Abstract
Background and Aims
Phenotypic plasticity is based on the organism's ability to perceive, integrate and respond to multiple signals and cues informative of environmental opportunities and perils. A growing body of evidence demonstrates that plants are able to adapt to imminent threats by perceiving cues emitted from their damaged neighbours. Here, the hypothesis was tested that unstressed plants are able to perceive and respond to stress cues emitted from their drought- and osmotically stressed neighbours and to induce stress responses in additional unstressed plants.
Methods
Split-root Pisum sativum, Cynodon dactylon, Digitaria sanguinalis and Stenotaphrum secundatum plants were subjected to osmotic stress or drought while sharing one of their rooting volumes with an unstressed neighbour, which in turn shared its other rooting volume with additional unstressed neighbours. Following the kinetics of stomatal aperture allowed testing for stress responses in both the stressed plants and their unstressed neighbours.
Key Results
In both P. sativum plants and the three wild clonal grasses, infliction of osmotic stress or drought caused stomatal closure in both the stressed plants and in their unstressed neighbours. While both continuous osmotic stress and drought induced prolonged stomatal closure and limited acclimation in stressed plants, their unstressed neighbours habituated to the stress cues and opened their stomata 3–24 h after the beginning of stress induction.
Conclusions
The results demonstrate a novel type of plant communication, by which plants might be able to increase their readiness to probable future osmotic and drought stresses. Further work is underway to decipher the identity and mode of operation of the involved communication vectors and to assess the potential ecological costs and benefits of emitting and perceiving drought and osmotic stress cues under various ecological scenarios.
Keywords: Cynodon dactylon, Digitaria sanguinalis, drought stress, osmotic stress, phenotypic plasticity, Pisum sativum, plant communication, root communication, root signalling, Stenotaphrum secundatum, stress cues
INTRODUCTION
Under natural conditions, organisms invariably experience significant environmental changes in both space and time. When heterogeneity is both fine-grained and relevant to fitness, selection is expected to favour environmentally induced changes in the phenotype, i.e. phenotypic plasticity (Bradshaw, 1965; Levins, 1968; Schlichting, 1986; Alpert and Simms, 2002). However, as phenotypic modifications require time, the environment can change before the products of plastic changes are functional, which might result in mismatching of the phenotype to the immediate environment (DeWitt et al., 1998). Although this limitation may reduce the adaptive value of some plastic responses, it also implies that selection is expected to favour anticipatory responses, i.e. plastic modifications induced by cues and signals tightly correlated with future rather than prevalent environmental conditions (Aphalo et al., 1995; Novoplansky, 2009; Shemesh et al., 2010a). For example, plants typical of open habitats are known to demonstrate similar responses to shade and to low red/far-red spectral cues, which are tightly correlated with imminent shade, regardless of prevalent levels of photosynthetic light (Franklin, 2008; Keuskampa et al., 2010). Anticipatory responses have been demonstrated to additional environmental factors such as nutrient availability (Forde and Zhang, 1998; Zhang and Forde, 1998; Shemesh et al., 2010a, b), drought (Passioura, 1988), root competition (Novoplansky and Goldberg, 2001), neighbour proximity (Pierik et al., 2009) and salinity (Ackerson and Youngner, 1975). In some cases, information regarding probable future conditions is transmitted between neighbouring plants, with the most studied example being the ‘talking trees’ phenomenon. In response to herbivory, some plants not only increase their local and systemic (e.g. Orians, 2005; Gómez and Stuefer, 2006; Miller et al., 2009) resistance, they also release various compounds, such as methyl jasmonate and green leaf volatiles, which induce defence and defensive priming responses in their undamaged neighbours (reviewed in Heil and Karban, 2010). A recent study has demonstrated that unstressed Pisum sativum plants not only swiftly closed their stomata in response to cues emitted by the roots of their osmotically stressed neighbours, but also induced stomatal closure in additional unstressed plants located further away from the stressed plant (Falik et al., 2011).
The purpose of the current study was to test whether the communication of stress cues between the roots of neighbouring Pisum sativum plants, demonstrated under highly controlled laboratory conditions (Falik et al., 2011), could be detected under more realistic settings. Specifically, the study focused on the following aspects of root communication:
Root communication in soil
Although various aqueous media and hydroponics are widely used in both physiological studies and agriculture, root development and responsiveness to environmental stimuli might be significantly affected by root structure and the physical properties of their growth medium (e.g. Takahashi, 1994; Kozlowski, 1999; Clark et al., 2003). Therefore, the study of root communication should take into account the potential alteration and attenuation of root signals and cues by soil aggregates, organic matter, air spaces, microbial activity and externally induced fluctuations in the levels of oxygen, CO2, ion concentrations and temperature. Here, root communication of stress cues was tested by following the responses of osmotically stressed Pisum sativum plants and their intact and unstressed neighbours when grown in soil.
Communication of stress cues in response to drought
Osmotic stress is prevalent in many ecosystems but it is typically caused and accompanied by salt and/or drought stresses (Zhu, 2002; Chaves et al., 2009). Besides osmotic stress, salt stress also causes severe Cl− and Na+ toxicity (e.g. Greenway and Munns, 1980). Here we tested the communication of drought stress cues by following the stomatal aperture in Pisum sativum plants that were subjected to drought and in their unstressed neighbours. Specifically, we tested the hypothesis that unstressed plants are able to perceive and respond to stress cues emitted by their drought-stressed neighbours and also relay these cues to additional unstressed plants.
Differential effects of stress and stress cues
Prolonged or repeated exposure to stress has been often demonstrated to result in acclimation, expressed in a pronounced decrease in responsiveness to continued stress, and increased tolerance to increased stress levels (e.g. in humans, Nielsen et al., 1993; bacteria, Hall et al., 2010; plants, Hughes and Dunn, 1996). For example, a 3-week exposure of Sorgum bicolor plants to sub-lethal NaCl levels, induced resistance to 0·3 m NaCl, a concentration which is invariably lethal for non-acclimated plants (Amzallag et al., 1990). Similar acclimation reactions have been observed in response to freezing conditions (Gilmour et al., 1988), excessive heat (Larkindale and Vierling, 2008) and heavy metals (Punshon and Dickinson, 1997). Here, we tested the hypothesis that plants differentially respond to direct stress and communicated stress cues. We predicted that in response to osmotic stress or drought, stressed plants would demonstrate prolonged and consistent stress responses, and relatively mild acclimation but their unstressed neighbours would habituate and cease to respond to the communicated stress cues relatively shortly after stress infliction. Testing for plant acclimation to direct stress and habituation to communicated stress cues was conducted by quantifying the kinetics of stomatal aperture after stress induction in the aforementioned experiments.
Communication of stress cues in wild plants
Despite the widespread usage of model cultivars in physiological and developmental studies, evaluating the ecological relevance and implications of root communication for plant responses and performance under natural settings calls for the study of these phenomena in wild plants. Accordingly, the communication of drought stress cues was tested in three wild species typical of xeric environments, where water limitation is a dominant determinant of plant survival and performance.
