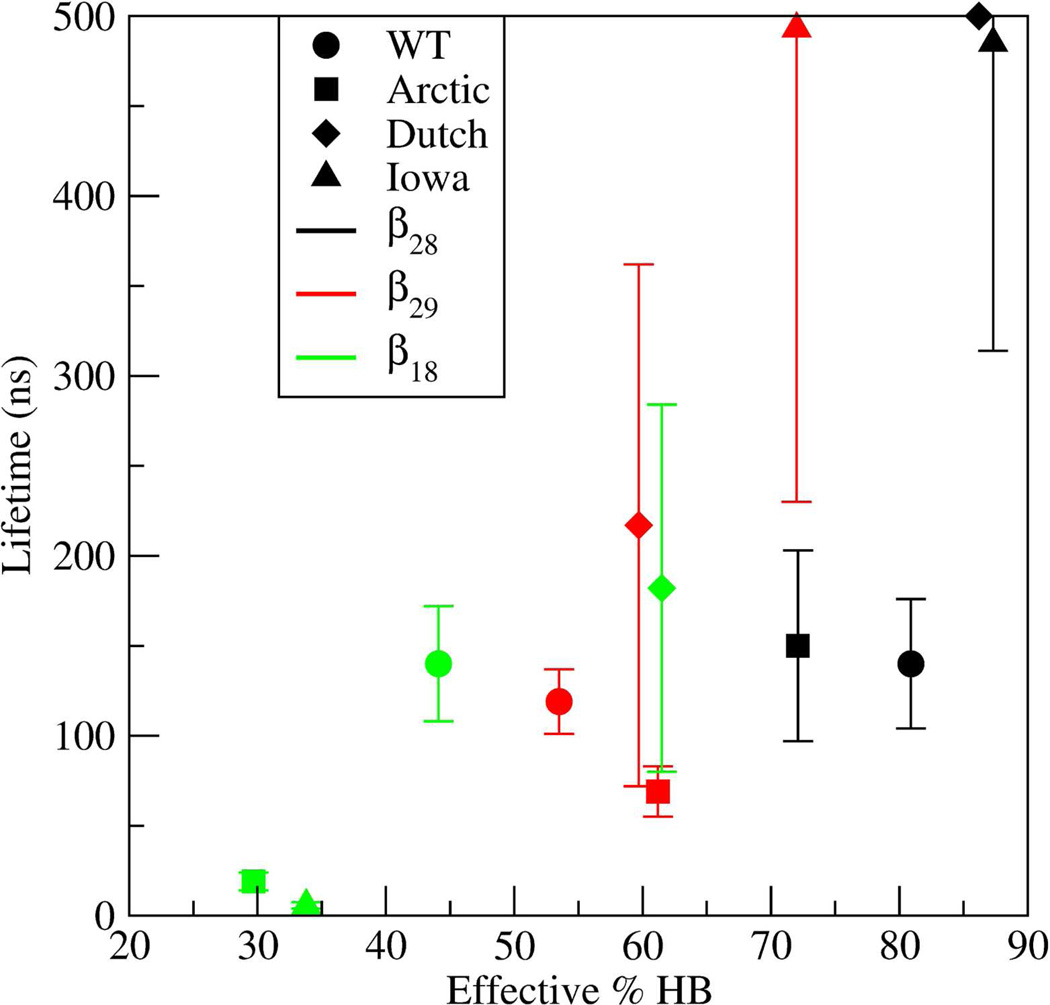
The distinct lifetimes of the three β-hairpin structures could be associated with S(2–4,6–8), the combined SASA of the decapeptide excluding the glycines and the two terminal alanines. Specifically, low S(2–4,6–8) values could potentially indicate a more stable structure with more hydrophobic contacts. To evaluate this possibility, average values for S(2–4,6–8) were calculated for each of the decapeptide and β-hairpin types (SM Table E) by: calculating the average Si(2–4,6–8) per trajectory (10 per β hairpin type), averaging over all Si to obtain <S(2–4,6–8)>, and normalizing this average by the values of each stretched decapeptide variant, Sext(2–4,6–8). This normalization was necessary because the range of S(2–4,6–8) values was variant-dependent (e.g., Arctic had a smaller range of S(2–4,6–8) values than the WT because of the [Glu22Gly] substitution). Sext(2–4,6–8) for each variant was obtained by using fully stretched decapeptides. The total times over which each average was determined are listed in SM Table F. To test whether the lifetimes were correlated with S(2–4,6–8), the dependence of lifetimes vs S(2–4,6–8) was plotted (Figure 8). The lifetime of the β28 in the Dutch mutant was set here to 500 ns. The data in Figure 8 demonstrated that the β28 (black) and β18 (green) lifetimes significantly decreased with increasing S(2–4,6–8), thus a higher exposure to solvent decreased their lifetimes. However, this correlation was not evident for the β29 type. In the following, the nature of the lifetimes of each of the β28, β29, and β18 hairpins is discussed.
