Abstract
Objectives
Compounds characterized by a peroxidic skeleton are an interesting starting point for antischistosomal drug discovery. Previously a series of 3-alkoxy-1,2-dioxolanes, which are chemically stable cyclic peroxides, demonstrated significant in vitro activity against Plasmodium falciparum. We aimed to evaluate the potential of these compounds against Schistosoma mansoni and elucidate the roles of iron and peroxidic groups in activity.
Methods
Drugs were tested against juvenile and adult stages of S. mansoni in vitro and in vivo. Selected structures were assessed in vitro against schistosomes in the presence of additional iron sources. In addition, drugs were tested in vitro and in vivo against Echinostoma caproni, a non-blood-feeding intestinal fluke. Finally, the activity of non-peroxidic analogues was evaluated.
Results
Three dioxolanes displayed IC50s ≤20.1 μM against adult schistosomes and values as low as 4.2 μM against newly transformed schistosomula. Nonetheless, only moderate, non-significant worm burden reductions were observed after treatment of mice harbouring adult infections. Drugs lacked activity against juvenile schistosomes in vivo. Two selected dioxolanes showed in vitro activity against E. caproni down to concentrations of 5 mg/L, but none of the compounds revealed in vivo activity. All tested non-peroxidic analogues lacked activity in vitro against both parasites.
Conclusions
Selected dioxolanes presented interesting in vitro activity, but low in vivo activities have to be overcome to identify a lead candidate. Although the inactivity of non-peroxidic analogues underlines the necessity of a peroxide functional group, incubation of adult schistosomes with additional iron sources did not alter activity, supporting an iron-independent mode of activation.
Keywords: chemotherapy, peroxides, non-peroxidic analogues, schistosomiasis
Introduction
Schistosomiasis is a neglected tropical disease caused by trematode flatworms of the genus Schistosoma. Five species of schistosomes infect humans, with Schistosoma haematobium, Schistosoma japonicum and Schistosoma mansoni being responsible for the main burden of schistosomiasis.1 Approximately 200 million people are affected by schistosomiasis, mainly in sub-Saharan Africa.2 Praziquantel is currently the gold standard for the treatment of schistosome infections. Because of the threat of drug resistance and the limitations in the activity profile of praziquantel (the drug lacks activity against the juvenile schistosome stages), identification of potential drug candidates has a high priority.
The antischistosomal potential of the antimalarials artemisinin and its semisynthetic derivatives artesunate and artemether has been studied thoroughly in the past three decades, in in vitro and in vivo studies, and in clinical trials.3–5 Given the promising activity profile of the artemisinins, with particularly high activities observed against juvenile schistosomes, different groups of fully synthetic peroxides have been studied in vivo and in vitro in recent years.6,7 For example, the synthetic trioxolane OZ418 was recently introduced as a promising drug candidate showing high worm burden reductions (WBRs) of 80% and 86% against S. mansoni and S. haematobium, respectively.8
The high antimalarial activity of 1,2,4-trioxolanes triggered investigations with 1,2-dioxolanes, which are structurally analogous five-membered ring peroxides offering enhanced chemical stability. However, the dioxolanes proved much less active than the corresponding 1,2,4-trioxolanes against Plasmodium falciparum in vitro and Plasmodium berghei in vivo.9 This reduction in activity has been attributed to a decreased tendency for scission of the alkoxy radicals derived from Fe(II) activation of the 1,2-dioxolane peroxide;10 β-scission to generate carbon radicals is considered important for the activity of peroxide antimalarials.11 3-Alkoxy-1,2-dioxolanes, which undergo activation by Fe(II) to generate oxygen-substituted alkoxy radicals closely related to the intermediates derived from trioxolanes, generate products indicative of efficient β-scission and have been shown to have promising antimalarial efficacy.10
To our knowledge the antischistosomal activity of the 3-alkoxy-1,2-dioxolanes has not been studied to date. In the present work 18 selected 3-alkoxy-1,2-dioxolanes were tested on S. mansoni in vitro and active candidates were followed up in vivo. To investigate whether the peroxide pharmacophore is an essential requirement for antischistosomal activity, non-peroxidic analogues of active compounds were synthesized and their antischistosomal potential was determined. In addition, the relationship between the parasite's haemoglobin consumption and the antischistosomal activity of compounds was studied in vitro by testing active compounds on the non-blood-feeding foodborne trematode Echinostoma caproni and evaluating the in vitro activity of active compounds under different incubation conditions, in media containing haemin, haemoglobin or red blood cells.
Materials and methods
Drugs
The 18 3-alkoxy-1,2-dioxolane substrates illustrated in Table 1 were prepared based upon methods described by Schiaffo et al.10 Two non-peroxidic analogues were prepared as illustrated in Figure S1 (available as Supplementary data at JAC Online).
Table 1.
