Abstract
Background
There is conflicting evidence regarding the effects of breast-feeding on maternal mortality from human immunodeficiency virus type 1 (HIV-1) infection, and little is known about the effects of breast-feeding on markers of HIV-1 disease progression.
Methods
HIV-1–seropositive women were enrolled during pregnancy and received short-course zidovudine. HIV-1 RNA levels and CD4 cell counts were determined at baseline and at months 1, 3, 6, 12, 18, and 24 postpartum and were compared between breast-feeding and formula-feeding mothers.
Results
Of 296 women, 98 formula fed and 198 breast-fed. At baseline, formula-feeding women had a higher education level and prevalence of HIV-1–related illness than did breast-feeding women; however, the groups did not differ with respect to CD4 cell counts and HIV-1 RNA levels. Between months 1 and 24 postpartum, CD4 cell counts decreased 3.9 cells/µL/month (P< .001), HIV-1 RNA levels increased 0.005 log10 copies/mL/month (P = .03), and body mass index (BMI) decreased 0.03 kg/m2/month (P< .001). The rate of CD4 cell count decline was higher in breast-feeding mothers (7.2 cells/µL/month) than in mothers who never breast-fed (4.0 cells/µL/month) (P = .01). BMI decreased more rapidly in breast-feeding women (P = .04), whereas HIV-1 RNA levels and mortality did not differ significantly between breast-feeding and formula-feeding women.
Conclusions
Breast-feeding was associated with significant decreases in CD4 cell counts and BMI. HIV-1 RNA levels and mortality were not increased, suggesting a limited adverse impact of breast-feeding in mothers receiving extended care for HIV-1 infection.
During the past several years, there has been substantial effort to determine the association between infant feeding and postnatal maternal health in HIV-1–infected women. The first study on this subject, conducted in Nairobi, Kenya, suggested that breast-feeding could be detrimental to HIV-1–infected women [1]. In that randomized clinical trial, HIV-1–infected women randomized to breast-feed had a significantly increased risk of maternal mortality, compared with women randomized to formula feed. This observation suggested that metabolic, immunologic, or hormonal changes associated with breast-feeding may accelerate HIV-1 disease progression in postpartum mothers. However, 4 subsequent studies conducted in Tanzania, Zambia, South Africa, and Zimbabwe did not find significant differences in mortality risks attributable to breast-feeding among HIV-1–infected mothers [2–5].
Current policies on infant feeding in the context of HIV-1 infection are based entirely on infant factors [6]. It is important to concurrently consider the potential effects of the mode of infant feeding on maternal health, because maternal health is an important independent outcome and because maternal survival and good health positively influence child survival and development. When a mother dies, the risk of her <5-year-old child dying more than triples, and when a mother is sick, care of her child is compromised [1, 7]. In developing countries, breast-feeding by HIV-1–infected women is the norm because of factors that hamper formula feeding, such as stigma, expense, and safety [8, 9]. It is therefore important to obtain comprehensive information to guide the counseling of HIV-1–infected mothers with respect to infant feeding.
To date, studies of the effects of breast-feeding on maternal HIV-1 disease progression have focused on the risk of mortality and have had different strengths and limitations. Our previous Nairobi study [1] reported a 3-fold increase in mortality risk associated with breast-feeding and is the only study that has had the benefit of random allocation to breast-feeding versus formula feeding. It has been noted that, in that cohort, the breast-feeding women had nonsignificantly higher baseline HIV-1 loads and that the formula-feeding women may have had more interaction with the clinical team than did the breast-feeding women. Although these differences may have led to increased mortality in the breast-feeding arm, the randomized study design was the optimal way to overcome potential bias [1, 10]. The 4 subsequent studies that failed to find adverse effects of breast-feeding on maternal survival during the postpartum follow-up period were observational cohort studies. Two of these studies included only breast-feeding women, with no non–breast-feeding comparison group [3, 4]. In the South African and Tanzanian cohorts—and in a large meta-analysis compiling data from several African studies—there was evidence of selection bias, with sicker mothers opting either not to breastfeed or to shorten the duration of breast-feeding, compared with those in better health [2, 4, 5]. This selection bias may have confounded the outcome assessment. Four of the 5 studies solely evaluated mortality as the outcome measure reflecting HIV-1 disease progression. In all of the studies, assessment of maternal outcomes was post hoc, following cohorts designed primarily for assessment of mother-to-child HIV-1 transmission. Hence, we conducted a study to prospectively compare immunological and viral markers of HIV-1 disease progression, as well as the risk of mortality between breast-feeding and formula-feeding HIV-1–infected women.
METHODS
Study setting and population
A prospective cohort study was conducted in Nairobi, Kenya, from October 2000 to June 2005. Pregnant women attending 4 Nairobi City Council clinics were offered counseling and HIV-1 screening before 28 weeks of gestation, and HIV-1–seropositive women were referred to the study clinic at Kenyatta National Hospital. Women were eligible to participate in the study if they planned to reside in Nairobi for 2 years after delivery. Written, informed consent was obtained from all subjects. The human-experimentation guidelines of the US Department of Health and Human Services were followed, and approval was obtained from the Institutional Review Board of the University of Washington and the Ethical Review Committee the Kenya Medical Research Institute.
Clinical procedures
At enrollment, information on sociodemographic characteristics and medical history was collected using a standardized questionnaire. Women were counseled on safe infant-feeding options and were given at least 2 weeks to decide on how they planned to feed their infants. Women were informed of the availability of free formula and the potential risks of breast-milk transmission of HIV-1 to the baby. Depending on each mother’s feeding choice, further relevant guidance was provided to minimize the risks associated with each method.