Figure 8.
Dependence of the β-hairpin lifetimes on the normalized total SASA of the decapeptide excluding the glycines and two terminal alanines, <S(2–4,6–8)> / Sext. The symbols correspond to WT - circle; Arctic - square; Dutch - diamond; and Iowa - triangle. The different β-hairpins are denoted by the color of the symbol, where β28 is black, β29 is red, and β18 is green. Error bars correspond to the standard error of the mean.
For the first case, having established that for the β28 hairpin type the WT and Arctic decapeptides had shorter lifetimes (~150 ns) than the Iowa (485 ns) and Dutch (≥500 ns) because their side chains were more exposed to the solvent (Figure 8), specific amino acids were probed to examine their role in reducing the solvent exposure. In SM Figure XI, contributions from different SASA combinations of amino acids are plotted for all four decapeptide variants. For the β28 type, minima in S(2–4,6–8) were caused by decreased S(2,4,8) (in the Dutch mutant) or decreased S(3,7) and S(3,6) (in the Iowa mutant). By examining representative structures (using the cluster analysis described in the Methods section but now only using β-hairpin structures), it was observed that whereas the side chains of Val24 and Lys28 were on the same side of the hairpin loop (SM Figure VII), the Gln22 side chain in the Dutch mutant closely packed with the Val24 and Lys28 side chains, thus lowering the effective S(2,4,8) (SM Figure XII). In contrast, in the WT and Iowa mutant, the Glu22 side chain was solvated. In the Arctic mutant this packing was also missing as the Gly22 lacked a side chain. This observed Gln22-Val24-Lys28 packing in the Dutch mutant also enhanced the lifetime of the structure by reducing the fluctutations between the side chains of Val24 and Lys28, which was consistent with a narrower distribution of the dot product D between the vectorial directions of the Val24 and Lys28 side chains (SM Figure XIII). By examining a representative structure of the Iowa β28 type structure, it was observed that the dip in S(3,7) (SM Figure XI, top) was caused by the Asn23–Asn27 hydrophobic packing (SM Figure XIV). The additional reduction in the value of S(3,6) for the Iowa mutant (SM Figure XI, top) did not independently contribute to a longer lifetime since the Asp23 and Ser26 side chains were on opposite sides of the β28 hairpin, but rather reflected the decrease in the R(3,7) distance resulting from the Asn23–Asn27 hydrophobic packing discussed above.
Additional contributions to the increased lifetime of the Dutch and Iowa β28 hairpin type structures came from their backbone HBs. In Figure 9, the lifetimes of β-hairpins are plotted as a function of the "effective HB percentage". These effective HB percentages were derived from Table A by adding up all in-register backbone HBs (middle section of that Table) while subtracting the percentage of "competing" HBs. Specifically, for the β28 type, the effective HB percentages were obtained by adding up all Glu22-Lys28, Val24-Ser26, and Ala21–Ala30 contributions while subtracting the contribution from Lys28(O)-Ala21(H) that competed with the formation of the Lys28(O)-Glu22(H). For the β29 type, all of the backbone HBs were included except Ala30(O)-Glu22(H), which was subtracted due to the competition with Gly29(O)-Glu22(H). Similarly, for the β18 type, the contributions from Gly29(O)-Ala21(H) and Ala30(O)-Ala21(H) HBs were subtracted because they competed with formation of the Lys28(O)-Ala21(H) HB. In Figure 9, the β28 in the Dutch and Iowa mutants had larger effective HB percentages than the WT and Arctic. These results demonstrated that both reduced SASA and an increase in backbone HBs contributed to longer lifetimes of the β28 hairpin in the Dutch and Iowa mutants.
Figure 9.
Dependence of β-hairpin lifetimes on the effective backbone hydrogen bonding propensity. The symbols correspond to WT - circle; Arctic - square; Dutch - diamond; and Iowa - triangle. The different β-hairpins are denoted by the color of the symbol, where β28 is black, β29 is red, and β18 is green.
For the case of the β29 hairpins, the lifetimes of the Arctic (<70 ns) and Iowa (≈500 ns) mutants were respectively lower and higher than the lifetimes of the WT and Dutch mutants (100–200 ns). In terms of SASA, the small lifetime of the β29 in the Arctic mutant corresponded to an increased value of S(2–4,6–8), indicating an increased exposure to the solvent relative to the other variants (SM Figure XI, middle). On the other hand, the long lifetime in the β29 Iowa hairpin could not be attributed to a reduced overall SASA, as none of the S(x,y) values (SM Figure XI) was significantly smaller than those for the WT peptide. Also, examination of representative structures in the Iowa mutant did not reveal any other hydrophobic contacts that could lengthen the lifetime of the hairpin. The long lifetime of the Iowa β29 type was most likely caused by the backbone HBs, consistent with the highest value of the effective HB percentage (Figure 9). Interestingly, this higher effective HB percentage in the Iowa occurred even in the absence of the Asp23-Lys28 SB (Table A), suggesting that the absence of this SB was compensated by an increase in the backbone HBs, resulting in a longer lifetime.
