Abstract
Treatment of HEK293 cells expressing the δ-opioid receptor with agonist [d-Pen2,5]enkephalin (DPDPE) resulted in the rapid phosphorylation of the receptor. We constructed several mutants of the potential phosphorylation sites (Ser/Thr) at the carboxyl tail of the receptor in order to delineate the receptor phosphorylation sites and the agonist-induced desensitization and internalization. The Ser and Thr were substituted to alanine, and the corresponding mutants were transiently and stably expressed in HEK293 cells. We found that only two residues, i.e. Thr358 and Ser363, were phosphorylated, with Ser363 being critical for the DPDPE-induced phosphorylation of the receptor. Furthermore, using alanine and aspartic acid substitutions, we found that the phosphorylation of the receptor is hierarchical, with Ser363 as the primary phosphorylation site. Here, we demonstrated that DPDPE-induced rapid receptor desensitization, as measured by adenylyl cyclase activity, and receptor internalization are intimately related to phosphorylation of Thr358 and Ser363, with Thr358 being involved in the receptor internalization.
Prolonged exposure to opioid drugs often produces tolerance, dependence, and addiction, and the molecular mechanisms underlying these phenomena are still poorly understood. Opioid receptors belong to the G protein-coupled receptor (GPCR)1 superfamily (1, 2). Therefore, one component of opioid tolerance is likely to be mediated by a phosphorylation-dependent desensitization of the receptor. Phosphorylation of the prototypic β2-adrenergic receptor by protein kinases, including the G protein-coupled receptor kinases (GRKs), promotes the association with the receptor of inhibitory proteins called arrestins (3, 4). This association uncouples the receptor from the G proteins and promotes targeting of activated receptors to clathrin-coated pits for subsequent internalization, thus blunting receptor signaling.
Agonist-induced phosphorylation of opioid receptors has been demonstrated in different systems (5–10). Concrete demonstration of the phosphorylation of δ-opioid receptor (DOR) was first reported by Pei and colleagues (6). Although the sites of agonist-dependent phosphorylation have not yet been identified, truncation of the carboxyl tail (C-tail) of DOR showed that major phosphorylation sites are localized within this domain (11, 12). Phosphorylation of DOR appears to be the mechanism for agonist-induced receptor desensitization (6, 13, 14). [d-Pen2,5]enkephalin (DPDPE)-induced phosphorylation of DOR seems to involve one or more GRKs (6), and Ala substitution of the last four carboxyl-terminal Ser and Thr of the receptor impaired the GRK- and arrestin-mediated receptor desensitization (13). In Xenopus oocytes, co-expression of GRK3 and β-arrestin2 resulted in an increased rate of agonist-induced homologous desensitization of DOR (13). However, truncation of the COOH-terminal 31 amino acids of DOR did not affect the agonist-induced desensitization of the receptor in CHO cells (15), unlike what Zhao and colleagues (11) reported in NG108-15 cells. Opioid receptors are endocytosed in a dynamin-dependent manner by clathrin-coated pits (14, 16–18). However, the precise role of phosphorylation in the mechanism of opioid receptor endocytosis is still not fully understood. A truncated mutant δ-opioid receptor undergoes rapid agonist-induced internalization in HEK293 cells, but is not phosphorylated in the presence of agonist, whereas the same mutant remained predominantly in the plasma membrane of CHO cells, suggesting that cell type-specific differences may exist in the biochemical requirements for the agonist-induced endocytosis (12).
In light of these discrepancies, it is imperative to identify the agonist-induced phosphorylation sites and to investigate the role of the phosphorylation of these sites in the regulation of the δ-opioid receptor. We used series of receptor mutants to identify the phosphorylation sites at the C-tail of DOR. In this study, we reported that two sites (Thr358 and Ser363) are phosphorylated in the presence of DPDPE and, furthermore, that the phosphorylation of the receptor is hierarchical. Additionally, we investigated the role played by these two phosphorylation sites in internalization of the receptor. Here, we also demonstrated that internalization of the activated receptor plays a role in the loss of δ-opioid receptor-mediated inhibition of adenylyl cyclase activity.
EXPERIMENTAL PROCEDURES
Materials
Dulbecco’s modified Eagle’s medium and Geneticin (G418) were purchased from Life Technologies, Inc. [3H]Diprenorphine (58 Ci/mmol) was supplied by Amersham Pharmacia Biotech and [32P]orthophosphate (>400 Ci/ml) by ICN (Costa Mesa, CA). 125I-Acetylated cAMP (2200 Ci/mmol) was purchased from Linco Research Inc. (St. Charles, MO). Polyclonal antibodies that recognize the acetylated cAMP were purchased from Calbiochem (La Jolla, CA). NIDA (National Institutes on Health, Bethesda, MD) supplied the [d-Pen2,5]enkephalin ligand. All other chemicals were purchased from Sigma.
Generation of the Mutants of the δ-Opioid Receptor
The human influenza virus hemagglutinin (HA) epitope-tagged mouse δ-opioid receptor (described in Ref. 19) (Dortag) subcloned into the expression vector pcDNA3 (Invitrogen, Carlsbad, CA), was used to generate most of the point mutations. Ser and/or Thr present at the C-tail of the receptor (from Thr335 to Ser363) were point-mutated to Ala or Asp by oligonucleotide-directed mutagenesis using a QuickChange site-directed mutagenesis kit from Stratagene (La Jolla, CA) according to the manufacturer’s directions, except for the following mutants. The T358A, S363A, and S363D mutants were constructed using the Altered Sites™ in vitro mutagenesis system provided by Promega Corp. (Madison, WI), using DOR-1 cDNA subcloned into the phagmid pAlter-1 as template. The nucleotide sequences of all mutants were confirmed by dideoxynucleotide sequencing using Sequenase II. The Eco47III-XbaI fragments of the different plasmids were excised and ligated to the Dortag in pCDNA3 with the same fragment removed.
