Abstract
Arsenic methylation is an important cellular metabolic process that modulates arsenic toxicity and carcinogenicity. Biomethylation of arsenic produces a series of mono-, di- and tri-methylated arsenic metabolites that can be detected in tissues and excretions. Here we report that zebrafish exposed to arsenite (AsIII) produces organic arsenicals, including MMAIII, MMAV and DMAV with characteristic tissue ratios, demonstrating that an arsenic methylation pathway exists in zebrafish. In mammals, cellular inorganic arsenic is methylated by a SAM-dependent arsenic methyltransferase, AS3MT. A zebrafish arsenic methyltransferase homologue, As3mt, was identified by sequence alignment. Western blotting analysis showed that As3mt was universally expressed in zebrafish tissues. Prominent expression in liver and intestine correlated with methylated arsenic metabolites detected in those tissues. As3mt was expressed in and purified from E. coli for in vitro functional studies. Our results demonstrated that As3mt methylated AsIII to DMAV as an end product and produced MMAIII and MMAV as intermediates. The activity of As3mt was inhibited by elevated concentrations of the substrate AsIII as well as the metalloid selenite, which is a well-known antagonistic micronutrient of arsenic toxicity. The activity As3mt was abolished by substitution of either Cys160 or Cys210, which correspond to conserved cysteine residues in AS3MT homologues, suggesting that they are involved in catalysis. Expression in zebrafish of an enzyme that has a similar function to human and rodent orthologues in catalyzing intracellular arsenic biomethylation validates the applicability of zebrafish as a valuable vertebrate model for understanding arsenic-associated diseases in humans.
Keywords: zebrafish, arsenic, arsenite, selenite, methylation, SAM, GSH, HPLC-ICP-MS, MMAIII, MMAV, DMAV
Introduction
Arsenic is an environmental toxicant, carcinogen as well as therapeutic drug (Tseng, 2008; de The and Chen, 2010; Kitchin and Conolly, 2010). Mechanisms of arsenic metabolism have been extensively studied. It has been found that trivalent arsenite (AsIII) and pentavalent arsenate (AsV) uptake are facilitated by aquaglyceroporins (Liu et al., 2002; Hamdi et al., 2009) and phosphate transporters(Bun-ya et al., 1996; Beene et al., 2011) in prokaryotes and eukaryotes. The adventitious uptake and accumulation of arsenic via nutrient transporters drives the evolution of a variety of arsenic detoxification pathways. One of the major pathways is methylation, which has been studied in microbes, plants and mammals (Qin et al., 2006; Drobna et al., 2009; Ye et al., 2012). Methylation of inorganic arsenicals produces different organic forms, which vary in ease of excretion, reactivity and carcinogenicity. In human, arsenic methylation produces dimethylarsinic acid (DMAV) as a major product along with other species, including monomethylarsonous acid (MMAIII), monomethylarsonic acid (MMAV),dimethylarsonous acid (DMAIII ) and trimethylarsine oxide (TMAO) (Styblo et al., 2002). All inorganic and methylated arsenicals can be detected in urine with different ratios depending on individual methylation efficiencies (Styblo et al., 2002; Katsoyiannis et al., 2007). Methylation has been viewed as a detoxification process for a long time since pentavalent arsenic species are less toxic than inorganic arsenic (Styblo et al., 2002). However, the trivalent intermediates, MMAIII and DMAIII, which can also be produced and excreted as intermediates, are more toxic than inorganic AsV or AsIII, which means under certain circumstances methylation activity can exacerbate arsenic toxicity (Petrick et al., 2000; Hirano et al., 2004; Drobna et al., 2005). Therefore, arsenic methylation serves as one of decisive factor to determine the overall arsenic induced malignancy (Rehman, et al., 2012). However, it is not well understood what causes interindividual variation in methylation efficiencies and in what situations arsenic methylation is beneficial for human health (Chung et al., 2009; Kwong et al., 2010; Wnek et al., 2010).
The bona fide arsenic methyltransferase, AS3MT, was isolated from rat liver, the major site for arsenic methylation (Thomas et al., 2004; Tseng, 2007). Currently, several AS3MT homologues have been identified from microbes to plants, each with similar function in arsenic methylation (Qin et al., 2006; Meng et al., 2011). Based on sequence alignment, two AS3MT homologues were identified in zebrafish. In this study, a closer homologue, as3mt was cloned and the enzyme function in arsenic methylation was studied using purified recombinant protein.
The AsIII methylation by AS3MT is proposed to have two rounds of reaction; each round includes oxidative methylation followed by reduction. The first round reaction produces MMAV which is then reduced to MMAIII, followed by a second round of methylation to DMAV (Marapakala et al., 2012). Some microbial homologues undergo a third round of methylation to TMA (trimethylarsinic acid), a gaseous form of arsenic (Qin et al., 2009), or the oxidized form of TMAO (trimethylarsine oxide). It has been shown that intracellular glutathione (GSH) level regulates the formation of TMA and possibly serves as a broad determinant of the pattern and extent of formation of arsenic metabolites (Waters et al., 2004). However, it is not clear which reaction step is rate-limiting. The detailed mechanisms are still lacking and questions such as whether toxic trivalent intermediates formed by AS3MT and which conditions favor their accumulation remain unanswered. Currently reported biochemical experiments were done in conditions fostering a complete reaction using high enzyme concentrations and prolonged reaction times, thus the trivalent intermediate, MMAIII, has not been detected. In this study, under a well controlled condition dependent on SAM and a reductant (β-mercaptoethanol, βME), we demonstrated that As3mt undergoes two rounds of step-wise methylation, with initial product of mono-methylated MMAIII and MMAV, followed by the second and final round of step with product of DMAV.
