Abstract
Background and Scope
Plant responses to the toxic effects of soil contaminants, such as excess metals or organic substances, have been studied mainly at physiological, biochemical and molecular levels, but the influence on root system architecture has received little attention. Nevertheless, the precise position, morphology and extent of roots can influence contaminant uptake. Here, data are discussed that aim to increase the molecular and ecological understanding of the influence of contaminants on root system architecture. Furthermore, the potential of plant-associated bacteria to influence root growth by their growth-promoting and stress-relieving capacities is explored.
Methods
Root growth parameters of Arabidopsis thaliana seedlings grown in vertical agar plates are quantified. Mutants are used in a reverse genetics approach to identify molecular components underlying quantitative changes in root architecture after exposure to excess cadmium, copper or zinc. Plant-associated bacteria are isolated from contaminated environments, genotypically and phenotypically characterized, and used to test plant root growth improvement in the presence of contaminants.
Key Results
The molecular determinants of primary root growth inhibition and effects on lateral root density by cadmium were identified. A vertical split-root system revealed local effects of cadmium and copper on root development. However, systemic effects of zinc exposure on root growth reduced both the avoidance of contaminated areas and colonization of non-contaminated areas. The potential for growth promotion and contaminant degradation of plant-associated bacteria was demonstrated by improved root growth of inoculated plants exposed to 2,4-di-nitro-toluene (DNT) or cadmium.
Conclusions
Knowledge concerning the specific influence of different contaminants on root system architecture and the molecular mechanisms by which this is achieved can be combined with the exploitation of plant-associated bacteria to influence root development and increase plant stress tolerance, which should lead to more optimal root systems for application in phytoremediation or safer biomass production.
Keywords: Abiotic stress; metals; organic contaminant; plant-associated bacteria; cadmium; copper; zinc; ‘2,4-DNT’; root morphology; endophyte; green revolution; systemic response
INTRODUCTION
Optimization of plant growth is required to ensure food and feed supply, and to respond to the need for biomass for renewable energy production and industrial feedstock applications (Weyens et al., 2009a, b; Vangronsveld et al., 2009). However, plant productivity is greatly affected by environmental stresses such as drought and nutrient-deficiency (de Dorlodot et al., 2007; Den Herder et al., 2010). Plant roots have recently gained attention in strategies for improving plant growth and yield. In the so-called ‘second green revolution’, roots are considered as mediators of increased nutrient uptake efficiency and drought tolerance, which is essential as water and nutrients will become increasingly limiting in the future (Lynch, 2007; Den Herder et al., 2010; Ghanem et al., 2011; Hinsinger et al., 2011). Another problem is that food supply is endangered due to the use of large areas of agricultural land for the production of energy biomass (Weyens et al., 2009a; Den Herder et al., 2010). Indeed, it is estimated that agricultural land in the European Union devoted to biomass production for biofuel/energy use will increase to 190 000 km2 by 2030 (European Biofuels Technology Platform 2010, Innovation driving a sustainable biofuel industry, http://www.biofuelstp.eu/). With increased nutrient uptake efficiency and drought or salt tolerance, marginal lands [abandoned farmland, degraded (saline, arid, etc.) and (diffusely) contaminated land, excluding conservation areas] may be taken into use to counter this problem (Weyens et al., 2009a, b). Contaminated lands may be unsuitable for food production due to possible accumulation of toxic substances in the food chain, but taking moderately contaminated land into use for non-food biomass production may further relieve the problem, although obviously plant growth would be affected due to toxicity of the contaminants. There are up to 3 million potentially contaminated sites in the EU-15 countries and at least 250 000 sites require urgent remedial action (Bardos et al., 2008). Moreover, diffuse contamination affects large areas across the world, mainly as a result of mining activity, fallout from industrial processes such as smelting, agriculture, traffic, areas elevated with contaminated dredged sediments, former landfill sites and abandoned industrial sites. Re-using such contaminated land for non-food crops during and after soil (phyto)remediation could bring them back into beneficial and sustainable use and reduce detrimental environmental, social and economic impacts on affected communities (Mench et al., 2009; Vangronsveld et al., 2009). Research towards a better understanding and improving plant growth in the presence of contaminants could contribute to future use of these contaminated areas for safe and efficient biomass production. Plant growth and stress tolerance need to be improved to enhance biomass production. Uptake, translocation, sequestration and possible degradation of the contaminants need to be controlled according to the application of the biomass produced. For safe biomass production on moderately contaminated lands, minimum uptake and influence on biomass quality parameters is desirable (Shute and Macfie, 2006). For phytoremediation purposes, maximal uptake, translocation and, if possible, breakdown of the products is desired, while maintaining minimal biomass quality for downstream valorization (Vangronsveld et al., 2009).
Root system architecture is a major factor that can be influenced to increase tolerance to abiotic stresses such as nutrient-deficiency, drought and salinity (de Dorlodot et al., 2007; Den Herder et al., 2010; Galvan-Ampudia and Testerink, 2011; Ghanem et al., 2011; Hinsinger et al., 2011). Indeed, as roots are the uptake system for water and nutrients, sensing of the local environment and root growth in the vicinity of essential resources are essential. An example of the intimate link between transport systems and root development is functioning of the AtNRT1·1 transporter in both nitrate uptake and in a nitrate-sensing mechanism that directs root growth towards local nitrate enrichments (Remans et al., 2006; Krouk et al., 2010). Root growth in response to salt stress has been well studied (Galvan-Ampudia and Testerink, 2011), but the role of root system architecture to abiotic stresses related to excess metals or organic substances has not been investigated. Yet, root transporter proteins unavoidably take up excess essential elements, as well as toxic non-essential elements via the transport systems for essential elements (Palmer and Guerinot, 2009; Verbruggen et al., 2009). A stress-induced morphogenic response, consisting of reduced primary root elongation and increased lateral root density, has been observed in plants exposed to a number of abiotic stress conditions, for example excess metals, and it was postulated that this response redirects plant growth to diminish stress exposure (Potters et al., 2007). Therefore, these kinds of morphogenic responses should be considered as a determining factor in the avoidance of contaminated soil patches. However, beyond the avoidance mechanism, the ability to colonize non-contaminated areas when root systems are partly exposed to contaminants needs to be evaluated. It can be assumed that the combination of avoidance and colonization would enable the plant to grow and produce safe biomass. On the other hand, for phytoremediation purposes, positioning of roots in contaminated zones is desirable. Much research has been dedicated to identify molecular and physiological traits of metal uptake and sequestration in hyperaccumulators, with the aim to transfer these traits to high-biomass plants in phytoremediation strategies (Hassan and Aarts, 2011). Some hyperaccumulators are also able to direct root growth towards metals in the soil (Whiting et al., 2000; Alford et al., 2010; Liu et al., 2010). However, with regard to non-accumulators, only a limited number of studies have dealt with the influence of metals on root system architecture, and the underlying mechanisms remain largely unexplored (Lequeux et al., 2010; Petó et al., 2011; Zhao et al., 2011). Symbiosis between mycorrhizal fungi and plants has been shown to alleviate the adverse effects on plant growth by stress factors in the soil, such as excess metals (reviewed by Miransari, 2010). Here, we focus on non-mycorrhizal plants, with Arabidopsis thaliana as a model, for understanding root development in the presence of contaminants.
Root system architecture has been intensively studied in A. thaliana. Its rather small root system is easy to quantify, and the relatively early availability of genome information and annotation, and development of molecular tools has yielded fundamental insight into root development in this species (Malamy, 2005; Péret et al., 2009; Baluška et al., 2010; Den Herder et al., 2010). Because of the vast knowledge on intrinsic root developmental programmes, A. thaliana also remains an excellent model to study environmental influences on the basic root developmental pathways (Remans et al., 2006; Pérez-Torres et al., 2008; Nibau et al., 2008; Monshausen and Gilroy, 2009; Den Herder et al., 2010; Krouk et al., 2010). Although it cannot be considered a general rule that selected A. thaliana genes have a similar effect in other species, examples exist of genes selected for positive yield effects in A. thaliana (e.g. one promoting root growth, Park et al., 2005) that have a similar effect in other species (Gonzalez et al., 2009).
Beneficial plant-associated bacteria can play a key role in supporting and/or enhancing plant health and growth (Zhuang et al., 2007; Taghavi et al., 2009; van der Lelie et al., 2009; Weyens et al., 2009a, b; Yang et al., 2009; Francis et al., 2010). The presence and activity of micro-organisms in the rhizosphere can have a major effect on the nutrient uptake efficiency and stress tolerance of the plant (Weyens et al., 2009a, b; Ghanem et al., 2011). Plant growth-promoting (PGP) activity can be caused by direct or indirect mechanisms. Direct PGP mechanisms may involve nitrogen fixation by diazotrophs (both rhizospheric and endophytic), increased availability of highly unavailable nutrients such as phosphorus, iron and other mineral nutrients, production of plant growth regulators such as auxins, cytokinins and gibberelines, and suppression of ethylene production by 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity (Bloemberg and Lugtenberg, 2001; Cocking, 2003; Ryu et al., 2005; Mantelin et al., 2006; Contesto et al., 2008; Onofre-Lemus et al., 2009; Weyens et al., 2009a; Rashid et al., 2012; Gamalero and Glick, 2012). Plant-associated bacteria can indirectly benefit the growth of their host plant by preventing the growth or activity of plant pathogens through competition for space and nutrients, antibiosis, production of hydrolytic enzymes, inhibition of pathogen-produced enzymes or toxins, and induction of plant defence mechanisms (Selosse et al., 2004; Raaijmakers et al., 2009). Plant-associated bacteria can thus be very important in enabling plants to establish or to grow better on marginal and contaminated land, and could contribute to more economic and environmentally friendly production of biomass (Mastretta et al., 2006; Saleem et al., 2007; Zhuang et al., 2007; Weyens et al., 2009b).
Here, experiments aimed to increase our molecular and ecological understanding of changes in root system architecture in the presence of excess metals are discussed. Examples demonstrate the potential of plant-associated bacteria to positively influence root growth and to alleviate the negative effects of excess metals or organic contaminants. A large amount of molecular data on root development under normal conditions and stress conditions exists for A. thaliana. A fundamental part of our research is aimed at identifying interference of contaminants with these known molecular parameters of root development. In this regard, mutants for genes potentially involved can be selected from the large collection of available mutants of A. thaliana. This area of research will yield fundamental knowledge concerning the environmental impacts on root development, and can be transferred to strategies for optimization of plant growth in the presence of contaminants using economically interesting plants.