Chemical structures of investigated 3-alkoxy-1,2-dioxolanes and determined IC50 values (mean of three experiments) against newly transformed schistosomula (NTS) and adult S. mansoni 72 h post-compound exposure; r represents goodness of fit (conformity with r ≥ 0.85)
| Compound |
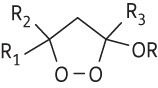 |
IC50 (μM) for S. mansoni |
||||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R | NTS | r | adult | r | |
| 1 | Me | Me | Me | 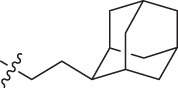 |
31.1 | 0.9 | 11.9 | 1.0 |
| 2 | Me | Bu | Me | CH2CH2Ph | 7.5 | 1.0 | 12.4 | 1.0 |
| 3 | -(CH2)5- | CH2CH2Ph | CH2CH2Ph | 4.2 | 0.9 | 20.1 | 0.8 | |
| 4 | Me | Me | CH2Ph | Me | 101.0 | 0.7 | 49.2 | 1.0 |
| 5 | 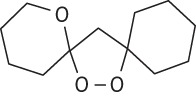 |
17.0 | 0.7 | 76.4 | 1.0 | |||
| 6 | Me | Me | Me | 5,6-epoxyhexyl | 35.9 | 0.9 | 101.5 | 1.0 |
| 7 | Me | Me | Me | CH2CH2CN | 89.2 | 1.0 | 137.0 | 1.0 |
| 8 | -(CH2)5- | Me | CH(OH)CH2OH | 90.9 | 0.9 | 138.4 | 1.0 | |
| 9 | Me | Me | Me | (CH2)9CO2Me | 89.7 | 0.9 | 169.3 | 0.9 |
| 10 | Me | Me | Me | CH2CH2CO2Me | 76.0 | 1.0 | 206.2 | 1.0 |
| 11 | Me | Me | Me | CH2CH2OMe | 69.2 | 0.8 | 207.4 | 0.9 |
| 12 | Me | Me | Me | CH2CH2 CH2OH | 42.6 | 0.8 | 235.8 | 0.9 |
| 13 | Me | Me | Me | (CH2)4CO2Me | 59.8 | 1.0 | 283.3 | 0.6 |
| 14 | Me | Me | Me | CH(OH)CH2OH | 102.5 | 0.9 | >436 | |
| 15 | Me | Me | Me | CH2CH2Ph | 2.7 | 0.9 | 58.4 | 0.9 |
| 16 | -(CH2)5- | Me | CH2CH2Ph | 2.8 | 0.9 | 49.4 | 0.9 | |
| 17 | -(CH2)5- | Me | 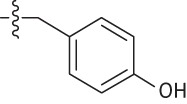 |
4.1 | 0.9 | 71.8 | 1.0 | |
| 18 | Me | Me | Me | 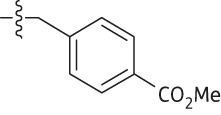 |
4.8 | 0.9 | 109.3 | 0.9 |
| Praziquantel | 2.2 | 0.9 | 0.08a | |||||
| Artesunate | 5.0 | 0.9 | 41.2 | 0.8 | ||||
aAs described by Keiser et al.26
Animals and parasites
Animal studies were conducted following Swiss regulations on animal welfare at the Swiss Tropical and Public Health Institute (Basel, Switzerland, permission no. 2070). Three-week-old (weight ∼14 g) female NMRI mice were purchased from Charles River (Sulzfeld, Germany) or Harlan Laboratories (Horst, The Netherlands). Before starting experiments, animals were allowed to adapt for 1 week under controlled conditions (temperature ∼22°C; humidity ∼50%; 12 h light and 12 h dark cycle; free access to rodent diet and water). Infection of mice with S. mansoni (Liberian strain) was performed subcutaneously by injection of 80–100 cercariae. Cercariae were harvested from infected intermediate host snails (Biomphalaria glabrata) by exposure to light for 3 h, following the standard procedures of our laboratory. For in vitro and in vivo studies with E. caproni, mice were infected intragastrically with 30 metacercariae harvested from infected B. glabrata snails.
In vitro screening
Preparation of NTS and adult schistosomes
NTS were obtained by mechanical transformation of S. mansoni cercariae.12,13 Cercariae were collected as described above. The schistosomula suspension was adjusted to a concentration of 100 NTS per 50 μL with Medium 199 (Invitrogen, Carlsbad, CA, USA) supplemented with 5% heat-inactivated fetal calf serum (iFCS), 100 U/mL penicillin and 100 mg/L streptomycin (Invitrogen). To ensure completed conversion from cercariae to NTS, suspensions were incubated at 37°C in an atmosphere of 5% CO2 in ambient air for a minimum of 12–24 h until use.14
Adult flukes were harvested from hepatic portal veins and mesenteric veins of infected NMRI mice (7–8 weeks post-infection) following standard procedures.6 Schistosomes were placed in RPMI 1640 culture medium supplemented with 5% iFCS, 100 U/mL penicillin and 100 mg/L streptomycin at 37°C in an atmosphere of 5% CO2 until use.
Preparation of adult E. caproni
Infected mice were killed 2 weeks post-infection. Trematodes were harvested from the excised small intestine and placed in RPMI medium supplemented with 100 U/mL penicillin, 100 mg/L streptomycin and 1% α-d-glucose (Sigma Aldrich, St Louis, MO, USA). Flukes were maintained at 37°C in an atmosphere of 5% CO2 in ambient air until use.
