Abstract
Background and Aims
Phosphorus commonly limits crop yield and is frequently applied as fertilizer; however, supplies of quality rock phosphate for fertilizer production are diminishing. Plants have evolved many mechanisms to increase their P-fertilizer use efficiency, and an understanding of these traits could result in improved long-term sustainability of agriculture. Here a mutant population is utilized to assess the impact of root hair length on P acquisition and yield under P-deficient conditions alone or when combined with drought.
Methods
Mutants with various root hair phenotypes were grown in the glasshouse in pots filled with soil representing sufficient and deficient P treatments and, in one experiment, a range of water availability was also imposed. Plants were variously harvested at 7 d, 8 weeks and 14 weeks, and variables including root hair length, rhizosheath weight, biomass, P accumulation and yield were measured.
Key Results
The results confirmed the robustness of the root hair phenotypes in soils and their relationship to rhizosheath production. The data demonstrated that root hair length is important for shoot P accumulation and biomass, while only the presence of root hairs is critical for yield. Root hair presence was also critical for tolerance to extreme combined P deficit and drought stress, with genotypes with no root hairs suffering extreme growth retardation in comparison with those with root hairs.
Conclusions
The results suggest that although root hair length is not important for maintaining yield, the presence of root hairs is implicit to sustainable yield of barley under P-deficient conditions and when combined with extreme drought. Root hairs are a trait that should be maintained in future germplasm.
Keywords: barley mutants, P-use efficiency, drought, rhizosheath, agricultural sustainability, root hairs, combined stress
INTRODUCTION
Phosphorus (P) is an essential nutrient required for plant growth and fecundity, but its low mobility in soil makes it one of the least phytoavailable elements. This presents a problem to agriculture of global proportions and, while the application of P fertilizer is a common solution for intensively managed agricultural systems, this has environmental repercussions in the form of the eutrophication of freshwater habitats (Tiessen, 2008). Moreover, the long-term sustainability of this practice is questionable, with world reserves of high-quality rock phosphate expected to be exhausted in the next 50 years at current rates of consumption (Gilbert, 2009).
The above scenario accentuates the need to improve the ability of crop plants to acquire P from existing sources in the soil and to utilize this P for growth. It is through an understanding of the plant mechanisms involved in these processes that progress can be made (Lynch, 2007). Plants have evolved a number of mechanisms to enhance P acquisition. These include modifications to root growth and architecture, the release of root exudates and associations with soil micro-organisms (White and Hammond, 2008). This paper focuses on changes to root system morphology that improve P acquisition, specifically through modifications to root hairs, and in particular root hair length.
It is through root hairs that the greatest proportion of P uptake occurs (Gahoonia and Nielsen, 1998). In P-deficient conditions, up to 90 % of total P acquired by plants can occur through root hairs, and in many species they can contribute almost 70 % of the total surface area of roots (Raghothama, 2005). Root hairs are tubular shaped cells specialized for nutrient uptake (Jungk, 2001). They arise from epidermal cells called trichoblasts, which undergo tip growth perpendicular to the root and, thereby, increase the root surface area in contact with the soil (Dolan, 2001). This maximizes the potential for P absorption (Peterson and Farquhar, 1996). It has been demonstrated that root systems with root hairs absorbed 78 % more P than those without (Barley and Rovira, 1970) and that plants increase root hair length and density when they are P deficient (Bates and Lynch, 1996; Ma et al., 2001). Newly formed root hairs are sites of expression for phosphate transporters (Mudge et al., 2002), and these specialized transporters operate at the root–rhizosphere interface taking up orthophosphate from the soil solution, transporting it across cell membranes and into the cytosol of roots (Raghothama, 2005). However, the physiological state of a P-deficient plant is complex, and the response is not limited to root hairs and is multigenic. For example, >1000 genes are differentially regulated under these conditions in Arabidopsis thaliana (Hammond et al., 2003; Wu et al., 2003; Morcuende et al., 2007). It has already been demonstrated that P acquisition is enhanced by the presence of root hairs (Gahoonia and Nielsen, 1998), and associations between root hair length and yield have also been studied (Gahoonia and Nielsen, 2004). However, it is not yet established whether this enhanced P acquisition is significant for the ability of a plant to yield (or reproduce) under P-limited conditions, whether or not any ability to improve growth in P-deficient conditions is compromised when plants are faced with additional drought stress or whether root hair length per se is critical for tolerance to combined stress or not.
Here we investigate the importance of the presence and length of root hairs for plant growth and crop yield under P-limited conditions and when P limitation is combined with drought stress. Our use of mutants allows us to control for many confounding effects seen in other studies. First, a mutant population of barley (Hordeum vulgare) ‘Optic’ was screened to identify mutants with differing root hair length. Phenotypes exhibiting no root hairs (NRH), short root hairs (SRH) and long root hairs (LRH) were then grown in P-sufficient, P-deficient and drought conditions to determine the effect of root hair length on tolerance to combined stress.
MATERIALS AND METHODS
Initial mutant screen
A screen of 458 mutant lines from an ethylmethane sulfonate (EMS) mutant barley (Hordeum vulgare) population in an ‘Optic’ genetic background (Caldwell et al., 2004) was performed. Twelve seeds of each line were germinated in 90 mm diameter Petri dishes containing blue blotting paper (Anchor Paper Company, MN, USA) and 6 mL of distilled water. Petri dishes were wrapped in foil and placed in an incubator at 16 °C. After 4 d the dishes were removed, photographed, and any variation from the wild type (WT) was recorded (Gregory et al., 2009). Thirteen different phenotypes were observed, including variants in root length, root hair length and root hair density (Gregory et al., 2009; White et al., 2009). Mutant lines in the phenotype categories relating to root hair length were selected for a growth experiment and comprised three genotypes of each of the following phenotypes: NRH, SRH and LRH. These lines were not genetic isolines as they were yet to be backcrossed and selected; therefore, they will probably possess multiple mutations. However, our use of three independent mutant lines for each phenotype gives us confidence that they are experimentally robust as representatives of the impact of root hairs on growth under a range of conditions.