All women received standard antenatal care as well as short-course zidovudine (Centers for Disease Control and Prevention Thai regimen) to reduce the risk of HIV-1 transmission to the baby [11]. At ~32 weeks of gestation, a physical examination was conducted, and blood was collected for measurement of CD4 cell counts, hemoglobin levels, and HIV-1 RNA levels. At delivery, blood was collected for measurement of HIV-1 RNA levels. Bioimpedance measurements were taken using a body cell mass analyzer (Biodynamics model 550; Biodynamics) at month 1 postpartum, to determine body cell mass and phase angle.
During the first postpartum year, women attended monthly clinic visits, during which they were assessed for intercurrent illnesses by use of a standard review tool. During the second postpartum year, women were followed quarterly. At months 1, 3, 6, 9, 12, 18, and 24 postpartum, weight and mid-arm circumference were assessed, and blood was collected for measurement of CD4 cell counts and HIV-1 RNA levels. All women received iron and multivitamin supplementation during the first 6 postpartum months. Women with severe immunosuppression (defined as a CD4 cell count <200 cells/µL) were provided with cotrimoxazole prophylaxis and were referred to HIV-1 treatment programs. After 2003, when highly active antiretroviral therapy (HAART) became available either free or at a highly subsidized cost, women referred to treatment programs received access to HAART.
Laboratory procedures
Lymphocyte counts and CD4 cell percentages were measured at the University of Nairobi by use of a FACScan flow cytometer (Becton Dickinson). Plasma HIV-1 RNA levels were quantified at the Fred Hutchinson Cancer Research Center by use of transcription-mediated amplification assays developed by Gen-Probe; these assays have been shown to quantify the subtypes of HIV-1 prevalent in Kenya [12].
Statistical analysis
Mothers who breast-fed at any time during follow-up were classified as breast-feeders, and those who never breast-fed were classified as formula feeders. Outcome measurements included changes in CD4 cell counts, HIV-1 RNA levels, and mortality. All data were analyzed using SPSS for Windows (version 11.5; SPSS Institute) and R (version 2.3.1; R Development Core Team; available at: http://www.r-project.org/). Maternal baseline characteristics were summarized using means for continuous variables and proportions for categorical variables. Continuous variables were compared using Student’s t tests for means, and categorical variables were compared using χ2 tests.
To examine nonlinear trends over time prenatally to 24 months postpartum in CD4 cell count, HIV-1 RNA level, and body mass index (BMI; calculated as weight in kilograms divided by the square of height in meters), we fit a linear mixed-effects model with cubic B splines with 2 interior knots and random intercepts and coefficients for the bases [13]. To assess the postpartum effects of breast-feeding on maternal health, we modeled CD4 cell count, HIV-1 RNA load, and BMI as predicted by time, duration of breast-feeding, and breast-feeding status and controlled for potential confounding variables by use of a linear mixed-effects model with random intercepts and slopes for time. We used the following fixed-effects model when adjusting for cumulative time of breast-feeding:
where yt is the value of the outcome variable at time t, BF indicates whether the subject ever breast-fed, ts is the time at which a subject weaned (0 for those who never breast-fed), and (t − ts)+ = t − ts when t> ts and 0 otherwise (i.e., time since weaning). The average change in y for a unit change in t is β1 while breast-feeding, β1+ β2 + β4 after breast-feeding, and β1 + β2 for those who never breast-fed. Confidence intervals and P values comparing specific groups were calculated using the bootstrap method.