Drug susceptibility assays with NTS
Drug dilution series with concentrations ranging from 0.37 to 90 mg/L (0.37, 1.1, 3.3, 10, 30, 90 mg/L) were prepared in 96-well flat-bottom plates (BD Falcon, USA) using supplemented medium (with iFCS and antibiotics). Prepared NTS suspension containing 100 NTS per 50 μL was added to each well to yield a total volume of 250 μL per well. The highest DMSO concentration (1.1%) used, diluted in Medium 199, served as control. Plates were incubated at 37°C in an atmosphere of 5% CO2. NTS were evaluated by microscopic readout (Carl Zeiss, Germany, magnification ×80) with regard to death, changes in motility, viability and morphological alterations 24, 48 and 72 h post-drug exposure. As described previously, drug effects were evaluated using a viability scale.12,13 Parasite fitness, morphology and motility were classified with scores ranging from 3 (normal activity, no morphological changes) to 0 (all worms dead). Duplicate examinations were performed for each concentration and experiments were repeated at least three times. IC50 values of the investigated drugs based on motility scale values obtained at the 72 h timepoint were determined using CompuSyn software (Version 3.0.1, 2007; ComboSyn, Inc.).
Drug susceptibility assay with adult schistosomes
In vitro screening on adult flukes was performed in 24-well flat-bottom plates (BD Falcon, USA). Supplemented RPMI 1640 medium (with iFCS and antibiotics) and drug stock solutions (10 mg/mL) were used to obtain final test concentrations of 1–90 mg/mL (1.1, 3.3, 10, 30, 90 mg/L) in wells with a final volume of 1.4 mL. Finally, three schistosomes of both sexes were added to each well. The highest concentration of DMSO (0.3%) in medium served as control. Twenty-four, 48 and 72 h post-drug exposure, phenotypes were monitored using the motility scale described by Ramirez et al.15 and an inverse microscope (Carl Zeiss, Germany, magnification ×80). Experiments were performed three times. IC50 values were calculated with CompuSyn software as described above for NTS (Version 3.0.1, 2007; ComboSyn, Inc.).
Drug susceptibility assay of adult S. mansoni in the presence of haemin, haemoglobin or red blood cells
Lead candidates were incubated as described above, but using different RPMI culture medium conditions. A haemin solution (1.5 mM) was prepared as follows: 50 mg haemin chloride (Fluka Analytical, The Netherlands) was dissolved in 10 mL of 0.1 M NaOH, 0.5 mL of 1 M HCl and 39.5 mL of PBS (pH = 7.4). The haemoglobin solution (0.23 mM) was prepared using 750 mg haemoglobin from bovine blood (Sigma Aldrich, USA) dissolved in the same amounts of NaOH, HCl and PBS as used above. Finally, supplemented RPMI media were prepared by adding 8% haemin solution, 10% haemoglobin solution or 2% red blood cells from red blood cell concentrate (blood group A Rhesus positive) to final concentrations in well plates of 120 μM for haemin, 23 μM for haemoglobin or 2% for red blood cells. At timepoints 24, 48 and 72 h post-exposure, phenotypes were monitored using the motility scale as described above using an inverse microscope (Carl Zeiss, Germany, magnification ×80) and data were compared between various incubation conditions.15
Isothermal microcalorimetry (IMC) drug assay with adult S. mansoni
Two non-peroxidic analogues (compounds 19, 20) and one selected dioxolane (compound 16) were further characterized using IMC as described by Manneck et al.16 Briefly, heat production and motility (derived from noise amplitudes) of schistosomes were measured using a 48-channel microcalorimeter (model TAM 48; TA Instruments, New Castle, DE, USA) over a period of 5 days. Samples were prepared in glass ampoules with 2900 μL of medium (supplemented RPMI 1640) containing four adult worms. Pre-warmed (37°C) ampoules were placed in channels and equilibration was performed for 12 h until a stable signal was observed. Drug suspensions (concentration of 900 mg/L) in supplemented medium (volume 100 μL) were injected, using 1 mL syringes (BD Plastipak, Becton Dickinson S.A., Madrid, Spain), to reach the final concentration of 30 mg/L per ampoule. Ampoules with dead worms served as the negative control and ampoules with worms treated with the highest concentration of DMSO (0.3%) served as the positive control. Heat flow was recorded as 1 data point per 1 min over at least 120 h. Tests at each concentration were performed three times.
Drug susceptibility assay with adult E. caproni
Assays were prepared in 24-well flat-bottom plates (Costar). Drug dilutions were prepared with drug stock solutions and supplemented RPMI medium (with antibiotics and glucose) to obtain final drug concentrations of 5–100 mg/L (5, 10, 50, 100 mg/L) in a total volume of 2 mL per well. Six to nine trematodes were used (one or two worms per well) for each experimental group. The highest concentration of DMSO (1%) served as control. Plates were incubated at 37°C in an atmosphere of 5% CO2. Twenty-four, 48 and 72 h post-drug exposure, phenotypes and mortality of worms were monitored as described elsewhere.12
In vivo screening
Studies with S. mansoni
Groups of four infected NMRI mice characterized by a patent schistosome infection (49 days post-infection) or a juvenile Schistosoma infection (21 days post-infection) were treated orally with the test drug using single oral doses of 400 mg compound per kg body weight. Seven to nine untreated mice served as controls. Animals harbouring an adult Schistosoma infection were killed by the CO2 method 14 days post-treatment, and mice treated at the juvenile infection stage were sacrificed 4 weeks post-treatment. Mice were then dissected and worms sexed and counted.6 Worm burdens of treated mice were compared with those of untreated animals and reductions of worm burden calculated.
