Abstract
Multidrug-resistance (MDR), a phenomenon in which cancer cells exhibit simultaneous resistance to chemically unrelated drugs, is a major factor in the failure of chemotherapy in cancer patients. Resistance to chemotherapy has been correlated to the overexpression of ABC drug transporters including P-glycoprotein (P-gp) that actively efflux chemotherapeutic drugs from cancer cells. Our previous study showed that bitter melon (Momordica charantia) leaf extract (BMLE) was able to reverse the MDR phenotype by increasing the intracellular accumulation of chemotherapeutic drugs. In the present study, bioguided fractionation was used to identify the active component(s) of BMLE, which is able to modulate the function of P-gp and the MDR phenotype in a human cervical carcinoma cell line (KB-V1). We found that kuguacin J, one of the active components in BMLE, increased sensitivity to vinblastine and paclitaxel in KB-V1 cells. A flow cytometry assay indicated that kuguacin J inhibits the transport function of P-gp and thereby significantly increase the accumulation of rhodamine123 and calcein AM in the cells. These results were confirmed by [3H]-vinblastine transport assay. Kuguacin J significantly increases intracellular [3H]-vinblastine accumulation and decreased the [3H]-vinblastine efflux in the cells. Kuguacin J also inhibited the incorporation of [125I]-iodoarylazidoprazosin into P-gp in a concentration-dependent manner, indicating that kuguacin J directly interacts with the drug-substrate-binding site on P-gp. These results indicate that kuguacin J modulates the function of P-gp by directly interacting at the drug-substrate-binding site and it appears to be an effective inhibitor of P-gp activity in vitro, and thus could be developed as an effective chemosensitizer to treat multidrug-resistant cancers.
Keywords: ABC transporter, Bitter melon, Chemosensitivity, Multidrug resistance, P-glycoprotein, Kuguacin J
1. Introduction
Drug resistance is a major impediment to the treatment of cancer patients receiving single or multiple drugs. Efforts to reverse the drug resistance of tumor cells have been largely unsuccessful [1]. In recent years, considerable research has been directed toward understanding the underlying mechanisms that confer drug resistance. One major cause of drug resistance is the overexpression of the 170 kDa plasma membrane, P-glycoprotein (P-gp). P-gp is a member of the highly conserved superfamily of ATP-binding cassette (ABC) transporter proteins. It acts as an ATP-driven efflux pump that decreases intracellular drug accumulation, thereby decreasing the effectiveness of many chemotherapeutic agents [2–5]. Several classes of synthetic compounds that inhibit P-gp-mediated efflux and enhance the accumulation and efficacy of anticancer agents have been identified. However, the efficacy of these compounds in animal studies and clinical trials has been disappointing due to dose-limiting toxicity [6]. MDR-reversing agents from natural products or plant-derived chemicals which have no toxicological effects are being investigated.
Momordica charantia L. or bitter melon is widely consumed as a vegetable and especially as a folk medicine in Asia. Extracts of bitter melon have been reported to possess antitumor activity such as inhibition of mouse spontaneous mammary tumourigenesis [7] and benzo(a)pyrene-induced mouse forestomach tumourigenesis [8]. Moreover, antioxidant activities [9], antiviral [10], antidiabetic and immunomodulating properties [11] of this plant also have been explored.
Our previous study demonstrated that bitter melon leaf extract (BMLE) reversed the MDR phenotype, increased the intracellular accumulation of [3H]-vinblastine, decreased the [3H]-vinblastine efflux in KB-V1 cells and increased their sensitivity to vinblastine [12]. In this study, bioguided fractionation was used to identify the active component(s) of BMLE that modulate the function of P-gp and the MDR phenotype in multidrug-resistant human cervical carcinoma KB-V1 cells. Our results revealed that kuguacin J, a known triterpeniod from BMLE, inhibits P-gp-mediated drug efflux, leading to an increase of the intracellular accumulation and cytotoxicity of chemotherapeutic drugs in drug-resistant human cervical carcinoma cells in vitro.
2. Materials and methods
2.1 Chemicals
[3H]-Vinblastine (10.8 Ci/mmol) was obtained from Amersham Bioscience (Cardiff, UK). Cyclosporin A (CsA), verapamil (Ver), vinblastine (Vin), paclitaxel (Pacl), 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma Chemical Company (St Louis, MO, USA). Dulbecco’s Modified Eagles Medium (DMEM) was purchased from Gibco BRL (Grand Island, NY, USA). Calcein-AM (C-AM) and Rhodamine123 (Rh123) were obtained from Molecular Probes, Inc (Eugene, OR, USA). [125I]Iodoarylazidoprazosin (IAAP) was purchased from Perkin Elmer Life Science (Wellesley, MA, USA).
2.2 Preparation and isolation of bitter melon extracts
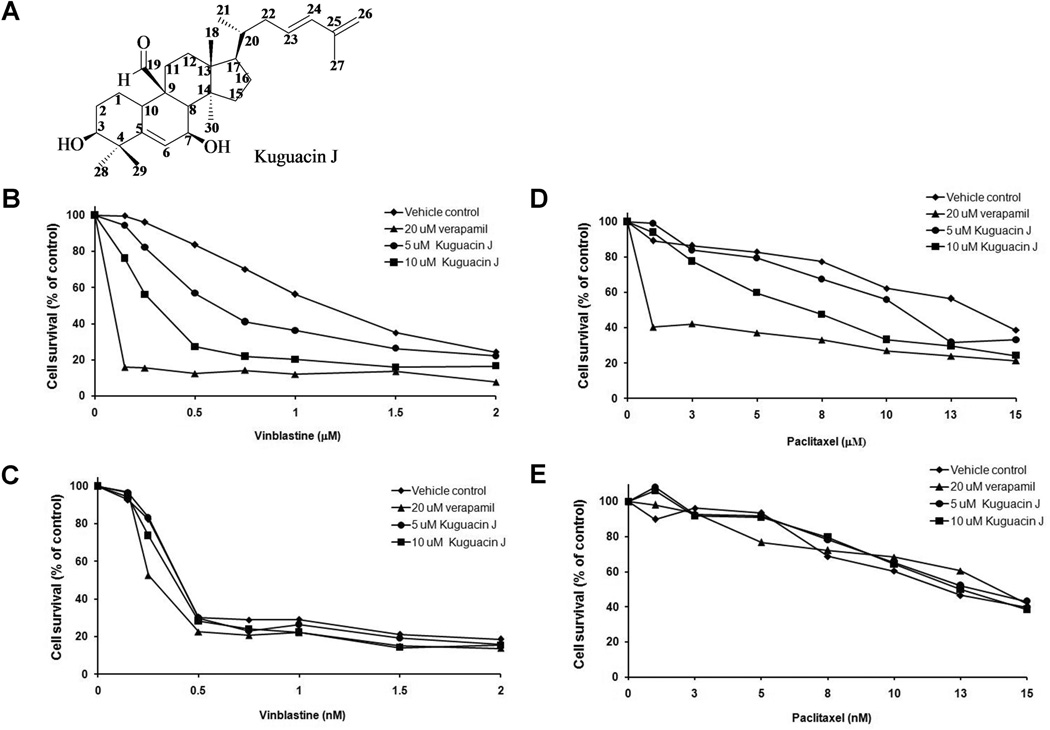
Leaves of bitter melon were collected in Lampang province, Thailand. A voucher specimen (BKF no. 15602) has been deposited at the Forest Herbarium, Department of National Park, Wildlife and Plant Conservation, Ministry of Natural Resources and Environment, Bangkok, Thailand. The fresh leaves were dried at 30–45°C and ground. Dried powdery plant samples (1 kilogram) were extracted exhaustively with 4 liters of 80% ethanol by maceration at 37°C for 16 h. The mixture was filtered and re-extracted with 4 L of ethanol. The combined filtrate was bleached with 160 g of active charcoal, filtered and concentrated by rotary evaporation to 120 ml before filtering to remove precipitates. The filtrate was rotary evaporated and lyophilized. 100 g of residue was re-dissolved in 1 L of 50% methanol and successively partitioned with hexane, diethyl ether, chloroform, ethyl acetate and an aqueous layer. Each fraction was dried under reduced pressure and then submitted for bioassays. From the bioassay-guided fractionation, the diethyl ether fraction was separated by column chromatography. The diethyl ether extract (15 g) was separated by column chromatography over silica gel (Merck, 70–230 mesh ASTM, cat. No. 7734) 100 g, eluting with various proportions of ethyl acetate-n-hexane, followed by the increasing volume of methanol in ethyl acetate and finally with pure methanol. Fractions (50 mL each) were collected and combined on the basis of TLC behavior. The solvents were evaporated to dryness to afford eight fractions (F1–F8). Fraction F2 (2.85 g) which eluted by 30% ethyl acetate-n-hexane, was obtained as white solids mixed with fat. Further separation by column chromatography over silica gel 50 g. Gradient elution was started from pure n-hexane, gradually enriched with ethyl acetate in hexane up to 20% methanol-ethyl acetate. Fractions were collected, combined and then solvent was removed under reduced pressure to afford subfraction A1. Further, the subfraction A1 (1.89 g) was separated by column chromatography over silica gel to yield white crystals of subfraction B1 (1.23 g). The crystals were recrystallized with 95% ethanol to give purified white crystals 1.10 g, which was identified as kuguacin J (Fig. 2A).