MATERIALS AND METHODS
Root communication in soil
Experimental design and set-up
A recent study has demonstrated that the communication of stress cues amongst neighbouring plants following the induction of osomtic stress was chiefly, if not solely, conducted between neighbouring roots rather than amongst shoots (Falik et al., 2011). Accordingly, rather than re-testing the possible roles of root and shoot communication of stress cues among neighbouring plants, the present experiment was designed to test the communication of stress cues between plants rooted in soil.
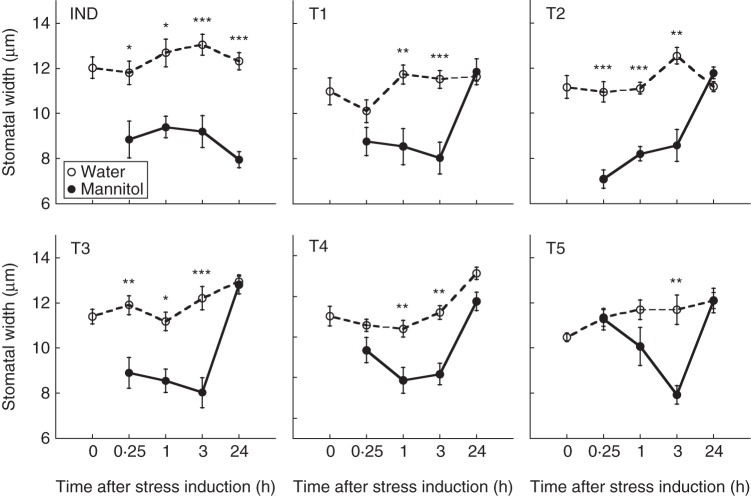
Split-root Pisum sativum L. var. Dunn plants were subjected to osmotic stress (IND, Fig 1A) while next to a row of five equidistant, intact and unstressed target plants. Each IND plant had three similarly sized roots, two of which were planted in an exclusive induction pot, which was subjected to either osmotic stress or a benign control treatment (yellow pot, Fig. 1A). The third root of each IND plant was planted in a second pot, sharing its rooting volume with the roots of five intact target plants (T1–T5; Fig. 1A). This configuration allowed the target plants to both perceive stress cues from the IND plant and exchange amongst themselves stress cues.
Fig. 1.
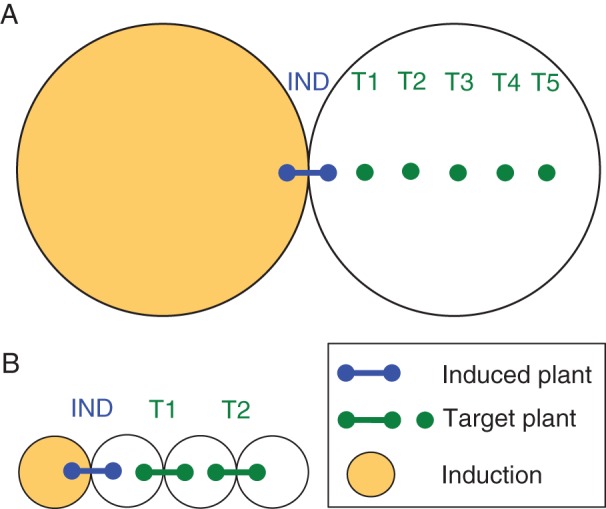
Testing for stress cuing: the experimental set-up. Circles represent pots (A) or rooting receptacles (B) and connector lines represent split-root plants. Plants neighbouring an externally induced plant (IND) shared their rooting volumes with their immediate unstressed (T1) neighbours, and target plants either shared the same rooting volume with all other target plants (T1–T5; A) or only with their immediate neighbours (T1 and T2; B). External induction was carried out by adding either mannitol (osmotic stress) or water (A), or by replacing the water by dry (drought) or wet (control) vermiculite-bentonite (VB) mixture to the induction (yellow) pot (A) or rooting receptacle (B). Stomatal width was destructively measured, in different experimental sets, immediately before (0 min) and at variable times after induction.
Osmotic stress was inflicted using mannitol, a natural sugar-alcohol osmoticum whose addition to the rooting medium is commonly used to elicit controlled osmotic stress in higher plants (e.g. Pandey et al., 2004; Falik et al., 2011). Responses to osmotic stress and stress cues were monitored by recording the stomatal aperture of the IND plant and its target neighbours, at variable intervals following the addition of mannitol or water to the induction pot (yellow, Fig. 1A).
The IND plants were grown so that they developed equal roots following removal of the tip of the seminal root (‘split-root plants’; Falik et al., 2003). Three days from germination, the seminal root was severed 2 mm below the hypocotyl and the plants were replanted in damp vermiculite. Seven days from germination, the stump of the seminal root typically regenerated three lateral roots. IND plants with three 25- to 30-mm-long roots were planted as described in 15-cm-diameter and 13-cm-high plastic pots filled with 1·5 L of commercial garden soil mixture (Deshanit, Beer Yaakov, Israel). The intact target plants were germinated 5 d after the split-root IND plants to ensure that their root sizes would be comparable at the time of their transplantation into the experimental pots. The induction and target pots were secured to each other using plastic soldering. Petroleum jelly was applied to the roots of the IND plants positioned above the meeting point between the induction and the target pots, and to the rims of the pots, in and around their contact point, to prevent seepage and capillary migration of mannitol between the pots. Using GC-MS analyses of the rooting media and a bioassay demonstrated that this protocol effectively prevented any direct effects of mannitol on the target plants (Falik et al., 2011). The same procedure was used in the water-control treatment, to account for possible confounding effects of petroleum jelly on the experimental plants.
Growth conditions and stress induction
The plants were grown in a naturally lit greenhouse, partially controlled by an automated pad-and-fan system (Termotecnica Pericoli, Albenga, Italy), under 30 % sunlight at the Sede Boqer campus, Israel (30 °52′N, 34 °47′E). Following transplantation to the experimental pots, plants were allowed to grow for 14 d before the onset of the experiment, during which time they were irrigated with tap water to field capacity every 3 or 4 d. Individual pots were bottom-drained into drip trays to prevent the seepage and capillary migration of root exudates between the pots.
The experiment was conducted on a cloudless day, 6 October 2008, between 0830 and 1030 h. External induction was carried out by slowly pouring 100 mL of either 0·8 m mannitol solution (Sigma, St Louis, MO, USA) or water into the induction pot (yellow pot, Fig. 1A).