The lifetimes of the β18 hairpins for the WT and Dutch mutants had values between 100 and 200 ns, whereas for the Arctic and Iowa mutants these lifetimes were below 20 ns (Figure 7). In the graph of the lifetime versus S(2–4,6–8) (Figure 8), the lifetime of the β18 hairpin decreased with increasing SASA. Thus, the Arctic and Iowa mutants with considerably larger SASA values had on average correspondingly smaller β18 hairpin lifetimes. An additional destabilizing factor was found when examining the effective HB percentages vs lifetimes (Figure 9), in which the Arctic and Iowa mutants with the shortest β18 hairpin lifetimes had the smallest effective HB percentages. This low value of the effective HB percentage was a result of the large number of HBs incompatible with the β18 hairpin, such as the Gly29/Ala30-Ala21 HBs (Table A).
In sum, increased lifetimes in the β28 hairpin were a result of strong hydrophobic contacts involving Gln22, Val24, and Lys28 side chains in the Dutch mutant, and the weaker, but relevant, Asn23–Asn27 contact in the Iowa mutant. For the case of the β29 hairpin lifetimes, the number of backbone HBs was the most influential factor where in particular the increased lifetime in the Iowa mutant was consistent with an increased number of backbone HBs originating from the absence of the Asp23-Lys28 SB. Lastly, the β18 hairpin lifetimes were also affected by hydrophobicity and by the competing HBs in the Iowa and Arctic mutants that destabilized the hairpin whereas in the WT and Dutch mutants, the Asp23-Lys28 SB helped sustain the β18 hairpin structure.
Discussion
Constant temperature MD simulations of the Aβ21–30 decapeptide and its Arctic, Dutch, and Iowa mutants in explicit water revealed three long-lived metastable β-hairpin structures (β28, β29, and β18) that differed in registers, number of backbone-backbone HBs, and appeared with different propensities in all four variants. Their lifetimes were also strongly variant-dependent. Results from the WT suggested that β-hairpin lifetimes may be an order of magnitude larger than lifetimes of loop conformations. Hydrophobic packing of side chains resulted in increased lifetime of the β28 in the Dutch (Gln22-Val24-Lys28) and Iowa (Asn23–Asn27) mutants, whereas in the β29 hairpins lifetimes were mostly influenced by backbone-backbone HBs. Overall, formation of SBs did not play a major role in β-hairpin lifetimes. Yet, the lack of a Asp23-Lys28 SB in the Iowa increased the number of backbone HBs resulting in an increased lifetime. Finally, the short lifetimes found in the β18 hairpin were attributed to the competition among the backbone HBs of different registers.
These β-hairpin structures comprised ≈5% to ≈12% of all structures in the WT, Arctic, and Dutch mutant, and up to ≈32% in the Iowa mutant, with uninterrupted structural persistence from tens to hundreds of nanoseconds. Nonetheless, the overwhelming total population of coil and loop structures in any given trajectory suggested that the free energy of the β-hairpins, with comparatively lower solvent exposure from the tightly packed side chains, increased intrapeptide contacts, and lower potential energy due to backbone-backbone HBs, could not compete with that from the coil and loop structures which benefited from the much larger entropic contribution of these structurally looser structures. Because of the majority of loop conformations, when considering whole-trajectory averages, these β-hairpins did not dominate the predominant structural characteristics. These predominant structural characteristics for most decapeptide variants showed instead a strong Asp23-Ser26 interaction involving at least two HBs, a loop structure with a turn comprising amino acids 23 through 27, and unstructured N- and C-termini, previously observed in other studies38–43,51,52,85. A low rate of SB formation was found (also seen in Ref.86), suggesting a relatively small contribution of SBs to the decapeptide conformational dynamics. Detailed whole-trajectory analysis of HBs also revealed that the Iowa mutant displayed a distinct HB network as compared with the WT, indicating that the absence of charge at position 23 profoundly changed the dynamics of HB formation whereas the absence of charge at position 22 left the HB network intact, also consistent with previous studies52,53. In contrast to previous work, clustering analysis including all conformations failed to show a dominating loop representative structure in the WT (largest cluster <8% of total trajectory), but rather a multitude of clusters. While these clusters of loop structures shared structural properties such as the R(3,6) distance and common HBs emanating from the Asp23(Oδ), they were structurally different from each other and displayed short lifetimes with a high rate of interconversion between them.
