Abstract
Background and Aims
During their domestication, maize, bean and squash evolved in polycultures grown by small-scale farmers in the Americas. Polycultures often overyield on low-fertility soils, which are a primary production constraint in low-input agriculture. We hypothesized that root architectural differences among these crops causes niche complementarity and thereby greater nutrient acquisition than corresponding monocultures.
Methods
A functional–structural plant model, SimRoot, was used to simulate the first 40 d of growth of these crops in monoculture and polyculture and to determine the effects of root competition on nutrient uptake and biomass production of each plant on low-nitrogen, -phosphorus and -potassium soils.
Key Results
Squash, the earliest domesticated crop, was most sensitive to low soil fertility, while bean, the most recently domesticated crop, was least sensitive to low soil fertility. Nitrate uptake and biomass production were up to 7 % greater in the polycultures than in the monocultures, but only when root architecture was taken into account. Enhanced nitrogen capture in polycultures was independent of nitrogen fixation by bean. Root competition had negligible effects on phosphorus or potassium uptake or biomass production.
Conclusions
We conclude that spatial niche differentiation caused by differences in root architecture allows polycultures to overyield when plants are competing for mobile soil resources. However, direct competition for immobile resources might be negligible in agricultural systems. Interspecies root spacing may also be too large to allow maize to benefit from root exudates of bean or squash. Above-ground competition for light, however, may have strong feedbacks on root foraging for immobile nutrients, which may increase cereal growth more than it will decrease the growth of the other crops. We note that the order of domestication of crops correlates with increasing nutrient efficiency, rather than production potential.
Keywords: ‘Three sisters’, polyculture, root architecture, SimRoot, functional–structural model, nutrient deficiency, maize, bean, squash, niche complementarity, root competition
INTRODUCTION
Small-scale subsistence farmers have cultivated maize (Zea mays), common bean (Phaseolus vulgaris) and squash (Cururbita pepo, C. ficifolia, C. maxima, C. mixta and C. moschata) since ancient times. The time and place of the initial domestication of these species is subject to debate, but currently scholars place the domestication of these crops in western Mexico around 10 thousand years ago for squash, 6·3 thousand years ago for maize and 2·3 thousand years ago for bean (Smith, 2006). It is likely that both maize and bean were domesticated by farmers who were already growing earlier-domesticated crops (Smith, 2006) and that intercropping of these crops in so-called ‘milpas’ (Emerson, 1953) was common practice early on (Scarry, 2008). Although many different planting schemes are possible, maize/bean and maize/bean/squash polycultures are the most well-known systems. Many subsistence farmers in Africa and Latin America still intercrop maize with bean and sometimes squash. In the Americas, the maize/bean/squash polyculture is commonly referred to as the ‘three sisters’ (Lewandowski, 1987).
Subsistence farmers in Latin America and Africa prefer the intercropping of maize and bean and sometimes squash over monoculture rotations for a variety of reasons. These include crop management benefits including better weed and pest suppression, and that maize provides physical support for climbing bean (Risch, 1981; Altieri, 1999; Scarry, 2008). These polycultures also often overyield on a per area basis in comparison with monoculture rotations (Gliessman, 1992; Tsubo et al., 2003). Overyielding may be a result of niche complementarity (Tilman et al., 2001). Maize, bean and squash have contrasting shoot architectures which may partially explain the niche complementarity effect. Maize has large long leaves while bean has small round leaves which easily occupy gaps in the maize canopy. Squash forms long vines along the ground with large round leaves, occupying the canopy understorey where it forms a living mulch which may conserve water and suppress weed germination (Liebman and Dyck, 1993). These synergies among shoot architectures may account for some of the overyielding of maize/bean and maize/bean/squash polycultures.
These species also have contrasting root architectures (Weaver and Bruner, 1927), which may be the basis for different, potentially complimentary, strategies for water and nutrient acquisition. Below-ground niche complementarity may explain the overyielding of these polycultures under conditions of limited soil fertility, which is prevalent in low-input smallholder farms. For example, bean can supply 20–60 % (Tsai et al., 1993) of its nitrogen through symbiotic nitrogen fixation, while the other two crops rely solely on the uptake of inorganic nitrogen from the soil. Bean and squash may produce more root exudates than maize, allowing them to mobilize sparingly soluble forms of phosphate (Pellet et al., 1995; Jones, 1998; Shen et al., 2002; Gent et al., 2005; Li et al., 2007). Recently, it has been suggested that these exudates may facilitate phosphorus uptake by maize in maize/faba bean intercrops (Li et al., 2007). Root architecture, however, has been overlooked in intercropping studies despite the recognition that root placement may be more important for competition than root physiology (Schwinning and Weiner, 1998). Maize, bean and squash differ strongly in root architecture (Weaver and Bruner, 1927) and we hypothesize that these differences in root architecture allow these crops to explore different soil domains with variable intensity.
To test this hypothesis we conducted a detailed study on the root architecture of these crops using SimRoot (Postma and Lynch, 2011a, b), a functional–structural plant model, and estimated competition for nitrate, potassium and phosphorus among roots of maize, bean and squash plants grown in monoculture or polyculture. Exploration of distinct soil domains may affect the relative competitiveness of these crops in low-fertility soils, as nutrient availability is often heterogeneous. We hypothesized that shallow-rooting species would grow better on low-phosphorus or low-potassium soils (Lynch and Brown, 2001; Zhu et al., 2005; Lynch, 2011) and grow worse on soils in which nitrate has leached to deeper layers (Dunbabin et al., 2004). We simulated different combinations of maize, bean and squash grown on soils varying in nitrogen, phosphorus or potassium availability.
MATERIALS AND METHODS
We used SimRoot (Lynch et al., 1997; Postma and Lynch, 2011b), a functional–structural plant model with focus on root architecture, nutrient uptake and resource utilization, to virtually reconstruct the root architecture of maize (Zea mays L.), bean (Phaseolus vulgaris L.) and squash (Cucurbita pepo L.) (Fig. 1 and Supplementary Data A). We simulated growth of these three species on soils with varying availability of nitrate, phosphorus or potassium, assuming that other nutrients were non-limiting. To test our hypothesis, we varied the simulated planting scheme, simulating both two different planting densities and different combinations of the three species.
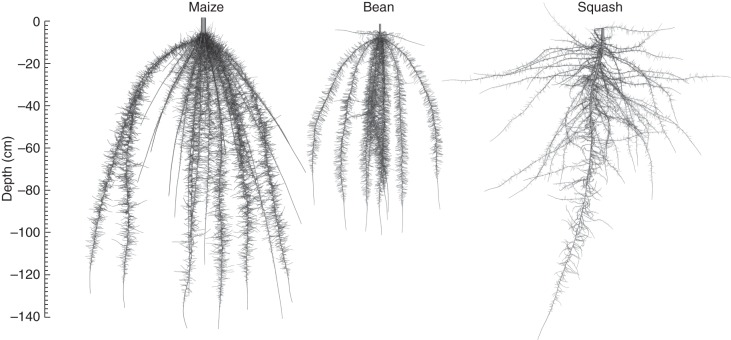
Fig. 1.
Visualization of maize, bean and squash root architecture 40 d after germination as simulated by SimRoot. Plants were not stressed. Thinner roots have been dilated (approx. ×2) for better visibility.
Runs
In total, we simulated eight different planting schemes: maize alone, bean alone, squash alone, three maize (MMM), three bean (BBB), three squash (SSS), maize/bean/bean (MBB) and maize/bean/squash (MBS). For each planting scheme we varied the nutrient availability of either nitrate (in two different soils, see below), phosphorus or potassium. Nitrate availability varied from 6·3 to 260 kg ha−1 across 28 levels and was varied by both changing the initial nitrate concentration in the profile, and the nitrate mineralization rate. Ammonium availability and uptake was not simulated, as in most agricultural soils ammonium is rapidly oxidized to nitrate by soil microbes. Phosphorus availability ranged from 0·17 to 5 kg ha−1 across 13 levels, and potassium availability ranged from 1·3 to 10·5 kg ha−1 across 11 levels. Phosphorus and potassium availability represent the nutrient content in the dissolved fraction, while the buffer capacity of the soil (i.e. the ratio between the dissolved and the absorbed fraction) was not varied. To separate niche complementarity as a result of nitrogen fixation in bean from other forms of complementarity, we simulated the BBB, MBB and MBS systems with and without biological nitrogen fixation. Additionally, we repeated the nitrate runs with the effect of root placement on nitrate uptake disabled. In these runs all roots experienced the average nitrate concentration in the soil column and the nutrient uptake by roots was spatial distributed over the whole soil column. As model results can vary due to variations in the random number generator, we ran all runs twice and show the average of these two repetitions. In total 2176 simulations <{8 planting schemes × [(28 N × 2 soils) + 13 P + 11 K] + 3 planting schemes without BNF × 28 N × 2 soils + 5 planting schemes without RSA × 28 N × 2 soils} × 2 repetitions> were run on the PennState clusters lionxi and lionxj (http://rcc.its.psu.edu/hpc/systems).
System description
We simulated 40 d of plant growth starting with seed germination. Plants were allowed to root in a 60 × 60 cm by 1·50-m-deep soil volume. The soil column had one or three plants planted in the middle. The three plants were planted in a triangle 10 cm apart. Roots that hit the boundary of the soil volume were mirrored back, so that the root density was similar to that under field conditions (Postma and Lynch, 2011a, b). Our planting scheme represented a traditional planting scheme in which maize, bean and squash are planted close together in small hills (Scarry, 2008). Farmers would often plant more than 3 plants in one hill and thin later. Planting scheme and distances between hills may vary considerably by farmer (Scarry, 2008). This planting scheme places plants close to each other, which may increase plant competition. However, planting in these hills makes it easier to walk through the field and control weeds. Furthermore, these hills allow farmers to heap fertile topsoil close to the plant. Similar planting schemes are still used by subsistence farmers today; for example, Tanzanian farmers plant 9–24 maize plants close together in locally fertilized spots (Malley et al., 2001).