Studies with E. caproni
Four NMRI mice were treated intragastrically with 400 mg/kg of test compounds 2 weeks post-infection with E. caproni. Four mice were left untreated and served as controls. One week post-treatment, mice were euthanized with CO2. At necropsy, the intestines were removed from the pylorus to the ileocaecal valve, placed in a Petri dish and opened longitudinally. All E. caproni worms were removed and counted.
Statistics
Parasite motility of treated and untreated NTS and adult trematodes was calculated as mean (±SD) using Microsoft Excel software. Motility data obtained from experiments with various media containing iron sources were compared using the Mann–Whitney test (considered significant at P ≤ 0.05). IC50 values were determined using the CompuSyn software (Version 3.0.1, 2007; ComboSyn, Inc.). For the comparison of IC50 values we used the Kruskal–Wallis test (considered significant at P ≤ 0.05). The Kruskal–Wallis test was also used for in vivo studies, comparing the medians of the responses between the treatment and control groups. A difference in median was considered to be significant at a significance level of 5% (StatsDirect statistical software, version 2.7.2.; StatsDirect Ltd, UK). Noise amplitudes and heat flows observed in calorimetric in vitro assays with adult S. mansoni were analysed using R software and Microsoft Excel. As described by Manneck et al.,17 noise amplitude values follow an exponential decay. Endpoints of worm motility were determined by the intersection of the sample amplitude curve with the background signal noise of dead worms.
Results
In vitro screening against S. mansoni
In a first step, 14 3-alkoxy-1,2-dioxolanes were studied against adult S. mansoni in vitro. Three of the compounds (1, 2 and 3) demonstrated good antischistosomal activity with IC50s of 11.9, 12.4 and 20.1 μM, respectively, against adult flukes. The remaining 11 substances revealed only minor activity (IC50s between 49.2 and 283.3 μM) or lacked activity (compound 14). Good activities were observed against the juvenile schistosome stage (NTS) for compounds 2 (IC50 7.5 μM) and 3 (IC50 4.2 μM). Moderate activity was detected for compounds 1 (IC50 31.1 μM), 5 (IC50 17.0 μM) and 6 (IC50 35.9 μM) against NTS. Finally, only minor activity was recorded for the remaining nine compounds against NTS (IC50 42.6–102.5 μM). In vitro findings are summarized in Table 1. For comparison, activities of standard antischistosomal drugs, praziquantel and artesunate, are also shown in Table 1.
Influence of haemin, haemoglobin or red blood cells on in vitro antischistosomal activity
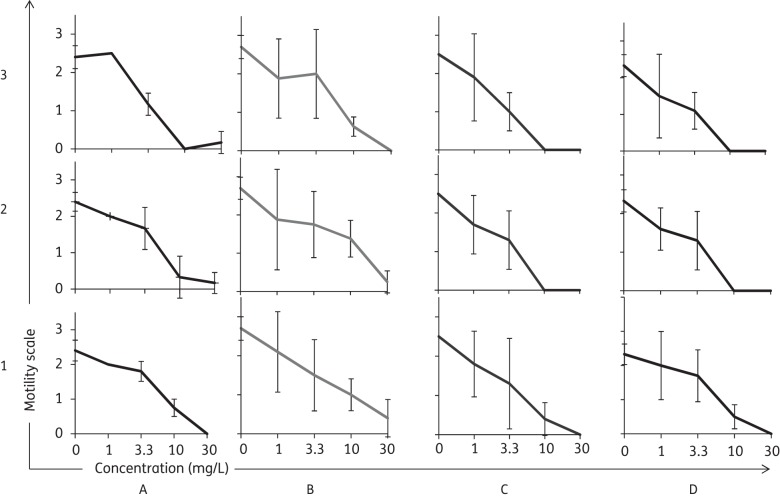
Incubation of adult schistosomes with various concentrations (1.1, 3.3, 10, 30 mg/L) of the three active alkoxydioxolanes (1–3) in supplemented media containing haemin, haemoglobin or red blood cells showed no significant differences in activity. The motilities recorded 72 h post-exposure of S. mansoni with compound 1 at different concentrations did not vary among the different media tested (Figure 1). Slightly higher, though not significant (P > 0.05) activities were detected for compounds 2 and 3 when incubated in the presence of haemin or haemoglobin. In the presence of these media all worms had died 72 h post-exposure to the tested compounds at a concentration of 10 mg/L and 30 mg/L.
Figure 1.
Motility of adult schistosomes 72 h post-treatment with various concentrations (1.1, 3.3, 10 and 30 mg/L) of compounds 1, 2 or 3 in four different incubation settings. (a) Standard incubation with supplemented RPMI. (b) Addition of 2% red blood cells from red blood cell concentrate. (c) Addition of 120 μM haemin. (d) Addition of 23 μM haemoglobin.
In vivo activity of selected alkoxydioxolanes against S. mansoni
The three lead structures 1–3 identified by prior in vitro screening, were tested in a juvenile as well as an adult S. mansoni infection mouse model (Table 2). All studied alkoxydioxolanes lacked in vivo activity against juvenile S. mansoni (Table 2; WBRs 0%–4%). However, moderate, non-significant activities were observed with compounds 1 and 2 against adult S. mansoni with WBRs of 42.5% and 37.0%, respectively. Compound 3 showed only low activity against adult S. mansoni in vivo with a WBR of 15.1%. Dead worms were detected in mouse livers after treatment of adult infections with compounds 1, 2 and 3. The presence of a patent schistosoma infection in treated animals was confirmed by observing granulous tissue and Schistosoma eggs within all livers.