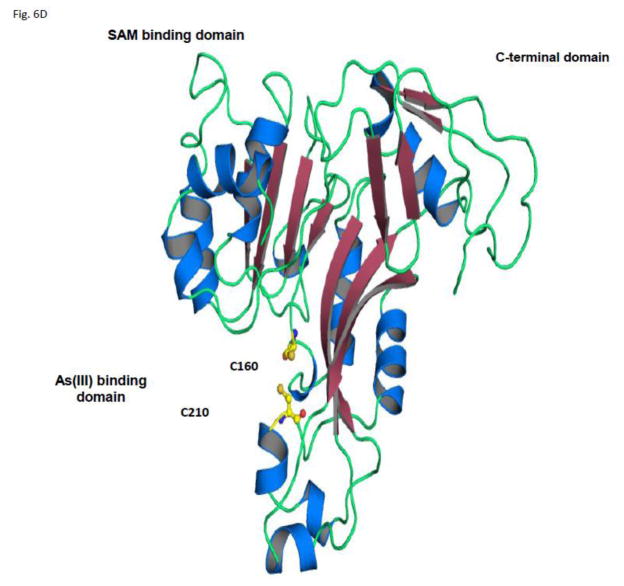
The lack of a three dimensional protein structure of AS3MT homologues prevented the complete elucidation of enzymatic mechanisms such as initial substrate binding and connection between different catalytic cycles. Currently, conserved cysteines have been identified in the sequences of a wide range of AS3MT homologues, and mutation of these cysteines led to the loss of zAs3mt function (Song et al., 2010). By homology modeling of As3mt using a recently available structure of an algal ArsM without (PDB ID 3P7E) and with bound AsIII (PDB ID 3QNH), we identified conserved two residues, C160 and C210, that we suggest are required for initial AsIII binding.
Zebrafish is an emerging model organism in toxicological studies with advantages in ease of toxicant exposure, high embryo availability and amenability of genetic modification. Additionally, zebrafish genome has conserved genes closely related to arsenic metabolism in humans thus providing an ideal animal model for investigating mechanisms of pathogenesis induced by arsenic exposure. In this study, we used zebrafish model to understand further the biomethylation of arsenic both in vivo and in vitro.
The methylation of arsenic in humans is influenced by many factors. For example, the individual difference, such as Body Mass Index (BMI), is found to be associated with altered arsenic methylation (Gomez-Rubio et al., 2011). Environmental factors, including the types of arsenic exposure and availability of micronutrients, for example selenium, may lead to variation in arsenic methylation. The presence of selenium will interfere with arsenic methylation given its general chemical properties similar to arsenic and potential to inhibit AS3MT (Song et al., 2010). Epidemiological studies demonstrated that low serum selenium is associated with increased MMA in urine (Basu et al., 2011). In the current study we found that selenium, in the form of selenite, is a potent inhibitor for As3mt function and is predicted to compete with AsIII for its initial binding. Since trivalent arsenite is also a clinical approved drug for leukemia treatment (Antman. et al., 2001), factors modulating As3mt function will be correlated to the interindividual difference in arsenic pathology and pharmacology. Detailed understanding of the enzymatic mechanisms of As3mt will promote application of zebrafish in the screening of efficient pharmacological intervention in the methylation process. The analogy of arsenic metabolism in zebrafish validates the use of this model in future arsenic toxicology and carcinogenesis studies.
Materials and Methods
Zebrafish husbandry and arsenic exposure
All studies involved zebrafish has been approved by Oakland University IACUC. Zebrafish ZF-1 line was maintained in an automatic 14:10 light:dark cycle at 28°C. Static waterborne arsenic exposure was carried out in adult zebrafish using 300 ppb or 5 ppm sodium arsenite for 120 hours. After treatment, zebrafish were rinsed for 1 hour and euthanized with 0.3 mM tricaine (MS-222 (Ethyl 3-aminobenzoate methanesulfonate), Sigma). Tissues were isolated and pooled (30 fish tissues used as one sample) and mass of each sample was determined. All tissue samples were homogenized in phosphate buffered saline (PBS buffer, pH 7.4) and adjusted to a final concentration of 100 mg wet tissue/mL buffer, and then centrifuged at 13,000 × g for 10 minutes. The resulting supernatants were filtered with a Microcon Ultracel YM-3 centrifugal filter (Millipore, MA) for speciation anaylysis by HPLC-ICP-MS (Hamdi et al., 2009).
Chemicals
The MMAIII was obtained through Dr. Styblo’s lab (North Carolina University). All other chemicals were obtained through commercial sources at analytical grade.
Cloning of zebrafish as3mt and site-directed mutagenesis
Zebrafish as3mt was cloned from a cDNA mixture synthesized from mRNA that has been extracted from whole zebrafish, and amplified using a pair of PCR primers with BglII and XhoI restriction sites (forward: 5′-GCAGATCTATGGCACCACGTCCAAAGCAGG-3′ and reverse: 5′-GCCTCGAGTCTATAAAGATGTTGCCTTCAG-3′). The amplified as3mt was initially cloned into pGEMT-easy (Promega) and a second round of PCR is applied to add 6XHis tag, followed by subcloning into pMAL-cX2 expression vector (NEB) (Liu et al., 2006). The resulting plasmid pMal-as3mt was transformed into E. coli BL21 (NEB) for overexpression and purification. The mutants of C165S and C210S were created by site-directed mutagenesis (Stratagene) using following primers: C165S forward: 5′-GATATTATCATA TCAAATTCTGTGGTGAATCTG-3′; reverse: 5′-CAGATTCACCACAGAATTTGATATGATAATATC-3′; C210S forward: 5′-CTTTATGGGGCGAGAGCCTCAGTGGAGCATTG-3′; reverse: 5′-CAATGCTCCACTGAGGCTCTCGCCCCATAAAG-3′. Both the WT gene and the mutants were verified by nucleotide sequencing.
