Abstract
Background and Aims
Apoplasmic barriers in plants fulfil important roles such as the control of apoplasmic movement of substances and the protection against invasion of pathogens. The aim of this study was to describe the development of apoplasmic barriers (Casparian bands and suberin lamellae) in endodermal cells of Arabidopsis thaliana primary root and during lateral root initiation.
Methods
Modifications of the endodermal cell walls in roots of wild-type Landsberg erecta (Ler) and mutants with defective endodermal development – scarecrow-3 (scr-3) and shortroot (shr) – of A. thaliana plants were characterized by light, fluorescent, confocal laser scanning, transmission and cryo-scanning electron microscopy.
Key Results
In wild-type plant roots Casparian bands initiate at approx. 1600 µm from the root cap junction and suberin lamellae first appear on the inner primary cell walls at approx. 7000–8000 µm from the root apex in the region of developing lateral root primordia. When a single cell replaces a pair of endodermal and cortical cells in the scr-3 mutant, Casparian band-like material is deposited ectopically at the junction between this ‘cortical’ cell and adjacent pericycle cells. Shr mutant roots with an undeveloped endodermis deposit Casparian band-like material in patches in the middle lamellae of cells of the vascular cylinder. Endodermal cells in the vicinity of developing lateral root primordia develop suberin lamellae earlier, and these are thicker, compared wih the neighbouring endodermal cells. Protruding primordia are protected by an endodermal pocket covered by suberin lamellae.
Conclusions
The data suggest that endodermal cell–cell contact is required for the spatial control of Casparian band development. Additionally, the endodermal cells form a collet (collar) of short cells covered by a thick suberin layer at the base of lateral root, which may serve as a barrier constituting a ‘safety zone’ protecting the vascular cylinder against uncontrolled movement of water, solutes or various pathogens.
Keywords: Apoplasmic (apoplastic) barriers, Arabidopsis thaliana, Casparian band, development, endodermis, lateral roots, lignin, suberin, suberin lamellae
INTRODUCTION
The primary root of Arabidopsis thaliana has a simple tissue and cell organization. Its radial pattern is a result of stereotypical meristematic cell divisions (Dolan et al., 1993). The quiescent centre occurs in a distal region of the meristem and consists of a group of four cells. These cells produce, by anticlinal division, the four groups of initials from which one cell forms the basis for the development of initials of the primary cortex. Cortical initials undergo asymmetric periclinal divisions resulting in two daughter cells. The outer cell forms a file of outer cortical cells, and the inner cell, the proendodermal cell, forms a one-layered file of inner cortical cells, the endodermis (Costa and Dolan, 2000). The asymmetric cell division is regulated by the SCARECROW (SCR) and SHORTROOT (SHR) genes (Di Laurenzio et al., 1996; Helariutta et al., 2000). Their loss of function results in the absence of the typical endodermal layer in the root and development of a single cortical layer. This layer possesses the features of both the outer cortex and endodermis (Di Laurenzio et al., 1996). A single layer of endodermis in plants is defined by an evolutionarily conserved mechanism, where the SCR sequesters the SHR protein and delimits its movement. Furthermore the SHR–SCR complex positively activates the transcription of SCR and in this way a positive feedback loop that promotes endodermal development in a single cell layer forms (Cui et al., 2007).
The endodermis is the innermost layer of primary cortex, which represents a boundary between the vascular cylinder and peripheral root tissues (von Guttenberg, 1968). The number of cells in the endodermis in a cross-section of an A. thaliana primary root is typically eight (Rost et al., 1996). Endodermal cells of vascular plants may develop in three developmental states resulting in primary, secondary or tertiary endodermis, respectively (von Guttenberg, 1968). Primary endodermis is characterized by a close contact between the cell wall and the cytoplasmic membrane in the centre of anticlinal walls (Bryant, 1934; Bonnet, 1968) and it is subsequently extended toward the tangential cell walls. Consequently the electron-dense material deposits in the net of the primary cell wall and a Casparian band is formed continually along the inner surface of anticlinal cell walls (Ma and Peterson, 2003). The exact Casparian band composition of several species is known. For example, the Casparian bands of Clivia miniata consist of lignins, approx. 10 times less suberin, and also of arabinose, hydroxyproline, proline, serine, lysine and other amino acids (Schreiber et al., 1999). The exact chemical composition of A. thaliana Casparian bands is unknown yet due to the difficulties in extracting enough of the cell wall material for chemical analysis.