Table 2.
In vivo activity following a single oral dose of 400 mg/kg of selected 3-alkoxy-1,2-dioxolanes in mice harbouring juvenile or adult S. mansoni
| Group | No. of mice investigated | No. of mice that died | No. of mice cured | TWR (%) | P value | FWBR (%) | P value |
|---|---|---|---|---|---|---|---|
| Control | 8 | 0 | 0 | − | − | − | − |
| Juvenile infection | |||||||
| 1 | 4 | 0 | 0 | 0 | 0.73 | 0 | 0.86 |
| 2 | 4 | 0 | 0 | 4.1 | 0.67 | 5.9 | 0.38 |
| 3 | 4 | 0 | 0 | 0 | 0.87 | 0 | 0.44 |
| Adult infection | |||||||
| 1 | 4 | 0 | 0 | 42.5 | 0.27 | 47.1 | 0.23 |
| 2 | 4 | 0 | 0 | 37.0 | 0.35 | 41.2 | 0.35 |
| 3 | 4 | 0 | 0 | 15.1 | 0.86 | 17.7 | 0.73 |
TWR, total worm burden reduction; FWBR, female worm burden reduction.
In vitro and in vivo activity of modified lead dioxolanes against S. mansoni
Given the low in vivo activity of the three test drugs, we were motivated to investigate four additional chemically related alkoxydioxolanes in vitro against NTS and adult schistosomes, followed by in vivo studies on a patent S. mansoni infection. All four compounds (15–18) showed very high activities against NTS, represented by low IC50 values (2.7–4.8 μM) (summarized in Table 1). However, the four drugs revealed only low activities against adult schistosomes in vitro, with IC50s between 49.4 and 109.3 μM. Moderate, but non-significant, in vivo WBRs of 32.0% and 21.3% were achieved with compounds 15 and 17, respectively. Low activities with WBRs of 12.0% and 16.4% were observed with compounds 16 and 18, respectively (summarized in Table 3).
Table 3.
In vivo activity following a single oral dose of 400 mg/kg of selected 3-alkoxy-1,2-dioxolanes in mice harbouring adult S. mansoni
| Group | No. of mice investigated | No. of mice that died | No. of mice cured | TWR (%) | P value | FWBR (%) | P value |
|---|---|---|---|---|---|---|---|
| Controla | 7 | 0 | 0 | − | − | − | − |
| Controlb | 9 | 0 | 0 | − | − | − | − |
| 15a | 4 | 1 | 0 | 32.0 | 0.1 | 37.8 | 0.1 |
| 16a | 4 | 1 | 0 | 12.0 | 0.7 | 5.4 | 0.7 |
| 17a | 4 | 1 | 0 | 21.3 | 0.4 | 35.1 | 0.3 |
| 18b | 4 | 0 | 0 | 16.4 | 0.9 | 11.9 | 0.7 |
TWR, total worm burden reduction; FWBR, female worm burden reduction.
aVersus control.
bVersus control.
In vitro and in vivo effect against E. caproni
Lead structures 1–3 as well as the four related structures (15–18) were tested in vitro on freshly harvested E. caproni. Data are summarized in Table 4. Six of seven compounds (1, 3, 15–18) showed 100% worm mortality 24 h post-drug exposure at a concentration of 50 mg/L. Two of the compounds (16 and 17) killed all worms 24 h post-incubation at a 5-fold lower concentration of 10 mg/L. Incubation of adult E. caproni with three spirocyclohexyl compounds (3, 16 and 17) at 5 mg/L for 72 h resulted in death of all worms. Compounds 15–17 were followed up in vivo. Treatment of E. caproni-infected mice with a single oral dose of 400 mg/kg of compound 15 resulted in a low WBR of 17.4%. Compounds 16 and 17 lacked in vivo activity (Table 5).
Table 4.
In vitro mortality of E. caproni worms at timepoints 24, 48 and 72 h post-drug treatment with selected compounds
| Drug | Drug concentration (mg/L) | No. of worms observed | Percentage of worms that died after indicated time |
|||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | |||
| Control | − | 20 | 0 | 0 | 0 | 0 |
| 1 | 100 | 8 | 0 | 100 | ||
| 50 | 8 | 0 | 100 | |||
| 10 | 7 | 0 | 43 | 100 | ||
| 5 | 8 | 0 | 25 | 75 | 88 | |
| 2 | 100 | 6 | 0 | 100 | ||
| 50 | 6 | 0 | 0 | 100 | ||
| 10 | 6 | 0 | 17 | 67 | 100 | |
| 5 | 7 | 0 | 0 | 29 | 57 | |
| 3 | 100 | 6 | 0 | 100 | ||
| 50 | 7 | 0 | 100 | |||
| 10 | 7 | 0 | 57 | 100 | ||
| 5 | 9 | 0 | 22 | 100 | ||
| 15 | 100 | 6 | 0 | 100 | ||
| 50 | 6 | 0 | 100 | |||
| 10 | 7 | 0 | 86 | 100 | ||
| 5 | 8 | 0 | 0 | 38 | 75 | |
| 16 | 100 | 6 | 0 | 100 | ||
| 50 | 6 | 0 | 100 | |||
| 10 | 7 | 0 | 100 | |||
| 5 | 6 | 0 | 50 | 67 | 100 | |
| 17 | 100 | 6 | 0 | 100 | ||
| 50 | 7 | 0 | 100 | |||
| 10 | 6 | 0 | 100 | |||
| 5 | 7 | 0 | 0 | 100 | ||
| 18 | 100 | 6 | 0 | 100 | ||
| 50 | 7 | 0 | 100 | |||
| 10 | 6 | 0 | 67 | 83 | 100 | |
| 5 | 6 | 0 | 0 | 17 | 33 | |
Table 5.
