Abstract
Therapy of mitochondrial respiratory chain diseases is complicated by limited understanding of cellular mechanisms that cause the widely variable clinical findings. Here, we show that focal segmental glomerulopathy-like kidney disease in Pdss2 mutant animals with primary coenzyme Q (CoQ) deficiency is significantly ameliorated by oral treatment with probucol (1% w/w). Preventative effects in missense mutant mice are similar whether fed probucol from weaning or for 3 weeks prior to typical nephritis onset. Furthermore, treating symptomatic animals for 2 weeks with probucol significantly reduces albuminuria. Probucol has a more pronounced health benefit than high-dose CoQ10 supplementation and uniquely restores CoQ9 content in mutant kidney. Probucol substantially mitigates transcriptional alterations across many intermediary metabolic domains, including peroxisome proliferator-activated receptor (PPAR) pathway signaling. Probucol's beneficial effects on the renal and metabolic manifestations of Pdss2 disease occur despite modest induction of oxidant stress and appear independent of its hypolipidemic effects. Rather, decreased CoQ9 content and altered PPAR pathway signaling appear, respectively, to orchestrate the glomerular and global metabolic consequences of primary CoQ deficiency, which are both preventable and treatable with oral probucol therapy.
Keywords: coenzyme Q, kidney, mitochondria, mouse, probucol
INTRODUCTION
Primary mitochondrial respiratory chain (RC) disease represents a complex compilation of multi-systemic disorders caused by pathogenic mutations in more than 60 nuclear and all 37 mitochondrial DNA-encoded genes (Haas et al, 2008). Therapy for this heterogeneous group of often devastating diseases is largely relegated to vitamin and supplement cocktails of theoretical utility whose objective efficacy is difficult to monitor but which commonly appear to have minimal clinical benefit (Parikh et al, 2009). A translational mouse model of primary mitochondrial RC disease caused by a homozygous amino acid substitution affecting the nuclear-encoded prenyl diphosphate synthase subunit 2 (Pdss2) enzyme required for coenzyme Q (CoQ) biosynthesis (Dell et al, 2000; Peng et al, 2004) develops a lethal kidney disease by 4 months of life (Lyon & Hulse, 1971).
CoQ is a highly lipophilic molecule that consists of a benzoquinone ring joined to a polyisoprenoid side chain that is comprised predominantly of 9 or 10 isoprenoid groups in mouse and humans, respectively. The isoprenyl side chain is synthesized by a heterotetramer of two subunits (Saiki et al, 2005), Pdss1 and Pdss2. In addition to serving an essential role in electron shuttling within the mitochondrial RC, CoQ has both antioxidant and oxidant functions (Linnane et al, 2007). Severe, collapsing glomerulopathy variant, focal segmental glomerulosclerosis (FSGS)-like renal disease in B6.Pdss2kd/kd missense mutant mice results from CoQ deficiency within glomerular podocytes (Peng et al, 2008). High-dose CoQ10 supplementation mitigates renal disease in B6.Pdss2kd/kd mice through approximately 140 days of life, but does not normalize albuminuria to control levels, prevent nephritis, nor restore CoQ9 or CoQ10 content in renal tissue (Saiki et al, 2008).
Here, we report the efficacy and results of detailed mechanistic investigations for both preventative and therapeutic trials in Pdss2 mutant mice with the oral lipophilic drug probucol.
RESULTS
Probucol therapy prevents renal disease in Pdss2kd/kd missense mutant mice
As probucol was previously reported to be beneficial in a mouse model of mitochondrial-based glomerular disease caused by mutations in Mpv17 (Binder et al, 1999), we added 1% probucol to standard mouse chow (TestDiet) fed to B6.Pdss2kd/kd missense mutant mice. Probucol effects were compared to those of long-term CoQ10 supplementation, since high-dose CoQ10 was previously observed to delay manifestations of renal disease in young B6.Pdss2kd/kd mice (Saiki et al, 2008). Urine albumin immediately preceding, and histological nephritis score immediately following sacrifice were determined in 4–5-month-old missense mutant littermates placed on standard un-supplemented diet (controls), probucol supplementation from birth, weaning or day 100 of life, high-dose CoQ10 supplementation from birth, weaning or day 100 of life, or vitamin E from weaning (Table 1). Probucol therapy significantly prevented renal disease whether administered from the time of weaning through at least 6 months of age or for only approximately 3 weeks prior to sacrifice. While all of the probucol-treated missense mutant groups showed significantly higher urine albumin than gender-matched healthy B6 controls (p < 0.01 in each case), no significant differences were seen in their mean nephritis score relative to gender-matched healthy B6 controls (p > 0.05). Representative examples of normal renal histology seen in probucol-treated missense mutant animals are illustrated in Fig 1. Vitamin E long-term administration from weaning had modest beneficial effect in female B6.Pdss2kd/kd mice but no significant effect in males (Table 1). Long-term CoQ10 therapy also significantly prevented renal disease manifestations when provided from weaning through 6 months of age. However, significantly improved renal histology and function was seen when CoQ10 treatment was initiated at birth rather than upon weaning (Table 1 and Supporting information File 1). The most dramatic rescue of the renal phenotype occurred in B6.Pdss kd/kd males that were treated with probucol from birth to 206 days of age (Table 1). Overall, probucol provided more long-lasting and effective prevention of renal disease than did supplemental CoQ10.
Table 1.
Effects of probucol or CoQ10 supplementation on renal disease manifestations of B6.Pdss2kd/kd missense mutant mice
| Strain | Gender | Treatment group | Treatment duration | N | Age at sacrifice (Days) | Nephritis score | Urine albumin (mg/24 h) | Albumin comparison group | Albumin p-value |
|---|---|---|---|---|---|---|---|---|---|
| B6 | M | No treatment | n/a | 14 | 156 ± 48.58 | 0.43 ± 0.46 | 0.46 ± 0.61 | ||
| B6 | F | No treatment | n/a | 12 | 156 ± 59.57 | 0.63 ± 0.62 | 0.22 ± 0.12 | ||
| B6.Pdss2kd/kd | M | No treatment | n/a | 24 | 135 ± 18.37 | 1.61 ± 0.93 | 10.60 ± 9.12 | ||
| B6.Pdss2kd/kd | F | No treatment | n/a | 20 | 136 ± 20.49 | 2.69 ± 0.99 | 31.47 ± 30.12 | B6.Pdss2kd/kd males | p < 0.001 |
| B6.Pdss2kd/kd | M | CoQ10 | Birth to sacrifice | 7 | 145 ± 8.29 | 0.69 ± 0.43 | 3.64 ± 4.85 | B6.Pdss2kd/kd untreated males | p < 0.05 |
| B6.Pdss2kd/kd | F | CoQ10 | Birth to sacrifice | 13 | 144 ± 16.29 | 0.71 ± 0.70 | 2.24 ± 1.79 | B6.Pdss2kd/kd untreated females | p < 0.001 |
| B6.Pdss2kd/kd | M | CoQ10 | Weaning to sacrifice | 6 | 125 ± 4.5 | 1.67 ± 0.75 | 4.11 ± 2.04 | B6.Pdss2kd/kd untreated males | p < 0.01 |
| B6.Pdss2kd/kd | F | CoQ10 | Weaning to sacrifice | 4 | 124 ± 3.19 | 2.26 ± 0.92 | 1.75 ± 0.43 | B6.Pdss2kd/kd untreated females | p < 0.001 |
| B6.Pdss2kd/kd | M | CoQ10 | Day 100 to sacrifice | 6 | 121 ± 0.94 | 1.42 ± 0.45 | 10.47 ± 3.47 | B6.Pdss2kd/kd untreated males | p > 0.05 |
| B6.Pdss2kd/kd | F | CoQ10 | Day 100 to sacrifice | 5 | 121 ± 0 | 1.30 ± 0.40 | 4.08 ± 1.15 | B6.Pdss2kd/kd untreated females | p < 0.001 |
| B6.Pdss2kd/kd | M | Probucol | Birth to sacrificea | 5 | 206 ± 0 | 0.6 ± 0.49 | 0.76 ± 0.14 | B6.Pdss2kd/kd untreated males | p < 0.001 |
| B6.Pdss2kd/kd | F | Probucol | Birth to sacrifice | 3 | 206 ± 0 | 1.67 ± 0.47 | 19.01 ± 9.50 | B6.Pdss2kd/kd untreated females | p > 0.05 |
| B6.Pdss2kd/kd | M | Probucol | Weaning to sacrifice | 14 | 156 ± 30.36 | 0.57 ± 0.82 | 1.15 ± 1.12 | B6.Pdss2kd/kd untreated males | p < 0.001 |
| B6.Pdss2kd/kd | F | Probucol | Weaning to sacrifice | 11 | 136 ± 9.43 | 0.55 ± 0.99 | 1.42 ± 0.91 | B6.Pdss2kd/kd untreated females | p < 0.001 |
| B6.Pdss2kd/kd | M | Probucol | Day 100 to sacrifice | 5 | 122 ± 0 | 1.40 ± 0.37 | 0.64 ± 0.17 | B6.Pdss2kd/kd untreated males | p < 0.001 |
| B6.Pdss2kd/kd | F | Probucol | Day 100 to sacrifice | 4 | 122 ± 0 | 0.88 ± 0.55 | 1.53 ± 0.36 | B6.Pdss2kd/kd untreated females | p < 0.001 |
| B6.Pdss2kd/kd | M | Vitamin E | Weaning to sacrifice | 9 | 122 ± 2.85 | 1.56 ± 0.50 | 11.58 ± 4.23 | B6.Pdss2kd/kd untreated males | p > 0.05 |
| B6.Pdss2kd/kd | F | Vitamin E | Weaning to sacrifice | 10 | 120 ± 2.20 | 1.20 ± 0.40 | 11.38 ± 4.72 | B6.Pdss2kd/kd untreated females | p < 0.01 |
Probucol therapy significantly prevented renal sequelae that are typically manifest by 4 months of age whether administered either from the time of weaning or for only 3 weeks prior to sacrifice, with significant disease prevention still evident through approximately 6 months of age. CoQ10 therapy significantly prevented renal manifestations only when provided from weaning through 4 months of age, although significantly greater effects were seen with probucol than CoQ10 at all treatment durations tested (p < 0.01 at younger age when treated from weaning; p < 0.001 at younger age when treated for 3 weeks prior to sacrifice; and p < 0.001 at older age). All values are reported as mean ± standard deviation. p-Values were calculated based on urine albumin by Mann–Whitney U-test relative to designated (male or female) age-matched untreated B6.Pdss2kd/kd animals.
Probucol treated missense mutant males showed the greatest therapeutic response in urine albumin at all ages examined, from the earliest age (122 days) through the oldest (206 days).
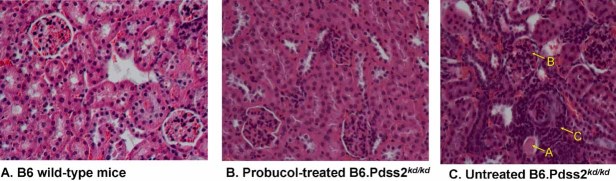
Figure 1. Comparison of probucol-treated and untreated B6.Pdss2kd/kd mouse kidney pathology.
Near-normal glomerular histology is evident upon haematoxylin and eosin staining of renal sections from male probucol-treated missense mutant mice shown at 100X magnification.
- Wild-type B6 control.
