Abstract
Cannabinoids suppress nocifensive behaviors in rodents. We presently investigated peripheral endocannabinoid modulation of itch- and pain-related behaviors elicited from facial vs. spinally-innervated skin of rats. Intradermal (id) injection of the pruritogen serotonin (5-HT) elicited significantly more hindlimb scratch bouts, and longer cumulative time scratching, when injected in the rostral back compared to the cheek. Pretreatment of skin with inhibitors of degrading enzymes for the endocannabinoids anandamide (URB597) or 2-arachidonoylglycerol (JZL184) significantly reduced scratching elicited by 5-HT in the rostral back. These effects were prevented by co-treatment with antagonists of the CB1 (AM251) or CB2 receptor (AM630), implicating both receptor subtypes in endocannabinoid suppression of scratching in spinally-innervated skin. Conversely, pretreatment with either enzyme inhibitor, or with AM630 alone, increased the number of scratch bouts elicited by id 5-HT injection in the cheek. Moreover, pretreatment with JZL184 also significantly increased pain-related forelimb wipes directed to the cheek following id injection of the algogen, allyl isothiocyanate (AITC; mustard oil). Thus, peripheral endocannabinoids have opposite effects on itch-related scratching behaviors in trigeminally- vs. spinally-innervated skin. These results suggest that increasing peripheral endocannabinoid levels represents a promising therapeutic approach to treat itch arising from the lower body, but caution that such treatment may not relieve, and may even exacerbate, itch and pain arising from trigeminally-innervated skin of the face or scalp.
Keywords: endocannabinoid, itch, scratch, C-fiber, cannabinoid receptor, CB1, CB2, G-protein-coupled receptor, serotonin
INTRODUCTION
Itch (pruritus) is defined as an unpleasant sensation which elicits the desire to scratch. Conditions associated with itch include insect bites, contact dermatitis, side effects of certain drugs, dry skin, cholestasis, uremia, certain cancers, neuropathies, and psychogenic conditions (Twycross et al., 2003). Antihistamines are ineffective for most types of itch except urticaria, and other common topical or systemic treatments include emollients, immunosuppressants, and opioid antagonists, all of which have negative side effects (Hoare et al., 2000).
Endocannabinoids may be a good candidate for peripherally-targeted itch suppression. Endocannabinoids bind the same G-protein-coupled cannabinoid receptors as Δ9-THC1, the main active component of cannabis. The two most studied endocannabinoids are 2-arachidonoylglycerol (2-AG) and anandamide (AEA). Systemic (Walker& Hohmann, 2005) and peripheral (Clapper et al., 2010; Spradley et al., 2010) modulation of 2-AG and AEA levels induced analgesia in models of acute, inflammatory, and neuropathic pain. These prior studies and the present experiments modulated endocannabinoids via administration of inhibitors of: the synthesizing enzyme of 2-AG, diacyglycerol lipase (tetrahydrolipstatin, THL); the degrading enzyme of 2-AG, monoacylglycerol lipase (JZL184); or the degrading enzyme of AEA, fatty acid amide hydrolase/FAAH (URB597) (Lichtman et al., 2004; Long et al., 2009). Additionally, the cannabinoid system is known to be involved in immunomodulation (Klein et al., 1998) and both CB1 and CB2 cannabinoid receptor subtypes are present on keratinocytes, skin sensory nerve fibers, and mast cells (Stander et al., 2005).
While numerous studies have demonstrated antinociceptive and antihyperalgesic effects of exogenous and endogenous cannabinoids (e.g., Calignano et al., 1998; Richardson et al., 1998; Johanek et al., 2001; Malan et al., 2001; Johanek & Simone, 2004; Patil et al., 2011), fewer have investigated their modulation of itch. A role for the endocannabinoid system in attenuation of allergic inflammation in a murine model of contact dermatitis has been reported (Oka et al., 2006; Karsak et al., 2007). Systemic administration of cannabinoid agonists or inhibition of FAAH reduced scratching behavior elicited by subcutaneous injection of the mast cell degranulating compound 48/80 (Schlosburg et al., 2009). Systemic cannabinoid agonists reduced ear-scratching elicited by systemic administration of a 5-HT-2A/C agonist (Darmani, 2001). However, only one brief clinical study reported reduced itch in uremic pruritus patients following topical treatment with an emollient containing endocannabinoids, although the emollient itself may have contributed to the antipruritic effect (Szepietowski et al., 2005). Thus, modulation of peripheral endocannabinoids may prove to be a useful approach to treat itch.
We presently investigated the effect of peripheral endocannabinoid modulation on itch-and pain-related behaviors in the rat. In this species, 5-HT is the most effective pruritogen (Thomsen et al., 2001; Jinks & Carstens, 2002). 5-HT activates putative itch-signaling cutaneous C-fibers (Hachisuka et al., 2010) that project to the superficial spinal dorsal horn to excite second-order neurons (Jinks and Carstens, 2002; Akiyama et al., 2009) via release of glutamate (Koga et al., 2011), substance P (Carstens et al., 2010) and/or gastrin releasing peptide (Sun and Chen, 2007; Sun et al., 2009). Dorsal horn neurons responsive to itch mediators function as scratch-reflex interneurons and/or project to higher centers via ascending pathways such as the spinothalamic tract (Andrew and Craig, 2001; Simone et al., 2004; Davidson et al.,2009).
To assess itch we used two rat behavioral models. In the first, traditional, model, intradermal (id) injection of pruritogens in the rostral back elicits bouts of directed hindlimb scratching (Nojima and Carstens, 2003). However, this model lacks the ability to distinguish between itch- and pain-related behaviors, since biomechanical limitations restrict the response to hindlimb scratches. In the second model, id injection of pruritogens into the cheek selectively elicited hindlimb scratch bouts directed to the injection site, whereas injection of an algogen elicited unilateral singular wiping motions of the ipsilateral forepaw across the injected cheek (Shimada and LaMotte, 2008; Akiyama et al., 2010; Klein et al., 2011; Spradley et al., 2012). We used these models to address the following: 1) if there are differences in pruritogen-evoked scratching behavior in trigeminally- (cheek) vs. spinally-innervated body regions (rostral back), 2) the relative ability of peripheral endocannabinoid modulation to affect itch-related scratching behaviors in each body region , and 3) to identify the role of CB1 and/or CB2 receptors in peripheral endocannabinoid modulation of itch- and pain-related behavior.
