Abstract
Resveratrol (3, 5, 4′-trihydroxy- trans- stilbene), a polyphenol compound, is derived from natural products such as the skin of red grapes, blueberries and cranberries. Resveratrol not only exhibits antioxidant, cardioprotection, and anti-aging properties, but can also inhibit cancer cell growth and induce apoptosis. It has been shown that resveratrol inhibits the activation of Nf-kB and subsequently down regulates the expression of Nf-kB regulated genes such as interleukin-2 and Bcl-2, leading to cell cycle arrest and increased apoptosis in multiple myeloma cells. In the skin, resveratrol has been reported to sensitize keratinocytes to UVA induced apoptosis. However, the effect of resveratrol on opening of the mitochondrial permeability transition pore has not been previously examined. Our data show that UVA (14J/cm2) along with resveratrol causes massive oxidative stress in mitochondria. As a consequence of oxidative stress, the mitochondrial membrane potential decreases which results in opening of the mitochondrial pores ultimately leading to apoptosis in human keratinocytes. These results may have clinical implications for development of future chemotherapeutic treatment for tumors of the skin.
Keywords: resveratrol; mitochondria; keratinocytes; UVA induced apoptosis; mitochondrial membrane potential; mitochondrial permeability transition pore, reactive oxygen species
1. Introduction
The major cause of skin carcinogenesis is ultraviolet (UV) light from the sun. Most studies of the three stages of skin tumor carcinogenesis (initiation, promotion and progression) have been done with UVB (280-320 nm) because it acts mainly on the epidermis, directly causing DNA damage in skin cells [1] including keratinocytes [2]. In addition to DNA damage, UVB induces other cellular responses. For example, PTEN (phosphatase and tensin homolog deleted on choromosome 10) is a tumor suppressor. Increasing PTEN expression enhances apoptosis of keratinocytes leading to tumor suppression. However, UVB-induced ERK/AKT-dependent PTEN suppression promotes survival of epidermal keratinocytes, resulting in skin carcinogenesis [3].
However, UVA (320-400 nm) is the predominant UV band that reaches the earth’s surface. In addition, UVA penetrates the skin more deeply than UVB, having an effect on both the epidermal and the dermal compartments. Studies have shown that UVA causes oxidative damage to the skin through lipid peroxidation, protein peroxidation and, most importantly, oxidative DNA damage, resulting in formation of 8-oxoguanine [4]. UVA causes Nf-kB activation at doses insufficient to cause DNA damage to skin fibroblasts [5]. Nf-kB, a nuclear transcriptional factor for regulating expression of many genes, has anti-apoptotic activities for regulating cell survival [6]. In addition, physiological doses of UVA were shown to induce p53 mutations in engineered human skin, demonstrating that UVA as well as UVB is mutagenic to keratinocytes [7]. Therefore, chronic exposure to UVA irradiation of keratinocytes could induce acquired apoptosis resistance resulting in malignant transformations [8].
Some studies have revealed roles for mitochondria in cells irradiated with UVA. One study used flow cytometry to show that a time-dependent decrease in the mitochondrial membrane potential (MMP) of cells exposed to UVA causes subsequent activation of caspase-3, resulting in apoptosis [9]. One hour after UVA irradiation of NCTC 2544 keratinocytes, a dose dependent decrease in cellular oxygen consumption and ATP content was observed when succinate or malate/glutamate were used as substrates for mitochondria electron transport [10]. These results strongly suggest that UVA irradiation decreases the activity of the mitochondrial respiratory chain. However, there are no studies on the changes in mitochondria due to exposure to UVA in the presence of resveratrol.
Resveratrol, a naturally occurring polyphenol compound, is present in the skin of red grapes, blueberries, and cranberries. Resveratrol’s anti-cancer properties have been proposed to be attributable to its activities on cell cycle control and on apoptosis induction. Resveratrol acts on the three stages of tumor carcinogenesis (initiation, promotion and progression), leading to suppression of tumor carcinogenesis [11]. For example, resveratrol inactivates Nf-kB and inhibits expression of COX-2 (Cyclooxygenase 2) induced by the tumor promoter agent: 12-O-tetradecanoylphorbol-13-acetate (TPA) in mouse skin [12]. COX-2 is an oncoprotein which is over expressed in many cancer cells. A study of Zykova et al. found that resveratrol directly binds to COX-2 and suppresses anchorage independent growth and colony formation in soft agar in colon HT-29 cells [13]. Resveratrol also inhibits UVB-mediated activation of Nf-kB in a dose-dependent manner in normal human keratinocytes [14]. In addition, resveratrol induces p53 expression by increasing cellular p53 content, serine-15 phosphorylation of p53, and p53 binding to DNA, leading to apoptosis in many types of tumor cells such as cancers of the prostate, stomach, colon, pancreas, thyroid, melanocytes, ovary carcinoma, and cervix [15]. The expression of Bcl2 was gradually decreased in cultured human promyelocytic leukemia (HL-60) cells treated with resveratrol [16], leading to increased apoptosis. In addition to these mechanisms, resveratrol has been shown to sensitize HaCaT cells to UVA induced apoptosis by increasing oxidative DNA damage [17].
The goal of our investigation was to explore the effects of resveratrol in combination with UVA exposure on mitochondria in human keratinocytes as a potential therapeutic tool to treat skin malignancy.
2. Material and methods
2.1. Materials and Cell Culture
The HaCaT cells, the immortalized keratinocytes [18] were cultured in DMEM medium (Invitrogen, San Diego, CA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and with penicillin and streptomycin (Invitrogen, San Diego, CA). The HEKa cells, adult human epidermal keratinocytes were obtained from Invitrogen (San Diego, CA) and cultured in EpiLife medium supplemented with calcium and human keratinocyte growth supplement (Invitrogen, San Diego, CA). Both keratinocyte cell lines were maintained in a humidified incubator with 5% CO2 at 37° C. For all assays, different dishes containing seeded cells were initially incubated either with resveratrol (dissolved in 95% ethanol) or vehicle control (95% ethanol) only or in specific combinations with CsA (dissolved in 95% ethanol). The CsA, resveratrol and ethanol were purchased from Sigma-Aldrich, St. Louis, MO. The final concentration of resveratrol was 5uM for HaCaT cells and 50uM for HEKa cells. In experiments where CsA was tested, the final concentration of CsA was 0.2uM.