At the start of the simulations most of the available nutrients were in the upper 30 cm of the soil (for exact values, see Supplementary Data B). During the simulations nitrate and potassium were allowed to leach down to deeper soil layers (however, downward movement of potassium in the soil hardly occurred). The phosphorus model does not simulate leaching of phosphorus, which should be negligible in the time span of the simulations. To vary the leaching rate of nitrate, we simulated a silt–loam soil with high water-holding capacity (moisture content varied between 20 and 30 %, v/v), and a loamy-sand with low water holding capacity (8–14 %, v/v, water content).
Parameterization of the model
All the simulation model parameters were based on measurements of actual plants; we did not estimate values for the parameters by fitting (calibrating) the model to a dataset (Supplementary Data B). Given the large number of parameters, and the already large computational demand of the study, we were not able to do further sensitivity analysis for the parameters employed. This may mean that although the theoretical principles, which we consider the main outcome of the study, are valid, the magnitude of the effects of various factors could potentially be sensitive to parameter choices. Such sensitivity may be useful in understanding the functional impacts of root phenotypes, but is beyond the scope of this study.
Model description
SimRoot is a functional–structural plant model that has been developed to simulate nutrient uptake and resource utilization in crops. The various components of SimRoot have been described in detail by Lynch et al. (1997), and Postma and Lynch (2011a, b). SimRoot simulates the growth of one or more plants over time. Growth is a function of photosynthate allocation to the different organs of the plant. The available carbon for growth comes from either carbon storage, such as seed reserves, or from photosynthesis. Carbon costs of respiration, nutrient uptake, nitrogen fixation and exudates are explicitly accounted for. Detailed description of the calculation of the carbon costs and the carbon allocation rules for growth is provided in Postma and Lynch (2011b). The root system is simulated in three-dimensional vector space, where the root architecture is represented by connected root nodes. The shoot is simulated non-geometrically, and, as in well-known crop models, is represented by integral parameters such as leaf area, leaf mass and stem mass. Photosynthesis by the shoot is simulated by estimating light interception multiplied with the light-use efficiency of the crop (see Postma and Lynch, 2011b).
SimRoot simulates nutrient uptake by the root system while it is growing in three-dimensional space. Phosphorus uptake is simulated using the Barber–Cushman model for each root segment while nitrate and potassium uptake are simulated by linking SimRoot to SWMS3D (Postma and Lynch, 2011a). A comparison between these two submodels showed that Barber–Cushman was better at simulating phosphorus-depletion zones which are only a few millimetres thick while SWMS3D was better at simulating mass flow of mobile nutrients and root competition in three dimensions (Postma and Lynch, 2011a). Less nutrient acquisition than is required for optimal plant growth results in nutrient stress which reduces photosynthesis and leaf area expansion and, thereby carbon availability for root growth (Postma and Lynch, 2011a).
Competition for nutrients in the model occurs when roots in proximity to each other are depleting the same soil resources. SWMS3D simulates competition in three dimensions, while the Barber–Cushman model simulates nutrient competition in one dimension based on the average root density within 1 cm of the root (Postma and Lynch, 2011a). In our simulations, plants competed for soil resources only, not for light. The non-geometric shoot model in SimRoot makes it difficult to simulate the shading in the layered canopy of the maize/bean/squash system. Instead, we simulated each plant as if it was an individual of a monoculture crop, even when the plant was competing with other species for nutrients below ground. The lack of interspecific shoot competition in the model allowed us to estimate root competition for soil resources independently from shoot competition for light.
New modules added to SimRoot
As mentioned in the Introduction, and further discussed in the Discussion, both biological nitrogen fixation and root exudates have been suggested in the literature as possible mechanisms through which polycultures could overyield on low-fertility soils. We added both a nitrogen fixation module and a root exudate module to SimRoot to simulate these processes. We included these processes by both including a carbon cost and nutrient gain. The nitrogen gain from biological nitrogen fixation was proportional to the nitrogen demand of the plant, necessary for optimal growth, while the phosphorus gain from exudates was simulated by assuming that the exudates increased the dissolved phosphorus concentration in the phosphorus-depletion zones around the root at the cost of reducing the solid-phase phosphorus concentration. Exudation rates were dependent on the age of each root segment. We considered adding a third module, which would simulate cluster root formation as it has been suggested that squash can form cluster roots (Waters and Blevins, 2000). Cluster root formation may give squash a competitive advantage in low-phosphorus soils (Shane and Lambers, 2005). We could not confirm the reports of cluster root formation in any of the low-phosphorus or low-iron environments in which we grew curcurbits (Fita et al., 2008; J. A. Postma and J. P. Lynch, pers. obs.). A more detailed description of the new modules added to SimRoot, including a discussion of the literature, is found in Supplementary Data C.
Post-simulation data analysis
To evaluate the simulated growth of the polycultures with the growth of the monocultures, we calculated the land equivalent ratios (LERs). LERs were calculated as the average of the ratios in plant dry biomass between the polycultures and the monocultures. For example, for the maize/bean/squash system the LER is (MMBS/MMMM + BMBS/BBBB + SMBS/SSSS)/3 where capital letters M, B, S denote the plant dry biomass of maize, bean and squash growing in the system indicated by the subscripts. The change in biomass production between the average of the monocultures and the polyculture was partitioned into niche complementarity effects and the selection effect as described by Loreau and Hector (2001).
RESULTS
Nutrient uptake by maize, common bean and pepo squash
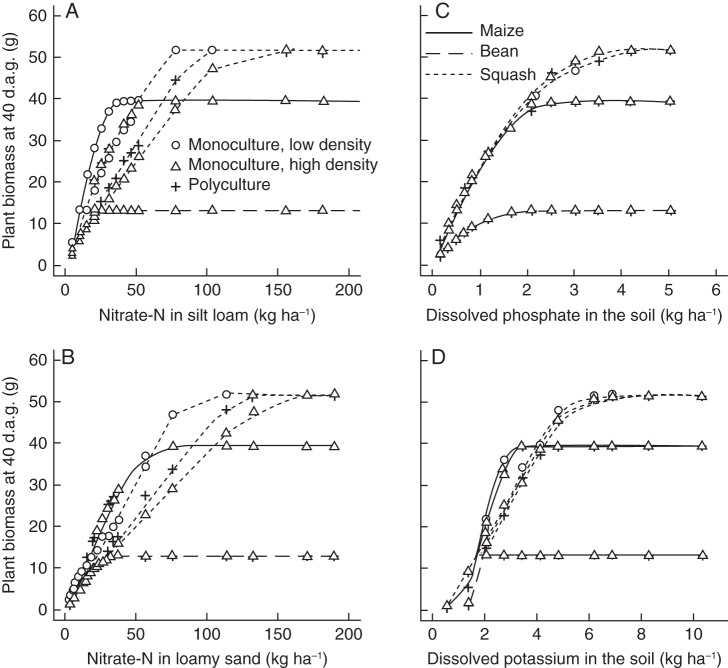
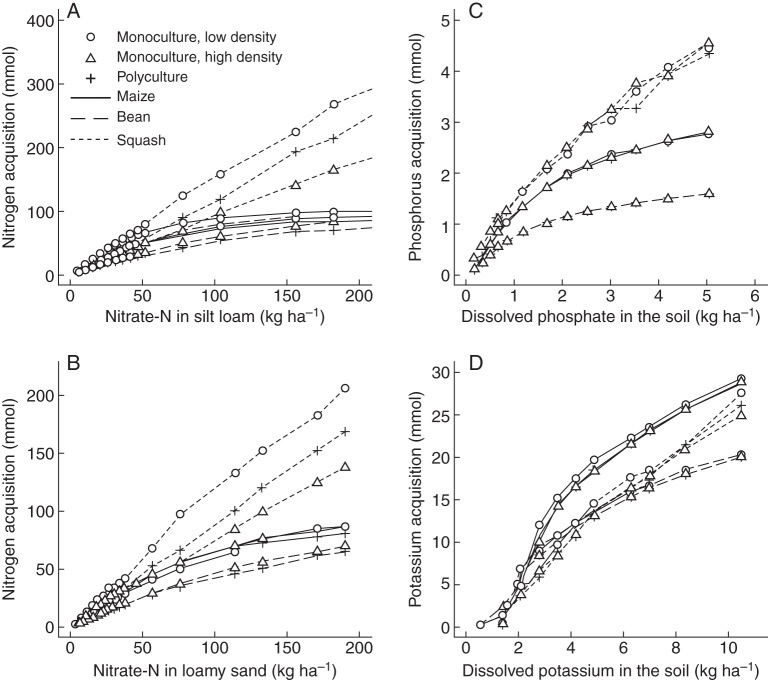
The model predicted asymptotic growth responses to nutrient availability of all three macronutrients (Fig. 2) similar to observed growth responses in the field. Squash had the greatest phosphorus and nitrogen uptake in fertile soils, compared with maize and bean (Fig. 3A–C), but maize took up more potassium (Fig. 3B). For all three macronutrients, squash had the greatest critical soil-nutrient level, defined as the nutrient availability below which plant growth is reduced (Fig. 2). Bean had the least nutrient uptake on fertile soils of all crops (Fig. 3) but also the smallest critical soil-nutrient level for all three macronutrients (Fig. 2). Bean acquired 11–66 % of its nitrogen through symbiotic fixation (data not shown). Maize had the steepest nutrient response curves while bean and squash had more gentle slopes (Fig. 2). Maize also had the lowest shoot : root ratio and its shoot : root ratio declined more on low-nitrogen and low-phosphorus soils than that of bean or squash (Fig. 4A, B). On low-potassium soils, shoot :root ratios did not decrease and, for squash, even increased when potassium availability was intermediate (Fig. 4C).