Cox regression analysis and Kaplan-Meier models were used to compare breast-feeding and formula-feeding women for time-dependent outcomes. These outcomes of interest included time to death, time to death or HAART initiation, and time to CD4 cell count <200 cells/µL during the postpartum period.
RESULTS
Baseline characteristics of women
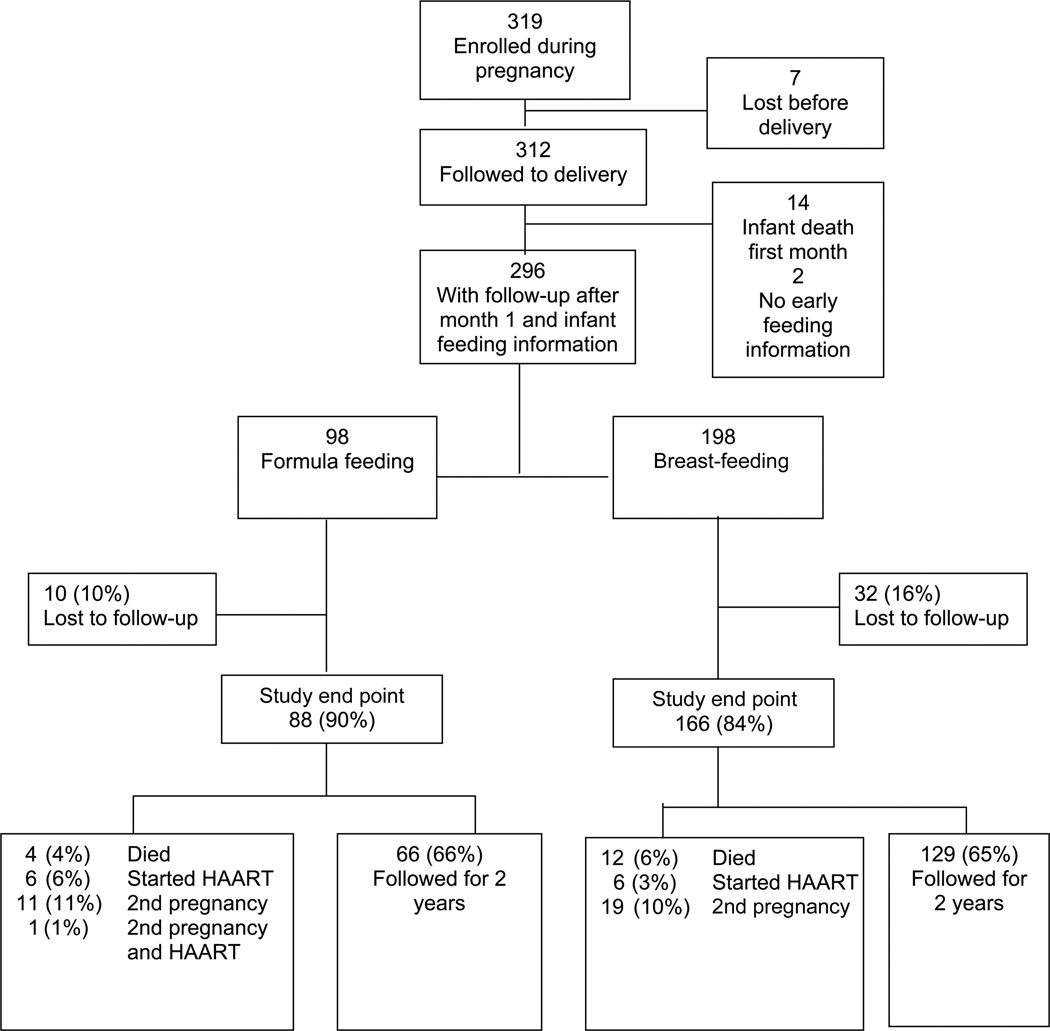
Of 319 women enrolled, 312 (98%) were followed to delivery, and 296 (93%) had information on infant feeding and CD4 cell counts (figure 1). Ninety-eight women (33%) elected to formula feed their infants, and 198 (67%) elected to breast-feed them. The median age of the women was 25 years, and the majority (90%) were married (table 1). The median duration of education for the cohort was 8 years, with a significantly larger proportion of the formula-feeding women having attained education beyond the primary level than the breast-feeding women (59% vs. 36%; P = .0003).
Figure 1.
Flow chart outlining maternal follow-up from pregnancy to 2 years postpartum
Table 1.
Characteristics of women at enrollment during pregnancy (baseline), delivery, and month 1 postpartum.
| Category, characteristic | Formula feeders (n = 98) |
Breast-feeders (n = 198) |
P |
|---|---|---|---|
| Sociodemographics | |||
| Age, years | 26.2 (25.4 to 27.0) | 25.4 (24.8 to 26.0) | .13 |
| Ever married, no. (%) | 86 (88) | 182 (92) | .35 |
| More than primary education, no. (%) | 58 (59) | 72 (36) | .00032 |
| Residential conditions | |||
| Living in 1 room, no. (%) | 68 (69) | 148 (75) | .40 |
| Use flush toilet, no. (%) | 61 (62) | 88 (44) | .006 |
| Room density, no. of people/room | 2.9 (2.7 to 3.1) | 3.2 (3.0 to 3.3) | .10 |
| Sexual and obstetric history | |||
| Age at first sex, years | 18.0 (17.5 to 18.6) | 17.4 (17.1 to 17.7) | .054 |
| No. of lifetime sex partners (nff = 97) | 4.0 (2.0 to 6.0) | 3.3 (2.7 to 3.8) | .48 |
| Primigravid mothers, no. (%) (nbf = 194) | 20 (20) | 36 (19) | .82 |
| Ever used contraceptives, no. (%) (nff = 97, nbf = 192) | 42 (43) | 70 (36) | .32 |
| Medical history | |||
| History of STI, no. (%) | 17 (17) | 26 (13) | .43 |
| History of tuberculosis, no. (%) | 10 (10) | 18 (9) | .92 |
| HIV-1–related illness,a no. (%) | 28 (29) | 35 (18) | .045 |
| Laboratory measurements | |||
| During pregnancy (32 weeks of gestation) | |||
| WBC count, 103 cells/µL (nff = 95, nbf = 195) | 6.3 (5.9 to 6.6) | 6.2 (6.0 to 6.4) | .75 |
| Lymphocyte percentage (nff = 95, nbf = 195) | 32.1 (30.3 to 33.9) | 32.1 (31.0 to 33.3) | .98 |
| CD4 cell count, cells/µL (nff = 95, nbf = 195) | 467 (410 to 525) | 465 (428 to 501) | .97 |
| CD4 cell percentage (nff = 95, nbf = 195) | 23.2 (21.3 to 25.0) | 23.4 (22.1 to 24.6) | .93 |
| CD8 cell percentage (nff = 95, nbf = 195) | 48.2 (45.7 to 50.6) | 47.3 (45.6 to 49.0) | .57 |
| Hb level, g/dL (nff = 98, nbf = 196) | 10.6 (10.3 to 10.9) | 10.4 (10.2 to 10.6) | .37 |
| CD4 cell count <200 cells/µL, no. (%) (nff = 95, nbf = 195) | 10 (11) | 26 (13) | .62 |
| HIV-1 RNA level, log10 copies/mL (nff = 89, nbf = 188) | 4.7 (4.5 to 4.8) | 4.7 (4.5 to 4.8) | .96 |
| Weight, kg (nbf = 194) | 64.5 (62.4 to 66.6) | 63.8 (62.6 to 65.1) | .59 |
| BMI, kg/m2 (nff = 95, nbf = 193) | 24.8 (24.2 to 25.4) | 24.8 (24.4 to 25.2) | .95 |
| At delivery | |||
| HIV-1 RNA level, log10 copies/mL (nff = 84, nbf = 157) | 4.0 (3.8 to 4.2) | 4.0 (3.9 to 4.2) | .91 |
| Change from 32 weeks of gestation to month 1 postpartum | |||
| CD4 cell count, cells/µL | 49.5 (−1.5 to 100.5) | 76.2 (51.1 to 101.4) | .35 |
| HIV-1 RNA level, log10 copies/mL | 0.04 (−0.15 to 0.22) | 0.14 (0.04 to 0.23) | .35 |
| BMI, kg/m2 | −1.6 (−1.8 to −1.4) | −1.6 (−1.8 to −1.3) | .79 |
NOTE. Data are mean (95% confidence interval) values, unless otherwise indicated. BMI, body mass index; Hb, hemoglobin; nbf, sample size for breast-feeders; nff, sample size for formula feeders; STI, sexually transmitted infection; WBC, white blood cell.