For the Annexin V-FITC apoptosis detection assay, we used a kit purchased from Biovision, Mountain View, CA. For the mitochondrial membrane potential, MitoSOX™ and MPTP opening assays we used kits purchased from Invitrogen, San Diego, CA. Mito Tracker Green and Hoechst 33342, used for live cell imaging, were also purchased from Invitrogen, San Diego, CA.
2.2. Irradiation of keratinocytes
The cells were initially exposed to different doses of UVA ranging from 1 to 20 J/cm2. The response to the various UVA doses was typically either no cell death or massive cell killing. One of our trial UVA irradiation doses, 14J/cm2, resulted in a relatively modest cell killing (80% cell survival) and was therefore used for our analysis. Twenty four hours after initial incubation, dishes designated to receive UVA exposure were irradiated with UVA at 14J/cm2. The UVA panel (Ultra-lite, Inc.) we used is fitted with lamps (six F72T12-BL-HO UVA) that delivered broad band UVA from 320-400 nm. The UVA dose was monitored with a UVA meter (National Biological Corporation, Beachwood, OH). Control cells (non-irradiated cells) were incubated inside the cell culture hood at room temperature at the same time the irradiated cells were being exposed to UVA.
2.3. Annexin V-FITC Apoptosis Detection Assay
HaCaT and HEKa cells were seeded at the concentration of 5 × 105 cells in 60mm dishes and received either a single or a combination treatment, as previously described, for the initial incubation period of 24 hours. Next, cells were treated with UVA exposure three-times in 12 hours intervals, then incubated for an additional 12 hours, trypsinized, collected, placed into conical tubes, spun down and washed with PBS, and then binding buffer was added to each tube. Fluorescence conjugate Annexin V and Propidium Iodide (PI) from the Biovision (Mountain View, CA) kit were next added to each tube and all tubes were then incubated in the dark for 5 minutes. The conjugate of Annexin V, a protein with a high affinity for phosphatidylserine (PS), allows detection of cells undergoing early to late stages of apoptosis and analysis by flow cytometry. PI was used to stain cells with altered membrane integrity which occurs in the later stages of apoptosis or necrosis. Finally, the apoptosis rate was detected by flow cytometry using a BD Fascan II at the excitation wavelength of 488 nm with different detectors in accordance with the manufacturer’s (Biovision, Mountain View, CA) protocol.
2.4. Mitochondrial Membrane Potential Assay (JC-1 Assay)
HaCaT and HEKa were seeded at the concentration of 5 × 105 cells in 60mm dishes and received a single dose of UVA irradiation after the initial incubation period of 24 hours. Next, cells were collected immediately afterward and placed into the tubes. For each tube, JC-1 (2μM) was added and then cells were incubated at 37°C for 30 minutes. Cells were then washed once by adding 2mL of warm phosphate-buffered saline (PBS) to each tube and re-suspended in a final volume of 500μl PBS for flow cytometry according to manufacturer’s protocol (Invitrogen, San Diego, CA). Flow cytometry was performed using 488nm excitation, green and red filters for detection in accordance with the manufacturer’s protocol (Invitrogen, San Diego, CA).
2.5. MitoSox Assay
HaCaT cells were seeded at the concentration of 5 × 105 cells in 60mm dishes and received either a single or a combination treatment, as previously described, for the initial incubation period of 24 hours. Cells were treated with a single dose of UVA exposure, collected immediately afterward and placed into the tubes. All cells, including controls, were stained with MitoSOX™ Red, incubated at 37°C in the dark for 15 minutes, then washed with PBS, trypsinized and resuspended. MitoSOX™ Red mitochondrial superoxide indicator selectively detects superoxide in mitochondria. MitoSOX™ Red fluorescence was analyzed by flow cytometry following the methods published previously by Norman et al. [19].
2.6. Live cell imaging by deconvolution microscopy
Approximately 1×105 HaCaT cells were seeded into Delta T dishes (Thermo Fisher Scientific, Waltham, MA) and received either a single or a combination treatment, as previously described, for the initial incubation period of 24 hours. After a single irradiation with UVA, cells were immediately stained with Mito Tracker Green and Hoechst 33342 following the manufacturer’s protocol. Deconvolution microscopy was then used to image the structure of the mitochondria in the live HaCaT cells. Deconvolution microscopic analysis was carried out using Olympus IX71 (Olympus America Inc, Center Valley, PA) with objective 60x W/1.4NA.
2.7. Mitochondrial Permeability Transition Pore (MPTP) Opening Assay
HaCaT cells were seeded at the concentration of 1.6 × 106 cells in 100mm dishes and received either a single or a combination treatment, as previously described, for the initial incubation period of 24 hours. Cells were treated with a single dose of UVA exposure and then two hours later trypsinized, collected and washed once with Hanks’ Balanced Salt Solution (HBSS, Fisher Scientific, Waltham, MA) with calcium prior to performing the MPTP assay. Cells from each of the 100mm dishes were divided into three populations and placed into the tubes. In the first population, cells were stained with calcein AM only. Cells in this first population will show calcein fluorescence in the cytosol as well as mitochondria. Cells in this first population treated with ethanol and exposed to UVA were used as a vehicle control and for normalization purposes. The second population of cells was stained with calcein AM followed by addition of CoCl2 which quenches the calcein fluorescence in the cytosol. This second population of cells was therefore used to measure the activation of the MPTP and subsequent pore opening. In the third population, ionomycin was added in addition to calcein AM and CoCl2. This third population of cells represents the residual calcein fluorescence in the mitochondria after ionomycin allowed entry of excess Ca2+ into the mitochondria, triggering MPTP activation and subsequent loss of mitochondrial calcein fluorescence. This third population of cells serves to determine the background fluorescence left in the mitochondria. Following the manufacturer’s protocol, calcein AM fluorescent dye was added to all of the tubes and then CoCl2 was additionally added to the tubes of second and third population. Then Ionomycin was added as a control to the third population of tubes already treated with calcein AM and CoCl2. All of the tubes were then incubated in the dark at 37°C for 15 minutes. Cells were then washed and again re-suspended in 500μl of HBSS buffer supplemented with calcium. Flow cytometry analysis was performed at a 488nm excitation wavelength for detection of the calcein signal in accordance with the manufacturer’s protocol (Invitrogen, San Diego, CA).
