Abstract
Chronic myelogenous leukemia (cml) is a disease characterized by the expression of Bcr/Abl, an oncogenic protein tyrosine kinase, and by evolution over time from a relatively benign chronic phase to a rapidly fatal cml blast crisis. Until recently, the standard of care included potentially curative therapy with allogeneic stem cell transplantation, available only to a minority (about 10%) of patients, or medical therapy with interferon-α with or without cytarabine, which helped to prolong the chronic phase of the disease in a minority of patients. The availability of imatinib mesylate, a selective inhibitor of Bcr/Abl approved by Health Canada in 2001, has profoundly altered the clinical and laboratory management of cml. This change in practice has been reviewed by the Canadian Consensus Group on the Management of Chronic Myelogenous Leukemia and has resulted in a new set of recommendations for the optimal care of cml patients.
Keywords: Chronic myeloid leukemia, clinical practice guideline, imatinib
1. INTRODUCTION
In 1924, the median survival time of patients with untreated chronic myeloid leukemia (cml) was about 2.4 years. Until the 1980s, cml was regarded as incurable and inexorably fatal. Within the past two decades, three pivotal discoveries have prolonged patient survival and led to cure in some patients 1:
Allogeneic stem cell transplantation (allogeneic sct) holds the promise of cure for selected patients with cml, but a lack of suitable donors and the higher incidence of graft-versus-host disease in older individuals have thwarted efforts to extend this procedure to all patients 1.
Interferon-α (ifnα) has expanded the therapeutic options for transplantation-ineligible patients. In a substantial minority, this agent clearly prolongs survival. However, this progress comes at a price. For more than 50% of patients, ifnα-related side effects are intolerable and lead to dose reduction 2,3.
The introduction of imatinib mesylate has ushered in the new era of molecularly targeted therapy 1. A prototype for modern oncologic therapeutics, imatinib mesylate targets the pathogenic basis of cml by inhibiting the Bcr/Abl protein tyrosine kinase 4.
Patients with chronic-phase (cp) cml who fail standard treatment have a high response rate to imatinib 5. Such dramatic, convincing progress in treatment is rare 2. Once again, the therapeutic approach to cml is changing to keep pace with the rapid evolution of biotechnology.
1.1 Therapeutic Dilemma
The key question facing many Canadian hematologists and their patients is how to capitalize on the therapeutic progress of imatinib mesylate without forfeiting the life-prolonging effects of ifnα or the curative benefits of allogeneic sct.
No information on long-term survival with imatinib mesylate is available. However, observations of imatinib mesylate resistance in patients in CP cml and of residual disease in complete cytogenetic responders casts doubt on its ability to prolong survival in every cml patient 2. Ultimately, only time can answer outstanding questions about the median survival of patients on imatinib mesylate. In the interim, the lack of long-term clinical data contributes to uncertainty about how to manage cml.
To resolve this therapeutic dilemma, the Canadian Consensus Group on the Management of Chronic Myelogenous Leukemia (ccgm-cml), a group of Canadian specialists in cml, met to review the strength of evidence and to formulate a consensus on general recommendations for the management of cml. The ultimate goal was to develop an evidence-based guideline for use in Canadian clinical practice.
2. METHODOLOGY
In November 2003, more than 70 Canadian hematologists and oncologists attended a series of regional meetings with the intention of developing a consensus on recommendations for the standard treatment of cml. Three regional consultations were held in Montreal, Toronto, and Vancouver. At each meeting, Canadian specialists in the management of cml (Appendix A) participated in a series of interactive workshops and developed consensus positions on key management issues.
Before these meetings, key opinion leaders had been invited to review the medical literature and clinical data on specific topics for presentation to the group. These leaders reviewed entries to medline (1985 to November 2003), The Cochrane Library, cancerlit, and cmlsupport.com, plus relevant abstracts and reports from the proceedings of the 1998–2003 annual meetings of the American Society of Hematology (ash) and the American Society of Clinical Oncology (asco). In addition, the National Comprehensive Cancer Network, National Institute for Clinical Excellence, National Guidelines Clearing-house, Cancer Care Ontario, and Canadian Medical Association databases were searched for relevant information on clinical practice guidelines for cml.
Subsequently, a steering committee (Appendix B), composed of representatives from each regional meeting, met to share the recommendations of their respective meetings and to reach a national consensus on key issues related to the management of cml. These meetings were funded by an unrestricted educational grant from Novartis Canada Inc.
The steering committee graded the levels of evidence according to National Cancer Institute of Canada practices for the evaluation of scientific evidence (Appendix C). Whenever possible, the steering committee incorporated newly emerging clinical data (since November 2003) into the recommendations to ensure the most up-to-date approach to the management of cml.
This consultative process led to the recommendations that follow. The recommendations describe current best practice in the management of cml. This paper presents recommended procedures for diagnosis and patient monitoring, and optimal therapeutic strategies for pharmacotherapy and transplantation.
Given that the understanding of and clinical research into cml is rapidly evolving, particularly in terms of the long-term effects of imatinib mesylate and newer drugs, the steering committee wishes to emphasize that these recommendations must be revisited as new data emerge.
3. DISCUSSION
3.1 Epidemiology of CML
The annual incidence of cml in North America is 1–2 cases per 100,000 population, which represents about 15% of all cases of leukemia 6. The incidence is slightly greater in men than in women (1.35:1 ratio). Median age at diagnosis is 45–55 years 6. Between 12% and 30% of patients are 60 years of age or older 6. Based on this incidence and Canadian cancer statistics, about 550 new cases of cml are estimated to be diagnosed annually in Canada 7 (Level III).
Chronic myelogenous leukemia is an acquired disorder with no known inherited predispositions. Disease concordance is absent in monozygotic twins. Exposure to ionizing radiation, as documented in Japanese survivors of the World War ii nuclear bomb attacks, is the only well-established risk factor for the development of cml, but this cause is seldom implicated in newly diagnosed patients 1.
3.2 Causative Factors in CML
The disease is characterized by the presence of the Philadelphia (Ph) chromosome, a shortened chromosome 22. The Ph chromosome is created when an apparently spontaneous and reciprocal, but unequal, transfer of genetic material occurs between chromosomes 9 and 22, which break in the long arms at q34 and q11 respectively. This translocation fuses the BCR gene on chromosome 22 to sequences of the ABL proto-oncogene from chromosome 9. The BCR/ABL fusion gene expresses a 210-kDa Bcr/Abl protein tyrosine kinase 1.
Chronic myelogenous leukemia is believed to originate when a single hematopoietic stem cell acquires a Ph chromosome carrying the BCR/ABL fusion gene. This dominant oncogene confers a proliferative advantage on its progeny, which gradually displace normal hematopoietic stem cells 1,8. Expression of the Bcr/Abl protein tyrosine kinase is thought to be the initiating event in the genesis of cml. The oncoprotein supports the proliferation of malignant cell clones.
The cellular effects of BCR/ABL expression are pleiotropic and include mitogenic stimulation of growth-factor signal transduction pathways, inhibition of apoptosis 9, altered adhesion to and regulation by bone marrow stromal cells 10, functional activation of ras 11, impairment of the cellular response to genotoxic stress, and enhancement of the rate of secondary mutagenesis 12.
3.3 Definition, Classification, and Natural History
Various clinical definitions are used to categorize patients into three stages of cml: cp, accelerated phase (ap), and blast crisis (bc). Today, the most widely used definition is based on the percentage of blasts in bone marrow. This definition, employed by investigators in the iris trial [International Randomized Study of Interferon versus ST1571 (ST1571 being now known as imatinib mesylate)] 13, was adopted by consensus at the ccgm-cml meetings.
This definition simplifies the classification of cml set out in National Comprehensive Cancer Network (nccn) Clinical Practice Guidelines 14 It replaces outdated recommendations such as the International Bone Marrow Transplant Registry criteria, which include benchmarks referring to chemotherapy—an outdated treatment modality for most cml patients 14 It is not as strict as World Health Organization criteria 14, which define bc as ≥20% blasts in blood or marrow and which insist on cytogenetic evidence of clonal evolution. The latter requirement may delay treatment at Canadian sites that lack immediate access to cytogenetic evaluation.
About 85% of patients with cml present in cp, which is characterized by clonal granulocytic hyperplasia of relatively mature, functional cells 15. Signs and symptoms may include painful splenomegaly, fever, night sweats, anorexia and weight loss, anemia, hyperuricemia and gout, or complications arising from leucocytosis in individuals who have a very high peripheral blood cell count. All symptoms usually resolve with normalization of the peripheral blood count. In fact, up to half of patients today are asymptomatic and are diagnosed on routine blood testing, or when testing is done for other reasons 8,15.
Before the advent of ifnα and imatinib mesylate, orally administered busulfan and hydroxyurea were commonly used to induce hematologic remissions 15. Busulfan or hydroxyurea can normalize the number of peripheral blood granulocytes, but circulating cells almost always remain Ph chromosome–positive 8. More importantly, treatment with busulfan and hydroxyurea does not prevent the evolution of cml from a stable cp to ap and finally to bc 8,15.
The bc stage is invariably fatal, with a median survival in the range 3–6 months. The median duration of ap is in the range 6–9 months, but a substantial number of patients (25%–40%) may progress abruptly from cp to bc without a clearly defined intervening ap 8,15. About 5%–10% of patients typically progress from cp to bc in the first 2 years, with a rate of progression of 20% in subsequent years 16. Disease progression is accompanied by clonal evolution, characterized in most patients by the appearance of secondary cytogenetic abnormalities; altered gene methylation; and mutation, suppression, or overexpression of a number of genes 17,18.
Recommendation 1
The ccgm-cml recommends the following categorization of cml stages, as defined by the iris investigators 13 (Level I.1iiDii):
Chronic phase The presence of less than 15% blasts, less than 20% basophils, and less than 30% blasts plus promyelocytes in peripheral blood and marrow.
Accelerated phase The presence of at least 15% blasts in blood or bone marrow, at least 30% blasts plus promyelocytes in blood or bone marrow, at least 20% peripheral basophils, or thrombocytopenia (platelets < 10×109/L).
Blast crisis The presence of at least 30% blasts in blood or bone marrow or extramedullary involvement—for example, chloromas.
3.4 Prognosis
A number of clinical prognostic scales have been developed to better estimate the prospective risk of cml progression and survival. Clinically, prognostic factors guide the selection of optimal treatment and the development of risk-adjusted treatments in patients awaiting therapy 19 (Level II-3.3iiiA).
The Sokal score was the first scale that achieved widespread use before the introduction of ifnα therapy. It works well as a prognostic discriminator of survival in patients treated with busulfan or hydroxyurea, but it is a poor discriminator in patients treated with ifnα.
The Sokal score categorizes patients into low [hazard ratio (hr): < 0.8], intermediate (hr: 0.8–1.2), or high risk (hr: >1.2) of death by rating these prognostic variables:
Patient age
Degree of splenomegaly
Platelet count
Percentage of peripheral blasts on a scale of increasing severity
Low-risk patients have a 2-year survival of 90%, a subsequent risk of less than 20% per year, and a median survival of 5 years. The high-risk group has a 2-year survival rate of 65%, a death rate of about 35% per year, and median survival of 2.5 years 3,20 (Level II-3.3iiiA).