- Probucol-treated B6.Pdss2kd/kd missense mutant mouse; 128-day old; 0.77 mg urine albumin.
- Untreated B6.Pdss2kd/kd missense mutant mouse; 132-day old; 7.3 mg urine albumin. Note tubular dilatation (arrow A), glomerular crescents (arrow B) and severe interstitial inflammation (arrow C) evident in kidney from untreated missense mutant mouse.
Data analysis suggested gender may influence drug treatment response. A gender effect was seen in response to short-term and long-term probucol treatments, as male B6.Pdss2kd/kd missense mutant mice had a significantly greater response in both cases than did females (p < 0.05). In contrast, short-term CoQ10 therapy for approximately 3 weeks prior to sacrifice had a significant effect in female B6.Pdss2kd/kd mice but no effect in males. When directly comparing probucol and CoQ10 treatment responses, significantly greater improvement following long-term treatment from weaning with probucol than CoQ10 was seen among male mice for albuminuria (p = 0.001 males and p = 0.1 females) and among both genders for nephritis score (p = 0.03 males and p = 0.06 females). Short-term treatment from day 100 to sacrifice resulted in significantly greater improvement in both genders treated with probucol relative to CoQ10 only in albuminuria (p = 0.002 males and p = 0.008 females) with equivalent effects on the more subjective nephritis score. Overall, male missense mutant mice tended to respond similarly or better to probucol therapy, whereas, females tended to respond similarly or better to CoQ10 supplementation.
Probucol therapy reverses renal disease manifestations in symptomatic Pdss2kd/kd missense mutant mice
A second cohort of B6.Pdss2kd/kd mice was maintained on normal chow until approximately 100 days of age and then tested in metabolic cages for urine albumin. Animals with urine albumin >1 mg in 24 h were placed on probucol-supplemented chow for approximately 2 weeks, retested in metabolic cages, and then sacrificed. As shown in Table 2, 24 h urine albumin in this cohort decreased from 11.46 +/− 12.3 mg before to 4.07 +/− 4.22 mg after probucol treatment (p < 0.01). A highly significant reduction occurred in both genders, with a greater reduction in albuminuria seen in males. Although considerable variation was present between animals especially prior to treatment, albuminuria was reduced by probucol treatment in every instance.
Table 2.
Renal disease phenotypes following short-term treatment of B6.Pdss2kd/kd missense mutant mice with probucol
| Urine albumin (mg/24 h) |
||||||
|---|---|---|---|---|---|---|
| Group | N | Age at first test | Probucol treatment duration | Pre-treatment test | Post-treatment test | p-Value |
| Males | 7 | 102 ± 0.49 | 14.6 ± 1.40 days | 14.40 ± 19.1 | 2.32 ± 1.73 | p < 0.05 |
| Females | 12 | 106 ± 1.37 | 13.4 ± 2.36 days | 10.30 ± 7.59 | 5.09 ± 5.10 | p < 0.05 |
| ALL | 19 | 104 ± 2.52 | 13.8 ± 2.13 days | 11.46 ± 12.3 | 4.07 ± 4.22 | p < 0.01 |
Elevated 24 h urine albumin levels suggestive of renal glomerular disease in 100-day-old untreated missense mutants (pre-treatment test) were significantly reduced in both males and females following approximately 2 weeks of oral probucol treatment (post-treatment test). Histological nephritis score was also lower following probucol treatment than typical of untreated age-matched missense mutants (Table 1). Non-parametric statistical analysis (Mann–Whitney U-test) of mean urine albumin in treated versus untreated missense mutant groups showed short-term probucol treatment significantly reduced albuminuria (p = 0.01). A trend towards greater reduction in urine albumin was seen in males, with a mean reduction by 71.1% +/− 14.1% in males and 47.1% +/− 27.6% in females (p = 0.07).
Probucol treatment lowers plasma cholesterol and hepatic phospholipid levels in B6.Pdss2kd/kd missense mutant and B6.Podocin/cre,Pdss2loxP/loxP glomerular-conditional knockout mice
We previously reported that Pdss2 missense mutants develop hypercholesterolemia that is partly attributable to nephritis and partly a direct consequence of primary CoQ deficiency, since blood cholesterol is also significantly increased in B6.Alb/cre,Pdss2loxP/loxP liver-conditional Pdss2 knockout mice that have normal renal function (Peng et al, 2008). Probucol has been previously reported to have potent lipid-lowering properties, which remain of uncertain mechanism (Stocker, 2009). To determine whether renal disease resolution on probucol therapy correlated with its lipoprotein effects, blood cholesterol and triglyceride levels were determined in both missense and glomerular podocyte-conditional knockout Pdss2 mutant mice following long-term probucol treatment (Table 3). We previously observed that, whereas, B6.Pdss2kd/kd mice with more advanced stage disease (152 +/− 16 days of age) had significantly elevated blood cholesterol, mutant mice up to 133 days of age did not (Madaio et al, 2005). Similarly, plasma lipids were not significantly elevated in 4-month-old (mean: 133-day old) B6.Pdss2kd/kd missense mutant mice that had clear evidence of renal disease (Table 3). Nonetheless, probucol treatment significantly decreased plasma cholesterol and triglyceride levels, as well as renal disease manifestations of albuminuria and nephritis relative to untreated B6.Pdss2kd/kd missense mutant littermates (consistent with data presented in Table 1). Although probucol treatment did normalize the significantly elevated blood cholesterol that was seen in B6.Podocin/cre,Pdss2loxP/loxP podocyte-conditional knockout animals, this lipid-lowering effect was insufficient to prevent or mitigate severe renal disease in these animals. To determine whether the hypolipidemic effects of probucol observed in plasma also extend to the cardiolipin-rich lipid milieu of the inner mitochondrial membrane in which the RC is located (Schlame & Ren, 2009), 31P-NMR analysis was performed on combined liver extracts from two animals per strain and treatment group (to provide sufficient tissue for analysis). Probucol significantly reduced liver membrane phospholipids in Pdss2 mice, including cardiolipin, sphingomyelin and phospholipids (Supporting information File 2). Thus, while probucol is clearly an effective anti-hyperlipidemic agent, this property was insufficient to rescue the renal sequelae of severe CoQ deficiency in mice without Pdss2 activity within renal glomerular podocytes. Interestingly, prophylactic therapy from birth with Simvastatin, an anti-hyperlipidemic agent that inhibits HMG-CoA reductase, neither prevented renal disease nor dramatically lowered blood cholesterol in Pdss2 missense mutant mice (data not shown), as was similarly observed following Simvastatin administration to apolipoprotein E-deficient mice (Ivanovski et al, 2008). Overall, the mechanism by which probucol prevents renal disease in missense mutant mice with some residual Pdss2 activity within podocytes (Peng et al, 2008) appears to be independent of its hypolipidemic effects.
Table 3.
Probucol effects on plasma lipids in B6.Pdss2kd/kd missense and B6.Podocin/cre,Pdss2loxP/loxP conditional knockout mice
| Mouse strain | N | Age (days) | Treatment | Cholesterol (mg/dl) | Triglycerides (mg/dl) | Urine albumin (mg/24 h) | Nephritis score (0–4) |
|---|---|---|---|---|---|---|---|
| B6 | 8 | 160 ± 13 | Control | 85.96 ± 17.0 | 75.94 ± 24.5 | 0.5 ± 0.1 | 0.07 ± 0.17 |
| B6.Pdss2kd/kd | 16 | 133 ± 7.2 | Control | 97.1 ± 24.8 | 67.0 ± 22.3 | 24.3 ± 17.2 | 3.4 ± 0.5 |
| B6.Pdss2kd/kd | 12 | 169 ± 23.4 | Probucol | < 12.5a | 42.7 ± 19.7 | 1.6 ± 1.0 | 1.0 ± 1.3 |
| (p < 0.001) | (p < 0.01) | (p < 0.001) | (p < 0.01) | ||||
| Pod/cre | 14 | 150 ± 9.8 | Control | 369.1 ± 168.4 | 114.5 ± 51.0 | 96.6 ± 31.1 | 3.7 ± 0.5 |
| Pod/cre | 11 | 164 ± 3.5 | Probucol | <63.2 ± 68.9b | 168.3 ± 77.4 | 45.6 ± 33.4 | 3.4 ± 0.7 |
| (p < 0.001) | (p = 0.069) | (p < 0.01) | (p = 0.178) |
Probucol therapy significantly lowered plasma cholesterol and triglycerides in B6.Pdss2kd/kd missense mutants, as well as plasma cholesterol in renal podocyte-conditional Pdss2 knockout mutants (‘pod/cre’). While renal disease manifestations of albuminuria and nephritis were prevented by probucol in missense mutants (consistent with data presented in Table 1), renal disease manifestations were largely unabated by probucol therapy in pod/cre conditional knockouts.
Cholesterol levels in all samples from this group were below the level of detection.
Cholesterol levels in four of the samples from this group were below the level of detection. All values are reported as mean ± standard deviation. p-Values were calculated by student's t-test for each mutation type relative to untreated mutant animals.
Resistance to oxidative stress is increased in Pdss2 mutant mice when treated long-term with CoQ10 but not when treated with probucol
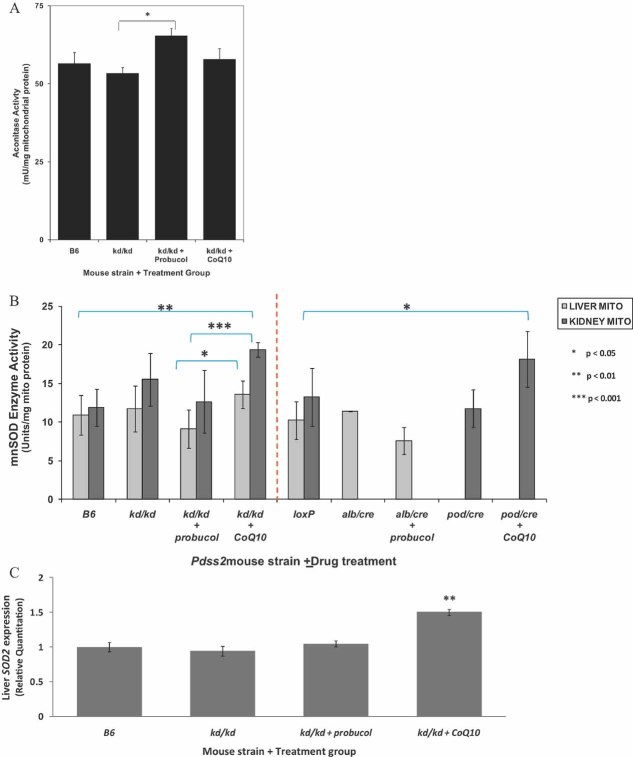
Although CoQ has both antioxidant and oxidant roles (Linnane & Eastwood, 2004), we observed no evidence of increased reactive species damage attributable to primary CoQ deficiency in either Pdss2 missense (kd/kd) or liver-conditional (Alb/cre) knockout mutants. Specifically, total lipid peroxidation of isolated liver mitochondria appeared unchanged in either mutant type relative to wild-type (WT) healthy controls both at baseline (Supporting information File 3A and B) and following long-term supplementation with either CoQ10 or probucol (Supporting information File 3C). To further evaluate oxidant stress in Pdss2 mice, aconitase activity was assayed in freeze-thawed liver mitochondria. Whereas, no significant difference was observed in Pdss2 mutants at baseline or following long-term CoQ10 therapy relative to B6 controls, aconitase activity was significantly increased by 15% in probucol-treated compared to untreated Pdss2 missense mutants (p < 0.05, Fig 2A).