In vivo activity following a single oral dose of 400 mg/kg of selected 3-alkoxy-1,2-dioxolanes against E. caproni in mice
| Group | No. of mice investigated | No. of mice cured | Worms | TWR (%) | P value |
|---|---|---|---|---|---|
| Control | 4 | 0 | 23.0 (0) | − | − |
| 15 | 4 | 0 | 19.0 (7.2) | 17.4 | 0.2 |
| 16 | 4 | 0 | 27.8 (4.5) | 0 | 0.2 |
| 17 | 4 | 0 | 23.0 (7.8) | 0 | 0.5 |
TWR, total worm burden reduction.
In vitro activity of non-peroxidic analogues
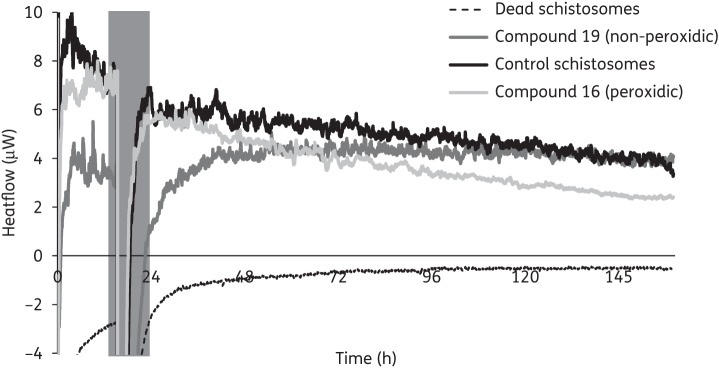
To elucidate the role and necessity of the peroxide core of the alkoxydioxolanes for trematocidal activity we tested the in vitro activity of two alkoxy-substituted tetrahydrofurans (19, 20) prepared as non-peroxidic analogues of the alkoxydioxolanes. Both derivatives lacked activity at concentrations of 30 and 90 mg/L against S. mansoni. Also, E. caproni was not affected at 50 and 100 mg/L. No effect against adult S. mansoni could be observed by microscopic examination 72 h post-treatment as well as by IMC 6 days after treatment. According to IMC, no loss of motility was detected for either non-peroxidic compound over an incubation period of 6 days (concentration 30 mg/L). For comparison, treatment of adult S. mansoni with 30 mg/L of the peroxidic analogue of compound 19 (compound 16) revealed a reduction in heat flow and complete loss of motility was detected 90 h post-exposure (Figure 2).
Figure 2.
Course of heat flow (μW) over time (h) after treatment of schistosomes with compound 19 (non-peroxidic) or compound 16 (peroxidic). Control schistosomes correspond to schistosomes treated with DMSO at the same concentration as used for drugs (0.3%). Amplitudes of curves represent the motility of schistosomes.
Discussion
The antischistosomal activity of semisynthetic artemisinins, frequently used in malaria treatment, synthetic trioxolanes and hybrid molecules of quinines and trioxanes has been well described.7,8 Compounds characterized by a peroxidic skeleton are therefore an interesting starting point for antischistosomal drug discovery. Hence, we were interested in elucidating the antischistosomal potential of recently introduced alkoxydioxolanes as well as their structural needs for activity against schistosomes.10
Three of 14 compounds tested (compounds 1–3) showed promising in vitro activity against adult S. mansoni flukes. Two of these compounds (2 and 3) also revealed very good efficacy against the juvenile stage (NTS) in vitro. The antischistosomal in vitro activity seems to be enhanced by the presence of a bulky substituent at C3, since the three lead compounds all feature a large alkoxide side chain at C3. The remaining non-active 11 compounds do not display this feature. This finding is in accordance with observations reported by Schiaffo et al.10 on the antimalarial structure–activity relationship of similar compounds. These studies revealed that antimalarial activity is enhanced by a spirocyclohexyl group at C5/C5′ and by the presence of a steric bulk at C3. However, for antischistosomal activity it does not seem to matter whether substitution at C5/C5′ is a dimethyl (compound 2) or spirocyclohexyl (compound 3) group. On the other hand, it is interesting to note that, with regard to E. caproni, the three most active compounds (3, 16 and 17) all display spirocyclohexyl units.
In our in vivo studies we observed only moderate, non-significant activities of alkoxydioxolanes in S. mansoni-infected NMRI mice. Compound 1 showed the highest in vivo activity against adult S. mansoni, achieving a total WBR of 42.5% and a female WBR of 47.1%. In contrast to results reported for artemisinins, surprisingly low WBRs were observed against juvenile S. mansoni harboured in mice.18 A recent study conducted with a library of dioxolanes revealed that the majority of the compounds tested against rat and human microsomes were metabolized rapidly. It was concluded that significant optimization of the groups attached to the dioxolane core is needed before a viable candidate for drug development could be identified.19 Hence, it is likely that the alkoxydioxolanes tested in this study also exhibit bioavailability problems which can likely play a role in limiting in vivo activities.