UNDERSTANDING ROOT DEVELOPMENT IN THE PRESENCE OF CONTAMINANTS
It was postulated that different abiotic stresses trigger similar morphological outcomes, and that at least some of the underlying molecular determinants are interchangeable between stress conditions (Potters et al., 2009). The underlying determinants of the stress-induced morphogenic responses (SIMRs) would be related to altered organismal gradients of plant hormones (e.g. auxin and ethylene), reactive oxygen species (ROS) and antioxidants, which at the cellular level result in altered cell division, elongation and/or differentiation (Potters et al., 2009; Ghanem et al., 2011). The implication of the above postulation is that, if any stress-specific perception and/or signalling exists, this would at some point be integrated into the general pathway that triggers the SIMRs causing reduced primary root growth and increased density of lateral roots. Description of the similarities between SIMRs caused by different stress conditions is useful for determining downstream events leading to similar changes in morphogenesis. These events are often linked to the influence on intrinsic root developmental programmes. For example, similar changes in auxin gradients have been revealed for a number of stress conditions that are linked to the same morphogenic response (Potters et al., 2007). Auxin is a key hormone that is part of intrinsic root developmental pathways (Nibau et al., 2008; Fukaki and Tasaka, 2009; Hodge, 2009) and it is therefore not surprising that changes in root architecture are likely to be connected to changes in auxin gradients and sensitivity.
However, much remains to be learned about more upstream pathways of perception and signalling of the stress factor and the actual interference mechanisms with the general intrinsic root developmental pathways. Furthermore, the description of similarities between SIMRs has often been limited to primary root growth inhibition and increases in lateral root densities in juvenile plants (Potters et al., 2007), but lateral root outgrowth has received little attention, although it is a major component defining root system architecture. Here, we investigate the following questions relating to root development in the presence of contaminants: (1) What are the upstream components that lead to changes in intrinsic root development programmes? (2) What is the mechanism of interference with intrinsic root developmental pathways? (3) To what extent are these components stress-specific and do they lead to stress-specific changes in root system architecture?
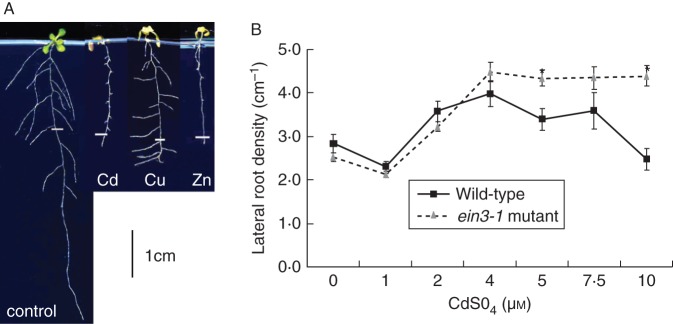
We elaborate on possible experimental procedures that can be used to solve these questions, in particular using A. thaliana in a vertical agar plate system. A. thaliana has a relatively simple root system for which root growth parameters can be easily quantified, and a number of programmes for semi-automatic analysis of root systems have been developed (Armengaud et al., 2009; Lobet et al., 2011). Furthermore, a large amount of knowledge on intrinsic root development is available, which is essential to our aim of connecting environmental triggers to the intrinsic pathways. As abiotic stress factors, we apply toxic amounts of cadmium (Cd), copper (Cu) and zinc (Zn) ions (as sulphate salts) to the roots and measure morphological responses of wild-type plants to describe the stress-specificity of the responses, and of selected mutants to discover underlying mechanisms in a reverse genetics approach. These three metals have different properties in plants: Cd is non-essential and non-redox active, Zn is essential but not redox-active, and Cu is essential and redox-active. Nevertheless, the three metals all lead to oxidative stress, but the underlying mechanisms of oxidative stress generation are different, with Cu being able to trigger direct production of ROS via Fenton-reactions, and Cd and Zn indirectly via inhibition of anti-oxidative defence, electron transport chains, or stimulation of pro-oxidative activities such as NADPH oxidase or lipoxygenase (Drazkiewicz et al., 2004; Broadley et al., 2007; Burkhead et al., 2009; Cuypers et al., 2009, 2011; Smeets et al., 2009; Remans et al., 2010). By comparing the effect of metals with different properties we aimed to increase the likelihood of encountering stress-specific effects. As such, we observed that exposure of A. thaliana roots to excess Cd, Cu and Zn in a vertical agar plate system led to inhibition of primary root growth, and to metal-specific changes in lateral root development (see Fig. 2A; T. Remans, unpubl. res.). Cd and Cu caused an increased lateral root density (similar to described SIMRs) but the lateral root elongation was much less inhibited by Cu than by Cd at concentrations that caused similar inhibition of primary root growth. When Zn was applied at the same effect concentration, both lateral root density and lateral root elongation were negatively affected. Thus, these three metals caused three different morphological outcomes, which contradicts the postulation by Potters et al. (2009) that different stresses cause similar morphological outcomes. Experiments with these three metals are used here to unravel the molecular mechanisms behind the observed responses and to gain more ecologically relevant insight into the responses. This is achieved by using A. thaliana mutants in a reverse genetics approach, and examples are given of the involvement of a lipoxygenase gene and a gene involved in ethylene signalling in primary root growth inhibition and in the regulation of lateral root density, respectively, when plants are exposed to Cd.
Fig. 2.
(A) Seven-day-old A. thaliana plants, with primary root length equal to the white mark, were exposed for another 7 d to 5 µm CdSO4, 10 µm CuSO4 or 75 µm ZnSO4. At a similar inhibition of primary root growth, lateral root density was increased by Cd and Cu, but decreased by Zn exposure. Lateral root elongation was less affected by Cu than by Cd. The effect on lateral root density and elongation may affect the ability of the lateral root tips to reach non-contaminated zones, for which the response to Cu may be more optimal. (Note: image contrast was enhanced to visualize roots, and shoot colour may look artificial). (B) Lateral root density (base to apex) of A. thaliana wild-type and ein3-1 mutant seedlings (as indicated) after 7 d exposure on vertical agar plates to CdSO4. Significant genotype effects within treatments are indicated (*P < 0·05, non-parametric Kruskal–Wallis test, n = 6).
Discovering metal-specific molecular determinants of primary root growth inhibition
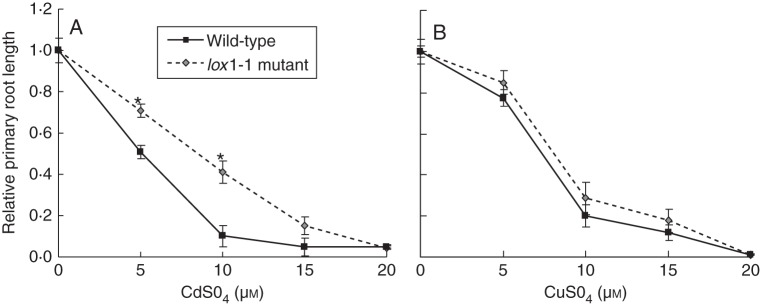
A first question is whether the primary root growth inhibition is determined by the same mechanisms, or whether metal-specific determinants contribute to the same outcome. Because exposure to excess Cd and Cu causes oxidative stress, we tested a number of mutants of genes involved in oxidative stress generation and oxidative signalling. These included NADPH oxidase mutants rbohC (Foreman et al., 2003), rbohD and rbohF (Torres et al., 2002), and lipoxygenase mutants lox1-1, lox5-2 (Vellosillo et al., 2007) and lox3D (Caldelari et al., 2011). Seedlings were germinated on control plates in a 50× diluted Gamborg's B5 nutrient background for 7 d; subsequently, a homogeneous set of plants was transferred to treatment plates covering a concentration range of Cd and Cu. Detailed methods for all original data are given in the Appendix. NADPH oxidase mutants rbohC, rbohD and rbohF did not show significantly altered sensitivity of primary root growth to Cd or Cu, and neither did lox3D or lox5-2 (data not shown). However, primary root growth of the lox1-1 mutant was less inhibited by moderate concentrations of Cd than wild-type plants, whereas no significant difference was observed for Cu (Fig. 1). This shows that the same morphological effect (primary root growth inhibition) can involve different stress-specific molecular determinants, which was shown here for the involvement of LIPOXYGENASE1 in Cd-induced primary root growth inhibition, but not in Cu-induced primary root growth inhibition. Two hypotheses can be posted concerning the possible metal-specificity of LOX1: (1) oxidative stress induces primary root growth inhibition (Potters et al., 2009) and this oxidative stress can be generated directly by Cu, but indirect oxidative stress generation by Cd needs LOX1; (2) alternatively, Cd-specific LOX1-derived oxylipin signalling may contribute to the onset of primary root growth inhibition. The fact that Cd and Cu both strongly induce LOX1 gene expression in roots (Remans et al., 2010; Cuypers et al., 2011) may favour the first hypothesis.
Fig. 1.
Primary root growth of A. thaliana wild-type and lox1-1 mutant seedlings (as indicated) during 7 d exposure on vertical agar plates to CdSO4 (A) or CuSO4 (B), expressed relative to the control (0 µm) for each genotype. Significant genotype effects within treatments are indicated (*P < 0·05, t-test; n = 20).