Plant growth experiments
Topsoil (0–10 cm depth) was collected from a site near JHI, Dundee, Scotland (NO 456 265). This was typical of arable soil of the region and defined as a Cambisol (FAO–Unesco classification). The soil was a sandy loam with a pH of 6·3 and contained total digestible P of 1475·0 mg P kg−1 of which 40·8 % was in organic moieties. Despite having a relatively high Olsen P of 84·5 mg P kg−1 (probably due to recent additions of pig manure), barley grown in this soil was responsive to the addition of P (George et al., 2011) and water-extractable P was relatively low (Pi = 6·3 mg P kg−1 and Po = 0·5 mg P kg−1). The soil was air-dried, mixed and passed through a 2 mm sieve to remove coarse material and vegetative matter. Soil was left unamended (P0), or fertilized with inorganic phosphate (KH2PO4) at 500 mg P kg−1 soil (P500) which was considered to be sufficient for plant growth based on a previously performed response curve for barley growth using the WT cultivar Optic (George et al., 2011). The soil was mixed with fertilizer in 20 kg lots in a cement mixer turning at approx. 75 rpm for 30 min. Filter papers, 90 mm in diameter (Whatman International Ltd, Maidstone, UK, cat no. 1001 090), were placed in the bottom of 13 cm diameter circular pots to prevent soil loss and the pots were filled with the equivalent of 1 kg of air-dried soil of the two soil P treatments. All soils were then watered daily using distilled water and maintained at 80 % field capacity (FC) as determined by gravimetric water content by weight. Pots were incubated at ambient temperature for 28 d in the glasshouse prior to planting.
Nine genotypes exhibiting variation in root hair length were selected from the mutant population, three from each phenotypic category, NRH, SRH and LRH, and five replicates were planted of each. For presentation purposes, the three genotypes representing each phenotype (NRH, SRH and LRH) were averaged, meaning that each mean data point consists of 15 replicates. Seeds of uniform size were selected and germinated on 0·5 % distilled water agar [1 g of BDH Agar (VWR, UK) per 200 mL of distilled water] prior to planting, until their radicles were between 5 and 10 mm long. Each pot was sown with a germinated seed of one of the nine mutant genotypes or the WT. The soils were maintained at approx. 80 % FC (equivalent of 40–50 kPa water potential) during the growth period by watering to weight with distilled water daily, and all required nutrients, except P, were provided weekly by addition of 25 mL per pot of a nutrient solution [25 mm (NH4)2SO4, 2 mm KNO3, 1 mm MgSO4, 10 mm Ca(NO3)2, 80 µm FeEDTA and micronutrients (30 nm H3BO3, 6 µm CuSO4, 6 µm MnSO4, 0·6 µm ZnSO4, 42 nm NH4Mo7, 12 µm Co4(NO3)2] This ensured plant growth was not limited by any nutrients other than P. Plants were grown in a randomized design in a glasshouse at 18/14 °C (day/night) with an approx. 16 h day length at minimum light intensity of 200 µE ensured by supplementary lighting. Pots were rotated between glasshouse benches regularly to reduce effects of possible environmental gradients. Plants were harvested after 7 d growth; roots were washed free of soil, with due care being taken to minimize any potential damage to root hairs. Root length was measured using an Epson Expression 1640XL flat bed scanner (Epson UK, London) and WINrhizo software (Regent Instruments, Quebec City, Canada). Ten fully elongated root hairs were measured at a set location, 4–6 cm from the root tip of the longest seminal root of each plant, using a compound light microscope (×5 magnification) with a graduated eye piece to determine an average root hair length (Haling et al., 2010). This also confirmed the absence of damage to root hairs potentially sustained during the harvest process. Root and shoot mass were measured by weighing oven-dried plant material, which had been dried at 70 °C for 4 d. A measurement of rhizosheath size was carried out by calculating the difference between the clean fresh root weight and the fresh root weight including attached soil (Haling et al., 2010).
The same nine genotypes were grown in a second parallel experiment in which leaf samples (newest fully extended leaf) were taken at flag leaf stage GS 49 – flag leaf unfurled and first awns visible – (Tottman, 1987), from each plant and frozen at –80 °C. Frozen samples were freeze dried, milled to a flour, and this was analysed to determine shoot P concentration. Powdered flag leaf samples (50 µg) were digested for 20 min at 180 °C in 3 mL of 15·8 m HNO3 (Aristar grade, VWR International, Poole, UK), followed by oxidation for 20 min at 180 °C with 1 mL of H2O2 in closed vessels using a MARSXpress microwave oven (CEM, Buckingham, UK). Digested samples were diluted to a final volume of 50 mL with de-ionized water and the concentrations of P in diluted digests were determined by reaction with malachite green (Irving and McLaughlin, 1990). After 14 weeks, plants were harvested, and grain number, grain weight and shoot dry weight (d. wt.) were recorded as measurements representative of yield.
In a third experiment, the same genotypes were grown for 8 weeks under a combination of P-deficit and water-deficit treatments. Soils and plant materials were treated exactly as above, in both preparation and throughout the growth experiment. The only variations from this were the nine experimental treatments which included three levels of P application, zero (P0), 250 mg P kg−1 (P250) and 500 mg P kg−1 (P500), and three levels of water availability, 50 % FC, 75 % FC and 100 % FC and all combinations thereof. As with previous experiments, these water conditions were maintained by weight on a daily or twice-daily basis (when required) and represent water potentials in the range between field capacity and permanent wilting point. At the end of the growth period plants were harvested by cutting stems at the soil surface and biomass weighed after oven drying at 70 °C for 4 d.
Data and statistical treatments
All data are presented as the mean of five replicates. Shoot P accumulation (mg P shoot−1) was calculated as the product of shoot P (μg P mg−1 d. wt.) concentration and total shoot biomass (g), and relative shoot biomass was calculated as the percentage biomass production relative to the optimal treatment which was set to 100 %. Differences between treatments for all recorded data were determined using general analysis of variance (ANOVA) and treatment means compared by least significant difference (l.s.d.; P = 0·05) using GenStat (Release 12, Rothamsted Experiment Station, UK).
Resampling techniques were used to calculate the statistics on P0 and P500 dry grain weight ratios as a measure of relative agronomic effectiveness (RAE), assuming P0 and P500 dry grain weight are independent variables (least favourable case). The yield of each phenotype under P-limited conditions was represented as a percentage of achievable yield of the same phenotype under P-fertilized conditions. Bootstrap analysis was used to estimate the standard error of the ratios, and random permutation tests of the ANOVA were carried out to analyse the influence of root hair length on dry grain weight ratios under P0 and P500 conditions (n = 1000). Analyses were performed using the statistical package R (R Foundation for Statistical Computing 2010, Vienna, Austria).