Communication of stress cues in response to drought
Experimental design and set-up
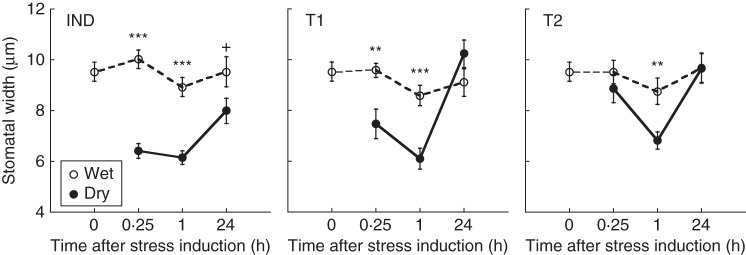
Split-root P. sativum plants were subjected to drought while neighbouring two unstressed target plants (Fig. 1B). The stressed (IND) plant shared one of its rooting receptacles with its nearest neighbour (T1), which shared its second rooting receptacle with another target plant (T2; Fig. 1B). This configuration allowed the T1 plant to exchange stress cues with both IND and T2 plants, while preventing direct root communication between IND and T2 plants.
Responses to drought and drought stress cues were monitored by recording the stomatal aperture of the IND plant and its target neighbours, following the induction of the IND plants by either a dry or a wet 4 : 1 mixture of no. 1 vermiculite (Agrekal, Habonim, Israel) and bentonite (VB) (Minerco, Netanya, Israel).
Split-root plants were prepared as described above and grown in 50-mL, 30-mm-diameter plastic receptacles (Miniplast, Ein Shemer, Israel) filled with distilled water. The rooting receptacles were secured to each other using plastic soldering. The top of each receptacle was covered by paraffin film (Parafilm, Chicago, IL, USA) through which the roots were inserted into the receptacles.
Growth conditions and stress induction
The plants were grown in a growth chamber, at 25 °C, under continuous 130 µE m−2 s−1 of cool-white fluorescent light, for 7 d, before they were treated with either dry or wet VB. Throughout this period, distilled water was injected through the paraffin film as needed to ensure that the roots were immersed in water.
Drought induction was carried out by carefully pumping the water from the induction receptacle (yellow; Fig. 1B) using a syringe and filling it with 8 g of dry VB. To account for handling effects, control sets were induced by filling the induction receptacle with a mixture of wet VB (5·5 g VB and 45 mL distilled water). Accordingly, differences in stomata aperture between the dry and wet induction treatments reflected the effects of drought induction rather than responses to the physical handing of the plants or the chemical components of VB.
Differential responses to stress and communicated stress cues
Monitoring the acclimation of stressed plants to direct osmotic and drought stresses and habituation of plants to communicated stress cues was done by quantifying stomatal aperture in the aforementioned experiments at different time intervals after stress induction. Plants were monitored immediately before the external induction (0 min), and 0·25, 1, 3 and 24 h following external induction with mannitol in the greenhouse experiment (Fig. 1A) and 0, 0·25, 1 and 24 h following external drought induction in the drought-induction experiment (Fig. 1B).
Communication of stress cues in wild plants
Plants
The studied species were selected based on the following criteria.
(a) Ease of monitoring stomata aperture: following a preliminary survey, four species were omitted from the study as we were unable to prepare measurable epidermal impressions for them.
(b) Stoloniferous grasses: using clonal plants allows minimally destructive preparation of multiple similarly sized replications with similar developmental background. In addition, cuttings of these plants readily regenerate new roots and shoots and the roots of neighbouring ramets can be planted in different pots, allowing a clear spatial separation between the rooting volumes of induced and target plants, without the need to damage the plants for the preparation of split-root plants.
(c) Xeric background: it was assumed that the emission and perception of drought-related cues would be more developed in plants from water-limited ecosystems but not necessarily from extreme deserts, where osmotic stress and drought events are the norm rather than the exception and thus call for constitutive rather than induced adaptations.
Following a preliminary survey, the following graminoid species were selected to be tested for root communication of drought cuing.
(a) Cynodon dactylon (Bermuda grass) is a prostrate perennial grass, which spreads by means of both stolons and rhizomes (Fernandez, 2003). Cynodon dactylon is common in warm ecosystems of Africa, Asia, Australia, southern Europe and America (Holm et al., 1991), where it occurs on most soil types in disturbed and overgrazed habitats, gardens, roadsides, uncultivated lands, patches with high levels of nitrogen, moist sites along rivers (Parker, 1972) and desert washes (Gould, 1951). Various C. dactylon cultivars are commonly used as turf and lawn grasses (Horowitz, 1996).
(b) Digitaria sanguinalis (hairy crabgrass) is a summer annual grass, native to both moist and dry ecosystems in the tropical and temperate regions of Africa, Asia and southern Europe (Holm et al., 1991; King and Oliver, 1994) and is widely naturalized outside of its natural distribution range, where it is typically found in cultivated habitats, gardens and disturbed habitats (Radosevich et al., 2007).
(c) Stenotaphrum secundatum (buffalo grass) is a perennial stoloniferous plant native to North America, West Indies and Australia, but is currently naturalized in most tropical regions (Sauer, 1972; Busey, 1995). In its native habitats, Stenotaphrum species are predominantly seashore colonizers but they are commonly found in a wide range of anthropomorphic and disturbed habitats (Sauer, 1972). Stenotaphrum secundatum is a strong competitor with high tolerance to low light, high salt and heavy grazing pressures. Numerous S. secundatum cultivars are commonly used for the prevention of soil erosion and as turf and lawn plants (Sauer, 1972; Judd, 1975; Busey 2003).
Cynodon dactylon and Digitaria sanguinalis plants were collected between June and September 2011 from natural populations in the vicinity of the Sede Boqer campus, Israel, and Stenotaphrum secundatum was acquired from a commercial nursery (Deshe-Itzhar, Kfar Monash, Israel) as sod.
Plants were vegetatively propagated from 10 C. dactylon, 30 D. sanguinalis and an unknown number of S. secundatum mother plants. Two-ramet cuttings were planted in moist no. 2 vermiculite and grown in the greenhouse (see above) for 14–21 d, during which each ramet regenerated three to five leaves and 4- to 6-cm-long roots.
Experimental design and set-up
The experimental set-up was based on a slightly modified version of the experimental design used to test communication of drought stress cuing in P. sativum (Fig. 1B). Triplets of similarly sized two-ramet plants were planted in a row of 0·2-L, 7-cm-diameter, 9-cm-high pots (Miniplast, Ein Shemer, Israel). In stoloniferous plants, resource translocation is commonly acropetal (e.g. Price and Hutchings, 1992) and in response to herbivory, systemic warning signals were shown to travel more rapidly acropetally than basipetally (Gutbrodt et al., 2011), implying that planting direction might affect the rate and effectiveness of signal transmission within and among plants. To increase uniformity and the probability of finding communicative cuing, potential differential effects of axis polarity were avoided by directing the plants so their proximal ramets were rooted in (IND) or nearer (T1–T2) the induction pot (yellow, Fig. 1B). To allow rapid and non-destructive drought induction, the induction pot (yellow, Fig. 1B) was initially filled with tap water and the other pots were filled with no. 2 vermiculite. Upon transplantation into the experimental pots, all roots were trimmed to 3 cm to encourage root regeneration and intermingling in the shared target pots. Plants were allowed to regenerate and habituate to the experimental systems for at least 7 d before the onset of the experiment, during which they were individually irrigated to field capacity with 100 mL nutrient solution (Ecogan, Caesarea, Israel) every 3 or 4 d. Target pots were bottom-drained into separate drip trays to prevent seepage and capillary migration of root exudates between the pots. Pots were individually wrapped with aluminium foil to block light from reaching the roots.