Overexpression and purification of As3mt and mutants in E. coli
The E. coli strain BL21 carrying pMAL-as3mt1 was grown in LB medium supplied with ampicillin at 37 °C to an OD600 0.6–1.0, followed by induction with 0.6 mM IPTG for 8 hours. The overnight culture was harvested by centrifugation and washed. The cells were resuspended in buffer A (50 mM MOPS, pH 7.5, 20% (wt/vol) glycerol, 0.5 M NaCl, 20 mM imidazole, and 10 mM β-ME) and lysed by a single pass through French-press at 20,000 psi. Membranes and unbroken cells were removed by ultracentrifugation. The supernatant was loaded at a flow rate of 0.5 ml/min onto a Ni(II)-NTA column (QIAGEN) preequilibrated with buffer A. After washing with the same buffer, protein was eluted with 60 ml of buffer B (50 mM MOPS, pH 7.5, 20% (wt/vol) glycerol, 0.5 M NaCl, 200 mM imidazole, and 10 mM β-ME). Fractions containing zAs3mt were pooled and loaded onto the Amylose column (NEB) preequilibrated with buffer C (200 mM NaCl, 20 mM Tris HCl, pH 7.4, 1 mM EDTA, 1 mM sodium azide, 10 mM β-ME) and washed. The zAs3mt protein was eluted with buffer D (200 mM NaCl, 20 mM Tris HCl, pH 7.4, 1 mM EDTA, 1 mM sodium azide, 10 mM β-ME, 10 mM maltose). The zAs3mt fractions were concentrated using a 30-kDa-cutoff Amicon Ultrafilter (Millipore). Protein concentrations were determined by Absorbance Assay (280 nm). The purity of As3mt1 was determined by SDS gel electrophoresis.
SDS-PAGE and Western Immunoblotting
Protein samples or zebrafish tissue samples (males and females) were processed at 100 °C for 5 minutes and loaded on 12% SDS-PAGE gel, followed by coomassie brilliant blue staining. A custom raised primary polyclonal antibody (Abmart) of zAs3mt was applied in the western-blotting at 1:1000 dilution. Band density was quantified using the ImageJ software after scanning. Mean value and standard errors were calculated using SigmaPlot 10.0.
Analysis of enzymatic reaction using purified As3mt
The in vitro reaction mixture contained 10 mM Tris-HCl (pH 7.4), 0.1 M NaCl, 0.5 mM EDTA, 5 mM β-ME, 1 mM SAM, 1 mM GSH, 100 μM sodium arsenite (AsIII) and 1 μM zAs3mt, unless other concentrations indicated. Reaction mixtures were incubated at 37 °C for indicated times and the methylation reaction was stopped by filtration with a cut off column. A negative control was performed in the absence of zAs3mt from the above mixture. Inhibitors were added at indicated concentrations at the beginning of the reaction.
Arsenic binding assay
Sodium arsenite at indicated concentrations was incubated with 2 μM purified WT or mutant proteins. After 30 minutes the mixture was passed through spin columns (Micro Bio-Spin 6, Biorad) to remove the unbound free arsenic. The protein bound arsenic was eluted and quantified by inductively coupled plasma-mass spectrometer (ICP-MS, ELAN 9000, PerkinElmer).
Structural modeling of zAs3mt
The 0.63 Å resolution structure Cyanidio ArsM (CmArsM, 1–323 residues, PDB ID 3P7E) (Barry P. Rosen, PNAS, 2012, in press) was used as a reference to construct a C-terminal truncated (1–299 residue) As3mt model. The two sequences shared 43.85% of identity and 285 residues were aligned.
Arsenic speciation by HPLC-ICP-MS
The speciation of samples from cell lysate, enzymatic reaction mixtures or the zebrafish tissues was achieved using ICP-MS coupled with HPLC (Series 2000, PerkinElmer) in the front end. For arsenic samples generated from the enzymatic reaction the reaction was halted by removing As3mt from the reaction samples by centrifugation using a 10-kDa-cutoff Amicon Ultrafilter (Millipore). The filtrate was then separated and analyzed by HPLC-ICP-MS using a reverse-phase C18 column (Jupiter 300) eluted isocratically with a mobile phase (3 mM malonic acid, 5 mM tetrabutylammonium hydroxide, and 5% methanol, pH 5.6, with a flow rate of 1.0 ml/min). Arsenic standards were purchased from commercial suppliers.
Results
Zebrafish methylate inorganic AsIII to MMAII, MMAV and DMAV under arsenic exposure
The arsenic biomethylation profile was examined in zebrafish (males and females) and compared under arsenic exposure. Two arsenic concentrations, 300 ppb and 5 ppm, were applied for 120 hours to model two different levels of non-lethal doses. The pattern of arsenic methylation was determined and compared in different tissues. Our results showed that, compared with acute exposure, little methylated arsenic can be detected following sublethal exposure, with AsIII as the dominant species in most tissues (Fig. 1). When 5 ppm was applied a significant amount of methylated arsenic species appeared. DMAV was found in brain, gill, and liver while MMAV was detected in all tissues at a relatively low level. The intestine showed a significant amount of MMAIII, and several unknown components, possibly involved the arsenic methylation through microbial activity. An unknown peak not corresponding to any arsenic standards that eluted after DMAV was detected in almost all tissues and is hypothesized to be some type of As-GSH conjugate or a metabolite of an uncharacterized pathway. In all tissues the inorganic form of AsIII persisted in many tissues as the dominant species, indicating the biomethylation in these tissues cannot be efficiently processed. Factors accounting for differences of arsenic retention patterns in specific tissues may be collectively determined by the combined activity of biomethylation, uptake and efflux. We noticed that skin displayed a minimal arsenic accumulation but with high ratio of biomethylation. Since the arsenic transporters that facilitate AsIII uptake were identified in skin (Hamdi et al., 2009), we predict skin could have either efficient extrusion systems or is able to metabolize arsenic via unknown mechanisms. During all AsIII exposures the other important inorganic arsenical, AsV, was not detected in any tissues. Our previous studies show that no AsV is detected when zebrafish were exposed to AsV, indicating efficient reducing systems exist in zebrafish (Beene et al., 2011).