RESULTS
Root hair determination in soil
There were significant differences in average root hair length between mutant phenotypes (P < 0·001) when examined after 7 d growth (Fig. 1A). The NRH phenotypes were confirmed to be hairless when grown in soil, with an average length of 0·007 mm, while root hairs of SRH and LRH mutant phenotypes were two orders of magnitude greater in length, root hairs averaging 0·543 and 0·691 mm, respectively. One of the genotypes exhibiting the NRH phenotype was observed to have bulges (Ishida et al., 2008) under the P-deficient treatment, and these contributed to the measurable length of root hairs associated with this phenotype. The root hairs of mutants grown in P-deficient conditions were 16 % longer than those grown in P-sufficient conditions. Figure 1B provides photographic evidence of the differences in root hair length between mutant phenotypes.
Fig. 1.
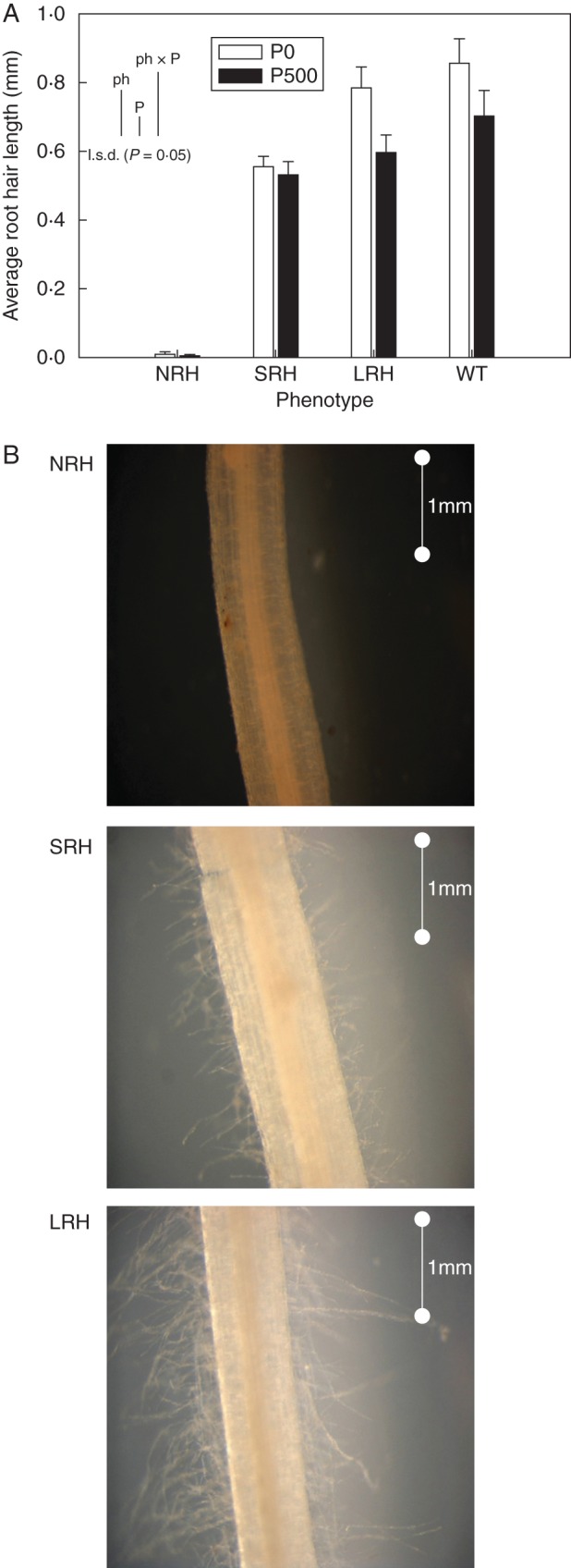
(A) Variation in average root hair length (mm) of contrasting root hair phenotypes grown in unamended soil (P0) and in soil to which 500 mg P kg−1 KH2PO4 had been added (P500). Data are the mean of five replicates, with error bars representing the s.e. Differences between phenotypes and P treatments were established using ANOVA and are shown by l.s.d. (P < 0·05) bars, with ‘ph’ representing phenotype, ‘P’ representing phosphorus treatment, and ‘phxP’ representing the interaction of phenotype and P treatment. NRH represent no root hair phenotypes; SRH, short root hair phenotypes; LRH, long root hair phenotypes; and WT, the wild type. (B) Images of different root hair phenotypes captured at ×5 magnification through a compound light microscope. Images are representative of the impact of EMS mutation on root hair phenotypes in nine mutant lines including NRH, SRH and LRH. The scale bar represents 1 mm. Images of WT roots were similar to those of LRH.
Rhizosheath weights were an order of magnitude greater in phenotypes in which root hairs were present (P < 0·001) than in phenotypes lacking root hairs (Fig. 2). The P-deficient treatment produced 18 % larger rhizosheaths than the P-sufficient treatment (P < 0·05). There was a positive relationship between rhizosheath weight and average root hair length (R2 value of 0·89; P < 0·01: data not presented).
Fig. 2.
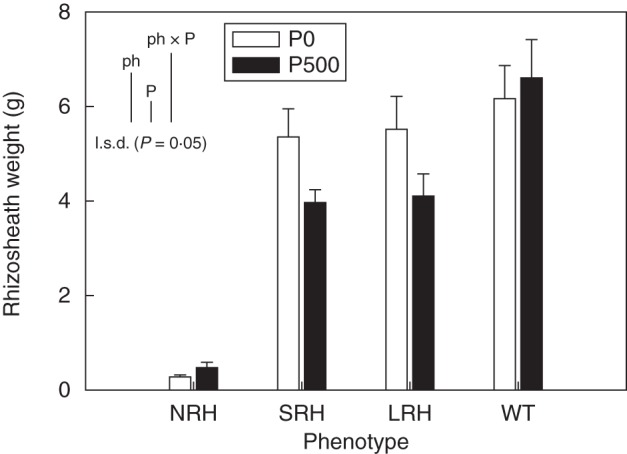
Variation in rhizosheath weight (g) of contrasting root hair phenotypes grown in unamended soil (P0) and in soil to which 500 mg P kg−1 KH2PO4 had been added (P500). Data show the means of five replicates, with error bars representing the s.e. Differences between phenotypes and P treatments were established using ANOVA and are shown by l.s.d. (P < 0·05) bars, with ‘ph’ representing phenotype, ‘P’ representing phosphorus treatment, and ‘phxP’ representing the interaction of phenotype and P treatment. NRH represent no root hair phenotypes; SRH, short root hair phenotypes; LRH, long root hair phenotypes; and WT, the wild type.