The existence and long lifetimes of the β-hairpin structures found here is not ubiquitous in all computational studies. Previous REMD studies on Aβ21–30 and its mutants have been characterized by a paucity or absence of β-hairpin structures39,52,53,85. On the other hand, a full-length Aβ1–40 and Aβ1–42 REMD study reported a β-turn formation in the Aβ1–4057. In addition, constant temperature MD of the full-length Aβ have shown evidence of a β-hairpin in the 21–30 region87 while discrete MD studies using a coarse-grained peptide model also found evidence for a β-hairpin structure in the decapeptide region within Aβ1–40, Aβ1–42, and their Arctic mutants60,61. A possible explanation for this discrepancy could be that whereas β-hairpin structures are metastable states with low occurrence probability, the mechanisms by which they fold are more readily accessible in simulations that examine dynamics and time evolution of the Aβ. Another possibility is that other mechanisms intrinsic to the formation of the β-hairpins presented here are at play, such as possible anti-Arrhenius behavior found in the folding rates of β-hairpins in some proteins88–90 that may need a special treatment when examined by REMD91,92. Other reasons could also include that simulation times used in REMD may have to be greater than the folding times of the β-hairpins studied here93 or that REMD might not show the folding94.
Our data, and that of others, demonstrate that the majority loop conformation observed in all four decapeptide variants was not static but rather comprised many short-lived conformations that interconvert with time52. This result raises the question of how could such a dynamic loop population nucleate the folding of the full-length Aβ. The present work elucidates the dynamics of three metastable β-hairpin structures that could play a critical role in nucleating full-length Aβ folding and affect the assembly pathway. Stability and structure of intermediates have been shown to affect protein folding, misfolding, and amyloid formation67. The β-hairpins studied here represent metastable states that could, in principle, nucleate the fold of the full-length Aβ by initially forming within the 21–30 region, then propagating into a β structure comprising the 17–22 and 28–37 regions54,95. This process is consistent with an experimental study that stabilized a β-hairpin of Aβ1–40 in the decapeptide region by binding Aβ1–40 to the affibody ZAβ396. This view is consistent also with studies that showed that a preformed lactam bridge in the decapeptide region of Aβ1–40 strongly enhanced the rate of fibrilization65,97 and produced toxic oligomeric species66. In our proposed scenario, the nucleation of a full-length Aβ fold by formation of the β-hairpin in the 21–30 region may be then followed by a subsequent loss of this same β-hairpin structure to become a turn, consistent with other full-length Aβ simulations58.
The results presented in this study suggest that longer β-hairpin lifetimes correlate with degree of aggregation. Clinically, Dutch mutation carriers suffer from recurrent strokes and exhibit diffuse plaques and massive cerebral amyloid angiopathy (CAA)49. In vitro studies have demonstrated that Dutch Aβ polymerizes into protofibrils and then into fibrils significantly faster than does WT Aβ98. This may be reflected in vivo by accelerated aggregation and deposition on the surface of endothelial cells and cerebrovasculature smooth muscle cells, causing damage99. Our results showed that the Dutch exhibited significantly longer β-hairpin lifetimes than the WT, Arctic, and Iowa mutants.
Similarly, the Iowa mutant had the largest propensity to form β-hairpins. These β-hairpins were also shown to have a substantially longer lifetime compared to the WT and Arctic mutants (but shorter than the Dutch). Clinically, the Iowa mutant also exhibits diffuse plaques with massive CAA similar to the Dutch, but rarely exhibits strokes or hemorrhage45,100, in contrast to the Dutch. It is plausible that the relatively large β-hairpin lifetimes (similarly to the Dutch) and the large propensity to form β-hairpins (different from the Dutch) in the Iowa mutant combine to produce the massive CAA but result in an assembly pathway distinct from the that of the Dutch mutant.
While there are differences between the Dutch and Iowa, there are also similarities. The β-hairpin lifetime results presented above separated the decapeptides into two classes: the WT and Arctic, with lifetimes less than 200 ns, and the Dutch and Iowa, with lifetimes of at least 500 ns. Experiments also show this separation. The Dutch and Iowa mutations are clinically, pathologically, and in vitro very similar45,101 leading to symptoms and pathologies distinct from AD102. On the other hand, the Arctic mutation exhibits clinical and pathological features typical of AD such as compact plaques, a fibrillization rate similar to the WT, and progressive dementia without stroke or hemorrhage. Also, the Arctic mutation does not exhibit the severe CAA that characterizes other mutations in the Aβ region of APP such as the Dutch and the Iowa mutations46,47.
Nonetheless, the Arctic stands out on its own. While it produces clinical and neuropathological features indistinguishable from those of late-onset sporadic AD it produces them prematurely46,47. In vitro studies suggested that increased generation of intermediate protofibrillar Aβ assemblies formed early during fibrillogenesis may play a role in the pathology compared to WT46,103,104. It is possible that the differences observed in this paper in the Arctic mutant regarding its intramolecular HB (enhanced over the other decapeptide types) play a role in these differences.