Figure 2. Assessment of mitochondrial oxidant stress and scavenging defenses in Pdss2 mutants following supplementation with probucol or CoQ10.
- Aconitase activity in isolated liver mitochondria is significantly increased in kd/kd mutants treated with probucol relative to untreated littermates (p < 0.05 relative to untreated kd/kd mutants). No significant increase in aconitase activity was seen in kd/kd mutants at baseline or upon CoQ10 supplementation. Results are the average ± standard error of five measurements per mitochondrial preparation from n = 3–5 female mice. Ages of mice ranged from 140 to 154 days for kd/kd and 215–216 days for B6.
- MnSOD enzyme activity is significantly increased in kidney mitochondria isolated from missense mutants (kd/kd) and podocyte-conditional knockouts (pod/cre) treated with CoQ10 relative to respective healthy controls. In contrast, a trend towards decreased mean MnSOD enzyme activity is observed in liver mitochondria from missense (kd/kd) and liver-conditional knockout (Alb/cre) mutant animals treated with probucol relative to respective healthy controls. Further, CoQ10-treated missense mutant (kd/kd) animals have significantly greater MnSOD enzyme activity in both liver and kidney relative to probucol-treated missense (kd/kd) animals. Brackets highlight statistically significant comparisons relative to either untreated controls or between treatment groups, as indicated. Column height and error bars indicate activity mean and standard deviation (SD), respectively. Dashed red line distinguishes missense mutant (kd/kd) animals (compared to WT B6 controls) from two different tissue-specific conditional knockout mutants (compared to LoxP controls). kd/kd, missense mutant; Alb/cre, liver-conditional knockout mutant; pod/cre, renal glomerular podocyte-conditional knockout mutant.
- SOD2 (mitochondrial MnSOD) hepatic gene expression is significantly increased in kd/kd mutants treated with CoQ10 relative to untreated littermates. In contrast, SOD2 expression is unchanged relative to B6 WT controls in kd/kd mutants on standard chow with or without probucol supplementation. Values are reported as mean ± standard error. Mean age of each group was B6, 216 days; kd/kd, 137 days; probucol-treated kd/kd, 144 days; and CoQ10-treated kd/kd 154 days. n = 5 for each group, except CoQ10-treated animals where n = 3. All animals were female and the same individuals in which global microarray expression profiling was performed.
Antioxidant defenses can be assessed by monitoring induction of manganese superoxide dismutase (MnSOD), the primary mitochondrial enzyme to scavenge matrix superoxide (Macmillan-Crow & Cruthirds, 2001). MnSOD activity in isolated kidney mitochondria was significantly increased only in missense mutant or renal podocyte-conditional (pod/cre) knockout mice following long-term treatment with CoQ10 relative to respective healthy controls (Fig 2B). In contrast, a trend towards decreased MnSOD enzyme activity in isolated liver mitochondria was seen in missense mutant or liver-conditional knockout mice treated long-term with probucol. Significantly greater MnSOD enzyme activity was seen following CoQ10 compared to probucol treatment in both liver and kidney mitochondria from missense mutant mice. Similarly, hepatic expression of SOD2 (mitochondrial MnSOD) was significantly increased only in CoQ10-treated missense mutants (Fig 2C), but unchanged in missense mutants on standard chow with or without probucol. These data suggest that, while long-term, high-dose CoQ10 supplementation induces mitochondrial oxidant scavenging capacity and oxidative stress resistance, long-term probucol supplementation does not.
Probucol reverses hyperglycemia in B6.Alb/cre,Pdss2loxP/loxP liver-conditional knockout mice
Given the central role of the liver in intermediary metabolism, we assessed major blood metabolites indicative of hepatic function and metabolic control in alb/cre liver-conditional knockout mice. B6.Alb/cre,Pdss2loxP/loxP mice exhibited no evidence of liver damage based on histologic (data not shown) or plasma ALT levels (Supporting information File 4), either at baseline or following long-term probucol therapy. Blood glucose was significantly elevated in Alb/cre liver-conditional knockout mutants (p < 0.05) and significantly decreased by feeding mice probucol from weaning. No significant difference due to either Pdss2 knockout in liver or long-term probucol therapy was observed in blood urea, which is exclusively synthesized in liver. Although blood ammonia was not increased in Alb/cre liver-conditional knockout mice relative to B6.Pdss2loxP/loxP (‘LoxP’) controls, probucol did significantly decrease blood ammonia relative to both untreated Alb/cre mutants and age-matched healthy controls (Supporting information File 4). Thus, probucol appears to have no adverse effect on hepatic function or biosynthetic capacity and actually appears to have potentially beneficial systemic metabolic effects by lowering blood levels of glucose and ammonia.
Probucol therapy reverses global transcriptional alterations across intermediary metabolic pathways in Pdss2 mice
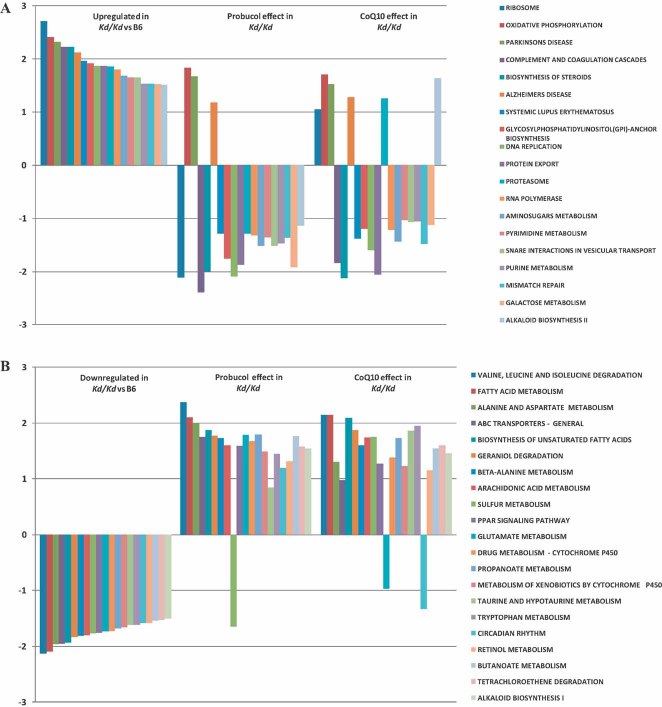
To discern whether the same transcriptional signature of mitochondrial dysfunction previously reported in B6.Alb/cre,Pdss2loxP/loxP liver-conditional knockout mice (Peng et al, 2008) was present regardless of mutation type, as well as to assess whether pharmacologic therapies modulate expression of particular pathways, genome-wide transcriptional profiling was performed in livers from B6.Pdss2kd/kd missense mutant mice on standard chow or supplemented long-term with either probucol or CoQ10. The influence of long-term probucol treatment on pathway-level expression alterations was also assessed in liver-conditional Pdss2 knockout mice. Significantly altered KEGG pathways as determined using Gene Set Enrichment Analysis (GSEA) to analyse relative genome-wide expression changes in both mutant types on standard chow and following supplementation with probucol or CoQ10 are visually represented by heatmap in Fig 3. Full details of all pathways analysed are provided in excel format in Supporting information File 5. Upregulated expression of oxidative phosphorylation (OXPHOS), the tricarboxylic acid cycle, directly interacting pathways involved in amino acid metabolism, and cell defense pathways was previously observed in liver-conditional Pdss2 knockout mice (Peng et al, 2008), as well as in several mitochondrial RC subunit missense mutant Caenorhabditis elegans strains (Falk et al, 2008). We now conclude that upregulation of OXPHOS, protein processing pathways, multiple immune pathways, and nucleotide metabolism occurs in both missense and liver-conditional knockout Pdss2 mice. However, Pdss2 missense mutants uniquely upregulate lipid biosynthesis and downregulate fatty acid metabolism, amino acid metabolism, glycolysis, cell defenses and peroxisome proliferator-activated receptor (PPAR) transcriptional signaling pathway expression. Regardless of the direction of transcriptional changes in either Pdss2 mutant type, probucol largely (but not completely, as detailed in Supporting information File 5) reversed the global pattern of metabolic pathway alterations (Fig 4). Indeed, long-term probucol therapy in both mutant types reversed expression alterations within the PPAR signalling pathway, fatty acid metabolism and glycolysis, despite these having been concordantly upregulated in knockouts and downregulated in missense Pdss2 mutants.
Figure 3. Pathway-level heatmap depicts metabolic pathways whose expression was significantly altered following chronic probucol or CoQ10 supplementation in missense and liver-conditional knockout Pdss2 mutants.
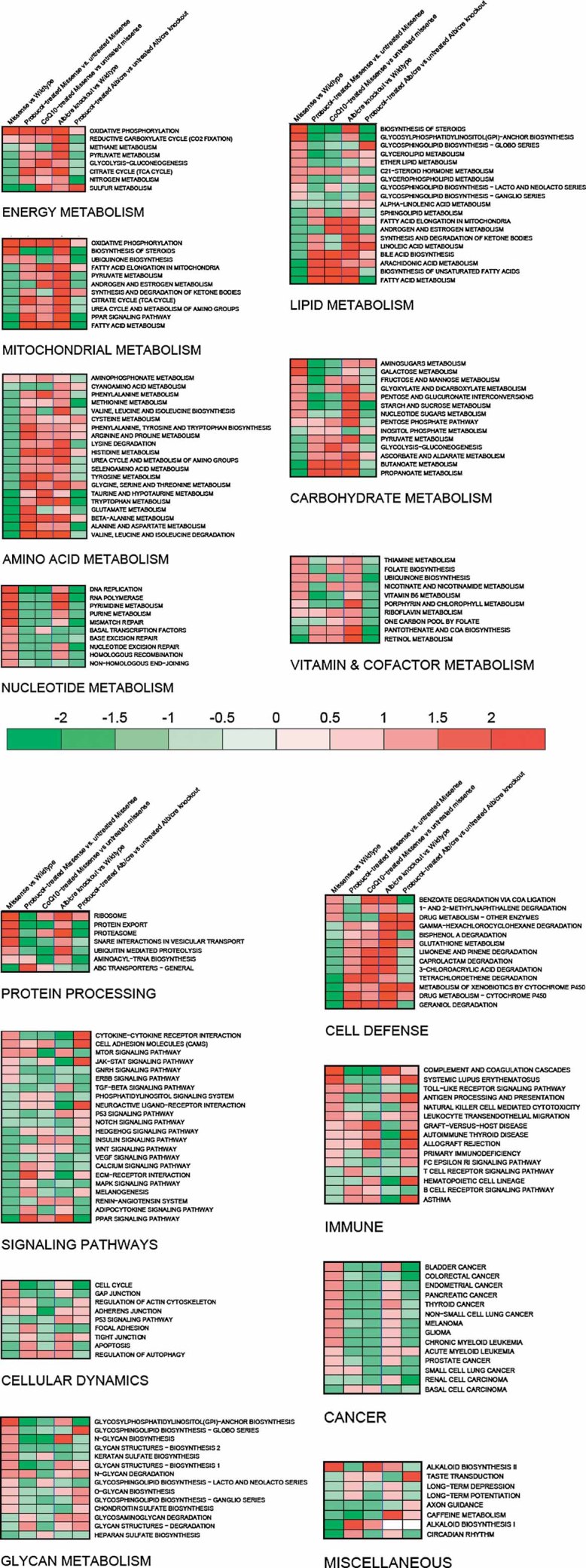
Heatmaps clustered by primary metabolic functions are provided for all KEGG-defined pathways in both mutant types on standard chow alone or with chronic supplementation of either probucol (both mutant types) or CoQ10 (missense mutants only). This view illustrates that the most significantly altered KEGG-defined pathways fall into several key areas of intermediary metabolism. Probucol therapy has a dramatic effect in normalizing observed expression alterations across many metabolic domains in both mutant types. Rows represent overall expression alterations among individual KEGG-defined pathways. Columns represent pair-wise comparisons, from left to right: (1) B6.Pdss2kd/kd versus B6 WT control; (2) probucol-treated B6.Pdss2kd/kd versus untreated B6.Pdss2kd/kd; (3) CoQ10-treated B6.Pdss2kd/kd versus untreated B6.Pdss2kd/kd; (4) B6.Alb/CrePdss2loxP/loxP versus LoxP WT control; and (5) probucol-treated B6.Alb/CrePdss2loxP/loxP versus untreated B6.Alb/CrePdss2loxP/loxP. Red or green colour indicates pathway up- or down-regulation in the first group relative to the second, where intensity conveys significance of overall pathway alterations for each pair-wise comparison. Illumina and Affymetrix 3′ expression microarrays were used for analysis of B6.Pdss2kd/kd and B6.Alb/CrePdss2loxP/loxP experiments, respectively. Cross-platform validation of probucol-treated B6.Alb/CrePdss2loxP/loxP versus untreated B6.Alb/CrePdss2loxP/loxP results was performed by replicate analysis of the same samples on three additional expression microarray platforms, including Illumina (Zhang et al, 2010). Complete GSEA data output including statistical significance for KEGG pathways analysed in all comparisons are provided in Supporting information File 5.