The Sokal score is calculated for patients aged 5–84 years as:
 |
The Hasford prognostic score has proven to be more reliable for patients who choose ifnα 3,19 (Level II-3.3iiiA). Until recently, Hasford was the most commonly used prognostic scoring system. It relies on these prognostic indicators:
Patient age
Blast count
Basophil count
Spleen size
Eosinophil count
Platelet count
It remains a reliable indicator of prognosis in the subset of patients on ifnα therapy. The Hasford score can be calculated for individual patients at the Web site www.pharmacoepi.de/cgi-bin/pharmacoepi/cmlscore.cgi.
The Sokal and Hasford prognostic scoring systems are both based on clinical and laboratory data obtained at the time of diagnosis; scores obtained after therapy initiation are less reliable. No prognostic scoring system has been specifically designed for patients on imatinib mesylate, although the Sokal and Hasford systems have both been used in major clinical trials of imatinib therapy 13. The Sokal system appears to have gained wider acceptance.
Marin et al. 21 devised a prognostic scoring system for patients on imatinib mesylate after failure of ifnα (Level II-3.3iiiDi). In a sample of 145 patients, these authors showed that failure to achieve at least partial cytogenetic response and the presence of neutrophilia at 3 months were the only independent predictors of progression-free survival (pfs). They constructed a risk score of 0, 1, or 2 points that classifies patients into three prognostic groups. On this scale, patients score 1 point if they fail to achieve a partial cytogenetic response at 3 months and 1 point if they develop neutrophilia—defined as more than 1×109 cells/L—between days 45 and 90 of therapy. The prognosis for survival at 2 years was 100% for the low-risk (0 points) group, 82% [95% confidence interval (ci): 70%–90%] for the intermediate-risk (1 point) group, and 40% (95% ci: 25%–58%) for the high-risk (2 points) group (p < 0.0001). The scoring system was validated in a small sample of 29 patients. Further validation of this prognostic scoring system is needed. At the time of writing, whether this scoring system can be applied reliably to patients on imatinib mesylate who have received no prior treatment with ifnα is unknown.
Recommendation 2
Until further investigation validates a more reliable prognostic score for patients who are considering imatinib mesylate, the ccgm-cml recommends the use of the Sokal index for newly diagnosed patients with cml.
3.5 Diagnosis and Baseline Investigations
At patient presentation, investigations should include assessment of prognosis with the Sokal index, a bone marrow (bm) biopsy, baseline bm G-banding cytogenetics, peripheral blood fluorescence in situ hybridization (fish), and quantitative real-time polymerase chain reaction (q-rt-pcr) determination. It is important to distinguish the latter test from qualitative or semi-quantitative pcr techniques.
The diagnosis of cml is established by any combination of positive results from bm cytogenetic studies, fish, and q-rt-pcr. Some of this testing appears redundant, but it serves different purposes.
Bone marrow morphology and blast counts remain the standard for assigning cml patients to the cp, ap, or bc stage of the disease.
The biopsy, besides characterizing cellularity, helps to determine if fibrosis is present at diagnosis.
Baseline bm cytogenetics are indicated to identify patients who may already show signs of clonal evolution at diagnosis. It also confirms whether the Ph chromosome is present or absent.
A baseline fish can determine if chromosome der9 deletions are present or absent. The presence of these deletions is a significant adverse prognostic factor in cp cml patients treated with ifnα 22, but appears to have less impact in patients treated with imatinib mesylate 23 (Level II-2.3iiiA). Nevertheless, a baseline fish study will help to establish if fish can subsequently reliably be used to follow the cytogenetic response to treatment in the subset of patients who currently use or may cross over to ifnα therapy.
A q-rt-pcr should be performed at baseline. It will help to diagnose the rare cml patient who is Ph-negative by conventional cytogenetic analysis and also fish-negative. More importantly, it also provides baseline data for later comparative studies to monitor treatment response to imatinib mesylate. Most cp patients will achieve a complete cytogenetic response (ccr) on imatinib mesylate (79% at 31 months) 24 (Level I-2.1iiDii). The q-rt-pcr is the only technique sufficiently sensitive to monitor and ensure that a biologic response to imatinib mesylate is sustained in ccr patients.
Recommendation 3
The ccgm-cml recommends the following investigations at diagnosis for all patients with suspected or confirmed cml:
Assessment of prognosis (Sokal)
Bone marrow aspirate and biopsy
Baseline bone marrow cytogenetics
Peripheral blood or bone marrow fish for deletions in chromosome 9
Peripheral blood or bone marrow q-rt-pcr
3.6 Therapeutic Considerations for CML
Before developing their recommendations for treatment of cml, the ccgm-cml reviewed the following evidence-based data and considerations from clinical practice:
Allogeneic sct for cml may be curative, but several difficulties independent of donor availability limit its application as universal therapy for cml. Many patients fail to qualify for transplantation because of age, health, disease status, and other factors. The risks of transplantation are relatively high and potentially life threatening.
Medical therapy is able to prolong the duration of cp cml, reduce the rate of transformation, and improve the annual rate of survival. If tolerated by most patients, it may produce a greater impact on overall cml survival than does allogeneic sct, even if not curative.
3.6.1 Allogeneic SCT
Allogeneic sct is the only known cure for cml. Until recently, it was the treatment of choice for patients 55 years of age and younger in generally good health who had a related donor matched for human lymphocyte antigens (hlas), or for those 40–45 years of age or younger with an hla-matched unrelated donor. These criteria, which determine eligibility for treatment, are used at most Canadian transplantation centres. Reduced-intensity allografting, which makes use of the graft-versus-leukemia effect of the allograft, is relatively new and may extend the age limit for transplantation to 79 years or more. Physicians should check with local transplant centres.
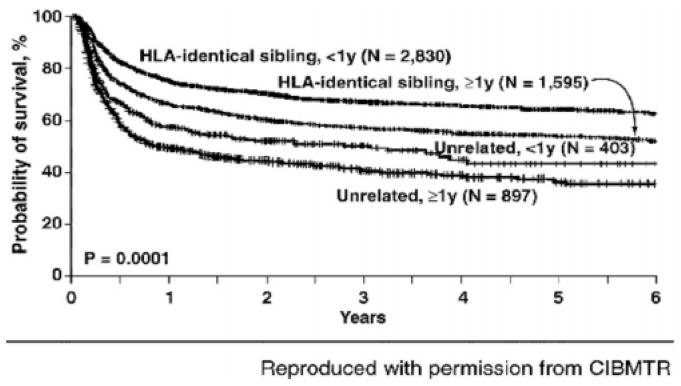
Data from the International Blood and Marrow Transplant Registry show that the 5-year survival for an hla-matched, related-donor allogeneic sct performed in cp cml less than 1 year after diagnosis ranges from 45% to 80% (see Figure 1) 3.
FIGURE 1.
Probability of survival after 5725 allogeneic stem cell transplants for chronic-phase chronic myelogenous leukemia by donor type and disease duration, 1991–19973. hla = human lymphocyte antigen
Survival is significantly lower if allogeneic sct is delayed beyond 1 year or if an hla-matched unrelated donor is used. Similarly, survival is considerably lower if cml progresses to ap before transplantation. Patients in bc seldom derive any benefit.
The choice of allogeneic sct in cp cml involves a tradeoff between exposure to early transplantation-related mortality and long-term disease-free survival. Moreover, the decision needs to be made early, when the patient is well, to minimize the chance that progression will occur and compromise the effectiveness of the procedure.
The risk:benefit ratio varies for individuals, depending on their specific disease-related and allogeneic sct–related risks. The most widely used method to evaluate risk factors and transplantation outcome in cml patients is described by Gratwohl et al. 25 (Level II-3.3iiiA), based on an analysis of 3142 patients transplanted between 1989 and 1997 by the European Group for Blood and Marrow Transplantation (Table I). Gratwohl and colleagues developed a clinical scoring system, ranging from 0 to 7, to estimate the 5-year transplantation-related mortality and overall survival for individual patients (Table II).
TABLE I.
Calculation of risk factor score for transplantation patients (Grawohl et al.) 25
| Points | |||
|---|---|---|---|
| 0 | 1 | 2 | |
| Stage | CP1 | AP | BP≥CP2 |
| Patient age (years) | <20 | 20–40 | >40 |
| Interval (months) | <12 | >12 | — |
| Patient/donor | Other | M/F | — |
| Donor | SD | UD | — |
CP1 = first chronic phase; AP = accelerated phase; BP = Blast phase; CP2 = second chronic phase; M/F = male/female; SD = sibling donor; UD = unrelated donor.
TABLE II.
Calculation of prognosis at 5 years by risk factor score (Gratwohl et al.) 25
| Score | Transplantation-related mortality (%) | Overall survival (%) |
|---|---|---|
| 0–1 | 20 | 70 |
| 2 | 30 | 60 |
| 3 | 45 | 50 |
| 4 | 50 | 40 |
| 5–7 | 70 | 20 |
Despite its effectiveness, allogeneic sct has had a relatively modest impact on cml survival. Because of age restrictions, absence of a suitable donor, health status, and other considerations, only about 10% of cml patients are eligible for the procedure. Survival in the range 80%–85% at 10 years has been reported by some institutions in young patients who are optimally conditioned with busulfan and cyclophosphamide therapy (as opposed to total body irradiation and cyclophosphamide), when the dose of busulfan is adjusted according to its measured clearance in individual patients 26 (Level II-2.3iiA). These results, which can be achieved in only a minority of cml patients, are roughly equivalent to overall survival rates predicted for patients on imatinib mesylate therapy, which is feasible in most cml patients.
As Goldman et al. 27 indicate, the advent of imatinib mesylate has greatly complicated the decision-making process for patients who are weighing the risks and benefits of transplantation versus medical therapy. Two divergent approaches to managing the newly diagnosed patient with chronic cml have emerged. One approach is to offer all such patients a 2- to 3-month trial of imatinib mesylate to determine whether they will respond to treatment. Responders would continue on imatinib mesylate indefinitely, while eligible non-responders would proceed to allogeneic sct.
The other approach advocates the identification of low-risk, eligible patients for whom allogeneic sct could be confidently recommended as primary treatment 27. This group would likely include patients under 45 years of age with an hla-identical sibling donor and patients under 35 years of age with an hla-matched unrelated donor. For those in the latter category, the cytomegalovirus status of patient and donor must be taken into account. Goldman et al. 27 also recommend the use of prognostic scores such as the Sokal index as a guide, suggesting that, for patients with a higher probability of survival with medical therapy (that is, those in the Sokol “good risk” category), the upper age limit for transplantation be lowered by 10 years. Conversely, for patients in the Sokol “poor risk” category, the upper age limit could be increased by 10 years.
The nccn guideline recommends the discussion of allogeneic sct as a first-line therapy with eligible candidates 14. It points out that the widespread application of this potentially curative therapeutic option is limited by donor availability and high toxicity in older patients, which limits the age of eligibility to less than 65 years at many centres.
