Abstract
Background and Aims
Silicon (Si) has been shown to ameliorate the negative influence of cadmium (Cd) on plant growth and development. However, the mechanism of this phenomenon is not fully understood. Here we describe the effect of Si on growth, and uptake and subcellular distribution of Cd in maize plants in relation to the development of root tissues.
Methods
Young maize plants (Zea mays) were cultivated for 10 d hydroponically with 5 or 50 µm Cd and/or 5 mm Si. Growth parameters and the concentrations of Cd and Si were determined in root and shoot by atomic absorption spectrometry or inductively coupled plasma mass spectroscopy. The development of apoplasmic barriers (Casparian bands and suberin lamellae) and vascular tissues in roots were analysed, and the influence of Si on apoplasmic and symplasmic distribution of 109Cd applied at 34 nm was investigated between root and shoot.
Key Results
Si stimulated the growth of young maize plants exposed to Cd and influenced the development of Casparian bands and suberin lamellae as well as vascular tissues in root. Si did not affect the distribution of apoplasmic and symplasmic Cd in maize roots, but considerably decreased symplasmic and increased apoplasmic concentration of Cd in maize shoots.
Conclusions
Differences in Cd uptake of roots and shoots are probably related to the development of apoplasmic barriers and maturation of vascular tissues in roots. Alleviation of Cd toxicity by Si might be attributed to enhanced binding of Cd to the apoplasmic fraction in maize shoots.
Keywords: Cadmium toxicity, Casparian band, environmental stress, maize (Zea mays L.), root anatomy, silicon, subcellular Cd distribution, suberin lamella, xylem lignification
INTRODUCTION
Due to several positive effects on the alleviation of different forms of biotic as well as abiotic stresses, silicon (Si) has been a focus of plant biology and agronomy research in recent decades. Besides the alleviation of the negative influence of various parasites and pathogens, there are also various positive effects of Si on growth of plants suffering from various kinds of abiotic stress, e.g. heavy and toxic metals, higher salinity, drought or higher radiation (Liang et al., 2007; Zargar et al., 2010). For example, the suggested mechanisms of alleviation of manganese (Mn) toxicity by Si involve not only increased Mn adsorption by cell walls but also active removal of excess Mn by soluble Si in the apoplasm and increased levels of enzymatic and non-enzymatic antioxidants (Horst et al., 1999; Iwasaki et al., 2002; Rogalla and Römheld, 2002; Shi et al., 2005). By contrast, the mechanisms of aluminium (Al) detoxication are based on the reduction of the Al3+ content in symplasm and the formation of hard soluble aluminosilicates and/or hydroxyaluminosilicates in the apoplasmic space, in particular cell walls of the outer epidermis (Hodson and Sangster, 1993; Hodson and Evans, 1995; Wang et al., 2004). Silicate precipitates with bound zinc (Zn) were found to be localized in the intercellular space, cytoplasm, nucleus and vacuolar vesicles of leaf mesophyll cells (Neumann and Zur Nieden, 2001; Cunha and Nascimento, 2009). This indicates that the formation of Zn–Si precipitates might be responsible, in part, for the alleviation of Zn toxicity in plants. Recently, Song et al. (2011) found that Si-mediated alleviation of Zn toxicity in rice is mainly due to Si-mediated antioxidant defence capacity and membrane integrity, with a possible role of Si in reduction of root–shoot translocation of Zn. Si was shown to increase plant biomass, decrease copper (Cu) uptake and leaf chlorosis, and increase the expression of free-radical-metabolizing enzymes (Nowakowski and Nowakowska, 1997; Li et al., 2008; Khandekar and Leisner, 2011).
The positive role of Si in alleviation of cadmium (Cd) toxicity has also been reported. Cd is a toxic metal that is released into the environment largely due to mining, agriculture and industrial activities. Contamination of agricultural soils by Cd is a serious environmental problem as Cd enters the human body through the food chain and might accumulate in animal and human tissues (Dorne et al., 2011). Several symptoms of Cd toxicity have been observed on plant shoots, for example reduced growth, decreased photosynthesis, enhanced oxidative stress and, at high concentrations, cell death and destruction of the whole plant (Benavides et al., 2005; Hasan et al., 2009; Nagajyoti et al., 2010). Similarly, various negative effects of Cd toxicity have been also observed on roots (Lux et al., 2011; Piršelová et al., 2011).
In recent years, several studies of Si effects on root and shoot biomass of plants exposed to Cd have been performed. Liang et al. (2005) found that maize plants grown in the soils containing both Cd and Si had significantly higher root and shoot biomass when compared with plants grown in non-Si-containing soil. Cunha et al. (2008) described that addition of Si into the soil experimentally polluted by Cd and Zn induced a significant increase in maize biomass. Also, from our previous experiments with hydroponically cultivated maize it is evident that Si enhances the growth of plants exposed to Cd (Vaculík et al., 2009). Lukačová Kuliková and Lux (2010) reported that Si either increased or decreased the root length and dry weight of five various Cd-treated maize hybrids; however, the effect was specific for a hybrid.
There is also evidence for an alleviating effect of Si on biomass production on other plant species exposed to elevated levels of Cd. Shi et al. (2005) found that after application of both Cd and Si to Yoshida nutrient hydroponics medium, root and shoot biomass increased significantly in rice plants as compared with medium lacking Si. Similarly, Zhang et al. (2008) demonstrated that Si enhanced the root and shoot biomass in rice plants treated with 2 µm Cd, but that the beneficial effect of Si decreased in plants treated with a two-fold higher Cd concentration (4 µm). Nwugo and Huerta (2008) observed no significant change in root and shoot length, dry weight as well as total leaf area in rice plants treated with Cd and Si together from the 6th day of experimentation when compared with plants treated only with Cd. However, these parameters were significantly improved in plants treated from the 6th day with Cd and from the 20th day also with Si (Nwugo and Huerta, 2008). Liu et al. (2009) found that foliar application of Si in the form of silica sols significantly increased dry weight of rice shoots and grains exposed to various soil Cd concentrations (0–30 mg kg−1). Recently, Gu et al. (2011) found that application of Si mitigated the negative effects of metals, including Cd, in rice grown on multi-metal-contaminated acidic soil.
The effect of Si on biomass production in plants suffering Cd toxicity was recently investigated in several non-monocotyledonous species, for example by Song et al. (2009) on pakchoi (Brassica chinensis). Similarly, Feng et al. (2010) reported Si-enhanced growth of root and shoot of Cd-treated cucumber (Cucumis sativus). Shi et al. (2010) observed the same alleviating effect of Si on both Cd-tolerant and Cd-sensitive cultivars of peanut (Arachis hypogaea).
Si clearly has a role in enhancing the growth of various plant species affected by Cd toxicity. However, the mechanism of this mitigation is not fully understood. Root, as the organ having first contact with the soil, is responsible for the uptake of various elements to the whole plant organism. Si-activated changes in the development of root tissues might influence the uptake and concentration of Cd in plants. On the other hand, Si might decrease the concentration of toxic Cd ions by binding them to the apoplasmic space, such as the cell wall, or sequestering them to vacuoles.
The aim of this study was to examine the effect of Si on root growth, and uptake and subcellular distribution of Cd in maize to provide a better understanding of the alleviating phenomenon of Si in plants exposed to Cd.
MATERIALS AND METHODS
Hydroponic cultivation of plants
Young maize plants (Zea mays, hybrid ‘Jozefina’) were cultivated hydroponically until the second fully developed leaf in a growth chamber with a 12-h photoperiod, a temperature of 25/18 °C (day/night), 75 % humidity and 200 µmol m−2 s−1 photosynthetically active radiation (PAR).
Caryopses were sterilized for 20 min in 4 % Wolfin Thiuran 75W or 5 % Savo (Biochemie, Czech Republic) and washed carefully several times with water before germination. Thereafter, they were imbibed in water for 4 h at room temperature and germinated in rolls of wet filter paper for 72 h at 25 °C in the dark.
Seedlings were transferred to 3-litre glass containers (ten plants per container) filled with half-strength Hoagland solution (Hoagland and Arnon, 1950) with or without Cd and/or Si. After 2 d of cultivation the medium was changed to full-strength Hoagland solution. The solutions were changed every second day. The frequent exchange of the nutrient solution was done instead of air bubbling to prevent mechanical disturbance to the roots, an important consideration for anatomical studies. Additionally, this process prevents depletion of Cd in the solution. In total, the plants in each treatment were cultivated for 10 d.