Figure 4. Probucol has a greater effect than CoQ10 supplementation in normalizing alterations among the most differentially expressed metabolic pathways in Pdss2 missense mutant mouse liver.
The most differentially regulated KEGG-defined pathways as determined in GSEA to have normalized enrichment score (NES) >1.5 are shown for B6.Pdss2kd/kd missense mutants relative to B6 healthy controls. Probucol has a more widespread and substantial effect than does CoQ10 in ‘reversing’, or preventing, expression alterations among pathway that are both upregulated (Panel A) and downregulated (Panel B) in missense mutants. Bar length represents NES for each pathway as determined in GSEA v2.0. Complete GSEA data output including statistical analyses of all KEGG-defined pathways analysed in missense animals, as well as in liver-conditional knockout mutants treated with probucol, are provided in Supporting information File 5.
Probucol had no significant effect on OXPHOS pathway upregulation that occurs in these primary mitochondrial RC mutants, consistent with its apparent inability to serve as an electron acceptor to replace deficient CoQ in the RC. Further support for this finding is provided by polarographic RC analysis of permeabilized skeletal muscle fibres (Oroboros), which showed no significant difference in RC capacity of probucol treated B6.Pdss2kd/kd missense mutants relative to untreated B6.Pdss2kd/kd controls (Supporting information File 6). Furthermore, probucol had no significant effect on gene expression of major pathways previously linked to its apparent mechanistic effects in other models, including antioxidant genes (Supporting information File 7) or heme oxygenase (HMOX1 and HMOX2; Supporting information File 8).
Branched-chain amino acid catabolism was the most upregulated KEGG metabolic pathway by either therapy in Pdss2 missense mutants (Fig 4B and Supporting information File 5). Steroid biosynthesis and the complement and coagulation cascade were the most downregulated pathways in missense mutants by CoQ10 and probucol therapy, respectively (Fig 4A and Supporting information File 5). Steroid biosynthesis was also the most down-regulated pathway in liver-conditional knockout mutants on probucol therapy (Supporting information File 5), a result technically validated on three additional microarray platforms (Zhang et al, 2010). CoQ10 supplementation in missense mutants caused a less global and pronounced ‘reversal’ of metabolic alterations than did probucol (Fig 4). Gene-level heatmap analysis among the most significantly altered KEGG pathways illustrates that a particular subset of a given pathway's genes may drive overall pathway significance (Supporting information File 9; Zhang et al, 2010). Overall, the KEGG Atlas overview of intermediary metabolism provides an integrated gestalt of the global metabolic expression alterations caused by both mutation types and their substantial normalization with probucol therapy (Supporting information File 10).
PPAR signaling mediates metabolic consequences of primary CoQ10 deficiency in Pdss2 mutant mice
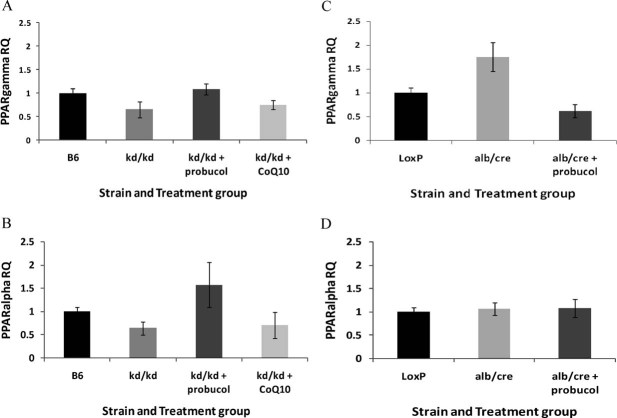
The PPAR family of nuclear transcription factors transcriptionally controls many aspects of metabolism through modulation of more than 50 target genes involved in lipid, glucose and amino acid homeostasis, as well as inflammation, cell proliferation and cell division (Feldman et al, 2008). Expression of PPAR pathway transcription factors and target genes was significantly altered as judged by global microarray-based transcriptional profiling analysis in liver from both missense and liver-conditional knockout mutants, and largely normalized with probucol therapy (Supporting information File 11). Quantitative Polymerase Chain Reaction (after reverse transcription) (RT-PCR) analysis confirmed that expression of the PPAR-γ and PPAR-α transcription factors were decreased in Pdss2 missense mutants and fully normalized following probucol treatment, but were unaltered by CoQ10 supplementation (Fig 5A and B). Interestingly, PPAR-γ expression was significantly increased in Pdss2 knockout mice but also normalized with probucol therapy (Fig 5C). PPAR-α expression was unchanged in Pdss2 knockout mice (Fig 5D). Overall, these data confirm that PPAR expression alterations occur in primary mitochondrial dysfunction caused by CoQ deficiency and normalize with probucol therapy.
Figure 5. PPAR transcription factor expression normalizes with probucol therapy in Pdss2 mutant mice.
- Hepatic expression of PPAR-γ and
- PPAR-α in Pdss2 missense mice is decreased by quantitative RT-PCR validation, but increased by long-term probucol supplementation either to (PPAR-γ) or exceeding (PPAR-α) expression levels seen in B6 WT controls. CoQ10 supplementation does not significantly alter expression of either PPAR transcription factor in missense mutants. n = 4 female mice per strain and treatment group aged 129–156 days.
- Hepatic expression of PPAR-γ is increased by quantitative RT-PCR validation in liver-conditional Pdss2 knockout mice relative to LoxP WT controls, but clearly decreased by long-term probucol therapy below expression levels seen in WT controls.
- In contrast, hepatic PPAR-α expression is unchanged in male liver-conditional Pdss2 knockout mice either on standard chow or supplemented long-term with probucol relative to LoxP WT controls. n = 3 male mice per strain and treatment group aged 120–141 days.
Probucol therapy increases tissue CoQ content in Pdss2kd/kd mice
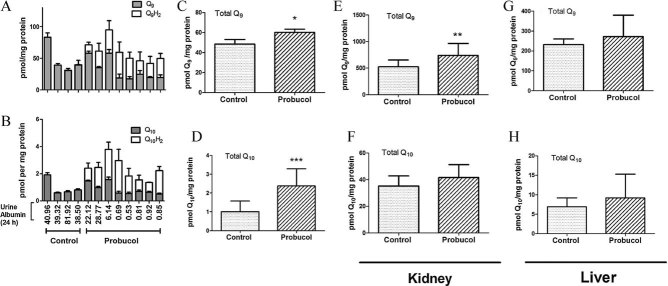
Peroxisomal inducers and PPAR-α signaling agonists are known to induce CoQ synthesis in mice (Turunen et al, 2004). The enhanced rescue of kidney disease in B6.Pdss2kd/kd mice achieved by both long- and short-term supplementation with probucol relative to CoQ10, and the restoration of PPAR-γ and PPAR-α expression in response to probucol treatment, raised the question of whether probucol alters CoQ tissue levels. To investigate this possibility, B6.Pdss2kd/kd mice were fed standard chow alone or supplemented with probucol from birth to age 207 days, when renal disease severity was assessed by measuring 24 h urine albumin levels and determining histological nephritis score (Supporting information File 12). Liver homogenates were analysed to quantify CoQ9, CoQ9H2, CoQ10 and CoQ10H2 levels (Fig 6A and B). CoQ9 and CoQ10 in lipid extracts prepared from the liver of un-supplemented mice were present exclusively in the oxidized quinone state. In contrast, lipid extracts from the liver of probucol-supplemented animals contained 20–80% of total CoQ content in the reduced or hydroquinone state (CoQH2). Although no precautions were taken to prevent auto-oxidation of CoQH2, liver extracts from the un-supplemented and probucol-supplemented animals were prepared and analysed at the same time. Thus, the lack of CoQ9H2 and CoQ10H2 is a unique characteristic of samples prepared from the un-supplemented B6.Pdss2kd/kd mice. Summation of the hydroquinone and quinone content for CoQ9 (Fig 6C) and CoQ10 (Fig 6D) indicated that chronic probucol supplementation significantly elevated total CoQ content, which correlated with a dramatic rescue of kidney disease as evidenced by significantly decreased urine albumin [Fig 6B; average urine albumin in un-supplemented = 50.18 vs. probucol-treated = 7.6 (p < 0.01) and nephritis score (average nephritis score in un-supplemented = 3.0 vs. probucol-treated = 1.0, p < 0.01)]. Unfortunately, kidneys from these animals were used for histological purposes and were not available for CoQ content analysis. Similarly as observed when comparing renal disease severity, a gender effect was evident when comparing CoQ9 and CoQ10 liver levels (Supporting information File 12). Both CoQ species were approximately two-fold greater in females than males at baseline and increased following long-term probucol treatment in both genders.
Figure 6. Probucol supplementation increases content of CoQ9 and CoQ10 in kidney and liver of Pdss2kd/kd missense mutant mice.
Long-term probucol supplementation
A–D. Pdss2kd/kd mice (3 females and 5 males) were fed diets supplemented with probucol (1% w/w) from birth and killed at 205–207 days of age. Control B6.Pdss2kd/kd mice (2 females and 2 males) were fed unsupplemented standard chow diet and sacrificed at 205–215 days of age. Each bar represents the average content of (A) CoQ9 and CoQ9H2 and (B) CoQ10 and CoQ10H2 measured in three aliquots of liver homogenates prepared from each mouse, calculated as the average of six measurements from three independent sample aliquots. Error bars indicate SD. Twenty-four hour urine albumin levels collected immediately prior to liver dissection are indicated for each animal. (C) Total CoQ9 content represents the average ± SD of the sum of CoQ9 plus CoQ9H2 (determined from panel A). (D) Total CoQ10 content represents the average ± SD of the sum of CoQ10 and CoQ10H2 (determined from panel B) *p < 0.05 and ***p < 0.001.