In summary, constant temperature all-atom molecular dynamics simulations to study the dynamics of monomeric Aβ21–30 in explicit water and its Dutch [Glu22Gln], Arctic [Glu22Gly], and Iowa [Asp23Asn] isoforms revealed a majority of loop conformers predominantly exhibiting a hydrogen bond network involving the Asp23 and Ser26 amino acids. A minority, but important, population of conformers forming metastable β-hairpin structures were also observed. Measurements of the lifetimes of the dominant β-hairpins indicated that the Dutch mutant had the longest β-hairpin lifetime (greater than 500ns), closely followed by the Iowa mutant (≈500ns) while the Aβ21–30 and the Arctic mutant had significantly lower lifetimes (≈200ns). Connection with clinical and pathological data suggest that the lifetimes and β-hairpin formation propensity may correlate with degree of aggregation. Stabilizing or preventing intermediates prone to amyloid formation may prevent toxic assembly67. Experimental studies have shown that targeting the central region of the Aβ using antibodies and fragments inhibited Aβ aggregation105–107. The results of our study suggest that targeting the stability of the metastable β-hairpin structures studied here could, in principle, be of therapeutic utility.
Supplementary Material
Acknowledgments
Financial support was provided by National Institutes of Health grants AG023661, NS038328, and AG027818, the Jim Easton Consortium for Alzheimer's Drug Discovery and Biomarkers at UCLA, and the California Alzheimer's Disease Research Fund grant #07-65806. Computational time was provided in part by TeraGrid computational resources grant TG-MCB110142.
Abbreviations
- Aβ
amyloid β-protein
- AD
Alzheimer's disease
- HB
hydrogen bond
- MD
molecular dynamics
- SASA
solvent accessible surface area
- SB
salt bridge
Footnotes
Supporting Information Available: Detailed simulation results are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hardy J, Selkoe DJ. Science. 2002;297:353. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Allsop D. Trends Pharmacol Sci. 1991;12:383. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 3.Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. Brain Res. 1991;563:311. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- 4.Hardy JA, Higgins GA. Science. 1992;256:184. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 5.Kirkitadze MD, Bitan G, Teplow DB. J. Neurosci. Res. 2002;69:567. doi: 10.1002/jnr.10328. [DOI] [PubMed] [Google Scholar]
- 6.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6448. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh DM, Klyubin I, Shankar GM, Townsend M, Fadeeva JV, Betts V, Podlisny MB, Cleary JP, Ashe KH, Rowan MJ, Selkoe DJ. Biochem Soc T. 2005;33:1087. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- 8.Klein WL, Stine WB, Teplow DB. Neurobiol. Aging. 2004;25:569. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Teplow DB, Lazo ND, Bitan G, Bernstein S, Wyttenbach T, Bowers MT, Baumketner A, Shea JE, Urbanc B, Cruz L, Borreguero J, Stanley HE. Accounts Chem. Res. 2006;39:635. doi: 10.1021/ar050063s. [DOI] [PubMed] [Google Scholar]
- 10.Haass C, Selkoe DJ. Nat. Rev. Mol. Cell Biol. 2007;8:101. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 11.Roychaudhuri R, Yang M, Hoshi MM, Teplow DB. J. Biol. Chem. 2009;284:4749. doi: 10.1074/jbc.R800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh DM, Selkoe DJ. J Neurochem. 2007;101:1172. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 13.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. Nature. 2006;440:352. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 14.Urbanc B, Cruz L, Teplow D, Stanley HE. Curr Alzheimer Res. 2006;3:493. doi: 10.2174/156720506779025170. [DOI] [PubMed] [Google Scholar]
- 15.Fink AL. Curr. Opin. Struct. Biol. 2005;15:35. doi: 10.1016/j.sbi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Uversky VN. Protein Sci. 2002;11:739. doi: 10.1110/ps.4210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitan G, Lomakin A, Teplow DB. J. Biol. Chem. 2001;276:35176. doi: 10.1074/jbc.M102223200. [DOI] [PubMed] [Google Scholar]
- 18.Teplow DB. Amyloid, Prions, and Other Protein Aggregates, Pt C. Vol. 413. San Diego: Elsevier Academic Press Inc; 2006. Preparation of amyloid β-protein for structural and functional studies; p. 20. [Google Scholar]