Six different treatments were applied:
Control (C) – Hoagland solution without Cd and Si;
Cadmium 5 (Cd5) – Hoagland solution with 5 µm Cd(NO3).4H2O;
Cadmium 50 (Cd50) – Hoagland solution with 50 µm Cd(NO3).4H2O;
Silicon (Si) – Hoagland solution with 5 mm Si in the form of sodium silicate solution (27 % SiO2 dissolved in 14 % NaOH);
Cd5 plus silicon (Cd5 + Si) – Hoagland solution with addition of both Cd and Si at the same concentrations as in the Cd5 and Si treatments; and
Cd 50 plus silicon (Cd50 + Si) – Hoagland solution with addition of both Cd and Si at the same concentrations as in the Cd50 and Si treatments.
The pH of each cultivation solution was adjusted to 6·2 using HCl. The Si concentration used in our experiments was based on our previous experiments with this maize cultivar. Note that no precipitation of Si in the solution was observed.
For experiments investigating the distribution of 109Cd in maize plants, experimental material was cultivated hydroponically in a growth chamber (Conviron, Winnipeg, Canada) at the Institute of Botany, Stockholm University, Sweden, at 16/8 h and 25/23 °C day/night regime, 75 % humidity and 300 µmol m−2 s−1 PAR. Sterilized seeds were soaked in water for 4 h and then germinated for 3 d rolled in wet filter paper in the dark at 25 °C. Plants were then transferred to 2·1-litre pots, six plants in each pot, containing 50 % Hoagland nutrient medium (pH 6·2). The medium also contained 34 nm 109Cd (3·7 kBq L−1; Perkin-Elmer, Boston, MA, USA). The medium was used with or without 5 mm Si in the form of sodium silicate solution (Sigma, St Louis, MO, USA; 27 % SiO2 dissolved in 14 % NaOH). Plants were harvested after 7 d of treatment.
Evaluation of the growth and elemental concentration
Plant material was harvested at the fully developed second leaf stage (13th day after imbibition, or 10th day of hydroponic cultivation). The plants were divided into below- and above-ground parts. The total length of primary seminal roots was measured. Fresh weights of below- and above-ground parts of the plants were determined. Thereafter, roots were washed three times in distilled water. Root and shoot material was dried at 70 °C for 72 h, and the dry weights of below- and above-ground parts were determined. The concentration of Cd and Si was determined in finely ground dried root and shoot tissue using atomic absorption spectrometry (AAS), or using inductively coupled plasma mass spectroscopy (ICP-MS) in the laboratories of the Institute of Geology, Faculty of Natural Sciences, Comenius University in Bratislava, Slovakia, or the AcmeLabs, Vancouver, Canada.
Determination of changes in root tissue development
For analysis of the changes in the lignification of xylem vessels caused by Cd and/or Si, cross-sections of roots were stained with phloroglucinol and hydrochloric acid. Casparian bands were visualized by staining with 0·2 % berberine hemisulphate and post-staining with 0·1 % toluidine blue, and suberin lamellae were stained with 0·2 % fluorol yellow 088 according to Brundrett et al. (1988, 1991) and Lux et al. (2005). All sections were observed with a Zeiss Axioskop 2 plus epifluorescence microscope (Jena, Germany) and images were capture with an Olympus DP-72 digital camera (Tokyo, Japan).
Comparison of apoplasmic and symplasmic distribution of Cd in roots
Differences in the distribution of radioactively labelled Cd isotopes between apoplasm and symplasm were determined in roots and shoots of maize plants treated with 34 nm 109Cd (3·7 kBq L−1; Perkin-Elmer) with or without 5 mm Si. The fractions of cell walls, organelles and soluble material were isolated in shoots according to Lozano-Rodríguez et al. (1997). In roots, the xylem sap and apoplasmic fluids were first isolated according to Lopez-Millan et al. (2000), and the cell-wall fractions, organelle-rich fractions and soluble fractions were isolated from the same material according to Lozano-Rodríguez et al. (1997). These fractions were mixed with scintillation cocktail (EmulsifierSafe, Perkin-Elmer) at a ratio of 1 : 9, and were analysed in a scintillation counter (WALLAC 1409 LS, Perkin-Elmer).
Statistical analysis
Statistical significance was assessed with Student's t-test using the Statgraphics Centurion XV v. 15·2·05 (StatPoint, Inc., Warrenton, VA, USA) and Excel (Microsoft Office 2003) programs and a single-step multiple comparisons of means was performed via Tukey test. A P-value <0·05 was defined as significant. The data presented (growth analysis) are from six different replicates; in each replicate ten plants were analysed. In total, 60 plants per treatment were analysed. Three independent repetitions of plant cultivation were done for the determination of the Cd and Si concentration in the below- and above-ground plant parts. For determination of changes in root tissue development, eight different roots from each treatment were analysed. For determination of radioactively labeled 109Cd in plants, six different replicates were analysed.
RESULTS
Effect of Si on root growth
Cd affected the length and branching of primary seminal roots. Roots treated with Cd5 were shorter (Table 1), yellowish and less branched when compared with controls (Fig. 1). Similarly, the length of lateral roots was shorter when compared with control plants (Fig. 1). Roots treated with a higher concentration of Cd (Cd50) showed the same symptoms of Cd toxicity; they were even shorter (Table 1) and had shorter lateral roots than Cd5-treated roots (Fig. 1). However, addition of Si partially mitigated the negative influence of Cd in roots. Primary roots treated with Cd5 + Si and Cd50 + Si were longer (Table 1) and had longer lateral roots when compared with Cd5- and Cd50-treated roots, respectively (Fig. 1). Addition of Si enhanced the branching of seminal roots when compared with controls (Fig. 1).
Table 1.
Length, and fresh and dry weight of primary seminal roots of young maize plants grown hydroponically for 10 d and exposed to various concentrations of Cd and/or Si
| Treatment | Root length (cm) | Root f. wt (mg) | Root d. wt (mg) |
|---|---|---|---|
| C | 15·03 ± 1·39a | 176·3 ± 19·1a | 7·6 ± 1·1a |
| Cd5 | 12·97 ± 0·54b | 152·9 ± 8·9b | 6·6 ± 0·4b |
| Cd5 + Si | 17·23 ± 1·53c | 174·7 ± 25·0a | 7·5 ± 0·7a |
| Cd50 | 9·49 ± 1·09d | 95·1 ± 3·6c | 6·0 ± 0·5c |
| Cd50 + Si | 10·45 ± 0·80e | 135·8 ± 18·6d | 7·7 ± 0·4a |
| Si | 17·53 ± 1·28c | 216·5 ± 27·3e | 10·1 ± 0·8d |
C, control; Cd5, 5 µm Cd; Cd5 + Si, 5 µm Cd with 5 mm Si; Cd50, 50 µm Cd; Cd50 + Si, 50 µm Cd with 5 mm Si; Si, 5 mm Si. Values are means ± s.d. (n = 15). Different letters indicate significant differences among the treatments at P < 0·05 %.
Fig. 1.
Appearance of roots of young maize plants grown hydroponically for 10 d and treated with Cd, Si or both elements together. C, control; Cd5, 5 µm Cd; Cd5 + Si, 5 µm Cd with 5 mm Si; Cd50, 50 µm Cd; Cd50 + Si, 50 µm Cd with 5 mm Si; Si, 5 mm Si. Scale bar = 10 mm.
Differences were observed in the fresh and dry weight of roots among the treatments. Cd at both applied concentrations (Cd5 and Cd50) significantly decreased root fresh as well as dry weight when compared with control plants (Table 1). However, this was alleviated by addition of Si in Cd5 + Si and Cd50 + Si plants. Si applied to plants grown without Cd increased the fresh as well as dry weight of root when compared with control plants (Table 1).
Si-induced changes in Cd uptake in young maize plants
The effect of exogenous Si application on the changes in the ionome of below- and above-ground parts of young maize plants treated with two different Cd concentrations was investigated. The concentration of Cd in maize roots positively correlated with increased Cd treatment. At low Cd, Si applied increased the Cd concentration and also the total Cd content in roots (Cd5 + Si versus Cd5; Fig. 2A). By contrast, at higher Cd the addition of Si in Cd50 + Si decreased the Cd concentration compared with Cd50 (Fig. 2A), but no significant difference in total root Cd content was observed between Cd50 and Cd50 + Si (Table 2).