Fig. 2.
Structure of Kuguacin J (A) and the effect of kuguacin J on vinblastine and paclitaxel cytotoxicity in KB-V1 (B, D) and KB-3-1 (C, E) cell lines. Cells were incubated in the presence or absence of kuguacin J in combination with vinblastine or paclitaxel. The number of viable cells was determined by an MTT assay. The Y-axis shows the percent of cell survival, and the X-axis shows varying concentrations of kuguacin J. Each point represents the mean of three independent experiments performed in triplicate.
2.3 Cells and cell culture
An MDR cervical carcinoma cell line (KB-V1) and a drug-sensitive cervical carcinoma cell line (KB-3-1) were generous gifts from Dr. Michael M. Gottesman (National Cancer Institute, Bethesda, MD, USA). Both cell lines were cultured in DMEM with 4.5 g of glucose/L plus 10% fetal bovine serum (FBS), 5 mM l-glutamine, 50 U/ml penicillin and 50 g/ml streptomycin; 1 g/ml of vinblastine was added only to the KB-V1 culture medium. These two cell lines were maintained in a humidified incubator with an atmosphere comprising 95% air and 5% CO2 at 37°C. When the cells reached 70–80% confluence, they were harvested and plated either for subsequent passages or for drug treatments.
2.4 Measurement of paclitaxel and vinblastine cytotoxicity
KB-V1 and KB-3-1 cells were plated at 2.0 × 103 cells per well in 96-well plates. After 24 h, BMLE or kuguacin J and various concentrations of vinblastine or paclitaxel were added. The cells were incubated for 48 h at 37°C, and then cell growth was assessed by means of an MTT colorimetric assay [12]. In each experiment, determinations were carried out in triplicate. Relative resistance was calculated as the ratio of the IC50 value of the KB-V1 cells to the IC50 value of the KB-3-1 cells.
2.5 Accumulation of fluorescent substrates
To measure accumulation of Rh123 [2], KB-V1 and KB-3-1 cells (5 × 105) were incubated with 200 ng/mL of Rh123 for 30 minutes at 37°C in DMEM without phenol red medium either with or without MDR1 modulators (5 µM CsA or 20 µM Ver or various concentration of kuguacin J). The cells were washed twice with ice-cold PBS and resuspended in 0.1% BSA PBS. Samples were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA) equipped with a 488-nm argon laser. The green fluorescence of Rh123 was measured by a 530 nm band-pass filter (hLi). To measure accumulation of C-AM [13], KB-V1 and KB-3-1 cells (5 ×105) were incubated with 0.1 µmol/L of C-AM for 15 minutes at 37°C in DMEM without phenol red medium either with or without the MDR1 modulators (5 µM CsA or 20 µM Ver or various concentrations of kuguacin J). The cells were washed twice with ice-cold PBS and resuspended in 0.1% BSA-PBS. Samples were immediately analyzed on a FACScan flow cytometer.
2.6 Radiolabeled drug transportation
The effect of BMLE on P-gp-mediated drug transport was confirmed by monitoring the intracellular radiolabeled drug accumulation using a [3H]-vinblastine incorporation assay [12]. The cells (6.0×105 cells/well) were cultured in complete DMEM for 24 h, and then the cells were treated with various concentrations of BMLE or kuguacin J or the MDR reversing agent (10 µM CSA or 20 µM Ver) in the presence of 0.05 Ci [3H]-vinblastine/ml for 60 min. After washing with ice-cold PBS (pH 7.4), the cells were harvested, and then the amount of intracellular radioactivity was measured.
To determine the drug efflux [14], the cells were seeded as described above in the drug accumulation experiments. Cells were incubated for 60 min at 37°C with 0.05 Ci [3H]-vinblastine/ml in the presence of 20 µM Verapamil in order to load the cells with radiolabeled drug. The cells were then washed with ice-cold PBS (pH 7.4), and then treated with various concentrations of BMLE or kuguacin J or the reversing agent (10 µM CSA or 20 µM Ver). After incubation at 37°C for 30 min, cells were washed with ice-cold PBS (pH 7.4) and the amount of intracellular radioactivity was measured as described above.
2.7 Preparation of crude membranes from P-gp-expressing High Five insect cells
High Five insect cells (Invitrogen, Carlsbad, CA, USA) were infected with the recombinant baculovirus carrying the human MDR1 cDNA with a 6X Histidine tag at the C-terminal end as described [15]. Crude membranes were prepared as described previously [16].
2.8 Photoaffinity Labeling with [125I]-IAAP
The crude membranes of P-gp expressing High Five insect cells (60 µg) were incubated with varying concentrations of kuguacin J (0–50 µM) in 100 µl of 50 mM Tris-HCl, pH 7.5 at room temperature for 5 min. Then, 4–6 nM [125I]-IAAP (2200 Ci/mmole) was added and incubated for 5 min under subdued light. The samples were photocrosslinked with a UV lamp (365 nm) for 5 min at room temperature and 20 µg of each sample was electrophoresed on a 7 % Tris-acetate gel. The gel was dried and exposed to X-ray film for 3–6 h at −80°C. The signals of [125I]-IAAP incorporated into P-gp were quantified using the STORM 860 phosphor imager system (Molecular Dynamics, Sunnyvale, CA, USA) and the software ImageQuant TL (GE Healthcare, Piscataway, NJ, USA) [16].
2.9 ATPase assay
Crude membrane protein (10 µg) from High-five cells expressing P-gp were incubated at room temperature for 5 min with varying concentrations of kuguacin J (0–25 µM) in the presence and absence of sodium orthovanadate (Vi) (0.3 mM) in 100 µl of ATPase assay buffer (50 mM Mes-Tris, pH 6.8, 50 mM KCl, 5 mM sodium azide, 10 mM MgCl2, 2 mM DTT, 1 mM EGTA, 1 mM ouabaine). The reaction was initiated by the addition of 5 mM ATP and incubated for 20 min at 37°C. SDS solution (0.1 ml of 5% SDS) was added to terminate the reaction and the amount of inorganic phosphate released was quantified with a colorimetric reaction, as described previously [17]. The specific activity was recorded as vanadate (Vi)-sensitive ATPase activity.
2.10 Statistical analysis
The results are presented as means ± SD from triplicate samples of three independent experiments. Differences between the means were analyzed by one-way ANOVA and Student t-test. Statistical significance was considered when P< 0.05 or P<0.01. All statistical analyses were performed using SPSS 10.0 software.