2.8. Statistical Analysis
Data were collected and analyzed to obtain the mean and standard deviation for three independent experiments. A two-tailed student T-test was used to analyze the statistical significance in comparison to the vehicle treatment.
3. Results
3.1. Treatment combination
Our results primarily explore combination treatments of UVA and resveratrol for HEKa cells and UVA, resveratrol and CsA for HaCaT cells. CsA, an immunosuppressor drug used in organ transplant patients, modulates calcineurin signaling as well as the MPTP opening resulting in decreased apoptosis [19]. We used CsA to determine if the pore opening by resveratrol and UVA could be blocked by the action of CsA through binding to cyclophilin D. Ethanol, a solvent for CsA and resveratrol, was used as a vehicle control.
3.2. Resveratrol sensitized UVA induced apoptosis
As shown in Figure 1A, there was no statistical difference in viability between the control and cells treated with only CsA, resveratrol, or the combination of CsA and resveratrol (i.e. no UVA). Therefore, there was no significant cytotoxicity effect on the HaCaT and HEKa cells induced by resveratrol, CsA, or their combination in cells not exposed to UVA. UVA dose of 14J/cm2 was chosen since this dose of irradiation causes a modest decrease in cell viability to 80% (Figure 1A). Cells receiving UVA exposure only (no ethanol), cells receiving UVA and incubated with ethanol, or cells receiving UVA and CsA, had a significant, but small increase in percentage of cells in all stages of apoptosis as observed in positive staining of Annexin V only as well as positive staining of Annexin V and PI (p<0.05) in comparison to the control cells (Figure 1B).
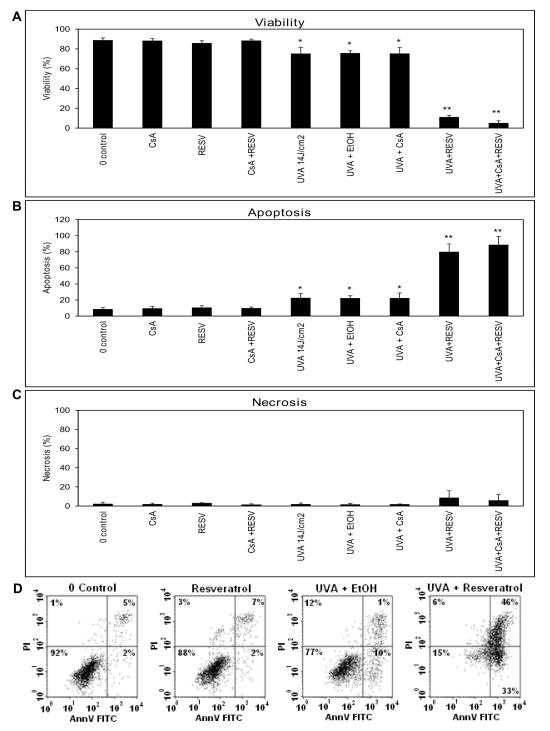
Figure 1. Statistically significant apoptosis induced by treatment with the combination of UVA exposure and resveratrol in HaCaT cells.
Results of the Annexin V and PI assay as the means of three independent experiments. Percentage of A) cell viability B) apoptosis and C) necrosis are shown. Asterisks (**p<0.01; *p<0.05) indicate that HaCaT cells incubated with resveratrol and then exposed to UVA have shown a significant decrease in cellular viability and an increase in overall apoptosis compared to their controls (0 Control and UVA+EtOH). D) Flow cytometry data showing examples of one of the three apoptosis experiments where the lower left quadrant always shows viable cells, the lower right and upper right quadrants shows cells undergoing apoptosis, the upper left quadrant shows necrotic cells.
However, cells that were incubated with resveratrol, then irradiated with UVA showed a substantial decrease in cell viability (Figure 1A, E) as well as a significant increase in percentage of overall apoptosis (either cells positive stained with Annexin V only or both Annexin V and PI positive stained cells) (Figure 1B, E) in comparison to the controls (0 Control and UVA plus ethanol). There was no significant difference in necrosis (positive staining of PI only) observed among the different groups (Figure 1C, E). To determine whether CsA would influence the resveratrol-sensitized UVA-induced apoptosis, we treated cells with a combination of CsA, resveratrol and UVA. Our data showed that under these conditions, CsA failed to block the massive increase in percentage of cells in all stages of apoptosis (Figures 1B).
The profound effect of resveratrol in sensitizing the HaCaT cells to UVA was found to occur in the finite time period during the actual irradiation treatment as shown in Figure 2. Figure 2C shows flow cytometry results for HaCaT cells treated with resveratrol exclusively before and after UVA exposure that did not result in increased apoptosis compared to UVA control as shown in Figure 2B (Figure 2A is a non UVA control). However, we observed that when resveratrol is present during the critical UVA irradiation period, then the HaCaT cells are sensitized to apoptosis resulting in a significant amount of cell death (Figure 2D).
Figure 2. UVA sensitization by resveratrol requires compound to be specifically present during the irradiation period.
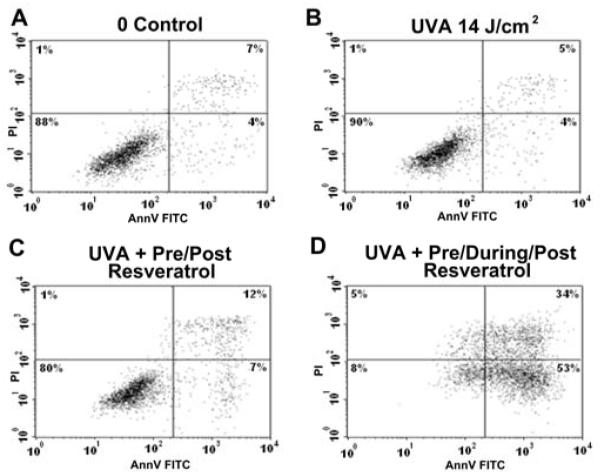
Flow cytometry data showing the sensitivity of HaCaT cells to resveratrol that is applied before, during and after the UVA irradiation. Panels show: A) the control; B) cells treated with UVA only; C) cells treated with resveratrol before and after but not during UVA exposure; and D) cells treated with resveratrol before, during and after UVA exposure.
In conclusion, CsA was found to have no protective effect in combination with resveratrol but resveratrol itself had a robust effect on enhanced apoptosis after UVA exposure.
