Abstract
Background and Aims
We sought to explore the interactions between roots and soil without disturbance and in four dimensions (i.e. 3-D plus time) using X-ray micro-computed tomography.
Methods
The roots of tomato Solanum lycopersicum ‘Ailsa Craig’ plants were visualized in undisturbed soil columns for 10 consecutive days to measure the effect of soil compaction on selected root traits including elongation rate. Treatments included bulk density (1·2 vs. 1·6 g cm−3) and soil type (loamy sand vs. clay loam).
Key Results
Plants grown at the higher soil bulk density exploited smaller soil volumes (P < 0·05) and exhibited reductions in root surface area (P < 0·001), total root volume (P < 0·001) and total root length (P < 0·05), but had a greater mean root diameter (P < 0·05) than at low soil bulk density. Swelling of the root tip area was observed in compacted soil (P < 0·05) and the tortuosity of the root path was also greater (P < 0·01). Root elongation rates varied greatly during the 10-d observation period (P < 0·001), increasing to a maximum at day 2 before decreasing to a minimum at day 4. The emergence of lateral roots occurred later in plants grown in compacted soil (P < 0·01). Novel rooting characteristics (convex hull volume, centroid and maximum width), measured by image analysis, were successfully employed to discriminate treatment effects. The root systems of plants grown in compacted soil had smaller convex hull volumes (P < 0·05), a higher centre of mass (P < 0·05) and a smaller maximum width than roots grown in uncompacted soil.
Conclusions
Soil compaction adversely affects root system architecture, influencing resource capture by limiting the volume of soil explored. Lateral roots formed later in plants grown in compacted soil and total root length and surface area were reduced. Root diameter was increased and swelling of the root tip occurred in compacted soil.
Keywords: Solanum lycopersicum, root elongation rate, root system architecture, soil compaction, X-ray micro-computed tomography, μCT
INTRODUCTION
Soil mechanical resistance influences root elongation rate (Barley et al., 1965). Taylor and Ratliff (1969) showed that root elongation rates in cotton (Gossypium hirsutum) and peanut (Arachis hypogaea) decreased with increasing soil strength. Interactions between soil physical stresses may decrease root elongation rates in maize to a greater extent than if the stresses were experienced independently, although this effect remains to be confirmed for other species (Bengough et al., 2006). However, moderate compaction of the seedbed may be beneficial for root growth and resource capture (Atkinson et al., 2009) and reduces the risk of lodging in cereals (Scott et al., 2005).
When roots encounter physical barriers, they initially attempt to maintain growth by exerting considerable turgor-dependent forces (Monshausen and Gilroy, 2009). Bengough et al. (1997) stated that the maximum soil strength that roots can overcome is determined by the maximum turgor generated within the elongation zone and the shape and frictional characteristics of the root tip. When individual roots encounter compacted soil, radial expansion is increased (Bengough et al., 2006), resulting in shorter, thicker roots. The increased radial expansion of cortical cells in roots is believed to explain why root elongation rate is reduced in compacted soil (Bengough and Young, 1993). However, it has also been reported that the thicker roots produced in response to compacted horizons can penetrate the soil more effectively and maintain their elongation rates (Materechera et al., 1992).
Passioura (2002) reported that root growth slowed markedly when soil resistance reached 1 MPa and ceased entirely at 5 MPa, while Bengough and Young (1993) concluded that a 2- to 5-d period was required for roots subjected to mechanical impedance to return to their unimpeded growth rate once they penetrated into softer soil. Bengough and MacKenzie (1994) showed that root elongation in pea (Pisum sativum ‘Helka’) decreased by 50 % within 30 min of imposing an applied force of 100 mN.
In a recent review, Bengough et al. (2011) claimed that root elongation is typically halved in soils with penetrometer resistances in the range 0·8–2 MPa. Differences between species in their ability to penetrate strong soil may be attributable to variation in root diameter and the tendency of roots to deflect or buckle rather than to differences in root growth pressure (Clark et al., 2003). Interestingly, the root elongation rates of dicotyledonous species are generally less affected by high impedance soil than those of monocotyledonous species (Materechera et al., 1991), perhaps explaining why thick-rooted dicot crops are used in rotations as they may provide more effective penetration of compacted subsoil layers (Clark et al., 2003). Reductions in root elongation rate resulting from difficulty in generating the force required to displace soil particles and extend may pose serious problems in agriculture.
Until recently, relatively few studies have examined the influence of soil strength and bulk density on root elongation rate (Goss and Russell, 1980; Bengough and Young, 1993; Bengough et al., 1994), even though growing roots often encounter a range of bulk densities within the intrinsically heterogeneous rooting medium as they penetrate through soil aggregates or explore soil pore space. The lack of experimental information on the in situ 4-D responses of roots to soil compaction has resulted primarily from the difficulty and time-consuming nature of procedures for extracting roots from soil to characterize root system architecture over extended periods (Imhoff et al., 2010). Indeed, as recently as 2010, Pagés et al. (2010) stated that measurements of the elongation rate of individual roots remained a challenge. The development of new imaging technologies, such as X-ray micro-computed tomography (μCT), allows the root systems of individual plants to be visualized over periods of days to measure root traits (Tracy et al., 2010). μCT is increasingly being employed in agricultural and environmental sciences as it offers unique benefits, not least its ability to provide non-destructive in situ visualization and quantification of root system architecture, as was recently reviewed by Mooney et al. (2012).
The objective of the present study was to visualize and quantify the impact of soil bulk density and soil type on root elongation rate and other rooting characteristics in tomato (Solanum lycopersicum) using X-ray μCT. The effect of soil compaction on the ability of root systems to explore and exploit the soil environment was examined using novel root architectural descriptors derived by image analysis.