Other measurements taken after 7 d growth are summarized in Table 1. Dry weights of shoots were greater in the NRH phenotypes than in the SRH and LRH phenotypes (P < 0·001). As expected, no treatment effect was found at this early stage of growth. Total root lengths were longer in the P-deficient treatment (P < 0·005) and varied between phenotype, with LRH mutants displaying 18 and 19 % shorter total root lengths than SRH and NRH phenotypes, respectively. The measurement of the longest seminal root at 7 d demonstrated a treatment effect, with P-deficient plants having longer (P < 0·005) seminal roots than those of P-sufficient plants. A similar treatment effect was found in fresh root weights (P < 0·005), with a 9 % increase in weight being found in the P-deficient treatment.
Table 1.
Summary of plant measurements taken after 7 d growth for mutant phenotypes grown in a range of P treatments
| Measurements | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dry shoot weight (g) |
Total root length (cm) |
Longest seminal root (cm) |
Fresh root weight (g) |
|||||
| Phenotypes | P0 | P500 | P0 | P500 | P0 | P500 | P0 | P500 |
| No root hairs | 0·03 | 0·04 | 181·00 | 154·30 | 22·31 | 19·51 | 0·40 | 0·35 |
| Short root hairs | 0·02 | 0·02 | 191·20 | 139·00 | 22·25 | 20·23 | 0·42 | 0·35 |
| Long root hairs | 0·03 | 0·03 | 146·10 | 125·70 | 19·69 | 19·55 | 0·36 | 0·32 |
| Wild type | 0·04 | 0·04 | 207·20 | 176·00 | 24·62 | 21·34 | 0·48 | 0·49 |
| ANOVA | ||||||||
| l.s.d. | P-value | l.s.d. | P-value | l.s.d. | P-value | l.s.d. | P-value | |
| Root phenotype | 0·01 | <0·001 | 26·62 | 0·02 | – | 0·10 | – | 0·05 |
| P treatment | – | 0·21 | 20·62 | 0·00 | 1·52 | 0·02 | 0·05 | 0·02 |
| Root phenotype × P treatment | – | 0·44 | – | 0·66 | – | 0·51 | – | 0·07 |
P0 (P-deficient) was unamended soil and P500 (P-sufficient) had KH2PO4 added at rate of 500 mg P kg−1 soil. Data are the mean of five replicates, with differences between root phenotypes and P treatments established using ANOVA from which the l.s.d. and P-value data are derived.
Growth to yield
A summary of measurements taken at the 14 week harvest (Table 2) demonstrates a number of significant responses in phenotype and treatment. P-sufficient plants produced more (P < 0·05) tillers, more heads, more grains and greater yield than P-deficient plants. Likewise, genotypes with root hairs produced greater (P < 0·05) numbers of tillers, heads and grains, and more yield. The exception to this was the LRH genotype which produced fewer (P < 0·05) grains and less yield than the other genotypes with root hairs. In one instance, the WT differed from the other genotypes by producing more heads. The total above-ground biomass was found to differ significantly (P < 0·001) between phenotypes, with the LRH mutants producing on average 18 % more above-ground biomass than the NRH phenotypes.
Table 2.
Summary of plant measurements taken after 14 weeks growth for mutant phenotypes grown in a range of P treatments
| Measurements | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grain weight (g) |
No. of grains |
No. of heads |
No. of tillers |
Above-ground biomass (g) |
Shoot P concentration (mg Pg−1) |
|||||||
| Phenotypes | P0 | P500 | P0 | P500 | P0 | P500 | P0 | P500 | P0 | P500 | P0 | P500 |
| No root hairs | 2·74 | 4·57 | 78·00 | 140·50 | 3·93 | 7·27 | 4·33 | 8·27 | 6·62 | 11·72 | 2·51 | 2·98 |
| Short root hairs | 4·05 | 4·71 | 109·40 | 125·50 | 5·93 | 9·40 | 6·71 | 11·00 | 8·85 | 12·48 | 2·57 | 3·34 |
| Long root hairs | 2·31 | 3·09 | 89·10 | 104·70 | 5·60 | 8·78 | 7·50 | 12·22 | 8·58 | 13·92 | 2·83 | 3·32 |
| Wild type | 4·37 | 4·38 | 119·80 | 119·00 | 6·20 | 10·80 | 6·80 | 16·20 | 9·81 | 14·65 | 2·07 | 2·75 |
| ANOVA | ||||||||||||
| l.s.d. | P-value | l.s.d. | P-value | l.s.d. | P-value | l.s.d. | P-value | l.s.d. | P-value | l.s.d | P-value | |
| Root phenotype | 1·01 | <0·001 | 22·04 | 0·03 | 0·65 | <0·001 | 0·81 | <0·001 | 1·16 | <0·001 | 0·54 | 0·02 |
| P treatment | 0·45 | <0·001 | 10·39 | <0·001 | 0·31 | <0·001 | 0·38 | <0·001 | 0·55 | <0·001 | 0·24 | <0·001 |
| Root phenotype × P treatment | – | 0·09 | 31·16 | <0·001 | – | 0·62 | 1·15 | <0·001 | – | 0·08 | – | 0·75 |
P0 (P-deficient) was unamended soil and P500 (P-sufficient) had KH2PO4 added at rate of 500 mg P kg−1 soil. Data are tde mean of five replicates, witd differences between root phenotypes and P treatments established using ANOVA from which tde l.s.d. and P-value data are derived.
While significant differences (P < 0·001) were found between the P treatments with respect to shoot P concentrations, with plants in the P-sufficient treatment having concentrations (3·10 mg P g−1) that were 19 % greater than those of the P-deficient treatment (2·50 mg P g−1), no significant differences were found between phenotypes. These concentrations fall on either side of critical for maximum growth as demonstrated by George et al. (2011) and thereby define P-sufficient and P-deficient treatments. Calculations using shoot P concentrations and total shoot biomass to produce shoot P accumulation data resulted in significant differences between treatments and phenotypes. There was a 35 % increase (P < 0·001) in average shoot P accumulation by LRH mutant phenotypes (Fig. 3) compared with NRH phenotypes. Under P-deficient conditions, shoot P accumulation in LRH mutants was almost twice that in NRH mutants. Average shoot P accumulation doubled with P fertilization (P < 0·001).