Fig. 2.
Dry weight of individual plants (g) at 40 d after germination (d.a.g.) at different levels of nitrate in silt loam (A), nitrate in loamy sand (B), phosphorus (C) or potassium availability (D). Nutrient availability is expressed in kg ha−1, which is the dissolved fraction in the top 60 cm of the soil at the start of the simulation. Lines show differences between crops and symbols show differences between cropping systems, one plant per 60 × 60 × 150 cm soil volume (monoculture low density), three plants of the same species per soil volume (monoculture high density) and three plants of different species per soil volume (polyculture).
Fig. 3.
Nutrient acquisition at 40 d after germination, otherwise as in Fig. 2. Nitrogen acquisition includes nitrogen obtained through biological nitrogen fixation. Note the different scales along the y-axes.
Fig. 4.
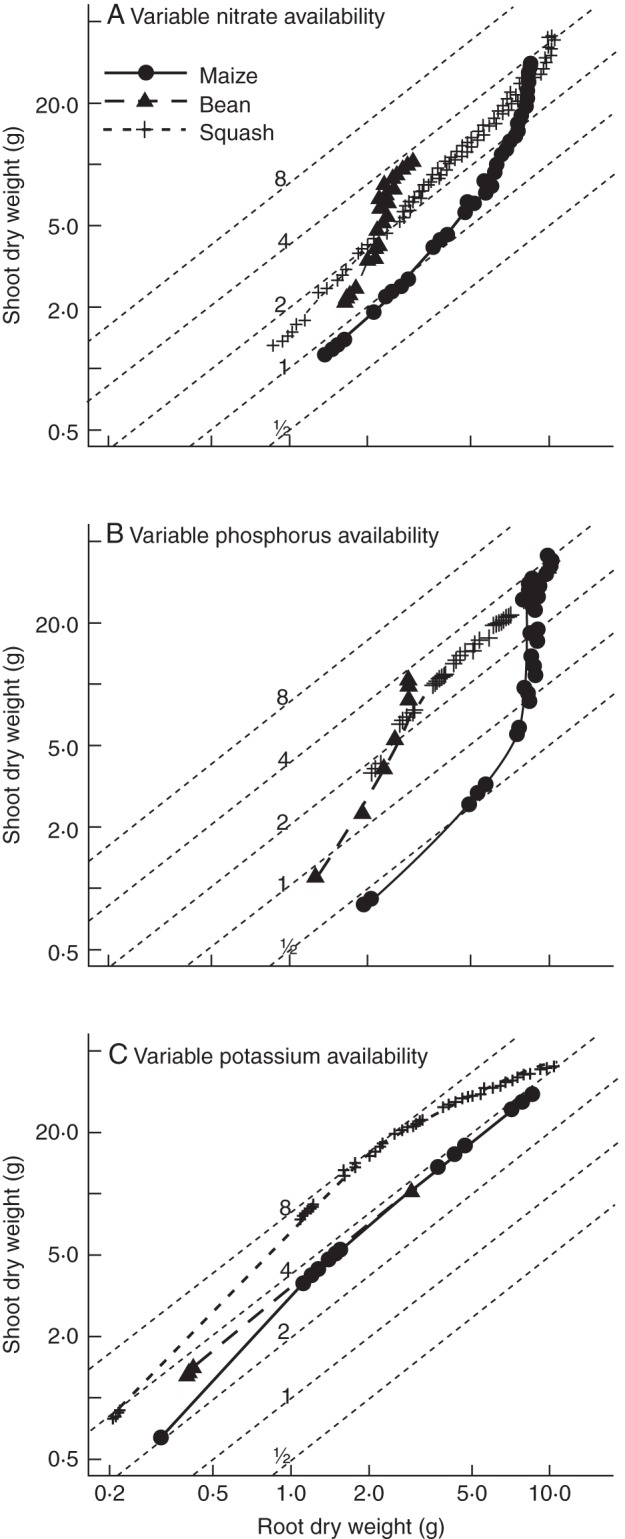
Allometry of maize, bean and squash plants at 40 d after germination for plants grown on soils varying in nitrate (A), phosphorus (B) or potassium availability (C). Grey iso lines show shoot : root ratios for 0·5, 1, 2, 4 and 8. Note the log scales along the x- and y-axes.
The vertical distribution of roots differed among the crops (Fig. 5). Maize placed the greatest proportion of roots in the top 20 cm of the soil, while squash placed the greatest proportion of roots in the 20- to 40-cm soil layer. Bean had a relatively equal vertical root distribution throughout the topmost 60 cm of soil. All species had fewer roots below 60 cm. Nitrogen deficiency reduced growth of deep roots in bean, but reduced growth of shallow roots in squash, while in maize it affected deep and shallow roots equally (Fig. 5A). In the model, root distribution is not affected by nutrient availability directly, but only indirectly through reduced carbon availability for root growth in nutrient-deficient plants. The root distribution of crops changes over time (data not shown) as growth rates change over time (Fig. 6). Root growth of maize was more exponential than root growth of bean and squash.
Fig. 5.
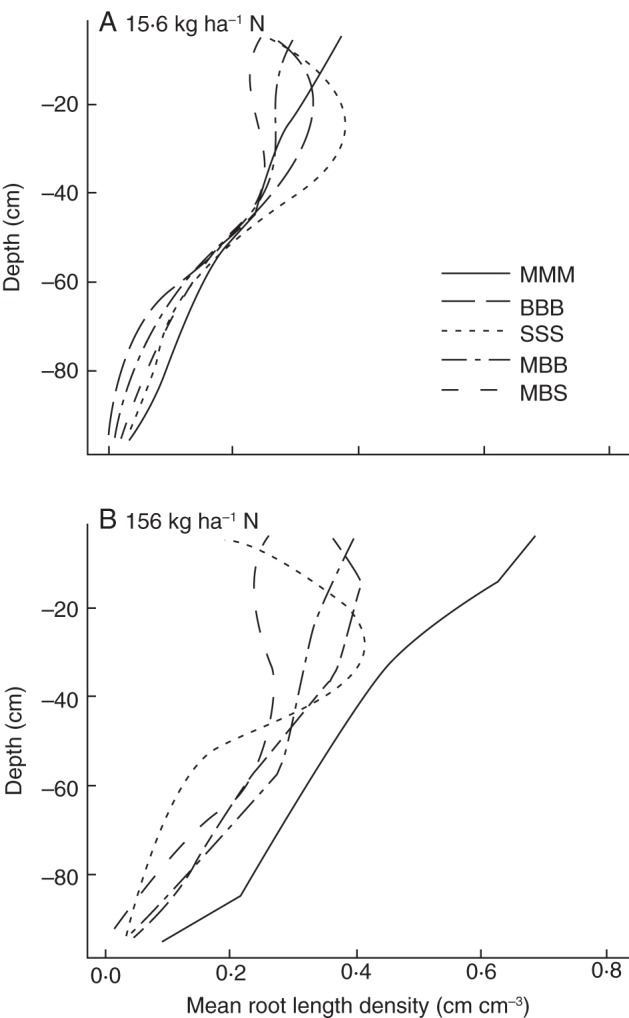
Root distribution by depth in five different systems: monocultures of maize (MMM), bean (BBB) and squash (SSS) and polycultures of maize and bean (MBB) and maize, bean and squash (MBS). Panels show root distribution of nitrate stressed or non-stressed plants at a soil-nitrate availability of 15·6 (A) and 156 kg ha−1 (B). For explanation of availability see Fig. 2.
Fig. 6.
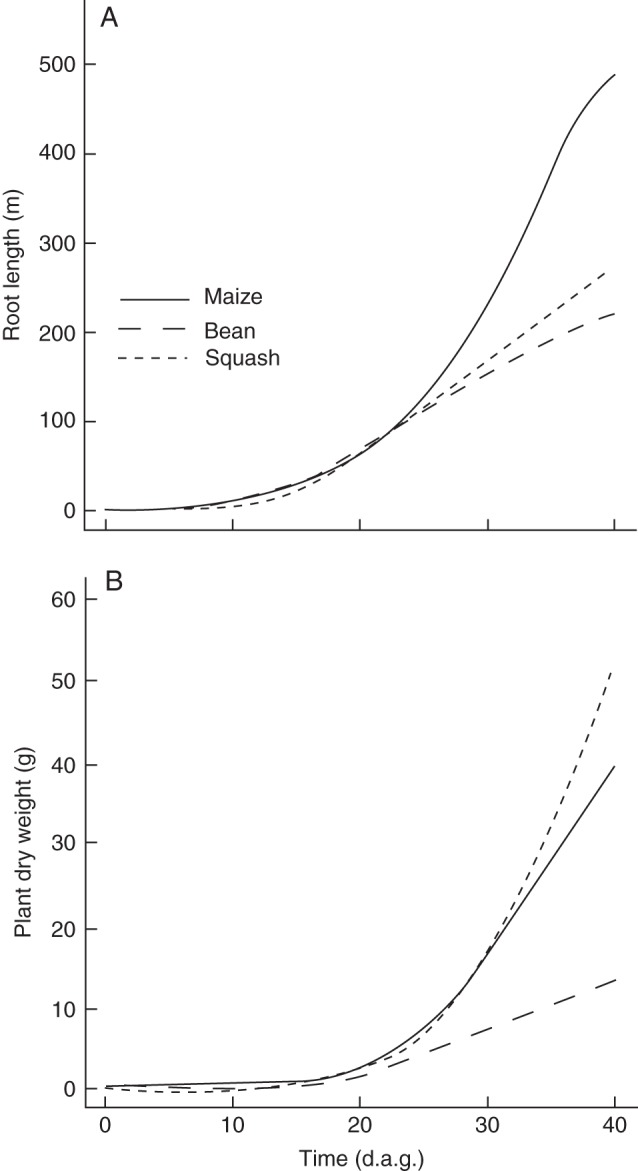
Root-length development (A) and dry-weight accumulation (B) for non-stressed maize, bean and squash plants grown in monoculture; d.a.g. = days after germination.