MATERIALS AND METHODS
Sample preparation and μCT scanning procedures
A Newport series loamy sand (brown soil) and a Worcester series clay loam soil (argillic pelosol) from the University of Nottingham farm at Bunny, Nottinghamshire, UK (52·52 °N, 1·07 °W) were air-dried and sieved to <2 mm. Columns (70 mm height × 30 mm diameter) were uniformly packed to provide bulk densities of 1·2 g cm−3 (uncompacted) or 1·6 g cm−3 (compacted). Three replicates were prepared for each soil type and treatment combination to give a total of 12 columns. These were packed with air-dry soil in approx. 1-cm-deep layers; after compacting each layer, the surface was lightly scarified before adding further material to ensure homogeneous packing (Lewis and Sjöstrom, 2010). The columns were then saturated, drained to field capacity and placed in a growth room under conditions of 28/22 °C day/night with a 12-h photoperiod and a photosynthetic photon flux density at plant level of 226 µmol m−2 s−1. Seeds of tomato Solanum lycopersicum L. (formerly Lycopersicon esculentum Mill.) ‘Ailsa Craig’ imbibed water for 48 h before being planted 5 mm below the soil surface. The columns were placed in a transparent propagator to maintain high relative humidity levels during germination and seedling growth. They were weighed daily and sufficient water was added to maintain soil moisture content close to field capacity. Two glass beads 1 mm in diameter were placed on either side of the columns, just below the soil surface to act as reference objects during image analysis. These highly attenuate the X-ray beam and were used to align the columns for the daily segmentation of the root system architecture within the imaging software (VG StudioMAX® 2·1).
All columns were scanned daily for 10 d after germination using a Phoenix Nanotom® (GE Measurement & Control Solutions, Wunstorf, Germany) X-ray μCT scanner set at 110 kV and 180 µA, with a 0·1-mm copper filter and an image averaging of 1. Pixel/voxel resolution was set at 24 µm and each scan took 20 min to complete. For each column, 1200 image projections were captured on all sampling dates; each stack of images had a file size of approx. 15 GB. The columns were scanned during the photoperiod in randomized order to ensure that all four treatment combinations were equally exposed to any diurnal variation in root growth that may have occurred and avoid systematic error. The same randomized sequence was used throughout the observation period to ensure that each column was scanned at 24-h intervals. The total number of individual scans was 120. Following the final scan on day 10, the roots were washed from the soil and analysed using WinRHIZO® 2002c scanning equipment and software. The images obtained were used to verify the X-ray μCT images.
Image processing and analysis
The original grey-level X-ray μCT images were processed using ImageJ 1·42 software (http://rsbweb.nih.gov/ij/) after resizing the images to 19·53 × 19·53 mm (850 × 850 pixels) to exclude the area outside the soil column (i.e. the container and air space). A uniform Contrast Enhancement filter was applied to normalize all slices and reduce the effect of differences in the pixel grey-level between neighbouring slices. This unwanted effect, known as beam hardening, occurs because the low energy (strongly attenuated) photons of a polychromatic beam are emitted with a higher velocity than the high energy (weakly attenuated) photons (Wildenschild et al., 2002). To separate pores from the solid matrix, the Maximum Entropy threshold algorithm was used for all soil samples. Binary images were analysed, using the Analyze Particles tool to provide information for number of pores, total pore area, total porosity and mean pore size for each individual image (900 for each soil column). Pore spaces <1 pixel were excluded from the analysis and care was taken to ensure that no root material was classified as pore space during porosity analysis. Connectivity of the pore space was analysed using the Defect Analysis module in VG StudioMAX® 2·1 software.
Root systems were non-destructively extracted from the grey-scale μCT images using the Region Growing selection tool in VG StudioMAX® 2·1 software. The root system models segmented from the μCT image data were used for quantitative determination of root length, volume, surface area, mean diameter, root tip diameter, maximum rooting depth and the angle of lateral roots to the main axis. Tortuosity of the root path (the ratio of actual path length compared with the shortest possible path) was measured by comparing the length of the tap root measured using the Polyline tool in VG StudioMAX® 2·1 software to the linear depth of the root system. Root angle was determined using Simpleware ScanIP software as this has the major advantage of allowing measurements to be made whilst roots are visualized in 3-D.
Several novel root characteristics derived by image analysis were also determined using the segmented root systems, including convex hull volume, centroid and minimum enclosing circle. The convex hull of the root system was obtained using the QuickHull algorithm (Barber et al., 1996) and its volume was estimated using the Monte Carlo Integration (Rubinstein, 1981). The convex hull of a root system can be used to compare root systems from different plants (Iyer-Pascuzzi et al., 2010). Figure 1A shows an image of the root system of a tomato plant enclosed by its convex hull. The centroid was also determined; this is the geometric centre of an object and corresponds to its centre of mass if mass per unit volume is constant throughout the object. This was achieved by computing the mean x, y and z co-ordinates for all extracted root data voxels. Figure 1B shows an example of the centroid, together with its vertical centre-line. Using Welzl's algorithm for the minimum enclosing circle (Welzl, 1991), it is possible to determine the maximum width of root systems (Fig. 1C), i.e. the maximum horizontal distance achieved. Maximum rooting depth was calculated by counting the number of cross-sectional image slices between the uppermost and lowermost voxels present in the μCT image stack. The number obtained was multiplied by voxel size to establish maximum rooting depth (Fig. 1D).
Fig. 1.
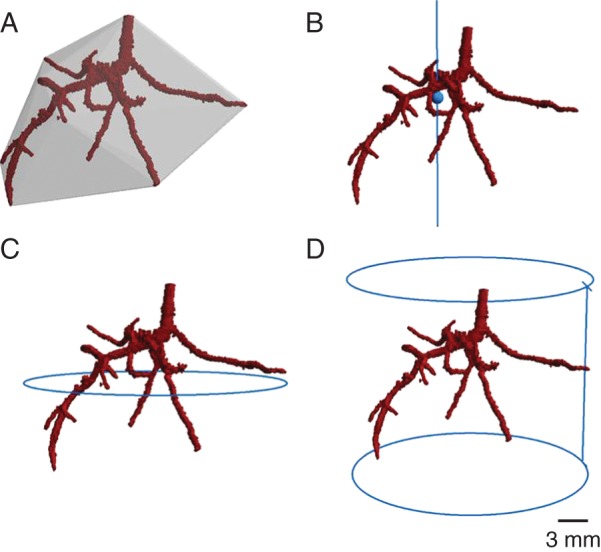
Example of (A) convex hull, (B) centroid, (C) maximum width and (D) rooting depth measurements for a 10-d-old tomato plant grown in compacted clay loam soil. Vertical lines represent vertical plane (B) and rooting depth (D).