Cell Culture and Transfections
Human embryonic kidney HEK293 cells were cultured in minimal essential medium supplemented with 10% fetal bovine serum, 100 µg/ml streptomycin, 100 IU/ml penicillin under humidified atmosphere at 5% CO2. Cells were transiently transfected by the calcium phosphate precipitation method, and the assays were performed 48 h after transfection. Pool of stably transfected cells expressing the wild-type or mutant receptors were isolated in the presence of 1 mg/ml G418, without selection of individual clones, to avoid any position effect due to the random integration of each cDNA into the chromosomes. Receptor expressions were determined by whole cell binding using [3H]diprenorphine in 25 mm HEPES buffer, pH 7.6. Specific binding is defined as the difference between the radioactivity bound to the cells in the absence and presence of 100 µm naloxone. HEK293 cells stably expressing the ecdysone receptor in the pVgRxR vector (EcR-293 cells) were purchased from Invitrogen and clones stably co-expressing the wild-type or S363A mutant receptors were described in Ref. 21. 0.2 µm or 2 µm ponasterone A (PA), used to induced respectively low (0.37 ± 0.14 pmol/mg of protein for the wild-type receptor; 0.14 ± 0.1 pmol/mg of protein for the S363A mutant) or high (1.03 ± 0.5 pmol/mg of protein for the wild-type receptor; 1.15 ± 0.7 pmol/mg of protein for the S363A mutant) receptor level, were added 48 h before assays.
Opioid Inhibition of Intracellular cAMP Level
HEK293 cells seeded in 100-mm dishes were transiently transfected with 10 µg of wild-type or mutant receptor cDNAs, as indicated. The next day, cells were washed, detached, and plated in 24-well plates. Assays were performed the following day. The cells were pretreated with 1 µm DPDPE for various time intervals (15 min to 6 h), and were challenged with the same concentration of DPDPE. The intracellular cAMP level was determined by radioimmunoassay using 125I-acetylated cAMP and rabbit polyclonal antibodies that recognize the acetylated cAMP, as described in Ref. 10. The amount of cAMP in each well was determined by comparing the ability of the diluted samples to compete for 125I-acetylated cAMP binding to the antibodies with that of standard concentrations of acetylated cAMP.
Receptor Phosphorylation and Immunodetection
Transiently or stably transfected HEK293 cells were seeded in 100-mm dishes, and proteins were solubilized as described previously in Ref. 10. Briefly, DOR and mutant receptors were immunoprecipitated using the rat monoclonal antibody 3F10 (Roche Molecular Biochemicals) and a 60-µl slurry (50%) of prewashed immunopure protein G-agarose beads (Pierce) overnight at 4 °C. Receptor proteins were dissociated from the beads by adding 60 µl of SDS-PAGE sample buffer (62.5 mm Tris buffer, pH 6.8, 2% SDS, 3 m urea, 10% glycerol, 5% 2-mercaptoethanol, and 0.001% bromphenol blue). The samples were heated at 42 °C for 1 h and then separated on a 10% SDS-PAGE. Phosphorylated proteins were visualized and quantified by using the PhosphorImager Storm 840 system (Molecular Dynamics), and immunodetections were performed with the peroxidase-conjugated monoclonal antibodies (12CA5) to the HA epitope tag (Roche Molecular Biochemicals). Detection was performed using the ECL Plus Western blotting detection system (Amersham Pharmacia Biotech). Immunoblots were quantitated by densitometric scanning of film exposed in the linear range (Molecular Analysis Software, Bio-Rad).
Quantitation of δ-Opioid Receptor Internalization by Fluorescence Flow Cytometry
Stably transfected HEK293 cells expressing wild-type or mutant HA epitope-tagged receptors were treated with 1 µm DPDPE for the indicated times. Cells were chilled on ice to stop membrane trafficking, and receptors were visualized by using a 1:400 dilution of the high affinity mouse monoclonal anti-HA antibody (HA.11 clone 16B12; Babco, Richmond, CA) and a 1:500 dilution of the secondary antibodies goat anti-mouse IgG conjugated with Alexa488 (Molecular Probes, Eugene, OR). Incubations were performed at 4 °C. Surface receptor staining intensity of antibody-labeled cells was analyzed using fluorescence flow cytometry (FACScan, Becton Dickenson, Palo Alto, CA).
Data Analysis
Data were analyzed using the GraphPad program. Mean values from individual treatment groups were statistically analyzed by a one-way analysis of variance with subsequent comparisons among treatment groups from their control by Student’s t test.
RESULTS
To investigate the in vivo phosphorylation properties of DOR, HEK293 cells expressing the wild-type receptor were radiolabeled with [32P]orthophosphate (32Pi), then treated with a saturating concentration (1 µm) of DPDPE, and finally receptors were immunopurified and analyzed by SDS-PAGE autoradiography (Fig. 1a). Nonspecific signal in these experiments was negligible (lane 1). In presence of DPDPE, phosphorylation of the receptor was strongly stimulated (lane 3) when compared with the basal phosphorylation in absence of agonist (lane 2) and revealed a diffuse phosphoprotein band migrating at approximately 50–60 kDa, corresponding to that of the HA epitope-tagged DOR (Fig. 1b). Phosphorylation of this protein band was time- and concentration-dependent (data not shown), and the phosphorylation patterns were similar in HEK293 cells either transiently or stably expressing the receptor (Figs. 1, 3, and 4). Additional immunoreactive species were observed in some experiments with an apparent molecular mass corresponding to the receptor dimers, consistently with previously described oligomers of DOR (19, 20).