Fig. 3.
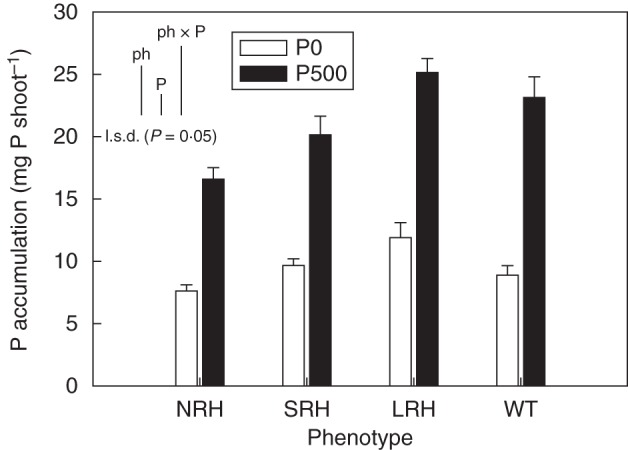
Variation in shoot P accumulation (mg P shoot−1) of contrasting root hair phenotypes grown in unamended soil (P0) and in soil to which 500 mg P kg−1 KH2PO4 had been added (P500). Data are the means of five replicates, with error bars representing the s.e. Differences between phenotypes and P treatments were established using ANOVA and are shown by l.s.d. (P < 0·05) bars, with ‘ph’ representing phenotype, ‘P’ representing phosphorus treatment, and ‘phxP’ representing the interaction of phenotype and P treatment. NRH represent no root hair phenotypes; SRH, short root hair phenotypes; LRH, long root hair phenotypes; and WT, the wild type.
Grain weight obtained from plants grown in pots for 14 weeks in the glasshouse (Table 2) was used to calculate the percentage relative effectiveness of phenotype on yield (Fig. 4). This analysis produced significant differences (P < 0·05) between the mutant phenotypes. Mutants with root hairs generally produced greater yield than mutants without, such that the NRH phenotype had a 40 % decline in yield compared with the WT.
Fig. 4.
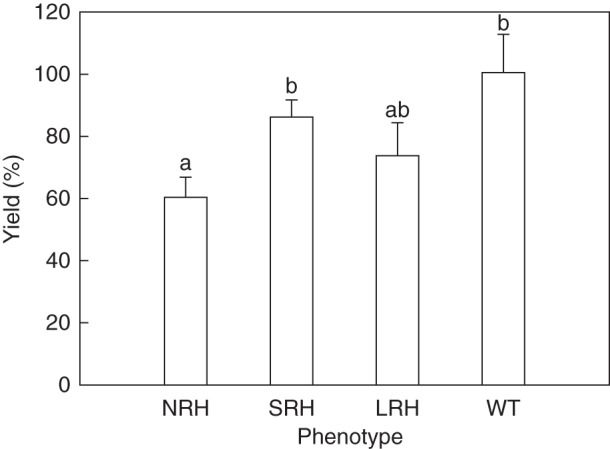
Relative agronomic effectiveness (RAE) of phenotypes on yield calculated from grain weight as a measure of yield. The yield of each phenotype under P-limited conditions is represented as a percentage of achievable yield for the same phenotype under unlimited P conditions. Bootstrap analysis was used to estimate the standard error of the ratios, and random permutation tests of the analysis of variance (ANOVA) were carried out to analyse the influence of root hair length on dry grain weight ratios under P0 and P500 conditions (n = 1000). Error bars represent the s.e. Different letters across all treatments indicate significant differences determined by l.s.d. (P = 0·05). NRH represent no root hair phenotypes; SRH, short root hair phenotypes; LRH, long root hair phenotypes; and WT, the wild type.
Growth under combined P deficit and water stress
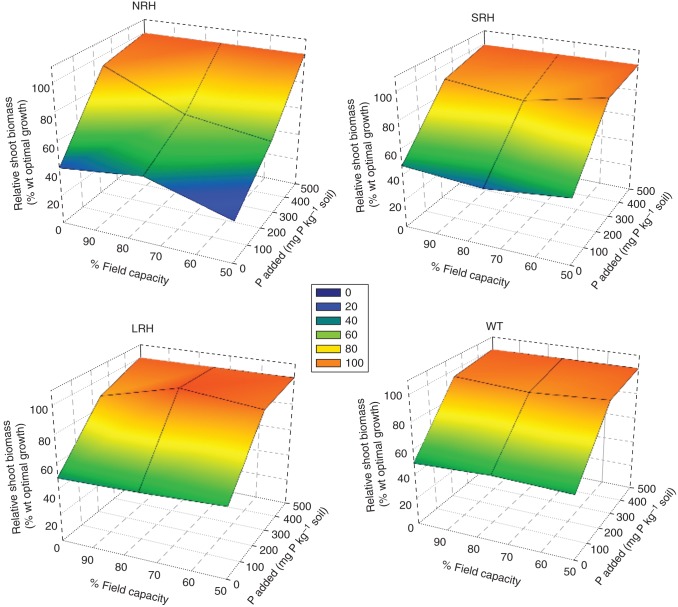
When grown under a range of treatments that combined P and water deficit, all genotypes responded (P < 0·001) positively to the addition of P and on average grew 2·1-fold bigger under P-sufficient conditions compared with P-deficient conditions (Fig. 5). Likewise, all genotypes responded positively (P < 0·001) to increasing water availability and grew on average 2·2-fold bigger at 100 % FC compared with those grown at 50 % FC.
Fig. 5.
Response planes of relative shoot biomass production (relative to optimal P condition) against combined water and phosphorus stress. Five replicates of plants including no root hair (NRH), short root hair (SRH), long root hair (LRH) and wild-type (WT) genotypes of barley were grown for 8 weeks in soils under controlled conditions before harvest and analysis. Soil treatments included three phosphorus additions 0, 250 and 500 mg P kg−1 soil and three soil water regimes 50, 75 and 100 % field capacity and all combinations of the two treatments.
All genotypes responded positively (P < 0·001) to the combined increase of P and water (Fig. 5). In all P treatments, biomass increased by 1·7-fold between 50 and 75 % FC and 2·2- to 2·3-fold between 50 and 100 % FC. In all water availability treatments, biomass increased by 1·8- to 1·9-fold between and P0 and P250 and 2·1- to 2·2-fold between P0 and P500. Where both P and water availability increased, biomass was bigger by between 3·1- and 4·7-fold (Fig. 5). It was apparent that the impact of increasing water availability and P availability separately was similar, while the impact of providing the two resources in combination was much greater.