The results were analysed by three-way general analysis of variance (ANOVA) containing bulk density, soil type, time and all possible interactions as explanatory variables using Genstat 13·1. Each soil column was analysed individually, with measurement date set as a polynomial contrast. The effect of treatments on root parameters was tested by including polynomial contrasts fitted to the day factor in the analysis; a significant treatment × linear interaction indicates that the root characteristic analysed differed among treatments. Analysing the data in this way facilitated the testing of whether the linear component of changes with time differed between treatments to a greater extent than expected from the residual variation, instead of simply considering the overall effect of time. Normality was tested by interpreting the plots of residuals; in all cases the data were normally distributed, satisfying the assumptions underlying general analysis of variance.
RESULTS
Root system architecture
Mean root volume increased with time and the interaction of bulk density × soil type was significant (Fig. 2A; P < 0·001). Root volume was greater in uncompacted than in compacted soil for both soil types i.e. 16·3 vs. 15·2 mm3 for the uncompacted vs. compacted clay loam and 30·5 vs. 17·3 mm3 for the uncompacted vs. compacted loamy sand. The effect of bulk density on root volume was greater in the loamy sand soil than the clay loam, as no significant differences were found for clay loam, whereas a significant treatment effect was found for loamy sand (Fig. 2A; P < 0·001). Standard errors of the mean for root volume increased with time and were greatest in the uncompacted loamy sand (Fig. 2A). No significant trends for root volume were detected from the WinRHIZO® analysis.
Fig. 2.
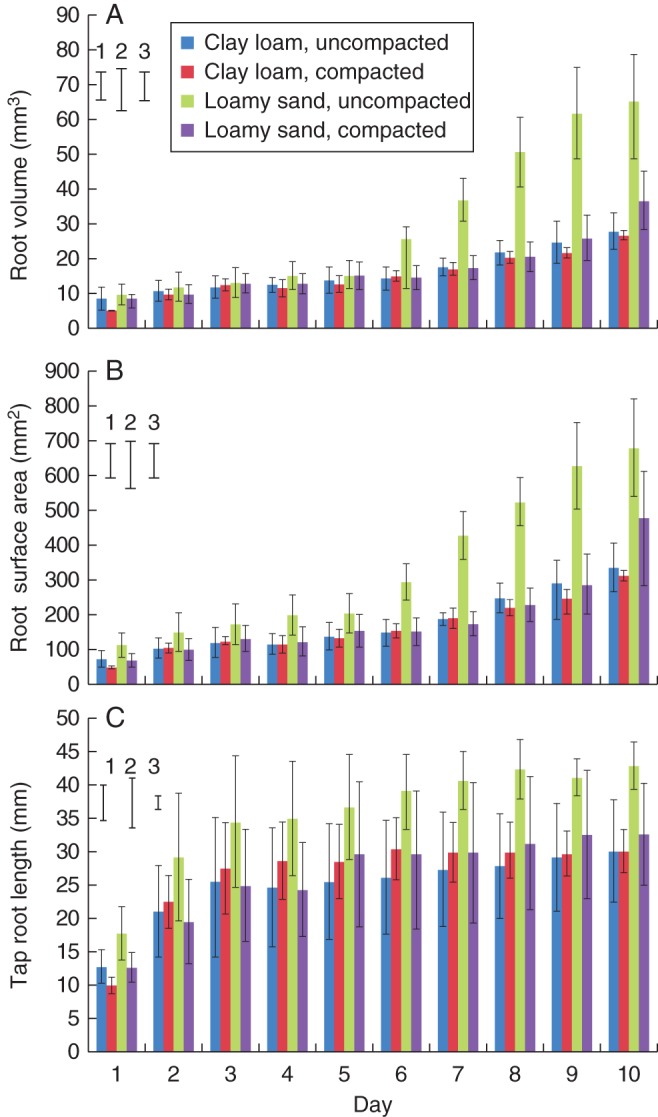
Semi-automated segmented mean values for (A) root volume, (B) root surface area and (C) tap root length during the 10-d observation period for both compaction treatments and soil types. Error bars associated with the histograms show double standard errors of the mean. Standard errors of the difference are shown for (1) soil type, (2) compaction treatment and (3) day.
The bulk density × soil type interaction was significant for root surface area (P < 0·001; Fig 2B), which was greater in the uncompacted treatment for both soil types (i.e. 176 vs. 164 mm2 for uncompacted vs. compacted clay loam and 338 vs. 189 mm2 for uncompacted vs. compacted loamy sand). The effect of bulk density on root surface area was greater in the loamy sand than in the clay loam (Fig. 2B; P < 0·001). WinRHIZO® analysis showed similar trends although, interestingly, no significant effects of soil type or compaction treatment were detected.
Root length was expressed in two ways, vertical length (i.e. maximum rooting depth) and tap root length determined using the Polyline tool in VGStudioMAX®. Parametric analysis revealed that overall values for both measures were lower in compacted soil and the difference between compaction treatments was greater for vertical rooting depth, although the differences between treatments were not significant. Compaction reduced vertical root depth in the loamy sand soil (16·0 vs. 27·1 mm for compacted and uncompacted loamy sand soil), although not significantly, but had no effect in the clay loam soil for which root length was very similar in both compaction treatments. Tap root length was slightly, although not significantly, lower in compacted soil (26·7 vs. 30·5 mm3), and was greater for plants grown in loamy sand compared with clay loam (31·3 vs. 25·9 mm; P < 0·05; Fig. 2C). The WinRHIZO® analyses showed that compaction reduced total root length relative to uncompacted soil (P < 0·05), and this effect was greater in the loamy sand soil (Fig. 3A). The bulk density × soil type interaction was significant (P < 0·05; Fig. 3A), as root length was greater for plants grown in uncompacted soil than for those grown in compacted soil for both soil types; this effect was more pronounced in the loamy sand soil (30·7 vs. 16·4 mm3 for the uncompacted and compacted treatments) than in the clay loam (8·0 vs. 17·1 mm3 for the uncompacted and compacted; P < 0·05; Fig. 3A). The development of the root systems over the observation period can be seen in Fig. 4, and a visual assessment made of the differences in root architectures across treatments. The final image is a destructive WinRHIZO® image included for verification with the X-ray μCT images (Fig. 4).