The France Intergroupe des Leucémies Myéloïdes Chroniques (filmc) recommends first-line allogeneic sct for young patients (<20 years) at low risk of disease progression 28.
The Ontario-based guidelines point out that the safety and efficacy of allogeneic sct has not been assessed in randomized clinical trials for the initial treatment of cp patients 29 It also notes that prior imatinib therapy does not appear to compromise allogeneic sct, except by delaying the procedure.
Recommendation 4
The option of allogeneic sct should be discussed with all transplantation-eligible patients. All transplantation-eligible patients should be referred to a transplantation centre for hla typing and matching of potential donors. Referral permits advanced preparation for allogeneic sct in the event that this option is chosen as first-line therapy or that imatinib mesylate therapy fails; in either case, the patient can proceed to transplantation in a timely manner. Allogeneic sct as first-line therapy is an acceptable option that may be selected by some patients based on personal preference after discussion of the pros and cons versus imatinib mesylate therapy. Patient choice is the determining factor in treatment decision-making.
3.6.2 Interferon-α
Interferon-α (with or without cytarabine therapy) was the first agent to alter the natural history of cml and prolong survival, as compared with conventional therapy with busulfan or hydroxyurea 3. Until recently, most patients ineligible for allogeneic sct were offered ifnα therapy 3.
An important surrogate endpoint in clinical trials of ifnα was the degree of cytogenetic response—that is, reduction in the percentage of Ph-positive cells—observed in patients who were induced into hematologic remission. A major improvement in survival, up to 72% at 10 years, was observed in 5%–33% of patients, who achieved a ccr (0% Ph-positive cells) within 12–24 months 3. Patients with low-risk Sokal scores and a ccr within 12–24 months fared the best, with a 10-year probability of survival of 89% 3. Based on this evidence, ifnα became the first-line medical therapy for cml, despite a considerable increase in toxicity as compared with conventional therapy.
Interferon-α therapy has several drawbacks. Treatment for one or more years (12–24 months) is required to establish the presence and degree of cytogenetic response, owing to the slow kinetics of the biologic response to the drug 3, and therapy offers prolonged survival to a limited number of patients 3. Overall, cp patients with a low-risk Sokal score have a 10-year survival rate of about 40% 3. Tolerability is poor, and dose adjustment is required in more than 50% of patients 3. Up to 25% of patients discontinue treatment because of severe adverse effects 3.
The use of ifnα has not significantly altered the practice of offering allogeneic sct up front to eligible patients who have suitable donors. The modest survival benefit conferred by ifnα (except in the small percentage of patients who achieve ccr), together with the significant treatment-related toxicity, slow cytogenetic response, and controversial reports of prior ifnα treatment adversely affecting survival after allogeneic sct, have maintained the status quo for allogeneic sct–eligible patients.
The nccn no longer recommends the use of ifnα as initial therapy for cml; however, and although the evidence is limited, ifnα may be considered for second-line therapy in transplantation-ineligible patients who fail to respond to imatinib mesylate 14.
The Canadian representatives recommend that the cytogenetic response of patients who are initially treated with and remain on ifnα should be assessed. All patients who have less than a ccr should switch to imatinib mesylate. Patients who achieve a ccr on ifnα and who are tolerant of therapy should remain on ifnα and continue to be monitored every 3 months, as patients on imatinib mesylate are.
This recommendation differs slightly from the Ontario clinical practice guidelines, which suggest that it is reasonable for physicians to recommend a switch from ifnα to imatinib mesylate, because many patients are unlikely to remain on long-term ifnα, and the survival benefit with imatinib mesylate is not inferior to that of ifnα 29.
The National Institute for Clinical Excellence recommends that the decision to switch to imatinib mesylate should be based on drug tolerance, disease response, and patient preference 16. The filmc recommends the continuation of ifnα for stable patients with tolerable side effects 28.
Recommendation 5
The ccgm-cml recommends that patients on a pre-existing regimen of ifnα with or without cytarabine who experience intolerable side effects switch to imatinib mesylate. The ccgm-cml recommends the assessment of cytogenetic response in patients who are initially treated with and remain on ifnα. All patients with less than a ccr should be switched to imatinib mesylate. Patients who achieve a ccr on ifnα and who are tolerant of therapy should remain on ifnα and be monitored.
3.6.3 Imatinib Mesylate
Clinical trials provide convincing evidence that imatinib mesylate, formerly called ST1571, an orally administered selective inhibitor of the Abl, Arg, Pdgfr, and Kit protein tyrosine kinases, is a useful treatment for all phases of cml 5,13,24 (Level I.1iDii). Most significantly, it is superior to ifnα plus cytarabine in slowing disease progression and improving survival in cp patients 13 (Level I.1iDii). Unfortunately, the results with more advanced disease are usually short-lived, and these patients should be considered for transplant at an earlier stage.
The pivotal clinical data come from the iris study, an open-label, phase iii, randomized study of 1106 patients with newly diagnosed cp cml 13 (Level I.1iiDii). These de novo patients were randomized to receive imatinib mesylate (400 mg daily) or ifnα plus cytarabine arabinoside. After a median follow-up of 19 months, the estimated rate of minor cytogenetic response (mcr) at 18 months was 85.2% for imatinib mesylate and 22.1% for combination therapy (p < 0.001). The estimated rates of ccr at 18 months were 76.2% (95% ci: 72.5%–79.9%) for imatinib mesylate and 14.5% (95% ci: 10.5%–18.5%) for combination therapy (p < 0.001). Freedom from progression to ap or bc was estimated at 96.7% for imatinib mesylate and 91.5% for combination therapy (p < 0.001) 13 (Level I.1iiDii).
An update of iris was presented at the asco Annual Meeting in June 2006 30,31. The trial is no longer randomized, because only 3% of the originally assigned cohort remain in the control arm; the remainder have crossed over to the imatinib arm. Hence, iris is now a long-term follow-up study. Reasons for crossover included intolerance, lack of response, and the fact that imatinib went on the market in the United States 13. No data for the control arm or crossover patients were presented at this update.
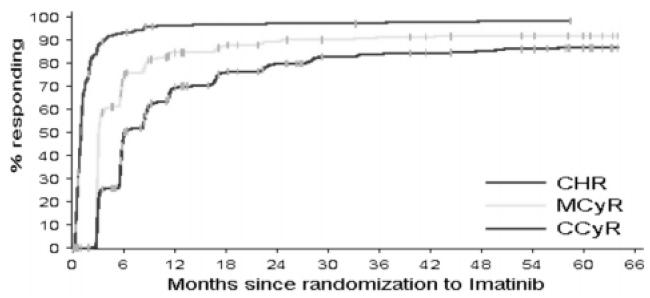
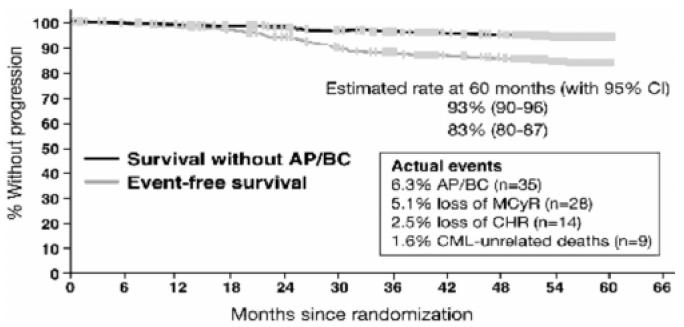
In the imatinib arm, at 60 months, 98% of patients had achieved a complete hematologic response (chr), 92% had an mcr, and 87% had a ccr (Figure 2) 30,31. At 60 months, 97% of patients who achieved a 3-log reduction in BCR/ABL transcripts at 12 months (ccr) remained progression-free. If they had achieved a <3-log but >2-log reduction in transcripts at 12 months (partial cytogenetic response), pfs was 93% 30,31. Among patients with no cytogenetic response at 12 months, pfs was 81% 30,31. Event-free survival was estimated at 83% (95% ci: 80%–87%) at 60 months, and pfs was estimated at 93% (95% ci: 90%–96%) at 60 months for patients on first-line imatinib (Figure 3) 30,31 (Level II-2.3iDii).
FIGURE 2.
Progression-free survival rates and survival without accelerated phase or blast crisis with first-line imatinib [from the International Randomized Study of Interferon versus ST1571 (imatinib)] 31. CHR = complete hematologic response; MCyR = major cytogenetic response; CCyR = complete cytogenetic response.
FIGURE 3.
Estimated response to first-line imatinib. AP = accelerated phase; BC = blast crisis; CI = confidence interval; MCyR = major cytogenetic response; CHR = complete hematologic response; CML = chronic myelogenous leukemia. (Reproduced with permission from B.J. Druker.)
Using a Kaplan–Meier model based on the intent-to-treat principle, the overall rate of survival for patients on initial imatinib therapy at 5 years was estimated at 89% (95% ci: 86%–92%), regardless of whether crossover had occurred 30,31. This analysis included deaths occurring after the discontinuation of imatinib therapy. Among patients initially randomized to imatinib, 57 deaths occurred: 14 after bm transplantation, 20 unrelated to cml, and 23 related to cml 30,31. When the analysis was censored for cml-related deaths, the estimated overall survival at 60 months was 95% 30,31 (Level II-2.3iDii). International experts have agreed that this survival outcome is better than that of any other reported treatment for cp cml 32.
A major issue in treating newly-diagnosed cp patients with imatinib mesylate is how long to wait before determining an adequate response. The analysis of the iris cytogenetic data has provided evidence that the degree of cytogenetic response in imatinib mesylate–treated patients measured at 3, 6, and 12 months is predictive of pfs at 24 months, as had been surmised when the trial was first conceptualized 33 (Level II-2.3iiiDii). The cytogenetic response observed at 3, 6, and 12 months can also predict the probability of achieving an mcr or ccr at 6, 12, and 24 months (Table III) 33 (Level II-2.3iiiDii). Recent iris data reported at asco 2006 indicate that a late response to imatinib may occur and that the timing of the response does not affect its durability 30,31.
TABLE III.