METHODS
2.1. Animals
Adult male Sprague Dawley rats from Simonsen and Charles River (weighing 305–634g) were doubly housed, and had free access to food and water. All procedures were approved by the UC Davis Institutional Animal Care and Use Committee and followed the guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals (2011). Every attempt was made to minimize the number of animals tested.
2.2 Behavioral Testing of the Rostral Back and Cheek Models
Prior to the day of testing, the fur from each rat’s cheek or rostral back was shaved. At the time of testing, rats were pretreated with an intradermal (id) injection of the vehicle, an inhibitor, an antagonist, or a cocktail in either the cheek or the rostral back. These first injections always were of a 50 µL volume and were verified by the presence of a bleb at the injection site. Injections were administered via a Hamilton syringe connected to PE50 tubing and a 30 gauge hypodermic needle. Then rats were habituated to an opaque Plexiglas chamber with a Plexiglas top on a clear glass table through which the animals were videotaped from below (for cheek testing) or above (for rostral back testing) for 15 minutes. Subsequently, rats were injected id in the cheek or rostral back (same injection site as the first id injection) 15 min after the pretreatment injection with 10 µL of the pruritogen serotonin (1%, 5-HT) or 10 µL of the algogen allyl isothiocyanate, the main pungent component of mustard oil (10%, AITC). Immediately following the second injection, rats were replaced in the Plexiglas testing chambers. Behaviors were recorded for 1 hr post-pruritogen/algogen injection. Videotapes were scored by viewers blinded to the experimental condition. Viewers scored the number and length of each bout (events) of facial grooming with both forelimbs, wiping with the forelimb, and hindlimb scratching for rats injected in the cheek, and only the hindlimb scratching for rats injected in the rostral back. Off-site scratches (such as ears or opposite cheek) were excluded from analyses. Scratch bouts consisted of episodes of rapid hindpaw movements across the injected cheek area, or directed to the rostral back injection site, and ending when the rat brought the claws of the hindpaw to the mouth (to gnaw/lick the claws) and/or placed the hindpaw on the floor. Also the length (rounded to the half-second) of each scratch and groom bout was quantified and used for various analyses. Wiping behavior consisted of single, isolated unilateral, caudal-to-rostral wiping movements of the inner aspect of the forepaw across the injected cheek. A bout of facial grooming consisted of a discrete episode of head- or face-washing by both forepaws. Wipes and grooming of non-injected body regions were excluded from analyses. To conserve animals, rats were tested in multiple experiments with at least one week between repeat testing involving the same injection site.
2.3. Chemicals
The pruritogen serotonin (5-hydroxytryptamine, 5-HT-HCl, Sigma, St. Louis, MO) was tested at a concentration of 1% (47 mM) in saline, and the algogen allyl isothiocyanate (AITC, the pungent ingredient in mustard oil; Sigma) was tested at a concentration of 10% (1 M) in a 7% Tween-80 solution (in 0.9% saline) based on doses that elicited maximal scratching or wiping responses (Klein et al., 2011).
The inhibitor of the AEA degrading enzyme FAAH, URB597, was tested at a concentration of 75 µg/50 µL, and the inhibitor of the 2-AG degrading enzyme monoacylglycerol lipase (MGL), JZL184, was tested at a concentration of 100 µg/50 µL (Lichtman et al., 2004; Long et al., 2009; Spradley et al., 2010). The CB1 inverse agonist AM251 was tested at 80 µg/50 µL, and the CB2 antagonist AM630 was tested at 25 µg/50 µL (Spradley et al., 2010). The inhibitor of the synthesizing enzyme of 2-AG diacylglycerol lipase (DGL), THL, was tested at 200 µg/50 µL. All inhibitors and cannabinoid receptor antagonists were purchased from Cayman Chemical (Ann Arbor, MI), and the doses chosen were based on the literature cited with the exception of THL (the dose of which was chosen based on preliminary dose response curves, data not shown). All compounds were dissolved in a vehicle containing DMSO, ethanol, Tween-80, and saline in a ratio of 1:1:1:17, and this vehicle was also administered id as a control. To make the cocktails (consisting of one of the degrading enzyme inhibitors plus one of the cannabinoid receptor antagonists), each component was made separately in double strength concentrations and then was combined with the other component to yield the original desired concentrations and volumes described above as when administered singly.
2.4. Statistics
All data are expressed as Mean ± SEM and data are significant when P ≤ 0.05. Data represented by line graphs were analyzed by repeated measures ANOVA. When Mauchly’s Test of Sphericity indicated significance, the Greenhouse-Geisser correction factor was applied to the interaction term of all repeated factors. When a significant interaction was present, 1-way ANOVAs were performed for each time point. The values represent the total amount of specified behavior quantified per 5-min bin either during the baseline period (time -15 up through 0 min) or during the post-pruritogen/algogen period (time 0 up through 60 min).
The data represented in bar graphs were analyzed via either 1-way ANOVAs and Tukey post-hocs as needed or unpaired two-tailed t-tests; these values represent the total amount of the specified behavior quantified over the entire 60-min post-pruritogen/algogen injection period.
Statistics were performed using the software SPSS 9.0, and for graphing purposes the software Graphpad Prism 5.0 was utilized.