Drought stress was inflicted to the proximal root of the IND plant, using a VB mixture as described above. All experiments were conducted in the greenhouse, on cloudless days in September and October 2011, between 0830 and 1030 h. Stomatal aperture of IND plants and their unstressed target neighbours was recorded 60 min after inducing the IND plants by either dry or wet VB, a time interval during which stress communication had been demonstrated in earlier experiments (Falik et al., 2011).
Stomata measurements
Stomatal aperture was used as a highly sensitive phenotypic expression of plant response to drought and osmotic stress (Neill et al., 2008). Throughout, stomatal aperture was estimated from epidermal impressions following Falik et al. (2011): the lower surfaces of one or two fully unfurled 20- to 30-mm2 leaflets or leaves of each sampled plant were copied using a fresh mixture of vinyl polysiloxane dental impression material (Elite HD + , Badia Polesine, Rovigo, Italy). Following hardening, the resulting imprints were further copied with clear nail polish, which resulted in transparent preparations suitable for microscopic examination. Because the preparation of the imprints involved a highly disruptive procedure, each plant set was only measured once, i.e. separate replication sets were sampled at different times and induction treatments.
Stomata measurements were carried out using AxioVision software (Carl Zeiss MicroImaging, Thornwood, NY, USA) on digital images of the nail-polish preparations. Average stomatal width was calculated from the data of at least ten stomata per plant, selected haphazardly from two to five 0·02 mm2 areas in the centre of each microscopic preparation. Accordingly, each data point (Figs 2–4) represents the average width of 100–150 stomata nested within 10–15 replication sets per treatment per time interval.
Fig. 2.
Stomatal width of induced P. sativum plants (IND) and their unstressed neighbours (T1–T5) immediately before (0), and 0·25, 1, 3 and 24 h after part of the root system of the IND plant was supplemented with either water or mannitol, as indicated. Data represent means ± s.e.m; n = 10. *, P < 0·05; **, P < 0·01; ***, P < 0·001.
Fig. 3.
Stomatal width of induced P. sativum plants (IND) and their unstressed neighbours (T1, T2) immediately before (0), and 0·25, 1 and 24 h after part of the root system of the IND plant was subjected to wet or dry VB, as indicated. Data represent means ± s.e.m.; n = 15. **, P < 0·01; ***, P < 0·001.
Fig. 4.
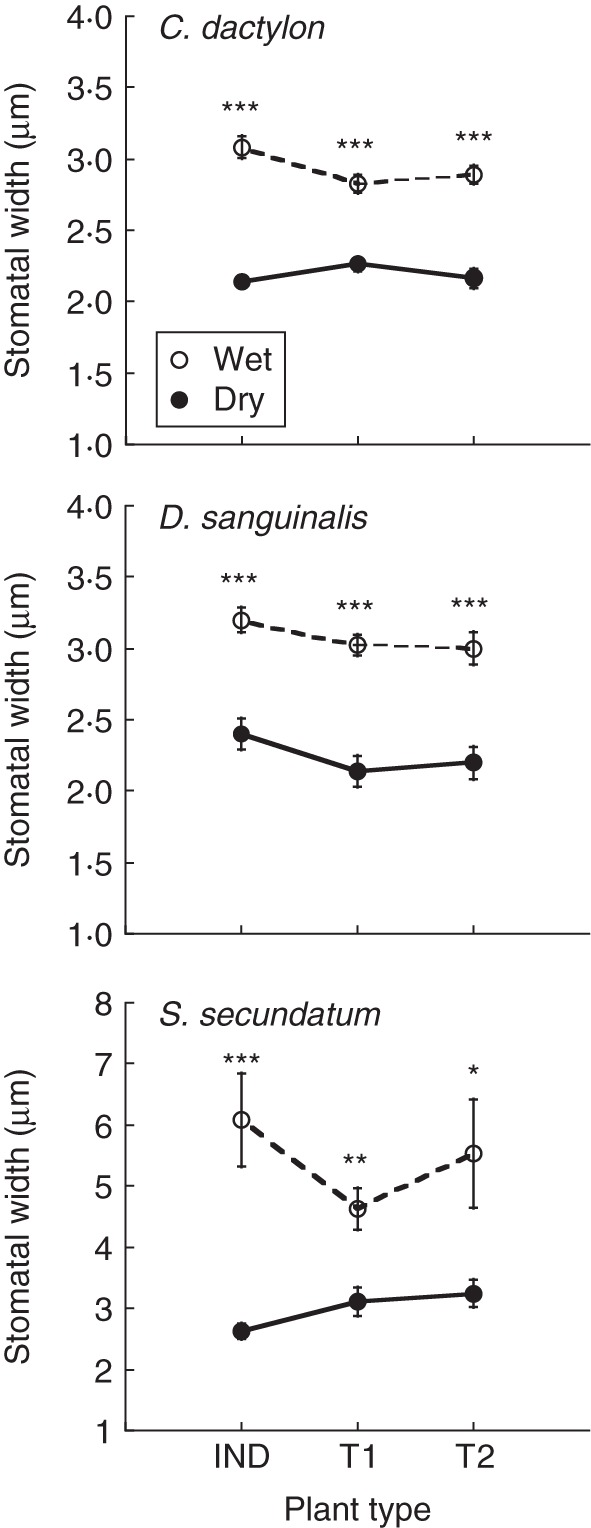
Stomatal width of induced C. dactylon, D. sanguinalis and S. secundatum plants (IND) and their unstressed neighbours (T1, T2) immediately before (0), and 60 min after part of the root system of the IND plant was subjected to wet or dry VB, as indicated. Data represent means ± s.e.m.; n = 10. *, P < 0·05; **, P < 0·01; ***, P < 0·001.
To avoid observer bias, all samples were handled and analysed using a single-blind protocol, whereby the observer could not know the identity of the samples.
The significances of treatment main effects (water versus mannitol) were analysed using one-way ANOVAs and the comparisons of stressed and control treatments was analysed using Tukey-corrected comparisons (SYSTAT 10; SPSS Inc., Chicago, IL, USA).