Fig. 2.
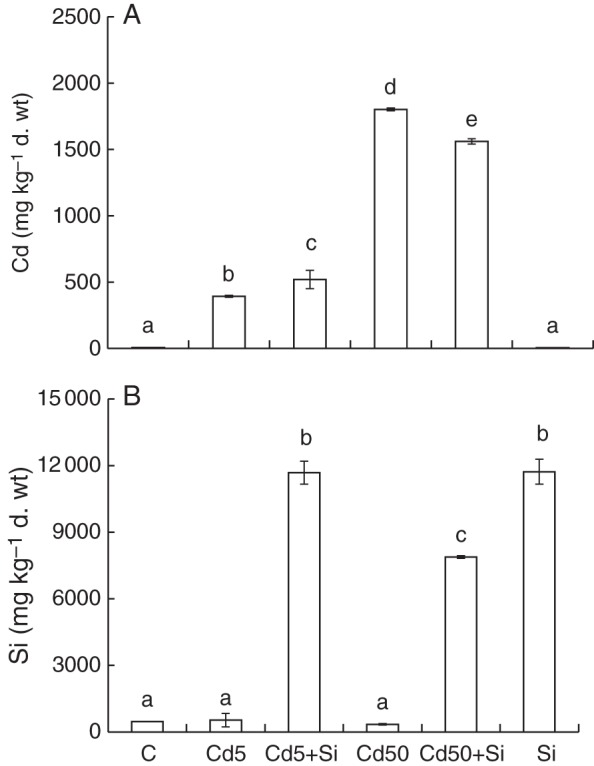
Concentration of (A) Cd and (B) Si in the below-ground part of young maize plants grown hydroponically for 10 d and treated with Cd, Si or both elements together. C, control; Cd5, 5 µm Cd; Cd5 + Si, 5 µm Cd with 5 mm Si; Cd50, 50 µm Cd; Cd50 + Si, 50 µm Cd with 5 mm Si; Si, 5 mm Si. Values are means ± s.d. (n = 3). Different letters indicate significant differences among the treatments at P < 0·05 %.
Table 2.
Total average content of cadmium (μg per plant) in the root, shoot, whole plant and [Cd]shoot/[Cd]root ratio in young maize plants grown hydroponically for 10 d and exposed to various concentrations of Cd and/or Si
| Cd5 | Cd5 + Si | Cd50 | Cd50 + Si | |
|---|---|---|---|---|
| Root | 7·73a | 11·73b | 35·2c | 36·1c |
| Shoot | 2·98a | 4·52b | 9·54c | 12·25d |
| Whole plant | 10·71a | 16·25b | 44·74c | 48·35d |
| [Cd]shoot/[Cd]root | 0·386 | 0·385 | 0·271 | 0·339 |
Cd5, 5 µm Cd; Cd5 + Si, 5 µm Cd with 5 mm Si; Cd50, 50 µm Cd; Cd50 + Si, 50 µm Cd with 5 mm Si (n = 3). Different letters indicate significant differences among the treatments at P < 0·05 %.
Si-treated plants accumulated approx. 25 times more Si in below-ground parts compared with control plants (Fig. 2B). No effect of Cd was found on Si concentration in maize roots treated without additional Si. However, an influence of Cd on Si concentration in Si-treated roots was found. In roots treated with lower Cd and Si (Cd5 + Si) there was no significant difference in Si concentration when compared with Si treatment. However, higher Cd decreased the Si concentration in roots of Cd50 + Si plants when compared with Si treatment (Fig. 2B).
Similarly, the concentration of Cd correlated positively with increased Cd treatment in shoots. However, the concentration of Cd in the shoot was approx. 10-fold lower than in the root. We found an increase in Cd concentration and also in total content of Cd accumulated in shoots treated with lower Cd and Si (Cd5 + Si) when compared with the Cd5 treatment (Fig. 3A, Table 2). No significant differences in shoot Cd concentration were observed between the Cd50 and Cd50 + Si treatments (Fig. 3A), although the total content of Cd was significantly higher in Cd50 + Si- than in Cd50-treated plants (Table 2).
Fig. 3.
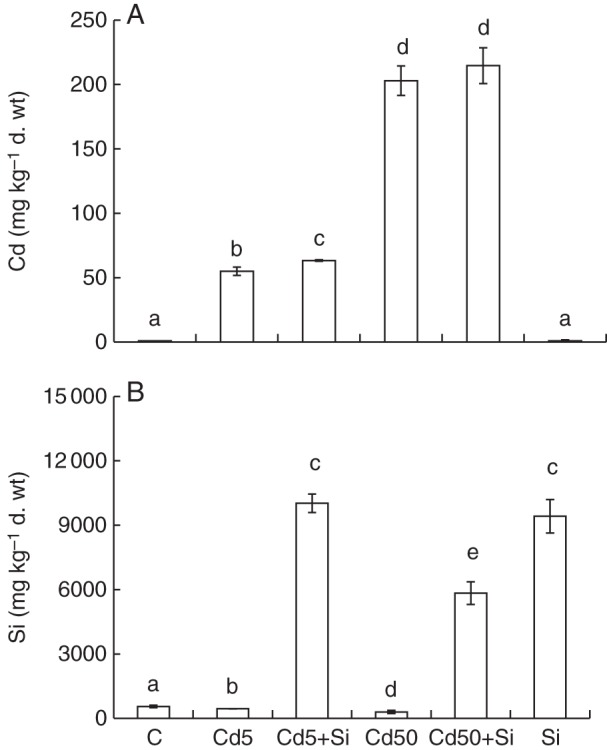
Concentration of (A) Cd and (B) Si in the above-ground part of young maize plants grown hydroponically for 10 d in and treated with Cd, Si or both elements together. C, control; Cd5, 5 µm Cd; Cd5 + Si, 5 µm Cd with 5 mm Si; Cd50, 50 µm Cd; Cd50 + Si, 50 µm Cd with 5 mm Si; Si, 5 mm Si. Values are means ± s.d. (n = 3). Different letters indicate significant differences among the treatments at P < 0·05 %.
Similar to data for roots, we observed that Si application in shoot increased the Si concentration about 17-fold compared with controls. However, Cd at increasing concentration decreased the concentration of Si in plants treated without additional Si (Fig. 3B). This decrease was about 20 % in Cd5-treated plants and about 45 % in Cd50-treated plants compared with controls. No difference in Si concentration was observed between Cd5 + Si- and Si-treated shoots (Fig. 3B). However, a higher Cd level decreased Si concentration in Cd50 + Si plants when compared with the Si treatment (Fig. 3B).
Effect of Si on the distribution of 109Cd in maize plants
In maize plants grown for 7 d hydroponically in the presence of a low concentration of radioactively labelled isotopes of Cd (109Cd), considerably higher levels were found in root tissues, and only a very low level was detected in shoots (Fig. 4).
Fig. 4.
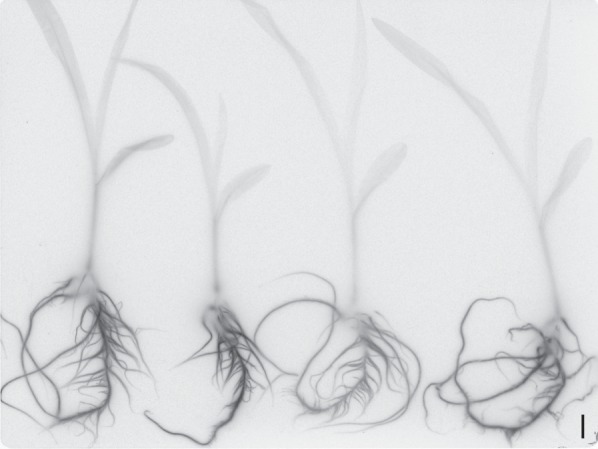
Distribution of 109Cd in roots and shoots of young maize plants grown hydroponically for 7 d in the presence of 34 nm 109Cd. Scale bar = 10 mm.
No differences in 109Cd distribution in apoplasm (xylem sap + apoplasmic fluids + cell-wall fraction) and symplasm (organelle-rich fraction + soluble fraction) were observed between 109Cd- and 109Cd + Si-treated roots (Fig. 5).
Fig. 5.