3. RESULTS
3.1. 5-HT-evoked scratching was greater in the rostral back vs. the cheek
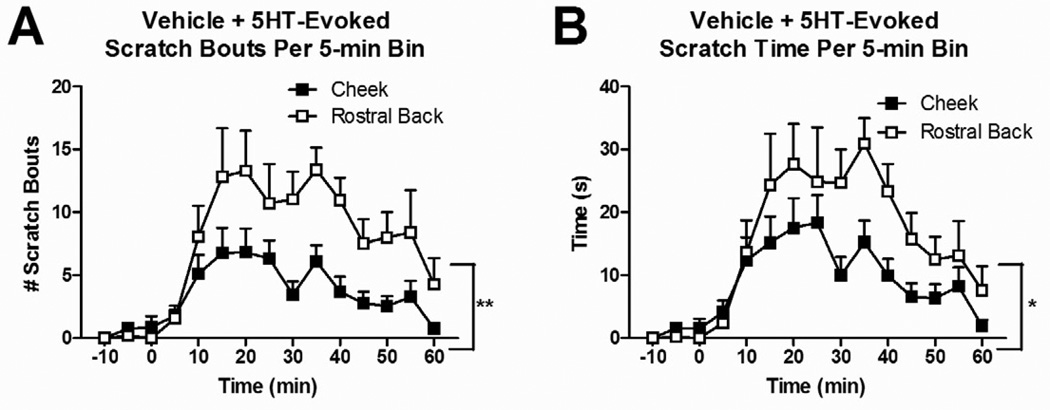
Id injections of the vehicle in rostral back or cheek elicited very little scratching behavior (0.06 ± 0.06 scratch bouts/15 min for rostral back; 0.5 ± 0.4 for cheek). Fifteen min after vehicle injection, 5-HT was injected id. 5-HT evoked significantly more scratch bouts (summed over the 60 min observation period) in rats injected in the rostral back compared to the cheek (Fig. 1A; F(1,23)=11.968, P=0.002). Similarly, the cumulative time spent scratching was significantly greater (Fig. 1B; F(1,23)=5.471, P=0.028) for 5-HT injections in the rostral back compared to the cheek. Finally, the mean duration of scratch bouts was significantly shorter for bouts directed to the rostral back vs. cheek (1.98 ± 0.1 vs. 2.5 ± 0.17 sec, respectively; t(24)=2.736, P=0.0115).
Fig. 1.
Effect of id 5-HT on scratching behavior in the cheek vs. rostral back over time. Rats were pretreated with vehicle 15 min prior to 5-HT. Data are Mean ± SEM. *P≤0.05, **P≤0.01 (cheek vs. rostral back).
3.2. Inhibition of endocannabinoid degradation in rostral back skin reduces scratching via CB1 and CB2 receptor mechanisms
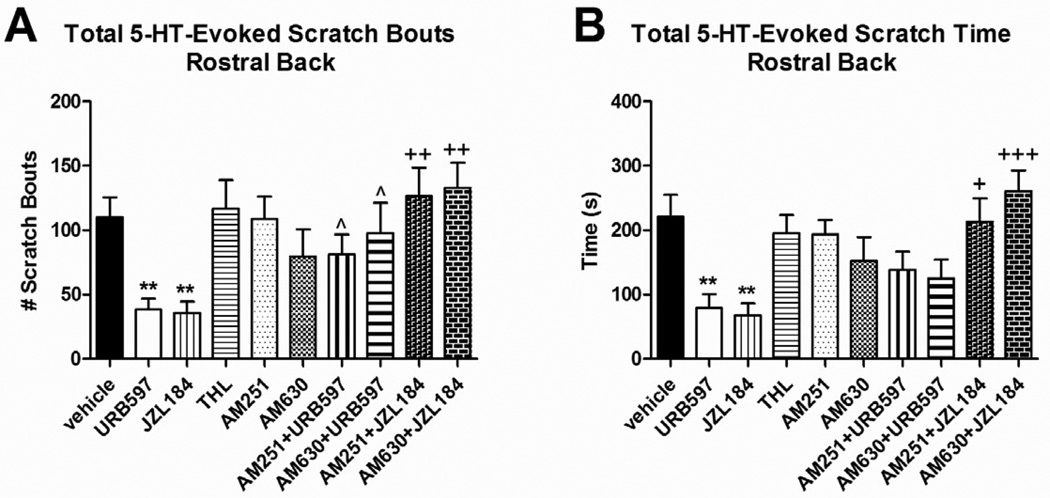
Pretreatment of skin in the rostral back with both URB597 (t(18)=3.287, P=0.0041) and JZL184 (t(18)=3.393, P=0.0032) resulted in a significant reduction in the total number of 5-HT-evoked scratch bouts (Fig. 2A) and cumulative scratch time (Fig. 2B) compared to vehicle-pretreated rats. Pretreatment with THL, AM251, or AM630 had no effect on any of these parameters of 5-HT-evoked scratching (Fig. 2A–B), indicating absence of endocannabinoid tone.
Fig. 2.
Effect of endocannabinoid modulation in the rostral back on the total 5-HT-evoked scratch bouts and cumulative scratch time when rats were pretreated 15 min prior to 5-HT. Data are Mean ± SEM. *P≤0.05, **P≤0.01 (vs. vehicle); ^P≤0.05 (vs. URB597); +P≤0.05, ++P≤0.01 +++P≤0.001 (vs. JZL184).
Rats pretreated with a cocktail containing the AEA degrading enzyme inhibitor URB597, plus either the CB1 inverse agonist AM251, or the CB2 antagonist AM630, exhibited a normal number of 5-HT-evoked scratch bouts (equivalent to vehicle-pretreated rats) that was significantly greater compared to rats pretreated only with URB597 (t(13)=2.317, P=0.0375 for AM251 + URB597, t(12)=2.369, P=0.0355 for AM630 + URB597) (Fig. 2A). Similar results were obtained in rats pretreated with the 2-AG degrading enzyme inhibitor JZL184, plus either AM251 (t(12)=3.855, P=0.0023) or AM630 (t(13)=4.39, P=0.0007) (Fig. 2A). Thus, both CB1 and CB2 antagonists reversed the antipruritic effects of URB597 and JZL184 pretreatment.
The data for JZL184, as assessed by total number of scratch bouts (Fig. 2A), was corroborated by measures of total cumulative time spent scratching (Fig. 2B). However, results with the AEA degrading enzyme inhibitor URB597 were more complex. While the antipruritic effect of URB597 was reversed by both CB1 and CB2 antagonists based on scoring of scratch bouts (Fig. 2A), analysis of the cumulative time spent scratching (Fig. 2B) indicated that neither antagonist reversed the effect of URB597 although there was a trend toward increased scratch time.