Short-term probucol supplementation
E–H. Female Pdss2kd/kd mice (100-day old) were fed standard diets plus (n = 3) or minus (n = 2) probucol for 15 days. Aliquots of kidney (E and F) and liver (G and H) homogenates were measured for content of total CoQ9 (E and G) or total CoQ10 (F and H). **p < 0.01. Both CoQ9 and CoQ9H2 and CoQ10 and CoQ10H2 were present in all extracts, with no differences between them as was observed in extracts prepared from the older mice (205–207 days of age) following long-term probucol treatment.
Given the ability of long-term probucol supplementation to increase tissue CoQ content, the effect of short-term (15 day) probucol treatment on tissue CoQ content was investigated in liver and kidney from female B6.Pdss2kd/kd mice already showing albuminuria (24 h albumin > 1 mg) when treatment was begun. Total CoQ9 content in kidney homogenates was significantly increased by short-term probucol supplementation (Fig 6E, p < 0.01). Total CoQ10 content in kidney was also marginally increased (Fig 6F; p = 0.0689). However, no significant differences in CoQ9 or CoQ10 levels were observed in liver (Fig 6G and H). Short-term probucol treatment also did not alter the proportion of hydroquinone in either tissue (data not shown). Thus, probucol supplementation throughout life increased hydroquinone and total quinone levels in liver even at advanced age, whereas, short-term treatment of symptomatic B6.Pdss2kd/kd mice significantly increased only the kidney total CoQ9 content.
DISCUSSION
Biological significance
This translational investigation of a potential oral therapy for a genetic-based mitochondrial RC disorder revealed that endogenous upregulation of CoQ content by probucol treatment is far more effective than attempts to replace the deficient metabolite (CoQ) by exogenous CoQ10 supplementation. Probucol treatment successfully improved disease manifestations whether initiated at birth, at weaning, for a short time (3 weeks) prior to onset of typical disease manifestations, or even for just 2 weeks after the onset of the pathophysiologic disease process in untreated Pdss2 mutant animals. Indeed, the mitochondria defects that are characteristic of this phenotype can be observed in most B6.Pdss2kd/kd mice before 100-days old by electron microscopy (Hallman et al, 2006), and B6.Pdss2kd/kd mice may manifest significant albuminuria as early as 96-days old (Madaio et al, 2005). Deficiencies in tissue CoQ content can be observed even earlier: the average CoQ9 concentration in kidney extracts from 40-day-old B6 control mice was 1,088 ± 59 pmol/mg protein, as compared to 292 ± 15 pmol/mg protein in 37-day-old B6.Pdss2kd/kd missense mutant mice (Saiki et al, 2008). Therefore, not only does probucol have preventative effects (Table 1), but it successfully treats mice after onset of the disease process, as evidenced by albuminuria (Table 2).
Prophylactic probucol supplementation long-term from birth to pre-symptomatic animals clearly increased CoQ9 and CoQ10 levels in liver (Fig 6) and prevented nephritis (Table 1). It is interesting that total CoQ content, particularly CoQ9, was also modestly but significantly increased in kidney (Fig 6) and all markers of nephritis (Table 2) were significantly decreased following only 2 weeks of probucol treatment to B6.Pdss2kd/kd mice already showing albuminuria (Fig 6). Strong correlation was similarly observed previously between high-kidney CoQ9 content and low urine albumin in B6.Pdss2kd/kd line G mice: two line G mice that had high-urine albumin also had low renal CoQ9 content, whereas six line G mice that did not manifest evidence of nephritis all had high-renal CoQ9 content (Saiki et al, 2008). The gene cluster, ‘ubiquinone biosynthesis’ was not, however, differentially expressed in liver following long-term probucol therapy (Fig 3). In particular, expression of ADCK3, the homologue of yeast COQ8 that appears to regulate Q content (Barros et al, 2005; Zampol et al, 2010), was not significantly different by microarray profiling in liver from B6.Pdss2kd/kd mutants either at baseline or following probucol treatment. It remains possible that CoQ content could be affected by the rate of turnover, or it may be that the ADCK3 polypeptide is regulated by either translational control or post-translational modifications. Further, while hepatic expression offers the benefit of assaying a homogenous tissue, it may not be reflective of CoQ pathway expression changes in renal podocytes. Although the mechanism by which short-term probucol treatment increases renal CoQ9 content in symptomatic B6.Pdss2kd/kd mice remains uncertain, it seems likely that this effect is important for kidney disease rescue. The increases detected in de novo content of CoQ (whether due to increased synthesis or stability) arising from probucol supplementation are more efficacious than can be attained with CoQ10 supplementation (Saiki et al, 2008). To this end, we have previously shown that although long-term CoQ10 treatment partially mitigates albuminuria and nephritis, it does not correct the CoQ9 or CoQ10 deficiency in B6.Pdss2kd/kd mouse kidney (Saiki et al, 2008). Similarly, defects in the de novo synthesis of lipoic acid in yeast cannot be corrected by simply providing lipoic acid exogenously (Hiltunen et al, 2010).
Beneficial effects of probucol administered before the onset of overt disease manifestations suggests that an effective application of this lipophilic agent may be as a prophylactic agent to prevent systemic sequelae of mitochondrial disease. In addition, significantly reduced albuminuria following onset of overt disease manifestations suggests that probucol therapy may also have value as a treatment modality for mitochondrial disease. Future studies of probucol are needed to discern whether similar benefit occurs in human subjects, whether its efficacy is multi-systemic or limited to the kidney, whether therapeutic effects are detectable if administered to individuals already manifesting severe disease sequelae, and whether it has a tolerable safety profile in human patients afflicted with the heterogeneous class of mitochondrial diseases. No evidence of overt liver disease (Supporting information File 4) was observed following long-term probucol administration to Pdss2 mutant mice, although a significant reduction was seen in blood cholesterol, glucose, and ammonia as well as in hepatic phospholipid content (Supporting information File 2). Patients with a multi-systemic infantile form of CoQ10 deficiency who harbour mutations in PDSS1, PDSS2, COQ2 or COQ9 may also exhibit renal disease, which in some cases has been reported to respond to treatment with CoQ10 (Quinzii & Hirano, 2010). It will be important to evaluate the efficacy of probucol therapy in these patients.
Investigation of additional possible mechanisms underlying probucol efficacy in Pdss2 mutants
Probucol is structurally comprised of two phenol rings joined by a disulfide bridge. It represents a chemical prototype from which newer generation analogues have since been generated that lack some potentially deleterious effects of probucol (Stocker, 2009), including lowering HDL cholesterol and prolonging cardiac repolarization (QTc interval). While neither of these parameters was specifically assessed in probucol-treated Pdss2 mice, we observed no incidence of sudden or unexplained death as might be seen with prolonged QTc in treated animals up to 8-months old. The mechanisms by which probucol lowers plasma lipid levels, acts as an antioxidant, and fights inflammation remain active areas of investigation (Stocker, 2009). Probucol has recently been reported to have renal protective effects during the administration of nephrotoxic agents (Yin et al, 2009), as well as beneficial effects on cardiac and neurologic function in the face of oxidative stress (Farina et al, 2009; Wu et al, 2006; Zhou et al, 2009). It has successfully been used to prevent restenosis after coronary angioplasty (Tardif et al, 1997) and also prevents atherosclerosis and related cardiovascular diseases associated with increased oxidative stress and inflammation in several animal models, where its efficacy has been attributed to induction of heme oxygenase (Wu et al, 2006). However, heme oxygenase gene expression was not significantly altered by probucol treatment in either missense or liver-conditional Pdss2 knockout mice when analysed by expression microarray profiling of liver (Supporting information File 8).
Probucol's ability to prevent renal disease in this model highlights the question of what aspect of CoQ function underlies the etiology of glomerular disease in Pdss2 mutants having primary CoQ deficiency. It does not appear likely that their renal disease results solely from decreased electron transport capacity in kidney (Peng et al, 2008). Although probucol supplementation clearly increased tissue CoQ content (Fig 6), it did not appear to improve mitochondrial respiratory capacity (Supporting information File 6). Nor does severely diminished RC capacity necessarily cause renal disease in many forms of primary mitochondrial disease.
Antioxidant properties attributed to probucol appear related to its sulfur atoms more so than its phenol rings (Wu et al, 2006). Resolution of renal disease with probucol, therefore, makes increased oxidative stress in the setting of primary CoQ deficiency an attractive candidate to explain the pathophysiologic mechanism of Pdss2-related renal disease. Oxidative stress has also been linked to other forms of primary renal glomerular disease that have diverse etiologies including infection, altered immune responses and altered lipid metabolism (Mayrhofer et al, 2009). However, we were unable to demonstrate evidence of increased oxidative stress, oxidative damage or major oxidant scavenging response in either liver or kidney mitochondria isolated from Pdss2 mutant animals. Rather, aconitase activity was significantly increased by 15% only following probucol treatment (Fig 3A). In addition, long-term treatment with only vitamin E, another lipophilic antioxidant, did not substantially ameliorate renal disease in B6.Pdss2kd/kd mutant mice. Finally, no significant expression alterations were seen in either individual antioxidant scavenging genes or at the level of concordant antioxidant pathways in Pdss2 missense or liver-conditional knockout mutants supplemented long-term with probucol (Supporting information File 7). Nonetheless, it is well recognized that gene expression changes are not necessarily indicative of cellular antioxidant response. It remains possible that the endogenous boost in tissue CoQ content elicited by probucol may function as a crucial antioxidant in podocyte cellular membranes. Analysis of a fibroblast cell line from the sole human patient reported to be affected with an autosomal recessive Pdss2-based disease (Lopez et al, 2006) identified nearly 90% CoQ10 deficiency and impaired ATP synthetic capability, but similarly as observed in the Pdss2 mutant mice, found no sign of increased oxidant stress as assessed by relative oxidant species production and antioxidant defense markers (Quinzii et al, 2008).
Inflammation may play a pathogenic role in Pdss2-related nephritis, as probucol has been previously shown to reduce mononuclear cell adhesion to endothelium in vivo as well as to inhibit macrophage infiltration. Indeed, we have previously observed substantial inflammatory infiltrates in kidneys of B6.Pdss2kd/kd mutants and rearing animals in germ-free conditions substantially mitigates renal disease (Hallman et al, 2006). We also found many immune pathways to have significantly upregulated expression in livers of Pdss2 mutant mice, alterations that were largely reversed with probucol therapy (Supporting information File 5).