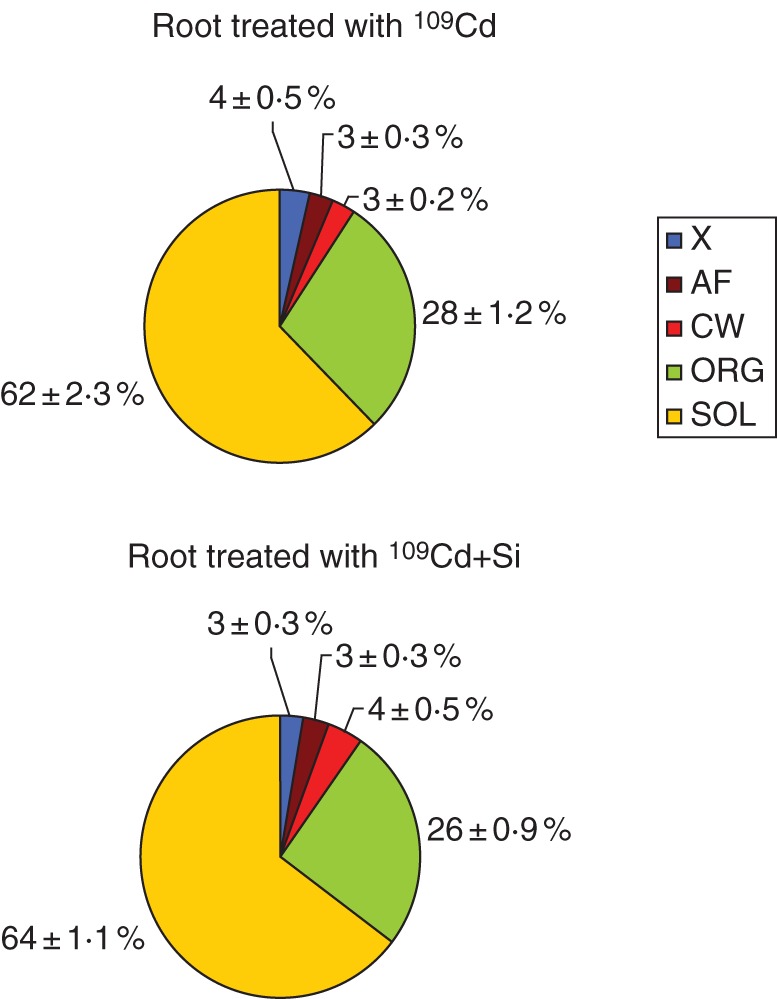
Distribution of 109Cd in different fractions of roots of maize plants grown hydroponically for 7 d; two treatments were used (109Cd, 34 nm 109Cd; 109Cd + Si, 34 nm 109Cd + 5 mm Si). Values are means of six different replicates. X, xylem sap; AF, apoplasmic fluids; CW, cell-wall fraction; ORG, organelle-rich fraction; SOL, soluble fraction.
Considerably greater differences were observed in the shoot distribution of 109Cd in three different cell compartments between 109Cd- and 109Cd + Si-treated plants (Fig. 6). The total content of 109Cd increased more than three-fold in the cell-wall fraction in the 109Cd + Si compared with 109Cd treatment. Conversely, a decrease in 109Cd content was determined in the soluble fraction in 109Cd + Si-treated compared with 109Cd-treated plants. No differences were observed in the 109Cd content in the organelle-rich fraction of shoot between 109Cd + Si- and 109Cd-treated plants (Fig. 6).
Fig. 6.
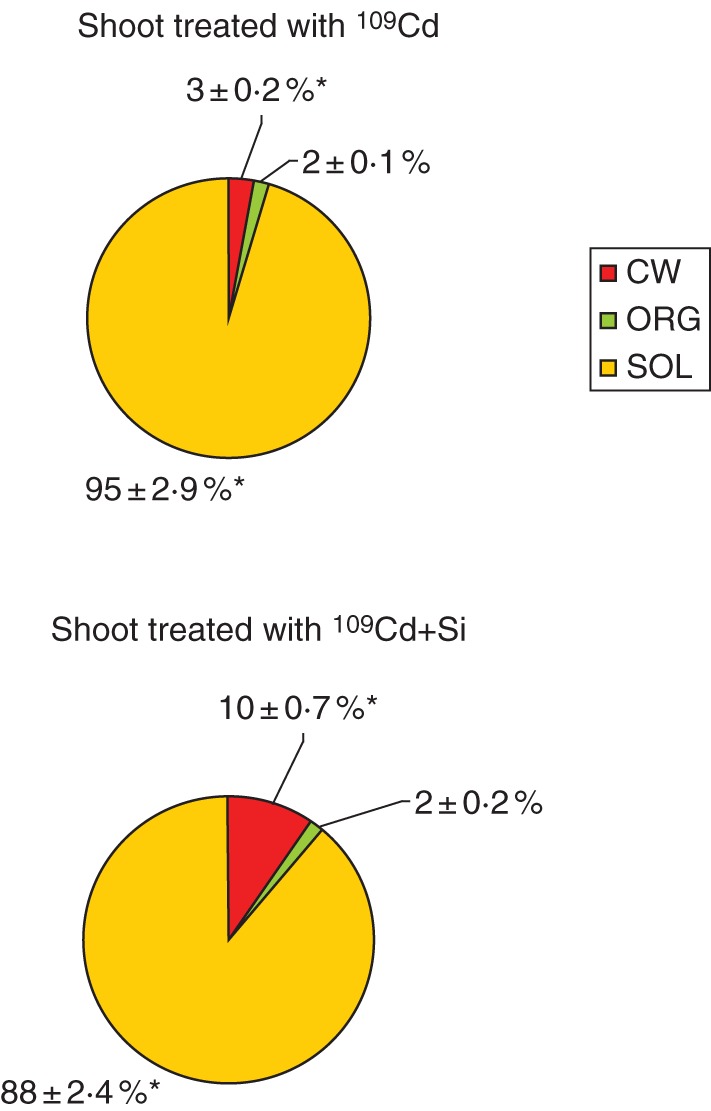
Distribution of 109Cd in different fractions of shoots of maize plants grown hydroponically for 7 d; two treatments were used (109Cd, 34 nm 109Cd; 109Cd + Si, 34 nm 109Cd + 5 mm Si). Values are means of six different replicates. CW, cell-wall fraction; ORG, organelle-rich fraction; SOL, soluble fraction. Asterisks indicate significant differences among the treatments at P < 0·05 %.
Development of apoplasmic barriers in roots
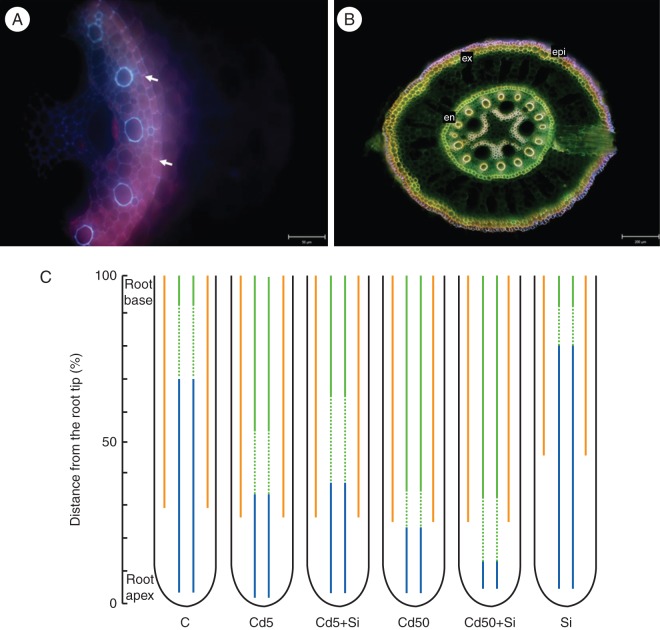
Casparian bands (Fig. 7A) developed in the endodermis relatively close to the root apex and only slight differences were observed between the treatments. In roots treated with Cd5 the Casparian bands developed closer to the root apex when compared with Cd5 + Si-treated roots. However, noticeable differences were observed in suberin lamellae development in exo- and endodermis. Suberin lamellae (Fig. 7B) developed closer to the root apex in exodermis than in endodermis in control plants. Cd applied either alone or in combination with Si did not influence the development of exodermis in all treatments. Application of Si alone shifted the development of suberin lamellae more distantly from the root apex in exodermis.
Fig. 7.