Figure 1. Arsenic methylation in zebrafish tissues.
Adult zebrafish were exposed to sodium arsenite at 300 ppb or 5 ppm for 120 hours in static water. Different tissues were isolated for speciation assays using HPLC-ICP-MS. Each sample contains a pool of thirty tissues. Species analyzed in the assay include AsIII, AsV, MMAIII, MMAV, and DMAV. The upper plots represent 5 ppm and the lower plots represent 300 ppb AsIII treated samples.
The As3mt is extensively expressed in zebrafish tissues
The expression of As3mt was ubiquitous in zebrafish tissues (Fig. 2). In contrast to mammalian AS3MT which is mainly expressed in liver, zebrafish As3mt is a broadly expressed protein detected in brain, eye, gill, intestine, liver, muscle and skin. Quantitative analysis showed that zAs3mt is mostly expressed in liver, muscle and intestine. As a result, arsenic methylation occurred in many tissues and methylated arsenic products were detected (Fig. 1). However, in tissues such as muscle with substantial As3mt expression, no methylated species were detected. It is possible that the activity of this enzyme is sensitive to tissue microenvironments, such as an absence of essential cofactors.
Figure 2. Expression of As3mt in different zebrafish tissues determined by western-blotting.
Zebrafish tissue was isolated and homogenized. All samples were suspended in PBS buffer at final concentration of 200 mg wet tissue/mL. Western blotting was performed using a custom raised antibody (Abmart). To quantify the expression of As3mt in different tissues, a commercial antibody that reacts with pan-actin (alpha-actin and beta-actin) was applied as a control. Lower panel: Quantification of As3mt protein expression from western-blotting analysis. Three replicates were obtained from western-blotting and quantified by ImageJ after scanning. Mean value and standard errors were derived with SigmaPlot 10.0.
Purified As3mt catalyzed step-wise methylation of AsIII, producing DMAV as the end product and MMAIII and MMAV as intermediates
His-tagged and MBP-tagged As3mt were purified as soluble recombinant proteins at approximately 80 kDa following Nickle-column and Maltose column purification (Fig. 3A). The enzymatic activity was assayed in buffer D, which contains SAM and β-ME, with an enzyme:substrate molar ratio of 1:100. The reaction was monitored in a time course fashion in order to detect step-wise formation and release of intermediates and products. The presence of MMAV and MMAIII were detected in the first round of methylation, followed by a second round of methylation to DMAV (Fig. 3). Our results showed that DMAV is the final product and MMAV and MMAIII are plausible intermediates. These in vitro results are consistent with our observations in vivo. DMAV is also the dominant biomethylation product detected in mammals and the final product for most AS3MT homologues, showing zebrafish and mammals showed similar arsenic biomethylation pathways. MMAIII is rarely observed in tissues, possibly due to the rate of second round of methylation following MMAIII formation is faster than first round. To our knowledge, this is the first experimental evidence showing the existence of MMAIII intermediate in an in vitro system using a purified arsenic methyltransferase.
Figure 3. Purification of As3mt protein and time-course of As3mt catalysis.
Left A: Double tagged As3mt was purified as a soluble protein. SDS-PAGE showed the purity of As3mt after elution from Ni(II)-NTA column and Maltose column (1. Protein ladder; 2. After Ni(II)-NTA column; 3. After maltose column). Right B: Time-course reaction of As3mt catalyzed arsenite methylation from AsIII to DMAV, producing MMAV and MMAIII as initial intermediates. The ratio of E:S is 1:100 (1μM: 100μM). Reaction time frame is indicated. Peaks indicated in the figure: 1. AsIII 2. MMAIII 3. DMAV, 4. MMAV 5. AsV.
Increased substrate concentration inhibits As3mt function
As AS3MT catalyzes multiple steps in arsenic methylation it is anticipated that the methylation pattern might be different according to varied substrate concentrations. To address this question in vitro, the effect of substrate doses on zAs3mt activity was investigated by applying various concentrations of AsIII concentrations in the enzymatic reaction mixture. As shown in Fig. 4, with an AsIII concentration range from 25 μM to 200 μM the As3mt activity declined. As3mt completely lost methylation ability at an AsIII concentration of 200 μM.
Figure 4. Inhibition of As3mt by increased substrate concentrations.
Sodium arsenite was added at indicated concentration (25, 50, 100, 200 μM) and incubated with 1 μM of purified As3mt and 100 μM of sodium arsenite. After reaction was finished (2 hours) arsenic speciation was determined by HPLC-ICP-MS.
Selenite is an inhibitor for As3mt
Selenium has been used to antagonize arsenic toxicity. In this study we found that selenium, in the form of selenite, inhibited As3MT activity efficiently. Addition of sodium selenite at 1:10 molar ratio with AsIII in the reaction mixture completely abolished the arsenic methylation (Fig. 5).
Figure 5. Inhibition of As3mt by selenite.
Sodium selenite was added at a final concentration of 2 μM causing abolished AsIII methylation catalyzed by As3mt.