3. Results
3.1 P-glycoprotein (ABCB1)-mediated multidrug resistance reversing properties of BMLE and bioguided fractionation
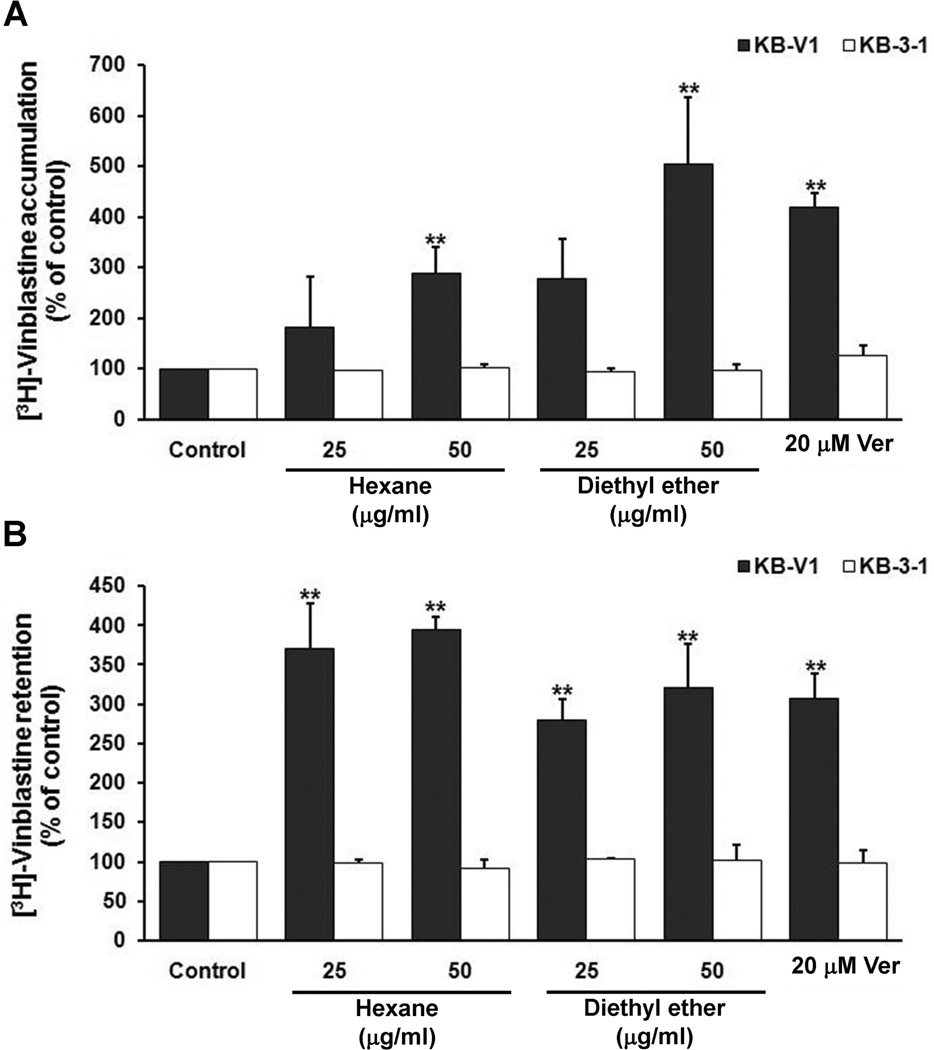
In order to isolate the compound(s) responsible for P-gp reversing properties, a bioguided fractionation strategy was performed throughout the separation procedure (see Preparation and isolation in Materials and Methods). We examined the modulation of the MDR phenotype of the total ethanol extract and the hexane, diethyl ether, chloroform, ethyl acetate and residue fractions combined with vinblastine. The results showed that the hexane and diethyl ether fractions (50 µg/ml; noncytotoxic concentration) had the highest MDR reversing properties, and decreased the IC50 of vinblastine in KB-V1 cells in a dose dependent manner while similar treatment of KB-3-1 cells showed no modulating effect. The MDR reversing properties of the total ethanol extract and the hexane and diethyl ether fractions are shown in Table 1. Moreover, these fractions were tested by [3H]-vinblastine incorporation assay. The results indicated that the hexane and diethyl ether fractions increased intracellular [3H]-vinblastine accumulation and decreased the [3H]-vinblastine efflux in KB-V1 cells in a concentration dependent manner, while no change was observed in KB-3-1 cells (Fig. 1B, C).
Table 1.
Modulation of resistance to vinblastine in KB cells by total ethanol, hexane and diethyl ether fractions from bitter melon leaves
| IC50a | Relative resistanceb | |
|---|---|---|
| Vinblastine Treatment | ||
| KB-3-1 | 0.7 ± 0.04 nM | 1 |
| KB-V1 | 1.5 ± 0.07 µM | 2,143 |
| Total ethanol extract | ||
| KB-V1 + 2.5 µg/ml of the extract | 1.2 ± 0.28 µM | 1,714 |
| KB-V1 + 5 µg/ml of the extract | 0.9 ± 0.35 µM | 1,286 |
| Hexane fraction | ||
| KB-V1 + 2.5 µg/ml of the extract | 0.9 ± 0.14 µM | 1,286 |
| KB-V1 + 5 µg/ml of the extract | 0.5 ± 0.11 µM | 714* |
| Diethyl ether fraction | ||
| KB-V1 + 2.5 µg/ml of the extract | 0.8 ± 0.19 µM | 1,142 |
| KB-V1 + 5 µg/ml of the extract | 0.6 ± 0.05 µM | 857* |
Determined by the MTT assay, and the values are means ± SD of three independent experiments.
IC50 of KB-V1/IC50 of KB-3-1. Each point represents the mean (±SD) of three independent experiments performed in triplicate.
P<0.05,
P<0.01, vs control treated without hexane, and diethyl ether fractions.
Fig. 1.
The effect of BMLE on [3H]-vinblastine accumulation (B) and retention (C) in KB-V1 and KB-3-1 cell lines. The amount of intracellular radioactivity in the presence of verapamil (Ver) and various concentrations of BMLE were examined as described in Materials and Methods. The Y-axis shows the percent of [3H]-vinblastine accumulation, and the X-axis shows varying concentrations of BMLE. The mean value from three independent experiments is shown, and error bars indicate SDs. *P<0.05, **P<0.01, vs vehicle control.
As the percent yield of diethyl ether fraction was higher than hexane fraction, so we therefore purified the active ingredient(s) which play important roles in P-gp modulation using the diethyl ether fraction as a staring material (Materials and Methods). Eight fractions were collected, and it was found that Fraction F2 provided the highest MDR reversing property (Data not shown). The Fraction F2 was further separated according to Materials and Methods, and yielded subfraction B2, a purified white crystal.
3.2 Structure identification of kuguacin J, an active component form BMLE
Kuguacin J (Fig. 2A) was obtained as an amorphous powder. The EIMS spectrum exhibited a [M+] ion peak at m/z 454 corresponding to the molecular formula C30H46O3. Its IR spectrum displayed aldehyde carbonyl absorption at 1709 cm−1. In addition, the strong absorption band at 3464 cm−1 revealed O-H stretching. The fragmentation ions in the mass spectrum at m/z 454 (M+, 7), 442(9), 425 (68), 414(10), 396 (23), 323(100), 175(48), 135(75), 121(81), 55(30), were also useful to obtain the structure of kuguacin J [18]. The ions at m/z 109 were resulted from the olefinic side chain in the structure. Furthermore, independent evidence that the angular aldehyde group is readily eliminated from triterpenes was provided by the EIMS spectrum, which exhibited an intense M-29 peak (425). The high resolution nuclear magnetic resonance 1H and 13C-NMR (1D and 2D) spectra were recorded at 400 MHz and 150 MHz respectively using a DPX on a BrÜker DPX 400 spectrometer in deuteron-chloroform as internal standard. The 1H-NMR spectrum of kuguacin J exhibited characteristic proton signals of the aldehyde group, a downfield singlet at δ 9.75 and a broad singlet due to exomethylene protons at δ 4.85. The doublet at δ 6.13 (J=14.7) showed the geometry trans with proton at δ 5.61 (H-23). The 13C-NMR spectrum data of δC 208.7, the most downfield signal, confirmed the presence of the carbonyl of the aldehyde group. In order to obtain more information about the location of the aldehyde group and double bonds in the structure of kuguacin J, a 2D-NMR HMBC and DQF-COSY were carried out. Furthermore, the purity of this compound was confirmed by the HRESIMS technique and exhibited m/z: 477.3339 [M+Na]+ (calc. for C30H46O3Na, 477.3344). Finally, this compound was identified as the previously known triterpenoid, kuguacin J by comparing its physical and HPLC, IR, MS and NMR data with those reported in the literature [18–21].