As a next step, we tested HEKa cells treated with resveratrol only in order to confirm whether another keratinocyte cell line has the same response to resveratrol treatment in combination with UVA irradiation. We have observed that 5uM resveratrol did not affect apoptosis of HEKa cells, the effect seen in HaCaT cells. However, treatment of HEKa cells with 50uM resveratrol led to enhanced apoptosis after UVA exposure while having no toxic effect on these cells. As shown in Supplementary Figure 1, HEKa cells treated with 50uM resveratrol showed significantly enhanced apoptosis compared to UVA treated control – the effect similar to HaCaT cells as shown in Figure 1A.
3.3. Resveratrol and UVA induce an immediate MMP change
In order to find out the effect of resveratrol and UVA on mitochondria, we performed an assay to determine the MMP change using the lipophilic fluorochrome JC-1. Uptake of JC-1 into mitochondria is driven by the MMP. The accumulation of JC-1 in mitochondria leads to the formation of JC-1 aggregates detected in the red channel by flow cytometry. JC-1 does not accumulate in mitochondria with depolarized MMP, and remains in the cytoplasm as monomers which are detectable in the green channel by flow cytometry.
As shown in Figure 3, the combination of UVA and 5uM resveratrol induced a significant MMP change in HaCaT cells (p<0.01) compared to control treated with UVA and ethanol only. The combination of CsA with resveratrol and UVA also induced a significant MMP change (p<0.01) in comparison to the control (UVA and ethanol). However, cells treated without the combination of resveratrol and UVA did not show any significant MMP change. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP), a mitochondrial depolarizing agent, was used as a positive control for MMP reduction. CsA was investigated for its ability to modulate these MMP changes induced by UVA and resveratrol and was again found to have no impact on the alteration of MMP. In advance, we tested the effect of 50uM resveratrol in combination with UVA exposure on HEKa cells in order to confirm the combined effect of UVA and resveratrol on keratinocytes and we obtained the same results as we did using HaCaT cells treated with 5uM resveratrol. As shown in Supplementary Figure 2, MMP dropped significantly in HEKa cells treated with the combination of 50uM resveratrol and UVA irradiation compared to UVA treated control.
Figure 3. Combination treatment of HaCaT cells with UVA and resveratrol induced an immediate MMP change.
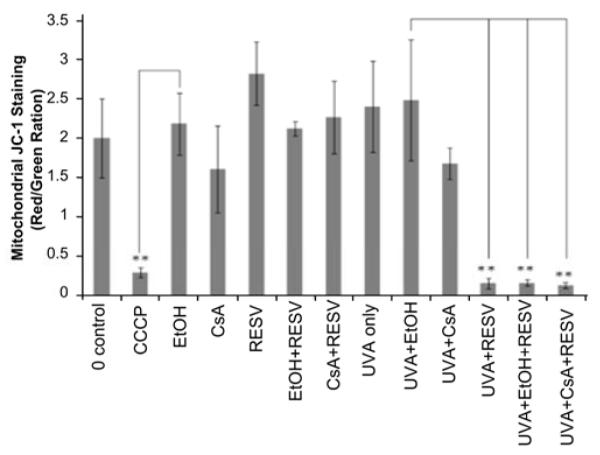
Flow cytometry analysis with JC-1 staining in HaCaT cells exposed to various chemicals or control before or after UVA (14J/cm2 and cells collected immediately after UVA treatment). CCCP, a disruptor of mitochondrial membrane potential, is used as a positive control. Data are represented as a ratio of red to green fluorescence, and represent the mean of three independent experiments (**p<0.01).
3.4. Resveratrol enhances oxidative stress in mitochondria after UVA treatment
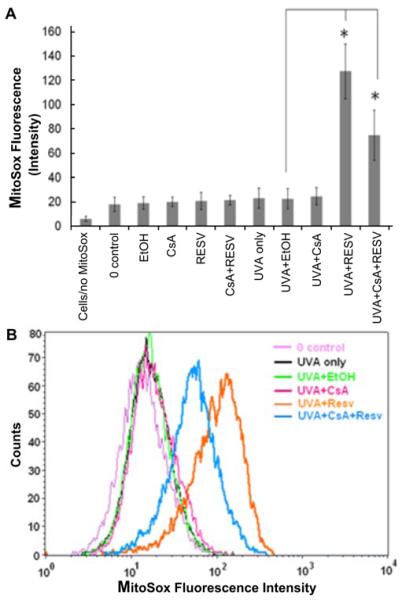
Exposure to UVA and resveratrol has already been shown to cause oxidative stress in genomic DNA in HaCaT cells [17]. In order to measure oxidative stress specific to mitochondria, we performed the MitoSOX™. MitoSOX™ Red reagent is oxidized by superoxide once inside the mitochondria, and is converted to a fluorogenic oxidation product upon binding to nucleic acids. Our results in Figure 4 show that the cells treated with 5uM resveratrol and UVA, as well as resveratrol with CsA and UVA have significantly (p<0.05) increased oxidative stress in mitochondria when compared to the control (UVA with ethanol only). In other controls shown in Figure 4A, HaCaT cells not exposed to UVA, but incubated with ethanol, CsA, resveratrol, or CsA and resveratrol respectively did not show any significant increase in oxidative stress of the mitochondria. In addition, treatment with CsA and UVA, or ethanol and UVA, as well as UVA only, did not cause any significant change in oxidative stress of the mitochondria in comparison to the control (Figure 4A). Figure 4B shows flow cytometry data for the combination treatments of one of the three experiments.
Figure 4. Oxidative stress in mitochondria is increased in HaCaT cells treated with resveratrol and UVA.
A) Bar graph of MitoSox fluorescence intensity measured by flow cytometry is shown for combination treatments (e.g. UVA and resveratrol) and represents the mean of three independent experiments (*p<0.05). B) Flow cytometry data are shown for combination treatments of one of the three experiments.