Probability of achieving a complete cytogenetic response (ccr) and a minor cytogenetic response (mcr) at 6, 12, and 24 months by cytogenetic response at 3, 6, and 12 months
| Response level | ||||
|---|---|---|---|---|
| >95% | 66%–95% | 36%–65% | 1%–35% | |
| Cytogenetic response at 3 months: | ||||
| Probability (range) of achieving a ccr at | ||||
| 6 Months | 3 (0–10) | 8 (0–20) | 11 (0–22) | 55 (47–63) |
| 12 Months | 25 (9–41) | 29 (10–48) | 37 (21–54) | 84 (78–90) |
| 24 Months | 48 (30–66) | 56 (33–79) | 61 (44–78) | 91 (86–96) |
| Probability (range) of achieving an mcr at | ||||
| 6 Months | 9 (0–20) | 38 (18–57) | 60 (43–77) | |
| 12 Months | 47 (29–65) | 60 (39–80) | 83 (70–96) | |
| 24 Months | 57 (39–75) | 70 (50–90) | 86 (74–98) | |
| Cytogenetic response at 6 months: | ||||
| Probability (range) of achieving a ccr at | ||||
| 12 Months | 7 (0–20) | 19 (0–38) | 5 (0–16) | 59 (48–71) |
| 24 Months | 14 (0–32) | 46 (20–72) | 50 (26–73) | 77 (67–87) |
| Probability (range) of achieving an mcr at | ||||
| 12 Months | 13 (0–29) | 50 (25–75) | 79 (60–98) | |
| 24 Months | 34 (9–60) | 64 (40–89) | 93 (80–100) | |
| Cytogenetic response at 12 months: | ||||
| Probability (range) of achieving a ccr at | ||||
| 24 Months | 9 (0–27) | 14 (0–41) | 20 (0–56) | 57 (36–71) |
| Probability (range) of achieving an mcr at | ||||
| 24 Months | 27 (0–54) | 14 (0–41) | 67 (28–100) | |
Recent evidence also suggests that achieving a ccr is an important factor that influences pfs. Among 370 patients who had achieved a ccr by q-rt-pcr at 3 months, those who had a major molecular response (mmr), defined as 3-log or greater reduction in BCR/ABL mrna levels, had a 100% probability of remaining progression-free at 24 months, regardless of medication 34 (Level II-2.3iiiDii). Imatinib mesylate was more efficient than ifnα plus cytarabine was in eliciting a mmr 34 (Level II-2.3iiiDii). At 12 months, about 39% of imatinib mesylate patients as compared with 2% of combination-therapy patients achieved a mmr 34 (Level II-2.3iiiDii).
The 60-month iris data, presented at asco in June 2006, indicate that a greater degree of cytogenetic response appears to convey a greater degree of protection from progression to ap or bc, regardless of when the cytogenetic response is achieved 30,31. Patients who had a ccr early or at 12, 18, or 24 months achieved similar levels of protection against progression.
The effects of imatinib appeared to be durable: Patients who achieved a ccr within the first 24 months of imatinib therapy had a 99% survival rate at 60 months. No patient with a ccr and mmr at 18 months had progressed to ap or bc at 60 months 30,31. However, all patients with a ccr at 18 months did exceptionally well regardless of their molecular response; in patients with a <3-log reduction in BCR/ABL, the estimated rate of survival at 60 months was 98% 30,31. Notably, those who failed to achieve a ccr by 18 months had a significantly poorer outcome, with an estimated rate of survival of 87% at 60 months 30,31 (Level II-2.3iiiDii).
Regarding how long to wait for an adequate response to imatinib, 64% of patients who achieved a partial cytogenetic response by 12 months achieved a ccr at 24 months. Half of patients with a partial response at 18 months later achieved a ccr 30,31. More than one third (36%) of patients with no cytogenetic response at 12 months eventually developed a ccr. Among those with no cytogenetic response at 18 months, 27% later developed a ccr 30,31 (Level II-2.3iDiii).
Overall rates of disease progression were low, and the rate of any progression for all patients on initial imatinib therapy declined over time (Table IV) 30,31 (Level II-2.3iDii). This rate of transformation is a major improvement over the traditional rate of 10%–15% observed in patients treated with conventional therapy and the 5%–10% observed in patients treated with ifnα plus cytarabine.
TABLE IV.
| Year | All events (%)a | Progression toap/bc(%) |
|---|---|---|
| 1 | 3.3 | 1.5 |
| 2 | 7.5 | 2.8 |
| 3 | 4.8 | 1.6 |
| 4 | 1.5 | 0.9 |
| 5 | 0.9 | 0.8 |
All deaths or loss of response, including progression to accelerated phase (ap) or blast crisis (bc).
The overall risk of transformation to ap or bc for patients on first-line imatinib was associated with the patient’s level of response, regardless of the timing of response achievement. Of particular interest, in patients who achieved a ccr on imatinib, the risk of any progression declined over time to less than 0.5% at 5 years 30,31. For patients on first-line imatinib, the annual percentages of progression to ap or bc declined from 2.1% in the first year to 0.8% in the second year, 0.3% in the third year, and 0% in the fourth year after achieving a ccr 30,31. The timing of the ccr appeared to have no impact on this result. Once ccr was achieved, the longer the patient remained on imatinib, the lower became the risk of any progression over time (Level II-2.3iDii).
Evidence from iris clearly shows that imatinib mesylate is more effective than ifnα plus cytarabine as first-line therapy for newly diagnosed patients with cp cml 13,31,32 (Level I.1iDii). Imatinib mesylate prolongs pfs and elicits a beneficial cytogenetic response in more patients without the toxicity of ifnα 13,31,32 (Level I.1iDii; Level II-2.3iiiDii). Based on this evidence, the ccgm-cml reached a consensus regarding the use of imatinib mesylate as first-line therapy in this patient group. Data from q-rt-pcr showing that the extent of molecular response is predictive of pfs and can be evaluated as early as 3 months further supports the group’s position27 (Level I.1iDii).
The nccn, the National Institute for Clinical Excellence, the filmc, Ontario-based guidelines, and the European LeukemiaNet expert panel recommend the use of imatinib mesylate as first-line therapy in newly diagnosed patients with cp, Ph chromosome–positive cml 14,16,28,29,32. The European LeukemiaNet recommends a trial of imatinib mesylate for any patient with newly diagnosed cp cml regardless of risk, given that a patient’s early response to imatinib can either reinforce or weaken the indication for allogeneic sct 32. Imatinib is also recommended as second-line therapy for patients who are refractory or intolerant to ifnα and for previously untreated patients with disease progression 14,29.
The best outcome for allogeneic sct is a 5-year survival rate of 75%–80% 14,35. Unfortunately, this result can be achieved only in a minority of cml patients. It is roughly equivalent to the overall survival rate predicted for patients on imatinib mesylate therapy, which is feasible in most cml patients.
The greater efficacy of medical therapy with imatinib mesylate has clearly shifted the established risk:benefit ratio with respect to allogeneic sct. Much discussion has ensued internationally and within the ccgm-cml consultative meetings about how to alter pre-existing recommendations for cml patients. At the regional and steering committee meetings, two major approaches, both of which accepted the premise that the survival benefit conferred by imatinib mesylate will likely be sustained on long-term follow-up, were considered.
The first approach is to redefine the indications for allogeneic sct to select younger patients who are at lowest risk for complications. The second is to offer imatinib mesylate as first-line therapy to all patients, to monitor the response to treatment, and to reserve allogeneic sct for patients who fail to respond to a degree predictive of prolonged disease-free survival. Patient preference between these two approaches, after full discussion of the relative risks and benefits, is to be respected and will often dictate the choice of treatment.
This choice was supported by evidence that the cytogenetic response to imatinib mesylate can be determined as early as 3 months after the start of treatment, well within the 1-year limit from diagnosis for optimal transplantation. Another key factor that contributed to this position was the comparison between the numbers of patients who are eligible and who stand to benefit from either treatment.
Many Canadian representatives strongly believe that it is essential to discuss the option of transplantation with their patients and to refer, at the time of diagnosis, any transplantation-eligible patient to a transplantation centre for standard evaluation. For any patient who fails to respond to imatinib mesylate, pre-assessment of transplantation risk will guide the decision to pursue transplantation or an alternative medical therapy, reducing further delays to transplantation. Having sibling matches or unrelated donors identified beforehand will reduce the delay significantly.
Recommendation 6
For all newly diagnosed patients with chronic-phase cml who do not elect related-donor allogeneic sct as first-line therapy, imatinib mesylate is recommended.
3.6.4 Initiation of Imatinib
Imatinib mesylate was approved by Health Canada in late 2001 for newly diagnosed, cp cml patients at a dose of 400–600 mg daily and is now approved for patients in ap and bc at a dose of 600–800 mg daily. Health Canada approved a dose increase to 600 mg or 800 mg from 400 mg daily in adult cml patients with cp disease, and practitioners may consider 600 mg to 800 mg (given as 400 mg twice daily) in adult cml patients in ap or bc in the absence of severe adverse drug reactions and severe non-leukemia-related neutropenia or thrombocytopenia in the following circumstances:
Disease progression (at any time)
Failure to achieve a satisfactory hematologic response after at least 3 months of treatment
Failure to achieve a cytogenetic response after 12 months of treatment
Loss of a previously achieved hematologic or cytogenetic response 36
Imatinib mesylate (400 mg daily) is effective in cp patients after failure of ifnα therapy, but few patients achieve a molecular remission. At least one study of 36 patients has shown that high-dose imatinib mesylate (up to 800 mg daily) induces a ccr in most patients in cp after ifnα failure. The ccr was accompanied by a high rate of molecular remission 37 (Level II-2.3iiiDiii).
In a study of 108 newly diagnosed patients in cp, Cortes et al. 38 (Level II-2.3iiiDiii) reported at ash 2003 that, as compared with the 400 mg daily dose, the 800 mg daily dose results in higher rates of ccr and molecular remission, with some increase in myelosuppression.
Higher doses of imatinib mesylate may overcome disease-poor response to conventional doses (400 mg daily) in cp patients. Limited experience suggests that up to one third of cp patients will achieve a major cytogenetic response with dose escalation 5. Ontario-based guidelines recommend dose escalation to 800 mg daily in cp patients who do not achieve a chr after 3 months—or at least an mcr after 12 months of therapy 29. The nccn recommends dose escalation to 600–800 mg daily after 6 months, as tolerated, in patients with a ccr, a partial cytogenetic response, or an mcr, and 800 mg daily as one therapeutic option in patients with no cytogenetic response 14.
In one study of 34 patients treated for cytogenetic resistance or relapse, 56% achieved a complete or partial cytogenetic response to higher doses (600–800 mg daily) of imatinib mesylate 39 (Level II-2.3iiiDiii). Among 20 patients who had hematologic resistance or relapse, 65% achieved a complete or partial hematologic response 39 (Level II-2.3iiiDiii). Doses of 800 mg daily are less well tolerated, with a higher incidence of fluid retention, skin rashes, and muscle cramps 5.
Preliminary research by Kantarjian et al. 40 (Level II-1.2A) indicates that a higher starting dose of 800 mg daily of imatinib mesylate elicits a significantly higher ccr, mmr, and complete molecular response in patients with Ph-positive cp cml than does a starting dose of 400 mg daily. Adverse events were similar in type and frequency for both dosages; however, at least one third of patients taking 800 mg daily required a dose reduction within 3–12 months.
At ash 2004, Cortes et al. 41 reported that initiating high-dose [hd (800 mg daily)] imatinib therapy results in higher rates of ccr and molecular remission in treatment-naïve cp patients than does standard-dose [sd (400 mg daily)] therapy (Level II- 2.3iDii). In that study, 222 patients were allocated among three clinical trials: one trial of sd therapy (n = 50) and two with a hd regimen (n = 172). The estimated pfs at 12 months was 92% in the sd trial and 99% in the hd trials (p = 0.42). Patients on hd therapy had some increase in myelosuppression, as shown by these findings: grade 3 or worse anemia (7% on hd vs. 4% on sd), neutropenia (39% vs. 20%), and thrombocytopenia (27% vs. 12%). At 12 months, more patients in hd trials required dose reduction (36% vs. 14%).