Genotypes with root hairs (SRH, LRH and WT) responded to increasing availability of P and water combined by increasing biomass by between 2·5- and 4·9-fold. In comparison, genotypes without root hairs (NRH) increased biomass to a much greater extent, by between 4·1- and 8·6-fold. Root hairless mutants are much more strongly affected by combined stress than those with root hairs (Fig. 5). NRH genotypes under the most extreme combined P and water deficit (P0 and 50 % FC) treatment were at least 1·5- and 2·3-fold smaller than those genotypes with root hairs (LRH, SRH and WT) under the same conditions, achieving only 30 % of the growth of the NRH genotype under P-sufficient conditions (P500 and 100 % FC) in comparison with the 50–53 % comparable growth achieved by the genotypes with root hairs. There were no significant differences between the genotypes with root hairs in their response to combined P and water deficit.
DISCUSSION
Root hairs are thought to play an important role in P acquisition by plants (Barley and Rovira, 1970), and the importance of root hair length for P acquisition has previously been established (Gahoonia et al., 2001). With large amounts of added P fertilizer required to achieve maximum growth by barley (George et al., 2011), the identification of genotypes that can access P in P-limited environments is of importance to improving the long-term sustainability of agriculture. Here we use mutants to identify the impacts of root hair length on P-limited yield in combination with drought stress, an approach which minimizes many of the confounding factors seen in other studies.
Through the use of a phenotypic screen of such a mutant barley population, we tested the hypothesis that genotypes exhibiting LRH could have an advantage over those with SRH and NRH with respect to P acquisition and, ultimately, yield. A selection of nine genotypes exhibiting three root hair phenotypes was made, and the root hair characteristics identified in the initial screen proved to be robust when plants were grown in soil. Average root hair length data confirmed the NRH phenotypes to be hairless, while the SRH and LRH phenotypes remained differentiated. Root hair lengths measured here were comparable with others measured in barley (Gahoonia and Nielsen, 2004), but it is noteworthy that in other species, such as maize, root hairs can be significantly longer (Zhu et al., 2010). One of the NRH genotypes, however, displayed small bulges (Ishida et al., 2008) instead of hairs in the P-deficient treatment only, perhaps indicating an upregulation of P acquisition mechanisms without any visible root hair formation. This particular mutant merits further investigation of its potential to acquire essential nutrients and its response to P-deficient conditions. Bulges are unable to increase the surface area of the plant root system to the same extent as fully developed root hairs, but could enhance P acquisition through other mechanisms associated with root hair development.
Root hair length was typically longer in P-deficient treatments for all phenotypes with root hairs present; consistent with the findings of Bates and Lynch (1996), root hair length was highly regulated by P availability. The strong positive correlation of rhizosheath weight with root hair length is of potential significance to nutrient acquisition and in particular P uptake, since it defines the interface between the soil and root. Rhizosheaths are formed as a result of a proliferation of root hairs that enmesh soil particles to form cylinders that remain attached to the root when it is removed from the surrounding soil (Bristow et al., 1985). They are permeated by root hairs, with the thickness of the sheath being defined by the length of the root hairs. Rhizosheaths were first thought to be special features of desert grasses, but also form on many mesophytic grasses, including all soil-grown cereals, maize and sorghum. Their development in relation to root development has been studied most extensively in the mesophytic grasses (McCully, 1999). The importance of root hairs in rhizosheath development has been established previously (Moreno-Espindola et al., 2007) where the relative importance of root hairs vs. fungal hyphae in the binding of sand particles was compared. Root hairs were found to be the main mechanism in rhizosheath formation. In our study, the rhizosheath mass of the NRH phenotypes was negligible in comparison with those formed in the presence of root hairs, with implications for P acquisition and yield resulting from the increased surface area of the root coming into contact with the rhizosphere.
Phosphorus accumulation increased significantly with root hair length, with LRH phenotypes accumulating almost 50 % more P under the P-deficient conditions than NRH phenotypes. This is consistent with the findings of other research (Gahoonia et al., 2001) which compared the brb (bald root barley) with the WT Pallas with respect to rhizosphere P depletion and plant P accumulation, and subsequent research (Gahoonia and Nielsen, 2004), which compared P accumulation of different barley genotypes with varied root hair lengths under low P field conditions. Indeed our shoot biomass data are further corroborated by the findings of Gahoonia et al. (2001), with significant increases in shoot biomass being consistent with increases in root hair length. Grain weight as a measure of yield produced some results contrasting with previous findings (Gahoonia and Nielsen, 2004), with our data suggesting that while the presence of root hairs is important to yield, the length of the root hairs did not have a significant impact. In contrast, the earlier research demonstrated that LRH genotypes maintained yield under P-deficient conditions whereas SRH genotypes did not (Gahoonia and Nielsen, 2004). Closer analysis of the data of Gahoonia and Nielsen (2004), however, reveals our data to be consistent with theirs. Both P accumulation and yield data were consistent when comparing genotypes with similar root hair length in both experiments. Genotypes described by Gahoonia and Nielsen (2004) with the equivalent root hair lengths to our SRH and LRH genotypes also had no significant differences in yield under low P conditions. The wider range of root hair lengths used in their study helps explain the observation of improved yield with increasing root hair length. It is possible, however, that the use of cultivars to compare the effects of root hair length on yield by Gahoonia and Nielsen (2004) may have compromised the results due to the lack of genetic similarity between the cultivar genotypes. Evidence for this can be seen when comparing cultivars with the same root hair length which have different responses in yield in the same P-deficient environments (Gahoonia and Nielsen, 2004). In our study the use of mutants reduces the likelihood of such pleiotropic effects.