3.3. Inhibition of endocannabinoid degradation in cheek skin increases scratching, wiping, and grooming behaviors
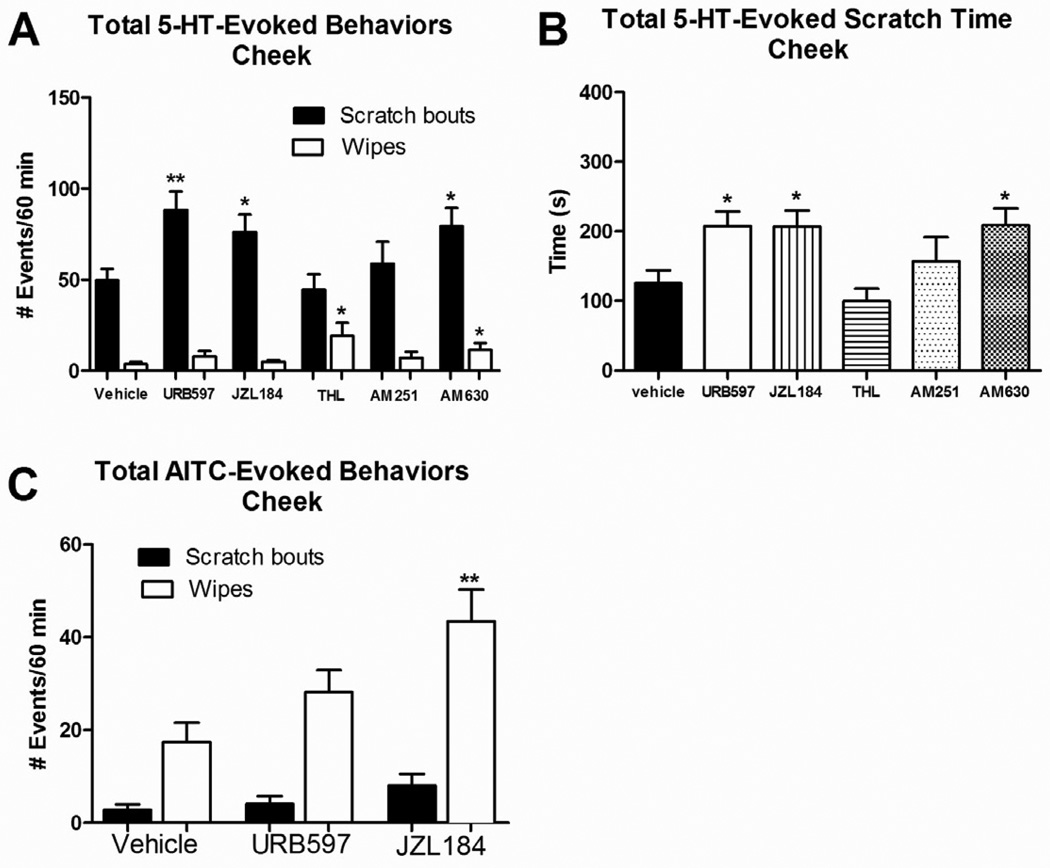
Rats pretreated with vehicle followed by id injection of 5-HT in the cheek exhibited many hindlimb scratch bouts but very few ipsilateral forelimb wipes directed to the cheek, as previously reported (Klein et al., 2011). Pretreatment with either the FAAH inhibitor URB597 or the MGL inhibitor JZL184 resulted in a significant increase in the number of 5-HT-evoked scratch bouts compared to vehicle-pretreated rats (Fig. 3A; URB597: t(17)=3.439, P=0.0031; JZL184: t(18)=2.427, P=0.0259). Pretreatment with AM630 or the DGL inhibitor THL both also increased the number of 5-HT-evoked forelimb wipes compared to vehicle pretreatment (Fig. 3A; AM630: t(18)=2.305, P=0.0333; THL: t(18)=2.537, P=0.0206).
Fig. 3.
Effect of endocannabinoid modulation in the cheek on the total 5-HT- and/or AITC-evoked scratch bouts and cumulative scratch time, as well as wiping behavior, when rats were pretreated 15 min prior to 5-HT or AITC. Data are Mean ± SEM. *P≤0.05, **P≤0.01 (vs. vehicle)
Pretreatment with the CB2 antagonist AM630 significantly increased the number of 5-HT-evoked scratch bouts (t(18)=2.65, P=0.0163). Pretreatment with the CB1 inverse agonist AM251 did not significantly affect 5-HT-evoked scratching (Fig. 3A). These results were corroborated by analysis of cumulative time scratching (Fig. 3B).
AITC evoked very few scratch bouts and many wipes (Fig. 3C). Pretreatment with JZL184 resulted in a significant increase in AITC-evoked wipes compared to vehicle pretreated rats (t(13)=3.323, P=0.0055). Pretreatment with URB597 resulted in a numeric increase in wipes that did not achieve statistical significance (Fig. 3C; t(14)=1.693, P=0.1125). AITC-evoked scratching behavior was not affected by either pretreatment (Fig. 3C, black bars).
The number of groom bouts following id cheek injection of 5-HT or AITC was not affected by pretreatment with either degrading enzyme inhibitor, except for one condition in which URB597 pretreatment significantly increased AITC-evoked groom bouts (t(18)=2.94, P=0.0088). JZL184 pretreatment resulted in a numeric increase in AITC-evoked groom bouts that was just short of statistical significance (t(18)=2.071, P=0.053).
4. DISCUSSION
5-HT elicited more scratch bouts and greater cumulative time spent scratching when injected in the rostral back compared to the cheek. Additionally, the presumed elevation of levels of the endocannabinoids 2-AG and AEA peripherally in skin, by pretreatment with inhibitors of their degrading enzymes MGL and FAAH, respectively, resulted in a CB1 and CB2 receptor-mediated decrease in scratching elicited by injection of 5-HT in the rostral back, but enhanced scratching elicited by cheek injection of 5-HT as well as wiping elicited by AITC. These results emphasize fundamental differences in the sensitivity of facial vs. lower-body skin to itch and pain mediators, and the ability of endocannabinoids to modulate itch and pain in these different body regions of rats. Although caution is warranted when translating basic science data in animals to clinical practice, particularly given the known species differences in itch mechanisms (Carstens & Kuraishi, 2004), our data nevertheless strongly suggest that effective clinical treatments may differ between facial vs. lower body skin.