RESULTS
Root communication in soil
Stress induction caused rapid though gradual stomatal closure in both the IND plant and its unstressed neighbours. Fifteen minutes after mannitol supplementation to the induction pot, the IND plant and its three nearest neighbours (T1–T3) closed their stomata by 13–30 % compared with their water controls, while target plants positioned further away from the IND plant (T4, T5) maintained opened stomata (Fig. 2). One hour after stress induction, the width of the stomata of the IND and its four nearest neighbours (T1–T4) was drastically reduced to a similar extent of 19–29 %, compared with their water controls, and 3 h after stress induction the differences between the stressed sets and their water controls were still maintained (Fig. 2). The furthest target plant (T5) demonstrated a relatively delayed though increasing response to stress induction starting 1 h after induction, reaching a maximal difference of 29 % between the osmotically stressed sets and their water controls 3 h after induction (Fig. 2). Twenty-four hours after induction, the osmotically stressed (IND) plants still demonstrated significant stomatal closure, comparable to their state 3 h after stress induction. In contrast, at this time, the unstressed neighbours opened their stomata to a similar level, which was insignificantly different from their water controls (Fig. 2).
Communication of stress cues in response to drought
Fifteen minutes after drought induction, the IND plant and its nearest neighbour (T1) closed their stomata by 22–36 %, compared with their water controls, while the T2 target, positioned further away from the IND plant, maintained opened stomata (Fig. 3). One hour after drought induction, the width of both the IND plant and its unstressed neighbours was reduced by 22–31 %, compared with their unstressed neighbours (Fig. 3). Twenty-four hours after drought induction, the stressed plant (IND) still maintained slightly (16 %; P = 0·058) closed stomata, compared with its wet control; however, the unstressed target plants (T1, T2) opened their stomata to the same extent as their water controls (Fig. 3).
Communication of stress cues in wild plants
Sixty minutes following drought induction, both the IND plants and their two unstressed neighbours closed their stomata to a similar extent of 20–30, 25–29 and 33–57 %, in C. dactylon, D. saguinalis and S. secundatum, respectively, compared with their wet controls (Fig. 4).
DISCUSSION
Phenotypic plasticity relies on the perception and integration of internal and external information regarding prevailing and expected physiological states and growth conditions. Although lacking any elaborate information-processing abilities, even simple organisms such as viruses, bacteria and plants are able to perceive and communicate environmental information, which may significantly affect their performance and fitness (Waters and Bassler, 2005; Weitz et al., 2008; Karban, 2010). Here, we studied the possibility that drought and osmotic-stress cues can be communicated between stressed and unstressed plants. In agreement with an earlier study (Falik et al., 2011), the results demonstrated that unstressed plants not only respond to communicated stress cues emitted by their stressed neighbours (T1, Figs 2–4), they also induce stress responses in additional unstressed plants (T2, Figs 3–4). Specifically, following an infliction of either osmotic stress or drought, both stressed plants, and their unstressed neighbours closed their stomata, even when not in an immediate neighbourhood with a stressed plant (Figs 2–4). An earlier study, conducted with P. sativum grown in aqueous media, has found that the communication of osmotic stress cues was based on root rather than shoot communication (Falik et al., 2011). Accordingly, the demonstration of communication of ostomotic stress cues between soil-rooted P. sativum (Fig. 2) and drought-stress cues in both P. sativum (Fig. 3) and three wild plants (Fig. 4) implies that the observed phenomena might be common and play an adaptive role in naturally grown plants. In addition, the results supported our hypothesis that plants differentially respond to direct stress and communicated stress cues. As predicted, continuous osmotic stress or drought-induced prolonged stomata closure and limited acclimation in stressed plants (IND, Figs 2–3). In contrast, by 24 h after stress infliction, unstressed plants neighbouring the stressed plants fully reopened their stomata (T1–T5, Fig. 2; T1–T2, Fig. 3).
Habituation to stress cues
The fact that stress-induced plants demonstrated stomatal closure even 24 h after the beginning of stress infliction was not surprising, although in all of the experiments, only half of the root system of each IND plant was subjected to stress while the other half was grown under benign conditions (Fig. 1). Because stomatal closure is both photosynthetically costly (e.g. Tezara et al., 1999) and may cause harmful increases in leaf temperature (e.g. Liu et al., 2011), it was expected that plants that perceived stress cues, which were not accompanied by true stress, would cease their emergency stomatal closure shortly after stress induction. Naturally, much more work is needed to decipher the identity and mode of operation of the involved communication vectors as well as the kinetics of their emission from the stressed plants; however, the faster recovery of stomatal opening in unstressed neighbours suggests that the unstressed cue receivers habituated to the stress cues emitted from their stressed neighbours. Alternatively, it is possible that stressed plants only release stress signals for a brief period after stress induction. The identification of the communication vector and its kinetics in the rhizosphere will help to disentangle between these possibilities.
Nonetheless, the results raise important questions as to the possible adaptive implications of the emission and perception of stress cues. Does the habituation to communicated stress cues merely reflect a ‘cry-wolf’ response? For how long does the perception of drought or osmotic-stress cues induce and/or prime unstressed plants to these subsequent stress episodes? These questions exemplify a multifaceted evolutionary conundrum (for reviews related to the evolutionary aspects of induced defences against herbivores, see Heil and Karban, 2010; Agrawal, 2011; Karban, 2011; Kessler and Heil, 2011). Regardless of their mode of operation, for such communication to be evolutionarily stable, both the emitters and the receivers of stress cues must benefit, or at least not incur fitness losses, from sharing the environmental information. In the following sections the potential adaptive implications of the communication of stress cues between stressed and unstressed plants are briefly discussed.
Why respond to stress cues?
On the receiver (unstressed plant) end, plastic responsiveness to anticipatory cues regarding imminent stress may help plants to avoid potentially significant costs associated with constitutive stress adaptations (Heil and Karban, 2010, and references therein). In the case of drought and osmotic stress, both constitutive and induced adaptions may include costly allocation to specific attributes (e.g. Skirycz and Inze, 2010), which, in and of themselves, might significantly limit plant performance under benign conditions (Sambatti and Caylor, 2007). Much like in the case of the induction of defences against herbivores (Kessler and Heil, 2011), responses to stress cues may result in reduced performance. For example, Falik et al. (2011) found that unstressed plants, which shared their rooting volume with osmotically stressed plants (T1; Fig. 1), had lower biomass compared with plants that neighboured unstressed plants. It is expected that unstressed cue receivers will also incur long-term costs related to increased stress readiness (priming), which may come at the expense of fitness losses under benign conditions, i.e. where the anticipated stress does not materialize (Fig. 5). In addition, due to allocation trade-offs, both induced and primed plants might be more vulnerable to additional challenges such as competition and herbivory. Ongoing research is aimed at studying the potential adaptive consequences of responsiveness to communicated stress cues with an emphasis on separating the potential induction of (a) elevated stress adaptation and (b) increased latent readiness to develop full-scale stress adaption (priming) to forthcoming stress conditions. Although a recent study has demonstrated clear fitness costs to induction of resistance against pathogens but no costs to priming for the same adaptations (van Hulten et al., 2006), it can be expected that both increased stress tolerance and priming would come at performance cost under benign conditions (Fig. 5), as otherwise all plants would be expected to be constituently ready to develop heightened adaptations (as in primed plants) to a wide spectrum of perils and stresses (Karban, 2011).