HIV-1–related illness was defined as fever >1 month, oral thrush, weight loss >5 kg, chronic cough, or persistent diarrhea.
The formula feeders were more likely to have a flush toilet at home than were the breast-feeders (62% vs. 44%; P = .006). The formula feeders and breast-feeders were similar with respect to reported number of lifetime sex partners, parity, and contraceptive use. The formula-feeding women were more likely to report a history of HIV-1–related illness (fever >1 month, oral thrush, weight loss >5 kg, chronic cough, or persistent diarrhea) than were their breast-feeding counterparts (29% vs. 18%; P = .045). Overall, 43 women (15%) reported a history of sexually transmitted infection, and 28 (10%) had had tuberculosis during the previous year; however, there were no significant differences in the prevalences of these conditions between the 2 groups.
Immunologic, virologic, and nutritional parameters at baseline
At 32 weeks of gestation, the mean CD4 cell count was 467 cells/µL among formula feeders and 465 cells/µL among breast-feeders (P = .97) (table 1). HIV-1 RNA levels were 4.7 log 10 copies/µL among both formula feeders and breast-feeders (P = .96). At delivery, HIV-1 RNA levels decreased after short-course zidovudine in both groups, to 4.0 log 10 copies/µL. The mean hemoglobin level for the cohort was 10.6 g/dL and did not differ significantly between the groups (P = .37). Changes in CD4 cell counts, HIV-1 RNA levels, and BMI between 32 weeks of gestation and month 1 postpartum were not significantly different between the formula feeding and breast-feeding women (table 1).
Follow-up in the cohort
Follow-up after delivery is out-lined in figure 1. Over the 2-year postpartum follow-up period, 10% of formula feeders and 16% of breast-feeders were lost to follow-up (P = .23). Women lost to follow-up had nonsignificantly higher CD4 cell counts at baseline than did those who remained in follow-up (533 vs. 454 cells/µL; P = .17). For analyses of disease markers, data were censored if women started HAART or had a second pregnancy. Sixty-six percent of the formula-feeding women and 65% of the breast-feeding women were followed for 2 years.