In the present work we also studied the activity of alkoxydioxolanes in the presence of various additional iron sources, since haemoglobin metabolism, the potential target of these drugs, is a common feature of both Schistosoma and Plasmodium spp.20 Earlier studies investigated the relationship between Fe(II) reactivity, the efficiency of haem alkylation and antimalarial activity for various peroxides in vitro. The authors stated that Fe(II) reactivity for the tested peroxide heterocycles is a necessary, but insufficient, property of antimalarial peroxides,21 and the alkoxydioxolanes have been shown to undergo cleavage to alkoxy radicals in the presence of iron(II).10 Interestingly, we did not observe significant differences in susceptibility of the tested agents in the different iron-source-containing media. This finding is in contrast to the haemin-dependent antischistosomal in vitro effect of the trioxolane OZ78 on S. mansoni and S. japonicum, whereas similar haemin-independent activity was described for OZ209.6,22 Similarly, it was recently shown that the antimalarial arylmethanol mefloquine, a drug class also described to interfere with haemoglobin degradation in Plasmodium, revealed a 57-fold lower IC50 in the presence of haemoglobin against adult S. mansoni in vitro.23 Our results suggest that the alkoxydioxolanes possess an iron-independent mechanism of action on schistosomes in vitro. This discovery is supported by the observed high in vitro activities of three compounds (3, 16 and 17) at concentrations as low as 5 mg/L against the non-haematophagous intestinal fluke E. caproni.
Nonetheless, the results with the isosteric compounds that lack a peroxide functional group underline the necessity of the peroxidic core for trematocidal activity. Both compounds lacked in vitro activity against adult S. mansoni and E. caproni. Similar results were recently demonstrated in studies with the liver fluke Fasciola hepatica. While OZ78 has excellent in vitro and in vivo activity against F. hepatica, its non-peroxidic analogue failed to show an effect against the fluke.24 The peroxidic feature seems therefore to play a role in the iron-independent mode of action. The basis for the iron-independent activity of the alkoxydioxolanes is unclear. These molecules, like all peroxides, are oxidants, and in principle capable of reaction with strongly nucleophilic or reducing agents. Alternatively, it is possible that the alkoxydioxolanes undergo activation via acid-catalysed ring opening of the peroxyacetal core to generate a more reactive 3-hydroperoxyketone; a similar model has been proposed to account for the antimalarial activity of artemisinin.25
In conclusion, we have demonstrated that a number of alkoxydioxolanes are characterized by good in vitro antischistosomal activity and non-significant in vivo effects on S. mansoni, with compound 1 being the most promising candidate. Similarities, but also differences, exist between antimalarial and antischistosomal activity of alkoxydioxolanes. The peroxidic bond is essential to antischistosomal activity, but activation of the molecules seems to be independent of iron. The low in vivo activity of this drug class, which may result from limited bioavailability, represents a challenge that would need to be overcome in order to identify an antischistosomal lead candidate.
Funding
This work was supported by the Swiss National Science Foundation (project no. PPOOA3_114941 and PPOOP3_135170 to J. K.), the Scientific & Technological Cooperation Programme Switzerland-Russia, the Medicines for Malaria Venture (P. H. D. and C. E. S.), the Nebraska Research Initiative (P. H. D.) and the University of Nebraska-Lincoln (W. S. and E. B.). Parts of this research were conducted in facilities remodelled with National Institutes of Health support (RR016544).
Transparency declarations
None to declare.
Supplementary data
Figure S1 is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
References
- 1.Gryseels B, Polman K, Clerinx J, et al. Human schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. doi:10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Steinmann P, Keiser J, Bos R, et al. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25. doi: 10.1016/S1473-3099(06)70521-7. doi:10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 3.Li HJ, Wang W, Li YZ, et al. Effects of artemether, artesunate and dihydroartemisinin administered orally at multiple doses or combination in treatment of mice infected with Schistosoma japonicum. Parasitol Res. 2011;109:515–9. doi: 10.1007/s00436-011-2474-5. doi:10.1007/s00436-011-2474-5. [DOI] [PubMed] [Google Scholar]
- 4.Keiser J, Utzinger J. Artemisinins and synthetic trioxolanes in the treatment of helminth infections. Curr Opin Infect Dis. 2007;20:605–12. doi: 10.1097/QCO.0b013e3282f19ec4. doi:10.1097/QCO.0b013e3282f19ec4. [DOI] [PubMed] [Google Scholar]
- 5.Keiser J, N'Guessan NA, Adoubryn KD, et al. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis. 2011;50:1205–13. doi: 10.1086/651682. doi:10.1086/651682. [DOI] [PubMed] [Google Scholar]