FIG. 1. In vivo DPDPE-induced phosphorylation of wild-type δ-opioid receptor.
HEK293 cells transiently transfected with 10 µg of HA epitope-tagged DOR cDNA or empty vector (mock) were labeled with 32Pi and stimulated with 1 µm DPDPE for 30 min. The receptors were purified as described under “Experimental Procedures” and analyzed by phosphorimaging (a) and immunoblotting (b).
FIG. 3. Analysis of mutant δ-opioid receptors DPDPE-dependent phosphorylation.
Cells transiently transfected with 10 µg of each cDNA were labeled with 32Pi and stimulated with 1 µm DPDPE for 30 min. a, extracted phosphoreceptors from wild type and various Ser/Thr mutant receptors. Pictured is the result of a single experiment representative of at least three performed. b, receptor proteins detected by immunoblot of the corresponding phosphorylated gel. c, receptor phosphorylated bands were quantitatively analyzed with PhosphorImager, and band intensities were expressed as percentage of maximal phosphorylation obtained for DOR in presence of 1 µm DPDPE. For each experiment, the amount of 32Pi incorporated into the various receptors was expressed as a function of the amount of receptor present in each lane (assessed by densitometric analysis of the immunoblot). Data shown are means ± S.E.
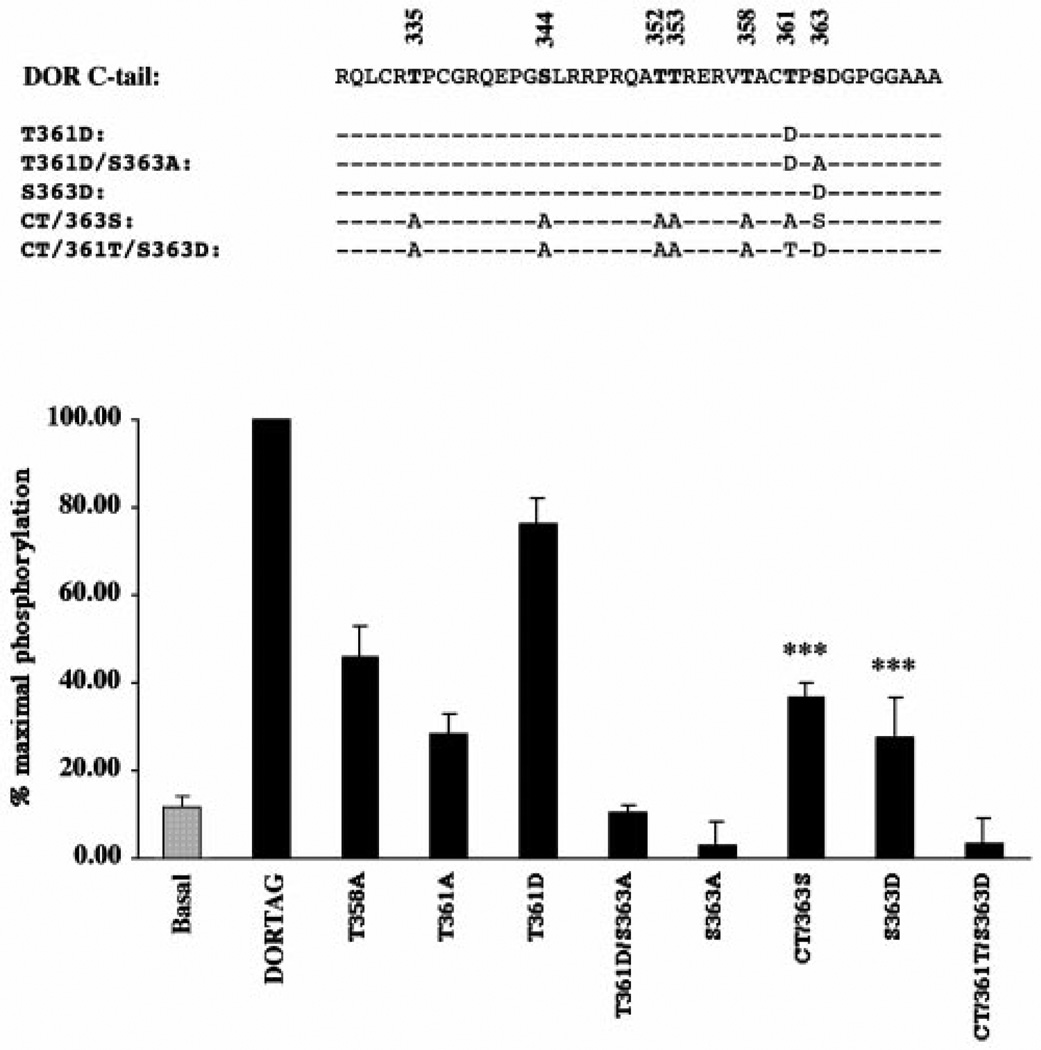
FIG. 4. Hierarchical DPDPE-dependent phosphorylation of DOR.
HEK293 cells stably expressing the wild type or mutant receptors were labeled, and the ability of 1 µm DPDPE to induce phosphorylation was determined as described under “Experimental Procedures.” Top, amino acid sequence of the intracellular C-tail of DOR. The different Ala or Asp substitutions are indicated, and the dashed lines represent no changes from the wild-type sequence. Bottom, receptors were purified and resolved on a SDS-PAGE. After quantification by phosphorimaging and immunoblotting, intensities of the phosphorylated bands were expressed as a percentage of DPDPE-induced phosphorylation of the wild-type receptor. Data shown represent mean ± S.E. of at least three separate experiments. ***, p < 0.001 compared with the phosphorylation pattern of the S363A mutant.