Fig. 3.
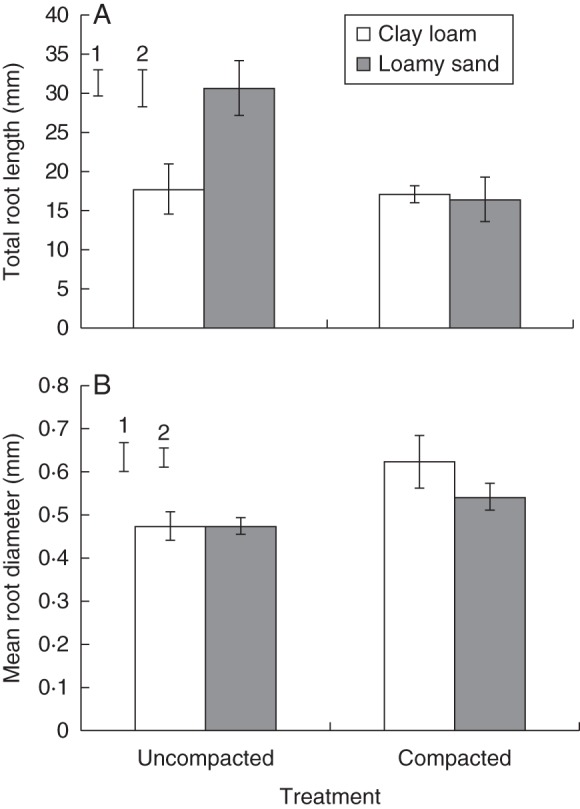
WinRHIZO® analysis values for (A) total root length and (B) mean root diameter for both compaction treatments and soil types. Error bars associated with the histograms show double standard errors of the mean. Standard errors of the difference are shown for (1) soil type and (2) compaction treatment.
Fig. 4.
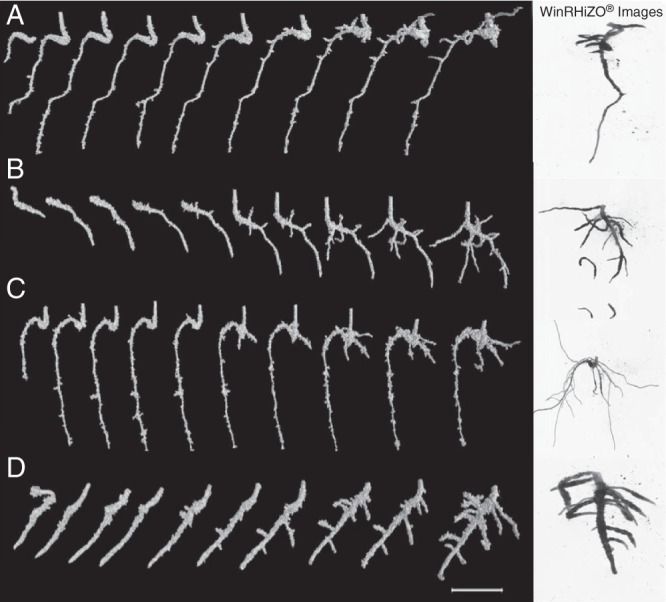
Root growth over 10 consecutive days and final destructive WinRHIZO® root images (white background) for uncompacted and compacted clay loam (A, B) and loamy sand soil columns (C, D). Scale bar = 10 mm.
WinRHIZO® analysis revealed that mean root diameter was greater in compacted soil for both soil types (0·58 vs. 0·47 mm2; P < 0·05; Fig. 3B), but there was no significant difference between soil types. Root tip diameter, derived from the X-ray μCT images, was also greater in compacted than in uncompacted soil (0·49 mm vs. 0·38 mm; P < 0·05; Fig. 5A) but did not differ significantly between soil types. The bulk density × soil type interaction was significant for tortuosity of the root path (P < 0·01; Fig. 5B) as the values were greater for plants grown in compacted soil for both soil types; however, this effect was much greater for the loamy sand (1·34 vs. 1·74 for the uncompacted and compacted treatments) than for the clay loam (1·30 vs. 1·33; P < 0·01; Fig. 5B).
Fig. 5.
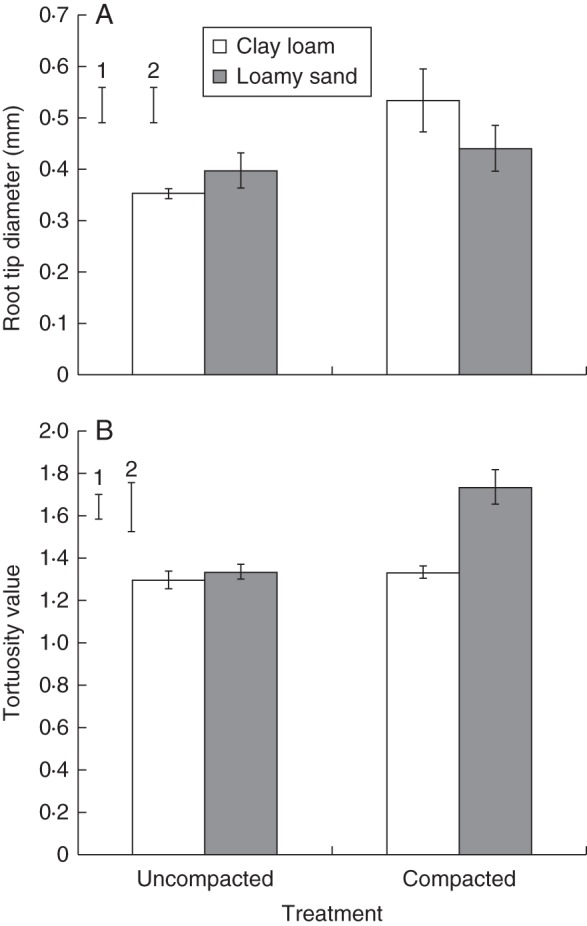
Mean root tip diameter (A) and tortuosity (B) values for both compaction treatments and soil types. Error bars associated with the histograms show double standard errors of the mean. Standard errors of the difference are shown for (1) soil type and (2) compaction treatment.