Development of apoplasmic barriers in roots. (A) Cross-section of the root of young maize plant grown hydroponically for 10 d with developed Casparian bands (white arrows) in the endodermis 6 mm from the root tip; scale bar = 50 µm. (B) Cross-section of the basal part of young maize plants grown hydroponically for 10 d with developed lateral root and suberin lamellae in exo- and endodermis; scale bar = 200 µm. Abbreviations: epi, epidermis; ex, exodermis; en, endodermis. (C) Scheme of development of apoplasmic barriers (Casparian bands and suberin lamellae) in exo- and endodermis of young maize plants grown hydroponically for 10 d and treated with Cd, Si or both elements together. C, control; Cd5, 5 µm Cd; Cd5 + Si, 5 µm Cd with 5 mm Si; Cd50, 50 µm Cd; Cd50 + Si, 50 µm Cd with 5 mm Si; Si, 5 mm Si. Different regions of the root can be distinguished: a region in which Casparian bands in endodermis are developed (solid blue lines), a region in which endodermal suberin lamellae are fully developed (solid green lines), a region in which the suberin lamellae in endodermis are partially developed (broken green lines), and a region in which suberin lamellae are fully developed in exodermis (solid orange lines). Because the length of roots grown in the absence and presence of Cd and/or Si differed, the distance from the root apex was expressed as a percentage of the total root length.
When plants were treated with Cd5, suberin lamellae in endodermis started to develop closer to the root apex. By contrast, in the Cd5 + Si treatment suberin lamellae in endodermis developed further from the root apex than in the Cd5 treatment. Suberin lamellae started to develop closer to the root apex in the Cd50 + Si treatment compared with the Cd50 treatment. However, no differences in the distance of fully developed suberin lamellae from the root apex were observed between the Cd50 and Cd50 + Si treatments. Similarly, the development of suberin lamellae was initiated earlier in control than in Si-treated plants, and no differences in the distance of fully developed suberin lamellae in endodermis from the root apex were later observed between these treatments. The effect of all treatments on development of exo- and endodermis is summarized in Fig. 7C.
Lignification of xylem vessels in roots
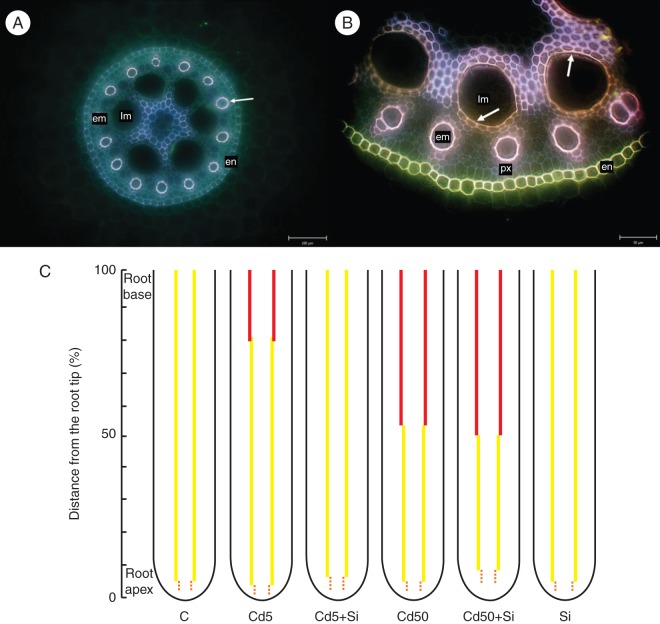
We observed a slight effect of Cd and/or Si on the development of protoxylem and early metaxylem elements (Fig. 8A) in roots. Cd at the lower concentration (Cd5) induced earlier protoxylem development when compared with control roots. This was alleviated by addition of Si in the Cd5 + Si treatment. However, the higher concentration of Cd (Cd50) caused no differences in protoxylem element development when compared with controls. Similarly, we found no significant changes in early metaxylem development in the two Cd treatments. Only application of Si in both combined Cd + Si treatments led to delayed early metaxylem lignification when compared with control or Cd-treated plants. Si itself did not enhance the lignification of early metaxylem vessels when compared with control plants.
Fig. 8.
Lignification of xylem elements in roots. (A) Cross-section of the central zone of apical part of a young maize plant root grown hydroponically for 10 d; scale bar = 100 µm. Arrow shows the lignified cell wall of early metaxylem vessel. (B) Detail of the central zone of basal part of young maize plants root grown hydroponically for 10 d; scale bar = 50 µm. Arrows show the lignified cell wall of late metaxylem vessel. Abbreviations: en, endodermis; px, protoxylem elements; em, early metaxylem vessel; lm, late metaxylem vessel. (C) Scheme of lignification of xylem elements in roots of young maize plants grown hydroponically for 10 d and treated with Cd, Si or both elements together. C, control; Cd5, 5 µm Cd; Cd5 + Si, 5 µm Cd with 5 mm Si; Cd50, 50 µm Cd; Cd50 + Si, 50 µm Cd with 5 mm Si; Si, 5 mm Si. Different regions of the root can be distinguished: a region in which protoxylem is developed (broken orange lines), a region in which early metaxylem is developed (solid yellow lines) and a region in which late metaxylem is developed (solid red lines). Because the length of roots grown in the absence and presence of Cd and/or Si differed, the distance from the root tip was expressed as a percentage of total root length.
An increase in Cd concentration correlated positively with earlier lignification of late metaxylem vessels (Fig. 8B) compared with controls. This early lignification was suppressed in the combined Cd5 + Si treatment. When roots were grown at higher Cd concentration (Cd50), late metaxylem vessels lignified even at 50 % of the total root length under the conditions used in our experiments. Addition of Si in the Cd50 + Si treatment had no influence on the development of late metaxylem vessels when compared with the Cd50 treatment. No effect on lignification was observed when roots were grown in the presence of Si when compared with the control treatment. The effect of all treatments on lignification of xylem vessels is summarized in Fig. 8C.
DISCUSSION
At increasing concentration Cd significantly decreased the length of primary seminal roots, root fresh and dry weight as well as root branching in our experiments. Application of Si increased all of these parameters. It has been shown that Si has an alleviating influence on the growth of many different plant species exposed to various abiotic stresses, including Cd (Liang et al., 2007). Similar to our results, Si-induced enhancement of below- and abovground biomass of maize plants treated with Cd was described by Liang et al. (2005) and also in our previous study (Vaculík et al., 2009). The mitigation of Cd toxicity by Si was observed also in other plant species, for example rice (Shi et al., 2005; Nwugo and Huerta, 2008), pakchoi (Song et al., 2009), peanut (Shi et al., 2010) and cucumber (Feng et al., 2010). Lukačová Kuliková and Lux (2010) found that some maize hybrids decreased whereas others increased their root and shoot biomass when treated simultaneously with Si and Cd as compared with plants treated solely with Cd. Taking all these data together, we might conclude that Si enhanced the root and shoot biomass in plants exposed to Cd, as observed in our experiments. However, the different responses of various Cd-treated species to Si treatment might be attributed to species and cultivar specificity.
We observed a positive correlation between Cd concentration in the growth medium and in maize root and shoot tissues. This is in agreement with other studies performed on various plants, including maize (e.g. Wang et al., 2007). Several studies have dealt with the effect of Si on Cd uptake in different plants. Total Cd or Cd concentration decreased due to Si addition in rice (Shi et al., 2005; Zhang, 2008; Liu et al., 2009; Gu et al., 2011), peanut (Shi et al., 2010), pakchoi (Song et al., 2009), cucumber (Feng et al., 2010) and strawberry (Treder and Cieslinski, 2005). In maize, addition of a higher Si concentration (400 mg kg−1) to Cd-contaminated soil decreased the Cd concentration and also total Cd content in above-ground parts, and decreased only Cd concentration but not total Cd content in below-ground parts. However, Si when applied at a lower concentration (50 mg kg−1) decreased the Cd concentration in shoot but not in root, and an increase in total Cd content was observed in root as well as in shoot (Liang et al., 2005). Similarly, Lukačová Kuliková and Lux (2010) found a decrease in Cd concentration caused by Si in shoots of various maize hybrids treated with high Cd (100 µm). On the other hand, we observed an increase in root and shoot Cd concentration and in total Cd content in maize plants treated simultaneously with Cd5 + Si when compared with the Cd5 treatment (Figs 2A and 3A, Table 1). However, at higher applied Cd concentration, addition of Si (Cd50 + Si) did not increase the concentration of Cd in root or in shoot (Figs 2A and 3A, Table 1), and total Cd content was higher only in shoot, probably due to Si-enhanced biomass production. Therefore, we conclude that effect of Si on Cd uptake varies between plant species, and in maize depends on the concentration of Cd in the medium.