Previous studies suggested that agonist-induced phosphorylation of DOR occurs at the carboxyl tail domain (11, 12). The intracellular C-tail of the mouse DOR contains seven Ser and Thr putative phosphorylation sites (Fig. 2). To identify which Ser and/or Thr are phosphorylated in response to DPDPE, individual or multiple Ser/Thr were substituted into Ala. All these mutant receptors were stably expressed in HEK293 cells, and no significant differences were detected in their ability to bind the antagonist diprenorphine or the agonist DPDPE, when compared with that of the wild-type receptor (Table I). Ala substitution of all Ser and Thr at the C-tail of the receptor (mutant DTS) completely blocked the DPDPE-induced phosphorylation (Fig. 3), indicating that the phosphorylation site(s) are likely located within the COOH terminus of the receptor. We systematically substituted all these carboxyl terminus Ser/Thr to Ala. When compared with the wild-type receptor, the T335A, S344A, T352A, or T353A mutants did not display any significant differences in agonist-induced receptor phosphorylation, indicating that none of these four residues are involved in the DPDPE-induced phosphorylation of the receptor (Fig. 3, lanes 3–6). In contrast, mutation of Thr358, Thr361, or Ser363 to Ala dramatically reduced the phosphorylation level to about 47.7%, 66.3%, or 91.8%, respectively, as compared with the wild-type receptor, indicating that these residues are involved in the agonist-induced phosphorylation of the receptor (Fig. 3). Interestingly, little or no agonist-induced phosphorylation could be detected with the S363A mutant, indicating that Ser363 is critical for the overall phosphorylation of DOR. The complete blockade of the DPDPE-induced phosphorylation of the receptor by the S363A mutation (Figs. 3 and 4), suggested that this residue is either a phosphorylation site or is a kinase recognition site. The actual phosphorylation of this site was demonstrated by the mutant receptor in which all the Ser/Thr residues at the C-tail, except Ser363, were substituted to Ala (mutant CT/363S, Fig. 4). This mutant showed a significant agonist-induced phosphorylation and reached 36.6 ± 3.2% (n = 9) of that of the wild-type receptor. This result showed clearly that Ser363 is the primary phosphorylation site, and that phosphorylation of DOR could be hierarchical with Ser363 being the first residue to be phosphorylated.
FIG. 2. Substitution mutagenesis of the δ-opioid receptor C-tail.
Potential phosphorylation sites (Ser/Thr) of the C-tail are indicated in bold. The numbers above indicate the amino acid positions in the receptor protein. The amino acid sequences of the mutants identical to the wild type are represented by dashed lines, and the changes are as indicated.
Table I. Characterization of the δ-opioid receptor and mutant receptors lacking putative phosphorylation sites.
Radioligand binding studies were performed on membrane preparation as described in Ref. 35. KD values of diprenorphine were determined by saturation binding. KH and KL represent high (H) and low (L) affinity dissociation constants for DPDPE; RH (%) represents the percentage of receptor in the high affinity binding state for the agonist. KH, KD, and RH were determined by competition assay. Data from competition and saturation binding were fitted by non-linear regression curve fitting using the data analysis program GraphPad. Data shown are mean ± S.D. of at least two independent experiments. Wild type and mutant receptors have comparable affinities for [3H]diprenorphine and DPDPE, and mutation of the Ser/Thr at the C-tail did not affect the proportion of receptors in the agonist-detected high affinity states.
|
Kd diprenorphine |
KD DPDPE | RH | ||
|---|---|---|---|---|
| KH | KL | |||
| nM | nM | % | ||
| DOR | 0.9 ± 0.1 | 2.3 ± 0.9 | 298.6 ± 37 | 65.2 ± 7.0 |
| T335A | 0.9 ± 0.1 | 4.9 ± 1.3 | 278.7 ± 104.8 | 57.9 ± 2.8 |
| S344A | 1.0 ± 0.1 | 2.2 ± 1.2 | 166.8 ± 76.9 | 55.3 ± 8.7 |
| T352A | 1.35 ± 0.1 | 2.4 ± 1.6 | 287.7 ± 116.3 | 55.4 ± 5.4 |
| T353A | 1.1 ± 0.1 | 4.2 ± 1.5 | 303.3 ± 40.4 | 65.2 ± 0.7 |
| T358A | 0.9 ± 0.1 | 1.5 ± 0.5 | 189.0 ± 49.4 | 57.3 ± 3.9 |
| T361A | 0.8 ± 0.1 | 1.4 ± 0.1 | 137.1 ± 54.0 | 57.3 ± 7.6 |
| S363A | 0.8 ± 0.2 | 2.5 ± 1.5 | 282.7 ± 60.1 | 60.1 ± 8.4 |
| T361A/S363A | 1.1 ± 0.1 | 2.7 ± 0.1 | 205.3 ± 52.1 | 57.5 ± 7.8 |
| DTS | 0.9 ± 0.1 | 3.4 ± 0.9 | 319.2 ± 77.8 | 62.7 ± 7.9 |
| T361D | 1.3 ± 0.2 | 3.5 ± 1.5 | 485.3 ± 100.3 | 69.2 ± 1.5 |
| T361D/S363A | 1.2 ± 0.2 | 3.5 ± 1.5 | 192.9 ± 114.3 | 63.2 ± 8.6 |
| CT/A363S | 0.8 ± 0.2 | 2.1 ± 0.8 | 97.6 ± 31.3 | 53.7 ± 5.0 |
| S363D | 2.4 ± 0.1 | 3.5 ± 0.9 | 185.0 ± 13.1 | 65.0 ± 3.3 |
| CT/361T/S363D | 1.0 ± 0.1 | 3.2 ± 0.9 | 260 ± 54.1 | 69.1 ± 0.03 |