4.1 Differences in trigeminal vs. spinal processing and modulation of itch and pain
Limited evidence suggests differences in itch and pain modulation between trigeminally-versus spinally-innervated body regions. The incidence and magnitude of itch elicited by id microdialysis of histamine and compound 48/80 were much higher when delivered to the forearm than to the scalp of human subjects (Ruckwied et al., 2002). Moreover, plasma protein extravasation elicited by both itch mediators was much lower for scalp vs. forearm skin (Ruckwied et al., 2002). The authors suggested that the differences in physiological and sensory responses are attributable to structural tissue differences between scalp and forearm skin, and to a lower density and/or higher central itch threshold for scalp vs. forearm skin (Ruckwied et al., 2002). This is supported by a recent study reporting that histamine-evoked itch sensation was weaker on the face compared to distal extremities (Truini et al., 2011). Shingles patients affected in regions such as the head, face, or neck tend to experience postherpetic itch more frequently compared to patients affected in lower body regions (Oaklander et al., 2003). Additionally, neuroselective transcutaneous electric stimulation was reported to elicit itch in the hand and ankle but not head or neck (Ozawa et al., 2006). The intensity of histamine-evoked itch did not differ across different lower body regions (forearm, upper arm, or upper back) (Wahlgren and Ekblom, 1991). In contrast, the algogen capsaicin evoked irritation at significantly lower concentrations when applied to facial vs. forearm skin (Green, 2000). Peripheral opioid receptor activation differentially suppressed muscle pain elicited from the masseter (trigeminally-innervated) but not gastrocnemius or triceps muscles (spinally-innervated) (Sanchez et al., 2010). Also, it is often noted that spinally-administered opiates induce pruritus that is preferentially localized to the face (Ballantyne et al., 1988). These observations suggest that there are major differences in trigeminal vs. spinal signaling and modulation of itch and pain.
The above-noted findings support our present data showing opposing effects of endocannabinoid modulation of itch and pain in facial vs. rostral back skin. Also, a previous study reported that pretreatment of the rat hindpaw with URB597 suppressed capsaicin-evoked mechanical allodynia via the CB1 receptor, and pretreatment with JZL184 suppressed capsaicin-evoked nocifensive behavior and thermal hyperalgesia via both CB1 and CB2 receptors (Spradley et al., 2010). In the present study, similar pretreatments of the rostral back reduced 5-HT-evoked scratching behavior. In contrast, however, the same pretreatments in cheek skin significantly increased forelimb wiping elicited by cheek injection of AITC. We believe that wiping reflects a nocifensive response, since forelimb wiping elicited by cheek microinjection of AITC was significantly reduced by systemic pretreatment with morphine, but not naltrexone (Spradley et al., 2012). Therefore, assuming that AITC-evoked facial wiping, and capsaicin-evoked hindpaw nocifensive responses reflect pain, then the presumed increase in peripheral levels of 2-AG has a pronociceptive effect in facial skin that is opposite to the antihyperalgesic and antinociceptive effects of increased levels of 2-AG in hindpaw skin. The implication is that topical endocannabinoid agonists may be useful analgesics in spinally-innervated skin but may exacerbate pain arising from trigeminally-innervated skin of the face or scalp.
4.2 Endocannabinoid Suppression of Itch and Pain
Pretreatment of skin of the rostral back with the 2-AG or AEA degrading enzyme inhibitors resulted in a reduction in 5-HT-evoked scratching behavior. This is consistent with previous studies reporting that itch-related behaviors were suppressed by systemic administration of exogenous cannabinoids or inhibition of FAAH (Darmani, 2001; Schlosburg et al., 2009). In the rostral back model hindlimb scratching is the only biomechanically viable option. Rostral back injection of AITC elicited very little scratching or other pain-related behaviors such as freezing or vocalization (authors’ unpublished observations). Assuming that itch and pain processing in skin on the rostral back and hindpaw are similar, it is to be expected that rostral back pretreatment with the degrading enzymes for 2-AG or AEA would produce analgesia similar to the hindpaw (Spradley et al., 2010). Our findings are consistent with reports that topical application of the synthetic cannabinoid agonist, HU210, significantly attenuated itch elicited by histamine delivered to forearm skin in human subjects (Dvorak et al., 2003), as well as capsaicin-evoked pain and hyperalgesia/allodynia (Ruckwied et al., 2003), and support the concept that activation of peripheral cannabinoid receptors may be useful to relieve both itch and pain in skin of the lower body.
In the rostral back, pretreatment with neither the CB1 inverse agonist AM251, nor the CB2 antagonist AM630, nor THL which inhibits synthesis of 2-AG, had any effect on 5-HT-evoked scratching behavior (Fig. 2). This implies that there was no significant amount of tonic endocannabinoid activity. Both AM251 and AM630 completely reversed the antipruritic effect of pretreatment with JZL184, indicating that the presumed increase in levels of 2-AG in the skin suppresses itch via both CB1 and CB2 receptors. The antipruritic effect of URB597, which increased AEA levels in the skin, was also reversed by both CB antagonists according to some, but not all, measures of scratching (Fig. 2). Our data are consistent with a previous report that compound 48/80-evoked scratching was attenuated by systemic pretreatment with the FAAH inhibitor URB597, as well as in mice lacking CB1 receptors (Schlosburg et al., 2009), and extend them by showing that in addition to AEA, 2-AG is also involved in peripheral modulation of itch via both CB1 and CB2 receptors.
In contrast to the rostral back, pretreatment of cheek skin with URB597 or JZL184 significantly enhanced 5-HT-evoked scratching, with JZL184 also significantly increasing AITC-evoked wiping (Fig. 3D) and URB597 also significantly increasing grooming. Curiously, the CB2 antagonist AM630 increased both 5-HT-evoked facial scratching and wiping, presumably by blocking tonic CB2-mediated antipruritic and antinociceptive actions. THL pretreatment also increased 5-HT-evoked wiping (Fig. 3A), suggesting the presence of tonic 2-AG-mediated antinociception. Overall, these results indicate complex interactions of endocannabinoids with peripheral itch- and pain-processing mechanisms, whereby tonic levels of antipruritic and antinociceptive activity are overcome by pro-pruritic and pro-nociceptive effects of increasing levels of AEA and 2-AG.