We found that 109Cd was predominantly localized in the root of maize plants. It has been reported that in most vascular plants Cd is taken up and deposited in below-ground plant parts, and only a smaller part is translocated to the shoot (Lux et al., 2011). However, this contrasts with some plants hyper-accumulating Cd (Baker, 1981). In root tissues, most Cd has been localized in the apoplasm, especially in cell walls, with a lower Cd content within root cells (e.g. Seregin et al., 2004; Liu et al., 2007; Vázquez et al., 2007). By contrast, we found considerably more radioactively labelled 109Cd in the symplasmic (90 %) than in the apoplasmic (10 %) fraction of maize roots as well as in shoots. Similarly, several authors described a higher Cd concentration in symplasm than in apoplasm of roots and shoots in various plants treated with Cd at up to 50 µm (Lozano-Rodríguez et al., 1997; Shi et al., 2005; Fu et al., 2011). However, Shi et al. (2010) found a higher Cd concentration in symplasm than in root apoplasm but not in shoot apoplasm of peanut plants treated with 200 µm Cd, and Redjala et al. (2009) found more 109Cd in the symplasm of maize root tissues treated with lower Cd (0·25 µm), and a higher Cd concentration in the medium (50 µm) resulted in an increase in total Cd content in apoplasm. Therefore we suggest that in plants treated with a lower level of Cd, most of this element is bound to the symplasm, and that the distribution of Cd in apoplasm/symplasm might be modified after plants are exposed to a higher level of this heavy metal.
Addition of 5 mm Si does not affect the distribution of apoplasmic and symplasmic Cd in maize roots. However, Si decreased the symplasmic and increased the apoplasmic concentration of Cd in maize shoots. Similar results were observed in rice by Shi et al. (2005), who also found no differences between apoplasmic and symplasmic Cd concentration in roots of Si-treated peanut plants. By contrast, in leaves the addition of 1·8 mm Si decreased the Cd content in the organelle fraction of a Cd-sensitive cultivar and decreased it in the cell-wall fraction of a Cd-tolerant cultivar of peanut (Shi et al., 2010). It was also found that addition of 1·8 mm Si to Mn-treated plants increased the concentration of Mn in the cell wall and considerably reduced available Mn in the cytoplasm (Rogalla and Römheld, 2002). Therefore, we suggest that the alleviating effect of Si might be partially attributed to binding of Cd to the apoplasmic fraction, thereby reducing the availability and toxicity of Cd for maize leaf cells. Similarly to previous findings, we also consider that the effect of Si on the subcellular distribution of Cd might vary among different species and with applied concentration of Si.
Knowledge of root anatomy and physiology is essential for a better understanding of the uptake and accumulation of elements into shoots. Elements are transported radially from the rhizodermis through apoplasm or symplasm across the cortex to the xylem and the shoot. Uptake is controlled by apoplasmic barriers in the endo- and exodermis (White, 2001; Ma and Peterson, 2003; Baxter et al., 2009; Schreiber, 2010; Ranathunge et al., 2011). The development of these apoplasmic barriers is variable and often differs between plant species and environmental conditions (Zimmerman and Steudle, 1998; Seago et al., 1999; Enstone and Peterson, 2005; Meyer et al., 2009; Redjala et al., 2011).
In the maize plants used in our experiments, both Casparian bands and, in particular, suberin lamellae developed closer to the root apex in Cd5-treated roots than in control plants. These observations are consistent with several studies showing that roots exposed to Cd develop apoplasmic barriers closer to the root apex (Schreiber et al., 1999; Martinka and Lux, 2004; Zelko and Lux, 2004; Lux et al., 2011). Development of apoplasmic barriers closer to the root apex was also induced by other abiotic stresses, e.g. by higher salinity (Reinhardt and Rost, 1995; Karahara et al., 2004; Krishnamurthy et al., 2009) and by drought stress (North and Nobel, 1995).
Casparian bands and suberin lamellae developed further from the root apex in Cd5 + Si- than in Cd5-treated plants. This was in agreement with our previous observations, and we suggest that the greater distance of suberin lamellae development from the root apex in endodermis caused by Si is probably related to higher Cd uptake in below- and above-ground parts of plants treated with Cd5 + Si- compared with Cd5-treated plants (Vaculík et al., 2009). Conversely, in roots treated with a higher level of Cd with Si (Cd50 + Si), suberization of individual endodermal cells started closer to the root apex than with the Cd50 treatment, but the suberin lamellae in Cd50 and Cd50 + Si were fully developed at the same distance from the root apex. The decrease in root Cd concentration, probably caused by the beginning of endodermis suberization closer to the root apex in Cd50 + Si, might explain the lack of significant differences in total Cd between Cd50- and Cd50 + Si-treated roots. No difference in Cd concentration was also observed between Cd50- and Cd50 + Si-treated shoots, and therefore the higher total content of Cd with the Cd50 + Si treatment than with Cd50 can be attributed to Si-induced increase in biomass production. The suberization of individual endodermal cells started more distant from the root apex in Si-treated than in control roots; however, no difference in fully developed suberin lamellae in endodermis was observed between control and Si-treated roots, in agreement with our previous study (Vaculík et al., 2009).
In exodermis the suberin lamellae developed closer to the root apex compared with endodermis under control conditions. It is known that exodermis usually develops later than endodermis (Ma and Peterson, 2003), but environmental conditions can modify the barrier chemical composition and fate of exodermis development (Hose et al., 2001; Lux et al., 2011; Redjala et al., 2011). In contrast to results for endodermis, we found that the presence of different levels of Cd and Cd in combination with Si did not influence the development of exodermal suberin lamellae when compared with control plants. But exodermal suberin lamellae developed further from the root apex in maize roots treated only with Si (Si treatment) than in controls. Recently, Fleck et al. (2011) found that Si enhanced the suberization and lignification of root tissues both in exodermis and in endodermis when compared with non-treated rice plants. Therefore, we conclude that the effect of Si on processes of cell-wall modifications in exo- and endodermis might vary among species and with growth conditions.
The changes in development of the apoplasmic barriers indicate that, in young maize plants grown hydroponically, the endodermis is more sensitive to Cd than exodermis, the latter is developed relatively close to the root apex (30 % of the root length) even under control conditions. This is further supported by the fact that in roots exposed to a higher Cd concentration, application of Si did not influence the development of suberin lamellae in endodermis as compared with roots treated with a lower Cd concentration. Therefore, we consider that endodermis serves as a more efficient barrier to apoplasmic Cd transport than exodermis in maize roots.
Cd, as well as other mineral elements and toxic compounds, is transported from root to shoot by longitudinal translocation via the system of xylem vessels. This includes transport by primary xylem elements in all vascular plants, and in species where secondary thickening occurs, secondary xylem is also involved. Maize, as a monocot, first develops the protoxylem elements responsible for elemental translocation in the apical part of the root. Later, the transport function is taken over by early metaxylem vessels of larger diameter and with greater transport capacity. In the older part of roots the transport of water and solutes is realized mostly by late metaxylem vessels of large diameter and with several times higher transport efficiency (Esau, 1965; Luxová and Lux, 1971; St Aubin et al., 1986). These developmental processes follow the same sequence in the hybrid used for our experiments.
The two levels of Cd used by us enhanced the development of xylem elements, although the difference was more evident when higher Cd stress was applied. The development of xylem elements depends on environmental conditions and species. It is known that Cd accelerates root maturation, including the development of xylem vessels in radish root (Vittória et al., 2001). Similarly, Schutzendübel et al. (2001) found an accelerated lignification of protoxylem elements closer to the root apex in Scots pine (Pinus sylvestris), and Ďurčeková et al. (2007) reported premature xylogenesis in barley roots exposed to Cd. Enhancement of metaxylem lignification due to the influence of various environmental stresses, including Cd, was also observed in barley (Valentovičová et al., 2009).