Mean values for convex hull volume for both soil types were much lower in compacted than in uncompacted soil (1083 vs. 2805 mm3; P < 0·05; Fig. 6), but were not significantly affected by soil type. Figure 6A, B show examples of convex hull volumes for plants grown in uncompacted and compacted loamy sand soil; the corresponding values for maximum rooting depth and convex hull volume were, respectively, 32·5 vs. 10·5 mm and 1804 vs. 625 mm3.
Fig. 6.
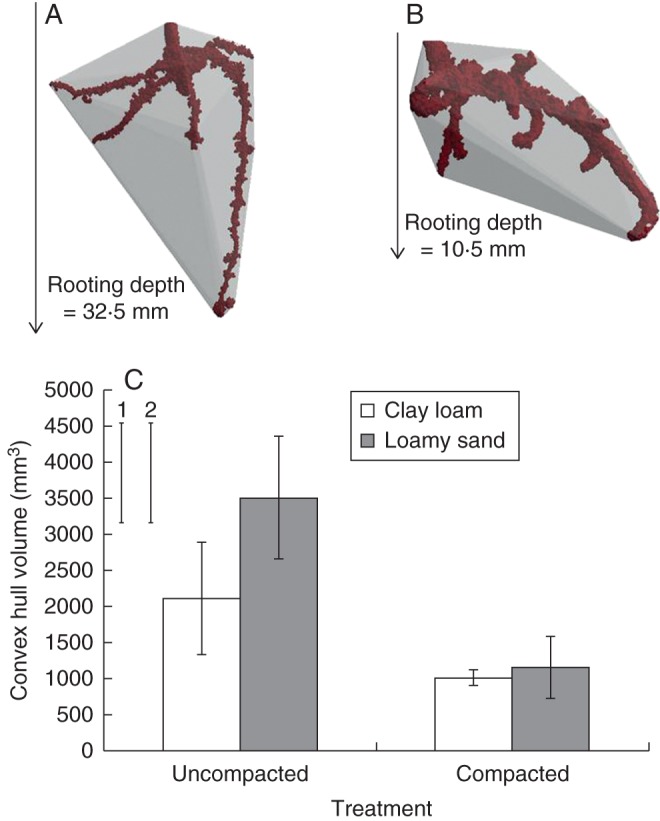
Convex hull volumes for 10-d-old plants grown in uncompacted (A) and compacted loamy sand soil (B). Vertical lines with arrow heads illustrate maximum rooting depth. (C) Mean convex hull volumes for both compaction treatments and soil types. Error bars associated with the histograms in (C) show double standard errors of the mean; standard errors of the difference (SED) are shown for (1) soil type and (2) compaction treatment.
The mean maximum horizontal spread of root systems grown in compacted soil was smaller in plants grown in uncompacted soil (19·8 vs. 27·4 mm; P > 0·05), but no significant effects of bulk density or soil type were detected. The horizontal expansion of the root systems was not constrained by the column walls as the columns were 30 mm in diameter. The centre of mass of the root system, calculated by the centroid value (Fig. 1C), was deeper for plants grown in uncompacted soil than for those grown in compacted soil (9·96 vs. 7·15 mm; P < 0·05), but there was no significant effect of soil type.
Mean daily elongation rate of the tap root varied greatly during the 10-d observation period (P < 0·001; Fig. 7), increasing to a maximum at day 2 and decreasing to a minimum after day 4; root elongation subsequently showed little variation with time. Differences between compaction treatments were small and inconsistent. Mean root elongation rate over the 10-d experimental period did not differ significantly between the clay loam and loamy sand soils (2·45 vs. 3·11 mm d−1, respectively, for both bulk densities), or between uncompacted and compacted soil (2·86 vs. 2·69 mm d−1, respectively, for both soil types).
Fig. 7.
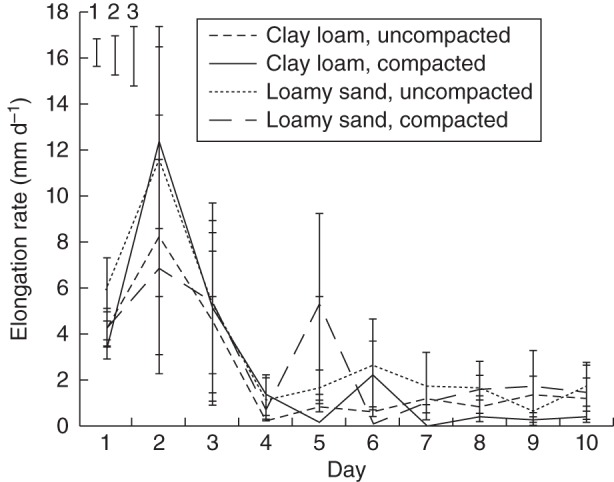
Mean daily tap root elongation rates for both compaction and soil type treatments during the 10-d experimental period. Error bars show double standard errors of the mean; standard errors of the difference are shown for (1) soil type, (2) compaction treatment and (3) day.
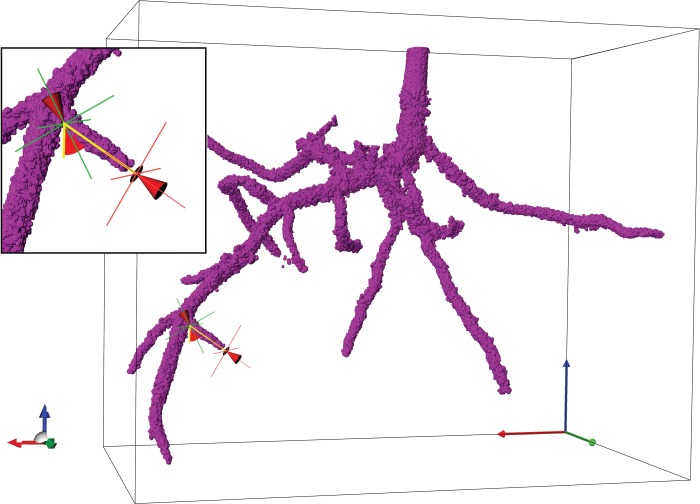
Lateral root number did not differ significantly between the uncompacted and compacted treatments (7·3 vs. 8·3 plant−1), although the increase in compacted soil was greater in loamy sand than in the clay loam (15 vs. 10 % increase; P < 0·05). No significant differences were found for lateral root angle relative to the vertical plane determined using 3-D analysis of segmented root X-ray μCT images (Fig. 8), although values tended to be greater in compacted than in uncompacted soil (means of 69·2 vs. 52·9°). Lateral roots emerged sooner in uncompacted than in compacted soil (mean of 3·5 vs. 5·7 d for both soil types; P < 0·01; Fig. 4). This trend was apparent for both soil types, i.e. 3·3 vs. 5·0 d for the uncompacted and compacted clay loam treatments and 3·7 vs. 6·3 d for the uncompacted and compacted loamy sand treatments.