Our finding that endocannabinoids appear to activate pruriceptive afferents in facial skin, while inhibiting pruriceptors and nociceptors in rostral back skin, is puzzling and the underlying molecular mechanisms are currently unclear. Cannabinoid receptors, endocannabinoids, and their synthesizing/degrading machinery have been identified in keratinocytes and sensory nerve fibers (Hohmann & Herkenham, 1999; Maccarrone et al., 2003; Stander et al., 2005) and possibly also mast cells (Facci et al., 1995; Lau and Chow, 2003; but see Maccarrone et al., 2000; Croxford & Yamamura, 2005). Thus, endocannabinoids have multiple sites of action to potentially modulate peripheral transduction and transmission of itch and pain.
Considerable evidence indicates that cannabinoids act directly on nociceptive nerve endings via both CB1 and CB2 receptors (for recent review, see Kress & Kuner, 2009). Intradermal AEA directly excited nociceptors in a TRPV1-dependent manner (Potenziere et al., 2009) but also inhibited nociceptor responses to noxious stimuli (Sokal et al., 2003). Peripheral application of another agonist, arachidonyl-2'-chloroethylamide (ACEA), inhibited mechanically-evoked responses of nociceptors in a CB1 –receptor dependent manner (Kelly & Donaldson, 2008; Potenzieri et al., 2008). Knockout of CB1 in Nav1.8-expressing nociceptors reduced the analgesic effect of peripheral and systemic cannabinoids (Agarwal et al., 2007). Cannabinoids acting via CB1 indirectly inhibit TRPV1, TRPM8 and P2X2 and P2X2/3 receptors, directly inhibit a variety of potassium, sodium, calcium and thermosensory TRP channels (TRPV1, TRPA1 and TRPM8), and also directly activate thermosensory TRP channels (TRPV1,2,4 and TRPA1) (Guo & Ikeda, 2004; van der Stelt and Di Marzo, 2005; Reis et al., 2011; Todorovic and Jevtovic-Todorovic, 2011; reviewed in Kress & Kuner, 2009). Moreover, FAAH is found in TRPV1-expressing dorsal root ganglion cells, raising the possibility of a peripheral autocoid action of AEA (Lever et al., 2009). That histamine-evoked scratching behavior requires TRPV1 (Imamachi et al., 2009), and histamine-independent scratching requires TRPA1 (Wilson et al., 2011), raises the possibility that endocannabinoids may act peripherally at these TRP channels expressed in pruritogen-sensitive nerve endings to suppress itch-related scratching behavior, as observed presently. What is surprising is that while presumed elevation of peripheral endocannabinoids reduced both itch and pain behaviors in the lower body, it enhanced itch behavior in the cheek. To our knowledge, the only relevant study reported that cannabinoids elicited nocifensive eye-wiping behavior and evoked CGRP release (and did not prevent capsaicin-evoked CGRP release) from trigeminal ganglion neurons (Price et al., 2004), supporting a ronociceptive action of cannabinoids in trigeminally innervated tissues.
Cannabinoids may also act indirectly to peripherally modulate itch and pain. For example, AEA was reported to either elicit (Lau & Chow, 2003) or inhibit (Vannacci et al., 2004) release of histamine from mast cells. An intriguing possibility is that cannabinoids acting via the CB2 receptor induce the release of β-endorphin from skin keratinocytes, to inhibit pain by local action at µ-opioid receptors (Ibrahim et al., 2005). Intradermal injection of µ-receptor agonists elicited scratching behavior (Yamamoto & Sugimoto, 2010). We speculate that cannabinoid-mediated release of endogenous opioids in the skin is capable of eliciting itch and also suppressing pain. The differential effects of peripheral endocannabinoid modulation, i.e., antipruritic and antinoceptive in lower body but pro-pruritic in the face, may depend on differential distributions of cannabinoid and µ-opioid receptors in these different skin regions. Cannabinoids inhibit monoamine oxidase (MAO) activity (Fisar, 2010), and MAO is reported to exist in the skin (Slominski et al., 2003; Nordlind et al., 2008). We speculate that cannabinoid inhibition of MAO in facial skin could decrease 5-HT degradation and thus increase the intensity and duration of itch and scratching behavior, as presently observed. Such an effect was insufficient to overcome the antipruritic effect of topical application of enzyme inhibitors in lower body skin, again possibly due to differential levels of MAO expression in the facial vs. lower body skin.
In conclusion, the present data show that the presumed increase in levels of the endocannabinoids AEA and 2-AG in rostral back skin reduces 5-HT-evoked scratching behavior via CB1 and CB2 receptors, while the same treatments in skin of the cheek enhance 5-HT-evoked scratching and AITC-evoked wiping indicative of itch and pain, respectively. These results indicate fundamental differences in endocannabinoid modulation of itch and pain in skin innervated by the trigeminal nerve, compared to skin of the lower body innervated by spinal nerves. These results hold promise for use of drugs that modulate peripheral endocannabinoid levels to treat both itch and pain arising from skin of the lower body, with the added advantage that this approach is likely to induce fewer negative side effects compared to systemic endocannabinoid treatment. However, our results caution that such treatments may be ineffective against, or even worsen, itch and pain arising from facial skin.