- 19.Ono K, Condron MM, Teplow DB. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14745. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JP, Stimson ER, Ghilardi JR, Mantyh PW, Lu YA, Felix AM, Llanos W, Behbin A, Cummings M, Vancriekinge M, Timms W, Maggio JE. Biochemistry. 1995;34:5191. doi: 10.1021/bi00015a033. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Iwata K, Lachenmann MJ, Peng JW, Li S, Stimson ER, Lu Y, Felix AM, Maggio JE, Lee JP. J. Struct. Biol. 2000;130:130. doi: 10.1006/jsbi.2000.4288. [DOI] [PubMed] [Google Scholar]
- 22.Riek R, Guntert P, Dobeli H, Wipf B, Wuthrich K. Eur J Biochem. 2001;268:5930. doi: 10.1046/j.0014-2956.2001.02537.x. [DOI] [PubMed] [Google Scholar]
- 23.Hou LM, Shao HY, Zhang YB, Li H, Menon NK, Neuhaus EB, Brewer JM, Byeon IJL, Ray DG, Vitek MP, Iwashita T, Makula RA, Przybyla AB, Zagorski MG. J. Am. Chem. Soc. 2004;126:1992. doi: 10.1021/ja036813f. [DOI] [PubMed] [Google Scholar]
- 24.Serpell LC. Biochim. Biophys. Acta-Mol. Basis Dis. 2000;1502:16. doi: 10.1016/s0925-4439(00)00029-6. [DOI] [PubMed] [Google Scholar]
- 25.Wei GH, Shea JE. Biophys. J. 2006;91:1638. doi: 10.1529/biophysj.105.079186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanuy D, Ma BY, Nussinov R. Biophys. J. 2003;84:1884. doi: 10.1016/S0006-3495(03)74996-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klimov DK, Thirumalai D. Structure. 2003;11:295. doi: 10.1016/s0969-2126(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 28.Favrin G, Irback A, Mohanty S. Biophys. J. 2004;87:3657. doi: 10.1529/biophysj.104.046839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santini S, Wei GH, Mousseau N, Derreumaux P. Structure. 2004;12:1245. doi: 10.1016/j.str.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Ma BY, Nussinov R. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14126. doi: 10.1073/pnas.212206899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balbach JJ, Ishii Y, Antzutkin ON, Leapman RD, Rizzo NW, Dyda F, Reed J, Tycko R. Biochemistry. 2000;39:13748. doi: 10.1021/bi0011330. [DOI] [PubMed] [Google Scholar]
- 32.Baumketner A, Shea JE. J. Mol. Biol. 2007;366:275. doi: 10.1016/j.jmb.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Melquiond A, Dong X, Mousseau N, Derreumaux P. Curr Alzheimer Res. 2008;5:244. doi: 10.2174/156720508784533330. [DOI] [PubMed] [Google Scholar]
- 34.Baumketner A, Shea JE. J. Mol. Biol. 2006;362:567. doi: 10.1016/j.jmb.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Baumketner A, Krone MG, Shea JE. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6027. doi: 10.1073/pnas.0708193105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chebaro Y, Mousseau N, Derreumaux P. J. Phys. Chem. B. 2009;113:7668. doi: 10.1021/jp900425e. [DOI] [PubMed] [Google Scholar]
- 37.Wise-Scira O, Xu L, Kitahara T, Perry G, Coskuner O. J. Chem. Phys. 2011;135 doi: 10.1063/1.3662490. [DOI] [PubMed] [Google Scholar]
- 38.Lazo ND, Grant MA, Condron MC, Rigby AC, Teplow DB. Protein Sci. 2005;14:1581. doi: 10.1110/ps.041292205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumketner A, Bernstein SL, Wyttenbach T, Lazo ND, Teplow DB, Bowers MT, Shea JE. Protein Sci. 2006;15:1239. doi: 10.1110/ps.062076806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, Mousseau N, Derreumaux P. J. Chem. Phys. 2006;125:084911. doi: 10.1063/1.2337628. [DOI] [PubMed] [Google Scholar]
- 41.Cruz L, Urbanc B, Borreguero JM, Lazo ND, Teplow DB, Stanley HE. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18258. doi: 10.1073/pnas.0509276102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borreguero JM, Urbanc B, Lazo ND, Buldyrev SV, Teplow DB, Stanley HE. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6015. doi: 10.1073/pnas.0502006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fawzi NL, Phillips AH, Ruscio JZ, Doucleff M, Wemmer DE, Head-Gordon T. J. Am. Chem. Soc. 2008;130:6145. doi: 10.1021/ja710366c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami K, Irie K, Morimoto A, Ohigashi H, Shindo M, Nagao M, Shimizu T, Shirasawa T. J. Biol. Chem. 2003;278:46179. doi: 10.1074/jbc.M301874200. [DOI] [PubMed] [Google Scholar]
- 45.Grabowski TJ, Cho HS, Vonsattel JPG, Rebeck GW, Greenberg SM. Ann Neurol. 2001;49:697. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- 46.Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Naslund J, Lannfelt L. Nat Neurosci. 2001;4:887. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 47.Kamino K, Orr HT, Payami H, Wijsman EM, Alonso ME, Pulst SM, Anderson L, Odahl S, Nemens E, White JA, Sadovnick AD, Ball MJ, Kaye J, Warren A, McInnis M, Antonarakis SE, Korenberg JR, Sharma V, Kukull W, Larson E, Heston LL, Martin GM, Bird TD, Schellenberg GD. Am. J. Hum. Genet. 1992;51:998. [PMC free article] [PubMed] [Google Scholar]