In ap and bc studies to date, the main impact of higher imatinib doses is on time-to-progression and early survival 5.
After reviewing the evidence, the ccgm-cml reached a consensus that the minimum starting doses of imatinib mesylate should be 400 mg daily in patients in cp, 600 mg daily in patients in ap, and up to 800 mg daily in patients in bc.
Recommendation 7
The ccgm-cml recommends the following minimum starting dosages of imatinib mesylate for patients with cml:
Chronic phase 400 mg daily
Accelerated phase 600 mg daily
Blast crisis Up to 800 mg daily
In patients who switch to imatinib mesylate after failure of ifnα with or without cytarabine, the ccgm-cml recommends a starting dose of 400 mg daily of imatinib mesylate.
3.6.5 Monitoring Patient Response to Imatinib Mesylate
The ccgm-cml representatives felt that it was important to develop specific guidelines for patient monitoring to track therapeutic efficacy and development of resistance in patients on imatinib mesylate. The representatives strongly felt that a lack of routine monitoring compromises patient care, because decisions about adjusting or choosing alternative therapy cannot be made in a timely fashion to benefit patients.
Disease response to imatinib mesylate should be evaluated at 3-month intervals by q-rt-pcr or fish. Annual bm cytogenetic analysis should be performed in patients to detect evidence of clonal evolution and to document the presence of secondary cytogenetic changes that may be present in patients who achieve ccr. The appearance of secondary chromosomal abnormalities is often transient, and their prognostic significance has yet to be determined 15.
The Canadian representatives defined the major treatment-response milestones for patients in cp as the achievement of chr at 3 months, mcr at 12 months, ccr at 18 months, and mmr at 24 months. Patients who reach all of these milestones should continue on the same dose of imatinib mesylate as long as their response is sustained. At present, no step-down protocols have been developed for long-term maintenance therapy with imatinib mesylate. Failure to achieve the recommended therapeutic milestones or loss of responsiveness to imatinib mesylate on continued monitoring indicates a need to consider a change of therapy.
While acknowledging that the optimal guidelines for imatinib mesylate monitoring are unclear, the nccn suggests that cytogenetic testing may begin as early as 3 months after the start of therapy 14. In Ph-positive patients, bone marrow cytogenetics are recommended at 6 and 12 months after the start of therapy. After the patient achieves a ccr, the nccn recommends fish or q-rt-pcr to monitor patients every 3–6 months. If the patient has a >1-log increase on q-rt-pcr or a positive fish, bone-marrow cytogenetics are recommended. If no sign of disease progression is found, annual bone-marrow cytogenetics are recommended to detect clonal abnormalities 14.
The expert panel of the European LeukemiaNet recommends evaluation of hematologic response every 2 weeks until a chr is achieved and confirmed. They recommend cytogenetic evaluation before treatment, at least every 6 months until a ccr is achieved and confirmed, and then once every 12 months. They recommend checking the patient’s molecular response every 3 months 32. After the patient attains a mmr, conventional cytogenetic evaluations may be performed less frequently, depending on the patient’s clinical, hematologic, and molecular findings 32. The expert panel recommends fish only prior to treatment to identify Ph-negative patients 32. They state that chr, ccr, and mmr must be confirmed on two subsequent occasions 32.
Recommendation 8
The ccgm-cml recommends the following tests and frequency of testing to monitor hematologic, cytogenetic, and molecular response in all patients on imatinib mesylate and to guide therapeutic decision-making:
bm cytogenetic testing
At diagnosis
Annually, if a major cytogenetic response is maintained
q-rt-pcr
At diagnosis and every 3 months (fish may be substituted every 3 months until ccr); q-rt-pcr and fish should be performed at standardized laboratories
If a ≥ 0.5-log increase occurs, the test should be repeated within 4 weeks; mutational analysis is recommended
ABL-kinase sequencing for mutations
At confirmed increase of 0.5 log in the q-rt-pcr; ABL sequencing should be performed at standardized laboratories
The ccgm-cml recommends the achievement of the following therapeutic milestones, to indicate the successful progression of imatinib mesylate therapy in cp patients with cml:
chr at 3 months
Major cytogenetic response at 12 months
ccr at 18 months
mmr at 24 months
Failure to achieve these therapeutic milestones within the specified time limits indicates a need to reconsider the therapeutic strategy. The ccgm-cml recommends these alternatives:
Allogeneic sct, if an option
Increasing the dose of imatinib mesylate up to 800 mg daily
On continued lack of response despite maximum dose escalation, discontinuation of imatinib mesylate treatment and consideration of alternative therapy is justifiable. Once the milestones have been achieved, treatment should be maintained indefinitely as long as the patient continues to respond.
3.6.6 Emergence of Resistance in Imatinib Mesylate-Treated Patients
Resistance to imatinib mesylate has emerged in a proportion of patients with cml who are treated with this drug as first-line therapy. Acquired resistance to imatinib mesylate is almost always associated with reactivation of Bcr/Abl kinase activity 42. In most cases, resistance is attributable to the presence of point mutations in the BCR/ABL kinase domain 43. Most patients who are prospectively monitored by q-rt-pcr and who have as little as a doubling in the level of BCR/ABL transcripts are shown to harbour ABL-kinase mutations 44 (Level II-2.3iiiDiii).
A spectrum of ABL-kinase mutations has been described that variably abrogates the binding of imatinib mesylate and confers partial-to-complete resistance to imatinib mesylate. The presence of BCR/ABL kinase domain mutations has important prognostic and therapeutic implications. The location of mutations—within rather than outside the P-loop of the kinase domain—appears to have a major impact on the natural history of the disease 45 (Level II-2.3iD). Patients with mutations in the P-loop have a particularly poor prognosis 43,44.
In an Australian study, 12 of 13 patients (92%) with P-loop mutations died within a median of 4.5 months after mutation detection, but only 3 of 14 patients (21%) with non-P-loop mutations had died after a median follow-up of 11 months 44. In that study, a longer time from diagnosis to the start of imatinib therapy and a failure to achieve an mcr by 6 months were associated with a higher incidence of mutation. The development of ABL mutations in cml patients confers varying degrees of resistance to imatinib mesylate, adversely affecting survival 45.
For optimal clinical management of patients on imatinib mesylate, early detection of treatment-resistant ABL mutations is crucial. Testing for ABL mutation should be requested for all cp cml patients who fail imatinib mesylate therapy (see the next subsection). Mutation testing should be restricted to a limited number of specialized laboratories. Routine screening of other responding or stable patients should not be done, because these patients almost never have a mutation.
3.6.7 Failure of Imatinib Mesylate Therapy
In the event that therapeutic milestones are not reached or that disease progression is documented by q-rt-pcr or fish, transplantation eligibility becomes a major variable in decision-making. Canadian representatives agreed that all patients who fail imatinib mesylate therapy and who are eligible for transplantation should be referred for allogeneic sct.
Ontario-based guidelines suggest that, for transplantation-eligible patients who may choose allogeneic sct as second-line therapy in the advent of imatinib mesylate failure, cytogenetic analysis should be performed within 12 months of the start of imatinib therapy 29.
The filmc recommends allogeneic sct for eligible patients who have no cytogenetic response to imatinib mesylate at 3 months or who have a hematologic relapse within 12 months (5% of patients). They feel that the absence of a ccr at 12 months justifies discussion of allogeneic sct in transplantation-eligible patients under the age of 50 years 28. Similarly, the nccn recommends bm transplantation, if feasible, when no hematologic response to imatinib mesylate has occurred at 3 months 14.
The expert panel of the European LeukemiaNet defines the failure of imatinib at various stages of treatment: no hematologic response at 3 months, no cytogenetic response or partial hematologic response at 6 months, less than partial cytogenetic response at 12 or 18 months, and loss of chr, ccr, or development of mutation at any time 32.
In transplantation-ineligible patients who do not have P-loop ABL mutations or who have mutations associated with only partial resistance, the recommended approach is to escalate the dose of imatinib mesylate to 800 mg daily in an attempt to re-establish responsiveness. This practice is supported by evidence of a greater, more rapid response in patients who are initially treated with doses of imatinib mesylate greater than 400 mg daily and, more importantly, after dose escalation, of salvage of up to 50% of patients who initially progress on 400 mg daily 38.
Failing this strategy, transplantation-ineligible patients could be treated with ifnα with or without cytarabine or on a phase ii protocol of newer generation drugs, other experimental drugs, immunologic manipulations, or combination therapy, if available. Patients who fail both medical treatments should be offered palliative therapy with hydroxyurea.
Recommendation 9
The ccgm-cml recommends the following definition of disease progression in compliant patients on imatinib mesylate therapy:
Transformation from cp to ap or bc
Cytogenetic (clonal) evolution in Ph-positive cells
Loss of ccr
Confirmed increase of 0.5 log or more (q-rt-pcr) for patients in ccr or better
Detection of ABL mutations with loss of response
For patients in whom imatinib mesylate fails, the ccgm-cml recommends these treatment strategies:
Allogeneic sct for all transplantation-eligible patients
Dose escalation up to 800 mg daily of imatinib mesylate in transplantation-ineligible patients who do not have ABL mutations that confer complete resistance to imatinib mesylate
Therapy with ifnα (with or without cytarabine) for transplantation-ineligible patients who fail to respond to dose escalation within 3 months or who have mutations of ABL that confer resistance
Institutional research board–approved therapeutic protocols for clinical trials of new, experimental agentsa
Treatment with hydroxyurea or busulfan in patients in whom ifnα may be deemed inappropriatea
3.7 Outstanding Issues
The recommendations of the ccgm-cml should lead to a major improvement in the treatment of patients with cml. For the few patients who are eligible for allogeneic sct, the recommendation for first-line therapy strikes a better balance—avoiding premature mortality and morbidity, while still providing the transplantation option for those who are unlikely to benefit from medical therapy or who choose a more aggressive approach up front.
The key to successful implementation of the guidelines in Canada is the availability of adequate laboratory support to monitor patient response. Without rigorous monitoring, a substantial number of patients may, over time, receive expensive and ineffective therapy, and transplantation-eligible patients may miss the opportunity to receive potentially lifesaving treatment.
Consequently, one of the ccgm-cml’s strongest recommendations is that these guidelines be implemented concurrently with the deployment of readily available standardized fish and q-rt-pcr testing for all Canadian patients. A total of seven standardized laboratories now provide q-rt-pcr cml testing in Canada. Another program to standardize up to 13 q-rt-pcr laboratories across Canada is underway. Efforts are also currently being made to establish long-term funding for Canadian referral centres to provide these services for all patients as the availability of this technology becomes more widespread.
Recommendation 10
The ccgm-cml strongly recommends standardized q-rt-pcr testing for all Canadian patients with cml. Mutational analysis should be regionalized in even fewer centres.
4. DISCLOSURE
The following physicians disclosed that they have received honoraria from Novartis Pharmaceuticals Canada Inc. for consultancy: Pierre Laneuville, Jeffrey H. Lipton, Michael Barnett, Robert Belanger, Stephen Couban, Donna Forrest, Denis-Claude Roy. Dr. Lipton also disclosed that he has received honoraria from Novartis Pharmaceuticals Canada Inc. as a participant in a speaker’s bureau.