Kuguacin J:, m.p. 165.7–166.8°C. (Lit[22] m.p. 166–169 °C). 13C-NMR 150 MHz,CDCl3: δ 20.57 (C-1), 29.94 (C-2), 76.28 (C-3), 42.30 (C-4), 145.42 (C-5), 125.09 (C-6), 76.13 (C-7), 47.51 (C-8), 50.74 (C-9), 40.54 (C-10), 23.11 (C-11), 29.86 (C-12), 45.40 (C-13), 48.48 (C-14), 33.46 (C-15), 28.19 (C-16), 49.60 (C-17), 14.69 (C-18), 207.86 (C-19), 38.1 (C-20), 19.65 (C-21), 39.67 (C-22), 138.8 (C-23), 134.27 (C-24), 142.13 (C-25),114.14 (C-26), 19.40 (C-27), 27.09 (C-28), 27.69 (C-29). 1H-NMR 400 MHz,CDCl3: δ 1.24 (H-1), 1.31 (H-2), 3.55 (H-3), 5.6 (H-6), 3.41 (H-7), 2.09 (H-8), 2.5 (H-10), 1.63 (H-11), 1.31 (H-12), 1.4 (H-15), 1.31 (H-16), 1.49 (H-17), 0.91 (H-18), 9.75 (H-19), 1.53 (H-20), 0.89 (H-21), 2.25 (H-22), 5.61 (H-23), 6.13 (H-24), 4.85 (H-26), 1.84 (H-27), 1.06 (H-28), 1.39 (H-29).
3.3 Effect of kuguacin J on cytotoxicity of paclitaxel and vinblastine in KB-3-1 and KB-V1 cells
To examine the MDR reversing property of kuguacin J on paclitaxel and vinblastine cytotoxicity, the growth inhibition of cells was investigated in response to increasing concentrations of paclitaxel or vinblastine with or without kuguacin J. The results showed that 5 and 10 µM of kuguacin J increased sensitivity of KB-V1 cells to both vinblastine and paclitaxel, 1.9- and 4.3-fold, respectively for vinblastine, and 1.9- and 3.2-fold, respectively for paclitaxel, while similar treatment of KB-3-1 cells provided no modulating effect (Table 2, Fig. 2B–D).
Table 2.
Modulation of resistance to vinblastine and paclitaxel in KB cells by kuguacin J
| IC50a | Relative resistanceb | |
|---|---|---|
| Vinblastine Treatment | ||
| KB-3-1 | 0.4 ± 0.12 nM | 1 |
| KB-V1 | 1.3 ± 0.28 µM | 3,023 |
| Kuguacin J | ||
| KB-V1 + 5 µM Kuguacin J | 0.7 ± 0.38 µM | 1,595* |
| KB-V1 + 10 µM Kuguacin J | 0.3 ± 0.03 µM | 700** |
| KB-V1 + 20 µM Verapamil | 0.1 ± 0.04 µM | 464** |
| IC50a | Relative resistanceb | |
| Paclitaxel Treatment | ||
| KB-3-1 | 7.3 ± 0.16 nM | 1 |
| KB-V1 | 13.3 ± 1.16 µM | 1,821 |
| Kuguacin J | ||
| KB-V1 + 5 µM Kuguacin J | 8.3 ± 0.58 µM | 950* |
| KB-V1 + 10 µM Kuguacin J | 5.2 ± 0.29 µM | 579** |
| KB-V1 + 20 µM Verapamil | 4.5 ± 0.50 µM | 545** |
Determined by the MTT assay, and the values are means ± SD of three independent experiments.
IC50 of KB-V1/IC50 of KB-3-1. Each point represents the mean (±SD) of three independent experiments performed in triplicate.
P<0.05,
P<0.01, vs control treated without kuguacin J.
3.4 Effect of kuguacin J on C-AM and Rh123 accumulation
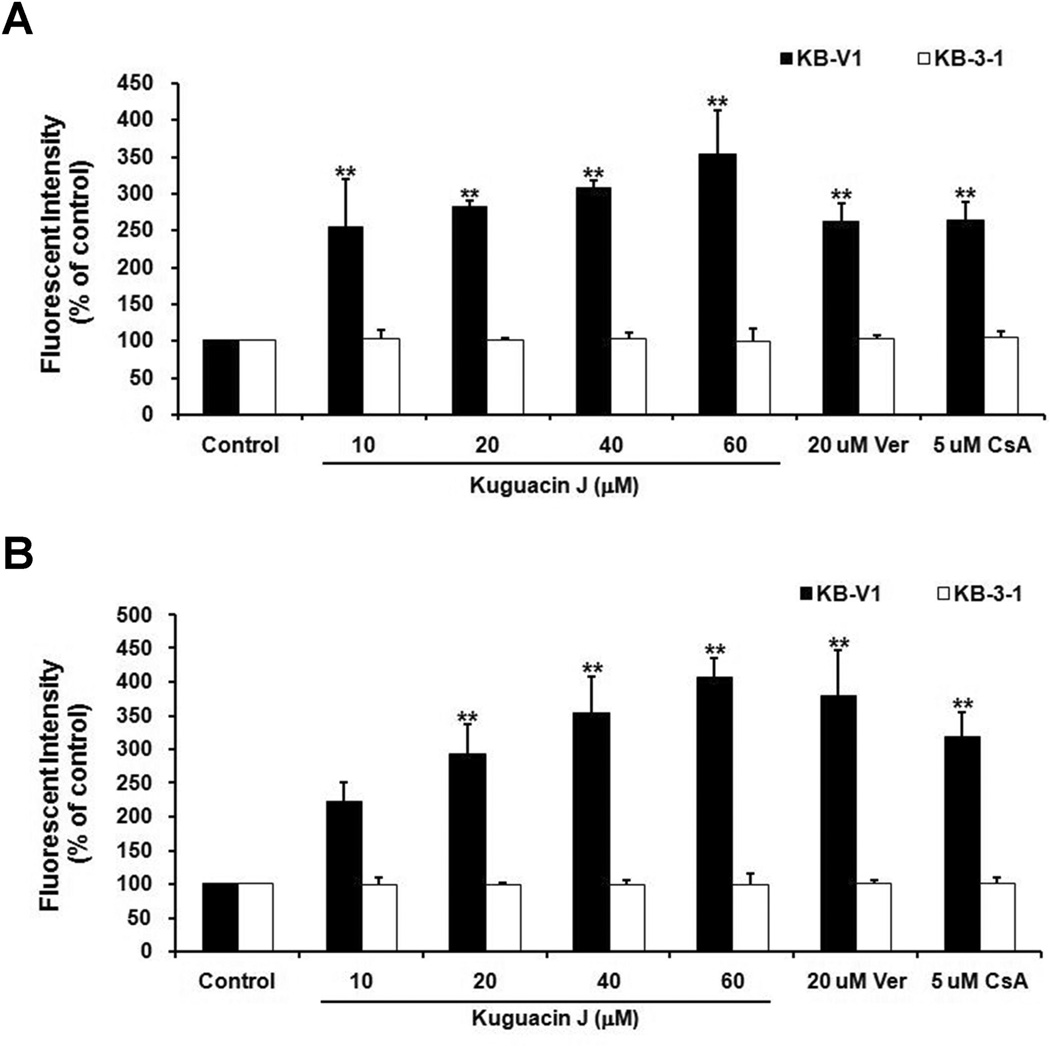
We next studied the effect of kuguacin J on P-gp function. Accumulation of fluorescent substrates of P-gp (C-AM and Rh123) was determined using flow cytometry. These fluorescent substrates were chosen to determine whether the inhibitory effects of kuguacin J are substrate specific or not. The results demonstrated the accumulation of C-AM was increased by 2.2-, 2.9-, 3.5- and 4.1-fold when KB-V1 cells were treated with 10, 20, 40 and 60 µM of kuguacin J, respectively (Fig. 3A). The same treatment of kuguacin J showed that the accumulation of Rh123 was also significantly increased by 2.5-, 2.8-, 3.1- and 3.5-fold, respectively (Fig. 3B). These effects were not found in wild type KB-3-1 cells (Fig. 3A, B).
Fig. 3.