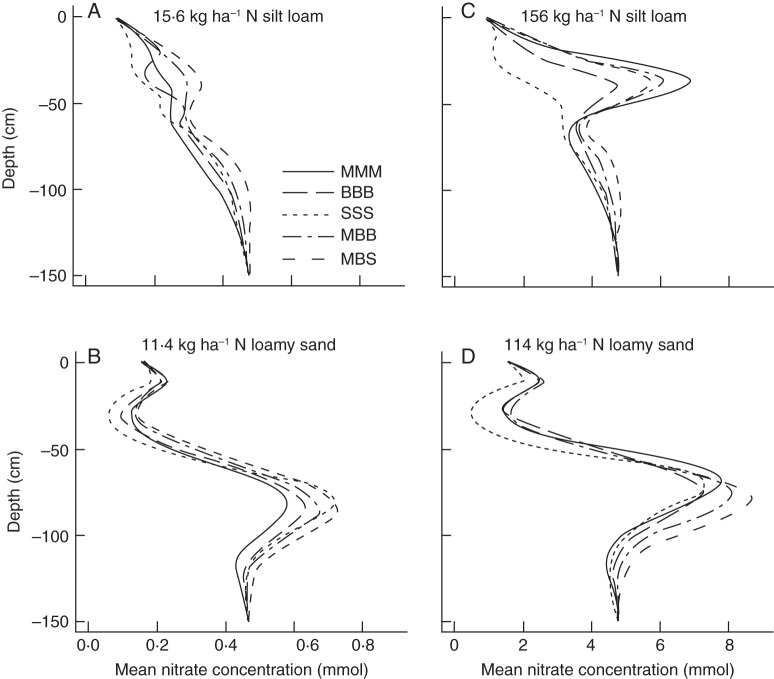
The nitrate fertilizer, which caused high nitrate concentrations in the top 30 cm of the soil at the beginning of the simulation, had leached to a depth of 30–60 cm after 40 d in the silt loam (Fig. 7A, C) and to a depth of 50–110 cm in the loamy sand (Fig. 7B, D). The monoculture squash took up most of the nitrate in the fertilizer peak in the silt loam, while other crop systems only partly depleted the nitrate peak. Nitrate uptake in the faster-leaching loamy sand (Fig. 3B) was less than in the silt loam (Fig. 3A), demonstrating that nitrate may leach before it can be taken up by the crop roots. In all systems, monoculture bean left the most nitrate in the soil.
Fig. 7.
Nitrate concentration at different depths in five different systems growing in soils differing in soil type and nitrate availability (see legend to Fig. 2). For abbreviations of labels see legend to Fig. 5. Note the different x-axis scales for panels on the left and right.
Competition in maize bean and maize/bean/squash intercropping
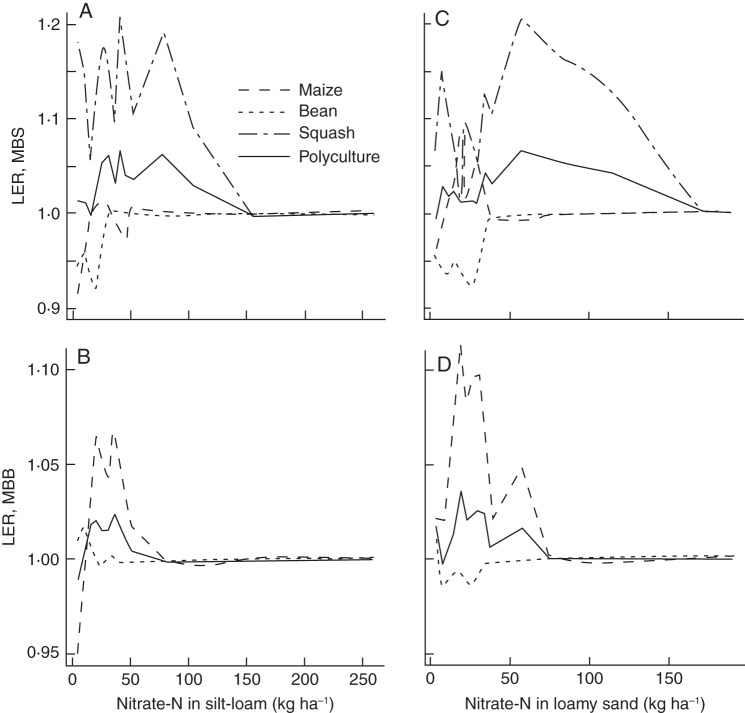
Inter-plant root competition affected plant growth negatively on low-nitrogen soils (Fig. 2A, B), but not on low-phosphorus soils (Fig. 2C) and affected plant growth on low-potassium soils marginally (Fig. 2D). Increased plant density (additive design, going from 28 to 83 thousand plants per hectare) had greater effects on individual plant growth than changes in plant community (replacement design, replacing neighbours of the same species with neighbours of different species). The LER for biomass production was 1 or >1 for both polycultures when grown in low-nitrogen soils (Fig. 8). On low-phosphorus and on low-potassium soils the LER was one for both polycultures (data not shown). The partial LERs for maize and squash were nearly always 1 or >1, while the partial LER for bean was nearly always 1 or <1.
Fig. 8.
Land equivalent ratios (LER) for the biomass production in maize/bean/bean polyculture (MBB) and the ‘three sisters’ polyculture (MBS) at different levels of nitrate availability in a silt loam (A, B) and loamy sand (C, D) (see legend to Fig. 2). Partial LERs, normalized to 1, are given for the different crops using broken lines. Note the different x- and y-axis scales for the different soils and polycultures.
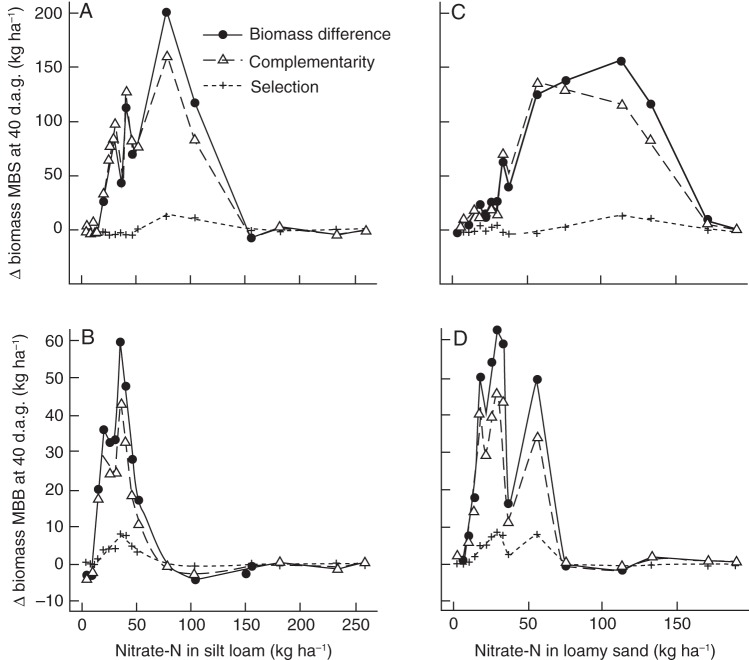
The polycultures had greater biomass production on low nitrate soils at many of the tested fertility levels and the largest part of the greater biomass production was due to niche complementarity, while the selection effect was relatively small (Fig. 9). When soil-nitrate availability was low, the complementarity effect was only positive when the model simulated both root architectural effects on nitrate uptake and biological nitrogen fixation. This was true for both the ‘three sisters’ polyculture (Figs 9 and 10) and the maize/bean/bean polyculture (data not shown) and was true for both soils (data not shown). On soils with medium nitrate availability, the ‘three sisters’ polyculture had a positive complementarity effect, also when effects of root architecture on uptake or biological nitrogen fixation were not simulated. When root architecture was eliminated (in other words, when all roots experienced the average nutrient concentration in the entire soil column) plants took up more nitrate and grew more. As a result, the complementarity graphs for runs in which root architecture did not limit nitrate uptake are shifted to the left (Fig. 10).
Fig. 9.
Difference in biomass production between the polycultures and the average of the monocultures, partitioned over a complementarity and a selection effect; d.a.g. = days after germination. The panels are as in Fig. 8 and the x-axis as in Fig. 2. Note the different x- and y-axis scales for the different soils and polycultures.
Fig. 10.