Molecular determinants of lateral root growth effects
Under abiotic stress conditions, lateral root development is also modified. Plants exposed to increasing CdSO4 concentrations showed an increased lateral root density that returned back to control levels at higher concentrations (Fig. 2B). In our reverse genetics approach, at higher Cd exposure concentrations, the increased lateral root density is maintained in the ein3-1 mutant (Solano et al., 1998), whereas it returns to normal levels in control plants (Fig. 2B). This suggests that ethylene signalling is involved in the decrease of lateral root outgrowth under high Cd exposure. Increasing the lateral root density would allow the plant to explore the soil more thoroughly for non-contaminated zones, but at high Cd concentrations the response would be lost again due to high toxicity levels or general growth inhibition, by which investment of the plant in lateral roots in these zones would be avoided. The upstream regulation of the EIN3 transcription factor and its downstream targets that regulate lateral root development when plants are exposed to Cd remain to be revealed to confirm the involvement of ethylene in this response. Further studies using a reverse genetics approach could reveal more factors involved in lateral root responses, and evaluation of selected mutants under multiple stress conditions could reveal stress-specific determinants. Focus should be given to both lateral root density (number of lateral roots) and lateral root outgrowth (elongation), as both factors determine root architecture and whether a plant will be able to colonize non-contaminated zones. Indeed, as illustrated in Fig. 2A, lateral root outgrowth may be crucial to reach non-contaminated zones. In this regard, the lateral root growth response observed for Cu may be more beneficial than the response to Cd or Zn. Clearly, determination of the molecular parameters underlying observed morphological responses is important to link environmental factors to intrinsic root developmental pathways. However, understanding root developmental responses is another aspect that deserves attention. The next section describes our experimental set-up aimed at deciphering responses in a more ecological context.
What more can the agar plate system reveal? Mimicking heterogeneous conditions to study avoidance and colonization responses
Identifying the molecular determinants of morphological responses is worthwhile for gaining fundamental knowledge on how environmental triggers interact with intrinsic root development pathways. However, focusing only on root growth effects in homogeneous conditions may not be sufficient to understand plant responses in a more ecological context. Root foraging responses to nutrients have been discovered in conditions of heterogeneous nutrient distribution (reviewed by Hodge, 2009) and has led to the identification of the molecular parameters involved (Nibau et al., 2008). By analogy, we applied heterogeneous growth conditions to study avoidance/colonization responses, i.e. the ability of the roots to avoid contaminated zones and at the same time to colonize non-contaminated zones. Stress avoidance only without efficient colonization could be insufficient for survival of the plant. We use a vertical split-root system to mimic heterogeneous growth conditions, by positioning 8-d-old seedlings such that the growing root tip experiences the opposite condition (contaminated or non-contaminated) than the rest of the plant (Fig. 3A). This allows us to interpret the responses in a context that is more ecologically relevant as it permits (1) distinguishing local and systemic effects, and (2) describing avoidance and colonization responses.
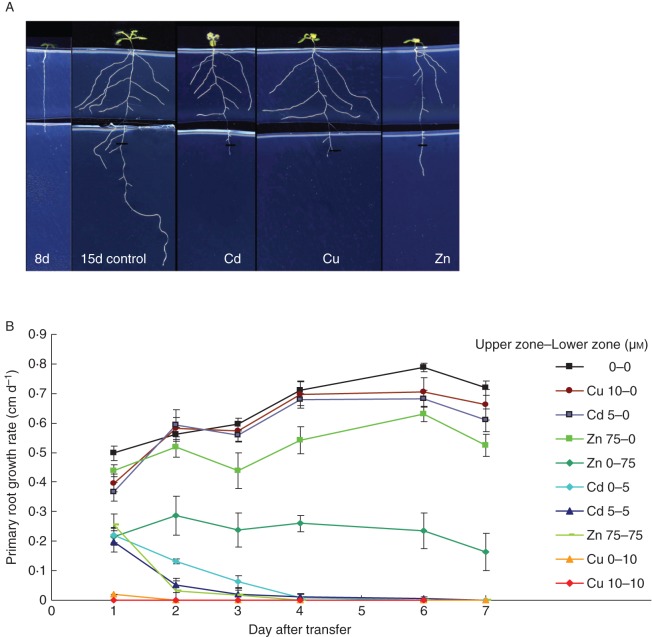
Fig. 3.
(A) In the vertical split root-system, 8-d-old seedlings grown under control conditions were positioned such that the primary root tip was exposed to a different treatment from the rest of the root system (control conditions have the same treatment in the upper and lower zone). Visualized here are plants exposed at the growing root tip only to 5 µm CdSO4, 10 µm CuSO4 or 75 µm ZnSO4. Lateral root growth in the upper non-contaminated zone is visually compromised by Zn exposure of the primary root tip, which is not the case for plants exposed to Cd or Cu at the primary root tip. (B) Primary root growth rates of A. thaliana wild-type seedlings 7 d after transfer of the seedlings to vertical split-root plates containing heterogeneous conditions of CdSO4, CuSO4 or ZnSO4. Indicated in the legend is the metal concentration (μm) in the top zone and the concentration in the bottom zone (n = 6–14).
The effect on primary root growth rate of heterogeneous application of Cd, Cu and Zn to different parts of the root system was studied (Fig. 3B). Homogeneous conditions where the full root system was exposed or not served as controls. The concentrations of the metals were chosen such that the homogeneous exposure had a similar inhibiting effect on primary root growth (Figs 3B and 4A). In heterogeneous conditions, exposure of the primary root tips only to 5 µm CdSO4 or 10 µm CuSO4 was sufficient to induce an avoidance response consisting of a complete inhibition of primary root growth that occurred between days 1 and 4 after transfer, depending on the metal and the exposure condition of the top zone (Fig. 3B). However, primary root tips exposed to 75 µm Zn did not stop growing when the rest of the plant experienced control conditions, but continued at a reduced but steady growth rate (Fig. 3B; Zn 0–75). This suggests that the avoidance mechanism induced by Zn is not as strong as that induced by Cd or Cu, something which was not found in homogeneous conditions as similar growth inhibition is observed for plants exposed to a homogenous medium containing 5 µm Cd or 75 µm Zn (Fig. 3B; Cd 5-5 and Zn 75-75). In the inverse exposure of only the top part of the root system to Cd or Cu, primary root growth in the non-contaminated zone was unaffected. However, the primary root of Zn-exposed plants grew more slowly (Fig. 3B, Zn 75-0), resulting 7 d after transfer in a small but statistically significant inhibitory effect (Fig. 4A; Zn 75-0). In summary, Cd and Cu exert a local effect on primary root growth, leading to maximal avoidance and colonization responses, whereas exposure of plants to Zn revealed systemic effects that lead to decreased avoidance and colonization responses.
Fig. 4.
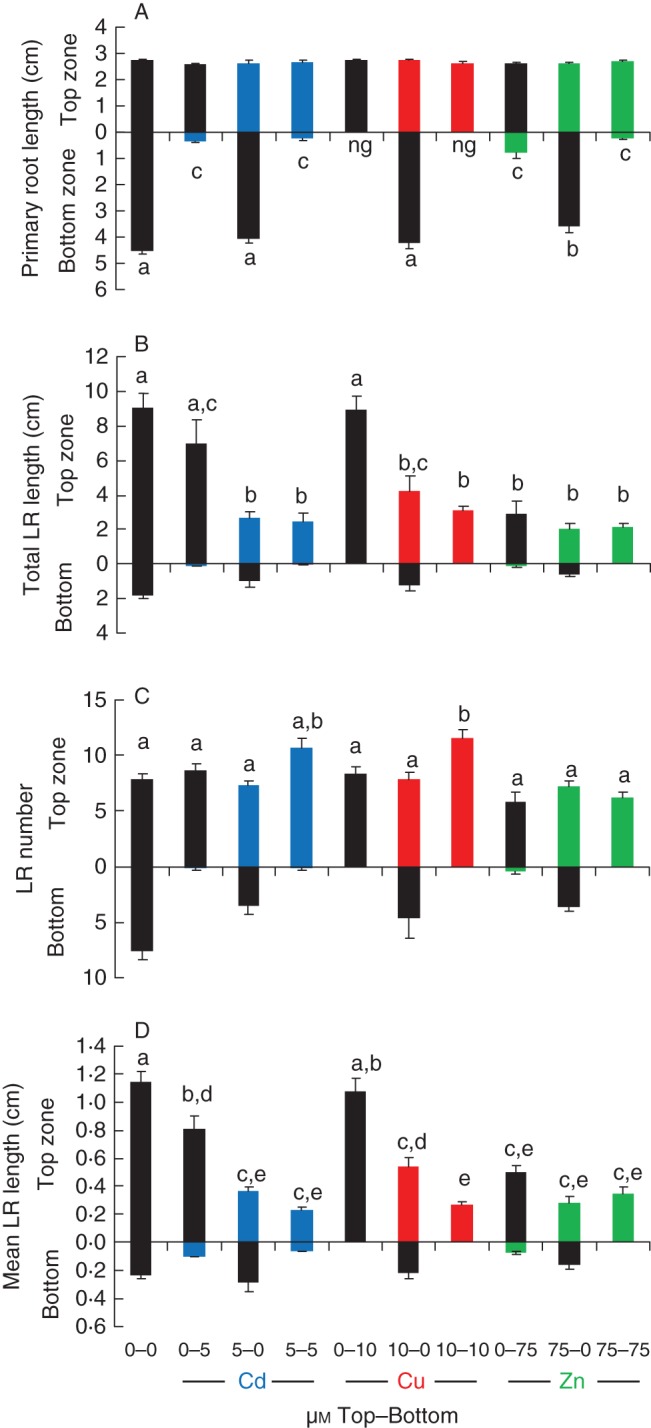
Primary root length (A), total lateral root (LR) length (B), number of laterals (C) and mean lateral root length (D) 7 d after transfer of 8-d-old A. thaliana seedlings to vertical split-root plates, in which the primary root tip was positioned in the bottom zone and the rest of the root system in the top zone. The x-axis values indicate the exposure concentrations (μm) as ‘top zone–bottom zone’. Plants were exposed to CdSO4, CuSO4 or ZnSO4, colour-coded on the figure; black indicates zones with zero exposure. Different letters indicate statistically significant differences (P < 0·05) after one-way ANOVA and Tukey correction (n = 6–14) (ng, no growth detected).