CD4 cell count, HIV-1 RNA level, BMI, and mortality during the postpartum period and postpartum differences in HIV-1 disease progression markers between breast-feeding and formula-feeding mothers
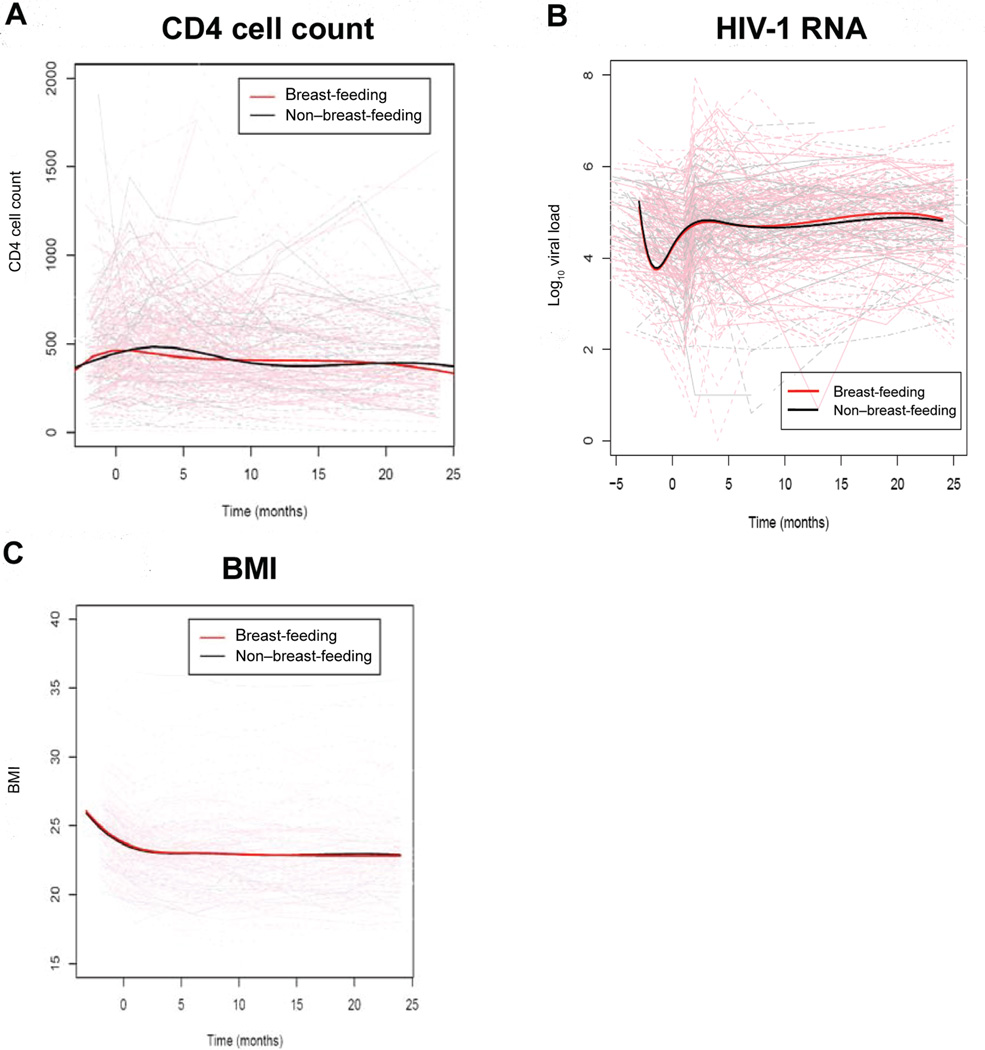
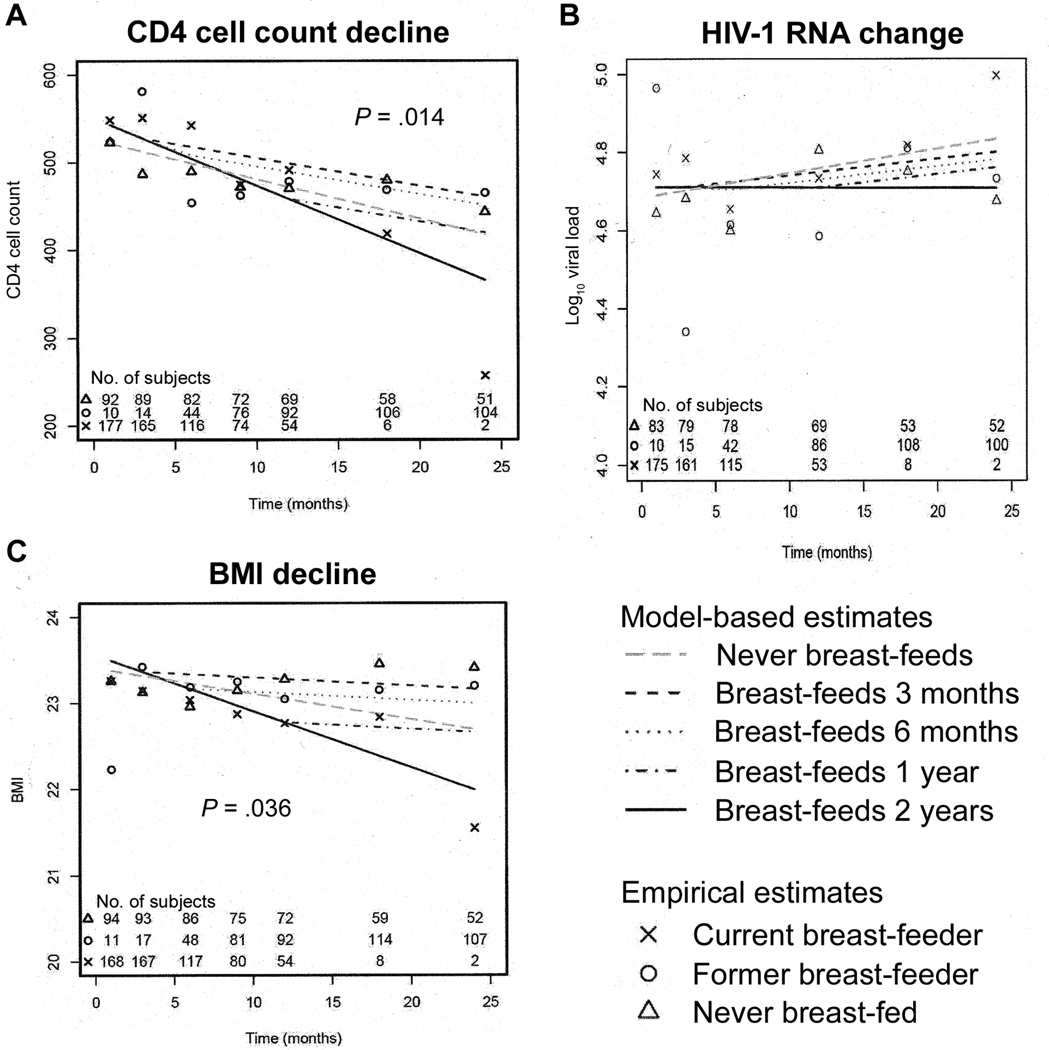
In linear-mixed effects models for all women, CD4 cell counts declined 3.9 cells/µL/month between months 1 and 24 (P< .001), and CD4 cell percentages declined 0.11% per month (P< .001). In spline models adjusting for baseline covariates that differed between the breast-feeding and formula-feeding women, CD4 cell counts increased in both the breast-feeding and formula-feeding women during the first month postpartum, after which CD4 cell counts decreased without a significant difference between the 2 groups during the 2-year postpartum follow-up period (figure 2A). To overcome the limitation of the spline models, which allocated women as ever versus never breast-feeder and, therefore, did not take into account duration of breast-feeding, mixed-effects models that adjusted for baseline confounding variables and that incorporated duration of breast-feeding were used to estimate differences between current, former, and never breast-feeding mothers (figure 3 and table 2). The current breast-feeding mothers had a significantly higher rate of CD4 cell count decline than did the mothers who never breast-fed (−7.7 vs. −4.4 cells/µL/month; P = .014) (figure 3A and table 2). Lines illustrating the rates of decline among women who ceased breast-feeding after 3, 6, and 12 months versus those who never and whose who continued breast-feeding are shown in figure 3A. After cessation of breast-feeding, former breast-feeders had a significantly lower rate of CD4 cell count decline (−3.2 cells/µL/month) than did current breast-feeders (P = .003) and a rate similar to that of never breast-feeders (P = .3).
Figure 2.
Spline models of CD4 cell counts (A), HIV-1 RNA levels (B), and body mass index (BMI) (C) during 2 years of follow-up. Solid bold lines indicate summary data from spline models for breast-feeding and non–breast-feeding mothers, and faint lines indicate the change in the parameter for each woman.
Figure 3.