APPENDIX A PARTICIPANTS IN REGIONAL CONFERENCES
Thierry Alcindor md abim
Specialty: Internal Medicine, Hematology, Oncology
Medical Oncologist, Montreal General Hospital and Royal Victoria Hospital
Assistant Professor, Departments of Medicine and Oncology, McGill University
Montreal, Quebec
Joffre Allard md
Specialty: Internal Medicine (QC), Hematology (QC), Medical Oncology
Active Staff Member, Department of Hematology, Centre Hospitalier Regional de Rimouski
Rimouski, Quebec
Peter Anglin md frcpc
Specialty: Internal Medicine, Hematology, Medical Oncology (Clinical Hematology)
Active Staff Member, The Credit Valley Hospital Consultant, University Health Network—Princess Margaret Hospital Site
Courtesy Staff, Dufferin–Caledon Healthcare Corp.
Instructor, University of Toronto
Toronto, Ontario
Nizar Bahlis md
Specialty: Internal Medicine, Hematology, Oncology
Assistant Professor, University of Calgary and Tom
Baker Cancer Centre
Calgary, Alberta
Isabelle Bence–Bruckler md frcpc
Specialty: Internal Medicine, Hematology (Bone Marrow Transplantation)
Staff Member, Hematology, The Ottawa Hospital—General Campus
Associate Professor, Department of Medicine, University of Ottawa
Ottawa, Ontario
Normand Blais md frcpc
Specialty: Internal Medicine, Hematology, Medical Oncology
Hematologist and Medical Oncologist, Department of Medicine, CHUM—Hôpital Notre-Dame
Laval, Quebec
Lambert Busque md frcpc
Specialty: Internal Medicine, Hematology
Staff Member, Hôpital Maisonneuve–Rosemont
Director, Molecular Hematology
Associate Professor, Department of Medicine, Université de Montréal
Montreal, Quebec
Stephen Caplan md frcpc
Specialty: Hematology (Lymphoma)
Director, Division of Hematology, Sir Mortimer
B. Davis Jewish General Hospital
Associate Professor, Departments of Medicine and Oncology, McGill University
Montreal, Quebec
Hak Ming Chiu md frcpc
Specialty: Hematology, Internal Medicine, Medical Oncology
Active Staff Member, Oshawa Lakeridge Health Corporation—Oshawa Site
Oshawa, Ontario
Kathy Chun phd fccmg
Specialty: Cancer Cytogenetics
Assistant Professor, Department of Laboratory Medicine and Pathobiology, University of Toronto
Head, Cancer Cytogenetics, University Health
Network Toronto, Ontario
Denis Cournoyer md frcpc
Specialty: Hematology, Internal Medicine
Staff Member, cusm–Montreal General Hospital
Associate Professor, Departments of Medicine and Oncology, McGill University
Montreal, Quebec
Nazim Damji md frcpc dabim
Specialty: Hematology, Oncology, Internal Medicine
Staff Member, Trillium Health Centre—Mississauga Site
Etobicoke, Ontario
Robert Delage md frcpc
Specialty: Hematology, Internal Medicine
Active Staff Member, Centre Hospitalier Affilié
de Québec—Hôpital du St-Sacrement
Associate Professor, Université Laval
Sainte-Foy, Quebec
Yvan Drolet md
Specialty: Hematology (QC), Medical Oncology (QC), Pediatrics
Staff Member, Centre Hospitalier Universitaire de
Québec—Hôtel-Dieu de Québec
Clinical Professor, Université Laval
Quebec City, Quebec
Peter Duggan md frcpc
Specialty: Hematology, Internal Medicine
Staff Member, Health Sciences Centre
St. John’s, Newfoundland
Alain Filion md frcpc
Specialty: Hematology, Internal Medicine, Medical Oncology (Hematology/Oncology)
Staff Member, Department of Hematology/Oncology, Centre Hospitalier Universitaire de Québec—Hôtel-Dieu de Québec
Hematology Oncologist, Hôtel-Dieu de Lévis
Quebec City, Quebec
Kuljit Grewal md frcpc
Specialty: Hematology, Internal Medicine
Chief Division of Hematology, Department of Medicine, Health Sciences Centre
Associate Professor, Department of Medicine, Memorial University
St. John’s, Newfoundland
Marlene Hamilton md frcpc
Specialty: Hematology, Internal Medicine, Medical Oncology
Assistant Professor, University of Alberta
Edmonton, Alberta
Wanda Hasegawa md frcpc
Specialty: Internal Medicine, Hematology (Bone Marrow Transplant)
Staff Physician, Royal University Hospital
Clinical Associate Professor, University of Saskatchewan
Saskatoon, Saskatchewan
Josée Hébert md frcpc
Specialty: Hematology, Internal Medicine
Staff Member, Hôpital Maisonneuve–Rosemont
Montreal, Quebec
Donna Hogge md Phd frcpc
Specialty: Hematology, Internal Medicine (Hematology/Oncology)
Active Staff Member, Vancouver Hospital and Health Sciences Centre, British Columbia Cancer Agency—Vancouver Cancer Centre
Clinical Professor, Department of Medicine, University of British Columbia
Vancouver, British Columbia
Kang Howson–Jan md frcpc
Specialty: Internal Medicine (Bone Marrow Transplant)
Staff Member, Department of Hematology, London Health Sciences Centre—Victoria Campus Site Assistant Professor, University of Western Ontario Consultant, London Regional Cancer Centre
London, Ontario
Sabir Hussain md frcpc
Specialty: Internal Medicine, Hematology, Oncology
Staff Member, Department of Oncology, Dr. Everett Chalmers Regional Hospital (SH)
Fredericton, New Brunswick
Suzanne Kamel–Reid Phd
Director, Molecular Diagnostics, Department of Pathology, The University Health Network
Head, Genetics Program, Toronto Medical Laboratories/UHN
Scientist, Ontario Cancer Institute
Professor, Laboratory Medicine and Pathobiology, University of Toronto
Toronto, Ontario
Henry Krieger md frcpc
Specialty: Internal Medicine, Hematology (Medical Oncology)
Staff Member, The Scarborough Hospital—General Division, Sunnybrook and Women’s College Health Sciences Centre
Lecturer, University of Toronto
Toronto, Ontario
George Kutas md frcpc
Specialty: Internal Medicine (Hematology)
Active Staff Member, Sunnybrook and Women’s
College Health Sciences Centre
Associate Professor, University of Toronto Physician, Toronto–Sunnybrook Regional Cancer Centre
Toronto, Ontario
Sylvie Lachance md frcp
Specialty: Internal Medicine, Hematology
Staff Hematologist–Oncologist, Stem Cell Transplant Unit, Montreal General Hospital and Royal Victoria Hospital
Associate Professor, McGill University
Montreal, Quebec
Wendy Lam Bsc (Pharm) md frcpc
Specialty: Internal Medicine, Hematology
Hematologist/Medical Oncologist, Burnaby Hospital Regional Cancer Centre
Burnaby, British Columbia
Loree Larratt md frcpc
Specialty: Internal Medicine, Hematology Staff Member, University of Alberta Hospital—Stollery Children’s Centre WCM, Cross Cancer Institute
Associate Professor, University of Alberta
Edmonton, Alberta
Brian Leber md frcpc
Specialty: Internal Medicine, Hematology
Attending Physician, Hamilton Health Sciences—McMaster University Medical Centre
Associate Professor, Medicine, and Associate Member, Biochemistry, McMaster University
Hamilton, Ontario
Heather Leitch md Phd frcpc
Specialty: Internal Medicine, Hematology
Hematologist, St. Paul’s Hospital
Clinical Associate Professor, University of British Columbia
Vancouver, British Columbia
Charles Li md frcpc
Specialty: Internal Medicine, Hematology
Staff Member, St. Paul’s Hospital, University of British Columbia
Clinical Assistant Professor, Department of Medicine, University of British Columbia
Vancouver, British Columbia
Michel Maheu md frcpc
Specialty: Internal Medicine, Hematology/Pathology, Medical Oncology (QC)
Active Staff Member, Centre Hospitalier Pierre–Le Gardeur
Lachenaie, Quebec
Kelly McNeil Bsc Clsp (mb)
Senior Molecular Technologist, Cancer Genetics
Laboratory, B.C. Cancer Agency
Vancouver, British Columbia
Jacinta Meharchand mb Chb frcpc
Specialty: Internal Medicine, Hematology, Oncology
Division Director, Hematology–Oncology, Toronto
East General Hospital
Toronto, Ontario
Krishan Mohindra md frcpc
Specialty: Internal Medicine, Hematology
Active Staff Member, Peterborough Regional
Health Centre
Peterborough, Ontario
Jean–Pierre Moquin md frcpc
Specialty: Internal Medicine, Hematology, Medical Oncology (QC)
Staff Member, Hôpital du Sacre-Coeur de Montréal
Associate Professor, Université de Montréal
Montreal, Quebec
Thomas Nevill md frcpc
Specialty: Internal Medicine, Hematology (Bone Marrow Transplant)
Active Staff Member, Vancouver Hospital and Health Sciences Centre, British Columbia Cancer Agency—Vancouver Cancer Centre
Associate Professor, University of British Columbia
Vancouver, British Columbia
Michael Noble md frcpc CertABIM (Medicine) CertABIM (Hematology)
Specialty: Internal Medicine, Hematology (Hematology/Oncology)
Active Staff Member, Royal Columbian Hospital, Eagle Ridge Hospital and Health Care Centre
Clinical Assistant Professor, University of British Columbia
Vancouver, British Columbia
Harold J. Olney md frcpc
Specialty: Internal Medicine, Hematology (QC), Medical Oncology
Staff Member, Centre Hospitalier de l’Université
de Montréal Hôpital Notre-Dame
Montreal, Quebec
Jaroslav Prchal md frcpc
Specialty: Hematology, Medical Oncology (QC)
Chief, Department of Oncology, St. Mary’s Hospital
Active Staff Member, McGill University Health Centre—Royal Victorial Hospital
Associate Professor, McGill University
Montreal, Quebec
Yasmin Rahim md frcpc facp
Specialty: Internal Medicine, Hematology, Medical Oncology
Staff Member, Department of Medical Oncology, Toronto East General and Orthopaedic Hospital Inc. Part-time Staff Member, Department of Hematology, Sunnybrook Health Science Centre
Part-time Teaching Staff Member, University of Toronto
Toronto, Ontario
Marcel Rochon md
Specialty: Hematology (QC), Medical Oncology (QC)
Member, Department of Hematology, Centre Hospitalier Universitaire de Sherbrooke—Hôpital Fleurimont
Consultant, Centre Hospitalier Universitaire de
Sherbrooke—Hôpital Hôtel-Dieu
Sherbrooke, Quebec
Sheldon Rubin md frcpc
Specialty: Internal Medicine, Hematology
Active Staff Member, Department of Internal Medicine, The Moncton Hospital
Courtesy Staff Member, Dr. Georges-L. Dumont Regional Hospital
Moncton, New Brunswick
Morel Rubinger md frcpc
Specialty: Internal Medicine, Hematology, Medical Oncology
Staff Member, Department of Medical Oncology, Health Sciences Centre
Associate Professor, Department of Internal Medicine, University of Manitoba
Acting Director, Blood and Marrow Transplant Program—Manitoba
Chair, Lymphoma Working Group
Winnipeg, Manitoba
Mitchell Sabloff md frcpc
Specialty: Haematology
Staff Member, The Ottawa Hospital—General
Campus Ottawa, Ontario
Sandeep Sehdev md frcpc
Specialty: Internal Medicine, Medical Oncology (Hematology)
Staff Member, William Osler Health Centre—Brampton Memorial Hospital Campus
Brampton, Ontario
April Shamy md frcpc
Specialty: Internal Medicine, Hematology
Staff Member, Sir Mortimer B. Davis Jewish General Hospital
Assistant Professor, Department of Medicine, McGill University
Montreal, Quebec
David Sheridan md frcpc
Specialty: Internal Medicine, Hematology
Professor, Departments of Medicine and Pathology, University of Saskatchewan
Saskatoon, Saskatchewan
Chaim Shustik md
Specialty: Internal Medicine, Hematology (QC), Medical Oncology (QC)