Fig. 8.
Illustration showing how lateral root angle relative to a vertical plane (yellow line) was determined; inset image shows the angle measurement tool in Simpleware ScanIP software. Red cones indicate the ends of the measurement tool. Tripod shows direction of x, y and z planes. Angle measurement shown is 60°.
Soil porous architecture
Total pore volume, at the scanner resolution used in the present study, was greater in uncompacted than in compacted soil (794 vs. 586 mm3 averaged over both soil types; P < 0·01), and was lower in the clay loam than in the loamy sand (580 vs. 799 mm3 averaged over both bulk densities; P < 0·01). Figure 9 shows connected pore spaces were more extensive in uncompacted soil, although these were frequently located close to the column walls. Mean pore diameter for both soil types was greater in uncompacted soil (0·036 vs. 0·016 mm; P > 0·05), but the mean number of pores was greater in compacted soil (46 vs. 29 cm−3 of soil; P > 0·05).
Fig. 9.
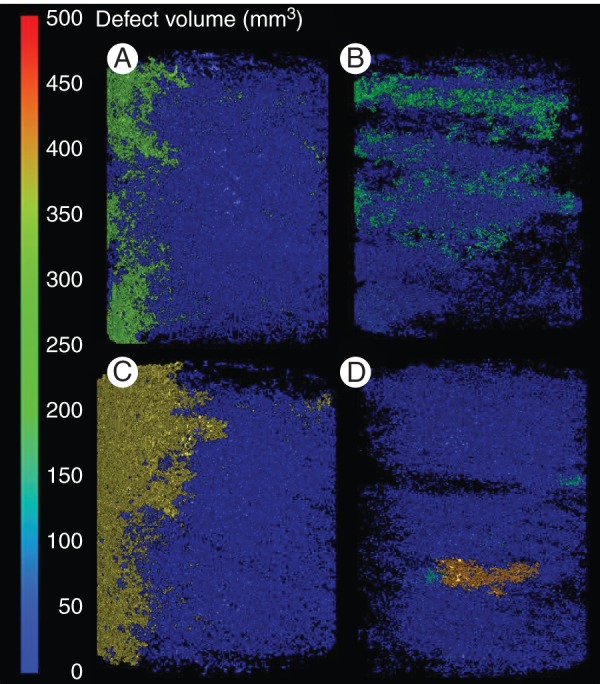
Soil pore architecture for uncompacted and compacted clay loam (A, B) and loamy sand soils (C, D) visualized using the Defect analysis tool in VGStudioMAX®. Blue areas show small unconnected pore space; green and orange areas are volumes of connected pores and equal the volume value on the scale bar. The intensity of colouration reflects the volume of connected pores.
DISCUSSION
The results clearly show that some root traits were directly affected by bulk density; for example, total root length was reduced and root diameter was increased in compacted soil. It is likely that root growth in compacted soil was restricted by the consequent increase in bulk density, which roots must overcome to extend. This may account for the larger standard errors obtained for mean values for the root characteristics of plants growing in uncompacted soil (Fig. 2), suggesting that they exhibited greater plasticity of root growth than was possible for plants growing in compacted soil. Variation between replicates also increased with time, implying that the initial stages of seedling growth are strictly programmed by constraints imposed by the finite supply of seed reserves, but that the root system becomes increasingly capable of establishing itself within the soil matrix from approximately day 7 onwards, as assimilate supplies from the shoot increase (Copeland and McDonald, 2001).
The greater bulk density and less well-connected and continuous pore space in compacted soil are likely to have been responsible for the greater tortuosity of roots (Fig. 5B), as they may have buckled as a result of physical impedance imposed by the soil and been forced to follow more convoluted pathways. In areas where soil water and nutrient supplies are limiting, failure to explore deeper soil horizons rapidly to exploit available reserves will be detrimental to early growth and establishment, and may ultimately reduce productivity. In cereals such as barley (Hordeum vulgare), poor penetration to depth may also increase the likelihood of lodging as the root system provides insufficient anchorage and physical support (Berry et al., 2006).
Compaction decreased the maximum spread of lateral roots and total root length. Although plants growing in compacted soil may have attempted to overcome this reduction in root length, and hence the surface area available for resource capture, by producing increased numbers of lateral roots, total root length was nevertheless lower than in uncompacted soil (Fig. 3A). No significant differences were found between compaction treatments for vertical rooting depth or tap root length, perhaps due to the young age of the plants and lack of differences in root elongation rates discussed later. Interestingly, the effect of soil compaction was markedly greater in the coarse-grained sandy soil than in the finer clay loam soil; however, as the soil was sieved to <2 mm, textural effects may have contributed to root architectural differences. Lateral roots may have an optimum root angle to achieve the most efficient distribution and maximize the volume of soil explored for water and/or nutrient uptake (Lynch and Brown, 2001). Although not statistically significant, compaction appeared to influence lateral root angle, as this was closer to 90 ° than in plants grown in uncompacted soil.
The surface area of root systems crucially affects the volume of soil with which roots are in contact and from which edaphic resources may be extracted. The observation that surface area was reduced in compacted soil clearly demonstrates the important influence of soil physical characteristics on root growth, soil colonization and resource capture. The longer and thinner roots produced by plants growing in uncompacted soil relative to those produced in compacted soil would have increased the surface area : volume ratio (Fig. 2), so enhancing resource capture by increasing the surface area of roots in contact with soil particles and organic material and thereby providing a more effective investment of photoassimilates in root production (Paula and Pausas, 2011).
