Abstract
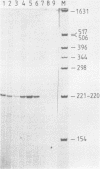
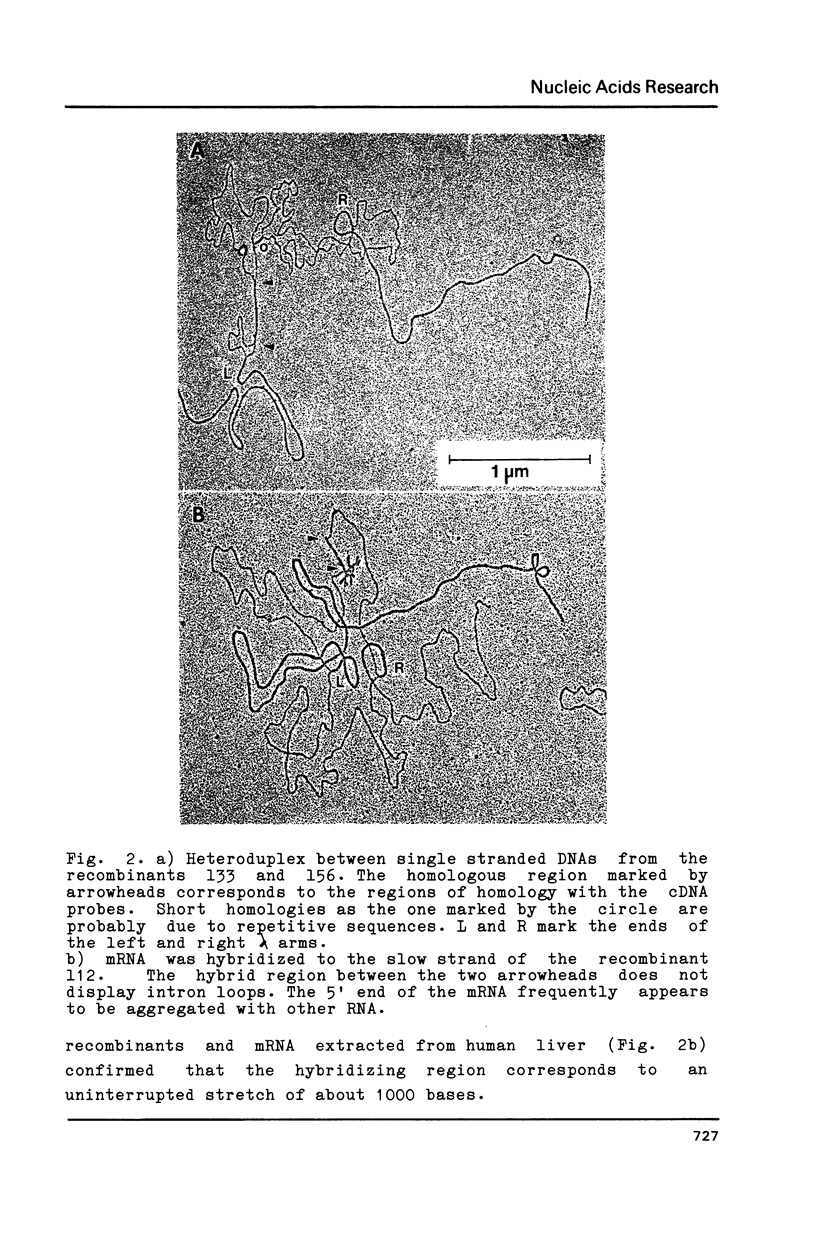
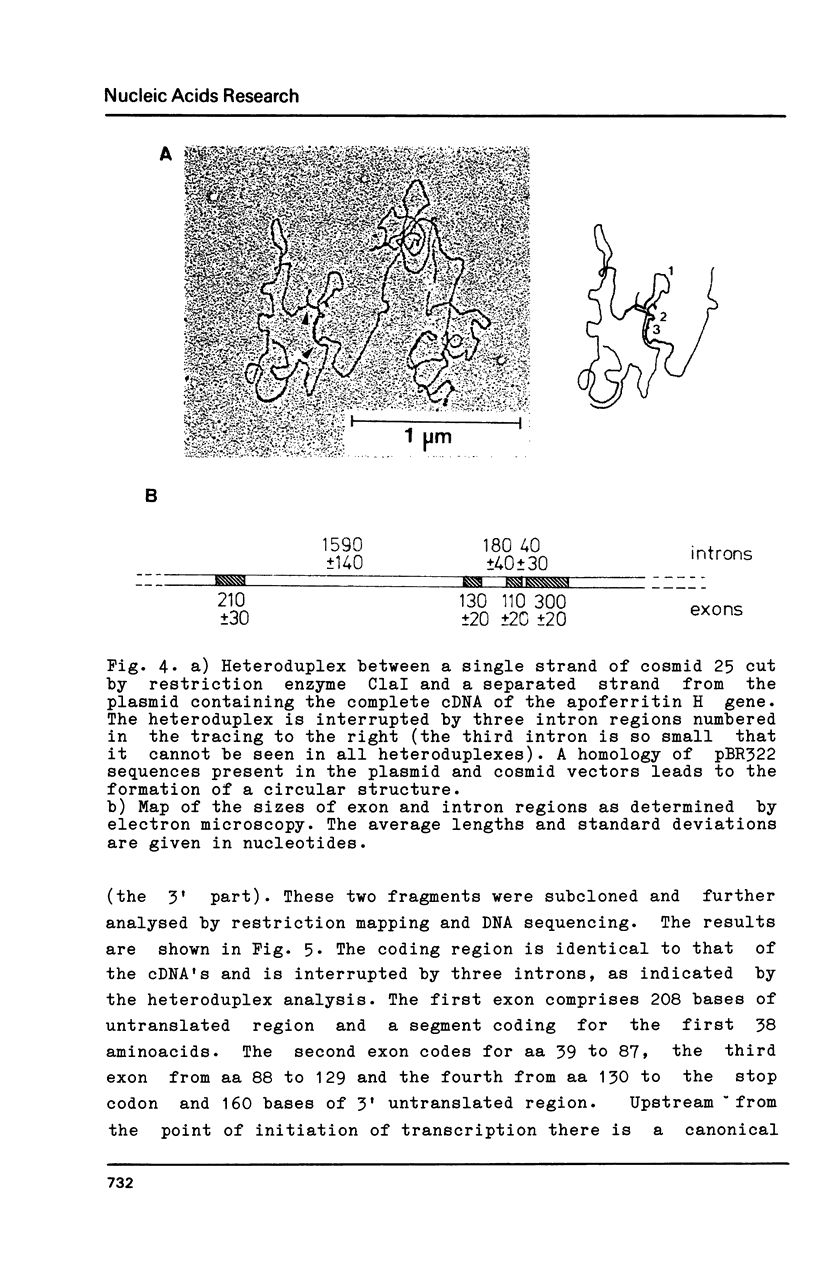
Ferritin is composed of two subunits, H and L. cDNA's coding for these proteins from human liver (1,2,3), lymphocytes (4) and from the monocyte-like cell line U937 (5) have been cloned and sequenced. Southern blot analysis on total human DNA reveals that there are many DNA segments hybridizing to the apoferritin H and L cDNA probes (1,2,4,6). In view of the tissue heterogeneity of ferritin molecules (7,8), it appeared possible that apoferritin molecules could be coded by a family of genes differentially expressed in various tissues (1,2). In this paper we describe the cloning and sequencing of the gene coding for human apoferritin H. This gene has three introns; the exon sequence is identical to that of cDNA's isolated from human liver, lymphocytes, HeLa cells and endothelial cells. In addition we show that at least 15 intronless pseudogenes exist, with features suggesting that they were originated by reverse transcription and insertion. On the basis of these results we conclude that only one gene is responsible for the synthesis of the majority of apoferritin H mRNA in various tissues examined, and that probably all the other DNA segments hybridizing with apoferritin cDNA are pseudogenes.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Benham F. J., Hodgkinson S., Davies K. E. A glyceraldehyde-3-phosphate dehydrogenase pseudogene on the short arm of the human X chromosomes defines a multigene family. EMBO J. 1984 Nov;3(11):2635–2640. doi: 10.1002/j.1460-2075.1984.tb02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensi G., Raugei G., Klefenz H., Cortese R. Structure and expression of the human haptoglobin locus. EMBO J. 1985 Jan;4(1):119–126. doi: 10.1002/j.1460-2075.1985.tb02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Jain S. K., Crampton J., Barrett K. J., Drysdale J. Isolation and characterization of a cDNA clone for human ferritin heavy chain. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4751–4755. doi: 10.1073/pnas.81.15.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Vecoli C., Belcher D. M., Jain S. K., Drysdale J. W. Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J Biol Chem. 1985 Sep 25;260(21):11755–11761. [PubMed] [Google Scholar]
- Brown A. J., Leibold E. A., Munro H. N. Isolation of cDNA clones for the light subunit of rat liver ferritin: evidence that the light subunit is encoded by a multigene family. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1265–1269. doi: 10.1073/pnas.80.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce C. F., Magnusson C. G., Crichton R. R. A reappraisal of the electrophoretic patterns obtained from ferritin and apoferritin in the presence of denaturants. FEBS Lett. 1978 Dec 15;96(2):257–262. doi: 10.1016/0014-5793(78)80413-x. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Cortese R., Harland R., Melton D. Transcription of tRNA genes in vivo: single-stranded compared to double-stranded templates. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4147–4151. doi: 10.1073/pnas.77.7.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese R., Melton D., Tranquilla T., Smith J. D. Cloning of nematode tRNA genes and their expression in the frog oocyte. Nucleic Acids Res. 1978 Dec;5(12):4593–4611. [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Santoro C., Colantuoni V., Bensi G., Raugei G., Romano V., Cortese R. Cloning and sequencing of a full length cDNA coding for a human apoferritin H chain: evidence for a multigene family. EMBO J. 1984 Jan;3(1):23–27. doi: 10.1002/j.1460-2075.1984.tb01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg S. J., Drysdale J., Worwood M. Genes for the 'H' subunit of human ferritin are present on a number of human chromosomes. Hum Genet. 1985;71(2):108–112. doi: 10.1007/BF00283363. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Millar J. A., Cumming R. L., Bryce C. F. The organ-specificity of ferritin in human and horse liver and spleen. Biochem J. 1973 Jan;131(1):51–59. doi: 10.1042/bj1310051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., van Heerikhuizen H., Clarke J., Koller B. Separation of complementary strands of plasmid DNA using the biotin-avidin system and its application to heteroduplex formation and RNA/DNA hybridizations in electron microscopy. Nucleic Acids Res. 1985 Aug 12;13(15):5457–5469. doi: 10.1093/nar/13.15.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W. Microheterogeneity in ferritin molecules. Biochim Biophys Acta. 1970 Apr 28;207(1):256–258. doi: 10.1016/0005-2795(70)90158-3. [DOI] [PubMed] [Google Scholar]
- Dörner M. H., Salfeld J., Will H., Leibold E. A., Vass J. K., Munro H. N. Structure of human ferritin light subunit messenger RNA: comparison with heavy subunit message and functional implications. Proc Natl Acad Sci U S A. 1985 May;82(10):3139–3143. doi: 10.1073/pnas.82.10.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish W. W. Ferritin structure: possible models for apoferritin subunit arrangement. J Theor Biol. 1976 Aug 7;60(2):385–392. doi: 10.1016/0022-5193(76)90064-3. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Harrison P. M., Banyard S. H., Hoare R. J., Russell S. M., Treffry A. The structure and function of ferritin. Ciba Found Symp. 1976 Dec 7;(51):19–40. doi: 10.1002/9780470720325.ch2. [DOI] [PubMed] [Google Scholar]
- Hollis G. F., Hieter P. A., McBride O. W., Swan D., Leder P. Processed genes: a dispersed human immunoglobulin gene bearing evidence of RNA-type processing. Nature. 1982 Mar 25;296(5855):321–325. doi: 10.1038/296321a0. [DOI] [PubMed] [Google Scholar]