Fig. 5.
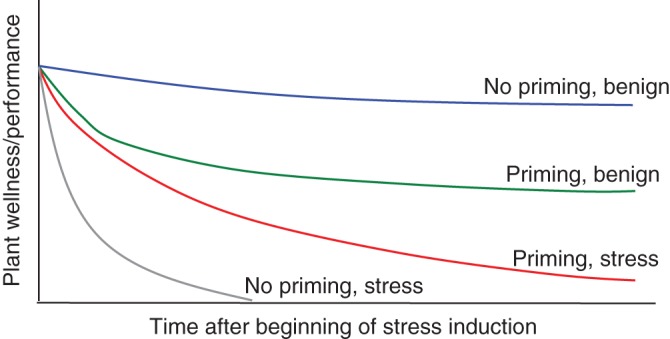
Hypothetical implications of communicative priming on long-term plant performance. Following an early induction by communicative cues, the best long-term performance is expected in control target plants that have not been subjected to communicative-stress cues (blue line) and the poorest performance is expected in unprimed plants, which are subjected to stress (grey line). Early induction by communicative-stress cues is expected to induce stress adaptions or priming, which significantly improve plant wellness and performance under subsequent exposure to stress (red line), but incur performance costs if post-induction conditions are benign (green line).
At this early stage, the relative roles of most of the above-mentioned factors are still poorly understood; however, considering its potential cost and benefits, responsiveness to communicated stress cues is expected to be tightly dependent on the reliability of the stress cues and thus to increase with the coefficient of correlation between the incidence of anticipatory stress cues and the probability of occurrence of subsequent stressful conditions (Novoplansky, 2009). As to osmotic stress and drought, it is expected that responsiveness to anticipatory cues would be more prevalent in plants that live where early bouts of drought and salinity are tightly correlated with subsequent occurrences of prolonged periods of severe drought or osmotic stress. Similarly, heightened responsiveness to communicated stress cues is expected wherever tight autocorrelations exist in space in water limitations or high salinity levels, which is expected to be created near and around drying vernal pools and other seasonal or fluctuating aquatic habitats (e.g. Kozlowski and Pallardy, 2002). In addition, responsiveness to stress cues might also depend on genetic relatedness and kin recognition between the communicating plants (Karban and Shiojiri, 2009; but see Milla et al., 2011).
Why emit stress cues?
Perhaps the simplest explanation for the emission of honest and useful stress cues might not necessarily be based on an adaptive rationale but rather on the fact that drought and osmotic stress cause direct damage, which may result in involuntary and possibly non-adaptive release of various damage products, which are later perceived (‘eavesdropped on’) by unstressed neighbours. Indeed, such cuing has been demonstrated in a few prey–predator systems, where chemical cues emitted from the excrement of predators or prey wounds are perceived by conspecific prey as warning signals (e.g. Ferrari et al., 2007; Moir and Weissburg, 2009). Although this explanation might be relevant to the communication of herbivory-warning cues (Heil and Karban, 2010), our findings suggest that it might not present a viable interpretation for the communication of stress cues in our system. The fact that the unstressed plants (T1, Figs 3–4) were as affective as the stressed plants (IND, Figs 3–4) in inducing stress responses in additional unstressed neighbours (T2, Figs 3–4) strongly indicates that the observed stress communication could not be based on the emission and perception of damage products alone. An arguably more plausible interpretation of the emission of osmotic and drought stress cues might be related to direct and indirect selective advantages conferred to emitters of stress cues. Selection is only expected to prefer ‘information leakiness’ from stressed plants where, at least on average, the fitness benefits of cue-emission outweigh the costs associated with the production and emission of potentially costly metabolites, and the provision of honest and useful warning cues to potential enemies and competitors (e.g. Bruin and Dicke, 2001). Accordingly, the emission of stress cues is expected to be more prevalent in (a) large plants, where external signalling amongst organs of the same plant might increase signalling speed and/or effectiveness (Rodriguez-Saona et al., 2009), (b) in plants with strict vascular orthostrichy (Orians, 2005), anatomical segmentation or sectoriality (e.g. Espino and Schenk, 2009), where restricted physiological integration limits or totally prevents internal communication between stressed and yet unstressed organs (Karban et al., 2000), and (c) in clonal plants and other plants, where kin or clone-mates are spatially aggregated and where non-random spatial distributions of whole plants or individual ramets increase the probability of kin neighbourhood and interactions (Cheplick, 1993; Herben and Novoplansky, 2008).
Concluding remarks
The results demonstrate a novel mode of communication whereby environmental information can affect and be relayed via multiple plants. Further work is underway, aiming at the mechanisms and adaptive implications of the observed phenomena. Besides studying the possible effects of communicative stress cuing on the induction of adaptations and priming to subsequent droughts and osmotic stresses, ongoing work focuses on the following questions. (a) To what extent is early exposure to communicative stress cues retained? Although long-term memory is possible both within the lifetime of plants (Trewavas, 2003; Novoplansky, 2009) and across generations (Paszkowski and Grossniklaus, 2011), it is generally expected that the memory of mere stress cues will be shorter than the memory of tangible stressful events, although the particular responsiveness levels of any plant are expected to be highly dependent on its natural history, including the levels and dynamics of various risks and opportunities in its natural environment. (b) What might be the role of mycorrhiza and other microorganisms in the facilitation of information networks among plants? Although much attention has been given to the role of mycorrhizal networks in enabling nutrient translocation among plants (e.g. Deslippe and Simard, 2011), less attention has been given to the possibility that micorrhizal networks and perhaps also bacterial biofilms (Danhorn and Fuqua, 2007) may serve as communication networks among plants (but see Song et al., 2010). (c) How specific are the communication vectors of stress cues? Amongst the best examples for warning cuing following herbivory are those to inter-specific communication (e.g. Karban et al., 2000). Although it can be generally expected that communicative stress cuing between roots is based on generic vectors such as abcisic acid (Trouverie et al., 2003), further work is needed to test whether inter-specific communication of stress cues is possible and to what extent it is affected by genetic relatedness (Karban and Shiojiri, 2009; Milla et al., 2011). Finally, the ultimate challenge of this study will be to evaluate the significance and implications of stress cuing in naturally grown plants. How far is communicative cuing effectively transmitted and acted upon by unstressed plants under natural settings, where plants are continuously subjected to multiple stresses and noisy cues while neighbouring multiple plants, which often belong to diverse taxa, experience different growth conditions?
ACKNOWLEDGEMENTS
We thank Ishai Hoffman for technical help and two anonymous reviewers for their helpful comments on an earlier version of the manuscript. The study was partially supported by a research grant from the Israel Science Foundation to A.N. This is publication no. 763 of the Mitrani Department of Desert Ecology.