ACKNOWLEDGEMENTS
We thank Clifford Chiu, Connie Duong, Candeline Grossett, Kelsey Hogeman, Alexander Horwitz, Margaret Ivanov, and Catherine Lin for their assistance with watching the videotapes for behavioral quantification, and Connie Duong, and Margaret Ivanov for their assistance with operating the behavioral experiments and rodent handling. This work was supported by NIH grant #AR-057194 and DE021183.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Δ9-THC: Δ9-tetrahydrocannabinol; 2-AG: 2-arachidonoylglycerol; AEA: anandamide; THL: tetrahydrolipstatin; JZL184: degrading enzyme of 2-AG; FAAH: fatty acid amide hydrolase; URB597: degrading enzyme of AEA; CB1/CB2: cannabinoid receptor subtypes 1 and 2, respectively; id: intradermal; 5-HT: serotonin; AITC: allyl isothiocyanate; MGL: monoacylglycerol lipase; AM251: CB1 inverse agonist; AM630: CB2 antagonist; DGL: diacylglycerol lipase; ANOVA: analysis of variance; HU210: synthetic cannabinoid agonist
REFERENCES
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J. Neurosci. 2009;29:6691–6699. doi: 10.1523/JNEUROSCI.6103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and µ-opoid modulation in mice. Acta Derm Venereol. 2010;90:575–581. doi: 10.2340/00015555-0962. [DOI] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat. Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33:149–160. doi: 10.1016/0304-3959(88)90085-1. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E, Kuraishi Y. Animal Models of Itch: Scratching Away at the Problem. In: Yosipovitch G, Greaves MW, Fleischer AB, McGlone F, editors. Itch: Basic Mechanisms and Therapy. Monticello, NY: Marcel Dekker; 2004. pp. 35–50. [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat. Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166(1–2):3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacol. Biochem. Behav. 2001;68:311–317. doi: 10.1016/s0091-3057(00)00477-9. [DOI] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat. Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak M, Watkinson A, McGlone F, Rukwied R. Histamine induced responses are attenuated by a cannabinoid receptor agonist in human skin. Inflamm. Res. 2003;52:238–245. doi: 10.1007/s00011-003-1162-z. [DOI] [PubMed] [Google Scholar]
- Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci U S A. 1995;92(8):3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisar Z. Inhibition of monoamine oxidase activity by cannabinoids. Nautn-Schmied Arch Pharmacol. 2010;381:563–572. doi: 10.1007/s00210-010-0517-6. [DOI] [PubMed] [Google Scholar]
- Green BG. Measurement of sensory irritation of the skin. Am. J. Contact Dermat. 2000;11:170–180. doi: 10.1053/ajcd.2000.7185. [DOI] [PubMed] [Google Scholar]
- Guo J, Ikeda SR. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol. Pharmacol. 2004;65:665–674. doi: 10.1124/mol.65.3.665. [DOI] [PubMed] [Google Scholar]
- Hachisuka J, Furue H, Furue M, Yoshimura M. Responsiveness of C neurons in rat dorsal root ganglion to 5-hydroxytryptamine-induced pruritic stimuli in vivo. J. Neurophysiol. 2010;104:271–279. doi: 10.1152/jn.00938.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technology Assessment. 2000;4(37) [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90(3):923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106(27):11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to id serotonin and other irritants: comparison with scratching behavior. J. Neurophysiol. 2002;87:1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Heitmiller DR, Turner M, Nader N, Hodges J, Simone DA. Cannabinoids attenuate capsaicin-evoked hyperalgesia through spinal and peripheral mechanisms. Pain. 2001;93:303–315. doi: 10.1016/S0304-3959(01)00336-0. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Simone DA. Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain. 2004;109:432–442. doi: 10.1016/j.pain.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, Starowicz K, Steuder R, Schlicker E, Cravatt B, Mechoulam R, Buettner R, Werner S, Di Marzo V, Tuting T, Zimmer A. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–1497. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- Kelly S, Donaldson LF. Peripheral cannabinoid CB1 receptors inhibit evoked responses of nociceptive neurones in vivo. Eur J Pharmacol. 2008;586(1–3):160–163. doi: 10.1016/j.ejphar.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton C, Friedman H. Cannabinoid receptors and immunity. Immunol. Today. 1998;19:373–381. doi: 10.1016/s0167-5699(98)01300-0. [DOI] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J. Neurophysiol. 2011;106:1078–1088. doi: 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Chen T, Li XY, Descalzi G, Ling J, Gu J, Zhuo M. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol. Pain. 2011;7:47. doi: 10.1186/1744-8069-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M, Kuner R. Mode of action of cannabinoids on nociceptive nerve endings. Exp Brain Res. 2009;196(1):79–88. doi: 10.1007/s00221-009-1762-0. [DOI] [PubMed] [Google Scholar]
- Lau AH, Chow SS. Effects of cannabinoid receptor agonists on immunologically induced histamine release from rat peritoneal mast cells. Eur. J. Pharmacol. 2003;464:229–235. doi: 10.1016/s0014-2999(03)01430-4. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Robinson M, Cibelli M, Paule C, Santhe P, Yee L, Hunt SP, Cravatt BF, Elphick MR, Nagy I, Rice AS. Localization of the endocannabinoid-degrading enzyme fatty acid amide hydrolase in rat dorsal root ganglion cells and its regulation after peripheral nerve injury. J. Neurosci. 2009;29:3766–3780. doi: 10.1523/JNEUROSCI.4071-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, Cravatt BF. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J. Pharmacol. Exp. Ther. 2004;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Di Rienzo M, Battista N, Gasperi V, Guerrieri P, Rossi A, Finazzi-Agro A. The endocannabinoid system in human keratinocytes. Evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J. Biol. Chem. 2003;278:33896–33903. doi: 10.1074/jbc.M303994200. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Fiorucci L, Erba F, Bari M, Finazzi-Agrò A, Ascoli F. Human mast cells take up and hydrolyze anandamide under the control of 5-lipoxygenase and do not express cannabinoid receptors. FEBS Lett. 2000;468(2–3):176–180. doi: 10.1016/s0014-5793(00)01223-0. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A, CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. eighth ed. Washington, D.C.: 2011. [PubMed] [Google Scholar]
- Nojima H, Carstens E. Quantitative assessment of directed hind limb scratching behavior as a rodent itch model. J. Neurosci. Methods. 2003;126:137–143. doi: 10.1016/s0165-0270(03)00074-8. [DOI] [PubMed] [Google Scholar]