Although a lower level of Cd (Cd5) enhanced the lignification of metaxylem vessels, no deposition of lignin into the cell walls of late metaxylem vessels was observed in Cd5 + Si-treated and control roots. Hashemi et al. (2010) also observed that an increased content of lignin in rapeseed (Brassica napus) plants exposed to higher salinity was ameliorated by addition of Si. By contrast, a higher level of Cd (Cd50) enhanced the lignification of metaxylem vessels too, and no difference between the Cd50 and Cd50 + Si treatments was found. Additionally, no effect on lignification was observed when roots were grown in Si when compared with the control treatment. Therefore, we conclude that Si delays metaxylem development in roots exposed to a lower Cd concentration, and that this effect is lost when roots are treated with a higher Cd concentration.
Conclusions
Si improves the growth of young maize plants exposed to 5 or 50 µm Cd under the cultivation conditions used in the present work. Differences in Cd uptake of root and shoot are at least partially related to the development of apoplasmic barriers and maturation of vascular tissues in root. The addition of Si to a very low 109Cd concentration does not affect the distribution of apoplasmic and symplasmic Cd in maize roots, but decreases symplasmic and increases apoplasmic concentration of Cd in maize shoots. These results indicate a decreased availability and toxicity of Cd for leaf cells, which might partially explain the phenomenon of Si-induced mitigation of Cd toxicity in maize plants.
ACKNOWLEDGEMENTS
The work was supported by the Slovak Research and Development Agency under contract nos. APVV-0140-10 and APVV SK-FR-0020-11, by grants VEGA 1/0472/10, VEGA 2/0024/10, VEGA 1/0817/12, and is a part of COST FA 0905 Action. M.V. thanks the SPP Foundation for a traveling grant. This study was also supported by the Kurt and Alice Wallenberg Foundations of Sweden.
LITERATURE CITED
- Baker AJM. Accumulators and excluders – strategies in the responses of plants to heavy metals. Journal of Plant Nutrition. 1981;3:643–654. [Google Scholar]
- Baxter I, Hosmani PS, Rus A, et al. Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PloS Genetics. 2009;5:e1000492. doi: 10.1371/journal.pgen.1000492. http://dx.doi.org/10.1371/journal.pgen.1000492 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides MP, Gallego SM, Tomaro ML. Cadmium toxicity in plants. Brazilian Journal of Plant Physiology. 2005;17:21–34. [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA. A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma. 1988;146:133–142. [Google Scholar]
- Brundrett MC, Kendric B, Peterson CA. Efficient lipid staining in plant material with Sudan red 7B or Fluorol yellow 088 in polyethylene glycol-glycerol. Biotechnic Histochemistry. 1991;66:111–116. doi: 10.3109/10520299109110562. [DOI] [PubMed] [Google Scholar]
- Cunha KPV, Nascimento CWA. Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) gown on a cadmium and zinc enriched soil. Water, Air and Soil Pollution. 2009;197:323–330. [Google Scholar]
- Cunha KPV, Nascimento CWA, Silva AJ. Silicon alleviates the toxicity of cadmium and zinc for maize (Zea mays L.) grown on a contaminated soil. Journal of Plant Soil Science. 2008;171:849–853. [Google Scholar]
- Dorne JLCM, Kas GEN, Bordajandi LR, et al. Human risk assessment of heavy metals: principles and applications. In: Sigel A, Sigel H, Sigel RKO, editors. Metal ions in toxicology: effects, interactions, interdependencies. Cambridge: Thomas Graham House, Science Park; 2011. pp. 27–60. [Google Scholar]
- Ďurčeková K, Huttová J, Mistrík I, Ollé M, Tamás L. Cadmium induces premature xylogenesis in barley roots. Plant and Soil. 2007;290:61–68. [Google Scholar]
- Enstone DE, Peterson CA. Suberin lamella development in maize seedlings roots grown in aerated and stagnant conditions. Plant, Cell and Environment. 2005;25:444–455. [Google Scholar]
- Esau K. Plant anatomy. 2nd edn. New York: John Wiley; 1965. [Google Scholar]
- Feng J, Shi Q, Wang X, Wei M, Yang F, Xu H. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Scientia Horticurturae. 2010;123:521–530. [Google Scholar]
- Fleck AT, Nye T, Repenning C, Stahl F, Zahn M, Schenk MK. Silicon enhances suberinization and lignification in roots of rice (Oryza sativa) Journal of Experimental Botany. 2011;62:2001–2011. doi: 10.1093/jxb/erq392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Dou Ch, Chen Y, Chen X, Shi J, Yu M, Xu J. Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. Journal of Hazardous Material. 2011;186:103–107. doi: 10.1016/j.jhazmat.2010.10.122. [DOI] [PubMed] [Google Scholar]
- Gu HH, Qiu H, Tian T, et al. Mitigation effect of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere. 2011;83:1234–1240. doi: 10.1016/j.chemosphere.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Hasan SA, Fariduddin Q, Ali B, Hayat S, Ahmad A. Cadmium: toxicity and tolerance in plants. Journal of Environmental Biology. 2009;30:165–174. [PubMed] [Google Scholar]
- Hashemi A, Abdolzadeh A, Sadeghipour HR. Beneficial effects of silicon nutrition in alleviating salinity stress in hydroponically grown canola, Brassica napus L., plants. Soil Science and Plant Nutrition. 2010;56:244–253. [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Berkeley, CA: California Agricultural Experiment Station, Circular 347.; 1950. [Google Scholar]
- Hodson MJ, Evans DE. Aluminium/silicon interactions in higher plants. Journal of Experimental Botany. 1995;46:161–171. doi: 10.1093/jxb/eraa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson MJ, Sangster AG. The interaction between silicon and aluminium in Sorghum bicolor (L.) Moench: growth analysis and X-ray microanalysis. Annals of Botany. 1993;72:389–400. [Google Scholar]
- Horst WJ, Fecht M, Naumann A, Wissemeier A, Maier P. Physiology of manganese toxicity and tolerance in Vigna unguiculata (L.) Walp. Journal of Plant Nutrition and Soil Science. 1999;162:263–274. [Google Scholar]
- Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W. The exodermis: a variable apoplastic barrier. Journal of Experimental Botany. 2001;52:2245–2264. doi: 10.1093/jexbot/52.365.2245. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Maier P, Fecht M, Horst WJ. Effects of silicon supply on apoplastic manganese concentrations in leaves and their relation to manganese tolerance in cowpea (Vigna ustulata (L.) Walp.) Plant and Soil. 2002;238:281–288. [Google Scholar]
- Karahara I, Ikeda A, Kondo T, Uetake Y. Development of the Casparian strip in primary root of maize under salt stress. Planta. 2004;219:41–47. doi: 10.1007/s00425-004-1208-7. [DOI] [PubMed] [Google Scholar]
- Khandekar S, Leisner S. Soluble silicon modulates expression of Arabidopsis thaliana genes involved in copper stress. Journal of Plant Physiology. 2011;168:699–705. doi: 10.1016/j.jplph.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Schreiber L, Mathew MK. The role of apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.) Planta. 2009;230:119–134. doi: 10.1007/s00425-009-0930-6. [DOI] [PubMed] [Google Scholar]
- Li J, Frantz J, Leisner S. Alleviation of copper toxicity in Arabidopsis thaliana by silicon addition to hydroponic solutions. Journal of American Society of Horticultural Science. 2008;133:670–677. [Google Scholar]
- Liang Y, Wong JWC, Wei L. Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere. 2005;58:475–483. doi: 10.1016/j.chemosphere.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Liang Y, Sun W, Zhu YG, Christie P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environmental Pollution. 2007;147:422–428. doi: 10.1016/j.envpol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Liu Ch, Li F, Luo Ch, et al. Foliar application of two silica sols reduced cadmium accumulation in rice grains. Journal of Hazardous Material. 2009;161:1466–1472. doi: 10.1016/j.jhazmat.2008.04.116. [DOI] [PubMed] [Google Scholar]
- Liu D, Kottke I, Adam D. Localization of cadmium in the root cells of Allium cepa by energy dispersive X-ray analysis. Biologia Plantarum. 2007;51:363–366. [Google Scholar]