Probucol's well-described effects on lipid metabolism, which include reduction of LDL and HDL levels (Stocker, 2009), prompted us to investigate whether altered lipid metabolism that occurs in the setting of Pdss2-related CoQ deficiency correlates with their renal disease. Expression profiling in Pdss2 missense mutant liver demonstrates these animals do have aberrant lipid metabolism, involving upregulation of lipid biosynthesis and downregulation of fatty acid oxidation (Fig 3 and Supporting information File 10). Plasma lipid abnormalities have also been previously observed to develop with age in both missense and liver-conditional knockout Pdss2 mutants (Peng et al, 2008), and here we report that significant hypercholesterolemia occurs in podocyte-conditional Pdss2 knockouts (Table 3). Our data show that both plasma lipids (Table 3) and membrane phosphoplipids in liver (Supporting information File 2) are significantly decreased by probucol therapy. Thus, it is possible that the dramatic prevention of renal disease by probucol supplementation may relate to its role in reversing the lipid abnormalities caused by Pdss2 deficiency. Indeed, a combination of altered lipid metabolism and oxidative stress was recently shown to underlie puromysin aminonucleoside nephrosis. Downregulation of enzymes involved in antioxidant pathways and fatty acid oxidation was detected at the protein level in cultured podocytes from puromycin aminonucleoside-treated animals (Mayrhofer et al, 2009), similar to what we observed at the expression level for fatty acid oxidation in Pdss2 mutant mice. However, we found renal disease and cholesterol levels were both unaltered when Pdss2 mutants were treated long-term with a classic anti-hyperlipidemic agent that inhibits HMG-CoA reductase, Simvastatin. Furthermore, efficient anti-hyperlipidemic activity of probucol in mice without Pdss2 activity within renal glomerular podocytes was insufficient to rescue their severe nephritis. Thus, the mechanism(s) by which probucol prevents renal disease in missense mutants with reduced or no Pdss2 activity within podocytes (Peng et al, 2008) are likely not fully dependent on the drug's hypolipidemic effects.
Finally, we show here that the redox state of CoQ is altered in liver of older Pdss2kd/kd mice (Fig 6). Several reports have indicated that CoQH2/CoQ redox status may decrease in various disease states (Kontush et al, 1997; Lim et al, 2006; Mabuchi et al, 2005; Vasta et al, 2011; Wada et al, 2007). Such differences were not noted in our prior analyses of tissue CoQ levels in 180-day-old Pdss2kd/kd mice, as we had previously utilized an oxidizing pre-column electrode to oxidize hydroquinones and generate just the quinone species (Peng et al, 2008; Saiki et al, 2008). Thus, it will be important to monitor CoQH2/CoQ ratios as a function of disease progression and response to probucol in future studies of this model of FSGS-like renal disease.
It is interesting to compare Pdss2 mutants with the other mouse mutant whose kidney disease is ameliorated by probucol, the Mpv17 knockout mouse. Both models involve primary mitochondrial defects and have severe proteinuria caused by a kidney disease similar to FSGS. Kidney disease occurred before 6 months of age in Mpv17−/− mice that were initially identified as a result of insertional inactivation (Weiher et al, 1990). Subsequently, no trace of proteinuria or renal pathology could be observed through at least 12 months of age after backcrossing the transgene to C57BL/6 (Spinazzola et al, 2006), although the renal pathology was again observed when the observation period was extended to 2 years of age (Viscomi et al, 2009). Apparently, the original mixed CFW background enhanced the renal phenotype to a greater extent than did the inbred C57BL/6 background. The Pdss2kd/kd genotype has so far been studied only two backgrounds, CBA/CaJ and C57BL/6 (B6), which have appeared equivalent in their effects on the renal phenotype (unpublished observations). The fact that kidney disease in both Mpv17−/− and Pdss2kd/kd mice can be ameliorated by probucol is puzzling, but may relate to an exquisite sensitivity of glomerular podocytes to mitochondrial dysfunction. Precisely how probucol may remedy the effects of primary mitochondrial deficiency in renal podocytes remains to be determined.
Effects of chronic CoQ10 supplementation in primary CoQ deficiency
Primary CoQ deficiency in humans has been shown to represent a rare form of mitochondrial RC disease that has four major clinical presentations, which are often but not universally ameliorated by CoQ10 supplementation (Quinzii et al, 2007). As mice and humans differ in their major CoQ isoform (CoQ9 and CoQ10, respectively), it remains possible that the failure of high-dose CoQ10 supplementation to prevent or cause sustained improvement of disease manifestations in Pdss2 mutant mice may relate to the minor CoQ10 isoform we supplied. It may also relate to relatively low tissue bioavailability of the formulation used. While CoQ10 is generally a first-line supplement for all forms of human RC disease (Parikh et al, 2009), little in vivo evidence either supports or refutes its use despite concerns that CoQ10 plays an important role as both an antioxidant and pro-oxidant (Haas, 2007; Linnane et al, 2007). This study provides evidence that chronic high-dose CoQ10 supplementation of Pdss2 mutant mice in equivalent doses to that provided to many human patients does induce antioxidant defenses, as assessed by both activity and relative expression assays of the prime mitochondrial antioxidant defense enzyme, MnSOD (Fig 2). Aconitase activity as a marker of oxidative stress (Fig 2) and qualitative analysis of 4-HNE-based mitochondrial lipid peroxidation (Supporting information File 3) were unchanged in both untreated and CoQ10-treated mutant mice relative to age-matched controls. Future study will be required to determine whether increased oxidant scavenging capacity ultimately represents a pro-oxidant or antioxidant effect.
Metabolic pathway expression profiling as a tool to survey cellular consequences and treatment response in mitochondrial disease
Applying metabolic pathway clustering to global transcriptional profiling presents a powerful opportunity to reduce the complexity of describing metabolic regulation in mitochondrial disease. We previously reported that mice harbouring conditional homozygous hepatic deletion of Pdss2 have altered intermediary metabolism characterized by amino acid abnormalities and upregulated expression of 15 basic intermediary metabolic and cellular defense pathways (Peng et al, 2008). Here, we show that while these mice manifest mild but significant hyperglycemia, they do not have obvious abnormalities of liver transaminase (ALT) or synthetic function (urea; Supporting information File 4). We further now report that significantly altered hepatic expression of many metabolic pathways also occurs in spontaneous Pdss2 missense mutants (Fig 3). We conclude that while the transcriptional signatures of these two mutant types are in many ways distinct, several metabolic pathways are similarly upregulated in both missense and knockout Pdss2 mutants, including OXPHOS, protein processing, immune pathways and nucleotide metabolism. Future study of intermediary metabolic flux alterations by isotopic profiling in both mutant types will be necessary to dissect whether the direction, rather than mere occurrence, of transcriptional alterations in multiple metabolic pathways is indicative of a specific adaptive response to primary mitochondrial dysfunction. Interestingly, the direction of altered expression for multiple intermediary metabolic pathways directly correlated in both mutant types with the direction of altered PPAR pathway signaling, and in particular, with PPAR-γ expression (Figs 3 and 5).
Probucol supplementation largely reversed transcriptional alterations not only globally across many aspects of intermediary metabolism but also in PPAR pathway signaling (Fig 4 and Supporting information Files 5 and 11). Altered expression of key PPAR pathway transcription factors expressed in liver was confirmed by quantitative RT-PCR, showing that PPAR-γ was upregulated in the liver-conditional knockout mice while both PPAR-α and PPAR-γ were downregulated in missense mutants, all of which normalized on probucol therapy (Fig 5). While high-dose supplemental CoQ10 therapy normalized expression of several pathways in Pdss2 missense mutants, its effect was less pronounced than that of probucol (Fig 4). Furthermore, CoQ10 supplementation neither normalized PPAR signalling (Fig 5) nor prevented kidney disease (Table 1). PPAR pathway activation by oral bezafibrate therapy has also been demonstrated to improve a myopathy caused by mitochondrial complex IV dysfunction in a COX10 mutant mouse (Wenz et al, 2008). Our findings suggest that PPAR pathway signaling alterations may orchestrate the global metabolic consequences of primary mitochondrial RC dysfunction. It is possible that probucol enhances PPAR signaling in Pdss2 missense mice, which then induces CoQ content. Indeed, there is considerable evidence for PPAR-induction of CoQ biosynthesis in response to peroxisome proliferators (Turunen et al, 2004). Alternatively, the increase in CoQ (and CoQH2) levels in response to probucol may be the switch that elicits PPAR signalling. PPAR induction in response to CoQ10H2 supplementation has been reported in SAMP1 mice (Schmelzer et al, 2010).
Metabolic pathway profiling thus appears to offer a sensitive means to elucidate mechanisms and to monitor treatment effects in primary mitochondrial disease. Pathway expression profiling in individual human mitochondrial disease patients who have widely different genetic causes for their disease may offer a route to effectively tailor clinical treatment regimens. In particular, it would be of interest to discern whether the global metabolic and transcriptional sequelae of RC dysfunction we observed in the mouse model of Pdss2 deficiency (Peng et al, 2008) are evident in the sole reported Pdss2 patient cell line, and whether probucol has any discernible modifying effect. Furthermore, elucidation of common pathway alterations across different classes of RC dysfunction may permit the development of pathway-targeted therapies for the difficult to treat class of primary mitochondrial disorders (Parikh et al, 2009).
The paper explained
PROBLEM
Mitochondrial RC defects are inherited diseases for which effective therapy has remained elusive. Simply providing a missing metabolite frequently has no clinical efficacy even when the specific enzymatic defect is well defined. For example, while some children born with defective CoQ biosynthesis respond to CoQ10 supplementation, many do not. We study a mouse model of a fatal renal glomerular disease caused by primary CoQ deficiency. These Pdss2 mutant mice show only limited therapeutic response to lifelong CoQ10 supplementation. During our investigation of other potential therapeutic agents, we have discovered that probuocol, a lipid-soluble antioxidant drug with lipid-lowering and anti-inflammatory activities of uncertain mechanism previously used to treat hyperlipidemia, has considerably greater therapeutic efficacy for the renal and metabolic disease sequelae in these mice than CoQ10 supplementation. Understanding the basis for the effectiveness of this drug could provide a valuable lead towards designing novel therapies for human mitochondrial diseases.
RESULTS
We demonstrate that long-term oral administration of probucol prevented an otherwise lethal glomerulopathy in B6.Pdss2kd/kd mice through at least 6 months of life. By contrast, long-term prophylactic CoQ10 supplementation was comparatively less effective for renal disease prevention, despite its induction of mitochondrial oxidant scavenging capacity. Short-term administration of probucol limited to just 2–3 weeks before the onset of nephritis also prevented renal disease, whereas, short-term supplementation with CoQ10 had no effect in males and only a modest therapeutic effect in female mutant mice. Remarkably, probucol treatment also significantly mitigated renal disease when fed for only 2 weeks to already symptomatic B6.Pdss2kd/kd animals. Mechanistic investigations demonstrated that despite a modest induction of oxidative stress, probucol administration substantially prevented renal disease and largely reversed global metabolic pathway transcriptional abnormalities. Probucol's beneficial effects on the renal and metabolic manifestations of Pdss2 disease appeared to be independent of its hypolipidemic properties. However, when administered from birth through 6 months of life, probucol significantly increased both the hydroquinone (CoQ9H2 and CoQ10H2) and total CoQ9 and CoQ10 content in liver. Furthermore, treating symptomatic animals with probucol for only 2 weeks beginning at day of life 100 significantly increased CoQ9 levels in mutant kidney. Finally, we observed that the mitochondrial RC dysfunction caused by primary CoQ deficiency in Pdss2 mutant mice elicited aberrant expression of many intermediary metabolic pathways and PPAR transcription factor pathway signaling, which were all significantly rescued by probucol treatment.
IMPACT
These data suggest that decreased CoQ9 content and altered PPAR pathway signaling may orchestrate the glomerular and global metabolic consequences of primary CoQ deficiency, respectively. Remarkably, these renal and metabolic sequelae of primary mitochondrial RC dysfunction were both preventable and treatable with oral probucol therapy. Thus, targeting the de novo supply of CoQ may be a more effective therapeutic strategy than supplementing the missing co-factor.