3.5. Disorganized mitochondrial structure was observed immediately after UVA and resveratrol treatment
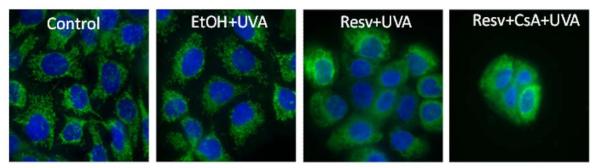
Deconvolution microscopy was used to visualize the mitochondrial structure of live HaCaT cells. Following staining with mitotracker green, imaging of mitochondria in cells without treatment (0 control) and cells treated with both UVA and ethanol showed an evenly distributed filamentous mitochondria in the cytoplasm. Immediately after combination treatment of UVA and resveratrol, mitochondria in these cells developed a disorganized, punctate and swollen appearance (Figure 5). Even after additional treatment with CsA, an inhibitor of MPTP, the disrupted pattern of mitochondria was still seen in the cells treated with UVA, resveratrol and CsA.
Figure 5. Disorganized mitochondrial structure was observed immediately after UVA and resveratrol treatment in HaCaT cells.
Cells undergoing combination treatments of resveratrol and UVA or resveratrol, CsA and UVA had swollen mitochondria, with a punctate, disorganized pattern as observed by live cell imaging using deconvolution microscopy. Nuclei were stained blue with Hoechst 33342. Mitochondria were stained with Mitotracker green and were visualized by green flourescence.
3.6. Resveratrol causes mitochondrial permeability transition pore (MPTP) opening after UVA treatment
The Mitoprobe™ transition pore assay kit was used along with HaCaT cells in order to determine whether MPTP opening occurred after treatment with resveratrol and UVA (Figure 6). The results indicated that MPTP opening of HaCaT cells treated with resveratrol in combination with UVA exposure was induced significantly (p<0.05) than the same population of HaCaT cells treated with UVA and ethanol only (i.e., vehicle). As described in the Material and Methods section, the fluorescence signal indicates the presence of calcein in the cells. Treatment with CoCl2 quenches the fluorescence signal in the cytosol but CoCl2 cannot enter mitochondria when the MPTP is in a closed status so almost the entire signal observed comes from the mitochondria. Therefore, smaller fluorescence intensity indicates relatively more cells with an MPTP status of “open”. When Ionomycin is added, the MPTP is forced to open resulting in almost complete quenching of the calcein fluorescence signal in both, the mitochondria and the cytosol. As shown in Figure 6, cells treated with resveratrol and UVA or resveratrol, UVA and CsA had a smaller calcein fluorescence signal indicating more cells with an MPTP status of “open”.
Figure 6. Combination treatment of resveratrol and UVA exposure in HaCaT cells caused the MPTP opening.
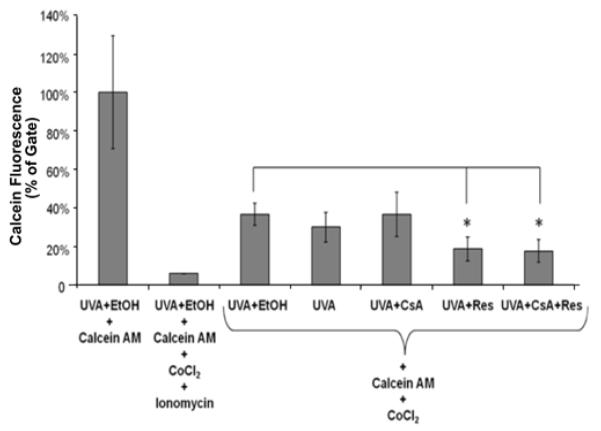
Flow cytometry analysis of MPTP opening in HaCaT cells exposed to 0.2μM CsA or 5μM resveratrol or their combination including UVA treatment (14 J/cm2) is shown. Cells were collected 2 hours after the UVA exposure. Data represent the mean of three independent experiments (*p<0.05).
4. Discussion
We have shown that resveratrol (5uM for HaCaT cells and 50uM for HEKa cells) sensitizes human keratinocytes to UVA induced apoptosis through a mitochondrial pathway. Immediately, after the UVA and resveratrol treatment, oxidative stress was detectable in mitochondria in HaCaT cells by using the Mitosox assay (Figure 4). This level of oxidative stress was not seen in the cells treated with UVA and ethanol. At the same time, MMP changes (depolarization as seen in Figure 3) were detected in the cells treated with UVA and resveratrol, but not in the cells treated with both UVA and ethanol or UVA and CsA, an inhibitor of the MPTP [19]. In addition, the structure of the mitochondria was altered at an early stage either immediately following or during treatment with resveratrol and UVA exposure; i.e., the mitochondria of the live keratinocytes treated with resveratrol and exposed to UVA show a swollen, punctate and disorganized pattern as observed using deconvolution microscopy (Figure 5). Moreover, an addition of CsA did not decrease the disrupted mitochondrial structure. Subsequently, MPTP opening occurred within 2 hours (Figure 6) of UVA and resveratrol treatment leading to apoptosis that was detected by apoptosis assay (Figure 1). Sensitization of keratinocytes to UVA in the presence of resveratrol required the compound to be present during the irradiation period as shown in Figure 2.
UVA is well known to induce oxidative DNA damage and DNA strand breaks [20]. Morliere et al. [21] demonstrated that UVA-induced oxidative stress in skin fibroblasts is mediated through iron by the Fenton reaction. Resveratrol is known for antioxidant properties [22; 23] as well as anticancer properties such as causing growth inhibition through G1 phase arrest and inducing apoptosis [24; 25]. However, a previous study showed that resveratrol can act as a pro-oxidant in HL-60 leukemia cells [26]. Recently, Hadi et al. [27] found that resveratrol can mobilize endogenous copper and lead to oxidative DNA breakage in human peripheral lymphocytes. Copper is one of the most active redox metal ions present in cells. Copper is also an important metal ion present in the chromatin and is closely associated with DNA bases, particularly guanine [28]. Hadi et al. [26] hypothesized that resveratrol not only binds copper but also catalyzes its redox cycling. Therefore, oxidative stress in mitochondria induced by resveratrol and UVA possibly is the result of effects by both, copper and iron. Verity and Gambell [29] also studied the effect of copper on mitochondria in vitro. They proposed that Cu++ induced mitochondrial swelling is mediated through a stoichiometric interaction with a thiol-containing membrane receptor. Changes of mitochondrial structure also could result from ATP reduction and calcium increase in mitochondria due to MMP dissipation.