- 48.Vanbroeckhoven C, Haan J, Bakker E, Hardy JA, Vanhul W, Wehnert A, Vegtervandervlis M, Roos RAC. Science. 1990;248:1120. doi: 10.1126/science.1971458. [DOI] [PubMed] [Google Scholar]
- 49.Levy E, Carman MD, Fernandezmadrid IJ, Power MD, Lieberburg I, Vanduinen SG, Bots G, Luyendijk W, Frangione B. Science. 1990;248:1124. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 50.Dahlgren KN, Manelli AM, Stine WB, Baker LK, Krafft GA, LaDu MJ. J. Biol. Chem. 2002;277:32046. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 51.Grant MA, Lazo ND, Lomakin A, Condron MM, Arai H, Yamin G, Rigby AC, Teplow DB. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16522. doi: 10.1073/pnas.0705197104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krone MG, Baumketner A, Bernstein SL, Wyttenbach T, Lazo ND, Teplow DB, Bowers MT, Shea JE. J. Mol. Biol. 2008;381:221. doi: 10.1016/j.jmb.2008.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray MM, Krone MG, Bernstein SL, Baumketner A, Condron MM, Lazo ND, Teplow DB, Wyttenbach T, Shea JE, Bowers MT. J. Phys. Chem. B. 2009;113:6041. doi: 10.1021/jp808384x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16742. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luhrs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Doeli H, Schubert D, Riek R. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17342. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flock D, Colacino S, Colombo G, Di Nola A. Proteins-Structure Function and Bioinformatics. 2006;62:183. doi: 10.1002/prot.20683. [DOI] [PubMed] [Google Scholar]
- 57.Sgourakis NG, Yan YL, McCallum SA, Wang CY, Garcia AE. J. Mol. Biol. 2007;368:1448. doi: 10.1016/j.jmb.2007.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang MF, Teplow DB. J. Mol. Biol. 2008;384:450. doi: 10.1016/j.jmb.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitternacht S, Staneva I, Hard T, Irback A. Proteins-Structure Function and Bioinformatics. 2010;78:2600. doi: 10.1002/prot.22775. [DOI] [PubMed] [Google Scholar]
- 60.Urbanc B, Cruz L, Yun S, Buldyrev SV, Bitan G, Teplow DB, Stanley HE. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17345. doi: 10.1073/pnas.0408153101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lam AR, Teplow DB, Stanley HE, Urbanc B. J. Am. Chem. Soc. 2008;130:17413. doi: 10.1021/ja804984h. [DOI] [PubMed] [Google Scholar]
- 62.Urbanc B, Betnel M, Cruz L, Bitan G, Teplow DB. J. Am. Chem. Soc. 2010;132:4266. doi: 10.1021/ja9096303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anand P, Nandel FS, Hansmann UHE. J. Chem. Phys. 2008;128:165102. doi: 10.1063/1.2907718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baumketner A, Bernstein SL, Wyttenbach T, Bitan G, Teplow DB, Bowers MT, Shea JE. Protein Sci. 2006;15:420. doi: 10.1110/ps.051762406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sciarretta KL, Gordon DJ, Petkova AT, Tycko R, Meredith SC. Biochemistry. 2005;44:6003. doi: 10.1021/bi0474867. [DOI] [PubMed] [Google Scholar]
- 66.Sandberg A, Luheshi LM, Sollvander S, de Barros TP, Macao B, Knowles TPJ, Biverstal H, Lendel C, Ekholm-Petterson F, Dubnovitsky A, Lannfelt L, Dobson CM, Hard T. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15595. doi: 10.1073/pnas.1001740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma BY, Nussinov R. Biophys. J. 2006;90:3365. doi: 10.1529/biophysj.105.075309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hammarstrom P, Wiseman RL, Powers ET, Kelly JW. Science. 2003;299:713. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 69.Jorgensen WL, Madura JD. Mol. Phys. 1985;56:1381. [Google Scholar]
- 70.Kaminski GA, Friesner RA, Tirado-Rives J, Jorgensen WL. J. Phys. Chem. B. 2001;105:6474. [Google Scholar]
- 71.Berendsen HJC, Vanderspoel D, Vandrunen R. Comput. Phys. Commun. 1995;91:43. [Google Scholar]
- 72.Lindahl E, Hess B, van der Spoel D. J. Mol. Model. 2001;7:306. [Google Scholar]
- 73.Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR. J. Chem. Phys. 1984;81:3684. [Google Scholar]
- 74.Miyamoto S, Kollman PA. J. Comput. Chem. 1992;13:952. [Google Scholar]
- 75.Hess B, Bekker H, Berendsen HJC, Fraaije J. J. Comput. Chem. 1997;18:1463. [Google Scholar]
- 76.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. J. Chem. Phys. 1995;103:8577. [Google Scholar]