In order to identify the other phosphorylation sites, Ser363 was mutated to aspartic acid (Fig. 4), which mimics a phosphorylated form of this residue, and which should induce a recovery of the receptor phosphorylation, if the mechanism is hierarchical. As shown in Fig. 4, the S363D mutant restores the DPDPE-induced phosphorylation (compared with the S363A mutant) to about 27.5% of that of the wild-type receptor. This result confirmed, therefore, that Ser363 has to be phosphorylated before another site, and that Thr358 and/or Thr361 is (are) the other phosphorylation site(s). No phosphorylation, however, could be detected with a mutant leaving only Thr361 as a potential phosphorylation site along with the Asp-substituted Ser363 (mutant CT/361T/S363D), suggesting, therefore, that Thr361 is not phosphorylated right after Ser363, but rather Thr358 is. Indeed, the extent of phosphorylation in the T358A mutant is not significantly different (p > 0.1) from the extent of phosphorylation in the mutant leaving Ser363 as the only potential phosphorylation site (mutant CT/363S). Consistently, the lower level of phosphorylation in the S363D mutant (reaching only about 27.5% of that of the wild-type receptor) confirms that only one site is phosphorylated along with Ser363 and that this other residue is Thr358. Nevertheless, the T361A mutation affected the agonist-induced phosphorylation of the receptor. Thr361 could participate in the phosphorylation of the receptor as a kinase recognition/binding site but not as a phosphorylation site. The T361D mutant, which was originally constructed to define the phosphorylation mechanism of DOR, exhibiting almost but not complete recovery of the phosphorylation level as compared with that of the wild-type receptor, might affect to a lesser extent the kinase’s recognition/binding to the receptor. This observed recovery was, however, blocked when Ser363 was mutated into Ala along with the T361D substitution (mutant T361D/S363A, Fig. 3), consistently with the critical role of Ser363 in the phosphorylation of the receptor.
Next, we examined the role of phosphorylation in the agonist-induced desensitization of DOR in HEK293 cells. The kinetics of the loss of DPDPE inhibition of the adenylyl cyclase activity were monophasic, and the apparent rate of desensitization was relatively slow, with a t1/2 of about 1.3 ± 0.25 h (n = 6, data not shown), consistently with previous study (10). In contrast, DPDPE-induced phosphorylation of the receptor followed much faster kinetics, showing a maximum within 10 min of DPDPE exposure (data not shown). The difference in these two rates suggests that receptor phosphorylation does not directly lead to DOR desensitization. The failure to correlate phosphorylation to desensitization of DOR could be due to the relatively high level of receptor expression (2.2 ± 0.8 pmol/mg of protein) in HEK293 cells. In an earlier study, by controlling the expression level of the receptor in HEK293 cells with an ecdysone-inducible expression system (21), we were able to demonstrate that the rate of DOR desensitization is dependent on the receptor level expressed at the cell surface. Using the same inducible-expression system to control the expression of the wild type and S363A mutant receptors, in HEK293 cells (see “Experimental Procedures”), we could demonstrate that receptor desensitization kinetics in the presence of DPDPE were receptor concentration-dependent (Fig. 5). At high levels of receptor expression (>1 pmol/mg of protein), the desensitization kinetics of both receptors were relatively slow and indistinguishable, suggesting that the absence of receptor phosphorylation did not affected the observed rate of desensitization. Similar results were obtained when the wild-type receptor and a mutant receptor incapable of being phosphorylated (mutant T361A/S363A) were transiently expressed in HEK293 cells (data not shown). At low levels of expression of the wild type receptor (<300 fmol/mg of protein), when receptor concentration was limited, the DPDPE-induced loss of response was significantly faster, and the receptor desensitized completely 40 min following DPDPE treatment. The elimination of receptor phosphorylation (S363A mutant) reduced dramatically the extent of desensitization, when expressed at similar levels as the wild-type receptor. At 40 min following agonist treatment, 50% of the response remained. Thus, the hierarchical phosphorylation of DOR at Ser363 is an important but not obligatory event in the DPDPE-induced desensitization.
FIG. 5. Receptor density affected the DPDPE-induced desensitization of the wild type and S363A mutant receptors.
Wild type (■, □) and S363A (▲, △) mutant receptor expression levels were controlled using the ecdysone-inducible expression system as described previously (21), and the ability of 1 µm DPDPE to inhibit the forskolin-activated adenylyl cyclase activity was performed accordingly. Cells were pretreated respectively with 2 µm PA (□, △) or 0.2 µm PA (■, ▲) to induce a high and low expression of the receptors. These PA concentrations were chosen so as to induce similar expression of the wild type and mutant receptors. Values represented mean ± S.D. of at least three experiments performed in triplicate.