Resveratrol, a polyphenol compound derived from red grapes, blue berries and other fruits has many biological and pharmacological effects such as inhibiting cancer cell growth, reducing incidence of heart disease and prevention of myocardial infarction in vivo and in vitro. It has been shown that resveratrol can inhibit mRNA of COX-2 and subsequently inhibit angiogenesis of tumor cells and prevent cancer cell growth [30]. Resveratrol also has an effect on cell cycle regulation and induces apoptosis. Resveratrol down regulates cyclin D1 and up regulates p21 which results in cell cycle arrest and apoptosis [31]. In addition, resveratrol has antioxidant activity which overcomes oxidative stress and reduces oxidative DNA damage in cells. Resveratrol also inhibits inflammation and increases immunity by inhibiting COX-2 [30].
Our results confirm that resveratrol enhances UVA-induced apoptosis in human keratinocytes, consistent with the work of Seve et al. [17]. They also found a significant amount of 8-oxo-7,8-dihydro-2′-deoxyguanosine production in genomic DNA and induction of DNA strand breaks and cell death in keratinocytes receiving the combination treatment of UVA and resveratrol. In addition, here we show the specific effects of oxidative stress on mitochondria by the combination treatment of resveratrol and UVA exposure.
In mitochondria, ATP (energy) production requires a series of components such as F0F1-ATP synthase. F1 motor is a water-soluble protein complex which catalyzes ATP synthesis while F0 motor is membrane embedded and catalyses ion translocation across the membrane. During the respiratory chain reaction, participating protein complexes move protons across and along the surface of the membrane as well as push protons from the matrix out to the intermembrane space of the mitochondria. This creates a concentration gradient of protons which F0F1- ATP synthase uses to drive ATP synthesis [32]. Resveratrol was found to inhibit the F0/F1-ATP synthase proton pump of the mitochondria inner membrane, responsible for the synthesis of ATP from ADP in the oxidative phosphorylation pathway [33]. Dörrie et al. [34] found that resveratrol induces extensive apoptosis by depolarization of MMP and by activity of Caspase-9 in acute lymphoblastic leukemia cells and also suggested that the ability of resveratrol to interfere with the machinery of mitochondrial energy transport could be partially responsible for the depolarization of MMP and subsequent apoptosis [34].
Crompton et al. found that CsA inhibited heart mitochondrial pore opening which causes calcium accumulation in the mitochondria [35]. Also, CsA was found to completely block MPTP opening and prevent swelling of brain mitochondria induced by calcium exposure [36]. According to Malouitre et al. CsA was found to be associated with and inhibit cyclophilin D [37]. Over expression of cyclophilin D induces mitochondrial opening [38] while cyclophilin D ablation or inhibition reduces pore opening [39]. Therefore, the observed prevention of mitochondrial pore opening by CsA may occur through inhibition of cyclophilin D. In another study, UVA exposure and CsA treatment was able to prevent the MPTP opening and reduce the incidence of apoptosis [19]. However, our results showed that MPTP opening is at a late stage just before the onset of apoptosis. Once oxidative stress, MMP dissipation and mitochondrial swelling occur, CsA can neither block the MPTP opening nor reverse the onset of apoptosis. Thus, our results indicate that resveratrol enhances the apoptosis induced by UVA in keratinocyte cells through a mitochondrial pathway. This apoptosis is also p53 independent since p53 in HaCaT cells is mutated [40].
One of the interesting features of resveratrol is its pleomorphic and diverse activities including antitapoptotic ability reported in ischemia/reperfusion while in tumor cells it can act as an inductor of apoptosis. Resveratrol can protect against ischemia and thus against apoptosis by activation of sirtuins, highly conserved class III histone deacetylases. Through sirtuins activation, resveratrol has been shown to reduce neural cell toxicity by suppressing postsynaptic glutamatergic transmission and to increase glutamate uptake in cells experiencing oxidative stress. Thus, by preventing cytotoxicity, resveratrol may function to protect the ischemic brain tissue against energy depletion, oxidative stress, and death signaling [41; 42]. In contrast, resveratrol was reported as a compound which triggers cell death in various tumors. This polyphenol affects death receptor distribution in ceramide-enriched membrane platforms which serve to trap and cluster receptor molecules, and facilitates the formation of a death-inducing signaling complex in the cell. To induce apoptosis, resveratrol also activates the ceramide/sphingomyelin pathway, which promotes ceramide generation and the downstream activation of kinase cascades [43]. Resveratrol reportedly enhances TRAIL-induced apoptosis (one of three extrinsic pathways of cell death) in prostate, melanoma and colon cancer cells. Mechanisms proposed for the TRAIL-potentiating effects of resveratrol include inhibition of Akt phosphorylation, upregulation of TRAIL receptors, increased expression of proapoptotic proteins such as Bax, p53 upregulated modulator of apoptosis (PUMA), and down regulation of antiapoptotic proteins [44].
Resveratrol is also known for its cancer prevention abilities by exhibiting anti-inflammatory, cell growth-modulatory, and anticarcinogenic effects by targeting activation of transcriptional factor NF-κB, AP-1 and associated kinases [45]. Moreover, resveratrol has been also shown to decrease the levels of pro-inflammatory mediators like interleukins and TNF-α [46]. Resveratrol was also suggested as a potential non-toxic alternative drug for a cancer treatment. Although it is considered as an anti-oxidant, resveratrol was reported to induce apoptosis through the mitochondrial pathway where the key mediators of resveratrol-induced apoptosis in cancer are mitochondria, calcium, and calpain. Using human breast cancer cell lines, it was observed that resveratrol initiates a rapid dissipation of mitochondrial membrane potential followed by release of cytochrome c. Resveratrol also caused an early release of free intracellular Ca2+ presumably from the ER. These results indicate a critical role for mitochondria in the intrinsic death pathway as well as in the Ca2+ and calpain-dependent cell death [47]. It also should be pointed out that resveratrol has also been shown to have effects on other cell types such as human peripheral lymphocytes in which it has been shown to be capable of causing DNA breakage in cells [48]. Indeed, resveratrol’s cellular targets are relatively diverse including mitochondria, NF-κB and DNA breakage. Moreover, these findings demonstrate that the use of resveratrol for treatment of various cancers could be an alternative approach in cancer therapeutics.