As mentioned above, another important parameter determining the root architectural responses are lateral roots. For those plants whose primary roots encounter contaminated areas, will the upper lateral roots be able to continue their colonization of the uncontaminated zone (Fig. 3A)? Total lateral root length in the uncontaminated upper zone when primary root tips encounter metal-contaminated areas seems unaffected by Cu, slightly affected by Cd and strongly affected by Zn (Fig. 3A). Indeed, quantitative data show that total lateral root length in the upper control zone was unaffected when primary root growth was inhibited by 10 µm Cu (Fig. 4B; Cu 0-10). For plants whose primary root tips were exposed to 5 µm Cd a small but statistically insignificant reduction was observed (Fig. 4B; Cd 0-5). However, for plants exposed to Zn, total lateral root length in the upper control zone was significantly reduced, to one-third of the control level (Fig. 4B; Zn 0-75). In fact, the total lateral root length in the upper zone was the same regardless of which part of the root system was exposed to Zn, suggesting that Zn exerts a complete systemic effect on total lateral root length (cf. Zn 0-75, 75-0 and 75-75; Fig. 4B). This systemic effect reduces the colonization response in the non-contaminated zone, something which was not observed for Cu and Cd.
Total lateral root length can be dissected into lateral root number and mean lateral root length to reveal which of these parameters is systemically affected by excess Zn. The design of these experiments does not allow us to study any inhibitory effects of the metals on lateral root initiation, as the lateral root primordia had already been initiated during pre-culture. The number of visible lateral roots would therefore originate from the stimulated or inhibited outgrowth of pre-initiated lateral root primordia. The number of emerged lateral roots in the upper control zone was not significantly affected when primary root tips were exposed to 75 µm Zn (Fig. 4C; Zn 0-75), but the mean length of these lateral roots was strongly reduced (Fig. 4D; Zn 0-75), suggesting that the systemic effect of Zn acts on lateral growth rate rather than on lateral root outgrowth. Also for Cd a lower but statistically significant systemic effect on lateral root elongation was observed (Fig. 4D; Cd 0-5). Comparing the effects of the treatments on lateral root number (Fig. 4C) and lateral root length (Fig. 4D) shows that lateral root lengths are more significantly affected, again emphasizing the importance of quantifying lateral root lengths alongside lateral root density in the description of morphological responses to abiotic stress. It is also remarkable that homogeneous exposure to Cu triggers a morphological response similar to that of phosphorus (P)-deprivation, yet the P-deprivation response can be systemically triggered by exposure of the primary root tip only to low P (Svistoonoff et al., 2007), whereas the effect of Cu is entirely local as contact of the primary root tip with excess Cu did not change lateral root number or lateral root length in the upper control zone (Fig. 4C, D).
Screening for the molecular determinants of root architectural changes related to efficiency of avoidance and colonization
We have previously made the assumption that efficient avoidance mechanisms of contaminated zones, combined with an efficient colonization mechanism of non-contaminated zones, would enable the plant to produce sufficient and safe biomass. A systemic inhibitory response to contaminants would decrease this efficiency, and Zn exposure is an example of this. Future research to reveal the molecular parameters involved in avoidance/colonization responses should be conducted in split-root systems, using forward or reverse genetics, although the application of forward genetics will depend on automated root growth analysis (de Dorlodot et al., 2007; Iyer-Pascuzzi et al., 2010) and a high-throughput system. Reverse genetics may at first instance be more feasible and can be conducted with a selection of mutants used to test hypotheses. For example, the involvement of signal transduction pathways can be investigated to reveal the underlying mechanism of the systemic Zn effect. As such, growth of A. thaliana roots in two dimensions in the vertical agar plate system, with increased sophistication of heterogeneous zones, and in combination with the large collection of mutants, can be used to unravel growth-inhibiting and growth-activating mechanisms in avoidance and colonization responses of the root system. The responses will be evaluated in relation to plant fitness and provide understanding towards more safe and more efficient biomass production or phytoremediation applications.
IMPROVING THE DEVELOPMENT OF ROOTS EXPOSED TO CONTAMINANTS
On contaminated soils, natural populations of plant species or specific ecotypes can exist that are tolerant to the contamination. However, these tolerant plants are often low in biomass (Vangronsveld et al., 2009). Nevertheless, the tolerance mechanisms in these plants are an interesting subject of study, and genetic determinants of tolerance have been transferred to higher biomass species using transgenic technology (Kärenlampi et al., 2000; Pilon-Smits, 2005; Seth et al., 2012). Although this may be worthwhile in some instances, the technology has a number of disadvantages. Over-expression of a transgene may increase tolerance to the stress condition, but may have a metabolic impact leading to decreased growth (Wojas et al., 2010). Also, the transgene may not be able to overcome multiple stress conditions, in which case introduction of multiple genes would be necessary. Economic valorization of contaminated land may demand specific species and not all of these may be easily amenable to genetic modification.
Another way of assisting plants to overcome the contaminant-associated stress is by exploiting the properties of naturally occurring plant-associated bacteria. These properties can improve the resistance of the plant to the contaminant, but also to other stress factors such as drought and nutrient deficiency. In the following, two examples are presented of the application of plant-associated bacteria to improve growth under stress conditions perceived by the roots.
The use of plant-associated bacteria to improve root growth of A. thaliana exposed to Cd
Plant endophytic bacteria can be transferred to subsequent generations via the seeds (Mastretta et al., 2009), through direct vascular connections from the maternal parent (Block et al., 1998), by colonization of the meristems (Pirttilä et al., 2000) or transferred through gametes directly (Madmony et al., 2005). It can be expected that those endophytes that contribute to the increased ability of the plant to tolerate a certain stress would be transferred preferentially, probably as a result of the inability of the non-resistant endophytes to survive the stress. Therefore, seed endophytes can be considered as a source for isolation of plant-associated bacteria to improve plant growth under unfavourable conditions. It was observed that the cultivable species composition and phenotypic characteristics of these species was different after cultivating A. thaliana for several generations on 2 µm CdSO4. The microbial composition in these seeds differed from the A. thaliana line grown in parallel for the same number of generations on a control soil (data not shown). Some of the bacterial strains isolated from the Cd seeds were used to inoculate A. thaliana and test their ability to improve growth under Cd stress. For this, A. thaliana control seeds (originating from plants that were never exposed to Cd) as well as Cd seeds (originating from plants that were exposed to 2 µm Cd for several generations) were sown on vertical agar plates. After 1 week, half of the control plants were inoculated with a bacterial suspension (108 c.f.u. ml−1) representative for the endophytic population isolated from Cd seeds. It consisted of four non Cd-resistant seed endophytes (4·28 % Bacillus sp., 13·7 % Bacillus arsenicus, 1·59 % Bacillus niacini, 79·36 % Bacillus circulans) and one Cd-resistant seed endophyte (0·53 % Bacillus arsenicus). The non-Cd-resistant endophytes may possess stress-relieving properties improving plant growth under Cd stress. Inoculation was carried out over 1 week. The plants were exposed to 0, 2 or 10 µm Cd during the entire experiment and primary root lengths were measured.
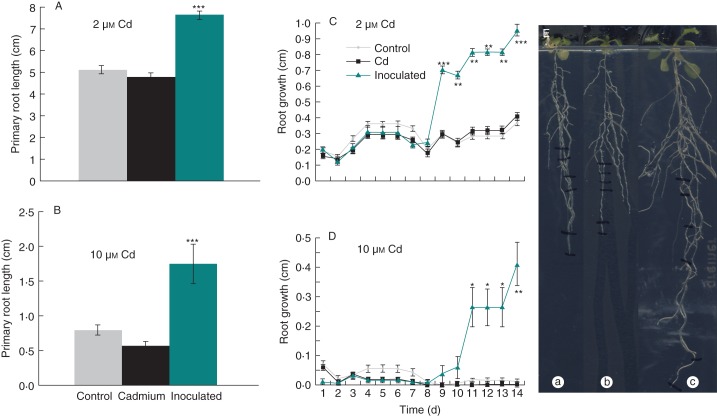
In the absence of Cd, total root length and growth rate of the root were the same for control plants, Cd plants and inoculated control plants. Upon exposure to 2 or 10 µm Cd, total root length (Fig. 5A, B, E) as well as growth rate (Fig. 5C, D) were significantly higher for inoculated control plants than for non-inoculated control plants or Cd plants. Apparently, the plants only benefited from the inoculation in the case of Cd stress. Under optimal conditions, when plants were not exposed to Cd stress, there was no measurable PGP effect, which may be due to the bacteria possessing characteristics that are stress-relieving and thus only promote growth under stress conditions. The lack of growth promotion in the Cd plants, even though containing the same bacteria as the inoculated control plants, can be explained by the fact that the growth-promoting effect only becomes apparent when the seed endophytes are present in high numbers, as is the case in the inoculated control plants (108 c.f.u. ml−1). This study provides evidence that inoculation of A. thaliana with seed endophytes can lead to the presence of bacteria in the plant that have a positive effect on root growth upon exposure to Cd.
Fig. 5.
Inoculation with seed endophytes can enhance root growth under Cd stress. Arabidopsis thaliana control seeds (from plants that were never exposed to Cd) as well as Cd seeds (from plants that were exposed to 2 µm Cd for several generations) were grown on vertical agar plates containing 0, 2 or 10 µm CdSO4 during the entire experiment. Half of the 7-d-old control plants were inoculated by transferring them to plates on which a bacterial suspension was streaked out (108 c.f.u. ml−1) representing the endophyte population isolated from Cd seeds (see text for species composition). (A, B) Primary root length of control plants, Cd plants and inoculated control plants after 21 d exposure to (A) 2 µm Cd or (B) 10 µm Cd (***P < 0·001, one-way ANOVA with Tukey correction, n = 20). (C, D) Primary root growth rate for control plants, Cd plants and inoculated plants upon exposure to (C) 2 or (D) 10 µm Cd. Day 1 on the graphs corresponds to the first day after inoculation (*P < 0·05, **P < 0·01, ***P < 0·001 within-day one-way ANOVA with Tukey correction, n = 20). (E) Vertical agar plate with a control plant (a), Cd plant (b) and inoculated plant (c).