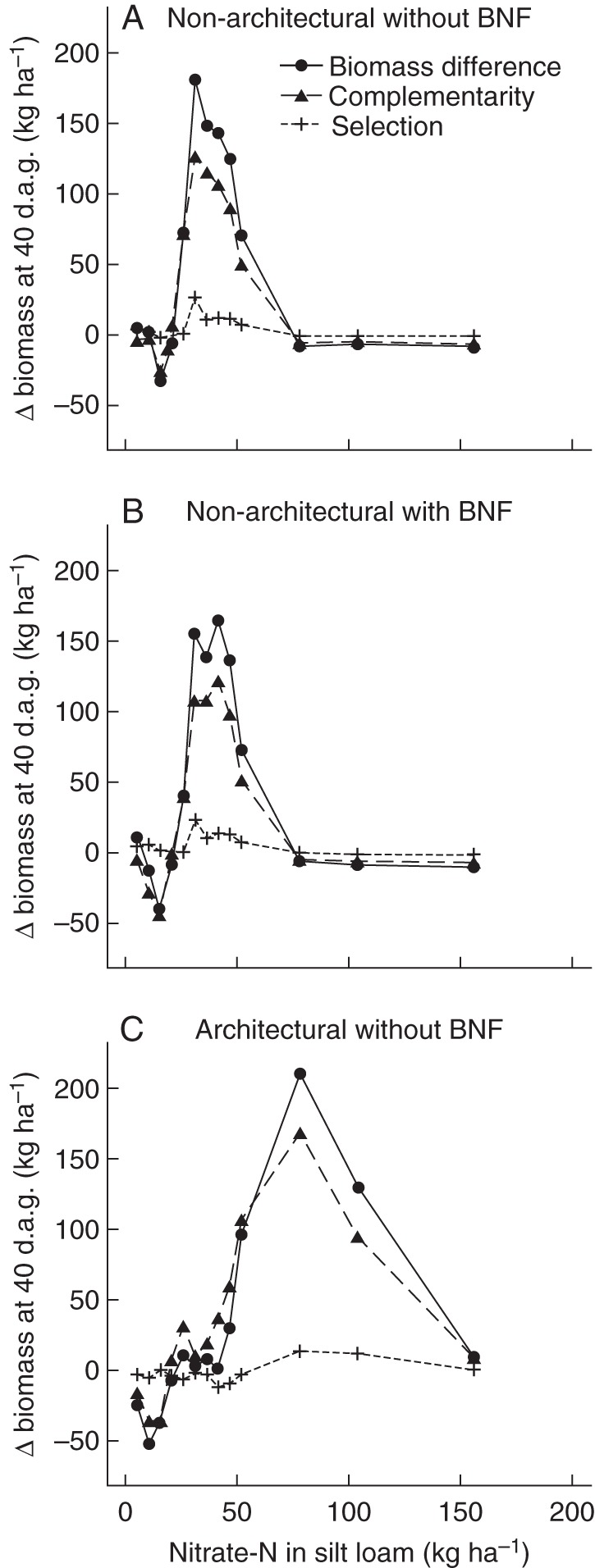
Differences in biomass production between the ‘three sisters’ polyculture and the average of the monocultures as in Fig. 9, but for simulations in which either the effect of root architecture on nutrient acquisition (B), or biological nitrogen fixation (BNF) (C) or both were turned off (A); d.a.g. = days after germination.
In comparison to the other species, maize had the greatest percentage of roots close to roots of other plants in the monoculture (Fig. 11A) and in the maize/bean/bean (Fig. 11C) polyculture but had the least roots close to other plants in the ‘three sisters’ polyculture (Fig. 11B). Squash had the smallest percentage of roots close to roots of other plants in the monocultures (Fig. 11A). Bean had the most roots close to roots of other plants in the ‘three sisters’ polyculture (Fig. 11B). All crops had greater proximity to the roots of other plants in monoculture than in polyculture (Fig. 11). However, the proximity between roots of the same plant was greater than the proximity between roots of different plants in all cropping systems.
Fig. 11.
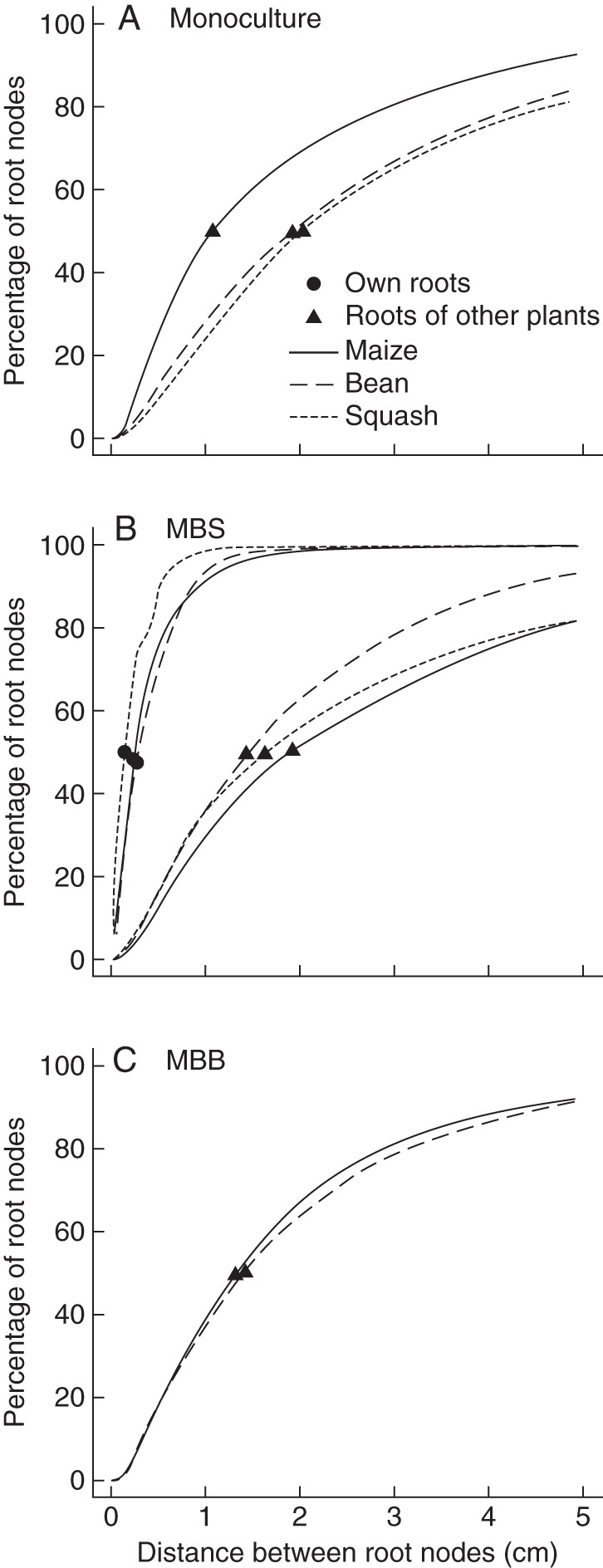
Cumulative distribution of the distance between a root node and the nearest neighbouring root node. Distributions are shown as a percentage of the root nodes of one plant and are shown for the distance between root nodes of different roots that belong to the same plant, labelled own roots, and roots that belong to a neighbouring plant. Distances to the roots of the same plant are only shown in the middle panel, as they are the same in all three systems. Distance to roots of other plants depends on the system. Results for different systems are plotted in different panels: (A) monocultures of three plants, (B) the ‘three sisters’ polyculture (MBS) and (C) the maize bean intercropping (MBB).
DISCUSSION
Biodiversity is thought to have important ecosystem functions which include greater productivity and resource utilization (Altieri, 1999; Clergue et al., 2009). These functions of biodiversity, in part, are a result of niche complementarity among species (Loreau and Hector, 2001). We hypothesized that differences in root architecture cause niche differentiation by allowing different species to explore distinct soil domains with varying intensity. Our simulation results predicted greater biomass production (Fig. 9), greater nitrate uptake (Fig. 3A, B) and greater distances between roots of different plants (Fig. 11) on low-nitrogen soils in the polycultures compared with the monocultures. On low-phosphorus and potassium soils root architecture created enough distance between the roots of different plants (Fig. 11) that inter-root competition for nutrients was low or absent. Consequently the polycultures did not have greater resource utilization (Fig. 3C, D) or greater productivity (Fig. 2C, D) on low-phosphorus or low-potassium soils.
The increase in productivity in the polycultures on low nitrate soils was mainly due to increased biomass production by the larger species, maize and squash (Fig. 2A, B), while the smaller species, bean, had unchanged or reduced growth (Fig. 8). Productivity of systems with greater plant diversity can be greater than the average of the monocultures when larger species benefit at the cost of smaller species (Loreau and Hector, 2001). However, this ‘selection effect’ was relatively small compared with the niche complementarity effect (Fig. 9). Therefore, we conclude that below-ground dominance of the larger species plays a relatively small role in maize/bean and maize/bean/squash polycultures. Complementarity effects were greater than the selection effect in most of the 44 species-richness studies reanalysed by Cardinale et al. (2007). The conclusion that complementarity effects rather than selection effects explain increased nitrate acquisition by polycultures agrees with the general thought that root competition is mainly size-symmetric, i.e. larger plants do not acquire a greater than proportional share of the available resources (Schwinning and Weiner, 1998; Schenk, 2006).
In our simulations, squash, the largest species, acquired the most nitrate (Figs 3A, B and 7) and benefited the most from growing in polyculture. This may suggest size-asymmetric competition for resources, in contrast to the general thought. However, even though squash is the largest plant on high nitrogen soils, it is not the largest plant on low-nitrogen soils (Fig. 2A, B) nor does it have the greatest root length (Figs 6A and 5). Therefore, our results are not explained strictly by size asymmetry. Similar conclusions were drawn by Schenk (2006) who suggests that much of the size (a)symmetry discussion in the literature is about root traits that are not strictly size related. In our simulations, squash monoculture took up a large proportion of the total nitrate that is available in the soil (Fig. 7), while the other two crops had larger amounts of residual nitrate left in the soil profile at 40 d after germination. Tilman (1990) found that superior competitors were plants that were able to take up more nitrate when they were growing in monoculture. Squash was able to take up more nitrate than maize and bean as its root length–density profile was more coincident with the nitrate-leaching profile (Figs 5 and 7) and as it had greater uptake capacity for nitrate [i.e. Imax of 6–15 µmol cm−2 d−1 (Wieneke, 1992) in comparison to maize which has an Imax of 2–6 µmol cm−2 d−1 (M. Silberbush, University Park, PA, USA, unpubl. res.; Pace and McClure, 1986)]. In the maize/bean/bean polyculture, the larger plant, maize, won the competition for nitrate over bean (Figs 8 and 9). In this system the larger root system of maize allows it to deplete deeper soil layers (Figs 1, 5 and 7). We conclude that although size may play a role in competition for nitrate, competition for nitrate is better understood in the context of root architecture and physiology traits which are only partly size dependent.