In general, plants produced more tillers and greater yield under P-sufficient conditions and in genotypes which had root hairs. One exception was the LRH genotype which produced similar yield to the NRH genotype. As a measure of relative agronomic effectiveness, the relative yield of the contrasting root hair length phenotypes over the two P treatments confirms the importance of root hairs in enhancing yield. This is in contrast to an analysis of mutants in maize (Wen and Schnable, 1994) which suggested that the presence of root hairs was not important. Moreover, our data also imply no increased benefit to yield between SRH and LRH phenotypes, i.e. phenotypes with SRH yield equally as well as those with LRH, despite there being a phenotypic effect of P accumulation. This does not appear to be due to a lack of biomass response in the LRH mutants related to luxury accumulation of P, as P concentrations are in the range that would be considered sufficient, not luxuriant (Table 2). Therefore, it may be considered that this could be a consequence of a greater metabolic cost incurred by the plant in producing longer root hairs, implying that while longer root hairs allow more efficient P accumulation, the benefits may be offset by the increased metabolic cost of achieving that extra length. Cost–benefit analyses of carbon respired for P acquisition (Bates and Lynch, 2000; Zhu et al., 2010) have suggested that long root hair mutants in A. thaliana and Zea mays benefited from increased P acquisition in response to P deficit. The A. thaliana study does not look at any implications for yield, nor does it account for the impact of mycorrhizal associations due to this species being non-mycorrhizal. Barley on the other hand has the ability to form complex symbiotic associations with micro-organisms, in particular mycorrhizas (Baon et al., 1993), and this can make a vital contribution to P acquisition particularly in P-limited environments (Clark and Zeto, 2000; Smith and Smith, 2011). This experiment did not measure mycorrhizal infection and, as such, the possible compensatory role of these relationships with respect to P uptake, as demonstrated by others (Schweiger et al., 1995; Jakobsen et al., 2005), cannot be ruled out. It is possible that increased mycorrhizal activity in the NRH mutants may compensate for a lack of hairs and reduced root–soil interface, and this relationship between plant, fungi and root hairs is the subject of ongoing research (L. K. Brown, T. S. George, G. E. Barrett, S. Mclaren, S. F. Hubbard and P. J. White, unpubl. res.).
Grain yield for the WT did not differ between the two P treatments, showing this genotype to be very efficient in P-deficient environments. This evidence suggests that the WT possesses traits that may have been altered or lost in the mutation process to the detriment of the mutants in their ability to acquire and conserve P under P-limited conditions. The results suggest that the WT already has efficient mechanisms which enable it to maintain yield under P-deficient conditions and further suggest that mutation may not be the best approach to identify more efficient genotypes with enhanced root hair phenotypes. While artificially mutated populations provide a valuable resource of genetic material for screening for certain phenotypic traits, the effects of other possible mutations on plant functionality are not always visible and remain unknown.
The importance of root hair length cannot be studied in isolation. Other root characteristics such as root hair density, mycorrhizal associations and total root length will also have an important role to play in determining the ability of a plant to acquire P and are currently being investigated. In this experiment, we have noted variation in total root length associated with the experimental treatments. It was apparent that plants under P-deficient conditions had longer heavier root systems. In addition, it was noted that the WT plants had significantly different root lengths and produced more heads than all the mutants. Our interpretation of this is that the WT is a poor control for this experiment due to it not having been through the mutational process in tandem with other genotypes. Of potential importance was the fact that the NRH and SRH mutant genotypes had longer total root length than the LRH mutants (Table 1), suggesting that genotypes with shorter or absent root hairs have compensated for this by producing longer root systems; this could, however, also be explained by the impact of a secondary mutation in these genotypes.
When challenged with a combination of P deficit and drought stress, the genotype with no root hairs showed a severe limitation to plant growth not seen in the genotypes with root hairs (Fig. 5). It is therefore apparent that the NRH genotypes suffered the greatest stress under these conditions, and this was almost completely ameliorated by the presence of root hairs of any length. If this is the case, then our data provide good evidence that the function of the root hairs, not just increased length, is particularly important in coping with extreme combined water and P stress. A likely explanation would be a combination of reduced surface area of the hairless roots and the increased tortuosity of the diffusional path in dry soils, both of which would greatly increase the time taken for resupply of P to the root surface for uptake by diffusion, and therefore limit P uptake.
In summary, we have used genotypes from a mutant population demonstrating phenotypic variation in root traits associated with resource capture to investigate the importance of root hair length to yield. The initial in vitro screen proved to be robust when plants were grown in soil. Root hair length was shown to be important in the acquisition of P in P-limited conditions, with LRH phenotypes accumulating significantly more P than those with SRH or NRH. However, there is a possibility of a metabolic cost to yield for LRH phenotypes, in that LRH phenotypes did not outperform SRH in yield. Moreover, it was demonstrated that the presence of root hairs of whatever length was important for tolerating severe stress caused by combined P and water deficit. Overall, the results suggest that although root hair length is not important for maintaining yield, the presence of root hairs is implicit to sustainable yield of barley under P-deficient conditions and where this stress is combined with drought. It is therefore imperative that the root hair trait be maintained in barley when breeding new cultivars, in order to ensure an ability to cope with increasing environmental stress and variability in future agro-environments.
ACKNOWLEDGEMENTS
This work was supported by various funding streams, as part of an MSc supported by the Scottish Crop Research Institute (now JHI) and the University of Dundee (L.K.B.) and under the auspices of the Royal Society of Edinburgh, Personal Research Fellowship (T.S.G.). The identification of barley mutants was supported by IAEA Technical Contract 113618/RO. Other aspects of the research were funded by the Scottish Government Work package 1·7 ‘Profitable and sustainable agriculture’.