The endodermis only rarely terminates development in the primary state (Casparian bands), and in almost all species secondary endodermis (suberin lamellae) is developed. The secondary state is characterized by deposition of suberin lamellae on the inner surface of primary cell walls (von Guttenberg, 1968), which is a more efficient apoplasmic barrier compared with the Casparian bands (Peterson et al., 1981; Schreiber et al., 1999; Ranathunge et al., 2005). Suberin lamellae possess a qualitatively similar chemical composition to that of Casparian bands, but the amount of lignins and suberin is reversed, with suberin being dominant (Schreiber et al., 1999). The monomer composition of suberin in A. thaliana consists mostly of 1-alcohols, ω-hydroxyacids, α,ω-diacids and 2-hydroxyacids (Höfer et al., 2008).
Endodermal development proceedes to a so-called tertiary state in some plant species through the deposition of thick, often lignified, non-suberized cell wall material with a small amount of proteins (Schreiber et al., 1999). The development of endodermal cells is not synchronous. An intermediate zone is formed where the endodermal cells in the primary state – passage cells – are surrounded by endodermal cells in the secondary state (Kroemer, 1903).
The endodermis plays several roles. Endodermal tissue controls the transport of water between the outer cortex and pericycle, and its plasma membrane contains large amounts of water-transporting aquaporins (Javot et al., 2003; Bramley et al., 2009). The endodermis also plays a role in maintaining the water pressure in the stele due to its high hydraulic resistance and low radial hydraulic conductivity when compared with stellar tissues (Joshi et al., 2009). It prevents the loss of compounds from the vascular cylinder and controls diffusion and transport of ions (von Guttenberg, 1968; Fernandez-Garcia et al., 2009). Vacuoles of endodermal cells are an iron storage compartment in embryos of A. thaliana (Roschzttardtz et al., 2009). The endodermis may also create a barrier preventing attacks of pathogens such as bacteria and fungi (Schreiber et al., 1999) or penetration of mycorhizal hyphae into the vascular cylinder (Strack et al., 2003). It mechanically supports the root and, in some species, it was shown that the endodermis affects the elasticity of the whole root (Hattori et al., 2003). The endodermis is the primary gibberellin-responsive tissue that regulates organ growth, and endodermal cell expansion is thought to be rate limiting for elongation of the whole root (Ubeda-Tamás et al., 2009).
Apoplasmic barriers develop at a regular distance from the root apex. This distance is species specific and its development is influenced by different environmental stimuli and stress factors. Roots develop Casparian bands and suberin lamellae closer to the root apex as a reaction to cadmium in the growth medium (Zelko and Lux, 2004; Vaculík et al., 2009). Increased suberin and lignin deposition is involved in abiotic stress-regulated responses (Vance et al., 1980; Mitchell et al., 1994; Franke et al., 2009; Lee et al., 2009). The roots of Ricinus communis increase the amount of aliphatic components of suberin after high salinity-induced stress (Schreiber et al., 2005). Extensive apoplasmic barriers develop in rice roots, correlated with reduced Na+ uptake and enhanced survival when challenged with high salinity (Krishnamurthy et al., 2009, 2011; Ranathunge et al., 2011). On the other hand, the endodermal apoplasmic barriers of Phragmites australis reacted to flooding and to hypoxia or anoxia by developing at a greater distance from the root apex. This apparently facilitates the supply of oxygen to the root apical part through the aerenchyma from stems (Soukup et al., 2002). Several genes involved in suberin biosynthesis have been described (Compagnon et al., 2009; Franke et al., 2009; Lee et al., 2009; Panikashvili et al., 2010; Ranathunge and Schreiber, 2011), but still some parts of the suberin biosynthetic pathway are not understood.