Niche complementarity resulted in an increase in biomass production on low nitrate soils in both polycultures, compared with the average of the monocultures (Fig. 9). As in these simulations species only compete for nitrate, but not for light, niche complementarity can be attributed to complementarity in nitrogen-acquisition strategies. Below-ground niche complementarity might be attributed to the ability of beans to fix nitrogen, to more complete nitrogen depletion in the whole soil domain, or to greater C : N ratios in the plant biomass, as species with greater C : N ratios may have greater growth responses in the polyculture than species with lower C : N ratios. More complete nitrate depletion of the whole soil domain can be the result of (a) the fact that the rate of nitrate uptake by roots with varying soil-nitrate concentrations is non-linear and varies among plant species and root classes, and (b) by differences in root architecture, which result in varying foraging intensity of distinct soil domains. The first complementarity effect is spatially independent, the second is spatially dependent. In the maize/bean/bean system the model simulated positive complementarity effects only when both root architecture was allowed to put constraints on soil exploration and symbiotic nitrogen fixation was active, which suggests a positive synergism between these two forms of niche differentiation. The synergism may be explained by the fact that in this polyculture, maize with its larger and deeper root system wins the ‘soil exploration competition’ over bean (Fig. 8) but bean is able to partly compensate with symbiotic nitrogen fixation. For the maize/bean/squash polyculture we see a similar result when nitrate availability is low (approx. <30 kg ha−1), namely that a positive complementarity effect only exists when both root architecture and symbiotic nitrogen fixation are simulated (Figs 9 and 10). However, at medium soil-nitrate availability, at which only squash is severely stressed (Fig. 2A, B), the model predicts a positive complementarity effect even when root architecture did not restrict soil exploration and symbiotic nitrogen fixation was inactive (Fig. 10A). In these runs, squash, which has the greatest demand for nitrate, benefited from the reduced nitrate uptake by maize and bean while maize and bean are still able to acquire sufficient nitrate as they have a lower critical soil-nutrient level. We conclude that, depending on the nitrate availability, symbiotic nitrogen fixation, spatial and non-spatial components are important for explaining niche complementarity. Roscher et al. (2008) also found that complementary nitrogen use in different plant communities did not solely depend on the presence of nitrogen-fixing species. Here we show that complementary nitrogen use may have, beside symbiotic nitrogen fixation, a spatial and a non-spatial component.
In many soils, including the ones we simulated, the greatest heterogeneity occurs with depth. Trade-offs exist for uptake of shallow and deep resources (Ge et al., 2000; Dunbabin et al., 2003; Ho et al., 2005) and as a consequence different species have evolved differences in root placement in shallower and deeper soil strata. Differences in vertical root distribution among species have led to the hypothesis that vertical niche segregation may occur in mixed stands (Mommer et al., 2010). Mommer et al. (2010) were not able to confirm this hypothesis experimentally since, although species did differ in vertical root distribution, strict separation of the rooting zones did not occur and differences in root placement between species, contrary to expectations, were less pronounced in the polyculture than in the monocultures. Strict segregation of the root foraging domains among the three species did not occur in our simulations either, but clear differences in the root distribution by depth did exist among the ‘three sisters’ (Fig. 5). These differences in vertical root distribution led to differences in nitrate depletion at different depths (Fig. 7). Maize had superior exploitation of deeper soil domains, while squash had superior exploitation of shallow soil domains. These differences may partially explain the niche complimentary effect caused by root architecture.
The increase in maize production at the expense of bean production (Fig. 8) agrees with the partial LERs for yield in both maize/bean and ‘three sisters’ polycultures reported in the literature (Risch and Hansen, 1982; Tsubo et al., 2003) although, after 40 d of growth, differences between the monoculture and polycultures were not as large as the differences in yield in these reports. The LERs for biomass production estimated here are close to those for 50-d-old plants (C. Zhang et al., University Park, PA, USA, unpubl. res.). The increase in squash growth in polyculture, as predicted by the model, has not been consistently observed across seasons (Risch and Hansen, 1982). Shoot competition and greater sensitivity to pests and diseases may have put squash at a disadvantage, despite its advantages in root competition.
Our results suggest that root competition for nitrate can explain observed differences in productivity of polycultures. Such a conclusion might be supported by Wilson (1988) who concluded from a large literature review that often root competition is more severe than shoot competition. However, we expect that a model of shoot competition might predict similar results as maize shoots overtop and shade bean and squash shoots, giving it an advantage over these crops, while squash, which has the largest shoot, may benefit from the more open canopy in the ‘three sisters’ polyculture. Therefore the agreement between our predictions and the reported experimental results cannot be used as an argument that root competition is stronger than shoot competition. Rather root and shoot competition may interact in such a way that an advantage below ground may lead to an advantage above ground and perhaps, more importantly, that an advantage above ground may result in greater root growth below ground and thereby greater soil exploration (Schenk, 2006). SimRoot does not simulate these feedbacks as it does not simulate shoot competition between species. While this is a limitation from the perspective of modelling actual polycultures, it permits the analysis of the direct effects of root competition apart from interactions with shoot competition.
Direct root competition for phosphorus and potassium was small or absent in nearly all our simulations when comparing monocultures with polycultures or very low planting densities to normal planting densities (Figs 2C, D and 3C, D). In previous studies with SimRoot (Ge et al., 2000; Rubio et al., 2001) it has been shown that there is overlap of phosphorus-depletion zones within a root system. This study shows that a root is more likely to be close to a root of the same plant than to a root of another plant (Fig. 11). Overlap between phosphorus-depletion zones of roots of different plants according to the model output does occur. However, most of the overlap is at the outer fringes of the phosphorus-depletion zones and occurs only in small portions of the root system. Phosphorus-depletion zones are usually <3 mm thick (Ge et al., 2000) and <15 % of root segments have a root of another plant within 6 mm (Fig. 11). Therefore the overlap of depletion zones for phosphorus and potassium has small effects on nutrient uptake and plant growth. We conclude that direct competition for buffered immobile resources is negligible in agricultural systems. This conclusion is supported by results of Nord et al. (2011) who found that the biomass of common bean plants was only affected by phosphorus availability and not by the presence of another bean plant.
Model choice, age of the plants and the agricultural setting could be used as arguments to critique this conclusion. The lack of direct root competition for immobile resources is not an artefact from using the Barber–Cushman model, which cannot simulate root competition in three dimensions (Postma and Lynch, 2011a), because our simulations of plant growth in low-potassium soils (which used SWMS3D, which does simulate root competition in three dimensions; Postma and Lynch, 2011a), also indicate that even for potassium, which has intermediate mobility, effects of direct root competition on potassium uptake and growth are nearly absent when comparing monocultures with polycultures or normal planting densities with low planting densities (Figs 2C, D and 3C, D). The plants that we simulated are young, 40 d after germination (d.a.g.). We expect that root growth will continue for some time after 40 d.a.g.; however, new root growth may not be in soil domains having the greatest root-length density. The root-length density in cores taken during the growing season of the ‘three sisters’ was greater at 50 d.a.g. than at 80 d.a.g. and did not exceed our simulated root length density at 40 d.a.g. (C. Zhang et al., University Park, PA, USA, unpubl. res.). The decline in root length density after 50 d.a.g. may be attributed to root turnover. Therefore, we conclude that the lack of direct competition we observed here is not a result of artificially low root densities. Depletion zones may increase over time; however, this increase slows down with the square root of time (Nye and Tinker, 1977; Fitter et al., 1991). Furthermore, nutrient uptake by older roots slows over time because of depletion of the resource in the vicinity of the root, and also because older roots often lose their cortex, either due to secondary growth or due to cortical senescence, or have suberized exoderma and no root hairs. As a result, competition for nutrients among older roots may not be important. Our results are based on agricultural crops planted in an empty field. Natural ecosystems often have much greater root densities and competition in these systems may be more severe. Determining the root length density at which competition for phosphorus or potassium would occur is difficult as it depends on several factors including root fineness, root hair development and the buffering capacity of the soil. Furthermore, average root-length density does not play an important role as significant clustering of roots occurs due to the limitations that root architecture creates for root placement (Grabarnik et al., 1998). Our results may therefore not be applicable to root competition for phosphorus or potassium in natural ecosystems.
The conclusion that direct competition for immobile resources is negligible agrees with Tilman's conclusion that nitrogen and soil disturbance were the most important factors determining species abundance and competition (Tilman, 1990), but contradicts the conclusion of Fitter et al. (2002) that competition for immobile resources may be more important than mobile resources. However, we suggest that these studies may have confounded root competition for nutrients, with asynchronous competition for light resulting in asynchronous carbon restriction of root growth, with adverse effects on nutrient uptake. Root growth may have greater effects than direct root competition on the uptake of immobile nutrients (Silberbush and Barber, 1983). Carbon limitations to root growth can cause further reduction in nutrient uptake (Lynch, 2007). Therefore the indirect effect of competition for light on phosphorus uptake may be greater than the effect of direct competition for phosphorus by roots. This conclusion may explain the experimental results of Fitter et al. (2002), in which plant competition was more severe in low-phosphorus soils and less severe in low-nitrogen soils.