Growth promotion and degradation of organic contaminants
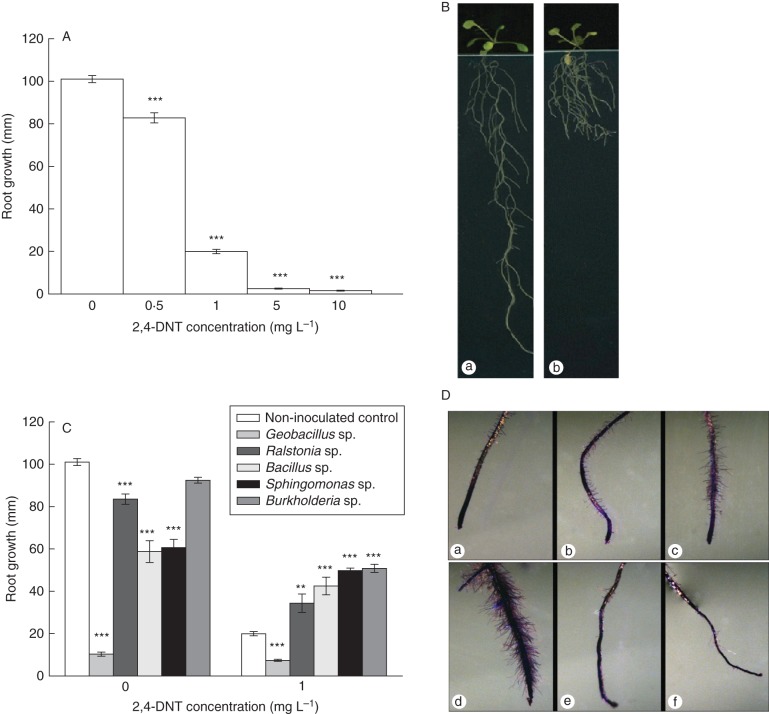
2,4-Di-nitro-toluene (2,4-DNT) is phytotoxic to A. thaliana seedlings, causing a severe reduction in primary root length at concentrations as low as 0·5 mg L−1. An 80 % reduction in root length was observed at a 2,4-DNT concentration of 1 mg L−1. Seedlings on the plates with 5 mg L−1 or higher 2,4-DNT concentrations did not grow at all (Fig. 6A). Primary root growth was inhibited in the presence of the nitroaromatic and a highly curled growth of the main root was also observed. Lateral root density was visually increased in the presence of 2,4-DNT (Fig. 6B) but the number of lateral roots and lateral root length could not be measured due to the dense growth.
Fig. 6.
Bacteria isolated from an explosives-polluted soil are capable of enhancing root growth in presence of 2,4-DNT contamination. (A) 2,4-DNT inhibits primary root growth when 7-d- old A. thaliana seedlings are grown over 9 d on plates amended with different 2,4-DNT concentrations (0 – 10 mg L−1, data are mean ± s.e.; ***P < 0·001, non-parametric Kruskal–Wallis test; n = 12). (B) Arabidopsis thaliana growth on 0 mg L−1 (a) and 1 mg L−1 (b) 2,4-DNT. (C) Primary root length 9 d after transfer of 7-d-old seedlings in response to 2,4-DNT (0 and 1 mg L−1) and in the presence or absence of bacteria tested for 2,4-DNT degradation and plant-growth promoting characteristics (IAA production and ACC-deaminase activity, see text). 103 c.f.u. was spread on a plate to inoculate the transferred seedlings (data are mean ± s.e.; ***P < 0·001, **P < 0·01, non-parametric Kruskal–Wallis test; n = 12). (D) Visualization of root hair formation by staining with Crystal Violet in 15-d-old A. thaliana seedlings exposed to 1 mg L−1 DNT and in the presence or absence of bacteria. (a) Non-inoculated control, (b) Burkholderia, (c) Sphingomonas sp., (d) Bacillus sp., (e) Ralstonia sp., (f) Geobacillus sp.
Numerous studies have investigated the toxicity of 2,4,6-trinitrotoluene (TNT) to plants using soil-based approaches, although little is known about specific root responses of plants towards aromatics (Gong et al., 1999; Travis et al., 2008). Active uptake of TNT in the roots has been observed and little is transported to the aerial parts of the plants (Sens et al., 1999; Yoon et al., 2006). Because of its resemblance to TNT, a similar transformation process for 2,4-DNT in plants is suggested. Bacteria are described to mineralize DNT (Spanggord et al., 1991; Snellinx et al., 2003) and RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine; Seth-Smith et al., 2002) but not TNT, and plants should be able to mineralize RDX, but not TNT or DNT (Van Aken et al., 2004; Rylott and Bruce, et al., 2009; Rylott et al., 2011). TNT and DNT are nitroaromatics while RDX is a nitramine. Detoxification of nitroaromatics by plants involves conjugation with glutathione or transformations catalysed by cytochrome P450 according to the green liver model (Burken, 2003; Gandia-Herrero et al., 2008). The glutathione conjugates are then transported to the vacuole or cell wall, protecting the plant from the toxic chemical. Metabolites of 2,4-DNT transformation have been reported as aminonitrotoluenes and unknown bound metabolites, suggesting its incorporation in cellulose and lignin (Yoon et al., 2006).
A collection of soil bacteria isolated from an explosives-contaminated site was tested for 2,4-DNT transformation abilities. Four bacteria showed high 2,4-DNT transformation capacity, with 10–50 % reduction in 2 weeks (Table 1). These bacteria were tested for their indole-3-acetic acid (IAA) production and ACC-deaminase activity. Four of five were positive for IAA production However, it is important to mention that Salkowski's reagent detects almost all indole ring compounds, including IAA and other IAA-like molecules. All of the bacteria displayed high levels of ACC-deaminase activity, ranging from 32 to 648 mm α-ketobutyrate (αKB) (mg protein)−1 h−1 (Table 1).
Table 1.
In vitro 2,4-DNT transformation rate and production rate of IAA and ACC-deaminase activity of the bacterial strains
| Taxon | 2,4-DNT transformation (% reduction after 2 weeks) | IAA [μm IAA (mg protein)−1 h−1] | ACC deaminase [mm αKB (mg protein)−1 h−1] |
|---|---|---|---|
| Geobacillus | 10·4 | 0 | 32 |
| Ralstonia | 21·3 | <10 | 361 |
| Bacillus | 10·3 | <10 | 37 |
| Sphingomonas | 35·4 | 65 | 648 |
| Burkholderia | 52·5 | 14 | 398 |
When the 2,4-DNT-transforming strains were inoculated on plant growth medium, main root length of the plants was significantly reduced in comparison with the non-inoculated control, except for Burkholderia sp. (Fig. 6C). Despite the high level of ACC-deaminase activity, these strains could not promote root growth better than the non-inoculated control under unpolluted conditions. However, when plants were exposed to 1 mg L−1 2,4-DNT, all strains except Geobacillus promoted primary root growth (Fig. 6C). The best root growth promotion was observed for Burkholderia sp., with a 150 % increase in main root length in comparison with the non-inoculated exposed control. This strain was most efficient in transforming 2,4-DNT in the flask cultures and displayed a high level of ACC-deaminase production [398 mm αKB (mg protein)−1 h−1]. A general trend was observed for IAA production, ACC-deaminase activity, 2,4-DNT-transforming abilities and the stimulatory effect on main root growth under DNT-polluting conditions. Only Geobacillus did not enhance root growth in control and exposed conditions despite its moderate level of ACC-deaminase activity. However, this strain did not produce IAA in vitro. At the end of the growth study, roots were stained to visualize root hair morphology. Most of the PGP bacteria increased root hair density and root length and the root hair formation started closer to the root tip (Fig. 6D). This effect is generally observed for PGP bacteria (Dobbelaere et al., 1999; Contesto et al., 2008).
As mentioned earlier, PGP rhizobacteria colonize the rhizosphere of many plants and stimulate plant growth either by fixing atmospheric nitrogen, making nutrients more available to plants or releasing phytohormones (Weyens et al., 2009a, b; Bloemberg and Lugtenberg, 2001). In addition, PGP rhizobacteria can also affect hormone levels in the plant. For example, ACC-deaminase activity of bacteria lowers ethylene levels in the host plant, releasing the growth-inhibiting effect exerted by this molecule (Glick et al., 1998; Glick, 2005). Much of the plant ACC produced in the roots is exuded in the rhizosphere where it is subsequently taken up by the bacteria and hydrolysed by ACC-deaminase. This generates a strong sink, thereby lowering internal ACC levels in the root. It has been demonstrated that transgenic ACC-deaminase plants show a higher tolerance to flooding and metal stress (Grichko and Glick, 2001; Stearns et al., 2005; Onofre-Lemus et al., 2009). In this study, we show that bacteria with ACC-deaminase activity and 2,4-DNT-degrading abilities promote root length of 2,4-DNT-stressed plants, probably due to lowering of ethylene levels and transformation of 2,4-DNT to less toxic compounds. However, pleiotrophic effects, including simultaneously signalling pathways elicited by the microbe, play a role in root elongation (Tanimoto et al., 1995; Pitts et al., 1998; Cao et al., 1999). Recently, two mechanisms were described to explain the effect of PGP rhizobacteria on root hair elongation, one ethylene-dependent signalling mechanism involving bacterial ACC-deaminase activity and one ethylene-independent mechanism explaining probably the largest part of the effect (Contesto et al., 2008; Desbrosses et al., 2009). Hormone-mutant studies can provide further insight into the regulation mechanisms of root growth under PGP and pollution conditions.
CONCLUSIONS
Understanding the molecular basis of root development can lead to the development of strategies that interfere with developmental and stress-related pathways with the aim to optimize root development. However, this may not be easily achieved as root growth seems to be determined by small-effect loci–environment interactions (Ghanem et al., 2011), and therefore plants selected with the best ability to adapt root development to one environmental condition may not be optimal for another condition (Den Herder et al., 2010). We have discussed here the potential of plant-associated bacteria to improve root growth. Is it still worthwhile studying the underlying molecular mechanisms of root developmental stress responses? Unravelling the molecular background of root development under stress conditions is worthwhile, both from a fundamental biological point of view and also from an applied point of view. The potential of plant-associated bacteria to improve root development is first estimated by screening phenotypic characteristics of production of PGP substances (e.g. IAA), stress-relieving properties (e.g. ACC-deaminase), increased nutrient uptake (organic acids, siderophores, nitrogen fixation, etc.) and sequestration or breakdown of the contaminant(s). Regarding the influence of the associated bacteria on plant growth, knowledge of the mechanisms by which plants alter root architecture may enhance the likelihood of finding the best plant–bacterium interaction by designing appropriate phenotypic screens. Fundamental knowledge gained in in vitro systems will have to be validated in growth systems and species that are more relevant to the eventual application in the field. Nevertheless, a better understanding of (1) the fundamental molecular basis and mechanisms of root development, (2) plant–bacteria interactions, (3) the interactions between different PGP bacteria (quorum sensing) and (4) an integration of environmental effects on all partners and their interactions is needed to optimize the approach and exploit the right plant–bacteria interaction.