Interestingly, although similar trends were apparent, WinRHIZO® analysis was unable to detect significant treatment effects on root volume and surface area, bringing into question the sensitivity of this well-established technique, perhaps due to the algorithms used to determine values for these variables. WinRHIZO® assumes that roots are perfectly cylindrical (Arseneault et al., 1995) and, in the case of root volume, the software determines 3-D values from 2-D images (unlike CT where the actual 3-D root volume in soil is measured). As some soil particles attached to the root surface could not be removed during root washing, pixels corresponding to these would have been included in the WinRHIZO® images during image analysis. This may have led to overestimation of volume, surface area and root diameter, and may explain why WinRHIZO® values were much greater than equivalent volumes derived from μCT scans.
The observed increase in the diameter of root tips and roots in compacted soil (Figs 3B and 5A) may reduce buckling of roots as they attempt to displace soil particles during extension growth (Potocka et al., 2011). The observation that lateral root formation began sooner in plants grown in uncompacted soil implies that these plants were more effective in obtaining resources to support seedling establishment and growth than those grown in compacted soil. Tap root elongation was most rapid 2–3 d after germination in all treatments (Figs 4 and 7), demonstrating the importance of forming a significant early root presence for anchorage and resource capture. However, as most lateral roots developed subsequently, it is likely that initial rapid tap root growth coincided with the period when seed reserves were still abundant prior to the development of significant photosynthetic capacity (Copeland and McDonald, 2001). The sharp decline in tap root elongation to a relatively stable rate after day 3 may reflect a transition between the initial establishment phase, supported primarily by seed reserves, and subsequent preferential allocation of remaining seed reserves and newly acquired water, mineral nutrients and photosynthate to support lateral root formation and shoot growth (Copeland and McDonald, 2001). Interestingly, this decrease in tap root elongation after day 3 occurred in both compaction treatments and soil types, suggesting that soil compaction may be a secondary rather than primary stress factor in terms of its impact on root growth during the early stages of seedling establishment.
Figure 9 highlights how the arrangement of soil particles and pore space may vary greatly over extremely short (approx. 1 µm) distances, especially in soils with a low bulk density. The images used to analyse soil pore architecture showed that roots growing in compacted soil potentially have thousands of discrete pore spaces to explore, but very little connected pore space to extend through (at the 24 µm resolution). This would restrict root elongation as this requires a continuous network of appropriately sized pores; Gregory (2006) suggested that a pore diameter of 10 µm is the minimum required. However, our observation that total pore volume was smaller in the clay soil than in the loamy sand suggests that a significant portion of porosity (as micropores) is not accounted for due to resolution limitations of the scans.
Although root volume is often used as a measure of root growth (Dupuy et al., 2010) and was affected by both bulk density and soil type, direct association of root volume with successful establishment of the root system may be misleading because it is possible that, although plants often produce shorter, thicker roots in response to compaction, they may nevertheless have total root volumes which are similar to plants possessing longer, thinner roots (in terms of voxel volume) (Tracy et al., 2012). However, the rooting depth and soil volume exploited for essential resources can be very different. We propose that other root structural descriptors, such as convex hull volume, should be considered in unison with traditional morphological measurements to determine root responses to soil compaction. For example, in the present study, plants grown in compacted soil consistently had smaller convex hull volumes than those grown in uncompacted soil (Fig. 6).
As supplies of essential nutrients such as phosphate for the manufacture of inorganic fertilizers are finite and rapidly declining (Cordell et al., 2009), it is vital to understand how roots function across a wide range of conditions and soil types, respond to stress, and establish the optimum root system architecture for resource capture. Technological advances, such as using X-ray μCT to study plant–soil interactions, permit repeated measurements of important rooting characteristics simultaneously and non-destructively over extended time periods. Here we have shown that many root morphological measurements are strongly influenced by soil compaction although, surprisingly, root elongation rate was unaffected by bulk density. This may be due to the presence of large areas of horizontally connected pore space (Fig. 9) in the compacted soil columns, which the lateral roots were able to exploit; this may also explain why average lateral root angles for plants grown in compacted soil were closer to 90 ° than in plants grown in uncompacted soil. The effects on root morphology were also more pronounced in the coarse-textured loamy sand than in the finer-textured clay loam soil, although the associated influence of soil structure and aggregation similar to those experienced in the field were not considered. Knowledge of which root characteristics are most important to quantify, and when during the growth cycle, is still urgently required and will be particularly valuable in enabling large high-tech plant phenotyping laboratories to accumulate substantial databases concerning the influence of environmental factors on root growth and function.
ACKNOWLEDGEMENTS
S.R.T. is funded by an Interdisciplinary Doctoral Training Centre award from the University of Nottingham. S.J.M. gratefully acknowledges the funding contribution made by Annals of Botany to present part of this research at the Rhizosphere 3 Conference in Perth, Australia in September 2011.