- Nordlind K, Azmitia EC, Slominski A. The skin as a mirror of the soul: exploring the possible roles of serotonin. Exp Dermatol. 2008;17(4):301–311. doi: 10.1111/j.1600-0625.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- Oaklander AL, Bowsher D, Galer B, Haanpaa M, Jensen MP. Herpes zoster itch: preliminary epidemiologic data. J. Pain. 2003;4:338–343. doi: 10.1016/s1526-5900(03)00637-0. [DOI] [PubMed] [Google Scholar]
- Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, Nasui M, Sugiura T. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J. Immunol. 2006;177:8796–8805. doi: 10.4049/jimmunol.177.12.8796. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Tsuchiyama K, Gomi R, Kurosaki F, Kawamoto Y, Aiba S. Neuroselective transcutaneous electric stimulation reveals body area-specific differences in itch perception. J. Am. Acad. Dermatol. 2006;55:996–1002. doi: 10.1016/j.jaad.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Patil M, Patwardhan A, Salas MM, Hargreaves KM, Akopian AN. Cannabinoid receptor antagonists AM251 and AM630 activate TRPA1 in sensory neurons. Neuropharmacology. 2011;61:778–788. doi: 10.1016/j.neuropharm.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenzieri C, Brink TS, Pacharinsak C, Simone DA. Cannabinoid modulation of cutaneous Adelta nociceptors during inflammation. J Neurophysiol. 2008;100(5):2794–2806. doi: 10.1152/jn.90809.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenzieri C, Brink TS, Simone DA. Excitation of cutaneous C nociceptors by intraplantar administration of anandamide. Brain Res. 2009;1268:38–47. doi: 10.1016/j.brainres.2009.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl- dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol. 2004;141(7):1118–1130. doi: 10.1038/sj.bjp.0705711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis GM, Ramos MA, Pacheco DF, Klein A, Perez AC, Duarte ID. Endogenous cannabinoid receptor agonist anandamide induces peripheral antinociception by activation of ATP-sensitive K+ channels. Life Sci. 2011;88:653–657. doi: 10.1016/j.lfs.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Rukwied R, Watkinson A, McGlone F, Dvorak M. Cannabinoid agonists attenuate capsaicin-induced responses in human skin. Pain. 2003;102:283–288. doi: 10.1016/S0304-3959(02)00401-3. [DOI] [PubMed] [Google Scholar]
- Rukwied R, Zeck S, Schmelz M, McGlone F. Sensitivity of human scalp skin to pruritic stimuli investigated by id microdialysis in vivo. J. Am. Acad. Dermatol. 2002;47:245–250. doi: 10.1067/mjd.2002.120461. [DOI] [PubMed] [Google Scholar]
- Sanchez EM, Bagues A, Martin MI. Contributions of peripheral and central opioid receptors to antinociception in rat muscle pain models. Pharmacol. Biochem. Behav. 2010;96:488–495. doi: 10.1016/j.pbb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Boger DL, Cravatt BF, Lichtman AH. Endocannabinoid modulation of scratching response in an acute allergenic model: a new prospective neural therapeutic target for pruritus. J. Pharmacol. Exp. Ther. 2009;329:314–323. doi: 10.1124/jpet.108.150136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J. Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur J Biochem. 2003;270(16):3335–3344. doi: 10.1046/j.1432-1033.2003.03708.x. [DOI] [PubMed] [Google Scholar]
- Sokal DM, Elmes SJ, Kendall DA, Chapman V. Intraplantar injection of anandamide inhibits mechanically-evoked responses of spinal neurones via activation of CB2 receptors in anaesthetised rats. Neuropharmacology. 2003;45(3):404–411. doi: 10.1016/s0028-3908(03)00195-3. [DOI] [PubMed] [Google Scholar]
- Spradley JM, Davoodi A, Iodi Carstens M, Carstens E. Opioid Modulation of Facial Itch- and Pain-related Responses and Grooming Behavior in Rats. Acta Dermatol. Venerol. 2012 doi: 10.2340/00015555-1364. (in press) [DOI] [PubMed] [Google Scholar]
- Spradley JM, Guindon J, Hohmann AG. Inhibitors of monoacylglycerol lipase, fatty-acid amide hydrolase and endocannabinoid transport differentially suppress capsaicin-induced behavioral sensitization through peripheral endocannabinoid mechanisms. Pharmacol. Res. 2010;62:249–258. doi: 10.1016/j.phrs.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stander S, Schmelz M, Metze D, Luger T, Rukwied R. Distribution of cannabinoid receptor 1 (C1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 2005;38:177–188. doi: 10.1016/j.jdermsci.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szepietowski JC, Reich A, Szepietowski T. Emollients with endocannabinoids in the treatment of uremic pruritus: discussion of the therapeutic options. Therapeutic Apheresis and Dialysis. 2005;9:277–279. doi: 10.1111/j.1774-9987.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Thomsen JS, Petersen MB, Benfeldt E, Jensen SB, Serup J. Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta. Derm. Venereol. 2001;81:250–254. doi: 10.1080/00015550152572868. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Jevtovic-Todorovic V. T-type voltage-gated calcium channels as targets for the development of novel pain therapies. Br. J. Pharmacol. 2011;163:484–495. doi: 10.1111/j.1476-5381.2011.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A, Leone C, Di Stefano G, Biasiotta A, La Cesa S, Teofoli P, Padua L, Cruccu G. Topographical distribution of warmth, burning and itch sensations in healthy humans. Neurosci Lett. 2011;494(2):165–168. doi: 10.1016/j.neulet.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: scratching more than the surface. Q. J. Med. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. Anandamide as an intracellular messenger regulating ion channel activity. Prostaglandins Other Lipid Mediat. 2005;77:111–122. doi: 10.1016/j.prostaglandins.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Vannacci A, Giannini L, Passani MB, Di Felice A, Pierpaoli S, Zagli G, Fantappiè O, Mazzanti R, Masini E, Mannaioni PF. The endocannabinoid 2-arachidonylglycerol decreases the immunological activation of Guinea pig mast cells: involvement of nitric oxide and eicosanoids. J Pharmacol Exp Ther. 2004;311(1):256–264. doi: 10.1124/jpet.104.068635. [DOI] [PubMed] [Google Scholar]
- Wahlgren CF, Ekblom A. Perception of histamine-induced itch elicited in three different skin regions. Acta. Derm. Venereol. 1991;71:205–208. [PubMed] [Google Scholar]
- Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb. Exp. Pharmacol. 2005;168:509–554. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptormediated itch. Nat Neurosci. 2011;14(5):595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Sugimoto Y. Involvement of peripheral mu opioid receptors in scratching behavior in mice. Eur J Pharmacol. 2010;649(1–3):336–341. doi: 10.1016/j.ejphar.2010.07.039. [DOI] [PubMed] [Google Scholar]