Since internalization is involved in the desensitization of several GPCRs (4), and since in our earlier study (21) we demonstrated that receptor internalization also participated in the rapid desensitization of DOR, we examined whether the DPDPE-induced phosphorylation of Thr358 and Ser363 would participate in the agonist-induced receptor internalization. The extent of internalization of the wild type and mutant receptors from the cell surface were examined using flow cytometry (Fig. 6). Kinetics analysis indicated that DOR internalized rapidly in presence of DPDPE and that about 80% of total cell surface receptor were internalized in response to 1 h agonist treatment, with a t1/2 = 10.8 ± 1.6 min (Table II). This rate compared favorably with previous reports (12, 17, 21). Substitution of the four first Ser or Thr at the C-tail into Ala (T335A to T353A) did not slow down the rate or lower the extent of internalization of the corresponding activated receptors. These results suggested, therefore, that none of these four residues are involved in the DPDPE-induced internalization of the receptor in HEK293 cells (Table II, group I). In contrast, the DTS mutant as well as the S363A mutant, both blocking the DPDPE-induced phosphorylation of the receptor, revealed a substantially slower rate and lower extent of internalization, strongly suggesting that phosphorylation of Ser363 and/or Thr358 contributed to the receptor internalization (Fig. 6 and Table II). Phosphorylation of Ser363 alone (mutant CT/363S) prevented the receptor from internalizing as efficiently as the wild-type receptor, suggesting that the phosphorylation of this Ser only is insufficient to induce receptor internalization. Ala substitution of Thr358 also blunted the agonist-induced internalization of the receptor. These results suggest that phosphorylation of Thr358 is critical for receptor internalization. Indeed, the S363D mutant (Table II, group I), allowing the DPDPE-induced phosphorylation of Thr358, exhibited a pattern of receptor internalization identical to the wild-type receptor. Therefore, internalization of DOR required the phosphorylation of Thr358, subsequent to the phosphorylation of Ser363.
FIG. 6. Kinetics of DPDPE-induced internalization of wild type and mutant receptors in HEK293 cells.
Receptor internalization was quantitated in stably transfected cells using fluorescence flow cytometry to measure the DPDPE-induced removal of HA epitope-tagged receptors from the plasma membrane. Cells were treated with 1 µm DPDPE for the indicated period of time and receptors were labeled with anti-HA antibody, as described under “Experimental Procedures.” Point represents average of mean fluorescence values (± S.E.) of at least three independent experiments performed in triplicate. The mean fluorescence after subtracting the autofluorescence of the cells (stained with the secondary antibody alone) without agonist treatment is taken as 100%.
Table II. Internalization parameters of the wild-type and mutant receptors in HEK293 cells.
The kinetics parameters of internalization of the wild type and mutant receptors were determined by one exponential curve fitting using the GraphPad program; internalization (%) represents the estimated plateau calculated using the GraphPad program. Mutant receptors could be organized into two groups, when comparing their internalization kinetics to that of the wild-type receptor. The first group comprised receptors showing similar properties as those of DOR, and the second group included receptors showing a substantially slower rate and/or slower extent of internalization.
| t1/2 | Internalization | |
|---|---|---|
| min | % | |
| DOR | 10.8 ± 1.6 | 80.2 ± 3.0 |
| Group I | ||
| T335A | 11.4 ± 0.2 | 80.6 ± 2.4 |
| S344A | 7.6 ± 1.4 | 78.2 ± 4.5 |
| T352A | 15.2 ± 1.3 | 76.8 ± 9.5 |
| T353A | 14.2 ± 2.6 | 70.8 ± 2.7 |
| S363D | 12.2 ± 0.6 | 74.6 ± 2.2 |
| Group II | ||
| T358A | 18.5 ± 3.5 | 56.0 ± 3.6** |
| S363A | 17.3 ± 2.0* | 63.3 ± 11.5** |
| DTS | 17.1 ± 3.1 | 56.4 ± 1.8** |
| CT/A363S | 12.3 ± 2.4 | 60.8 ± 2.6** |
p < 0.05;
p < 0.01, as compared to the wild-type receptor.
DISCUSSION
From our current mutagenesis studies, it is apparent that Ser363 is the primary phosphorylation site, that agonist-induced phosphorylation of DOR occurs in a hierarchical manner, and that Thr358 and Ser363 are the only two residues being phosphorylated. Thr361 participates in receptor phosphorylation, since its substitution into Ala reduced the extent of phosphorylation when compared with the wild-type receptor. Probably, the remaining phosphorylation observed with the T361A mutant comes from Ser363, since Ser363 is phosphorylated independently of Ser/Thr present at the C-tail of the receptor (mutant CT/363S, Fig. 4). Mutation of Thr361 could impair the recognition/binding of a kinase to phosphorylate Thr358, thereby reducing the receptor phosphorylation level. Hierarchical phosphorylation has been demonstrated for only a few GPCRs, including the rhodopsin and the N-formyl peptide receptor (22, 23). Whether this phosphorylation mechanism is conserved among members of the GPCR family, or specific to some of them, remains to be demonstrated. It is possible that phosphorylation of Ser363 creates a new acidic phosphoserine recognition sequence for the subsequent phosphorylation of Thr358 by the same or by another kinase. Several groups have proposed that members of the GRK family can phosphorylate DOR (6, 24). Although the consensus motif of GRK among various GPCRs has not been clearly defined, GRK normally phosphorylate Ser/Thr residues adjacent to an acidic or charged amino acid residue (25). Interestingly, Ser363 and Thr358 are immediately downstream of Pro and Val residues, respectively (Fig. 2). Ser363 is upstream from an aspartic residue, which could serve as a GRK recognition motif. However, Thr358 is upstream from an Ala residue. Whether phosphorylation of Ser363 and Thr358 involved a GRK remains to be demonstrated. Some reports suggested that agonist-induced phosphorylation of the opioid receptors could be mediated by Ca2+/calmodulin-dependent protein kinase II (26), or by mitogen-activated protein kinase (27), but the amino acid motif surrounding Thr358 or Ser363 does not correspond to that of either kinase. Thus, it is tempting to suggest that a yet unknown Ser/Thr kinase is responsible for the DPDPE-induced phosphorylation of DOR. The involvement of this unidentified kinase might be one of the reasons why overexpression of a dominant negative mutant of GRK could not completely block the agonist-induced phosphorylation of the receptor (6, 10).