The characterization of the development of the endodermal barriers at an ultrastructural level has not been carried out in A. thaliana. In the present study we characterized the development of endodermal apoplasmic barriers in the primary root of wild-type A. thaliana. We also investigated how this process proceeds during the development of lateral roots. Apoplasmic barrier development in the wild type was compared with plants with mutant genes shr and scr in which ground tissue is defective. Our hypothesis was that loss of SHR or SCR function would lead to morphological and topological changes in apoplasmic barrier development.
MATERIALS AND METHODS
Plant material and growth conditions
Wild-type plants of Arabidopsis thaliana [ecotype Landsberg erecta (Ler)] and the mutants shr and scr-3 were used for experiments. Bleach-sterilized seeds of A. thaliana were sown on full-strength MS medium (Murashige and Skoog, 1962), ten seeds per 120 mm round Petri dish with 20 mL of culture medium, and stratified at 4 °C for 2 d in the dark. Thereafter the seedlings were cultured under a 16/8 h light/dark photoperiod, with illumination of approx. 85 µmol m−2 s−1 provided by white fluorescent lamps, at 24 °C and 70 % air humidity. Samples from the apical region (0–10 mm) of seminal roots were collected from 7-day-old plants and processed specifically depending on the type of microscopic investigation.
Fixation and embedding
For light and electron microscopy, samples were fixed in glutaraldehyde (1·5 % v/v) in sodium caccodylate buffer (0·6 m, pH 7·0) for 2 h, rinsed in sodium caccodylate buffer, and post-fixed with an aqueous solution of osmium tetroxide (1·5 % v/v) for 2 h. The samples were then rinsed with buffer and dehydrated through an ethyl alcohol series and propylene oxide, and embedded in Spurr's resin (Spurr, 1969).
Light microscopy
To investigate histological features of endodermal cells, the Spurr-embedded samples were sectioned serially at 2 µm thickness using a microtome (Ultrotome Nova, LKB, Sweden). Sections were stained with toluidine blue (0·5 %) and basic fuchsin (0·1 %) according to Lux (1981). Sections were examined under a light microscope (Axioskop 2 plus, Carl Zeiss, Germany).
Fluorescent microscopy
Casparian bands and suberin lamellae were observed with fluorescent microscopy (filter set Carl Zeiss N. 25: excitation filter TBP 400 + 495 + 570, chromatic beam splitter TFT 410 + 505 + 585 and emission filter TBP 460 + 530 + 610; wavelengths are in nm) after staining the whole-mount roots or their cross-sections with berberine hemisulfate (0·1 % v/v) and toluidine blue (0·5 % v/v) (Brundrett et al., 1988), and Fluorol yellow 088 (0·01 % v/v) (Brundrett et al., 1991), respectively. The clearing method for the observation of the lateral root junction was also used (Lux et al., 2005).
Confocal microscopy
Intact plants of A. thaliana were stained for 90 s in a 10 µm aqueous solution of propidium iodide, rinsed three times with distilled water and investigated with a confocal laser scanning microscope (Olympus BX 62 FW1000, Olympus Corp., Japan) using an excitation beam (538 nm) and emission filter (560–640 nm) for the observation of endodermal cells in the seminal roots.
Transmission electron microscopy
Ultrathin sections of Spurr-embedded root samples were stained for 40 min with uranyl acetate (2 % v/v), 3 min with potassium permanganate (5 % v/v) and 5 min with lead citrate (2 % v/v). Photography was performed in a transmission electron microscope JEM 2000FX (JEOL, Japan).
Cryo-scanning electron microscopy
The root samples were mounted by Tissue-Tek (Agar Scientific Ltd, UK) onto the holder and deep frozen to –190 °C in an Alto 2500 cryostat (Gatan Inc., UK). After being broken at the required position, the samples were etched for 180 s at –90 °C, and thereafter covered with a 2 nm layer of platinum and palladium mixture, again cooled to –135 °C, and observed with a cryo-scanning electron microscope JSM 7401F (JEOL, Japan) at an accelerating voltage of 5 kV.