ACKNOWLEDGEMENTS
Funding was provided by Hasselt University Methusalem project 08M03VGRJ, and from the Research Foundation Flanders (FWO) doctoral (E.K., S.Tr., S.Th.) and post-doctoral (T.R. and N.W.) fellowships, and an FWO Individual Research Grant (1·5·103·09) to T.R., and by Hasselt University BOF funding (Bijzonder Onderzoeksfonds).
APPENDIX: METHODS
Seeds, sterilization and preparation of vertical agar plates
Arabidopsis thaliana seeds were surface-sterilized in 0·1 % (w/v) NaOCl and 0·1 % (v/v) Tween 80 for 5 min and washed four times with distilled water over 20 min. Seeds were sown on 12 × 12-cm vertical plates containing 50× diluted Gamborg's B5 macro- and micronutrients, except for CuSO4, which was added to a final concentration of 100 nm (to avoid Cu deficiency, see Burkhead et al., 2009). All media contained 0·5 g L−1 MES (2-[N-morpholino]ethanesulfonic acid; Sigma cat. no. M-8250) and 10 g L−1 plant tissue culture agar (Lab-M, Bury, UK) and were adjusted to pH 5·8 with KOH. Germination plates also contained 5 g L−1 sucrose. After incubation at 4 °C for 2–3 d in the dark, the germination plates were placed vertically in a culture room at 22 °C with 12/12-h light–dark cycle and a light intensity of 125–150 µmol m−2 s−1 delivered by fluorescent white lamps. After 7 or 8 d growth, depending on the experiment, plants were transferred to treatment plates, for which appropriate amounts of concentrated filter-sterilized (0·2 µm) CdSO4, CuSO4 or ZnSO4 and K2SO4 solutions were mixed into the medium. K2SO4 was added to complement growth media at different concentrations of metals to the same concentrations of SO42–. In all treatment plates 1 cm of agar was removed at the top to create an air gap for the shoots. Additionally, for split-root plates, a small section of agar was removed, using a scalpel blade, such that an upper zone of 2 × 12 cm was separated from the bottom zone. Concentrated (100×) solutions of CdSO4, CuSO4, ZnSO4 and K2SO4 were spread out (67 µL to the top zone and 300 µL to the bottom zone as these zones contain the equivalent of 6·7 and 30 mL of growth medium, respectively) and plates were allowed to dry in a laminar air-flow for another 10 min. Treatment plates containing 2,4-DNT (0, 0·5, 1, 5, 10 mg L−1) were prepared by spreading out 100× concentrated solutions.
Inoculation experiments and 2,4-DNT toxicity
In treatment plates containing various concentrations of 2,4-DNT (0, 1 mg L−1) and 0·5 g L−1 sucrose half of the plants were inoculated with either Geobacillus, Ralstonia, Bacillus, Sphingomonas or Burkholderia sp. The bacteria were isolated from an explosives-polluted soil and tested for 2,4-DNT degradation (see below) and plant-growth promoting characteristics (IAA production and ACC-deaminase activity, see below). Bacteria were grown overnight in rich medium and harvested in the late exponential phase. The cultures were centrifuged (4000 r.p.m., 30 min) and the pellets were resuspended in MgSO4 until the desired optical density was reached. For inoculation, 100 µL of 104 c.f.u. mL−1 was spread on the growth plates. Every day, the end of the root tip was marked. After 9 d growth under different 2,4-DNT concentrations in the presence or absence of bacteria, plant primary root length and root hair density was measured. For visualizing root hairs, roots were stained for 1 min in 0·075 % Crystal-Violet in 70 % ethyl alcohol and rinsed thoroughly with distilled water.
Inoculation experiments and cadmium toxicity
Arabidopsis thaliana control seeds (from plants that were never exposed to Cd) as well as Cd seeds (from plants that were exposed to 2 µm Cd for several generations) were sown on vertical agar plates. After 1 week, half of the control plants were inoculated with a bacterial suspension (108 c.f.u. ml−1) that represented the endophyte population isolated from Cd seeds. It consisted of four non Cd-resistant seed endophytes (4·28 % Bacillus sp., 13·7 % Bacillus arsenicus, 1·59 % Bacillus niacini, 79·36 % Bacillus circulans) and one Cd-resistant seed endophyte (0·53 % Bacillus arsenicus). Inoculation was carried out over 1 week. Plants were exposed to 0, 2 or 10 µm CdSO4 during the entire experiment. Seed endophytes were obtained according to Mastretta et al. (2009) and the species were counted and characterized genotypically according to Weyens et al. (2009c).
Bacterial IAA production and ACC-deaminase activity
Bacteria were inoculated from glycerol stock into 15-mL tubes with 1/10-strength medium supplemented with tryptophane (Patten and Glick, 2002). After 2 d growth, IAA production was assayed colorimetrically using ferric chloride acid reagent (Salkowski). IAA concentrations were calculated from an IAA standard curve (20–250 µm). Protein concentration was determined using the Bradford protein assay reagent (Bio-Rad) and a protein standard-curve was prepared from bovine serum albumin (BSA).
ACC-deaminase activity was determined by monitoring the amount of α-ketobutyrate (αKB) generated by the enzymatic hydrolysis of ACC (Belimov et al., 2005). SMN medium containing 5 mm ACC as sole source of N was inoculated with 20 µL cell suspension. Bacteria were incubated for 3 d at 30 °C, centrifuged at 4000 r.p.m. for 15 min, resuspended in 1 mL of 0·1 m Tris-HCl buffer (pH 8·5) and lysed by the addition of 30 µL toluene under vigorous vortexing. An aliquot of this suspension was stored on ice for protein determination. After reaction of mixtures containing 100 µL cell suspension, 10 µL 0·5 m ACC and 100 µL 0·1 m Tris-HCl buffer (pH 8·5) for 30 min at 30 ° C, 1 mL of 0·56 m HCl was added and incubated for 30 min at 30 °C and 150 r.p.m. Then, 500 µL of 0·56 m HCl and 150 µL of 0·2 % dinitrophenylhydrazine in 2 m HCl were added. The mixtures were reacted for 30 min at 30 °C, supplemented with 1 mL of 2 m NaOH and assayed for αKB by the formation of a brown colour. A standard curve of αKB was prepared in Tris-HCl buffer (pH 8·5). A stock solution of 100 mm was diluted ten-fold and this was used for preparing the dilution series. Protein concentration was determined using using the Bradford protein assay reagent (Bio-Rad) and a protein standard-curve was prepared from BSA.
2,4-DNT transformation
Cells were grown to late-log phase (48 h), harvested and rinsed in 10 mm MgSO4 buffer. Cells were used at a final concentration of 0·1 OD and incubated in flasks with 50 mL minimal medium with 2,4-DNT as sole nitrogen source and a mixture of carbon sources (per litre: 1·5 g fructose, 1·4 g glucose, 1·8 ml acetic acid, 8·1 g succinic acid). The minimal medium contained (per litre): 6 g NaH2PO4.7H2O, 3 g K2HPO4, 0·5 g NaCl, 0·52 g MgSO4, 2 mg Fe(III)citrate and 2·5 ml of a micronutrient stock solution (250× micronutrient stock, per litre: 300 mg HBO3, 50 mg ZnCl2, 30 mg MnCl2.4H2O, 200 mg CoCl2, 10 mg CuCl2.2H2O, 20 mg NiCl2.6H2O, 30 mg Na2MoO4.2H2O). Samples were taken at intervals, diluted with methanol and assayed for 2,4-DNT by high-performance liquid chromatography. For this, samples were separated with a Chromspher C18 reverse-phase column (5 µm, 250 × 4·6 mm) with a guard column preceding the main column. The mobile phase consisted of 35 : 65 (v/v) methanol/water in the beginning followed by 50 : 50 (v/v) methanol/water delivered at a flow rate of 1·3 ml min−1. 2,4-DNT was monitored at 254 nm.
Imaging and analysis
On each day of growth on vertical agar plates after transfer, the position of the primary root tip was marked on the plate to allow kinetic analysis. Plates were scanned on a Canoscan 4400F (Canon) at 600 d.p.i. and root growth was analysed using the Optimas 6·1 Image analysis program (Media Cybernetics).
Statistical analysis
Normal distribution of the data was tested using Shapiro–Wilk and the Kolmogorov–Smirnov tests. Logarithmic transformations were applied where necessary to obtain a normal distribution of the data. Treatment effects were determined by one-way ANOVA using SAS v9·1 software (SAS Institute Inc.). Statistical differences between group means were determined after Tukey's correction for multiple comparisons (concentration series compared with control and with each other).