The rapid and slow desensitization of DOR is dependent on the relative level of expression of the receptor. The slow rate of a loss of DPDPE inhibition of forskolin-stimulated adenylyl cyclase in cells expressing a relatively high level of receptors (Fig. 5) does not correlate with the rapid DPDPE-induced phosphorylation of the receptor (data not shown). The relatively high level of receptor expressed at the cell surface that is not phosphorylated and sufficiently high enough to maintain the agonist-mediated activity, along with the high efficient coupling between DOR and the adenylyl cyclase (28), could explain why the phosphorylation of the receptor did not correlate with the loss of response. The fact that the mutants showing no detectable agonist-induced phosphorylation, such as T361A/S363A and S363A mutants (data not shown and Fig. 5), which exhibit a similar rate and extent of desensitization as the wild-type receptor at relatively high receptor levels, suggests that events other than receptor phosphorylation e.g. internalization/sequestration/down-regulation could be involved in the slow desensitization of the receptor. At low levels of receptor expression, similar to the levels of expression observed in endogenously expressing cells, the blockade of the agonist-induced receptor phosphorylation dramatically attenuated the magnitude of rapid desensitization, implying that the receptor phosphorylation at Ser363 participates in receptor regulation. Nevertheless, the ability of the receptor to still desensitize indicates that the agonist-induced internalization contributes also to the loss of response, as it was shown for other GPCRs, like for example for the m3 mACh receptor (29) or the μ-opioid receptor (30, 31). We recently reported that, at low expression levels of μ- and δ-opioid receptors, a blockade of receptor internalization leads to a blockade of the agonist-induced rapid desensitization of these receptors (21, 30). Mutation of Thr358 attenuates the agonist-induced internalization of DOR, implying that phosphorylation of this residue is crucial for receptor internalization and hence contributed to the DPDPE-induced rapid desensitization. Down-regulation of DOR (32, 33), which involves receptor sequestration and degradation, likely contributes to the slow loss of receptor activity, regardless of whether or not the receptor is phosphorylated (Fig. 5). Thr353 has been reported to be required for the agonist-induced down-regulation of DOR in CHO cells (34). Since the DPDPE-induced phosphorylation of DOR in HEK293 cells does not involve Thr353 (Fig. 3), the ability of the receptor to down-regulate will not be affected by the inability of the receptor to be phosphorylated. Similarly, for the μ-opioid receptor, mutation of Ser363 to Ala attenuated agonist-induced down-regulation without being an agonist-induced phosphorylation site (35), showing that phosphorylation of a particular site is not necessarily a signal for the processing of receptor traffic at the early endosome. Whether or not Thr353 is involved in DPDPE-induced down-regulation of the receptor in HEK293 cells remains to be demonstrated, since a truncated δ-opioid receptor remaining predominantly in the plasma membrane of CHO cells, can still internalize in HEK293 cells (12). Alternatively, a NPXXY motif, common to many GPCRs including the opioid receptors (36), present at the interface between the seventh transmembrane and the C-tail of the receptor, or a di-Leu-based motif (37) present within the third cytoplasmic domain of DOR, could be implicated in the trafficking of the receptor.
We can propose that phosphorylation of Ser363 will promote the uncoupling of the activated receptor from its cognate G protein. Then, phosphorylation of Thr358 will regulate internalization of the receptor, further attenuating receptor-mediated signaling. Therefore, functional uncoupling of receptor and G protein, followed by endocytosis, will blunt the response to agonists. Native opioid peptides or opiate drugs are relatively resistant to proteolytic degradation in the extracellular environment. Therefore, opioid receptors may use this regulation mechanism to alter persistent activation. Whether the same mechanism persists in all in vitro cell models or in vivo remains to be demonstrated.
Acknowledgment
We thank Rachid El Kouhen for valuable discussions.
Footnotes
This work was supported in part by National Institutes of Health Grants DA00564, DA07339, DA01583, DA11806 and the F. and A. Stark Fund of the Minnesota Medical Foundation.
The abbreviations used are: GPCR, G protein-coupled receptor; DOR, δ-opioid receptor; GRK, G protein-coupled receptor kinase; DP-DPE, [d-Pen2,5]enkephalin; HA, hemagglutinin; PAGE, polyacrylamide gel electrophoresis; PA, ponasterone A; CHO, Chinese hamster ovary.