- 77.Bussi G, Donadio D, Parrinello M. J. Chem. Phys. 2007;126 doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 78.Marqusee S, Baldwin RL. Proc. Natl. Acad. Sci. U. S. A. 1987;84:8898. doi: 10.1073/pnas.84.24.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanner MF, Olson AJ, Spehner JC. Biopolymers. 1996;38:305. doi: 10.1002/(SICI)1097-0282(199603)38:3%3C305::AID-BIP4%3E3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 80.Humphrey W, Dalke A, Schulten K. J. Mol. Graph. 1996;14:33. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 81.Frishman D, Argos P. Proteins. 1995;23:566. doi: 10.1002/prot.340230412. [DOI] [PubMed] [Google Scholar]
- 82.Daura X, Gademann K, Jaun B, Seebach D, van Gunsteren WF, Mark AE. Angew. Chem.-Int. Edit. 1999;38:236. [Google Scholar]
- 83.Kumar S, Nussinov R. J. Mol. Biol. 1999;293:1241. doi: 10.1006/jmbi.1999.3218. [DOI] [PubMed] [Google Scholar]
- 84.Ruscio JZ, Fawzi NL, Head-Gordon T. J. Comput. Chem. 2010;31:620. doi: 10.1002/jcc.21355. [DOI] [PubMed] [Google Scholar]
- 85.Ball KA, Phillips AH, Nerenberg PS, Fawzi NL, Wemmer DE, Head-Gordon T. Biochemistry. 2011;50:7612. doi: 10.1021/bi200732x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tarus B, Straub JE, Thirumalai D. J. Mol. Biol. 2008;379:815. doi: 10.1016/j.jmb.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luttmann E, Fels G. Chem. Phys. 2006;323:138. [Google Scholar]
- 88.Munoz V, Thompson PA, Hofrichter J, Eaton WA. Nature. 1997;390:196. doi: 10.1038/36626. [DOI] [PubMed] [Google Scholar]
- 89.Karplus M. J. Phys. Chem. B. 2000;104:11. [Google Scholar]
- 90.Ferrara P, Apostolakis J, Caflisch A. J. Phys. Chem. B. 2000;104:5000. [Google Scholar]
- 91.Zheng WH, Andrec M, Gallicchio E, Levy RM. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15340. doi: 10.1073/pnas.0704418104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zuckerman DM. Equilibrium sampling in biomolecular simulations. In: Rees DC, Dill KA, Williamson JR, editors. Annual Review of Biophysics, Vol 40. Vol. 40. Palo Alto: 2011. p. 41. Annual Reviews: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Denschlag R, Lingenheil M, Tavan P. Chem. Phys. Lett. 2008;458:244. doi: 10.1021/ct8000365. [DOI] [PubMed] [Google Scholar]
- 94.Beck DAC, White GWN, Daggett V. J. Struct. Biol. 2007;157:514. doi: 10.1016/j.jsb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antzutkin ON, Leapman RD, Balbach JJ, Tycko R. Biochemistry. 2002;41:15436. doi: 10.1021/bi0204185. [DOI] [PubMed] [Google Scholar]
- 96.Hoyer W, Gronwall C, Jonsson A, Stahl S, Hard T. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5099. doi: 10.1073/pnas.0711731105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reddy G, Straub JE, Thirumalai D. J. Phys. Chem. B. 2009;113:1162. doi: 10.1021/jp808914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. J. Biol. Chem. 1997;272:22364. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 99.Eisenhauer PB, Johnson RJ, Wells JM, Davies TA, Fine RE. J. Neurosci. Res. 2000;60:804. doi: 10.1002/1097-4547(20000615)60:6<804::AID-JNR13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 100.Tomidokoro Y, Rostagno A, Neubert TA, Lu Y, Rebeck GW, Frangione B, Greenberg SM, Ghiso J. Am. J. Pathol. 2010;176:1841. doi: 10.2353/ajpath.2010.090636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Nostrand WE, Melchor JP, Cho HS, Greenberg SM, Rebeck GW. J. Biol. Chem. 2001;276:32860. doi: 10.1074/jbc.M104135200. [DOI] [PubMed] [Google Scholar]
- 102.Bornebroek M, Haan J, Roos RAC. Amyloid-Int. J. Exp. Clin. Investig. 1999;6:215. doi: 10.3109/13506129909007331. [DOI] [PubMed] [Google Scholar]
- 103.Paivio A, Jarvet J, Graslund A, Lannfelt L, Westlind-Danielsson A. J. Mol. Biol. 2004;339:145. doi: 10.1016/j.jmb.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 104.Whalen BM, Selkoe DJ, Hartley DM. Neurobiol Dis. 2005;20:254. doi: 10.1016/j.nbd.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 105.Zameer A, Schulz P, Wang MS, Sierks MR. Biochemistry. 2006;45:11532. doi: 10.1021/bi060601o. [DOI] [PubMed] [Google Scholar]
- 106.Legleiter J, Czilli DL, Gitter B, DeMattos RB, Holtzman DM, Kowalewski T. J. Mol. Biol. 2004;335:997. doi: 10.1016/j.jmb.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 107.Liu RT, Yuan B, Emadi S, Zameer A, Schulz P, McAllister C, Lyubchenko Y, Goud G, Sierks MR. Biochemistry. 2004;43:6959. doi: 10.1021/bi049933o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.