G&H In which patients and indications is endoscopic necrosectomy of the pancreas usually performed?
TB Endoscopic necrosectomy is nearly always performed in patients who have suffered an episode of clinically severe pancreatitis, as this group of patients has underlying pancreatic necrosis. Following the onset of severe pancreatitis, endoscopic necrosectomy cannot generally be performed earlier than 2–3 weeks later, and in most cases, we wait 4 weeks or more, as the initial attack of pancreatitis is managed medically. This later time frame is when patients develop a necrotic collection composed of dead pancreas tissue and fluid from their episode of pancreatitis, which appears radiographically as a defined collection on computed tomography (CT). The necrotic collection can then be accessed through the stomach or duodenum, depending upon the individual patient. The decision to intervene is based not only upon CT imaging results, but also on clinical symptoms, including ongoing severe abdominal pain, persistent symptoms of pancreatitis, pancreatic ascites, and infection. Thus, the use of endoscopic necrosectomy is determined by both the patient's symptoms and the imaging results; if the patient has minimal symptoms or is doing well, for example, we would likely wait prior to recommending an intervention. In addition, it is very important to stress that necrotic pancreatic collections are often confused radiographically with and mislabeled as pancreatic pseudocysts. Pseudocysts contain only liquid material, and if a pseudocyst drainage procedure is undertaken in a patient with a necrotic pancreatic collection, inadequate removal of solid material and subsequent infection will occur.
G&H Could you outline how endoscopic necrosectomy is performed?
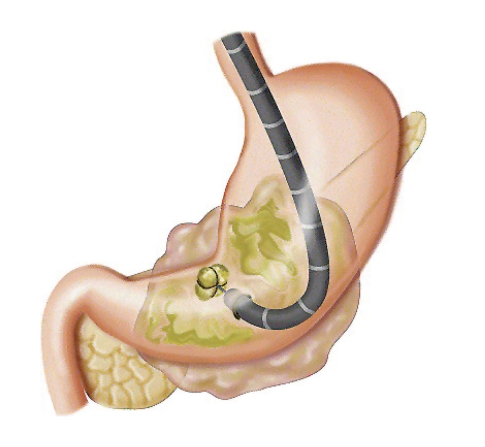
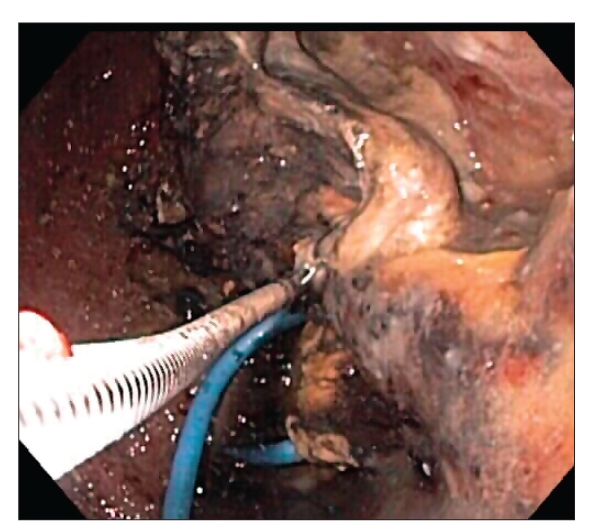
TB In endoscopic necrosectomy of the pancreas, the endoscope is first passed into the stomach or duodenum. From inside these organs, the necrotic cavity is usually visible pressing up against them. This cavity should not be confused with a cyst, as the cavity is a collection of dead pancreatic tissue, which is fairly solid material, in contrast to a cyst, which is usually liquid. Instruments are then passed through the endoscope to allow the endoscopist to puncture or enter directly through the wall of the stomach or duodenum to inside the cavity. A guidewire is then inserted through the stomach or duodenum and coiled inside the cavity. Over this wire, the wall of the stomach or duodenum is dilated over the guidewire to a diameter of at least 15 mm. A plastic stent is then inserted from the stomach (if the stomach was entered) across the opening to inside the cavity. This component of the procedure is usually performed with a side-viewing endoscope, as is used during endoscopic retrograde cholangiopancreatography (ERCP), which is then removed and replaced with a forward-viewing upper endoscope. The endoscope is driven through the hole created and inside the cavity. As the aim of this procedure is to remove the dead pancreas tissue, when the endoscope is passed directly into the cavity, the endoscopist utilizes various tools and accessories to remove the dead material from inside the cavity, dropping it into the stomach or duodenum.
G&H What have studies found regarding the outcomes and safety of endoscopic necrosectomy for treatment of pancreatitis?