Effect of kuguacin J on C-AM (A) and Rh123 (B) accumulation in KB-V1 and KB-3-1 cell lines. The fluorescence intensity in the presence of cyclosporin A (CsA), verapamil (Ver) and various concentrations of kuguacin J were analyzed using FACScan as described in Materials and Methods. The Y-axis shows the percent of the accumulation of fluorescent substrates, and the X-axis shows varying concentrations of kuguacin J. The mean value from three independent experiments is shown, and error bars indicate SDs. *P<0.05, **P<0.01, vs vehicle control.
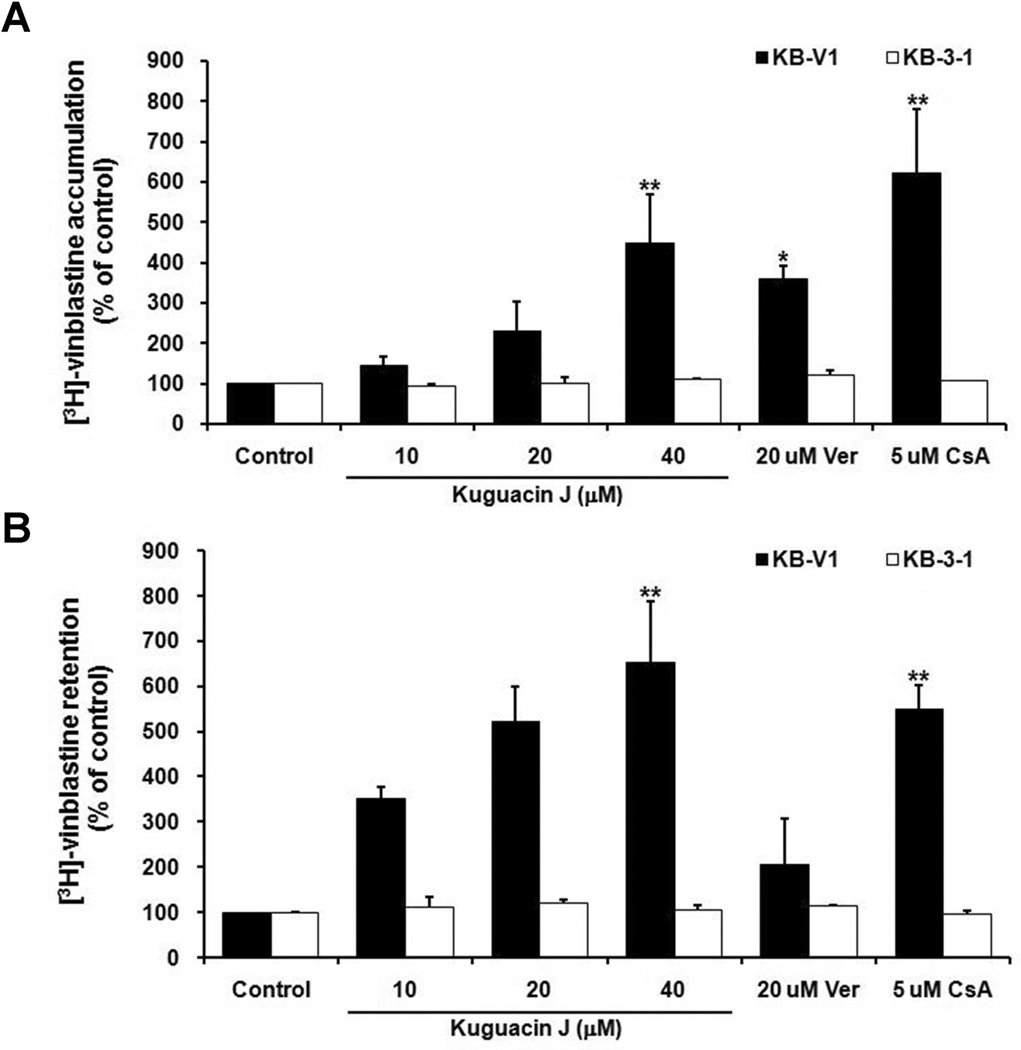
3.5 Effect of kuguacin J on [3H]-vinblastine accumulation and efflux
To confirm the P-gp-function inhibitory effect of kuguacin J, drug accumulation and efflux assays were performed. It was found that kuguacin J at 10, 20 and 40 µM increased [3H]-vinblastine accumulation in KB-V1 cells, by about 1.4-, 2.3- and 4.5-fold, respectively (Fig. 4A). Accumulation of [3H]-vinblastine in drug-sensitive KB-3-1 cells treated with kuguacin J, Ver or CsA did not differ from its accumulation in cells without any treatment (Fig. 4A).
Fig. 4.
Effect of kuguacin J on [3H]-vinblastine accumulation (A) and efflux (B) in KB-V1 and KB-3-1 cell lines. The amount of intracellular radioactivity in the presence of cyclosporin A (CsA), verapamil (Ver) and various concentrations of the kuguacin J was determined as described in Materials and Methods. The Y-axis shows the percent of [3H]-vinblastine accumulation and the X-axis shows varying concentrations of kuguacin J. The mean value from three independent experiments is shown, and error bars indicate SDs. *P<0.05, **P<0.01, vs vehicle control.
The effect of kuguacin J on the P-gp-mediated efflux of vinblastine showed that kuguacin J at concentrations 10, 20 and 40 µM caused a dose-dependent decrease in the amount of [3H]-vinblastine efflux from KB-V1 cells and resulted in an increase in intracellular [3H]-vinblastine retention by approximately 3.5-, 5.2- and 6.5-fold, respectively (Fig. 4B). These effects were not observed in KB-3-1 cells (Fig. 4B).
3.6 Effect of kuguacin J on photoaffinity labeling of P-gp with [125I]-IAAP
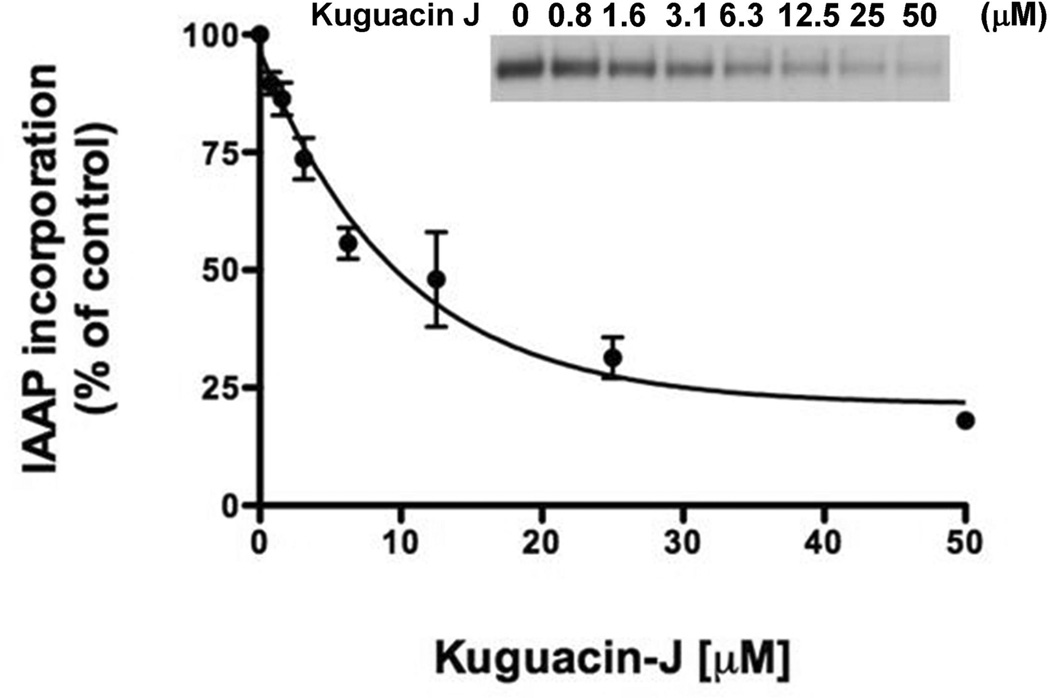
To study the interaction of kuguacin J with the substrate-binding sites of P-gp, we monitored the effect of kuguacin J on the crosslinking of [125I]-IAAP with P-gp, which is a transport substrate of P-gp [23]. This photoaffinity labeling with transport substrate is a useful tool to investigate the interaction of the drugs at the substrate-binding sites of P-gp. Fig. 5 clearly shows that kuguacin J inhibits the photocrosslinking of P-gp with [125I]-IAAP with an IC50 of 8.3 ± 5.4 µM (Fig. 5). This suggests that kuguacin J interacts directly with the substrate-binding site of P-gp.