LITERATURE CITED
- Baon JB, Smith SE, Alston AM. Mycorrhizal responses of barley cultivars differing in P-efficiency. Plant and Soil. 1993a;157:97–105. [Google Scholar]
- Barley KP, Rovira AD. The influence of root hairs on the uptake of phosphate. Communications in Soil Science and Plant Analysis. 1970;1:287–292. [Google Scholar]
- Bates TR, Lynch JP. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell and Environment. 1996;19:529–538. [Google Scholar]
- Bates TR, Lynch JP. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. American Journal of Botany. 2000;87:964–970. [PubMed] [Google Scholar]
- Bristow CE, Campbell GS, Wullstein LH, Neilson R. Water-uptake and storage by rhizosheaths of Oryzopsis hymenoides – a numeriacal-simulation. Physiologia Plantarum. 1985;65:228–232. [Google Scholar]
- Caldwell DG, McCallum N, Shaw P, Muehlbauer GJ, Marshall DF, Waugh R. A structured mutant population for forward and reverse genetics in barley (Hordeum vulgare L.) The Plant Journal. 2004;40:143–150. doi: 10.1111/j.1365-313X.2004.02190.x. [DOI] [PubMed] [Google Scholar]
- Clark RB, Zeto SK. Mineral acquisition by arbuscular mycorrhizal plants. Journal of Plant Nutrition. 2000;23:867–902. [Google Scholar]
- Dolan L. The role of ethylene in root hair growth in Arabidopsis. Journal of Plant Nutrition and Soil Science-Zeitschrift für Pflanzenernahrung und Bodenkunde. 2001;164:141–145. [Google Scholar]
- Gahoonia TS, Nielsen NE. Direct evidence on participation of root hairs in phosphorus 32P uptake from soil. Plant and Soil. 1998;198:147–152. [Google Scholar]
- Gahoonia TS, Nielsen NE. Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant and Soil. 2004;262:55–62. [Google Scholar]
- Gahoonia TS, Nielsen NE, Joshi PA, Jahoor A. A root hairless barley mutant for elucidating genetic of root hairs and phosphorus uptake. Plant and Soil. 2001;235:211–219. [Google Scholar]
- George TS, Brown LK, Newton AC, et al. Impact of soil tillage on the robustness of the genetic component of variation in phosphorus (P) use effeciency in barley (Hordeum vulgare L.) Plant and Soil. 2011;339:113–123. [Google Scholar]
- Gilbert N. The disappearing nutrient. Nature. 2009;461:716–714. doi: 10.1038/461716a. [DOI] [PubMed] [Google Scholar]
- Gregory PJ, Bengough AG, Grinev D, Schmidt S, Thomas WTB, Wojciechowski T, Young IM. Root phenomics of crops: opportunities and challenges. Functional Plant Biology. 2009;36:922–929. doi: 10.1071/FP09150. [DOI] [PubMed] [Google Scholar]
- Haling RE, Simpson RJ, Delhaize E, Hocking PJ, Richardson AE. Effect of lime on root growth, morphology and the rhizosheath of cereal seedlings growing in an acid soil. Plant and Soil. 2010;327:199–212. [Google Scholar]
- Hammond JP, Bennett MJ, Bowen HC, et al. Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiology. 2003;132:578–596. doi: 10.1104/pp.103.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving GCJ, McLaughlin MJ. A rapid and simple field-test for phosphorus in Olsen and Bray No 1 extracts of soil. Communications in Soil Science and Plant Analysis. 1990;21:2245–2255. [Google Scholar]
- Ishida T, Kurata T, Okada K, Wada T. A genetic regulatory network in the development of trichomes and root hairs. Annual Review of Plant Biology. 2008;59:365–386. doi: 10.1146/annurev.arplant.59.032607.092949. [DOI] [PubMed] [Google Scholar]
- Jakobsen I, Chen BD, Munkvold L, Lundsgaard T, Zhu YG. Contrasting phosphate acquisition of mycorrhizal fungi with that of root hairs using the root hairless barley mutant. Plant, Cell and Environment. 2005;28:928–938. [Google Scholar]
- Jungk A. Root hairs and the acquisition of plant nutrients from soil. Journal of Plant Nutrition and Soil Science-Zeitschrift für Pflanzenernahrung und Bodenkunde. 2001;164:121–129. [Google Scholar]
- Lynch JP. Roots of the second green revolution. Australian Journal of Botany. 2007;55:493–512. [Google Scholar]
- Ma Z, Bielenberg DG, Brown KM, Lynch JP. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant, Cell and Environment. 2001;24:459–467. [Google Scholar]
- McCully ME. Roots in soil: unearthing the complexities of roots and their rhizospheres. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:695–718. doi: 10.1146/annurev.arplant.50.1.695. [DOI] [PubMed] [Google Scholar]
- Morcuende R, Bari R, Gibon Y, et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant, Cell and Environment. 2007;30:85–112. doi: 10.1111/j.1365-3040.2006.01608.x. [DOI] [PubMed] [Google Scholar]
- Moreno-Espindola IP, Rivera-Becerril F, Ferrara-Guerrero MD, De Leon-Gonzalez F. Role of root-hairs and hyphae in adhesion of sand particles. Soil Biology and Biochemistry. 2007;39:2520–2526. [Google Scholar]
- Mudge SR, Rae AL, Diatloff E, Smith FW. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. The Plant Journal. 2002;31:341–353. doi: 10.1046/j.1365-313x.2002.01356.x. [DOI] [PubMed] [Google Scholar]
- Peterson RL, Farquhar ML. Root hairs: specialized tubular cells extending root surfaces. Botanical Review. 1996;62:1–40. [Google Scholar]
- Raghothama KG. Phosphorus and plant nutrition: an overview. 2005:355–378. Phosphorus: agriculture and the environment. American Society of Agronomy–Crop Science Society of America and Soil Science Society of America Monograph. [Google Scholar]
- Schweiger PF, Robson AD, Barrow NJ. Root hair length determines beneficial effect of a glomus species on shoot growth of some pasture species. New Phytologist. 1995;131:247–254. [Google Scholar]
- Smith FA, Smith SE. What is the significance of the arbuscular mycorrhizal colonisation of many economically important crop plants? Plant and Soil. 2011;348:63–79. [Google Scholar]
- Tiessen H. Phosphorus in the global environment. In: White PJ, Hammond JP, editors. The ecophysiology of plant–phosphorus interactions. Dordrecht, The Netherlands: Springer; 2008. pp. 1–7. [Google Scholar]
- Tottman DR. The decimal code for the growth-stages of cereals, with illustrations. Annals of Applied Biology. 1987;110:441–454. [Google Scholar]
- Wen TJ, Schnable PS. Analyses of mutants of three genes that influence root hair develpoment in Zea-mays (Gramineae) suggest that root hairs are dispensable. American Journal of Botany. 1994;81:833–842. [Google Scholar]
- White PJ, Hammond JP. Phosphorus nutrition of terrestrial plants. In: White PJ, Hammond JP, editors. The ecophysiology of plant–phosphorus interactions. Dordrecht, The Netherlands: Springer; 2008. pp. 51–81. [Google Scholar]
- White PJ, Bengough AG, Bingham IJ, George TS, Karley AJ, Valentine TA. Induced mutations affecting root architecture and mineral acquisition in barley. In: Shu QY, editor. Induced plant mutations in the genomics era. Rome: Food and Agriculture Organization of the United Nations; 2009. pp. 338–340. [Google Scholar]
- Wu P, Ma LG, Hou XL, Wang MY, Wu YR, Liu FY, Deng XW. Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiology. 2003;132:1260–1271. doi: 10.1104/pp.103.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JM, Zhang CC, Lynch JP. The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Functional Plant Biology. 2010;37:313–322. [Google Scholar]