LITERATURE CITED
- Alford E, Pilon-Smits E, Paschke M. Metallophytes – a view from the rhizosphere. Plant and Soil. 2010;337:33–50. [Google Scholar]
- Armengaud P, Zambaux K, Hills A, et al. EZ-Rhizo: integrated software for the fast and accurate measurement of root system architecture. Plant Journal. 2009;57:945–956. doi: 10.1111/j.1365-313X.2008.03739.x. [DOI] [PubMed] [Google Scholar]
- Bardos P, Chapman T, Andersson-Sköld Y, et al. Biomass production on marginal land. Biocycle. 2008;49:50–52. [Google Scholar]
- Baluška F, Mancuso S, Volkmann D, Barlow P. Root apex transition zone: a signalling-response nexus in the root. Trends in Plant Science. 2010;15:402–408. doi: 10.1016/j.tplants.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Belimov AA, Hontzeas N, Safronova VI, et al. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biology & Biochemistry. 2005;37:241–250. [Google Scholar]
- Block CC, Hill JH, McGee DC. Seed transmission of Pantoea stewartii in field and sweet corn. Plant Disease. 1998;82:775–780. doi: 10.1094/PDIS.1998.82.7.775. [DOI] [PubMed] [Google Scholar]
- Bloemberg GV, Lugtenberg BJJ. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Current Opinion in Plant Biology. 2001;4:343–350. doi: 10.1016/s1369-5266(00)00183-7. [DOI] [PubMed] [Google Scholar]
- Broadley M, White P, Hammond J, Zelko I, Lux A. Zinc in plants. New Phytologist. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Burken JG. Uptake and metabolism of organic compounds:green liver concept. In: McCutcheon SC, Schnoor JL, editors. Phytoremediation: transformation and control of contaminants. New Jersey: John Wiley & Sons; 2003. pp. 59–84. [Google Scholar]
- Burkhead J, Reynolds K, Abdel-Ghany S, Cohu C, Pilon M. Copper homeostasis. New Phytologist. 2009;182:799–816. doi: 10.1111/j.1469-8137.2009.02846.x. [DOI] [PubMed] [Google Scholar]
- Caldelari D, Wang G, Farmer E, Dong X. A. lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Molecular Biology. 2011;75:25–33. doi: 10.1007/s11103-010-9701-9. [DOI] [PubMed] [Google Scholar]
- Cao XF, Linstead P, Berger F, Kieber J, Dolan L. Differential ethylene sensitivity of epidermal cells is involved in the establishment of cell pattern in the Arabidopsis root. Physiologia Plantarum. 1999;106:311–317. doi: 10.1034/j.1399-3054.1999.106308.x. [DOI] [PubMed] [Google Scholar]
- Cocking EC. Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant and Soil. 2003;252:169–175. [Google Scholar]
- Contesto C, Desbrosses G, Lefoulon C, et al. Effects of rhizobacterial ACC deaminase activity on A. indicate that ethylene mediates local root responses to plant growth-promoting rhizobacteria. Plant Science. 2008;175:178–189. [Google Scholar]
- Cuypers A, Smeeets K, Vangronsveld J. Heavy metal stress in plants. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009. [Google Scholar]
- Cuypers A, Smeets K, Ruytinx J, et al. The cellular redox state as a modulator in cadmium and copper responses in A. thaliana seedlings. Journal of Plant Physiology. 2011;168:309–316. doi: 10.1016/j.jplph.2010.07.010. [DOI] [PubMed] [Google Scholar]
- de Dorlodot S, Forster B, Pages L, Price A, Tuberosa R, Draye X. Root system architecture: opportunities and constraints for genetic improvement of crops. Trends in Plant Science. 2007;12:474–481. doi: 10.1016/j.tplants.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Den Herder G, Van Isterdael G, Beeckman T, De Smet I. The roots of a new green revolution. Trends in Plant Science. 2010;15:600–607. doi: 10.1016/j.tplants.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Desbrosses G, Contesto C, Varoquaux F, Galland M, Touraine B. PGPR–A. interactions is a useful system to study signaling pathways involved in plant developmental control. Plant Signaling & Behavior. 2009;4:319–321. doi: 10.4161/psb.4.4.8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere S, Croonenborghs A, Thys A, Vande Broek A, Vanderleyden J. Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant and Soil. 1999;212:155–164. [Google Scholar]
- Drazkiewicz M, Skorzynska-Polit E, Krupa Z. Copper-induced oxidative stress and antioxidant defence in A. thaliana. Biometals. 2004;17:379–387. doi: 10.1023/b:biom.0000029417.18154.22. [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell J, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Francis I, Holsters M, Vereecke D. The Gram-positive side of plant–microbe interactions. Environmental Microbiology. 2010;12:1–12. doi: 10.1111/j.1462-2920.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M. Hormone interactions during lateral root formation. Plant Molecular Biology. 2009;69:437–449. doi: 10.1007/s11103-008-9417-2. [DOI] [PubMed] [Google Scholar]
- Galvan-Ampudia C, Testerink C. Salt stress signals shape the plant root. Current Opinion in Plant Biology. 2011;14:296–302. doi: 10.1016/j.pbi.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Gamalero E, Glick BR. Ethylene and abiotic stress tolerance in plants. In: Ahmad P, Prasad MNV, editors. Environmental adaptations and stress tolerance of plants in the era of climate change. New York: Springer; 2012. pp. 395–412. [Google Scholar]
- Gandia-Herrero F, Lorenz A, Larson T, et al. Detoxification of the explosive 2,4,6-trinitrotoluene in A.: discovery of bifunctional O- and C-glucosyltransferases. Plant Journal. 2008;56:963–974. doi: 10.1111/j.1365-313X.2008.03653.x. [DOI] [PubMed] [Google Scholar]
- Ghanem M, Hichri I, Smigocki A, et al. Root-targeted biotechnology to mediate hormonal signalling and improve crop stress tolerance. Plant Cell Reports. 2011;30:807–823. doi: 10.1007/s00299-011-1005-2. [DOI] [PubMed] [Google Scholar]
- Glick BR. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiology Letters. 2005;251:1–7. doi: 10.1016/j.femsle.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Glick BR, Penrose DM, Li J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. Journal of Theoretical Biology. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- Gong P, Wilke BM, Fleischmann S. Soil-based phytotoxicity of 2,4,6-trinitrotoluene (TNT) to terrestrial higher plants. Archives of Environmental Contamination and Toxicology. 1999;36:152–157. doi: 10.1007/s002449900455. [DOI] [PubMed] [Google Scholar]
- Gonzalez N, Beemster G, Inze D. David and Goliath: what can the tiny weed A. teach us to improve biomass production in crops? Current Opinion in Plant Biology. 2009;12:157–164. doi: 10.1016/j.pbi.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Grichko VP, Glick BR. Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiology and Biochemistry. 2001;39:11–17. [Google Scholar]
- Hassan Z, Aarts M. Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environmental and Experimental Botany. 2011;72:53–63. [Google Scholar]
- Hinsinger P, Brauman A, Devau N, et al. Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant and Soil. 2011;348:29–61. [Google Scholar]
- Hodge A. Root decisions. Plant Cell and Environment. 2009;32:628–640. doi: 10.1111/j.1365-3040.2008.01891.x. [DOI] [PubMed] [Google Scholar]
- Hodge A, Berta G, Doussan C, Merchan F, Crespi M. Plant root growth, architecture and function. Plant and Soil. 2009;321:153–187. [Google Scholar]
- Iyer-Pascuzzi A, Symonova O, Mileyko Y, et al. Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiology. 2010;152:1148–1157. doi: 10.1104/pp.109.150748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärenlampi S, Schat H, Vangronsveld J, et al. Genetic engineering in the improvement of plants for phytoremediation of metal polluted soils. Environmental Pollution. 2000;107:225–231. doi: 10.1016/s0269-7491(99)00141-4. [DOI] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, et al. Nitrate-regulated auxin transport by NRT1·1 defines a mechanism for nutrient sensing in plants. Developmental Cell. 2010;18:927–937. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Lequeux H, Hermans C, Lutts S, Verbruggen N. Response to copper excess in A. thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiology and Biochemistry. 2010;48:673–682. doi: 10.1016/j.plaphy.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Liu F, Tang Y, Du R, Yang H, Wu Q, Qiu R. Root foraging for zinc and cadmium requirement in the Zn/Cd hyperaccumulator plant Sedum alfredii. Plant and Soil. 2010;327:365–375. [Google Scholar]
- Lobet G, Pages L, Draye X. A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiology. 2011;157:29–39. doi: 10.1104/pp.111.179895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. Roots of the second green revolution. Australian Journal of Botany. 2007;55:493–512. [Google Scholar]
- Madmony A, Chernin L, Pleban S, Peleg E, Riov J. Enterobacter cloacae, an obligatory endophyte of pollen grains of Mediterranean pines. Folia Microbiologica. 2005;50:209–216. doi: 10.1007/BF02931568. [DOI] [PubMed] [Google Scholar]
- Malamy J. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell and Environment. 2005;28:67–77. doi: 10.1111/j.1365-3040.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- Mantelin S, Desbrosses G, Larcher M, Tranbarger T, Cleyet-Marel J-C, Touraine B. Nitrate-dependent control of root architecture and N nutrition are altered by a plant growth-promoting Phyllobacterium sp. Planta. 2006;223:591–603. doi: 10.1007/s00425-005-0106-y. [DOI] [PubMed] [Google Scholar]
- Mastretta C, Barac T, Vangronsveld J, Newman L, Taghavi S, van der Lelie D. Endophytic bacteria and their potential application to improve the phytoremediation of contaminated environments. Biotechnology and Genetic Engineering Reviews. 2006;23:175–207. doi: 10.1080/02648725.2006.10648084. [DOI] [PubMed] [Google Scholar]
- Mastretta C, Taghavi S, van der Lelie D, et al. Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. International Journal of Phytoremediation. 2009;11:251–267. [Google Scholar]
- Miransari M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biology. 2010;12:563–569. doi: 10.1111/j.1438-8677.2009.00308.x. [DOI] [PubMed] [Google Scholar]
- Mench M, Schwitzguébel JP, Schroeder P, Bert V, Gawronski S, Gupta S. Assessment of successful experiments and limitations of phytotechnologies: contaminant uptake, detoxification and sequestration, and consequences for food safety. Environmental Science and Pollution Research. 2009;16:876–900. doi: 10.1007/s11356-009-0252-z. [DOI] [PubMed] [Google Scholar]
- Monshausen G, Gilroy S. The exploring root – root growth responses to local environmental conditions. Current Opinion in Plant Biology. 2009;12:766–772. doi: 10.1016/j.pbi.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Nibau C, Gibbs D, Coates J. Branching out in new directions: the control of root architecture by lateral root formation. New Phytologist. 2008;179:595–614. doi: 10.1111/j.1469-8137.2008.02472.x. [DOI] [PubMed] [Google Scholar]