TB When performing endoscopic necrosectomy, more than 1 procedure is usually required to completely remove necrotic tissue. In most cases, complete endoscopic necrosectomy may be performed in as few as 2 procedures, but it occasionally takes 4 or 5 procedures, as it is not usually possible to remove all of the solid material from the cavity during the first procedure. When multiple procedures are required, the patients are then rescheduled for additional procedures ranging from several days to 1 or 2 weeks after the initial procedure with the removal of as much necrotic tissue as possible in each session. With multiple procedures, approximately 90% of patients experience complete resolution.
Some physicians think that endoscopic necrosectomy is either very extreme or dangerous, though we are finding that this is not true. However, I would not recommend that community gastroenterologists perform this procedure yet, as it requires endoscopic expertise and the ability to manage complications, which could be life-threatening, and it may require complex medical, surgical, and radiologic management of potential complications such as bleeding and perforation. Thus, we believe that endoscopic necrosectomy should be conducted in very specialized centers.
G&H How does endoscopic necrosectomy show a clinical benefit over open necrosectomy?
TB No studies have yet directly compared endoscopic necrosectomy to open surgical necrosectomy, and it will likely be difficult to conduct a comparative trial with an adequate number of patients. Nevertheless, the main advantage of endoscopic necrosectomy is that it is a nonsurgical procedure. Surgical necrosectomy, which has evolved over time to become the traditional standard of care in these patients, is not a simple operation, as appendectomy or cholecystectomy are, for example. Accessing the necrotic cavity surgically through the abdomen is quite technically difficult, as the cavity sits behind the stomach in the retroperitoneum. Frequently, the surgical approach also requires multiple operations to remove all of the dead tissue, similar to endoscopic necrosectomy. Overall, surgical necrosectomy carries modest complication rates and a large abdominal incision. Patients are frequently left with drains that extend from their abdomen and may also develop fistulous tracks to the skin. In addition, large ventral hernia defects may occur. These complications may require additional operations for closure at a later time. Thus, the endoscopic necrosectomy has many advantages over the open procedure.
Endoscopic necrosectomies are similar to natural orifice transluminal endoscopic surgery (NOTES) procedures; in fact, it could even be said that they are one of the earliest forms of NOTES procedures. An endoscopic necrosectomy is performed in a cavity or confined space; thus, when the procedure is complete, the hole that was created to provide access to the cavity closes by itself. In contrast, in a NOTES procedure, a hole that is created in the stomach, for example, cannot be left open to the abdomen.
G&H Could you discuss any complications associated with this procedure?
TB There is always a risk of a major bleeding complication with this procedure. When removing the dead pancreas tissue inside the necrotic cavity, blood vessels may be exposed and accidentally cut, which can lead to massive and difficult-to-control bleeding. Another potential risk is perforation, which, if it occurs, usually happens during the first procedure. The goal of endoscopic necrosectomy is to create a contained perforation into the necrotic cavity; however, a perforation may occur outside the cavity or in the wall of the cavity. Infection is another potential risk. As mentioned above, one of the indications for this procedure is the presence of an infection. However, even if there is no infection, infection may develop, as the procedure is not sterile and bacteria are introduced with the passage of the endoscope through the mouth. If the drainage is not adequate, there is a risk of clinical infection. Finally, standard sedation complications may also arise.
G&H Is endoscopic necrosectomy used most often as a primary therapy or in conjunction with other therapeutic procedures?
TB Endoscopic necrosectomy has evolved beyond being a secondary procedure that was used only in conjunction with other procedures. In the past, endoscopic drainage of pancreatic necrosis was performed transmurally with drains and stents but not with direct passage of the endoscope into the cavity (direct necrosectomy). Often, percutaneous drains were required as an adjunct to endoscopic drainage. In addition, endoscopic therapy was reserved for those who failed primary surgical or percutaneous drainage. Currently, direct endoscopic necrosectomy is used mainly as a primary therapy, and it is performed in patients who are not candidates for surgery as well patients who are candidates for surgery, at least at our institution. This is in contrast to many high-risk endoscopic procedures that are reserved only for patients who cannot undergo an operation.
G&H Do you anticipate any technological advances in this procedure?