REFERENCES
- 1.Evans CJ, Keith DE, Morrison H, Magendzo K, Edwards RH. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- 2.Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. Proc. Natl. Acad. Sci. U. S. A. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ. J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 4.Krupnick JG, Benovic JL. Annu. Rev. Pharmacol. Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 5.Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. J. Neurochem. 1995;65:1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- 6.Pei G, Kieffer BL, Lefkowitz RJ, Freedman NJ. Mol. Pharmacol. 1995;48:173–177. [PubMed] [Google Scholar]
- 7.Zhang L, Yu Y, Mackin S, Weight FF, Uhl GR, Wang JB. J. Biol. Chem. 1996;271:11449–11454. doi: 10.1074/jbc.271.19.11449. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y, Zhang L, Yin X, Sun H, Uhl GR, Wang JB. J. Biol. Chem. 1997;272:28869–28874. doi: 10.1074/jbc.272.46.28869. [DOI] [PubMed] [Google Scholar]
- 9.Appleyard SM, Patterson TA, Jin W, Chavkin C. J. Neurochem. 1997;69:2405–2412. doi: 10.1046/j.1471-4159.1997.69062405.x. [DOI] [PubMed] [Google Scholar]
- 10.El Kouhen R, Maestri-El Kouhen O, Law P-Y, Loh HH. J. Biol. Chem. 1999;274:9207–9215. doi: 10.1074/jbc.274.14.9207. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Pei G, Huang YL, Zhong FM, Ma L. Biochem. Biophys. Res. Commun. 1997;238:71–76. doi: 10.1006/bbrc.1997.7242. [DOI] [PubMed] [Google Scholar]
- 12.Murray SR, Evans CJ, von Zastrow M. J. Biol. Chem. 1998;273:24987–24991. doi: 10.1074/jbc.273.39.24987. [DOI] [PubMed] [Google Scholar]
- 13.Kovoor A, Nappey V, Kieffer BL, Chavkin C. J. Biol. Chem. 1997;272:27605–27611. doi: 10.1074/jbc.272.44.27605. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Ferguson SSG, Barak LS, Bodduluri SR, Laporte SA, Law P-Y, Caron MG. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Zhou D, Cheng Z, Wei Q, Chen J, Li G, Pei G, Chi Z. Biochem. Biophys. Res. Commun. 1998;249:321–324. doi: 10.1006/bbrc.1998.9080. [DOI] [PubMed] [Google Scholar]
- 16.Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. J. Biol. Cell. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- 17.Trapaidze N, Keith DE, Cvejic S, Evans CJ, Devi LA. J. Biol. Chem. 1996;271:29279–29285. doi: 10.1074/jbc.271.46.29279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu P, Murray SR, Lissin D, von Zastrow M. J. Biol. Cell. 1997;272:27124–27130. doi: 10.1074/jbc.272.43.27124. [DOI] [PubMed] [Google Scholar]
- 19.Ko JL, Arvidson U, Williams FG, Law P-Y, Elde R, Loh HH. Mol. Brain Res. 1999;69:171–185. doi: 10.1016/s0169-328x(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 20.Jordan BA, Devi LA. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law PY, Maestri-El Kouhen O, Solberg J, Wang W, Erickson LJ, Loh HH. J. Biol. Chem. 2000;275:32057–32065. doi: 10.1074/jbc.M002395200. [DOI] [PubMed] [Google Scholar]
- 22.Ohguro H, Palczewski K, Ericsson LH, Walsh KA, Johnson RS. Biochemistry. 1993;32:5718–5724. doi: 10.1021/bi00072a030. [DOI] [PubMed] [Google Scholar]
- 23.Prossnitz ER, Kim CM, Benovic JL, Ye RD. J. Biol. Chem. 1995;270:1130–13137. doi: 10.1074/jbc.270.3.1130. [DOI] [PubMed] [Google Scholar]
- 24.Hasbi A, Polastron J, Allouche S, Stanalisa L, Massotte D, Jauzac P. J. Neurochem. 1998;70:2129–2138. doi: 10.1046/j.1471-4159.1998.70052129.x. [DOI] [PubMed] [Google Scholar]
- 25.Fredricks ZL, Pitcher JA, Lefkowitz RJ. J. Biol. Chem. 1996;271:13796–13803. doi: 10.1074/jbc.271.23.13796. [DOI] [PubMed] [Google Scholar]
- 26.Koch T, Kroslack T, Mayer P, Raulf E, Hollt V. J. Neurochem. 1997;69:1767–1770. doi: 10.1046/j.1471-4159.1997.69041767.x. [DOI] [PubMed] [Google Scholar]
- 27.Polakiewicz RD, Schieferl SM, Dorner LF, Kansra V, Comb MJ. J. Biol. Chem. 1998;273:12402–12406. doi: 10.1074/jbc.273.20.12402. [DOI] [PubMed] [Google Scholar]
- 28.Fantozzi R, Mullikin-Kilpatrick D, Blume AJ. Mol. Pharmacol. 1981;20:8–15. [PubMed] [Google Scholar]
- 29.Yang J, Williams JA, Yule DI, Logsdon CD. Mol. Pharmacol. 1995;48:477–485. [PubMed] [Google Scholar]
- 30.Pak Y, Kouvelas A, Scheideler MA, Rasmussen J, O’Dowd BF, George SR. Mol. Pharmacol. 1996;50:1214–1222. [PubMed] [Google Scholar]
- 31.Law PY, Erickson LJ, El-Kouhen R, Dicker L, Solberg J, Wang W, Miller E, Burd AL, Loh HH. Mol. Pharmacol. 2000;58:388–398. doi: 10.1124/mol.58.2.388. [DOI] [PubMed] [Google Scholar]
- 32.Tsao PI, von Zastrow M. J. Biol. Chem. 2000;275:11130–11140. doi: 10.1074/jbc.275.15.11130. [DOI] [PubMed] [Google Scholar]
- 33.Hasbi A, Allouche S, Sichel F, Stanalisa L, Massotte D, Landemore G, Polastron J, Jauzac P. J. Pharmacol. Exp. Ther. 2000;293:237–247. [PubMed] [Google Scholar]
- 34.Cvejic S, Trapaidze N, Cyr C, Devi LA. J. Biol. Chem. 1996;271:4073–4076. doi: 10.1074/jbc.271.8.4073. [DOI] [PubMed] [Google Scholar]
- 35.Burd AL, El-Kouhen R, Erickson LJ, Loh HH, Law P-Y. J. Biol. Chem. 1998;273:34488–34495. doi: 10.1074/jbc.273.51.34488. [DOI] [PubMed] [Google Scholar]
- 36.Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. EMBO J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- 37.Gabilondo AM, Hegler J, Krasel C, Boivin-Jahns V, Hein L, Lohse MJ. Proc. Natl. Acad. Sci..U. S. A. 1997;94:12285–12290. doi: 10.1073/pnas.94.23.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]