Intercropping of cereals with a legume often increases cereal production without substantial reductions in legume yield (Li et al., 2007). Our results show such a response for the maize/bean/bean polyculture in low-nitrogen soils but not in low-potassium or low-phosphorus soils (Figs 2 and 8). We have suggested that changes in shoot competition may affect nutrient uptake in low-phosphorus soils rather than direct competition for nutrients. In most cereal–legume polycultures, the cereal is the taller plant and may therefore be the stronger above-ground competitor. Li et al. (2007), however, have proposed an alternative hypothesis which includes an ‘interspecific rhizosphere effect’, meaning that in low-phosphorus soils the cereals may benefit from the root exudates of the legumes. However, we postulate that, if roots are not close enough to compete for phosphorus uptake, they are also not close enough to benefit from each other's exudates. These exudates, such as citrate and malate, are compared with phosphate, large molecules with slow diffusion rates in the soil (Jones et al., 1996). Furthermore, organic exudates may be rapidly broken down by microbes in the soil (for example, malate may have a half-life of 1·7 h; Jones et al., 1996) and are absorbed to the anion-exchange phase (Jones, 1998). Therefore the ‘exudation’ zones are small (Rovira, 1969). Raynaud (2010) estimated the required root-length density for having overlapping of exudation zones for 10 000 parameter combinations in his root exudation model. For facilitation, we need to divide these numbers by 2, since the root surface area of the cereal needs to be inside the exudation zone of the legume. At an average root length density of 0·3 cm cm−3 (Fig. 5) this means that only in 1·8 % of the possible parameter combinations could some degree of facilitation occur at the outer fringes of the exudation zones. These 1·8 % represent simulations of exudates with fast diffusion coefficients and a long half-life in wet soils with low adsorption. Even in these soils, the cereal roots would be at the outer fringes of the exudation zones where concentrations of exudates are very low. Sauer et al. (2006) showed with labelled carbon that exudation zones of several plants do not extend beyond 3 mm, a distance that corresponds to the size of phosphorus-depletion zones. Our results show that there is no significant overlap of phosphorus-depletion zones, and that <5 % of maize root length is within 3 mm of a bean or squash root (Fig. 11). Therefore, we have to question if a cereal crop can really benefit significantly from the exudates of legume crops in polyculture, without purposely aligning its roots with those of the cereal crop. We are, however, not aware of observations of such aggressive forms of root competition between agricultural crops and question if it would actually benefit the cereal crop, as opposed to avoiding competition. Recently, maize and bean have been shown to avoid each other (Fang et al., 2011) and so have roots of two neighbouring bean plants (Nord et al., 2011). We conclude that it is unlikely that maize can benefit from root exudates of either bean or squash in polyculture.
Within a plant community, the degree of colonization of different species of mycorrhizal fungi may differ by plant species (McGonigle and Fitter, 1990) and as a result the utility of the mycorrhizal networks for each species may differ. Mycorrhizae can change the balance between plant species in a community (Newman et al., 1992; van der Heijden et al., 1998). For example, Xiao et al. (2010) found that mungbean benefited more from arbuscular mycorrhizal fungal inoculation than rice in a mungbean/rice polyculture and Reeves (1992) found that bean nitrogen content was increased more by the presence of mycorrhizal fungi than maize nitrogen content in maize/bean intercropping. Colonization with mycorrhizal fungi also affects root architecture, plant size and root length (Zhu et al., 2005). They will thereby alter root competition for soil resources, possibly intensifying them as root length density increases. Our simulation model does not include mycorrhizae as there is, to our knowledge, no mycorrhizal model that can both simulate realistic carbon and nutrient exchange between the fungal and plant symbionts, and simultaneously can simulate the spatio-temporal dynamics of soil foraging and nutrient uptake by the fungal symbiont. We refer to Schnepf et al. (2008) for what is probably the best mycorrhizae model currently presented in the literature. Although the absence of mycorrhizae is a deficit in our modelling effort, we do not expect mycorrhizae to cause root architecture to be irrelevant for nutrient competition as, in previous studies, the presence of mycorrhizae did not negate the importance of root architecture for nutrient uptake (Lynch and Brown, 2008).
We can conclude that maize, bean and squash have root architectures that allow them to avoid direct root competition for immobile resources, and that differences in the root architectures of these species allow them to take up more mobile nitrate in polyculture than in monoculture (Figs 3A, B and 9). The root architecture of these species resulted in differences in nutrient acquisition that altered the size ratios among the plants (Fig. 2A, B). The relative reductions in plant growth on low-fertility soils were smallest for bean and largest for squash. Squash, the oldest domesticated crop of the ‘three sisters’ (Smith, 2006), has, in contemporary agriculture, less importance than maize and bean. This is especially so in Africa, where the adoption of maize and bean as staple food crops is widespread. It is possible that the sensitivity of squash to low soil fertility has played a role in its decline. It is interesting to note that the order of domestication (Smith, 2006) goes from crops requiring fertile soil to crops with increasing tolerance to infertile soil, rather than from low to high yield potential. This shift away from yield potential toward productivity on soils with low fertility might have been intentional as increasingly societies relied on continuous cropping of large fields in which maintenance of soil fertility can be challenging (Scarry, 2008). Growing the ‘three sisters’ may have been an effective strategy for increasing productivity on low-fertility soils.
Conclusions
Three-dimensional simulation of maize, bean and squash root architecture (Fig. 1) shows large differences in root architecture among these crops which reflect differences in nutrient foraging strategies (Bray, 1954; Fitter, 1987; Lynch, 1995). The three species differed strongly in nutrient acquisition and we note that the order of domestication of these crops in Mesoamerica corresponds with increasing tolerance to low soil fertility. Niche complementarity in polycultures resulted in greater nutrient uptake and biomass production on low-nitrogen soils. Niche complementarity was greatest when both root architecture and biological nitrogen fixation were simulated. Differences in root architecture altered the depth and intensity of soil foraging by different species, suggesting that vertical niche segregation may occur among the ‘three sisters’. In low-phosphorus and low-potassium soils, root competition for nutrients was nearly absent. The root architecture of these species caused enough spacing between the roots of different plants that phosphorus and potassium acquisition were not affected by neighbouring plants, independent of the nature of the neighbouring plants.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Choachun Zhang and Ana Fita Fernandez for helping us to collect the experimental data that laid a strong foundation for the parameterization of the model. We thank them for the great times we had studying the ‘three sisters’ in the greenhouse and the field and for the many interesting discussions. We acknowledge the important contributions of Dr. Annette Dathe to the development of both the Simunek- and Barber–Cushman-based modules that were used in this article. We are grateful to her for solving the underlying mathematics of the Barber–Cushman model and her intense work on the strategic planning, implementation and testing of the link between SimRoot and SWMS3D. This research was funded by the McKnight Foundation CCRP and the USAID-Pulse CRSP.