Linear mixed-effects models of CD4 cell count (A), HIV-1 RNA level (B), and body mass index (BMI) (C) during 2 years of follow-up among current breast-feeders, former breast-feeders, and never breast-feeders. Differences between the current and never breast-feeders were significant for CD4 cell count and BMI decline.
Table 2.
Change per month in outcomes, determined on the basis of linear mixed-effects models adjusting for baseline confounding variables.
| Breast-feeders |
|||
|---|---|---|---|
| Outcome | Never breast-fed | Current | Former |
| CD4 cell count, cells/µL/month | −4.4 (−6.0 to −2.7) | −7.7 (−9.8 to −5.6)a | −3.2 (−4.9 to −1.5) |
| HIV-1 RNA levels, log10 copies/mL/month | 0.006 (0.000 to 0.012) | 0.000 (−0.008 to 0.008) | 0.004 (−0.002 to 0.010) |
| BMI, kg/m2/month | −0.027 (−0.050 to −0.038) | −0.065 (−0.092 to −0.038)a | −0.011 (−0.031 to 0.009) |
NOTE. Data are mean (95% confidence intervals [CIs]) values. Baseline confounding variables were CD4 cell count, HIV-1 RNA level, body mass index (BMI), history of HIV-1–related illness, room density, years of education, marital status, age at first sex, and type of toilet. CIs were estimated using the bootstrap method.
Significant difference in change over time compared with those who never breast-fed.
In linear mixed-effects models for the overall cohort, HIV-1 RNA levels increased 0.005 log10 copies/month between months 1 and 24 postpartum (P = .04). In spline models adjusting for baseline confounding variables, HIV-1 RNA levels decreased from baseline to delivery after short-course zidovudine and increased to predelivery levels subsequently. After delivery, HIV-1 RNA levels changed minimally during the 2-year follow-up period, without discernable differences between the ever and never breast-feeding mothers (figure 2B). Mixed-effects models incorporating duration of feeding indicated no significant differences in HIV-1 RNA levels between current, former, or never breast-feeding mothers (figure 3B and table 2).
In linear mixed-effects models for the overall cohort, BMI decreased 0.03 kg/m2/month between months 1 and 24 postpartum (P< .0001). In spline models, the ever and never breast-feeding mothers had minimal differences in BMI decline (figure 2C). However, in mixed-effects models incorporating duration of breast-feeding, BMI decline was significantly higher in the current versus never breast-feeding mothers (−0.065/month vs. −0.027/month; P = .036). Body cell mass, body cell mass percentage, and phase angle did not differ between the ever and never breast-feeding mothers in either the spline models or the mixed-effects models (data not shown).
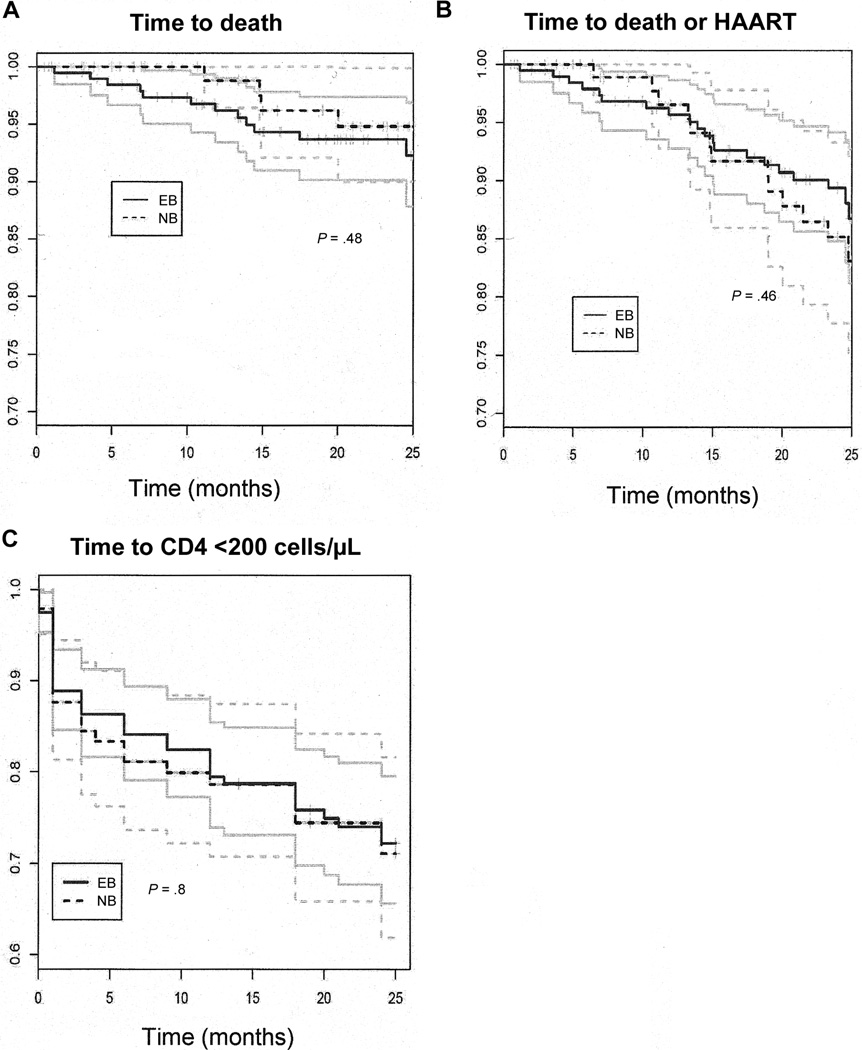
During the 2-year follow-up period, 4 (4%) of the women who never breast-fed died, all from HIV-1–related causes, and 12 (6%) of the women who ever breast-fed died, 8 from HIV-1–related causes and 4 from unknown causes. Mortality was significantly associated with baseline CD4 cell count (hazard ratio [HR] of 2.7 per 100-cell/µL decrease in CD4 cell count; P< .001). Women with baseline CD4 cell counts <200 cells/µL had an HR of 1.7 (P = .002) for death during the 2-year postpartum follow-up period. HIV-1 RNA levels at baseline and BMI at month 1 postpartum were associated with increased mortality, but these associations lacked statistical significance (HR of 1.4 per log HIV-1 RNA increase [P = .4]; HR of 1.3 per 1 U decrease in BMI [P = .07]). Kaplan-Meier survival analysis was used to estimate time to death, time to death or HAART initiation, and time to CD4 cell count <200 cells/µL (figure 4). In these analyses, there was no significant difference between the ever and never breast-feeders for time to death, time to death or HAART initiation, or time to CD4 cell count <200 cells/µL during the 2-year follow-up period. In extended tracing of 120 mothers in the cohort, we were able to extend the analysis to 30 months and again did not find significant differences between the 2 groups at this later time point (data not shown).