LITERATURE CITED
- Ackerson RC, Younger VB. Responses of bermudagrass to salinity. Agronomy Journal. 1975;67:678–681. [Google Scholar]
- Agrawal A. Plant defense evolution: synthesis and future directions. Functional Ecology. 2011;25:420–432. [Google Scholar]
- Alpert P, Simms EL. Relative advantages of plasticity and fixity in different environments: when is it good for a plant to adjust? Evolutionary Ecology. 2002;16:285–297. [Google Scholar]
- Amzallag N, Lerner HR, Poljakoff-Mayber A. Induction of increased salt-tolerance in Sorghum bicolor by NaCl pretreatment. Journal of Experimental Botany. 1990;41:29–34. [Google Scholar]
- Aphalo PJ, Ballare CL. On the importance of information-acquiring systems in plant–plant interactions. Functional Ecology. 1995;9:5–14. [Google Scholar]
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Bruin J, Dicke M. Chemical information transfer between wounded and unwounded plants: backing up the future. Biochemical Systematics and Ecology. 2001;29:1103–1113. [Google Scholar]
- Busey P. Genetic diversity and vulnerability of. St. Augustinegrass. Crop Science. 1995;35:322–327. [Google Scholar]
- Busey P. St. Augustinegrass, Stenotaphrum secundatum (Walt.) Kuntze. In: Casler MD, Duncan RR, editors. Biology, breeding, and genetics of turfgrasses. Hoboken, NJ: John Wiley & Sons; 2003. pp. 309–330. [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheplick GP. Sibling competition is a consequence of restricted dispersal in an annual cleistogmous grass. Ecology. 1993;74:2161–2164. [Google Scholar]
- Clark LJ, Whalley WR, Barraclough PB. How do roots penetrate strong soil? Plant and Soil. 2003;255:93–104. [Google Scholar]
- Danhorn T, Fuqua C. Biofilm formation by plant-associated bacteria. Annual Review of Microbiology. 2007;61:401–422. doi: 10.1146/annurev.micro.61.080706.093316. [DOI] [PubMed] [Google Scholar]
- Deslippe JR, Simard SW. Below-ground carbon transfer among Betula nana may increase with warming in Arctic tundra. New Phytologist. 2011;3:689–698. doi: 10.1111/j.1469-8137.2011.03835.x. [DOI] [PubMed] [Google Scholar]
- DeWitt TJ, Shi A, Wilson DS. Costs and limits of phenotypic plasticity. Trends in Ecology & Evolution. 1998;13:77–81. doi: 10.1016/s0169-5347(97)01274-3. [DOI] [PubMed] [Google Scholar]
- Espino S, Schenk HJ. Hydraulically integrated or modular? Comparing whole-plant-level hydraulic systems between two desert shrub species with different growth forms. New Phytologist. 2009;183:142–152. doi: 10.1111/j.1469-8137.2009.02828.x. [DOI] [PubMed] [Google Scholar]
- Falik O, Reides P, Gersani M, Novoplansky A. Self/non-self discrimination in roots. Journal of Ecology. 2003;91:525–531. [Google Scholar]
- Falik O, Mordoch Y, Quansah L, Fait A, Novoplansky A. Rumor has it … : relay communication of stress cues in plants. PLoS ONE. (e23625.) 2011;6 doi: 10.1371/journal.pone.0023625. http://dx.doi.org/10.1371/journal.pone.0023625 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez ON. Establishment of Cynodon dactylon from stolon and rhizome fragments. Weed Research. 2003;43:130–138. [Google Scholar]
- Ferrari MCO, Brown MR, Pollock MS, Chivers DP. The paradox of risk assessment: comparing responses of fathead minnows to capture-released and diet-released alarm cues from two different predators. Chemoecology. 2007;17:157–161. [Google Scholar]
- Forde BG, Zhang H. Nitrate and root branching. Trends in Plant Science. 1998;3:204–205. [Google Scholar]
- Franklin KA. Shade avoidance. New Phytologist. 2008;179:930–944. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Hajela RK, Thomashow MF. Cold acclimation in Arabidopsis thaliana. Plant Physiology. 1988;87:745–750. doi: 10.1104/pp.87.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez S, Stuefer JF. Members only: induced systemic resistance to herbivory in a clonal plant network. Oecologia. 2006;147:461–468. doi: 10.1007/s00442-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Gould FW. Grasses of the southwestern United States. Tucson, AZ: The University of Arizona Press; 1951. [Google Scholar]
- Greenway H, Munns R. Mechanisms of salt tolerance in nonhalophytes. Annual Review of Plant Physiology. 1980;31:149–190. [Google Scholar]
- Gutbrodt B, Mody K, Wittwer R, Dorn S. Within-plant distribution of induced resistance in apple seedlings: rapid acropetal and delayed basipetal responses. Planta. 2011;233:1199–1207. doi: 10.1007/s00425-011-1371-6. [DOI] [PubMed] [Google Scholar]
- Hall EK, Singer GA, Kainz MJ, Lennon JT. Evidence for a temperature acclimation mechanism in bacteria: an empirical test of a membrane-mediated trade-off. Functional Ecology. 2010;24:898–908. [Google Scholar]
- Heil M, Karban R. Explaining evolution of plant communication by airborne signals. Trends in Ecology & Evolution. 2010;25:137–144. doi: 10.1016/j.tree.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Herben T, Novoplansky A. Implications of self/nonself discrimination for spatial patterning of clonal plants. Evolutionary Ecology. 2008;22:337–350. [Google Scholar]
- Holm LG, Plunknett DL, Pancho JV, Herberger JP. The world's worst weeds: distribution and biology. Malabar, FL: Krieger Publishing Company; 1991. [Google Scholar]
- Horowitz M. Bermudagrass (Cynodon dactylon): a history of the weed and its control in Israel. Phytoparasitica. 1996;24:305–320. [Google Scholar]
- Hughes MA, Dunn MA. The molecular biology of plant acclimation to low temperature. Journal of Experimental Botany. 1996;47:291–305. [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CM, Ton J. Costs and benefits of priming for defense in Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 2006;103:5602–5607. doi: 10.1073/pnas.0510213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd BI. New World tropical forage grasses and their management. 4. Bermudagrass, giant stargrass, St. Augustinegrass, and jaraguagrass. World Crops. 1975;27:69–72. [Google Scholar]
- Karban R. Neighbors affect resistance to herbivory – a new mechanism. New Phytologist. 2010;186:564–566. doi: 10.1111/j.1469-8137.2010.03263.x. [DOI] [PubMed] [Google Scholar]
- Karban R. The ecology and evolution of induced resistance against herbivores. Functional Ecology. 2011;25:339–347. [Google Scholar]
- Karban R, Shiojiri K. Self-recognition affects plant communication and defense. Ecology Letters. 2009;12:502–506. doi: 10.1111/j.1461-0248.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT, Baxter KJ, Laue G, Felton GW. Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia. 2000;125:66–71. doi: 10.1007/PL00008892. [DOI] [PubMed] [Google Scholar]
- Kessler A, Heil M. The multiple faces of indirect defences and their agents of natural selection. Functional Ecology. 2011;25:348–357. [Google Scholar]