Fig. 5.
Effect of kuguacin J on the incorporation of the photoaffinity substrate, [125I]-IAAP, into P-gp. The photolabeling of P-gp with [125I]-IAAP to P-gp was monitored as described in Materials and Methods in the presence of increasing concentrations of kuguacin J (0–50 µM). The Y-axis shows the percent of [125I-IAAP incorporation, and the X-axis shows varying concentrations of kuguacin J. The mean value from three independent experiments is shown, and error bars indicate SDs. The IC50 value for the inhibition of [125I-IAAP incorporation into P-gp was 8.3 ± 5.4 µM. The autoradiogram of a representative experiment from three independent experiments is shown.
3.7 Effect of kuguacin J on ATPase activity of P-gp
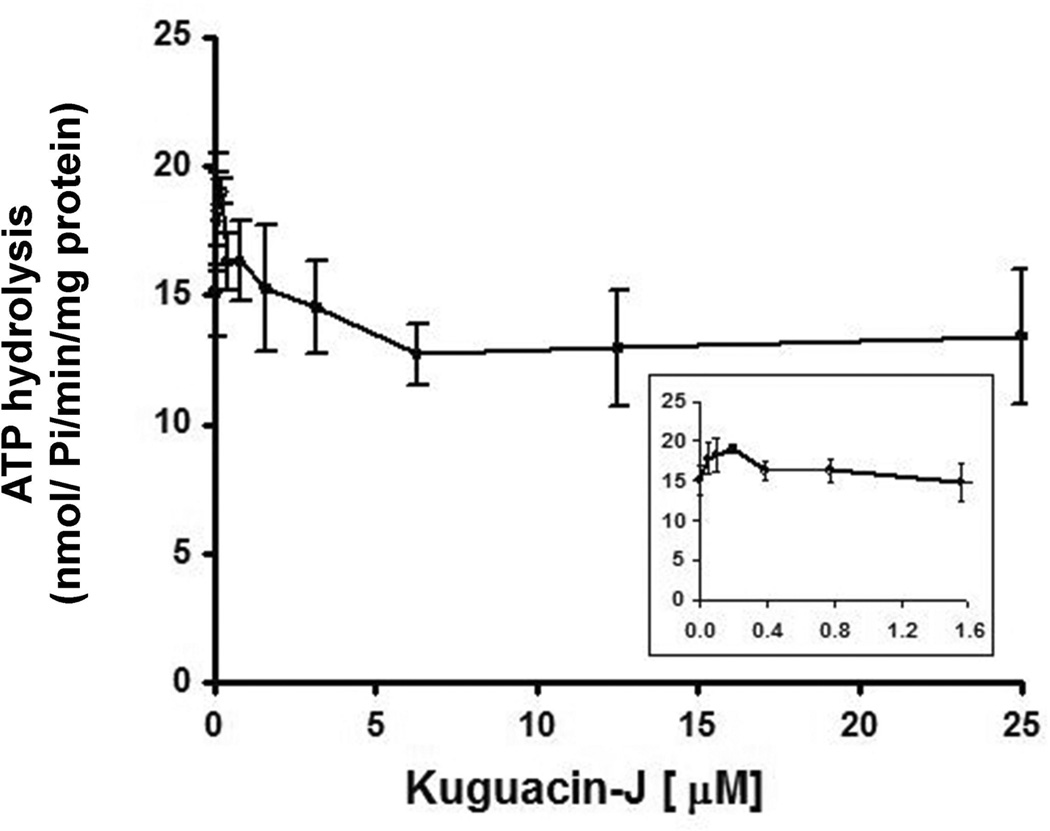
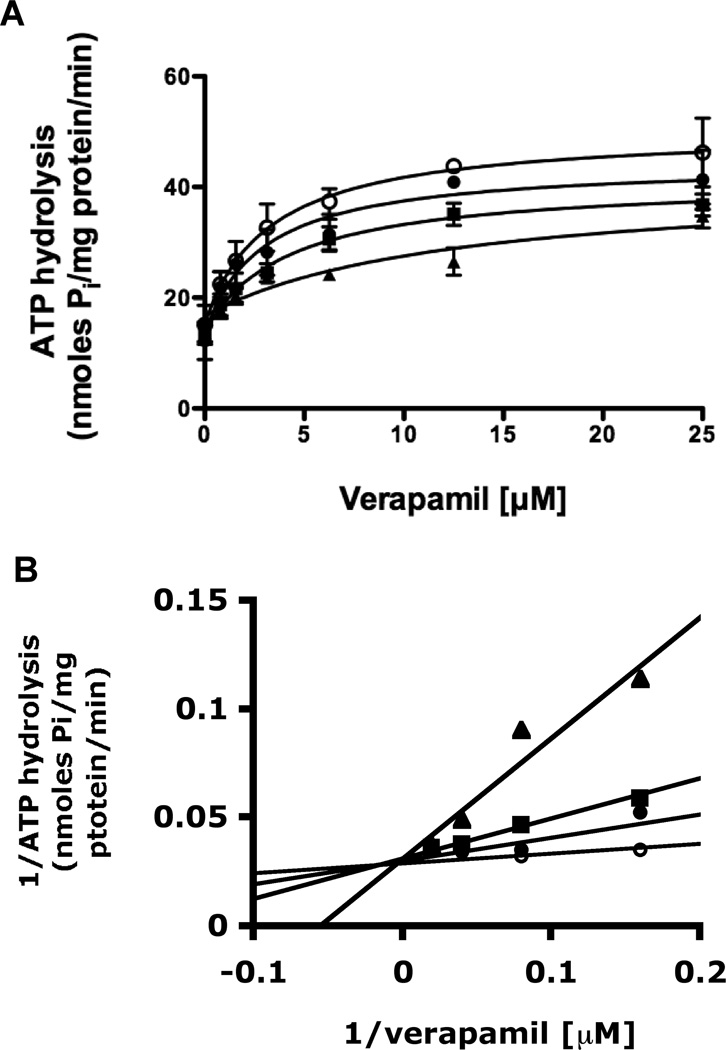
An ATPase assay was carried out to determine whether kuguacin J by interacting at the transport-substrate site influences ATPase activity of P-gp, since this pump utilizes the energy of ATP to transport a substrate from inside to outside the cell, and transport-substrates often stimulate P-gp-mediated ATP hydrolysis. As shown in Fig. 6, kuguacin J had no effect on the ATP hydrolysis except that a lower concentration of kuguacin J (50–100 nM) produced a 1.1–1.3-fold stimulation of ATP hydrolysis (17.8–20.9 nmoles Pi/mg protein/min) compared with basal activity (15.7 nmoles Pi/mg protein/min; Fig. 6). To further characterize the nature of kuguacin J interaction, we investigated the effect of Kuguacin-J on verapamil-stimulated ATPase activity. As shown in Fig. 7A, Kuguacin J inhibited verapamil-stimulated ATPase activity in a dose-dependent manner (Fig. 7A). The same data was transformed to the linear form of 1/V versus 1/S (Lineweaver-Burke plot) (Fig. 7B) to analyze the type of inhibition (ie competitive or non-competitive). Fig. 7B clearly showed that Kuguacin-J acts as competitive inhibitor of verapamil-stimulated ATPase activity (Fig. 7B). From these plots, the apparent Ki of Kuguacin-J was determined to be 2.4 ± 1.1 µM (n=3).
Fig. 6.
Effect of kuguacin J on ATP hydrolysis by P-gp. The vanadate-sensitive ATPase activity of P-gp was determined by using the Pi release assay, as described in Materials and Methods in the presence of increasing concentrations of kuguacin J (0–25 µM). The Y-axis shows the ATP hydrolysis (nmoles Pi/mg protein/min), and X-axis shows varying concentrations of kuguacin J. The mean value from three independent experiments is shown, and error bars indicate SDs. The inset shows ATPase activity in the presence of low concentrations of kuguacin J (0–1.6 µM).
Fig. 7.