Attending Physician, McGill University Health Centre—Royal Victoria Hospital
Professor of Medicine, McGill University
Montreal, Quebec
Raynald Simard md frcpc
Specialty: Internal Medicine, Hematology, Medical Oncology (QC)
Active Staff Member, Department of Hematology, Complexe Hospitalier de la Sagamie
Chicoutimi, Quebec
Denis Soulières md Msc frcpc
Specialty: Hematology, Medical Oncology
Staff Member, Centre Hospitalier de l’Université
de Montréal—Hôpital Notre-Dame
Director, Hematology Laboratory of Molecular Biology and Special Hematology
Assistant Professor, Université de Montréal
Montreal, Quebec
Douglas Stewart md frcpc
Specialty: Internal Medicine, Medical Oncology
Staff Member, Tom Baker Cancer Centre, Foothills Medical Centre
Associate Professor, University of Calgary
Calgary, Alberta
Claude Tessier md
Specialty: Hematology (QC), Medical Oncology (QC) (Ovarian Disorders)
Staff Member, Centre Hospitalier Universitaire de Québec—Hôtel-Dieu de Québec
Active Staff Member, Hôtel-Dieu de Lévis
Quebec City, Quebec
Cynthia Toze md frcpc abim abc (Internal Medicine) mhsc (Epidemiology)
Specialty: Internal Medicine, Hematology
Active Staff Member, Division of Hematology, Vancouver Columbia Cancer Agency, Vancouver Hospital and Health Sciences Centre
Associate Professor, University of British Columbia
Member, Leukemia/BMT Program of British Columbia
Vancouver, British Columbia
Robert Turner md mdcm frcpc
Specialty: Hematology/Oncology
Staff Member, Clinical Hematology, University of Alberta Hospital
Senior Specialist, Cross Cancer Institute
Professor, University of Alberta
Edmonton, Alberta
Paul D. Walde md frcpc lrcp mrcs mrcp abim
Specialty: Internal Medicine, Medical Oncology
Active Consulting Staff Member, Algoma District Cancer Program, Sault Area Hospitals
Sault-Ste-Marie, Ontario
Parveen Wasi md frcpc
Specialty: Internal Medicine, Hematology
Staff Member, Hamilton Health Sciences—McMaster Children’s Hospital
Associate Professor, Department of Medicine, McMaster University
Hamilton, Ontario
Hilary Wass md frcpc
Specialty: Hematology
Staff Member, British Columbia Cancer Agency—Vancouver Island Cancer Centre
Staff Member, Vancouver Island Health Authority
Victoria, British Columbia
Jonathan Wilson md frcpc facp
Specialty: Internal Medicine, Hematology, Medical Oncology
Staff Member, Humber River Regional Hospital—Church Site
Courtesy Staff Member, University Health Network—Toronto Western Hospital Site, West Park Healthcare Centre
Lecturer, University of Toronto
Toronto, Ontario
Anargyros Xenocostas md frcpc
Specialty: Internal Medicine, Hematology
Assistant Professor of Medicine and Consultant in Hematology, London Health Sciences Centre and the University of Western Ontario
London, Ontario
Sean Young Phd
Laboratory Geneticist, Cancer Genetics Laboratory, B.C. Cancer Agency
Vancouver, British Columbia
Appendix b CML FACULTY/STEERING COMMITTEE
Pierre Laneuville md frcpc (Committee Chair)*
Specialty: Internal Medicine, Hematology (Hematology/Oncology)
Division of Hematology, McGill University Health Centre—Royal Victoria Hospital
Associate Professor, Department of Medicine and Oncology, McGill University
President, Canadian Hematology Society Montreal, Quebec
Chair, Quebec and Atlantic Provinces Regional Committee
Michael Barnett bm frcpc frcp Frcpath*
Specialty: Hematology
Head of Hematology, University of British Columbia and Vancouver General Hospital
Member, Leukemia/Bone Marrow Transplant Program of British Columbia, British Columbia Cancer Agency, and Vancouver General Hospital
Vancouver, British Columbia
Chair, Western Provinces Regional Committee
Robert Bélanger md
Specialty: Hematology (QC), Hematology/Pathology
Staff Member, Department of Hematology, Hôpital Maisonneuve-Rosemont
Assistant Clinical Professor, Université de Montréal Montreal, Quebec
Member, Quebec and Atlantic Provinces Regional Committee
Stephen Couban md frcpc
Specialty: Internal Medicine, Hematology
Director, Blood and Marrow Transplant Program
Queen Elizabeth II Health Sciences Centre
Associate Professor, Dalhousie University
Halifax, Nova Scotia
Member, Quebec and Atlantic Provinces Regional Committee
Donna Forrest md frcpc
Specialty: Internal Medicine, Hematology
Associate Member, Leukemia/BMT Program of British Columbia, British Columbia Cancer Research Centre
Staff Member, Vancouver Hospital and Health Sciences Centre
Vancouver, British Columbia
Member, Western Provinces Regional Committee
Hans Messner Phd md frcpc
Specialty: Internal Medicine
Staff Member, Ontario Cancer Institute, University Health Network—Princess Margaret Hospital Site
Professor, Department of Medicine, University of Toronto
Toronto, Ontario
Member, Ontario Regional Committee
Denis-Claude Roy md frcpc abim abim (HEMATOLOGY)
Specialty: Internal Medicine, Hematology (Leukemia and Lymphoma)
Director, Cellular Therapy Laboratory and Program, Hôpital Maisonneuve–Rosemont
Professor of Medicine, Université de Montréal
Montreal, Quebec
Member, Quebec and Atlantic Provinces Regional Committee
Jeffrey H. Lipton Phd md frcpc*
Specialty: Internal Medicine, Medical Oncology (Bone Marrow Transplant)
Head, Chronic Myelogenous Leukemia Group, and Member, Allogeneic Blood and Marrow Transplant Unit, University Health Network—Princess Margaret Hospital Site
Associate Professor of Medicine, University of Toronto
Toronto, Ontario
Chair, Ontario Regional Committee
Appendix c LEVELS OF EVIDENCE
In keeping with the National Cancer Institute of Canada’s practice of evaluating scientific evidence using the guidelines of recognized organizations, such as the Canadian Task Force on Preventive Health Care and the United States National Cancer Institute, the Canadian Consensus Group on the Management of Chronic Myeloid Leukemia evaluated the evidence according to these classification systems.
C.1 Research Design Rating
The Research Design Rating set forth by the Canadian Task Force of Preventive Health Care assigns a rating of i to iii as presented in the table that follows A:
| Rating | Levels of evidence |
| I | Evidence from one or more randomized controlled trials |
| II-1 | Evidence from one or more controlled trials without randomization |
| II-2 | Evidence from cohort or case–control analytic studies, preferably from more than one centre or research group |
| II-3 | Evidence from comparisons between times or places with or without the intervention; dramatic results in uncontrolled experiments could be included here |
| III | Opinions of respected authorities, based on clinical experience; descriptive studies or reports of expert committees |
C.2 Therapeutic Endpoints
Similarly, the United States National Cancer Institute ranks human cancer treatment studies reporting one or more therapeutic endpoints on a scale in decreasing order of strength from 1iA to 4 according to the following criteria B:
Strength of study design
-
Randomized controlled clinical trials Studies in which participants are assigned by chance to separate groups for the comparison of different treatments. It is the patient’s choice to be in a randomized trial, but neither the researchers not the patient can choose the group in which the patient will be placed. Using chance to assign people helps to ensure that the groups will be similar and that the treatments they receive can be compared objectively. At the time of a trial, there is uncertainty about which of the treatments is best. These trials can be “double-blinded” or “non-blinded.” Double-blinded trials have a stronger study design.
Double-blinded Neither the patients nor the researchers know which patients are receiving the therapy under study or the comparison (i.e., control) treatment.
Nonblinded The researchers and the patients know which treatment is being given.
Nonrandomized controlled clinical trials Studies in which participants are assigned to a treatment group based on criteria that may be known to the researchers, such as the patient’s birth date, chart number, or day of clinic appointment. With this type of study design, there is less confidence that the group receiving the treatment under study and the control group are comparable.
-
Case series Studies that describe results from a group or series of patients who all received the treatment that is being investigated. These studies have a weak design, due in part, to the absence of a control group. Types of case series, in descending order of strength, are these:
Population-based, consecutive case series The study population is well-defined and is either the entire population of interest or a representative random sample of the larger population from which it is drawn. The study subjects receive treatment in the order in which they are identified by the researcher.
Consecutive case series Studies describing a series of patients who were not limited to a specific population and who received treatment in the same order in which they were identified by the researchers.
Non-consecutive case series Studies describing a series of patients who were not limited to a specific population and who do not represent a consecutive series of patients identified and treated by the researchers.
Strength of endpoints measured
Total mortality The proportion of the study population that dies. Frequently called the death rate. Measured from a defined point in time, such as the time of diagnosis or the time since treatment was initiated. This is the most easily defined and objective endpoint. The inverse of total mortality, that is, overall survival, may be the reported value.
Cause-specific mortality Death from a specified cause in the population under study, for example, death from cancer versus death from side effects of therapy versus death from other causes. This endpoint is more subjective than total mortality. When death from disease (cancer, heart disease, etc.) is the measured endpoint, the inverse value, that is, disease-specific survival, may be reported instead.
Carefully assessed quality of life Although a very subjective endpoint, quality of life is an extremely important endpoint to patients. The strength of a quality of life assessment depends on the validity of the instruments—that is, the questionnaires, psychological tests, and so on—used.
-
Indirect surrogates These are measures that substitute for actual health outcomes, and they are subject to investigator interpretation. In descending order of strength, indirect surrogates include these measures:
Disease-free survival Length of time no cancer was detected after treatment.
Progression-free survival Length of time disease was stable or did not get worse after treatment.
Tumour response rate The proportion of patients whose tumours responded to treatment and the degree or extent to which the tumours responded.