LITERATURE CITED
- Arseneault JL, Pouleur S, Messier C, Guay R. WinRHIZOTM, a root-measuring system with a unique overlap correction method. HortScience. 1995;30 906 (Abstract) [Google Scholar]
- Atkinson BS, Sparkes DL, Mooney SJ. Effect of seedbed cultivation and soil macrostructure on the establishment of winter wheat (Triticum aestivum) Soil & Tillage Research. 2009;103:291–301. [Google Scholar]
- Barber CB, Dobkin DP, Huhdanpaa H. The quickhull algorithm for convex hulls. ACM Transactions on Mathematical Software. 1996;22:469–483. [Google Scholar]
- Barley KP. Effect of localized pressure on growth of maize radicle. Australian Journal of Biological Sciences. 1965;18:499–503. [Google Scholar]
- Bengough AG, Mackenzie CJ. Simultaneous measurement of root force and elongation for seedling pea roots. Journal of Experimental Botany. 1994;45:95–102. [Google Scholar]
- Bengough AG, Young IM. Root elongation of seedling peas through layered soil of different penetration resistances. Plant and Soil. 1993;149:129–139. [Google Scholar]
- Bengough AG, Mackenzie CJ, Elangwe HE. Biophysics of the growth responses of pea roots to changes in penetration resistance. Plant and Soil. 1994;167:135–141. [Google Scholar]
- Bengough AG, Croser C, Pritchard J. A biophysical analysis of root growth under mechanical stress. Plant and Soil. 1997;189:155–164. [Google Scholar]
- Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA. Root responses to soil physical conditions: growth dynamics from field to cell. Journal of Experimental Botany. 2006;57:437–447. doi: 10.1093/jxb/erj003. [DOI] [PubMed] [Google Scholar]
- Bengough AG, McKenzie BM, Hallett PD, Valentine TA. Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. Journal of Experimental Botany. 2011;62:59–68. doi: 10.1093/jxb/erq350. [DOI] [PubMed] [Google Scholar]
- Berry PM, Sterling M, Mooney SJ. Development of a model of lodging for barley. Journal of Agronomy and Crop Science. 2006;192:151–158. [Google Scholar]
- Clark LJ, Whalley WR, Barraclough PB. How do roots penetrate strong soil? Plant and Soil. 2003;255:93–104. [Google Scholar]
- Copeland LO, McDonald MB. Principles of seed science and technology. The Netherlands: Kluwer Academic Publishers; 2001. 4th edn. Dordrecht. [Google Scholar]
- Cordell D, Drangert J-O, White S. The story of phosphorus: global food security and food for thought. Global Environmental Change. 2009;19:292–305. [Google Scholar]
- Dupuy L, Gregory PJ, Bengough AG. Root growth models: towards a new generation of continuous approaches. Journal of Experimental Botany. 2010;61:2131–2143. doi: 10.1093/jxb/erp389. [DOI] [PubMed] [Google Scholar]
- Goss MJ, Russell RS. Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.). III. Observations on the mechanism of response. Journal of Experimental Botany. 1980;31:577–588. [Google Scholar]
- Gregory PJ. Plant roots: growth, activity and interaction with soils. Oxford: Blackwell Publishing; 2006. [Google Scholar]
- Imhoff S, Kay BD, da Silva AP, Hajabbasi MA. Evaluating responses of maize (Zea mays L.) to soil physical conditions using a boundary line approach. Soil and Tillage Research. 2010;106:303–310. [Google Scholar]
- Iyer-Pascuzzi AS, Symonova O, Mileyko Y, et al. Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiology. 2010;152:1148–1157. doi: 10.1104/pp.109.150748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Sjostrom J. Optimizing the experimental design of soil columns in saturated and unsaturated transport experiments. Journal of Contaminant Hydrology. 2010;115:1–13. doi: 10.1016/j.jconhyd.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. Topsoil foraging: an architectural adaptation of plants to low phosphorus availability. Plant and Soil. 2001;237:225–237. [Google Scholar]
- Materechera SA, Dexter AR, Alston AM. Penetration of very strong soils by seedling roots of different plant species. Plant and Soil. 1991;135:31–41. [Google Scholar]
- Materechera SA, Alston AM, Kirby JM, Dexter AR. Influence of root diameter on the penetration of seminal roots into a compacted subsoil. Plant and Soil. 1992;144:297–303. [Google Scholar]
- Monshausen GB, Gilroy S. The exploring root: root growth responses to local environmental conditions. Current Opinion in Plant Biology. 2009;12:766–772. doi: 10.1016/j.pbi.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Mooney SJ, Pridmore TP, Helliwell J, Bennett MJ. Developing X-ray Computed Tomography to non-invasively image 3-D root systems architecture in soil. Plant and Soil. 2012 in press. http://dx.doi.org/10.1007/s11104-011-1039-9 . [Google Scholar]
- Pagés L, Serra V, Draye X, Doussan C, Pierret A. Estimating root elongation rates from morphological measurements of the root tip. Plant and Soil. 2010;328:35–44. [Google Scholar]
- Passioura JB. Soil conditions and plant growth. Plant, Cell & Environment. 2002;25:311–318. doi: 10.1046/j.0016-8025.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- Paula S, Pausas JG. Root traits explain different foraging strategies between resprouting abilities. Oecologia. 2011;165:321–331. doi: 10.1007/s00442-010-1806-y. [DOI] [PubMed] [Google Scholar]
- Potocka IJ, Szymanowska-Pułka J, Karczewski J, Nakielski J. Effect of mechanical stress on Zea root apex. I. Mechanical stress leads to the switch from closed to open meristem organization. Journal of Experimental Botany. 2011;62:4583–4593. doi: 10.1093/jxb/err169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein RY. Simulation and the Monte Carlo methods. New York, NY: John Wiley and Sons; 1981. [Google Scholar]
- Scott DI, Tams AR, Berry PM, Mooney SJ. The effects of wheel-induced soil compaction on anchorage strength and resistance to root lodging of winter barley (Hordeum vulgare L.) Soil and Tillage Research. 2005;82:147–160. [Google Scholar]
- Taylor HM, Ratliff lF. Root elongation rates of cotton and peanuts as a function of soil strength and soil water content. Soil Science. 1969;108:113–119. [Google Scholar]
- Tracy SR, Roberts JA, Black CR, McNeill A, Davidson R, Mooney SJ. The X-factor: visualizing undisturbed root architecture in soils using X-ray computed tomography. Journal of Experimental Botany. 2010;61:311–313. doi: 10.1093/jxb/erp386. [DOI] [PubMed] [Google Scholar]
- Tracy SR, Black CR, Roberts JA, et al. Quantifying the effect of soil compaction on three varieties of wheat (Triticum aestivum L.) using X-ray Micro Computed Tomography (CT) Plant and Soil. 2012 in press. http://dx.doi.org/10.1007/s11104-011-1022-5 . [Google Scholar]
- Welzl E. Smallest enclosing disks (balls and ellipsoids) In: Maurer H, editor. New results and new trends in computer science. New York, NY: Springer-Verlag; 1991. pp. 359–370. Lecture Notes in Computer Science no. 555. [Google Scholar]
- Wildenschild D, Hopmans JW, Vaz CMP, Rivers ML, Rikard D, Christensen BSB. Using X-ray computed tomography in hydrology: systems, resolutions and limitations. Journal of Hydrology. 2002;267:285–297. [Google Scholar]