Mutation of two cysteines (C160 and C210) lead to a loss of As3mt function and arsenic binding
In this study we directly tested if two cysteines (Fig. 6A) are involved in AsIII binding. The C165S and C210S mutants of As3mt were created and ectopically expressed in E. coli. The arsenic methylation activity of these mutants was studied and compared with the wild type (WT) As3mt. The cysteine mutation in these sites completely abolished the function of zAs3mt and no methylated arsenic species were produced (Fig. 6B). To further determine if these cysteines are required for substrate AsIII binding a direct assay was performed to investigate the properties of C165S and C210S in arsenic binding. Our results showed that WT As3mt binds AsIII substrates and saturated at an approximately 1:1 stoichiometry ratio, suggesting one AsIII binding site in the enzyme. However, both C160S and C210S mutants lost capability to bind AsIII, again indicating these two residues are required for substrate binding (Fig. 6C). In support of this hypothesis, a structure model was constructed based on a thermophilic alga Cyanidioschyzon sp. 5508 ArsM. As suggested in As3mt structure, the cysteine C160 and C210 are in close proximity and form a reasonable AsIII binding site (Fig. 6D).
Figure 6. Cys165 and Cys210 are predicted AsIII binding sites.
A). Sequence alignment of hsAS3MT, zAs3mt and CmArsM. Three conserved cysteines were indicated in the figure (boxed). B) Mutants of C160S and C210S were unable to methylate AsIII. Upper panel: cytosol from E. coli expressing mutant C160S and C210S with IPTG induction. Lower panel: Arsenic speciation in the culture medium of WT, negative control, mutant C160S and C210S, respectively after IPTG induction. Inside the figure showed the As3mt expression in E. coli after IPTG induction. C). C160S and C210S were unable to bind AsIII. Upper panel, WT, C160S and C210S were purified as recombinant proteins. Lower panel, binding assay of zAs3mt WT, C160S and C210S indicate that mutation of cysteines at 160 and 210 disabled the AsIII binding (plotted with SigmaPlot 10.0 with a hyperbolic curve). D). Structure model of zebrafish As3mt. The 0.63 Å CmArsM (1–323 residues) structure was used to construct As3mt model (1–299 residues). Cys160 and Cys210 were labeled in yellow color. Two addition functional domains, SAM binding domain and C-terminal domain were indicated in the structure.
Discussion
Due to the abundance of arsenic in the environment and diversity of mechanisms through which arsenic causes toxicity, it is important to understand the regulation of chemical metabolism, distribution, and bodily clearance of arsenic. The methylation of arsenic is one of the key variables and thus detailed understanding of mechanism of arsenic methyltransferases is crucial. Despite the possibility of other enzymes that catalyze arsenic biomethylation, such as N-6 adenine-specific DNA methyltransferase (N6AMT1), which was recently found to methylate MMAIII to DMAV (Ren et al., 2011), AS3MT is considered the major enzyme in arsenic biomethylation. This is consistently supported by many independent clinical studies which link AS3MT variants with altered profiles and ratios of arsenic methylated species (Valenzuela et al., 2009; Engstrom et al., 2011). It is clear that AS3MT transforms arsenic into less toxic DMAV which is more easily cleared from the body. However, it remains an open question whether AS3MT is responsible for the formation of more toxic intermediates such as MMAIII. This study showed zAs3mt transforms AsIII to DMAV similar to mammalian AS3MT homologues (Fig 3). The absence of significant MMAIII in zebrafish tissue after arsenic exposure can be explained by through MMAIII not being rate limiting in the second round of under these exposure conditions.
The unique nature of arsenic speciation may influence the pathogenesis of certain diseases such as cancer. The carcinogenic potential of arsenic is related to the sum of total arsenicals, including endogenously generated organic species. Therefore, function of AS3MT is a key factor in arsenic-induced carcinogenesis by affecting arsenic metabolism and bodily retention. This assumption is corroborated by a correlation between arsenic methylation and cancer incidence in epidemiological studies carried out in arsenic contaminated areas (Kwong et al., 2010; Wnek et al., 2010). Moreover, it has been shown by independent reports that AS3MT polymorphisms influence arsenic metabolism and individual cancer risk (Agusa et al., 2009; Kojima et al., 2009; Valenzuela et al., 2009). Analysis of arsenic speciation in urine shows that the concentration of monomethylated species is highly correlated with increased cancer risk(Chung et al., 2009).
Possible carcinogenic mechanisms include arsenic induced oxidative and DNA damage (Kojima et al., 2009) and pathogenesis via the generation of reactive oxygen species (ROS) (Jomova et al., 2011). Although all forms of inorganic arsenic as well as certain organic forms have been shown to generate ROS, the magnitude of arsenic induced oxidative stress is highly speciation dependent (Hughes, 2009). Arsenic also deregulates oncogenic signaling pathways and this is also likely to be speciation dependent. Given arsenic biomethylation via AS3MT activity produces intermediates with different capacity in elevating oxidative stress and ROS, it is reasonable to assume AS3MT activity may link to overall arsenic-induced carcenogenesis via controlling OS during arsenic exposure. Thus, the biologic action of AS3MT is predicted to play important roles in arsenic induced oxidative stress, DNA damage and ultimately, carcinogenesis. It follows that factors that modify AS3MT activity will affect overall arsenic-induced carcenogenesis. Therefore it is critical to elucidate the fundamental mechanisms of arsenic-induced methylation by AS3MT, and investigate the factors that may modify the enzymatic activities.