Figure 4.
Survival analysis comparing ever breast-feeders (EB) and never breast-feeders (NB) with respect to time to death (A), time to death or highly active antiretroviral therapy (HAART) initiation (B), and time to CD4 cell count <200 cells/µL (C). Solid lines indicate survival plots, and faint lines indicate 95% confidence intervals.
DISCUSSION
In this study of 296 HIV-1–infected postpartum women, there were significant differences in CD4 cell count decline and BMI between breast-feeding and non–breast-feeding women but no differences in HIV-1 RNA levels or mortality over the 2-year postpartum period. Thus, although lactation appeared to have effects on CD4 cell count, there were no long-term effects of lactation on either HIV-1 RNA level or mortality. Our finding of a more rapid CD4 cell count decline during breast-feeding may explain our earlier observation of significantly increased mortality in a randomized clinical trial of breast-feeding [1]. Our finding of negligible long-term effects of breast-feeding on HIV-1 RNA levels and mortality are consistent with the findings of other observational studies [2–5].
The mothers in this study had a significant decrease in CD4 cell count between months 1 and 24 postpartum (an estimated decrease of 3.9 cells/µL/month, or ~48 cells/µL/year). This is comparable with findings in cohorts of nonimmunosuppressed US men and women (~30 to >1100 cells/µL/year) [14]. CD4 cell count decline was highest among the current breast-feeding women in the present study (−7.7 cells/µL/month), and this decrease was significantly higher than that for the never breast-feeding women (−4.4 cells/µL/month). Women who stopped breast-feeding did not continue at the same rate of decline (−3.2 cells/µL/month) as during breast-feeding, suggesting that the breast-feeding effect on CD4 cell count decline is a non-sustained process related to active breast-feeding. The accelerated CD4 cell count decline during breast-feeding may result from hormonal effects, nutritional/metabolic effects, or the numeric loss of CD4 cells as part of increased cellular egress to infants via breast milk.
We were not surprised to observe significant decreases in BMI in current versus never breast-feeding mothers. The process of lactation entails caloric costs that translate into increased energy requirements and/or weight loss. Demonstration of this effect in the breast-feeding duration–adjusted mixed-effects model but not in the spline models comparing ever versus never breast-feeding mothers supports the superiority of statistical models that include duration of breast-feeding to determine the effect of breast-feeding. Breast-feeding had an effect on BMI but was not associated with postpartum body cell mass changes in women. It is possible that changes in body cell mass may have more influence on HIV-1 progression than changes in weight or BMI. Despite significant decreases in CD4 cell count and BMI attributable to breast-feeding, HIV-1 RNA levels remained remarkably stable in this cohort of women overall, and there were no discernible differences between the 2 feeding groups over time in any of the models used. Thus, although breast-feeding modified CD4 cell count decline and BMI, viral replication was not significantly altered despite these immune and nutritional changes due to lactation. It is possible that the process of lactation may affect lymphocyte production in a way that does not alter the replication of HIV-1. In addition, the levels of CD4 cell count decline we observed, although significant, may not be clinically relevant, particularly for women with short breast-feeding durations. The CD4 cell count decline in women who breast-fed for 6 months did not differ significantly from the decline in those who never breast-fed. The World Health Organization currently recommends 6 months of exclusive breast-feeding for HIV-1–infected women who elect to breast-feed. For this duration of breast-feeding, our data suggest that breast-feeding would have a minimal adverse effect on CD4 cell count.
Mortality in the cohort did not differ between the women who ever breast-fed and the women who never breast-fed. Several factors may explain the differing results we observed regarding the effect of breast-feeding on mortality between our present and previous study [1]. Both studies were based in Nairobi and involved the same recruitment clinics and study clinic. The first study, a randomized clinical trial, had a more ideal study design for comparing feeding groups in that feeding was randomized. The importance of randomization is evident, given the highly significant differences at baseline between the 2 self-selected feeding groups in our present study. Here, although formula was provided, the women who chose to formula feed had more education, were more likely to have access to flush toilets, and were more likely to report a history of HIV-1–related illness. In addition, unmeasured differences in counseling, stress, and nutrition between the formula-feeding and breast-feeding women may have contributed to HIV-1 disease progression and confounded our assessment of the effect of breast-feeding. Our first study had greater power to assess mortality, with >200 women in each feeding group and higher postpartum mortality. In the first study, adverse effects of breast-feeding were most notable in severely immunosuppressed women. Our present cohort received interventions that were unavailable during the first study, including short-course zidovudine, CD4 cell count–based provision of cotrimoxazole prophylaxis, multivitamin supplementation for 6 months postpartum, and referral for provision of HAART when severely immunosuppressed. It is plausible that the availability of these options for mothers attenuated the potential adverse effects of breast-feeding on maternal HIV-1 disease progression.