Effect of Kuguacin J on verapamil-stimulated ATP hydrolysis. (A) The kinetics of inhibition of the verapamil-stimulated ATPase activity by Kuguacin J. The vanadate-sensitive ATPase activity of P-gp was determined by using the Pi release assay, as described in Materials and Methods, in the presence of increasing concentration of verapamil (0–25 µM) at four different concentrations of kuguacin J (0, 2.5, 5, and 10 µM). The curve was fitted to the Michaelis-Menten equation: V = V0 + (Vmax − V0)S/(S + Km). The Y-axis shows the ATP hydrolysis (nmoles Pi/mg protein/min), and X-axis shows varying concentrations of verapamil. The mean value from three independent experiments is shown, and error bars indicate SDs. (B) The Lineweaver-Burke plot (1/ATP hydrolysis versus 1/verapamil) of the data in (A). The apparent Ki (2.4 ± 1.1 µM; n=3) is obtained from 1/V = 1/Vmax + Km/Vmax (1 + I/Ki)(1/S) equation as described [36].
4. Discussion
Resistance to chemotherapy is a major problem in the management of cancer patients, and is caused by various molecular mechanisms. One of these mechanisms is the overexpression of MDR1/P-glycoprotein, which is the major cause of multidrug-resistance (MDR) of human cancers. Potent MDR modulators are being investigated in clinical trials. Verapamil, a calcium channel blocker, and cyclosporine A, an immunosuppressive agent are the most effective P-gp inhibitors in vitro, but they have limited clinical use. Many current studies are focused on dietary herbs due to the fact that these have been used for centuries without producing any harmful side effects [2, 14, 24–27].
Bitter melon (Momordica charantia) is a plant that grows in tropical areas of Asia, the Caribbean and South America, and has a long history of use as a folk medicine. It has been reported that bitter melon has antidiabetic, antiviral, antioxidative and antineoplastic effects [28]. Some functional components have already been identified such as vegetable insulin and charantin, having hypoglycemic effects [29], and MAP30, a 30-kDa protein from the seeds and fruits of bitter melon, having antiviral effects [10, 30]. In a previous study, we reported that BMLE was able to reverse the MDR phenotype [12] and to inhibit rat prostate cancer invasion and migration in vitro and decrease lung metastasis in vivo [27]. However, the active compound(s) still remains to be identified. In this study, bioguided fractionation was used to identify the active component(s) in BMLE which acts to modulate the function of P-gp and the MDR phenotype in multidrug-resistant human cervical carcinoma. The ethanolic fraction was subsequently extracted with solvents increasing in polarity (i.e., ethanol, hexane, diethyl ether, chloroform and ethyl acetate). These extraction samples were tested for their abilities to modulate the function of P-gp in the multidrug-resistant human cervical carcinoma KB-V1 cells (with overexpression of P-gp) in comparison with wild type drug sensitive KB-3-1 cells (with low expression of P-gp). Among the extracts tested, the hexane and diethyl ether fractions had the most effective MDR reversing properties (Table 1), increased intracellular [3H]-vinblastine accumulation and decreased the [3H]-vinblastine efflux in KB-V1 cells, while no change was observed in KB-3-1 cells (data not shown). As the percent yield of diethyl ether fraction was higher than hexane fraction, so we therefore purified the active component using the diethyl ether fraction as a staring material (Materials and Methods). Bioassay-guided fractionation led us to isolate a purified white crystal. The IR, NMR and MS data of the compound was compared with previous reports [18] and it was identified as kuguacin J (3,7,23-trihydroxycucurbita-5,24-dien-19-al), (Fig. 2A).
We further examined the MDR reversing property of kuguacin J on the cytotoxicity of vinblastine or paclitaxel in KB-V1 and KB-3-1 cells and found that kuguacin J increased the sensitivity of KB-V1 cells to vinblastine and paclitaxel, but did not have this effect on KB-3-1 cells (Table 2, Fig. 2B–D). Moreover, treatment of KB-V1 and KB-3-1 cells with kuguacin J yielded a marked increase in C-AM and Rh123 accumulations in a concentration-dependent manner in KB-V1 cells but had no effect on KB-3-1 cells. C-AM and Rh123 are known to be good substrates for P-gp, indicating that kuguacin J modulates intracellular drug levels by inhibiting P-gp activity. We also confirmed the inhibitory effect of kuguacin J on P-gp function by [3H]-vinblastine transportation assays. It was found that kuguacin J also increased [3H]-vinblastine accumulation in KB-V1 cells and decreased [3H]-vinblastine efflux from KB-V1 cells. As previous study showed that P-gp expression in KB-V1 cells was not affected by treating the cells with BMLE [12]. Taken together, the data suggests that kuguacin J inhibits P-gp activity but not its expression.
The photoaffinity labeled transport substrate of P-gp, [125I]-IAAP, has been used extensively to study the interaction of the modulators at the substrate-binding site of P-gp [23]. It is also known that P-gp can be specifically labeled with [125I]-IAAP, and drug-substrates of P-gp inhibit the photocrosslinking of [125I]-IAAP to P-gp [16]. As shown in Fig. 5, kuguacin J inhibited the photocrosslinking of [125I]-IAAP to P-gp in a concentration-dependent manner (Fig. 5). This is direct evidence that kuguacin J interacts with the substrate-binding site of P-gp. To further verify the interaction of kuguacin J with P-gp, an ATPase assay was employed, which is another useful assay to study the interaction of transport-substrates with P-gp, as ATP hydrolysis is coupled to the transport function of this transporter [17]. Although kuguacin J had a negligible effect on the basal ATPase activity of P-gp, a low concentration of kuguacin J (50–100 nM) slightly stimulated P-gp-mediated ATP hydrolysis (Fig. 6). This finding also implies that Kuguacin J interacts with the substrate-binding site of P-gp.
The published reports on P-gp drug-binding data and kinetic results have suggested the existence of multiple overlapping substrate-binding sites within the P-gp [31, 32]. This means that the substrates for P-gp may interact competitively as well as non-competitively (allosterically) for binding to this protein. To further characterize the nature of interaction of kuguacin J, we investigated the effect of Kuguacin-J on verapamil-stimulated ATPase activity. Previous studies have shown that verapamil stimulates P-gp mediated ATP hydrolysis approximately 3 to 4-fold [33, 34]. However when P-gp is treated with verapamil and other drug-substrates, a concentration-dependent inhibition of verapamil-stimulated ATPase activity is observed [35]. It has been postulated that this inhibition is a consequence of competition at and displacement of verapamil at the substrate-binding site of P-gp [35]. In Fig. 7A, kuguacin J indeed inhibited the verapamil-stimulated ATPase activity of P-gp (Fig. 7A). Furthermore, the kinetic analyses clearly showed that kuguacin J competes with verapamil for the substrate-binding site of P-gp (Fig. 7B). Taken together, these biochemical data suggest that kuguacin J inhibits the transport function of P-gp by interacting with the substrate-binding site of P-gp where verapamil also binds.
In conclusion, this study is the first to demonstrate that kuguacin J, a compound isolated from BMLE, is an effective inhibitor of P-gp activity, and could be a candidate molecule for treating cancers exhibiting P-gp-mediated MDR. The results reported here open the possibility for investigations of the effect of kuguacin J in animal experiments to determine if this compound has potential as an effective chemosensitizer to be used in combination with conventional chemotherapy.
Acknowledgements
This work was supported by the National Research Council of Thailand (NRCT), the Royal Golden Jubilee Scholarship PhD. Program (RGJ), and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (S.O. and S.V.A.). We thank George Leiman for editorial assistance in preparation of the manuscript.