We found that resveratrol, in the presence of UVA, dramatically alters mitochondrial function which massively increases oxidative stress leading to depolarization of MMP, subsequent mitochondrial permeability transition pore opening (MPTP) and apoptosis. Our results suggest a possible intriguing therapeutic avenue for the treatment of non-melanoma skin cancer involving loading of malignant skin cells with resveratrol and subsequent UVA irradiation. Specifically, limited exposure to a relatively low dose of UVA would cause significant apoptosis to the skin tumors treated with resveratrol. This would therefore be an efficacious and potentially safe, inexpensive and simple procedure that can be used as an alternative to surgery in treatment of skin tumors.
Supplementary Material
Figure S1. Combination treatment of resveratrol and UVA exposure significantly induced apoptosis in HEKa cells resulting in decreased viability. Bar graph showing the percentage of cell viability is shown (*P<0.005). Results of the Annexin V and PI assay as the means of three independent experiments.
Figure S2. Combination treatment of resveratrol and UVA exposure induced MMP change in HEKa cells. Bar graph is showing the flow cytometry analysis with JC-1 staining. Data are represented as a ratio of red to green fluorescence, and represent the mean of three independent experiments (**p<0.05).
-
5
Resveratrol in combination with UVA sensitized apoptosis in human keratinocytes
-
6
MMP was more dissipated in cells exposed to UVA and cultured with resveratrol
-
7
Combination treatment of UVA and resveratrol caused more active MPTP in cells
-
8
Higher ROS levels were detected in cells after the treatment with resveratrol and UVA
-
9
Disorganized mitochondrial structure was found after UVA and resveratrol treatment
Acknowledgements
We would like to thank Dr. Tim Bowden for providing the HaCaT cells used in our experiments, Dr. Harris Bernstein for his valuable insights and critique of the manuscript and Dr. Darryl Boyer for his assistance in preparing this manuscript.
This work was supported by a VA Cancer Development Award and a VA Merit Review Award to JES, as well as by NIH AR051552 to JES. Core service utilization at the Arizona Cancer Center is supported by Grant (P30 CA023074) from the National Cancer Institute.
Abbreviations
- CsA
Cyclosporine A
- MMP
Mitochondrial Membrane Potential
- MPTP
Mitochondrial Permeability Transition Pore
- mtDNA
Mitochondrial DNA
- PI
Propidium Iodide
- ROS
Reactive Oxygen Species
- UV
Ultraviolet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Olsen WM, Huitfeldt HS, Eggset G. UVB-induced (6-4) photoproducts in hairless mouse epidermis studied by quantitative immunohistochemistry. Carcinogenesis. 1989;10:1669–1673. doi: 10.1093/carcin/10.9.1669. [DOI] [PubMed] [Google Scholar]
- [2].Bernerd F, Asselineau D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev Biol. 1997;183:123–138. doi: 10.1006/dbio.1996.8465. [DOI] [PubMed] [Google Scholar]
- [3].Ming M, Han W, Maddox J, Soltani K, Shea CR, Freeman DM, He YY. UVB-induced ERK/AKT-dependent PTEN suppression promotes survival of epidermal keratinocytes. Oncogene. 2010;29:492–502. doi: 10.1038/onc.2009.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cooke MS, Mistry N, Ladapo A, Herbert KE, Lunec J. Immunochemical quantitation of UV-induced oxidative and dimeric DNA damage to human keratinocytes. Free Radic Res. 2000;33:369–381. doi: 10.1080/10715760000300911. [DOI] [PubMed] [Google Scholar]
- [5].Vile GF, Tanew-Ilitschew A, Tyrrell RM. Activation of NF-kappa B in human skin fibroblasts by the oxidative stress generated by UVA radiation. Photochem Photobiol. 1995;62:463–468. doi: 10.1111/j.1751-1097.1995.tb02369.x. [DOI] [PubMed] [Google Scholar]
- [6].Strozyk E, Poppelmann B, Schwarz T, Kulms D. Differential effects of NF-kappaB on apoptosis induced by DNA-damaging agents: the type of DNA damage determines the final outcome. Oncogene. 2006;25:6239–6251. doi: 10.1038/sj.onc.1209655. [DOI] [PubMed] [Google Scholar]
- [7].Huang XX, Bernerd F, Halliday GM. Ultraviolet A within Sunlight Induces Mutations in the Epidermal Basal Layer of Engineered Human Skin. Am J Pathol. 2009;174:1534–1543. doi: 10.2353/ajpath.2009.080318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].He YY, Pi J, Huang JL, Diwan BA, Waalkes MP, Chignell CF. Chronic UVA irradiation of human HaCaT keratinocytes induces malignant transformation associated with acquired apoptotic resistance. Oncogene. 2006;25:3680–3688. doi: 10.1038/sj.onc.1209384. [DOI] [PubMed] [Google Scholar]
- [9].Tada-Oikawa S, Oikawa S, Kawanishi S. Role of ultraviolet A-induced oxidative DNA damage in apoptosis via loss of mitochondrial membrane potential and caspase-3 activation. Biochem Biophys Res Commun. 1998;247:693–696. doi: 10.1006/bbrc.1998.8869. [DOI] [PubMed] [Google Scholar]
- [10].Djavaheri-Mergny M, Marsac C, Maziere C, Santus R, Michel L, Dubertret L, Maziere JC. UV-A irradiation induces a decrease in the mitochondrial respiratory activity of human NCTC 2544 keratinocytes. Free Radic Res. 2001;34:583–594. doi: 10.1080/10715760100300481. [DOI] [PubMed] [Google Scholar]
- [11].Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- [12].Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27:1465–1474. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- [13].Zykova TA, Zhu F, Zhai X, Ma WY, Ermakova SP, Lee KW, Bode AM, Dong Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol Carcinog. 2008;47:797–805. doi: 10.1002/mc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5:74–82. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- [16].Surh YJ, Hurh YJ, Kang JY, Lee E, Kong G, Lee SJ. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett. 1999;140:1–10. doi: 10.1016/s0304-3835(99)00039-7. [DOI] [PubMed] [Google Scholar]
- [17].Seve M, Chimienti F, Devergnas S, Aouffen M, Douki T, Chantegrel J, Cadet J, Favier A. Resveratrol enhances UVA-induced DNA damage in HaCaT human keratinocytes. Med Chem. 2005;1:629–633. doi: 10.2174/157340605774598144. [DOI] [PubMed] [Google Scholar]