The present study has several strengths and limitations. This study had the benefit (in contrast to previous studies of maternal HIV-1 disease progression) of being designed primarily to assess maternal HIV-1 disease progression. Thus, we comprehensively delineated HIV-1 disease progression by looking in detail at several key markers of progression in addition to mortality, including CD4 cell count, HIV-1 RNA level, weight, and body cell mass changes. Monthly assessment of feeding status by self-report and physical examination enhanced the accuracy of the assessment of true feeding status. Analytical techniques adjusting for baseline confounding variables were used to determine the effects of ever breast-feeding. In addition, because lactation effects were likely to differ between the women who breast-fed for only a few days and those who breast-fed for longer periods, additional mixed-effects models incorporating duration of breast-feeding enabled the comparison of effects between current breast-feeding women, former breast-feeding women, and women who never breast-fed.
The limitations of this study include the nonrandomized design; the relatively high prevalence of censoring in the study because of a second pregnancy or HAART initiation; differing rates of losses to follow-up, with a higher loss among the breast-feeding mothers than among the non–breast-feeding mothers; and evaluation of the rate of CD4 cell count decline starting at month 1 postpartum rather than at delivery. Higher losses to follow-up among the breast-feeding mothers may have led to the underestimation of adverse maternal effects due to breast-feeding if the losses were the result of mortality or illness. However, losses to follow-up among the breast-feeding mothers was not statistically significantly higher than among the never breast-feeding mothers, and baseline CD4 cell counts did not differ significantly between the women who were lost and those who remained in follow-up. Censoring because of second pregnancies and HAART initiation is a phenomenon that is not unique to our cohort of postpartum HIV-1–infected women, and these levels of censoring are likely to be similar to those in other postpartum maternal cohorts in Africa during the era of expanded HAART access. To gain from informative censoring due to HAART initiation, we analyzed both time to death and time to death or HAART initiation.
In summary, our interpretation of the data suggests that, with good maternal follow-up—including monitoring for immunosuppression and management of immunosuppressed women via both the use of cotrimoxazole prophylaxis and referral to HAART programs—the potential effects of breast-feeding on CD4 cell count and nutrition are attenuated and the risks of increases in HIV-1 RNA level or mortality are minimized. Our study suggests that, although breast-feeding may affect CD4 cell count and BMI in HIV-1–infected women, with extended maternal HIV-1 care, lactation may not be associated with a significant compromise in maternal health.
Acknowledgments
We thank the women in the cohort, for their participation in this study, and the clinic staff and the Kenyatta National Hospital, for their support.
Financial support: National Institutes of Health (NIH; research grants RO1 HD 23412 and AI27757); University of Washington Center for AIDS Research. P.A.O., E.M.O., R.K.B., and C.F. were scholars in the University of Washington AIDS International Research and Training Program, which is supported by the NIH Fogarty International Center (grant D43-TW00007).
Approval to publish this work was granted by the director of the Kenya Medical Research Institute.
Footnotes
Presented in part: XVIth International AIDS Conference, Toronto, Canada, 13–18 August 2006 (abstract MOPE0308).
Potential conflicts of interest: none reported.
References
- 1.Nduati R, Richardson BA, John G, et al. Effect of breastfeeding on mortality among HIV-1 infected women: a randomised trial. Lancet. 2001;357:1651–1655. doi: 10.1016/S0140-6736(00)04820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coutsoudis A, Coovadia H, Pillay K, Kuhn L. Are HIV-infected women who breastfeed at increased risk of mortality? AIDS. 2001;15:653–655. doi: 10.1097/00002030-200103300-00019. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn L, Kasonde P, Sinkala M, et al. Prolonged breast-feeding and mortality up to two years post-partum among HIV-positive women in Zambia. AIDS. 2005;19:1677–1681. doi: 10.1097/01.aids.0000186817.38112.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedgh G, Spiegelman D, Larsen U, Msamanga G, Fawzi WW. Breastfeeding and maternal HIV-1 disease progression and mortality. AIDS. 2004;18:1043–1049. doi: 10.1097/00002030-200404300-00013. [DOI] [PubMed] [Google Scholar]
- 5.Breastfeeding and HIV International Transmission Study Group. Mortality among HIV-1 infected women according to children’s feeding modality. J Acquir Immune Defic Syndr. 2005;39:430–438. doi: 10.1097/01.qai.0000148531.04706.c0. [DOI] [PubMed] [Google Scholar]
- 6.Geneva: World Health Organization; 1998. HIV and infant feeding: implementation of guidelines. A report of the UNICEF-UNAIDS-WHO technical consultation on HIV and infant feeding (document WHO/FRH/NUT/CHD/98.4) [Google Scholar]
- 7.Taha TE, Miotti P, Liomba G, Dallabetta G, Chiphangwi J. HIV, maternal death and child survival in Africa. AIDS. 1996;10:111–112. doi: 10.1097/00002030-199601000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Kiarie JN, Richardson BA, Mbori-Ngacha D, Nduati RW, John-Stewart GC. Infant feeding practices of women in a perinatal HIV-1 prevention study in Nairobi, Kenya. J Acquir Immune Defic Syndr. 2004;35:75–81. doi: 10.1097/00126334-200401010-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, Coovadia HM. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–387. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 10.Newell M. Does breastfeeding really affect mortality among HIV-1 infected women? Lancet. 2001;357:1634–1635. doi: 10.1016/s0140-6736(00)04857-1. [DOI] [PubMed] [Google Scholar]
- 11.Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 12.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice JA, Wu CO. Nonparametric mixed effects models for unequally sampled noisy curves. Biometrics. 2001;57:253–259. doi: 10.1111/j.0006-341x.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- 14.Anastos K, Gange SJ, Lau B, et al. Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr. 2000;24:218–226. doi: 10.1097/00126334-200007010-00004. [DOI] [PubMed] [Google Scholar]