C.3 References
Canadian Task Force of Preventive Health Care. Research Design Rating [online]. London, ON: Canadian Task Force of Preventive Health Care; n.d. [Available at: www.ctfphc.org/ctfphc&methods.htm#Table_2; cited September 2005]
United States, National Institutes of Health, U.S. National Cancer Institute. Levels of Evidence for Human Studies of Cancer Complementary and Alternative Medicine (PDQ). Health Professional Version [online]. Bethesda: U.S. National Cancer Institute; October 2, 2002. [Available at: www.cancer.gov/cancertopics/pdq/levels-evidence-cam/healthprofessional/allpages/print; cited September 2005]
Footnotes
The efficacy of ifnα after imatinib mesylate failure is unknown; no data are available.
Principal authors.
5. REFERENCES
- 1.Goldman JM, Melo JV. Chronic myeloid leukemia: advances in biology and new approaches to treatment. N Engl J Med. 2003;349:1451–64. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 2.Hehlmann R. Current cm therapy: progress and dilemma. Leukemia. 2003;17:1010–12. doi: 10.1038/sj.leu.2402951. [DOI] [PubMed] [Google Scholar]
- 3.Schiffer CA, Hehlmann R, Larson R. Perspectives on the treatment of chronic phase and advanced phase cml and Philadelphia chromosome positive all. Leukemia. 2003;17:691–99. doi: 10.1038/sj.leu.2402879. [DOI] [PubMed] [Google Scholar]
- 4.Sausville EA. Imatinib for chronic myelogenous leukaemia: a 9 or 24 carat gold standard. Lancet. 2003;36:1400–1. doi: 10.1016/S0140-6736(03)13145-5. [DOI] [PubMed] [Google Scholar]
- 5.Druker BJ, O’Brien SG, Cortes J, Radich J. Chronic myelogenous leukemia. Hematology (Am Soc Hematol Educ Program) 2002:111–35. doi: 10.1182/asheducation-2002.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. New Engl J Med. 1999;341:164–72. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Cancer Society and the National Cancer Institute of Canada. Canadian Cancer Statistics 2005. Toronto: Canadian Cancer Society; 2005.
- 8.Hill J, Meehan KR. Chronic myelogenous leukemia. Curable with early diagnosis and treatment. Postgrad Med. 1999;106:149–59. doi: 10.3810/pgm.1999.09.686. [DOI] [PubMed] [Google Scholar]
- 9.Cortez D, Stoica G, Pierce JH, Pendergast AM. The bcr-abl tyrosine kinase inhibits apoptosis by activating a ras-dependent signaling pathway. Oncogene. 1996;13:2589–94. [PubMed] [Google Scholar]
- 10.Salgia R, Brunkhorst B, Pisick E, et al. Increased tyrosine phosphorylation of focal adhesion proteins in myeloid cell lines expressing p210BCR/ABL. Oncogene. 1995;11:1149–55. [PubMed] [Google Scholar]
- 11.Tauchi T, Boswell HS, Leibowitz D, Broxmeyer HE. Coupling between p210BCR-ABL and Shc and Grb2 adaptor proteins in hematopoietic cells permits growth factor receptor-independent link to ras activation pathway. J Exp Med. 1994;179:167–75. doi: 10.1084/jem.179.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salloukh HF, Laneuville P. Increase in mutant frequencies in mice expressing the bcr-abl activated tyrosine kinase. Leukemia. 2000;14:1401–4. doi: 10.1038/sj.leu.2401855. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Chronic Myelogenous Leukemia. Version 2.2005 [online]. [Accessed at www.nccn.org/professionals/physician_gls/f_guidelines.asp; cited September 15, 2005]
- 15.Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207–19. doi: 10.7326/0003-4819-131-3-199908030-00008. [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Clinical Excellence. Guidance on the use of imatinib for chronic myeloid leukemia. Technology appraisal 70 [online]. London, U.K.: National Institute for Clinical Excellence; October 2003. [Accessed at www.nice.org.uk/page.aspx?o=89859; cited September 15, 2005]
- 17.Bernstein R. Cytogenetics of chronic myelogenous leukemia. Semin Hematol. 1988;25:20–34. [PubMed] [Google Scholar]
- 18.Clarkson B, Strife A, Wisniewski D, Lambek CL, Liu C. Chronic myelogenous leukemia as a paradigm of early cancer and possible curative strategies. Leukemia. 2003;17:1211–62. doi: 10.1038/sj.leu.2402912. [DOI] [PubMed] [Google Scholar]
- 19.Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative cml Prognostic Factors Project Group. J Nat Cancer Inst. 1998;90:850–8. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 20.Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good risk” chronic granulocytic leukemia. Blood. 1984;63:789–99. [PubMed] [Google Scholar]
- 21.Marin D, Marktel S, Bua M, et al. Prognostic factors for patients with chronic myeloid leukemia in chronic phase treated with imatinib mesylate after failure of interferon alfa. Leukemia. 2003;17:1448–53. doi: 10.1038/sj.leu.2402996. [DOI] [PubMed] [Google Scholar]
- 22.Huntly BJP, Bench A, Green AR. Double jeopardy from a single translocation: deletions of the derivative chromosome 9 in chronic myeloid leukemia. Blood. 2003;102:1160–8. doi: 10.1182/blood-2003-01-0123. [DOI] [PubMed] [Google Scholar]
- 23.Quintas–Cardama A, Kantarjian H, Talpaz M, et al. Deletion of derivative chromosome 9 has no prognostic impact on patients with chronic myeloid leukemia (cml) treated with imatinib mesylate (Gleevec) [abstract 640; online] Blood. 2003:102. [Accessed at www.bloodjournal.org/misc/ASH_Meeting_Abstracts_Info.shtml; cited October 30, 2006]. [Google Scholar]
- 24.Cervantes F on behalf of the Iris Study Group. Durability of responses to imatinib in newly diagnosed chronic phase chronic myeloid leukemia: 24-month update from the iris study [abstract 633; online] Blood. 2003;102 [online]. [Accessed at www.bloodjournal.org/misc/ASH_Meeting_Abstracts_Info.shtml; cited October 30, 2006]. [Google Scholar]
- 25.Gratwohl A, Hermans J, Goldman J, et al. Risk assessment for patients with chronic myeloid leukemia before allogeneic blood or marrow transplantation. Lancet. 1998;352:1087–92. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 26.Radich JP, Gooley T, Bensinger W, et al. hla-matched related hematopoietic cell transplantation for chronic phase cml using a targeted busulfan and cyclophosphamide preparative regimen. Blood. 2003;102:31–5. doi: 10.1182/blood-2002-08-2619. [DOI] [PubMed] [Google Scholar]
- 27.Goldman JM, Marin D, Olavarria E, Apperley JF. Clinical decisions for chronic myeloid leukemia in the imatinib era. Semin Hematol. 2003;40(suppl 2):98–103. doi: 10.1053/shem.2003.50049. [DOI] [PubMed] [Google Scholar]
- 28.Bories D, Devergie A, Gardembas–Pain M, et al. Stratégies thérapeutiques et recommandations pour la prise en charge des patients atteints de leucémie myéloïde chronique. Hématologie. 2003;9:497–512. [Google Scholar]
- 29.Walker I, Makarski J, Meyer RM, for the Hematology Disease Site Group. Treatment of chronic myeloid leukemia with imatinib. Practice guideline report #6–15 [online]. Toronto: Cancer Care Ontario; July 16, 2004. [Accessed at www.cancercare.on.ca/index_hematologyCancerguidelines.htm; cited September 16, 2005]
- 30.Druker BJ, Guilhot F, O’Brien S, Larson RA on behalf of the Iris Study Group. Long-term benefit of imatinib for patients newly diagnosed with chronic myeloid leukemia in chronic phase: 5-year update from the iris study [abstract 6506; online] J Clin Oncol. 2006;24(suppl) [Accessed at www.asco.org/portal/site/asco/menuitem.34d60f5624ba07fd506fe310ee37a01d/?vgnextoid=76f8201eb61a7010VgnVCM100000ed730ad1RCRD&vmview=abst_detail_view&confID=40&abstractID=32026; cited October 20, 2006] [Google Scholar]
- 31.Novartis Pharmaceuticals USA. The iris study: 5-year data [unpublished; data on file].
- 32.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia. Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 33.Druker BJ, Gathmann I, Bolton AE, et al. on behalf of the Iris study group. Probability and impact of obtaining a cytogenetic response to imatinib as initial therapy for chronic myeloid leukemia in chronic phase [abstract 634; online] Blood. 2003:102. [Accessed at www.bloodjournal.org/misc/ASH_Meeting_Abstracts_Info.shtml; cited October 30, 2006]. [Google Scholar]
- 34.Radich JP, Gathmann I, Kaeda J, et al. Molecular response to imatinib and interferon plus Ara-C in newly diagnosed cml: efficacy of imatinib on patients after crossover from interferon therapy and prognostic importance of molecular response on long-term outcomes [abstract 635; online] Blood. 2003;102 [Accessed at www.bloodjournal.org/misc/ASH_Meeting_Abstracts_Info.shtml; cited October 30, 2006]. [Google Scholar]
- 35.Silver RT, Woolf SH, Hehlmann R, et al. An evidence-based analysis of the effect of busulfan, hydroxyurea, interferon, and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the American Society of Hematology. Blood. 1999;5:1517–36. [PubMed] [Google Scholar]
- 36.Novartis Pharmaceuticals Canada Inc. Gleevec [product monograph]. Rev. Dorval, QC: Novartis Pharmaceuticals Canada Inc.; June 16, 2006.
- 37.Cortes JE, Talpaz M, Giles F, et al. Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood. 2003;101:3794–3800. doi: 10.1182/blood-2002-09-2790. [DOI] [PubMed] [Google Scholar]
- 38.Cortes JE, Talpaz M, O’Brien S, et al. High rates of major cytogenetic response in patients with newly diagnosed chronic myeloid leukemia in early chronic phase treated with imatinib at 400 mg or 800 mg daily [abstract 350; online] Blood. 2003;102 [Accessed at www.bloodjournal.org/misc/ASH_Meeting_Abstracts_Info.shtml; cited October 30, 2006]. [Google Scholar]
- 39.Kantarjian HM, Talpaz M, O’Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–5. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- 40.Kantarjian H, Talpaz M, O’Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome–positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–8. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 41.Cortes J, Talpaz M, O’Brien S, et al. High-dose imatinib mesylate treatment in patients with previously untreated early chronic phase chronic myeloid leukemia [abstract 999; online] Blood. 2004;104 [Accessed at www.abstracts2view.com/hem_sandiego2004/view.php?nu=HEM4L1_2741; cited September 15, 2005] [Google Scholar]
- 42.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 43.Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;1:117–25. doi: 10.1016/s1535-6108(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 44.Branford S, Rudzki Z, Parkinson I, et al. Real-time quantitative pcr analysis can be used as a primary screen to identify patients with cml treated with imatinib who have BCR-ABL kinase domain mutations. Blood. 2004;104:2926–32. doi: 10.1182/blood-2004-03-1134. [DOI] [PubMed] [Google Scholar]
- 45.Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with cml treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the atp phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–83. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]