- [18].Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Norman KG, Canter JA, Shi MJ, Milne GL, Morrow JD, Sligh JE. Cyclosporine A suppresses keratinocyte cell death through MPTP inhibition in a model for skin cancer in organ transplant recipients. Mitochondrion. 2010;10:94–101. doi: 10.1016/j.mito.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cadet J, Douki T, Ravanat JL, Di Mascio P. Sensitized formation of oxidatively generated damage to cellular DNA by UVA radiation. Photochem Photobiol Sci. 2009;8:903–911. doi: 10.1039/b905343n. [DOI] [PubMed] [Google Scholar]
- [21].Morliere P, Salmon S, Aubailly M, Risler A, Santus R. Sensitization of skin fibroblasts to UVA by excess iron. Biochim Biophys Acta. 1997;1334:283–290. doi: 10.1016/s0304-4165(96)00106-7. [DOI] [PubMed] [Google Scholar]
- [22].Afaq F, Adhami VM, Ahmad N, Mukhtar H. Botanical antioxidants for chemoprevention of photocarcinogenesis. Front Biosci. 2002;7:d784–792. doi: 10.2741/afaq. [DOI] [PubMed] [Google Scholar]
- [23].Quincozes-Santos A, Andreazza AC, Nardin P, Funchal C, Goncalves CA, Gottfried C. Resveratrol attenuates oxidative-induced DNA damage in C6 Glioma cells. Neurotoxicology. 2007;28:886–891. doi: 10.1016/j.neuro.2007.03.008. [DOI] [PubMed] [Google Scholar]
- [24].Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB, Yang K, Shen HF, Xie LP. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010;101:488–493. doi: 10.1111/j.1349-7006.2009.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, Carter B, Turkson J, Jove R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther. 2006;5:621–629. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- [26].Zheng LF, Wei QY, Cai YJ, Fang JG, Zhou B, Yang L, Liu ZL. DNA damage induced by resveratrol and its synthetic analogues in the presence of Cu (II) ions: mechanism and structure-activity relationship. Free Radic Biol Med. 2006;41:1807–1816. doi: 10.1016/j.freeradbiomed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- [27].Hadi SM, Ullah MF, Azmi AS, Ahmad A, Shamim U, Zubair H, Khan HY. Resveratrol mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for chemoprevention of cancer. Pharm Res. 2010;27:979–988. doi: 10.1007/s11095-010-0055-4. [DOI] [PubMed] [Google Scholar]
- [28].Kagawa TF, Geierstanger BH, Wang AH, Ho PS. Covalent modification of guanine bases in double-stranded DNA. The 1.2-A Z-DNA structure of d(CGCGCG) in the presence of CuCl2. J Biol Chem. 1991;266:20175–20184. doi: 10.2210/pdb1d39/pdb. [DOI] [PubMed] [Google Scholar]
- [29].Verity MA, Gambell JK. Studies of copper ion-induced mitochondrial swelling in vitro. Biochem J. 1968;108:289–295. doi: 10.1042/bj1080289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- [31].Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res. 2001;7:1466–1473. [PubMed] [Google Scholar]
- [32].von Ballmoos C, Wiedenmann A, Dimroth P. Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem. 2009;78:649–672. doi: 10.1146/annurev.biochem.78.081307.104803. [DOI] [PubMed] [Google Scholar]
- [33].Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dorrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001;61:4731–4739. [PubMed] [Google Scholar]
- [35].Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- [36].Hansson MJ, Mansson R, Mattiasson G, Ohlsson J, Karlsson J, Keep MF, Elmer E. Brain-derived respiring mitochondria exhibit homogeneous, complete and cyclosporin-sensitive permeability transition. J Neurochem. 2004;89:715–729. doi: 10.1111/j.1471-4159.2004.02400.x. [DOI] [PubMed] [Google Scholar]
- [37].Malouitre S, Dube H, Selwood D, Crompton M. Mitochondrial targeting of cyclosporin A enables selective inhibition of cyclophilin-D and enhanced cytoprotection after glucose and oxygen deprivation. Biochemical Journal. 2010;425:137–148. doi: 10.1042/BJ20090332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li YM, Johnson N, Capano M, Edwards M, Crompton M. Cyclophilin-D promotes the mitochondrial permeability transition but has opposite effects on apoptosis and necrosis. Biochemical Journal. 2004;383:101–109. doi: 10.1042/BJ20040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- [40].Lehman TA, Modali R, Boukamp P, Stanek J, Bennett WP, Welsh JA, Metcalf RA, Stampfer MR, Fusenig N, Rogan EM, et al. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993;14:833–839. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- [41].Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011;31:1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Raval AP, Lin HW, Dave KR, DeFazio RA, Della Morte D, Kim EJ, Perez-Pinzon MA. Resveratrol and ischemic preconditioning in the brain. Curr Med Chem. 2008;15:1545–1551. doi: 10.2174/092986708784638861. [DOI] [PubMed] [Google Scholar]
- [43].Delmas D, Solary E, Latruffe N. Resveratrol, a Phytochemical Inducer of Multiple Cell Death Pathways: Apoptosis, Autophagy and Mitotic Catastrophe. Curr Med Chem. 2011;18:1100–1121. doi: 10.2174/092986711795029708. [DOI] [PubMed] [Google Scholar]
- [44].Hsieh TC, Wu JM. Resveratrol: Biological and pharmaceutical properties as anticancer molecule. Biofactors. 2010;36:360–369. doi: 10.1002/biof.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- [46].Kumar A, Sharma SS. NF-kappaB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010;394:360–365. doi: 10.1016/j.bbrc.2010.03.014. [DOI] [PubMed] [Google Scholar]
- [47].Wenner CE. Targeting mitochondria as a therapeutic target in cancer. J Cell Physiol. 2012;227:450–456. doi: 10.1002/jcp.22788. [DOI] [PubMed] [Google Scholar]
- [48].Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Research. 2004;64:2627–2633. doi: 10.1158/0008-5472.can-03-0846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Combination treatment of resveratrol and UVA exposure significantly induced apoptosis in HEKa cells resulting in decreased viability. Bar graph showing the percentage of cell viability is shown (*P<0.005). Results of the Annexin V and PI assay as the means of three independent experiments.
Figure S2. Combination treatment of resveratrol and UVA exposure induced MMP change in HEKa cells. Bar graph is showing the flow cytometry analysis with JC-1 staining. Data are represented as a ratio of red to green fluorescence, and represent the mean of three independent experiments (**p<0.05).