- Keuskampa DH, Pollmannb S, Voeseneka LACJ, Peetersa AJM, Pierik R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proceedings of the National Academy of Sciences of the USA. 2010;107:22740–22744. doi: 10.1073/pnas.1013457108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CA, Oliver LR. A model for predicting Large Crabgrass (Digitaria sanguinalis) emergence as influenced by temperature and water potential. Weed Science. 1994;42:561–567. [Google Scholar]
- Kozlowski TT. Soil compaction and growth of woody plants. Scandinavian Journal of Forest Research. 1999;14:596–619. [Google Scholar]
- Kozlowski TT, Pallardy SG. Acclimation and adaptive responses of woody plants to environmental stresses. Botanical Review. 2002;68:270–334. [Google Scholar]
- Larkindale J, Vierling E. Core genome responses involved in acclimation to high temperature. Plant Physiology. 2008;146:748–761. doi: 10.1104/pp.107.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins R. Evolution in changing environments. Princeton, NJ: Princeton University Press; 1968. [Google Scholar]
- Liu Y, Subhash C, Yan J, Song C, Zhao J, Li J. Maize leaf temperature responses to drought: thermal imaging and quantitative trait loci (QTL) mapping. Environmental and Experimental Botany. 2011;71:158–165. [Google Scholar]
- Milla R, Escudero A, Iriondo JM. Congruence between geographic range distribution and local competitive ability of two Lupinus species. American Journal of Botany. 2011;98:1456–1464. doi: 10.3732/ajb.1000519. [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, et al. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Science Signaling. 2009;2:45. doi: 10.1126/scisignal.2000448. [DOI] [PubMed] [Google Scholar]
- Moir F, Weissburg MJ. Cautious cannibals: behavioral responses of juvenile and adult blue crabs to the odor of injured conspecifics. Journal of Experimental Marine Biology and Ecology. 2009;369:87–92. [Google Scholar]
- Neill S, Barros R, Bright J, et al. Nitric oxide, stomatal closure, and abiotic stress. Journal of Experimental Botany. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. Journal of Physiology. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoplansky A. Picking battles wisely: plant behaviour under competition. Plant, Cell & Environment. 2009;32:726–741. doi: 10.1111/j.1365-3040.2009.01979.x. [DOI] [PubMed] [Google Scholar]
- Novoplansky A, Goldberg D. Effects of water pulsing on individual performance and competition hierarchies in plants. Journal of Vegetation Science. 2001;12:199–208. [Google Scholar]
- Orians C. Herbivores, vascular pathways, and systemic induction: facts and artifacts. Journal of Chemical Ecology. 2005;31:2231–2242. doi: 10.1007/s10886-005-7099-7. [DOI] [PubMed] [Google Scholar]
- Pandey GK, Cheong YH, Kim KN, et al. The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in arabidopsis. The Plant Cell. 2004;16:1912–1924. doi: 10.1105/tpc.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KF. An illustrated guide to Arizona weeds. Tucson, AZ: The University of Arizona Press; 1972. [Google Scholar]
- Passioura JB. Root signals control leaf expansion in wheat seedlings growing in drying soil. Australian Journal of Plant Physiology. 1988;15:687–693. [Google Scholar]
- Paszkowski J, Grossniklaus U. Selected aspects of transgenerational epigenetic inheritance and resetting in plants. Current Opinion in Plant Biology. 2011;14:195–203. doi: 10.1016/j.pbi.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Pierik R, Djakovic-Petrovic T, Keuskampa DH, de Wit M, Voesenek LACJ. Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and DELLA proteins in Arabidopsis. Plant Physiology. 2009;149:1701–1712. doi: 10.1104/pp.108.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price EAC, Hutchings MJ. The causes and developmental effects of integration and independence between different parts of Glechoma hederacea clones. Oikos. 1992;63:376–386. [Google Scholar]
- Punshon T, Dickinson NM. Mobilisation of heavy metals using short rotation coppice. Aspects of Applied Biology. 1997;49:285–292. [Google Scholar]
- Radosevich SR, Holt JS, Ghersa CM. Ecology of weeds and invasive plants: relationship to agriculture and natural resource management. 3rd edn. New York, NY: John Wiley and Sons; 2007. [Google Scholar]
- Rodriguez-Saona CR, Rodriguez-Saona LE, Frost CJ. Herbivore-induced volatiles in the perennial shrub Vaccinium corymbosum, and their role in later-branch signaling. Journal of Chemical Ecology. 2009;35:163–175. doi: 10.1007/s10886-008-9579-z. [DOI] [PubMed] [Google Scholar]
- Sambatti JBM, Caylor KK. When is breeding for drought tolerance optimal if drought is random? New Phytologist. 2007;175:70–80. doi: 10.1111/j.1469-8137.2007.02067.x. [DOI] [PubMed] [Google Scholar]
- Sauer JD. Revision of Stenotaphrum (Gramineae: Paniceae) with attention to its historical geography. Brittonia. 1972;24:202–222. [Google Scholar]
- Schlichting CD. The evolution of phenotypic plasticity in plants. Annual Review of Ecology and Systematics. 1986;17:667–693. [Google Scholar]
- Shemesh H, Arbiv A, Gersani M, Ovadia O, Novoplansky A. The effects of nutrient dynamics on root patch choice. PLoS ONE. (e10824.) 2010a;5 doi: 10.1371/journal.pone.0010824. http://dx.doi.org/10.1371/journal.pone.0010824 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh H, Ovadia O, Novoplansky A. Anticipating future conditions via trajectory sensitivity. Plant Signaling and Behavior. 2010b;5:1501–1503. doi: 10.4161/psb.5.11.13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirycz A, Inze D. More from less: plant growth under limited water. Current Opinion in Biotechnology. 2010;21:197–203. doi: 10.1016/j.copbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Song YY, Zeng RS, Xu JF, Li J, Shen X, Yihdego WG. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS ONE. 2010;5:e13324. doi: 10.1371/journal.pone.0013324. http://dx.doi:10.1371/journal.pone.0013324 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H. Hydrotropism and its interaction with gravitropism in roots. Plant and Soil. 1994;165:301–308. [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature. 1999;401:914–917. [Google Scholar]
- Trewavas A. Aspects of plant intelligence. Annals of Botany. 2003;92:1–20. doi: 10.1093/aob/mcg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouverie J, Thévenot C, Rocher JP, Sotta B, Prioul JL. The role of abscisic acid in the response of a specific vacuolar invertase to water stress in adult maize leaf. Journal of Experimental Botany. 2003;54:2177–2186. doi: 10.1093/jxb/erg234. [DOI] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annual Review of Cell and Developmental Biology. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Weitz J, Mileyko Y, Joh R, Voit E. Collective decision making in bacterial viruses. Biophysical Journal. 2008;95:2673–2680. doi: 10.1529/biophysj.108.133694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]