References
- 1.Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol. 2000;12:450–458. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Anuchapreeda S, Leechanachai P, Smith MM, Ambudkar SV, Limtrakul PN. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol. 2002;64:573–582. doi: 10.1016/s0006-2952(02)01224-8. [DOI] [PubMed] [Google Scholar]
- 3.Larsen AK, Escargueil AE, Skladanowski A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol Ther. 2000;85:217–229. doi: 10.1016/s0163-7258(99)00073-x. [DOI] [PubMed] [Google Scholar]
- 4.Lehnert M. Chemotherapy resistance in breast cancer. Anticancer Res. 1998;18 [PubMed] [Google Scholar]
- 5.Schoenlein PV, Shen DW, Barrett JT, Pastan I, Gottesman MM. Double minute chromosomes carrying the human multidrug resistance 1 and 2 genes are generated from the dimerization of submicroscopic circular DNAs in colchicine-selected KB carcinoma cells. Mol Biol Cell. 1992;3:507–520. doi: 10.1091/mbc.3.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varma MV, Ashokraj Y, Dey CS, Panchagnula R. P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol Res. 2003;48:347–359. doi: 10.1016/s1043-6618(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 7.Nagasawa H, Watanabe K, Inatomi H. Effects of bitter melon (Momordica charantia l.) or ginger rhizome (Zingiber offifinale rosc) on spontaneous mammary tumorigenesis in SHN mice. Am J Chin Med. 2002;30:195–205. doi: 10.1142/S0192415X02000302. [DOI] [PubMed] [Google Scholar]
- 8.Deep G, Dasgupta T, Rao AR, Kale RK. Cancer preventive potential of Momordica charantia L. against benzo(a)pyrene induced fore-stomach tumourigenesis in murine model system. Indian J Exp Biol. 2004;42:319–322. [PubMed] [Google Scholar]
- 9.Shi H, Hiramatsu M, Komatsu M, Kayama T. Antioxidant property of Fructus Momordicae extract. Biochem Mol Biol Int. 1996;40:1111–1121. doi: 10.1080/15216549600201753. [DOI] [PubMed] [Google Scholar]
- 10.Lee-Huang S, Huang PL, Chen HC, Bourinbaiar A, Huang HI, Kung HF. Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene. 1995;161:151–156. doi: 10.1016/0378-1119(95)00186-a. [DOI] [PubMed] [Google Scholar]
- 11.Cunnick JE, Sakamoto K, Chapes SK, Fortner GW, Takemoto DJ. Induction of tumor cytotoxic immune cells using a protein from the bitter melon (Momordica charantia) Cell Immunol. 1990;126:278–289. doi: 10.1016/0008-8749(90)90321-h. [DOI] [PubMed] [Google Scholar]
- 12.Limtrakul P, Khantamat O, Pintha K. Inhibition of P-glycoprotein activity and reversal of cancer multidrug resistance by Momordica charantia extract. Cancer Chemother Pharmacol. 2004;54:525–530. doi: 10.1007/s00280-004-0848-4. [DOI] [PubMed] [Google Scholar]
- 13.Legrand O, Simonin G, Perrot JY, Zittoun R, Marie JP. Pgp and MRP activities using calcein-AM are prognostic factors in adult acute myeloid leukemia patients. Blood. 1998;91:4480–4488. [PubMed] [Google Scholar]
- 14.Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar SV. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell Biochem. 2007;296:85–95. doi: 10.1007/s11010-006-9302-8. [DOI] [PubMed] [Google Scholar]
- 15.Ramachandra M, Ambudkar SV, Chen D, Hrycyna CA, Dey S, Gottesman MM, et al. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry. 1998;37:5010–5019. doi: 10.1021/bi973045u. [DOI] [PubMed] [Google Scholar]
- 16.Sauna ZE, Ambudkar SV. Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc Natl Acad Sci U S A. 2000;97:2515–2520. doi: 10.1073/pnas.97.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–514. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- 18.Chen JC, Liu WQ, Lu L, Qiu MH, Zheng YT, Yang LM, et al. Kuguacins F-S, cucurbitane triterpenoids from Momordica charantia. Phytochemistry. 2009;70:133–140. doi: 10.1016/j.phytochem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Tian R, Qiu M, Lu L, Zheng Y, Zhang Z. Trinorcucurbitane and cucurbitane triterpenoids from the roots of Momordica charantia. Phytochemistry. 2008;69:1043–1048. doi: 10.1016/j.phytochem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Mekuria DB, Kashiwagi T, Tebayashi S, Kim CS. Cucurbitane triterpenoid oviposition deterrent from Momordica charantia to the leafminer, Liriomyza trifolii. Biosci Biotechnol Biochem. 2005;69:1706–1710. doi: 10.1271/bbb.69.1706. [DOI] [PubMed] [Google Scholar]
- 21.Puspawati N. Isolation and identification momordicin I from leaves extract of Momordica charantia L. JURNAL KIMIA. 2008;2:53–56. [Google Scholar]
- 22.Choi SZ, Yang MC, Choi SU, Lee KR. Cytotoxic terpenes and lignans from the roots of Ainsliaea acerifolia. Arch Pharm Res. 2006;29:203–208. doi: 10.1007/BF02969394. [DOI] [PubMed] [Google Scholar]
- 23.Maki N, Hafkemeyer P, Dey S. Allosteric modulation of human P-glycoprotein. Inhibition of transport by preventing substrate translocation and dissociation. J Biol Chem. 2003;278:18132–18139. doi: 10.1074/jbc.M210413200. [DOI] [PubMed] [Google Scholar]
- 24.Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem Pharmacol. 2004;68:2043–2052. doi: 10.1016/j.bcp.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Limtrakul P, Khantamat O, Pintha K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J Chemother. 2005;17:86–95. doi: 10.1179/joc.2005.17.1.86. [DOI] [PubMed] [Google Scholar]
- 26.Limtrakul P, Siwanon S, Yodkeeree S, Duangrat C. Effect of Stemona curtisii root extract on P-glycoprotein and MRP-1 function in multidrug-resistant cancer cells. Phytomedicine. 2007;14:381–389. doi: 10.1016/j.phymed.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Pitchakarn P, Ogawa K, Suzuki S, Takahashi S, Asamoto M, Chewonarin T, et al. Momordica charantia leaf extract suppresses rat prostate cancer progression in vitro and in vivo. Cancer Sci. 2010;101:2234–2240. doi: 10.1111/j.1349-7006.2010.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basch E, Gabardi S, Ulbricht C. Bitter melon (Momordica charantia): a review of efficacy and safety. Am J Health Syst Pharm. 2003;60:356–359. doi: 10.1093/ajhp/60.4.356. [DOI] [PubMed] [Google Scholar]
- 29.Miura T, Itoh C, Iwamoto N, Kato M, Kawai M, Park SR, et al. Hypoglycemic activity of the fruit of the Momordica charantia in type 2 diabetic mice. J Nutr Sci Vitaminol (Tokyo) 2001;47:340–344. doi: 10.3177/jnsv.47.340. [DOI] [PubMed] [Google Scholar]
- 30.Lee-Huang S, Huang PL, Nara PL, Chen HC, Kung HF, Huang P, et al. MAP 30: a new inhibitor of HIV-1 infection and replication. FEBS Lett. 1990;272:12–18. doi: 10.1016/0014-5793(90)80438-o. [DOI] [PubMed] [Google Scholar]
- 31.Safa AR. Photoaffinity labels for characterizing drug interaction sites of P-glycoprotein. Methods Enzymol. 1998;292:289–307. doi: 10.1016/s0076-6879(98)92023-7. [DOI] [PubMed] [Google Scholar]
- 32.Ambudkar SV, Kim IW, Sauna ZE. The power of the pump: mechanisms of action of P-glycoprotein (ABCB1) Eur J Pharm Sci. 2006;27:392–400. doi: 10.1016/j.ejps.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992;267:4854–4858. [PubMed] [Google Scholar]
- 34.Ambudkar SV, Lelong IH, Zhang J, Cardarelli CO, Gottesman MM, Pastan I. Partial purification and reconstitution of the human multidrug-resistance pump: characterization of the drug-stimulatable ATP hydrolysis. Proc Natl Acad Sci U S A. 1992;89:8472–8476. doi: 10.1073/pnas.89.18.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao US, Scarborough GA. Direct demonstration of high affinity interactions of immunosuppressant drugs with the drug binding site of the human P-glycoprotein. Mol Pharmacol. 1994;45:773–776. [PubMed] [Google Scholar]
- 36.Litman T, Zeuthen T, Skovsgaard T, Stein WD. Competitive, non-competitive and cooperative interactions between substrates of P-glycoprotein as measured by its ATPase activity. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1997;1361:169–176. doi: 10.1016/s0925-4439(97)00027-6. [DOI] [PubMed] [Google Scholar]