LITERATURE CITED
- Altieri MA. The ecological role of biodiversity in agroecosystems. Agriculture, Ecosystems & Environment. 1999;74:19–31. [Google Scholar]
- Bray RH. A nutrient mobility concept of soil–plant relationships. Soil Science. 1954;78:9–22. [Google Scholar]
- Cardinale BJ, Wright JP, Cadotte MW, et al. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proceedings of the National Academy of Sciences of the USA. 2007;104:18123–18128. doi: 10.1073/pnas.0709069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clergue B, Amiaud B, Pervanchon F, Lasserre-Joulin F, Plantureux S. Biodiversity: function and assessment in agricultural areas: a review. The Netherlands: Springer Verlag; 2009. pp. 309–327. Sustainable Agriculture. Dordrecht. [Google Scholar]
- Dunbabin V, Diggle AJ, Rengel Z. Is there an optimal root architecture for nitrate capture in leaching environments? Plant, Cell & Environment. 2003;26:835–844. doi: 10.1046/j.1365-3040.2003.01015.x. [DOI] [PubMed] [Google Scholar]
- Dunbabin V, Rengel Z, Diggle AJ. Simulating form and function of root systems: efficiency of nitrate uptake is dependent on root system architecture and the spatial and temporal variability of nitrate supply. Functional Ecology. 2004;18:204–211. [Google Scholar]
- Emerson RA. A preliminary survey of the milpa system of maize culture as practiced by the Maya indians of the northern part of the Yucatan peninsula. Annals of the Missouri Botanical Garden. 1953;40:51–62. [Google Scholar]
- Fang S, Gao X, Yan D, Chen X, Liao H. Crop root behavior coordinates phosphorus status and neighbors: from field studies to 3D in situ reconstruction of root system architecture. Plant Physiology. 2011;155:1277–1285. doi: 10.1104/pp.110.167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fita A, Postma J, Picó B, Nuez F, Lynch J. Proceedings of the IXth EUCARPIA meeting on genetics and breeding of Cucurbitaceae. Avignon, France: INRA; 2008. Root architecture variation in Cucurbita; pp. 487–491. [Google Scholar]
- Fitter AH. An architectural approach to the comparative ecology of plant root systems. New Phytologist. 1987;106:61–77. [Google Scholar]
- Fitter AH, Stickland TR, Harvey ML, Wilson GW. Architectural analysis of plant root systems. 1 Architectural correlates of exploitation efficiency. New Phytologist. 1991;118:375–382. [Google Scholar]
- Fitter A, Williamson L, Linkohr B, Leyser O. Root system architecture determines fitness in an Arabidopsis mutant in competition for immobile phosphate ions but not for nitrate ions. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2002;269:2017–2022. doi: 10.1098/rspb.2002.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge ZY, Rubio G, Lynch JP. The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: results from a geometric simulation model. Plant and Soil. 2000;218:159–171. doi: 10.1023/a:1014987710937. [DOI] [PubMed] [Google Scholar]
- Gent MP, Parrish ZD, White JC. Nutrient uptake among subspecies of Cucurbita pepo L. is related to exudation of citric acid. Journal of the American Society for Horticultural Science. 2005;130:782–788. [Google Scholar]
- Gliessman SR. Agroecology in the tropics: achieving a balance between land use and preservation. Environmental Management. 1992;16:681–689. [Google Scholar]
- Grabarnik P, Pagès L, Bengough AG. Geometrical properties of simulated maize root systems: consequences for length density and intersection density. Plant and Soil. 1998;200:157–167. [Google Scholar]
- van der Heijden MGA, Klironomos JN, Ursic M, et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature. 1998;396:69–72. [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP. Root architectural trade-offs for water and phosphorus acquisition. Functional Plant Biology. 2005;32:737–748. doi: 10.1071/FP05043. [DOI] [PubMed] [Google Scholar]
- Jones DL. Organic acids in the rhizosphere – a critical review. Plant and Soil. 1998;205:25–44. [Google Scholar]
- Jones DL, Prabowo AM, Kochian LV. Kinetics of malate transport and decomposition in acid soils and isolated bacterial populations: the effect of microorganisms on root exudation of malate under Al stress. Plant and Soil. 1996;182:239–247. [Google Scholar]
- Lewandowski S. Diohe'ko, the Three Sisters in Seneca life: implications for a native agriculture in the Finger Lakes region of New York State. Agriculture and Human Values. 1987;4:76–93. [Google Scholar]
- Li L, Li S-M, Sun J-H, et al. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proceedings of the National Academy of Sciences of the USA. 2007;104:11192–11196. doi: 10.1073/pnas.0704591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman M, Dyck E. Crop rotation and intercropping strategies for weed management. Ecological Applications. 1993;3:92–122. doi: 10.2307/1941795. [DOI] [PubMed] [Google Scholar]
- Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- Lynch J. Root architecture and plant productivity. Plant Physiology. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. Rhizoeconomics: the roots of shoot growth limitations. HortScience. 2007;42:1107–1109. [Google Scholar]
- Lynch JP. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology. 2011;156:1041–1049. doi: 10.1104/pp.111.175414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant and Soil. 2001;237:225–237. [Google Scholar]
- Lynch JP, Brown KM. Root strategies for phosphorus acquisition. In: PJ White, Hammond JP., editors. Plant ecophysiology: the ecophysiology of plant–phosphorus interactions. The Netherlands: Springer Verlag; 2008. pp. 83–116. Dordrecht. [Google Scholar]
- Lynch JP, Nielsen KL, Davis RD, Jablokow AG. SimRoot: modelling and visualization of root systems. Plant and Soil. 1997;188:139–151. [Google Scholar]
- McGonigle TP, Fitter AH. Ecological specificity of vesicular-arbuscular mycorrhizal associations. Mycological Research. 1990;94:120–122. [Google Scholar]
- Malley Z, Temu A, Kinabo N, Mwigune S, Mwageni A. Sowing maize in pits: farmer innovation in southern Tanzania. In: Reij C, Waters-Bayer A., editors. Farmer innovation in Africa: a source of inspiration for agricultural development. London: Earthscan Publications; 2001. pp. 267–280. [Google Scholar]
- Mommer L, Van Ruijven J, De Caluwe H, et al. Unveiling below-ground species abundance in a biodiversity experiment: a test of vertical niche differentiation among grassland species. Journal of Ecology. 2010;98:1117–1127. [Google Scholar]
- Newman EI, Eason WR, Eissenstat DM, Ramos MIRF. Interactions between plants: the role of mycorrhizae. Mycorrhiza. 1992;1:47–53. [Google Scholar]
- Nord EA, Zhang C, Lynch JP. Root responses to neighbouring plants in common bean are mediated by nutrient concentration rather than self/non-self recognition. Functional Plant Biology. 2011;38:941–952. doi: 10.1071/FP11130. [DOI] [PubMed] [Google Scholar]
- Nye PH, Tinker PB. Solute movement in the soil–root system. Berkeley, CA: University of California Press; 1977. [Google Scholar]
- Pace GM, McClure PR. Comparison of nitrate uptake kinetic parameters across maize inbred lines. Journal of Plant Nutrition. 1986;9:1095–1111. [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.) Planta. 1995;196:788–795. [Google Scholar]
- Postma JA, Lynch JP. Root cortical aerenchyma enhances the acquisition and utilization of nitrogen, phosphorus, and potassium in Zea mays L. Plant Physiology. 2011a;156:1190–1201. doi: 10.1104/pp.111.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JA, Lynch JP. Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Annals of Botany. 2011b;107:829–841. doi: 10.1093/aob/mcq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud X. Soil properties are key determinants for the development of exudate gradients in a rhizosphere simulation model. Soil Biology and Biochemistry. 2010;42:210–219. [Google Scholar]
- Reeves M. The role of VAM fungi in nitrogen dynamics in maize–bean intercrops. Plant and Soil. 1992;144:85–92. [Google Scholar]
- Risch SJ. Insect herbivore abundance in tropical monocultures and polycultures: an experimental test of two hypotheses. Ecology. 1981;62:1325–1340. [Google Scholar]
- Risch SJ, Hansen MK. Plant growth, flowering phenologies, and yields of corn, beans and squash grown in pure stands and mixtures in Costa Rica. Journal of Applied Ecology. 1982;19:901–916. [Google Scholar]
- Roscher C, Thein S, Schmid B, Scherer-Lorenzen M. Complementary nitrogen use among potentially dominant species in a biodiversity experiment varies between two years. Journal of Ecology. 2008;96:477–488. [Google Scholar]
- Rovira A. Plant root exudates. The Botanical Review. 1969;35:35–57. [Google Scholar]
- Rubio G, Walk T, Ge ZY, Yan XL, Liao H, Lynch JP. Root gravitropism and below-ground competition among neighbouring plants: a modelling approach. Annals of Botany. 2001;88:929–940. [Google Scholar]
- Sauer D, Kuzyakov Y, Stahr K. Spatial distribution of root exudates of five plant species as assessed by 14C labeling. Journal of Plant Nutrition and Soil Science. 2006;169:360–362. [Google Scholar]
- Scarry CM. Crop husbandry practices in North America's eastern woodlands. In: EJ Reitz, SJ Scudder, Scarry CM., editors. Interdisciplinary Contributions to Archaeology. Case studies in environmental archaeology. New York, NY: Springer Verlag; 2008. pp. 391–404. [Google Scholar]
- Schenk HJ. Root competition: beyond resource depletion. Journal of Ecology. 2006;94:725–739. [Google Scholar]
- Schnepf A, Roose T, Schweiger P. Impact of growth and uptake patterns of arbuscular mycorrhizal fungi on plant phosphorus uptake: a modelling study. Plant and Soil. 2008;312:85–99. [Google Scholar]
- Schwinning S, Weiner J. Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia. 1998;113:447–455. doi: 10.1007/s004420050397. [DOI] [PubMed] [Google Scholar]
- Shane M, Lambers H. Cluster roots: a curiosity in context. Plant and Soil. 2005;274:101–125. [Google Scholar]
- Shen H, Yan X, Zhao M, Zheng S, Wang X. Exudation of organic acids in common bean as related to mobilization of aluminum- and iron-bound phosphates. Environmental and Experimental Botany. 2002;48:1–9. [Google Scholar]
- Silberbush M, Barber S. Sensitivity of simulated phosphorus uptake to parameters used by a mechanistic-mathematical model. Plant and Soil. 1983;74:93–100. [Google Scholar]
- Smith BD. Eastern North America as an independent center of plant domestication. Proceedings of the National Academy of Sciences of the USA. 2006;103:12223–12228. doi: 10.1073/pnas.0604335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D. Constraints and tradeoffs: toward a predictive theory of competition and succession. Oikos. 1990;58:3–15. [Google Scholar]
- Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C. Diversity and productivity in a long-term grassland experiment. Science. 2001;294:843–845. doi: 10.1126/science.1060391. [DOI] [PubMed] [Google Scholar]
- Tsai SM, Silva PM, Cabezas WL, Bonetti R. Variability in nitrogen fixation of common bean (Phaseolus vulgaris L.) intercropped with maize. Plant and Soil. 1993;152:93–101. [Google Scholar]
- Tsubo M, Mukhala E, Ogindo HO, Walker S. Productivity of maize–bean intercropping in a semi-arid region of South Africa. Water SA. 2003;29:381–388. [Google Scholar]
- Waters B, Blevins D. Ethylene production, cluster root formation, and localization of iron(III) reducing capacity in Fe deficient squash roots. Plant and Soil. 2000;225:21–31. [Google Scholar]
- Weaver JE, Bruner WE. Root development of vegetable crops. New York, NY: McGraw-Hill Book Co; 1927. [Google Scholar]
- Wieneke J. Nitrate fluxes in squash seedlings measured with 13N. Journal of Plant Nutrition. 1992;15:99–124. [Google Scholar]
- Wilson JB. Shoot competition and root competition. Journal of Applied Ecology. 1988;25:279–296. [Google Scholar]
- Xiao TJ, Yang QS, Ran W, Xu GH, Shen QR. Effect of inoculation with arbuscular mycorrhizal fungus on nitrogen and phosphorus utilization in upland rice–mungbean intercropping system. Agricultural Sciences in China. 2010;9:528–535. [Google Scholar]
- Zhu J, Kaeppler SM, Lynch JP. Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays) Functional Plant Biology. 2005;32:749–762. doi: 10.1071/FP05005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.