- Lopez-Millan AF, Morales F, Abadia A, Abadia J. Effects of iron deficiency on the composition of the leaf apoplastic fluid and xylem sap in sugar beet. Implications for iron and carbon transport. Plant Physiology. 2000;124:873–884. doi: 10.1104/pp.124.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Rodríguez E, Hernández LE, Bonay P, Carpena-Ruiz RO. Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. Journal of Experimental Botany. 1997;48:123–128. [Google Scholar]
- Lukačová Kuliková Z, Lux A. Silicon influence on maize, Zea mays L., hybrids exposed to cadmium treatment. Bulletin of Environmental Contamination and Toxicology. 2010;85:243–250. doi: 10.1007/s00128-010-0046-5. [DOI] [PubMed] [Google Scholar]
- Lux A, Morita S, Abe J, Ito K. An improved method for clearing and staining free-hand sections and whole-mount samples. Annals of Botany. 2005;96:989–996. doi: 10.1093/aob/mci266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Martinka M, Vaculík M, White PJ. Root responses to cadmium in the rhizosphere: a review. Journal of Experimental Botany. 2011;62:21–37. doi: 10.1093/jxb/erq281. [DOI] [PubMed] [Google Scholar]
- Luxová M, Lux A. Notes on the origin and development of primary root tissues. In: Kozinka J, editor. Structure and function of primary root tissues. Proceedings of a symposium, Tatranská Lomnica. Publishing House of the Slovak Academy of Sciences; 1971. pp. 37–40. [Google Scholar]
- Ma F, Peterson CA. Recent insights into the development, structure and chemistry of the endodermis and exodermis. Canadian Journal of Botany. 2003;81:405–421. [Google Scholar]
- Martinka M, Lux A. Response of roots of three populations of Silene dioica to cadmium treatment. Biologia. 2004;59:185–189. [Google Scholar]
- Meyer ChJ, Seago JL, Peterson CA. Environmental effects on the maturation of the endodermis and multiseriate exodermis of Iris germanica roots. Annals of Botany. 2009;103:687–702. doi: 10.1093/aob/mcn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagajyoti PC, Lee KD, Sreekanth TVM. Heavy metals, occurrence and toxicity for plants: a review. Environmental Chemistry Letters. 2010;8:199–216. [Google Scholar]
- Neumann D, Zur Nieden U. Silicon and heavy metal tolerance of higher plants. Phytochemistry. 2001;56:685–692. doi: 10.1016/s0031-9422(00)00472-6. [DOI] [PubMed] [Google Scholar]
- North GB, Nobel PS. Hydraulic conductivity of concentric root tissues of Agave deserti Engelm. under wet and drying conditions. New Phytologist. 1995;130:47–57. [Google Scholar]
- Nowakowski W, Nowakowska J. Silicon and copper interaction in the growth of spring wheat seedlings. Biologia Plantarum. 1997;39:463–466. [Google Scholar]
- Nwugo ChC, Huerta AJ. Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice exposed to low-level cadmium. Plant and Soil. 2008;311:73–86. [Google Scholar]
- Piršelová B, Kuna R, Libantová J, Moravčíková J, Matušíková I. Biochemical and physiological comparison of heavy metal-triggered defense responses in the monocot maize and dicot soybean roots. Molecular Biology Reports. 2011;38:3437–3446. doi: 10.1007/s11033-010-0453-z. [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Schreiber L, Franke R. Suberin research in the genomics era – new interest for an old polymer. Plant Science. 2011;180:399–413. doi: 10.1016/j.plantsci.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Redjala T, Sterckeman T, Morel JL. Cadmium uptake by roots: contribution of apoplast and of high- and low-affinity membrane transport systems. Environmental and Experimental Botany. 2009;67:235–242. [Google Scholar]
- Redjala T, Zelko I, Sterckeman T, Legué V, Lux A. Relationship between root structure and root cadmium uptake in maize. Environmental and Experimental Botany. 2011;71:241–248. [Google Scholar]
- Reinhardt DH, Rost TL. Salinity accelerates endodermal development and induces an exodermis in cotton seedling roots. Environmental and Experimental Botany. 1995;35:563–574. [Google Scholar]
- Rogalla H, Römhled V. Role of apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environment. 2002;25:549–555. [Google Scholar]
- Seago JL, Peterson CA, Enstone DE, Scholey CA. Development of the endodermis and hypodermis of Typha glauca Godr. and Typha angustifolia. Canadian Journal of Botany. 1999;77:122–134. [Google Scholar]
- Seregin IV, Shpigun LK, Ivanov VB. Distribution and toxic effects of cadmium and lead on maize root. Russian Journal of Plant Physiology. 2004;51:525–533. [Google Scholar]
- Shi G, Cai Q, Liu C, Wu L. Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regulation. 2010;61:45–52. [Google Scholar]
- Shi X, Zhang Ch, Wang H, Zhang F. Effect of Si on the distribution of Cd in rice seedlings. Plant and Soil. 2005;272:53–60. [Google Scholar]
- Schreiber L. Transport barriers made of cutin, suberin and associated waxes. Trends in Plant Science. 2010;15:546–553. doi: 10.1016/j.tplants.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Schreiber L, Hartmann K, Skrabs M, Zeier J. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. Journal of Experimental Botany. 1999;50:1267–1280. [Google Scholar]
- Schutzendübel A, Schwanz P, Teichmann T, et al. Cadmium-induced changes in antioxidative systems, H2O2 content and differentiation in pine (Pinus sylvestris) roots. Plant Physiology. 2001;127:887–898. doi: 10.1104/pp.010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A, Li Z, Zhang J, Xue G, Fan F, Liang Y. Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. Journal of Hazardous Material. 2009;172:74–83. doi: 10.1016/j.jhazmat.2009.06.143. [DOI] [PubMed] [Google Scholar]
- Song A, Li P, Li Z, Fan F, Nikolic M, Liang Y. The alleviation of zinc toxicity by silicon is related to zinc transport and antioxidative reactions in rice. Plant and Soil. 2011;344:319–333. [Google Scholar]
- St Aubin G, Canny MJ, Mc Cully ME. Living vessel elements in the late metaxylem of sheathed maize roots. Annals of Botany. 1986;58:577–588. [Google Scholar]
- Treder W, Cieslinski G. Effect of silicon application on cadmium uptake and distribution in strawberry plants grown on contaminated soils. Journal of Plant Nutrition. 2005;28:917–929. [Google Scholar]
- Vaculík M, Lux A, Luxová M, Tanimoto E, Lichtscheidl I. Silicon mitigates cadmium inhibitory effects in young maize plants. Environmental and Experimental Botany. 2009;67:52–58. [Google Scholar]
- Valentovičová K, Halušková Ľ, Huttová J, Mistrík I, Tamás L. Effect of heavy metals and temperature on the oxalate oxidase activity and lignification of metaxylem vessels in barley roots. Environmental and Experimental Botany. 2009;66:457–462. [Google Scholar]
- Vázquez S, Fernandez-Pascual M, Sanchez-Pardo B, Carpena RO, Zornoza P. Subcellular compartmentalisation of cadmium in white lupine determined by energy-dispersive X-ray microanalysis. Journal of Plant Physiology. 2007;164:1235–1238. doi: 10.1016/j.jplph.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Vittória AP, Lea PJ, Azevedo RA. Antioxidant enzymes responses to cadmium in radish tissues. Phytochemistry. 2001;57:701–710. doi: 10.1016/s0031-9422(01)00130-3. [DOI] [PubMed] [Google Scholar]
- Wang M, Zou J, Duan X, Jiang W, Liu D. Cadmium accumulation and its effect on metal uptake in maize (Zea mays L.) Bioresource Technology. 2007;98:82–88. doi: 10.1016/j.biortech.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Wang YX, Stass A, Horst WJ. Apoplastic binding of aluminium is involved in silicon-induced amelioration of aluminium toxicity in maize. Plant Physiology. 2004;136:3762–3770. doi: 10.1104/pp.104.045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. The pathways of calcium movement to the xylem. Journal of Experimental Botany. 2001;52:891–899. doi: 10.1093/jexbot/52.358.891. [DOI] [PubMed] [Google Scholar]
- Zargar SM, Nazir M, Agrawal GK, Kim DW, Rakwal R. Silicon in plant tolerance against environmental stressors: towards crop improvement using omics approaches. Current Proteomics. 2010;7:135–143. [Google Scholar]
- Zelko I, Lux A. Effect of cadmium on Karwinskia humboldtiana roots. Biologia. 2004;59:205–209. [Google Scholar]
- Zhang Ch, Wang L, Nie Q, Zhang W, Zhang F. Long-term effects of exogenous silicon on cadmium translocation and toxicity in rice (Oryza sativa L.) Environmental and Experimental Botany. 2008;62:300–307. [Google Scholar]
- Zimmerman HM, Steudle E. Apoplastic transport across young maize roots: effect of the exodermis. Planta. 1998;206:7–19. [Google Scholar]