- 6.Xiao SH, Keiser J, Chollet J, et al. In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob Agents Chemother. 2007;51:1440–5. doi: 10.1128/AAC.01537-06. doi:10.1128/AAC.01537-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boissier J, Cosledan F, Robert A, et al. In vitro activities of trioxaquines against Schistosoma mansoni. Antimicrob Agents Chemother. 2009;53:4903–6. doi: 10.1128/AAC.00640-09. doi:10.1128/AAC.00640-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keiser J, Ingram K, Vargas M, et al. In vivo activity of next generation synthetic ozonides against Schistosoma species. Antimicrob Agents Chemother. 2012;56:1090–2. doi: 10.1128/AAC.05371-11. doi:10.1128/AAC.05371-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Dong Y, Wittlin S, et al. Spiro- and dispiro-1,2-dioxolanes: contribution of iron(II)-mediated one-electron vs two-electron reduction to the activity of antimalarial peroxides. J Med Chem. 2007;50:5840–7. doi: 10.1021/jm0707673. doi:10.1021/jm0707673. [DOI] [PubMed] [Google Scholar]
- 10.Schiaffo CE, Rottman M, Wittlin S, et al. 3-Alkoxy-1,2-dioxolanes: synthesis and evaluation as potential antimalarial agents. ACS Med Chem Lett. 2011;2:316–9. doi: 10.1021/ml100308d. doi:10.1021/ml100308d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin-the debate continues. Molecules. 2010;15:1705–21. doi: 10.3390/molecules15031705. doi:10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keiser J. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology. 2009;137:589–603. doi: 10.1017/S0031182009991739. doi:10.1017/S0031182009991739. [DOI] [PubMed] [Google Scholar]
- 13.Manneck T, Haggenmuller Y, Keiser J. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology. 2009;137:85–98. doi: 10.1017/S0031182009990965. doi:10.1017/S0031182009990965. [DOI] [PubMed] [Google Scholar]
- 14.Cousin CE, Stirewalt MA, Dorsey CH, et al. Schistosoma mansoni: comparative development of schistosomules produced by artificial techniques. J Parasitol. 1986;72:606–9. doi:10.2307/3281520. [PubMed] [Google Scholar]
- 15.Ramirez B, Bickle Q, Yousif F, et al. Schistosomes: challenges in compound screening. Exp Opin Drug Discov. 2007;2(Suppl 1):S53–61. doi: 10.1517/17460441.2.S1.S53. doi:10.1517/17460441.2.S1.S53. [DOI] [PubMed] [Google Scholar]
- 16.Manneck T, Braissant O, Haggenmuller Y, et al. Isothermal microcalorimetry to study drugs against Schistosoma mansoni. J Clin Microbiol. 2011;49:1217–25. doi: 10.1128/JCM.02382-10. doi:10.1128/JCM.02382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manneck T, Braissant O, Ellis W, et al. Schistosoma mansoni: antischistosomal activity of the four optical isomers and the two racemates of mefloquine on schistosomula and adult worms in vitro and in vivo. Exp Parasitol. 2011;127:260–9. doi: 10.1016/j.exppara.2010.08.011. doi:10.1016/j.exppara.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Utzinger J, Chollet J, Tu Z, et al. Comparative study of the effects of artemether and artesunate on juvenile and adult Schistosoma mansoni in experimentally infected mice. Trans R Soc Trop Med Hyg. 2002;96:318–23. doi: 10.1016/s0035-9203(02)90110-0. doi:10.1016/S0035-9203(02)90110-0. [DOI] [PubMed] [Google Scholar]
- 19.Martyn DC, Beletsky G, Cortese JF, et al. Synthesis and in vitro DMPK profiling of a 1,2-dioxolane-based library with activity against Plasmodium falciparum. Bioorg Med Chem Lett. 2009;19:5657–60. doi: 10.1016/j.bmcl.2009.08.024. doi:10.1016/j.bmcl.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 20.de Villiers KA, Egan TJ. Recent advances in the discovery of haem-targeting drugs for malaria and schistosomiasis. Molecules. 2009;14:2868–87. doi: 10.3390/molecules14082868. doi:10.3390/molecules14082868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Creek DJ, Schiaffo CE, et al. Spiroadamantyl 1,2,4-trioxolane, 1,2,4-trioxane, and 1,2,4-trioxepane pairs: relationship between peroxide bond iron(II) reactivity, heme alkylation efficiency, and antimalarial activity. Bioorg Med Chem Lett. 2009;19:4542–5. doi: 10.1016/j.bmcl.2009.07.013. doi:10.1016/j.bmcl.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Xiao SH, Xue J, Mei JY, et al. Effectiveness of synthetic trioxolane OZ78 against Schistosoma japonicum in mice and rabbits. Parasitol Res. 2011 doi: 10.1007/s00436-011-2765-x. doi:10.1007/s00436-011-2765-x. [DOI] [PubMed] [Google Scholar]
- 23.Ingram K, Ellis W, Keiser J. Antischistosomal activities of melfoquine related arylmethanols. Antimicrob Agents Chemother. 2012 doi: 10.1128/AAC.06177-11. doi:10.1128/AAC.06177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q, Vargas M, Dong Y, et al. Structure-activity relationship of an ozonide carboxylic acid (OZ78) against Fasciola hepatica. J Med Chem. 2010;53:4223–33. doi: 10.1021/jm100226t. doi:10.1021/jm100226t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes RK, Pai HH-O, Voerste A. Ring opening of artemisinin (qinghaosu) and dihydroartemisinin and interception of the open hydroperoxides with formation of N-oxides: a chemical model for antimalarial mode of action. Tetrahedron Lett. 1999;40:4715–8. doi:10.1016/S0040-4039(99)00830-8. [Google Scholar]
- 26.Keiser J, Manneck T, Vargas M. Interactions of mefloquine with praziquantel in the Schistosoma mansoni mouse model and in vitro. J Antimicrob Chemother. 2011;66:1791–7. doi: 10.1093/jac/dkr178. doi:10.1093/jac/dkr178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.