The function of AS3MT in arsenic methylation raises the question of how AS3MT produces methylated trivalent species and under which conditions the more toxic intermediates exist. It has been difficult to measure the activity of AS3MT in real-time. Some trivalent arsenic, such as MMAIII, can be detected in human urine; however, this plausible intermediate has not been observed using purified enzyme. Possible reasons for the lack of such results are: 1) most studies of methylation activity using purified human or rodent AS3MT were performed with high enzyme:substrate ratios, which fosters a complete reaction, but not detectable quantities of intermediates, i.e., the trivalent methylated species (Song et al., 2011); 2) Until recently, no three dimensional structure was available, which precluded structure-function analysis and elucidation of the catalytic mechanism. In our studies, using a purified zebrafish As3mt, we studied the time course of arsenic methylation, and demonstrated the existence of trivalent and pentavalent arsenic intermediates MMAIII and MMAV, which were formed in the first round of the cycle. The reaction ends up with DMAV and the third round of methylation is not achieved, therefore TMA gas is not detected (data not shown). These in vitro results are highly consistent with the arsenic distribution in vivo, with all these intermediates and end products detected under AsIII exposure. In rodents, the dominant product of AsIII biomethylation is DMAV, with a small portion of MMAV, which has been demonstrated in tissue culture and knockout mice (Drobna et al., 2004). The arsenic cellular retention is related to both biomethylation and membrane transport of substrates and products. For example, in human hepatocytes, DMAV is readily extruded while MMAV is mostly retained (Drobna et al., 2004). Our results in tissues of AsIII exposed zebrafish indicated that MMAV and DMAV could be detected in many tissues with different patterns which resemble those in rodents and humans. Interestingly, an uncharacterized arsenic compound which is not consistent with any known arsenic methylated compounds was identified in all tissues samples. Further studies are required to determine whether this arsenic species resulted from a different metabolic pathway other than biomethylation.
The AS3MT catalytic mechanisms were previously studied using biochemical approaches (Song et al., 2010) but the detailed mechanisms such as AsIII binding, and the continuous catalysis of two cycle of reactions have not been elucidated in AS3MT. By site-directed mutagenesis, function of conserved and non-conserved cysteines were investigated in human AS3MT where a cysteine, C72, is shown to be critical for the reaction and hypothesized to be involved in the initial substrate binding (Song et al., 2011). However, in our study using As3mt, we suggest C165 and C210 form substrate binding site. Single mutation of these cysteines disabled the As3mt function as well as binding ability to arsenic. Therefore the C165 and C210 may together form a coordinating site for arsenic binding. The coordinated arrangement of these two cysteines was indicated in the structure model of As3mt in this study (Fig. 6D). In this model, these two cysteines are close to each other and form a pocket for arsenic binding. Single mutation of C65S produced indistinguishable levels of AsIII binding to WT (data not shown), indicating its importance comes from a role in catalytic activity following binding. Results using CmArsM orthologue show that C74 (C65 in zebrafish As3mt) is required only for the second round of methylation but not the first round of methylation, while both Cys174 and Cys224 are involved in the entire reaction. Taken together our results are consistent with the information derived from CmArsM structure, showing that AsIII is bound by Cys160 and C210 (Cys174 and Cys224 in CmArsM) while Cys65 (Cys72 in CmArsM) moves toward the other two AsIII-binding cysteine residues when SAM is bound. The model also discloses a SAM binding domain and a C-terminal domain with unknown function. More studies are required to investigate the in depth coordination of the two rounds of reaction.
The function of AS3MT is affected by several factors, such as divalent metals zinc, copper, mercury and metalloid selenite (Song et al., 2010). Through different mechanisms these compounds all react with coordinated thiols. We report here that selenite is an efficient inhibitor for zAs3mt function. Given both its similar molecular structure to AsIII and ability to bind vicinal thiols, it is postulated that SeIV is competing for same binding sites as arsenic, similar to the binding of cysteines in zinc finger structure (Larabee et al., 2002). We also showed that elevated substrate concentration completely abolished the As3mt activity in the MMA or DMA production. Currently the basis is not clear and there are several possibilities: 1) Increased substrate will affect the initial binding and change the appropriate position of substrate and cofactors. 2) Increased substrate is toxic for the enzyme by modifying the overall enzyme conformation. More experiments are required to investigate the precise mechanisms of substrate inhibition in zAs3mt.
The in vitro activity of As3mt and other mammalian AS3MT is relatively low, which requires a higher enzyme:substrate ratio. Possible explanations for the reduced activity are that there are unknown cofactors absent from the in vitro system, such as endogenous reducing systems. The elevated AsIII retention in tissues indicates that the in vivo activity of AS3MT is also limited. From an evolutionary perspective, As3mt function requires SAM, which requires ATP during synthesis. On the other hand, it is reasonable that zebrafish or mammals do not require a highly efficient enzyme to metabolize commonly occurring low levels of environmental arsenic. An additional detoxification pathway, such as MRP-mediated GSH conjugate efflux can provide an alternative detoxification pathway (Liu et al., 2001).
Currently there are no studies on the regulation of As3mt and further studies are needed to understand the regulation of As3mt expression along with the role of As3mt in long-term chronic arsenic exposure. The zebrafish model can be well adapted to these purposes by the availability of as3mt transgenesis. Along with the advantage in conducting high throughput chemical exposure studies and dose responsive toxicity studies with large number of sample applied. Zebrafish can be an ideal animal model for translational research not only to understand the role of As3mt in overall arsenic-induced carcinogenesis but also to facilitate therapeutic drug discovery for treatment and prevention for arsenic related malignancy via pharmacological intervention (Lam et al., 2006; Amatruda and Patton, 2008; Nguyen et al., 2011).
Highlights.
Zebrafish methylated AsIII to MMAIII, MMAV and DMAV.
A zebrafish arsenic methyltransferase (As3mt) was purified in E. coli.
As3mt catalyzed biomethylation of AsIII to DMAV and produced toxic intermediates.
As3mt activity is inhibited by elevated substrate concentrations and selenite.
C160 and C165 are predicted as AsIII binding sites.