- Onofre-Lemus J, Hernández-Lucas I, Girard L, Caballero-Mellado J. ACC (1-aminocyclopropane-1-carboxylate) deaminase activity, a widespread trait in Burkholderia species, and its growth-promoting effect on tomato plants. Applied and Environmental Microbiology. 2009;75:6581–6590. doi: 10.1128/AEM.01240-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CM, Guerinot ML. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nature Chemical Biology. 2009;5:333–340. doi: 10.1038/nchembio.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Li J, Pittman J, et al. Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proceedings of the National Academy of Sciences of the USA. 2005;102:18830–18835. doi: 10.1073/pnas.0509512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Applied and Environmental Microbiology. 2002;68:3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, et al. A. lateral root development: an emerging story. Trends in Plant Science. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Pérez-Torres C, Lopez-Bucio J, Cruz-Ramirez A, et al. Phosphate availability alters lateral root development in A. by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell. 2008;20:3258–3272. doi: 10.1105/tpc.108.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petó A, Lehotai N, Lozano-Juste J, et al. Involvement of nitric oxide and auxin in signal transduction of copper-induced morphological responses in A. seedlings. Annals of Botany. 2011;108:449–457. doi: 10.1093/aob/mcr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits E. Phytoremediation. Annual Review of Plant Biology. 2005;56:15–39. doi: 10.1146/annurev.arplant.56.032604.144214. [DOI] [PubMed] [Google Scholar]
- Pirttilä AM, Laukkanen H, Pospiech H, Myllylä R, Hohtola A. Detection of intracellular bacteria in the buds of Scots pine (Pinus sylvestris L.) by in situ hybridization. Applied and Environmental Microbiology. 2000;66:3073–3077. doi: 10.1128/aem.66.7.3073-3077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Cernac A, Estelle M. Auxin and ethylene promote root hair elongation in A. The Plant Journal. 1998;16:553–560. doi: 10.1046/j.1365-313x.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- Potters G, Pasternak T, Guisez Y, Palme K, Jansen M. Stress-induced morphogenic responses: growing out of trouble? Trends in Plant Science. 2007;12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Potters G, Pasternak T, Guisez Y, Jansen M. Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant Cell and Environment. 2009;32:158–169. doi: 10.1111/j.1365-3040.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- Raaijmakers J, Paulitz T, Steinberg C, Alabouvette C, Moënne-Loccoz Y. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant and Soil. 2009;321:341–361. [Google Scholar]
- Rashid S, Charles TC, Glick BR. Isolation and characterization of new plant growth-promoting bacterial endophytes. Applied Soil Ecology. 2012 in press. http://dx.doi.org/10.1016/j.apsoil.2011.09.011 . [Google Scholar]
- Remans T, Nacry P, Pervent M, et al. The A. NRT1·1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proceedings of the National Academy of Sciences of the USA. 2006;103:19206–19211. doi: 10.1073/pnas.0605275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans T, Opdenakker K, Smeets K, Mathijsen D, Vangronsveld J, Cuypers A. Metal-specific and NADPH oxidase dependent changes in lipoxygenase and NADPH oxidase gene expression in A. thaliana exposed to cadmium or excess copper. Functional Plant Biology. 2010;37:532–544. [Google Scholar]
- Rylott EL, Bruce NC. Plants disarm soil: engineering plants for the phytoremediation of explosives. Trends in Biotechnology. 2009;27:73–81. doi: 10.1016/j.tibtech.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Rylott EL, Lorenz A, Bruce NC. Biodegradation and biotransformation of explosives. Current Opinion in Biotechnology. 2011;22:434–440. doi: 10.1016/j.copbio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Ryu C-M, Hu C-H, Locy RD, Kloepper JW. Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant and Soil. 2005;268:285–292. [Google Scholar]
- Saleem M, Arshad M, Hussain S, Bhatti A. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. Journal of Industrial Microbiology & Biotechnology. 2007;34:635–648. doi: 10.1007/s10295-007-0240-6. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Baudoin E, Vandenkoornhuyse P. Symbiotic microorganisms, a key for ecological success and protection of plants. Comptes Rendus Biologies. 2004;327:639–648. doi: 10.1016/j.crvi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Sens C, Scheidemann P, Werner D. The distribution of 14C-TNT in different biochemical compartments of the monocotyledonous Triticum aestivum. Environmental Pollution. 1999;104:113–119. doi: 10.1007/BF02986402. [DOI] [PubMed] [Google Scholar]
- Seth CS, Remans T, Keunen E, et al. Phytoextraction of toxic metals: a central role for glutathione. Plant Cell and Environment. 2012;35:334–346. doi: 10.1111/j.1365-3040.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- Seth-Smith HMB, Rosser SJ, Basran A, et al. Cloning, sequencing, and characterization of the hexahydro-1,3,5-trinitro-1,3,5-triazine degradation gene cluster from Rhodococcus rhodochrous. Applied and Environmental Microbiology. 2002;68:4764–4771. doi: 10.1128/AEM.68.10.4764-4771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shute T, Macfie S. Cadmium and zinc accumulation in soybean: a threat to food safety? Science of the Total Environment. 2006;371:63–73. doi: 10.1016/j.scitotenv.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Smeets K, Opdenakker K, Remans T, et al. Oxidative stress-related responses at transcriptional and enzymatic levels after exposure to Cd or Cu in a multipollution context. Journal of Plant Physiology. 2009;166:1982–1992. doi: 10.1016/j.jplph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Snellinx Z, Taghavi S, Vangronsveld J, van der Lelie D. Microbial consortia that degrade 2,4-DNT by interspecies metabolism: isolation and characterisation. Biodegradation. 2003;14:19–29. doi: 10.1023/a:1023539104747. [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker J. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes & Development. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanggord RJ, Spain JC, Nishino SF, Mortelmans KE. Biodegradation of 2,4-dinitrotoluene by a Pseudomonas sp. Applied and Environmental Microbiology. 1991;57:3200–3205. doi: 10.1128/aem.57.11.3200-3205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns JC, Shah S, Greenberg BM, Dixon DG, Glick BR. Tolerance of transgenic canola expressing 1-aminocyclopropane-1-carboxylic acid deaminase to growth inhibition by nickel. Plant Physiology and Biochemistry. 2005;43:701–708. doi: 10.1016/j.plaphy.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Svistoonoff S, Creff A, Reymond M, et al. Root tip contact with low-phosphate media reprograms plant root architecture. Nature Genetics. 2007;39:792–796. doi: 10.1038/ng2041. [DOI] [PubMed] [Google Scholar]
- Taghavi S, Garafola C, Monchy S, et al. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Applied and Environmental Microbiology. 2009;75:748–757. doi: 10.1128/AEM.02239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto M, Roberts K, Dolan L. Ethylene is a positive regulator of root hair development in A. thaliana. The Plant Journal. 1995;8:943–948. doi: 10.1046/j.1365-313x.1995.8060943.x. [DOI] [PubMed] [Google Scholar]
- Torres M, Dangl J, Jones J. A. gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proceedings of the National Academy of Sciences of the USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis ER, Bruce NC, Rosser SJ. Short term exposure to elevated trinitrotoluene concentrations induced structural and functional changes in the soil bacterial community. Environmental Pollution. 2008;153:432–439. doi: 10.1016/j.envpol.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Van Aken B, Yoon JM, Schnoor JL. Biodegradation of nitro-substituted explosives 2,4,6-trinitrotoluene, hexahydro-1,3,5-trinitro-1,3,5-triazine, an octahydro-1,3,5,7-tetranitro-1,3,5-tetrazocine by a phytosymbiotic Methylobacterium sp associated with poplar tissues (Populus deltoides × nigra DN34) Applied and Environmental Microbiology. 2004;70:508–517. doi: 10.1128/AEM.70.1.508-517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lelie D, Taghavi S, Monchy S, et al. Poplar and its bacterial endophytes: coexistence and harmony. Critical Reviews in Plant Sciences. 2009;28:346–358. [Google Scholar]
- Vangronsveld J, Herzig R, Weyens N, et al. Phytoremediation of contaminated soils and groundwater: lessons from the field. Environmental Science and Pollution Research. 2009;16:765–794. doi: 10.1007/s11356-009-0213-6. [DOI] [PubMed] [Google Scholar]
- Vellosillo T, Martinez M, Lopez M, et al. Oxylipins produced by the 9-lipoxygenase pathway in A. regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell. 2007;19:831–846. doi: 10.1105/tpc.106.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C, Schat H. Mechanisms to cope with arsenic or cadmium excess in plants. Current Opinion in Plant Biology. 2009;12:364–372. doi: 10.1016/j.pbi.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Weyens N, van der Lelie D, Taghavi S, Newman L, Vangronsveld J. Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends in Biotechnology. 2009a;27:591–598. doi: 10.1016/j.tibtech.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Weyens N, van der Lelie D, Taghavi S, Vangronsveld J. Phytoremediation: plant–endophyte partnerships take the challenge. Current Opinion in Biotechnology. 2009b;20:248–254. doi: 10.1016/j.copbio.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Weyens N, Taghavi S, Barac T, et al. Bacteria associated with oak and ash on a TCE-contaminated site: characterization of isolates with potential to avoid evapotranspiration of TCE. Environmental Science and Pollution Research International. 2009c;16:830–843. doi: 10.1007/s11356-009-0154-0. [DOI] [PubMed] [Google Scholar]
- Whiting S, Leake J, McGrath S, Baker A. Positive responses to Zn and Cd by roots of the Zn and Cd hyperaccumulator Thlaspi caerulescens. New Phytologist. 2000;145:199–210. [Google Scholar]
- Wojas S, Clemens S, Sklodowska A, Antosiewicz D. Arsenic response of AtPCS1- and CePCS-expressing plants – Effects of external As(V) concentration on As-accumulation pattern and NPT metabolism. Journal of Plant Physiology. 2010;167:169–175. doi: 10.1016/j.jplph.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Yang J, Kloepper J, Ryu C. Rhizosphere bacteria help plants tolerate abiotic stress. Trends in Plant Science. 2009;14:1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Yoon JM, Oliver DJ, Shanks JV. Phytotransformation of 2,4-dinitrotoluene in A. thaliana: toxicity, fate, and gene expression studies in vitro. Biotechnology Progress. 2006;22:1524–1531. doi: 10.1021/bp0601443. [DOI] [PubMed] [Google Scholar]
- Zhao F, Hu F, Han M, Zhang S, Liu W. Superoxide radical and auxin are implicated in redistribution of root growth and the expression of auxin and cell-cycle genes in cadmium-stressed rice. Russian Journal of Plant Physiology. 2011;58:851–863. [Google Scholar]
- Zhuang X, Chen J, Shim H, Bai Z. New advances in plant growth-promoting rhizobacteria for bioremediation. Environment International. 2007;33:406–413. doi: 10.1016/j.envint.2006.12.005. [DOI] [PubMed] [Google Scholar]