TB A major breakthrough would be to develop appropriate endoscopic technology and accessories for these procedures. A significant limitation of direct necrosectomy is the lack of accessories that are effective for grasping and removing necrotic tissue; endoscopists are currently using accessories designed for other indications. In addition, this work is quite tedious, as it requires scraping and pulling out dead tissue. Sometimes, this process goes smoothly with the currently available accessories, but often it is a prolonged and frustrating procedure with only small amounts of tissue removed with each pass of an accessory. It would be much easier to grasp, remove, and dissect the dead tissue if we had the proper tools. Having said that, after performing over 40 of these procedures, I believe there is a learning curve and my debridement skills have improved. I can more accurately choose what types of accessories work for a given patient. The same tools do not work as effectively among different patients. For example, in one patient, a polypectomy snare may be used effectively to grasp some of the dead tissue, and the endoscopist may think that because the snare was so effective in this patient that it will work just as well in the next. However, the next patient may have differences in the consistency and adherence of the necrotic tissue and so on, causing the polypectomy snare not to work as well as with the first patient. Or, grasping forceps may work better on one patient than another, for similar reasons.
G&H Could you discuss any important recent or upcoming research in this area?
TB Presently, most of the literature published in this area comes from Europe. However, several non-European studies will be released shortly, one of which was conducted by my colleagues and I. In this study, which is currently in press with Gastrointestinal Endoscopy, we used the direct necrosectomy technique outlined above, in which we enter the necrotic cavity directly and pull out the dead pancreas material, and compared it to the technique that was used in the past for many years. In the original technique, first performed and reported in 1996, I would not directly enter the necrotic cavity, but place stents and irrigation tubes from the stomach or duodenum into the cavity. The irrigation tube extended from inside the cavity to out of the patient's nose, and we would irrigate with large volumes of saline to break up the solid material. It was not known at that time that it was possible to safely and effectively enter directly into the cavity and remove necrotic solid tissue. In our upcoming study, our success rate for complete resolution without surgery and without the need for percutaneous drainage is much higher and it appears to be faster in terms of patient improvement and hospital discharge using direct necrosectomy.
G&H What are the next steps for future research?
TB One goal is to pool data together from several centers in the United States. In our upcoming article in Gastrointestinal Endoscopy, we examined only approximately 30 patients with the new endoscopic necrosectomy technique, though by now we have completed 40 or 50 of these procedures. We would like to pool data from endoscopic necrosectomies performed in multiple centers to analyze a larger number of procedures and their outcomes in the United States. Our upcoming study is actually the first one from the United States using the new method, so we would like to compile a larger experience from several centers.
Ultimately, it would also be helpful to conduct a comparative study of endoscopic necrosectomy with surgery. However, this trial will likely be difficult to arrange; although a modest number of patients undergo this procedure, it is not performed on an everyday basis (closer to 1 or 2 per month). Thus, randomizing patients to surgery or endoscopy in a comparative trial would take a long time to accrue and likely require a multicenter study with expertise in surgery and endoscopy. In addition, adequate funding would need to be raised. These obstacles may be difficult to overcome, though only the future will tell.
Figure 1.
Figure 2.
Suggested Reading
- Voermans RP, Bruno MJ, van Berge Henegouwen MI, Fockens P. Review article: Translumenal endoscopic debridement of organized pancreatic necrosis--the first step towards natural orifice translumenal endoscopic surgery. Aliment Pharmacol Ther. 2007;26(suppl 2):233–239. doi: 10.1111/j.1365-2036.2007.03489.x. [DOI] [PubMed] [Google Scholar]
- Voermans RP, Veldkamp MC, Rauws EA, Bruno MJ, Fockens P. Endoscopic transmural debridement of symptomatic organized pancreatic necrosis (with videos) Gastrointest Endosc. 2007;66:909–916. doi: 10.1016/j.gie.2007.05.043. [DOI] [PubMed] [Google Scholar]
- Seewald S, Groth S, Omar S, Imazu H, Seitz U, et al. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm (videos) Gastrointest Endosc. 2005;62:92–100. doi: 10.1016/s0016-5107(05)00541-9. [DOI] [PubMed] [Google Scholar]
- Charnley RM, Lochan R, Gray H, O'Sullivan CB, Scott J, Oppong KE. Endoscopic necrosectomy as primary therapy in the management of infected pancreatic necrosis. Endoscopy. 2006;38:925–928. doi: 10.1055/s-2006-944731. [DOI] [PubMed] [Google Scholar]
- Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–64. doi: 10.1053/gast.1996.v111.pm8780582. [DOI] [PubMed] [Google Scholar]
- Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7–17. doi: 10.1067/mge.2002.125106. [DOI] [PubMed] [Google Scholar]
- Bucher P, Pugin F, Morel P. Minimally invasive necrosectomy for infected necrotizing pancreatitis. Pancreas. 2008;36:113–119. doi: 10.1097/MPA.0b013e3181514c9e. [DOI] [PubMed] [Google Scholar]