Acknowledgments
This work was supported by NIH ES016856 to Zijuan Liu and NIH GM55425 to Barry P. Rosen (Florida International University). We appreciate Dr. Barry P. Rosen’s critical review of this manuscript.
Abbreviations
- SAM
S-adenosylmethionine
- MMAIII
monomethylarsonous acid
- MMAV
monomethylarsonic acid
- DMAV
dimethylarsinic acid
- β-ME
β-mercaptoethanol
- ICP-MS
Inductively-coupled plasma spectrometry
- GSH
Glutathione
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agusa T, Iwata H, Fujihara J, Kunito T, Takeshita H, Minh TB, Trang PT, Viet PH, Tanabe S. Genetic polymorphisms in AS3MT and arsenic metabolism in residents of the Red River Delta, Vietnam. Toxicol Appl Pharmacol. 2009;236:131–141. doi: 10.1016/j.taap.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Amatruda JF, Patton EE. Genetic models of cancer in zebrafish. Int Rev Cell Mol Biol. 2008;271:1–34. doi: 10.1016/S1937-6448(08)01201-X. [DOI] [PubMed] [Google Scholar]
- Antman KH. Introduction: the history of arsenic trioxide in cancer therapy. Oncologist. 2001;6(Suppl 2):1–2. doi: 10.1634/theoncologist.6-suppl_2-1. [DOI] [PubMed] [Google Scholar]
- Basu A, Mitra S, Chung J, Guha Mazumder DN, Ghosh N, Kalman D, von Ehrenstein OS, Steinmaus C, Liaw J, Smith AH. Creatinine, diet, micronutrients, and arsenic methylation in West Bengal, India. Environ Health Perspect. 2011;119:1308–1313. doi: 10.1289/ehp.1003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beene LC, Halluer J, Yoshinaga M, Hamdi M, Liu Z. Pentavalent Arsenate Transport by Zebrafish Phosphate Transporter NaPi-IIb1. Zebrafish. 2011 doi: 10.1089/zeb.2011.0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bun-ya M, Shikata K, Nakade S, Yompakdee C, Harashima S, Oshima Y. Two new genes, PHO86 and PHO87, involved in inorganic phosphate uptake in Saccharomyces cerevisiae. Curr Genet. 1996;29:344–351. [PubMed] [Google Scholar]
- Chung CJ, Hsueh YM, Bai CH, Huang YK, Huang YL, Yang MH, Chen CJ. Polymorphisms in arsenic metabolism genes, urinary arsenic methylation profile and cancer. Cancer Causes Control. 2009;20:1653–1661. doi: 10.1007/s10552-009-9413-0. [DOI] [PubMed] [Google Scholar]
- de The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10:775–783. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- Drobna Z, Naranmandura H, Kubachka KM, Edwards BC, Herbin-Davis K, Styblo M, Le XC, Creed JT, Maeda N, Hughes MF, Thomas DJ. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem Res Toxicol. 2009;22:1713–1720. doi: 10.1021/tx900179r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, Waters SB, Devesa V, Harmon AW, Thomas DJ, Styblo M. Metabolism and toxicity of arsenic in human urothelial cells expressing rat arsenic (+3 oxidation state)-methyltransferase. Toxicol Appl Pharmacol. 2005;207:147–159. doi: 10.1016/j.taap.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, Waters SB, Walton FS, LeCluyse EL, Thomas DJ, Styblo M. Interindividual variation in the metabolism of arsenic in cultured primary human hepatocytes. Toxicol Appl Pharmacol. 2004;201:166–177. doi: 10.1016/j.taap.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Engstrom K, Vahter M, Mlakar SJ, Concha G, Nermell B, Raqib R, Cardozo A, Broberg K. Polymorphisms in arsenic(+III oxidation state) methyltransferase (AS3MT) predict gene expression of AS3MT as well as arsenic metabolism. Environ Health Perspect. 2011;119:182–188. doi: 10.1289/ehp.1002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rubio P, Roberge J, Arendell L, Harris RB, O’Rourke MK, Chen Z, Cantu-Soto E, Meza-Montenegro MM, Billheimer D, Lu Z, Klimecki WT. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol Appl Pharmacol. 2011;252:176–182. doi: 10.1016/j.taap.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi M, Sanchez MA, Beene LC, Liu Q, Landfear SM, Rosen BP, Liu Z. Arsenic transport by zebrafish aquaglyceroporins. BMC Mol Biol. 2009;10:104. doi: 10.1186/1471-2199-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Kobayashi Y, Cui X, Kanno S, Hayakawa T, Shraim A. The accumulation and toxicity of methylated arsenicals in endothelial cells: important roles of thiol compounds. Toxicol Appl Pharmacol. 2004;198:458–467. doi: 10.1016/j.taap.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Hughes MF. Arsenic methylation, oxidative stress and cancer--is there a link? J Natl Cancer Inst. 2009;101:1660–1661. doi: 10.1093/jnci/djp437. [DOI] [PubMed] [Google Scholar]
- Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- Katsoyiannis IA, Hug SJ, Ammann A, Zikoudi A, Hatziliontos C. Arsenic speciation and uranium concentrations in drinking water supply wells in Northern Greece: correlations with redox indicative parameters and implications for groundwater treatment. Sci Total Environ. 2007;383:128–140. doi: 10.1016/j.scitotenv.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Kitchin KT, Conolly R. Arsenic-induced carcinogenesis--oxidative stress as a possible mode of action and future research needs for more biologically based risk assessment. Chem Res Toxicol. 2010;23:327–335. doi: 10.1021/tx900343d. [DOI] [PubMed] [Google Scholar]