Probucol is a lipophilic agent with previously reported antioxidant, anti-inflammatory and lipid-lowering properties (Stocker, 2009) that we found to prevent an otherwise lethal renal glomerular disease as well as expression alterations across many domains of intermediary metabolism in a Pdss2 mutant mouse model of mitochondrial RC disease caused by primary CoQ deficiency. The beneficial effects of probucol exceeded those of supplemental CoQ10, both in terms of ameliorating renal disease and increasing tissue CoQ content. Furthermore, probucol treatment reversed albuminuria in B6.Pdss2kd/kd missense mutant animals that already manifested renal disease. The renal efficacy of probucol appears to lie in its ability to increase tissue CoQ content, although it is not yet known whether this phenomenon is mediated through enhanced de novo synthesis or decreased degradation. Probucol's beneficial effects on the renal and metabolic manifestations of Pdss2 disease occur despite its modest induction of oxidant stress and appear to be independent of its hypolipidemic effects.
In summary, the data suggest that decreased CoQ9 content and altered PPAR pathway signaling orchestrate the glomerular and global metabolic consequences of primary CoQ deficiency, respectively, which are both preventable and treatable with oral probucol therapy. Thus, we conclude that it is more important to induce the de novo supply of CoQ in the Pdss2kd/kd CoQ deficient animals, than to directly supplement the missing co-factor (CoQ10).
MATERIALS AND METHODS
B6.Pdss2kd/kd missense mutant mice treatment groups
B6.Pdss2kd/kd mice were derived by backcrossing a recombinant chromosome derived by positional cloning onto the B6 background (Dell et al, 2000). B6.Pdss2kd/kd mice in this report were in the 13th backcross generation. Untreated mice were fed a standard LabDiet rodent chow obtained from PMI Nutrition International (Brentwood, MO). Probucol-treated mice were either given the same diet to which 1% w/w probucol was added (Animal Specialties and Provisions, Quakertown, PA), which we estimate resulted in a final daily dosage of approximately 95 mg per kg body weight. The gender, ages and lengths of time that different groups of mice were treated with probucol are shown in Tables 1 and 2, and Supporting information File 12. For the mice in Table 2, urine albumin was measured at 100 days of age and only those with significant albuminuria (>1 mg in 24 h) were treated with probucol. Mice fed diets supplemented with probucol from birth (Fig 6) were raised by mothers fed probucol supplemented diets 1% w/w until weaning, and, thereafter, fed a probucol supplemented 1% w/w chow diet. CoQ10-treated mice received a standard chow with CoQ10 (LiQsorb; Tishcon Corp., Westbury, NY) added to their drinking water from either birth, weaning or day of life 100, as specified in Table 1. The amount of CoQ10 added was 1 mg/ml, which we estimate resulted in a final daily dosage of approximately 400 mg CoQ10 per kg body weight. Vitamin E was provided from weaning in standard chow at a dose of 300 IU per kg chow (Animal Specialties and Provisions), which we estimate resulted in a final daily dosage of approximately 29 IU per kg body weight. Simvastatin treatment consisted of feeding mice chow with 0.1% w/w Simvastatin from weaning, which we estimate resulted in a final daily dosage of approximately 100 mg per kg body weight. All studies were carried out in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committees of The University of Pennsylvania and The Children's Hospital of Philadelphia.
Tissue-specific conditional Pdss2 knockout mice treatment groups
Liver-conditional and podocyte-conditional Pdss2 knockout mice were generated as previously described (Peng et al, 2008). Briefly, for liver-conditional knockout the mutation was targeted to hepatocytes utilizing mice homozygous for the floxed gene (B6.Pdss2loxP/loxP) crossed with partners that expressed cre under the control of an albumin/cre promoter (B6.Cg-Tg(Alb-cre)21 Mgn/J (Alb/cre)) obtained from The Jackson Laboratory. Two B6.Alb/cre,Pdss2loxP/loxP mice (one male and one female) were fed standard mouse chow supplemented with probucol (1% w/w) from weaning beginning on day of life 44 (Zhang et al, 2010). Untreated controls consisted of two B6.Alb/cre,Pdss2loxP/lox mice (one male and one female) fed standard mouse chow. Animals were sacrificed and liver specimens flash frozen for RNA extraction at 140–169-day old. Podocyte-specific knockouts (B6.Podocin/cre,Pdss2loxP/loxP) were obtained by crossing mice homozygous for the floxed gene with partners that expressed cre under the control of the Podocin promoter (Moeller et al, 2000). Probucol and CoQ10 supplementation were provided to podocyte-conditional knockouts from birth in the same fashion as described above for missense mutants. All procedures were carried out in accordance with NIH guidelines were approved by the Institutional Animal Care and Use Committee of both The University of Pennsylvania and The Children's Hospital of Philadelphia.
Kidney disease evaluation
B6.Pdss2kd/kd mice were placed in metabolic cages without food for 24 h with 0.45% NaCl and 2.5% sucrose in the drinking water. Total urine volumes were measured and aliquots of urine were tested for albumin concentration by enzyme-linked immunosorbent assay (ELISA). Upon termination of each experiment, mice were euthanized and their kidneys were fixed and stained with haematoxylin and eosin. Histologic sections were scored blindly according to the following scale: 0 = no tubular dilatation and no mononuclear cell infiltrates; 1 = small focal areas of cellular infiltration and tubular dilatation involving less than 10% of the cortex; 2 = involvement of up to 25% of the cortex; 3 = involvement of up to 50% of the cortex; and 4 = extensive damage involving more than 75% of the cortex.
Mechanistic investigations in Pdss2 mouse tissues
Detailed methods of all biochemical and genomic assays reported in this manuscript are described in Supporting information File 13.
Acknowledgments
We are grateful to Meera Rao, MS and Stephen Dingley, BA, for their assistance with RNA isolation and specimen handling; to Eric Rappaport, PhD, Stephen G. Mahoney, BS and Kristen Hunter, BS, for microarray hybridization; to Roger C. Helgeson, PhD, for synthesis of diethoxy-Q10; to Suzanne Wherli, PhD, for performance of 31P-NMR analysis; to Harry Ischiropoulos, PhD, for providing generous access to imaging and spectrofluorimetry equipment; and to our Anonymous Reviewers for their helpful suggestions. We thank the members of the Morphology Core for Molecular Studies in Digestive and Liver Diseases at The University of Pennsylvania for histologic preparations. This work was supported by the National Institutes of Health [R01-DK55852 to DLG, K08-DK073545 to MJF, R01-DK53761 to IN, GM45952 to CFC, and DK050306]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supporting information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
MJF and DLG conceived the study design; MP conducted genotyping and urine albumin assays; RK and JSM assisted with histologic analyses; LXX, JYC and BM wrote methods for and performed and analysed tissue CoQ levels; EP wrote methods for and performed qRT-PCR analyses, along with EO and MPEP wrote the methods for, and along with MP performed the MnSOD enzyme activity assay; EP wrote the methods for and performed both 4-HNE Western blot analysis and high-resolution polarography; ENO wrote the methods for and performed aconitase enzyme activity analysis; JO wrote the methods for and prepared liver specimens for 31P-NMR analysis; ZZ performed and assisted MJF in writing methods for and interpretation of microarray expression pathway analyses; OH and IN wrote the methods for and performed plasma glucose, ammonia and urea analyses; IN assisted with interpretation of lipid NMR data; MJF, DLG, BM and CFC wrote the manuscript.
For more information
Online Mendelian Inheritance in Man (OMIM)
Prenyl Diphosphate Synthase, Subunit 2
http://www.ncbi.nlm.nih.gov/omim/610564
Mitochondrial Medicine Society
Mitochondria Research Society
The United Mitochondrial Disease Foundation
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Barros MH, Johnson A, Gin P, Marbois BN, Clarke CF, Tzagoloff A. The Saccharomyces cerevisiae COQ10 gene encodes a START domain protein required for function of coenzyme Q in respiration. J Biol Chem. 2005;280:42627–42635. doi: 10.1074/jbc.M510768200. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Bernt E, Schidt F, Stork H. D-Glucose. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 1198–1201. [Google Scholar]
- Binder CJ, Weiher H, Exner M, Kerjaschki D. Glomerular overproduction of oxygen radicals in Mpv17 gene-inactivated mice causes podocyte foot process flattening and proteinuria: a model of steroid-resistant nephrosis sensitive to radical scavenger therapy. Am J Pathol. 1999;154:1067–1075. doi: 10.1016/S0002-9440(10)65359-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG, Bunney WE, Myers RM, Speed TP, Akil H, et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005;33:e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell KM, Li YX, Peng M, Neilson EG, Gasser DL. Localization of the mouse kidney disease (kd) gene to a YAC/BAC contig on Chromosome 10. Mamm Genome. 2000;11:967–971. doi: 10.1007/s003350010188. [DOI] [PubMed] [Google Scholar]
- Dingley S, Polyak E, Lightfoot R, Ostrovsky J, Rao M, Greco T, Ischiropoulos H, Falk MJ. Mitochondrial respiratory chain dysfunction variably increases oxidant stress in Caenorhabditis elegans. Mitochondrion. 2009;10:125–136. doi: 10.1016/j.mito.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund PO. Determination of coenzyme Q10, alpha-tocopherol and cholesterol in biological samples by coupled-column liquid chromatography with coulometric and ultraviolet detection. J Chromatogr. 1988;425:87–97. doi: 10.1016/0378-4347(88)80009-4. [DOI] [PubMed] [Google Scholar]
- Falk MJ, Zhang Z, Rosenjack JR, Nissim I, Daikhin E, Sedensky MM, Yudkoff M, Morgan PG. Metabolic pathway profiling of mitochondrial respiratory chain mutants in C. elegans. Mol Genet Metab. 2008;93:388–397. doi: 10.1016/j.ymgme.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Campos F, Vendrell I, Berenguer J, Barzi M, Pons S, Sunol C. Probucol increases glutathione peroxidase-1 activity and displays long-lasting protection against methylmercury toxicity in cerebellar granule cells. Toxicol Sci. 2009;112:416–426. doi: 10.1093/toxsci/kfp219. [DOI] [PubMed] [Google Scholar]
- Feldman PL, Lambert MH, Henke BR. PPAR modulators and PPAR pan agonists for metabolic diseases: The next generation of drugs targeting peroxisome proliferator-activated receptors. Curr Top Med Chem. 2008;8:728–749. doi: 10.2174/156802608784535084. [DOI] [PubMed] [Google Scholar]
- Gardner PR. Aconitase: sensitive target and measure of superoxide. Methods Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- Geyer JW, Dabich D. Rapid method for determination of arginase activity in tissue homogenates. Anal Biochem. 1971;39:412–417. doi: 10.1016/0003-2697(71)90431-3. [DOI] [PubMed] [Google Scholar]