- Houghton M., Jackson I. J., Porter A. G., Doel S. M., Catlin G. H., Barber C., Carey N. H. The absence of introns within a human fibroblast interferon gene. Nucleic Acids Res. 1981 Jan 24;9(2):247–266. doi: 10.1093/nar/9.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., Barrett K. J., Boyd D., Favreau M. F., Crampton J., Drysdale J. W. Ferritin H and L chains are derived from different multigene families. J Biol Chem. 1985 Sep 25;260(21):11762–11768. [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Lecocq J. P. New versatile cloning and sequencing vectors based on bacteriophage M13. Gene. 1983 Dec;26(1):91–99. doi: 10.1016/0378-1119(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Koller B., Delius H., Bünemann H., Müller W. The isolation of DNA from agarose gels by electrophoretic elution onto malachite green-polyacrylamide columns. Gene. 1978 Nov;4(3):227–239. doi: 10.1016/0378-1119(78)90020-3. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Nagata S., Mantei N., Weissmann C. The structure of one of the eight or more distinct chromosomal genes for human interferon-alpha. Nature. 1980 Oct 2;287(5781):401–408. doi: 10.1038/287401a0. [DOI] [PubMed] [Google Scholar]
- Nagylaki T. Evolution of multigene families under interchromosomal gene conversion. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3796–3800. doi: 10.1073/pnas.81.12.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T. Some models of gene conversion for treating the evolution of multigene families. Genetics. 1984 Mar;106(3):517–528. doi: 10.1093/genetics/106.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poustka A., Rackwitz H. R., Frischauf A. M., Hohn B., Lehrach H. Selective isolation of cosmid clones by homologous recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4129–4133. doi: 10.1073/pnas.81.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Kunz G., Daetwyler H., Telford J., Smith H. O., Birnstiel M. L. Genes and spacers of cloned sea urchin histone DNA analyzed by sequencing. Cell. 1978 Jul;14(3):655–671. doi: 10.1016/0092-8674(78)90249-0. [DOI] [PubMed] [Google Scholar]
- Stein J. P., Munjaal R. P., Lagace L., Lai E. C., O'Malley B. W., Means A. R. Tissue-specific expression of a chicken calmodulin pseudogene lacking intervening sequences. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6485–6489. doi: 10.1073/pnas.80.21.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Arsdell S. W., Denison R. A., Bernstein L. B., Weiner A. M., Manser T., Gesteland R. F. Direct repeats flank three small nuclear RNA pseudogenes in the human genome. Cell. 1981 Oct;26(1 Pt 1):11–17. doi: 10.1016/0092-8674(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Vanin E. F., Goldberg G. I., Tucker P. W., Smithies O. A mouse alpha-globin-related pseudogene lacking intervening sequences. Nature. 1980 Jul 17;286(5770):222–226. doi: 10.1038/286222a0. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cripe T. P., Gwo-Shu Lee M., Cowan N. J. Evidence that a human beta-tubulin pseudogene is derived from its corresponding mRNA. Nature. 1982 May 6;297(5861):83–84. doi: 10.1038/297083a0. [DOI] [PubMed] [Google Scholar]