- Kojima C, Ramirez DC, Tokar EJ, Himeno S, Drobna Z, Styblo M, Mason RP, Waalkes MP. Requirement of arsenic biomethylation for oxidative DNA damage. J Natl Cancer Inst. 2009;101:1670–1681. doi: 10.1093/jnci/djp414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong RC, Karagas MR, Kelsey KT, Mason RA, Tanyos SA, Schned AR, Marsit CJ, Andrew AS. Arsenic exposure predicts bladder cancer survival in a US population. World J Urol. 2010;28:487–492. doi: 10.1007/s00345-009-0477-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SH, Wu YL, Vega VB, Miller LD, Spitsbergen J, Tong Y, Zhan H, Govindarajan KR, Lee S, Mathavan S, Murthy KR, Buhler DR, Liu ET, Gong Z. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol. 2006;24:73–75. doi: 10.1038/nbt1169. [DOI] [PubMed] [Google Scholar]
- Larabee JL, Hocker JR, Hanas RJ, Kahn FM, Hanas JS. Inhibition of zinc finger protein-DNA interactions by sodium selenite. Biochem Pharmacol. 2002;64:1757–1765. doi: 10.1016/s0006-2952(02)01414-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen H, Miller DS, Saavedra JE, Keefer LK, Johnson DR, Klaassen CD, Waalkes MP. Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol Pharmacol. 2001;60:302–309. doi: 10.1124/mol.60.2.302. [DOI] [PubMed] [Google Scholar]
- Liu Z, Sanchez MA, Jiang X, Boles E, Landfear SM, Rosen BP. Mammalian glucose permease GLUT1 facilitates transport of arsenic trioxide and methylarsonous acid. Biochem Biophys Res Commun. 2006;351:424–430. doi: 10.1016/j.bbrc.2006.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci U S A. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marapakala K, Qin J, Rosen BP. Identification of Catalytic Residues in the As(III) S-Adenosylmethionine Methyltransferase. Biochemistry. 2012;51:944–951. doi: 10.1021/bi201500c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XY, Qin J, Wang LH, Duan GL, Sun GX, Wu HL, Chu CC, Ling HQ, Rosen BP, Zhu YG. Arsenic biotransformation and volatilization in transgenic rice. New Phytol. 2011;191:49–56. doi: 10.1111/j.1469-8137.2011.03743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Emelyanov A, Koh CH, Spitsbergen JM, Parinov S, Gong Z. An inducible krasV12 transgenic zebrafish model for liver tumorigenesis and chemical drug screening. Dis Model Mech. 2011 doi: 10.1242/dmm.008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163:203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Qin J, Lehr CR, Yuan C, Le XC, McDermott TR, Rosen BP. Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga. Proc Natl Acad Sci U S A. 2009;106:5213–5217. doi: 10.1073/pnas.0900238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Rosen BP, Zhang Y, Wang G, Franke S, Rensing C. Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proc Natl Acad Sci U S A. 2006;103:2075–2080. doi: 10.1073/pnas.0506836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman, Naranmandura H. Arsenic metabolism and thioarsenicals. Metallomics. 2012 doi: 10.1039/c2mt00181k. [DOI] [PubMed] [Google Scholar]
- Ren X, Aleshin M, Jo WJ, Dills R, Kalman DA, Vulpe CD, Smith MT, Zhang L. Involvement of N-6 adenine-specific DNA methyltransferase 1 (N6AMT1) in arsenic biomethylation and its role in arsenic-induced toxicity. Environ Health Perspect. 2011;119:771–777. doi: 10.1289/ehp.1002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Geng Z, Li X, Hu X, Bian N, Zhang X, Wang Z. New insights into the mechanism of arsenite methylation with the recombinant human arsenic (+3) methyltransferase (hAS3MT) Biochimie. 2010;92:1397–1406. doi: 10.1016/j.biochi.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Song X, Geng Z, Li X, Zhao Q, Hu X, Zhang X, Wang Z. Functional and structural evaluation of cysteine residues in the human arsenic (+3 oxidation state) methyltransferase (hAS3MT) Biochimie. 2011;93:369–375. doi: 10.1016/j.biochi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ Health Perspect. 2002;110(Suppl 5):767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Waters SB, Styblo M. Elucidating the pathway for arsenic methylation. Toxicol Appl Pharmacol. 2004;198:319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Tseng CH. Arsenic methylation, urinary arsenic metabolites and human diseases: current perspective. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25:1–22. doi: 10.1080/10590500701201695. [DOI] [PubMed] [Google Scholar]
- Tseng CH. Cardiovascular disease in arsenic-exposed subjects living in the arseniasis-hyperendemic areas in Taiwan. Atherosclerosis. 2008;199:12–18. doi: 10.1016/j.atherosclerosis.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Valenzuela OL, Drobna Z, Hernandez-Castellanos E, Sanchez-Pena LC, Garcia-Vargas GG, Borja-Aburto VH, Styblo M, Del Razo LM. Association of AS3MT polymorphisms and the risk of premalignant arsenic skin lesions. Toxicol Appl Pharmacol. 2009;239:200–207. doi: 10.1016/j.taap.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SB, Devesa V, Fricke MW, Creed JT, Styblo M, Thomas DJ. Glutathione modulates recombinant rat arsenic (+3 oxidation state) methyltransferase-catalyzed formation of trimethylarsine oxide and trimethylarsine. Chem Res Toxicol. 2004;17:1621–1629. doi: 10.1021/tx0497853. [DOI] [PubMed] [Google Scholar]
- Wnek SM, Jensen TJ, Severson PL, Futscher BW, Gandolfi AJ. Monomethylarsonous acid produces irreversible events resulting in malignant transformation of a human bladder cell line following 12 weeks of low-level exposure. Toxicol Sci. 2010;116:44–57. doi: 10.1093/toxsci/kfq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Rensing C, Rosen BP, Zhu YG. Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 2012 doi: 10.1016/j.tplants.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]