- Haas RH. The evidence basis for coenzyme Q therapy in oxidative phosphorylation disease. Mitochondrion. 2007;7:S136–S145. doi: 10.1016/j.mito.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, Wong LJ, Cohen BH, Naviaux RK. The in-depth evaluation of suspected mitochondrial disease. Mol Genet Metab. 2008;94:16–37. doi: 10.1016/j.ymgme.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman TM, Peng M, Meade R, Hancock WW, Madaio MP, Gasser DL. The mitochondrial and kidney disease phenotypes of kd/kd mice under germfree conditions. J Autoimmun. 2006;26:1–6. doi: 10.1016/j.jaut.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen JK, Autio KJ, Schonauer MS, Kursu VA, Dieckmann CL, Kastaniotis AJ. Mitochondrial fatty acid synthesis and respiration. Biochim Biophys Acta. 2010;1797:1195–1202. doi: 10.1016/j.bbabio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovski O, Szumilak D, Nguyen-Khoa T, Nikolov IG, Joki N, Mothu N, Maizel J, Westenfeld R, Ketteler M, Lacour B, et al. Effect of simvastatin in apolipoprotein E deficient mice with surgically induced chronic renal failure. J Urol. 2008;179:1631–1636. doi: 10.1016/j.juro.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Kontush A, Reich A, Baum K, Spranger T, Finckh B, Kohlschutter A, Beisiegel U. Plasma ubiquinol-10 is decreased in patients with hyperlipidaemia. Atherosclerosis. 1997;129:119–126. doi: 10.1016/s0021-9150(96)06021-2. [DOI] [PubMed] [Google Scholar]
- Lemieux H, Gneiger E. Preparation of permeabilized muscle fibers for diagnosis of mitochondrial respiratory function. Mitochondrial Physiol Netw. 2008;12.22:1–4. [Google Scholar]
- Lemieux H, Votion M-D, Gneiger E. Mitochondrial respiration in permeabilized fibers: needle Biopsies from horse skeletal muscle. Mitochondrial Physiol Netw. 2009;12.23:1–4. [Google Scholar]
- Lim SC, Tan HH, Goh SK, Subramaniam T, Sum CF, Tan IK, Lee BL, Ong CN. Oxidative burden in prediabetic and diabetic individuals: evidence from plasma coenzyme Q(10) Diabet Med. 2006;23:1344–1349. doi: 10.1111/j.1464-5491.2006.01996.x. [DOI] [PubMed] [Google Scholar]
- Linnane AW, Eastwood H. Cellular redox poise modulation; the role of coenzyme Q10, gene and metabolic regulation. Mitochondrion. 2004;4:779–789. doi: 10.1016/j.mito.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Linnane AW, Kios M, Vitetta L. Coenzyme Q(10)—its role as a prooxidant in the formation of superoxide anion/hydrogen peroxide and the regulation of the metabolome. Mitochondrion. 2007;7:S51–S61. doi: 10.1016/j.mito.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Lopez LC, Schuelke M, Quinzii CM, Kanki T, Rodenburg RJ, Naini A, Dimauro S, Hirano M. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet. 2006;79:1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF, Hulse EV. An inherited kidney disease of mice resembling human nephronophthisis. J Med Genet. 1971;8:41–48. doi: 10.1136/jmg.8.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi H, Higashikata T, Kawashiri M, Katsuda S, Mizuno M, Nohara A, Inazu A, Koizumi J, Kobayashi J. Reduction of serum ubiquinol-10 and ubiquinone-10 levels by atorvastatin in hypercholesterolemic patients. J Atheroscler Thromb. 2005;12:111–119. doi: 10.5551/jat.12.111. [DOI] [PubMed] [Google Scholar]
- Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- Madaio MP, Ahima RS, Meade R, Rader DJ, Mendoza A, Peng M, Tomaszewski JE, Hancock WW, Gasser DL. Glomerular and tubular epithelial defects in kd/kd mice lead to progressive renal failure. Am J Nephrol. 2005;25:604–610. doi: 10.1159/000089709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbois B, Xie LX, Choi S, Hirano K, Hyman K, Clarke CF. Para-aminobenzoic acid is a precursor in coenzyme Q6 biosynthesis in Saccharomyces cerevisiae. J Biol Chem. 2010;285:27827–27838. doi: 10.1074/jbc.M110.151894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer C, Krieger S, Huttary N, Chang MW, Grillari J, Allmaier G, Kerjaschki D. Alterations in fatty acid utilization and an impaired antioxidant defense mechanism are early events in podocyte injury: a proteomic analysis. Am J Pathol. 2009;174:1191–1202. doi: 10.2353/ajpath.2009.080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Moeller MJ, Kovari IA, Holzman LB. Evaluation of a new tool for exploring podocyte biology: mouse Nphs1 5′ flanking region drives LacZ expression in podocytes. J Am Soc Nephrol. 2000;11:2306–2314. doi: 10.1681/ASN.V11122306. [DOI] [PubMed] [Google Scholar]
- Nissim I, Weinberg JM. Glycine attenuates Fanconi syndrome induced by maleate or ifosfamide in rats. Kidney Int. 1996;49:684–695. doi: 10.1038/ki.1996.97. [DOI] [PubMed] [Google Scholar]
- Nissim I, Luhovyy B, Horyn O, Daikhin Y, Yudkoff M. The role of mitochondrially bound arginase in the regulation of urea synthesis: studies with [U-15N4]arginine, isolated mitochondria, and perfused rat liver. J Biol Chem. 2005;280:17715–17724. doi: 10.1074/jbc.M500607200. [DOI] [PubMed] [Google Scholar]
- Parikh S, Saneto R, Falk MJ, Anselm I, Cohen BH, Haas R, Medicine Society TM A modern approach to the treatment of mitochondrial disease. Curr Treat Options Neurol. 2009;11:414–430. doi: 10.1007/s11940-009-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JR, Rumley AG, Oldroyd KG, Tait GW, Smellie WS, Packard CJ, Shepherd J, Lorimer AR. Probucol reduces plasma lipid peroxides in man. Atherosclerosis. 1992;97:63–66. doi: 10.1016/0021-9150(92)90051-h. [DOI] [PubMed] [Google Scholar]
- Peng M, Jarett L, Meade R, Madaio MP, Hancock WW, George AL, Jr, Neilson EG, Gasser DL. Mutant prenyltransferase-like mitochondrial protein (PLMP) and mitochondrial abnormalities in kd/kd mice. Kidney Int. 2004;66:20–28. doi: 10.1111/j.1523-1755.2004.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Falk MJ, Haase VH, King R, Polyak E, Selak M, Yudkoff M, Hancock WW, Meade R, Saiki R, et al. Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 2008;4:e1000061. doi: 10.1371/journal.pgen.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii CM, Hirano M. Coenzyme Q and mitochondrial disease. Dev Disabil Res Rev. 2010;16:183–188. doi: 10.1002/ddrr.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii CM, DiMauro S, Hirano M. Human coenzyme Q10 deficiency. Neurochem Res. 2007;32:723–727. doi: 10.1007/s11064-006-9190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii CM, Lopez LC, Von-Moltke J, Naini A, Krishna S, Schuelke M, Salviati L, Navas P, DiMauro S, Hirano M. Respiratory chain dysfunction and oxidative stress correlate with severity of primary CoQ10 deficiency. FASEB J. 2008;22:1874–1885. doi: 10.1096/fj.07-100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose IA, O'Connell EL. Mechanism of aconitase action. I. The hydrogen transfer reaction. J Biol Chem. 1967;242:1870–1879. [PubMed] [Google Scholar]
- Saiki R, Nagata A, Kainou T, Matsuda H, Kawamukai M. Characterization of solanesyl and decaprenyl diphosphate synthases in mice and humans. FEBS J. 2005;272:5606–5622. doi: 10.1111/j.1742-4658.2005.04956.x. [DOI] [PubMed] [Google Scholar]
- Saiki R, Lunceford AL, Shi Y, Marbois B, King R, Pachuski J, Kawamukai M, Gasser DL, Clarke CF. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am J Physiol Renal Physiol. 2008;295:F1535–F1544. doi: 10.1152/ajprenal.90445.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Ren M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim Biophys Acta. 2009;1788:2080–2083. doi: 10.1016/j.bbamem.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer C, Kubo H, Mori M, Sawashita J, Kitano M, Hosoe K, Boomgaarden I, Doring F, Higuchi K. Supplementation with the reduced form of Coenzyme Q10 decelerates phenotypic characteristics of senescence and induces a peroxisome proliferator-activated receptor-alpha gene expression signature in SAMP1 mice. Mol Nutr Food Res. 2010;54:805–815. doi: 10.1002/mnfr.200900155. [DOI] [PubMed] [Google Scholar]
- Spinazzola A, Viscomi C, Fernandez-Vizarra E, Carrara F, D'Adamo P, Calvo S, Marsano RM, Donnini C, Weiher H, Strisciuglio P, et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet. 2006;38:570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- Stocker R. Molecular mechanisms underlying the antiatherosclerotic and antidiabetic effects of probucol, succinobucol, and other probucol analogues. Curr Opin Lipidol. 2009;20:227–235. doi: 10.1097/MOL.0b013e32832aee68. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Cote G, Lesperance J, Bourassa M, Lambert J, Doucet S, Bilodeau L, Nattel S, de Guise P. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med. 1997;337:365–372. doi: 10.1056/NEJM199708073370601. [DOI] [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Vasta V, Sedensky M, Morgan P, Hahn SH. Altered redox status of coenzyme Q9 reflects mitochondrial electron transport chain deficiencies in Caenorhabditis elegans. Mitochondrion. 2011;11:136–138. doi: 10.1016/j.mito.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Viscomi C, Spinazzola A, Maggioni M, Fernandez-Vizarra E, Massa V, Pagano C, Vettor R, Mora M, Zeviani M. Early-onset liver mtDNA depletion and late-onset proteinuric nephropathy in Mpv17 knockout mice. Hum Mol Genet. 2009;18:12–26. doi: 10.1093/hmg/ddn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Goto H, Hagiwara S, Yamamoto Y. Redox status of coenzyme Q10 is associated with chronological age. J Am Geriatr Soc. 2007;55:1141–1142. doi: 10.1111/j.1532-5415.2007.01209.x. [DOI] [PubMed] [Google Scholar]
- Weiher H, Noda T, Gray DA, Sharpe AH, Jaenisch R. Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell. 1990;62:425–434. doi: 10.1016/0092-8674(90)90008-3. [DOI] [PubMed] [Google Scholar]
- Wenz T, Diaz F, Spiegelman BM, Moraes CT. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab. 2008;8:249–256. doi: 10.1016/j.cmet.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, Croft KD, Mori TA, Tanous D, Adams MR, et al. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006;203:1117–1127. doi: 10.1084/jem.20052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Li GP, Liu T, Liu HM, Chen X, He M, Zheng XT, Liu EZ, Zhou LJ. Role of probucol in preventing contrast induced acute kidney injury after coronary interventional procedure: a randomized trial. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37:385–388. [PubMed] [Google Scholar]
- Zampol MA, Busso C, Gomes F, Ferreira-Junior JR, Tzagoloff A, Barros MH. Over-expression of COQ10 in Saccharomyces cerevisiae inhibits mitochondrial respiration. Biochem Biophys Res Commun. 2010;402:82–87. doi: 10.1016/j.bbrc.2010.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Gasser DL, Rappaport EF, Falk MJ. Cross-platform expression microarray performance in a mouse model of mitochondrial disease therapy. Mol Genet Metab. 2010;99:309–318. doi: 10.1016/j.ymgme.2009.10.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SX, Zhou Y, Zhang YL, Lei J, Wang JF. Antioxidant probucol attenuates myocardial oxidative stress and collagen expressions in post-myocardial infarction rats. J Cardiovasc Pharmacol. 2009;54:154–162. doi: 10.1097/FJC.0b013e3181af6d7f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.