Digital image analysis
The anatomical and cytological structures, and proportion of Casparian bands and suberin lamellae were compared after digital documentation (digital camera DP72, Olympus) by image analysis software Lucia (v. 4·80, 2002, Laboratory Imaging, Prague, Czech Republic).
Statistical analysis
In each experiment, at least ten primary roots of the same genotype were used for microscopic and all other analyses. The experiments were repeated three times. Data are presented as the mean ± s.e. and they were analysed by one-way analysis of variance (ANOVA; Statgraphics Centurion XV, v. 15·2·05, StatPoint, Inc.).
RESULTS
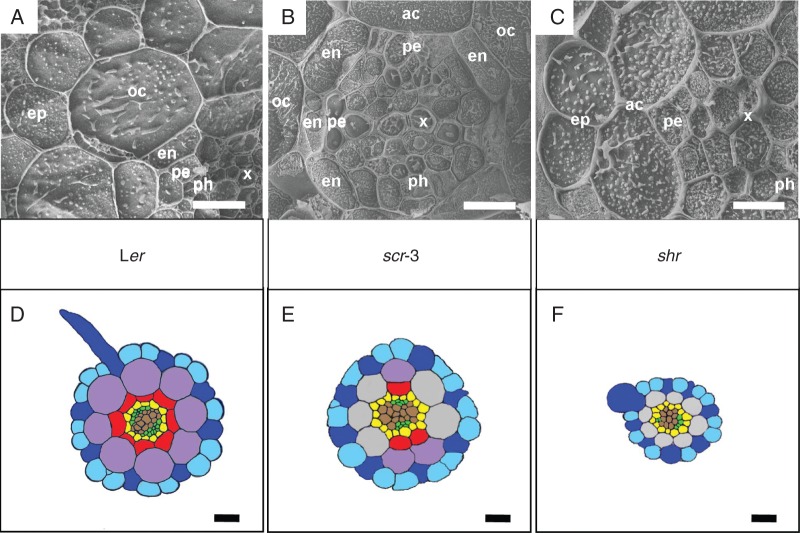
The primary structure of A. thaliana wild-type roots (shown here in 7-day-old ecotype Ler, Fig. 1A, D) has a simple and usually highly regular arrangement of tissues and cells. A single-layered epidermis (ep in Fig. 1A, blue colour in Fig. 1D) is formed by longitudinally arranged files of trichoblasts (dark blue) producing root hairs and files of atrichoblasts (light blue). The cortex consists of only two cell layers. The outer cortical layer (oc in Fig. 1A, violet colour in Fig. 1D) has the character of parenchyma cells without specific cell wall modifications, i.e. no exodermis is formed. The inner cortical layer differentiates as endodermis (en in Fig. 1A, red colour in Fig. 1D), arranged in cross-section as a regular circle of usually eight radially flattened cells. The vascular cylinder is encircled by a single-layered pericycle (pe in Fig. 1A, yellow colour in Fig. 1D), and vascular tissues are diarch, formed by two interconnected xylem poles (x in Fig. 1A, brown colour in Fig. 1D) and two separate phloem poles (ph in Fig. 1A, green colour in Fig. 1D).
Fig. 1.
Details of primary roots of three genotypes of A. thaliana by cryo-SEM (A–C) and the schemas of their anatomy drawn according to the semi-thin sections (D–F). (A, D) The wild-type Ler possesses a unilayered epidermis consisting of trichoblasts (dark blue) and atrichoblasts (light blue), a unilayered outer cortex (violet), a unilayered endodermis (red) and a vascular cylinder with a unilayered pericycle (yellow) and diarch arrangement of vascular tissues. (B, E) The scr-3 mutant possesses a unilayered epidermis consisting of trichoblasts (dark blue) and atrichoblasts (light blue), an incomplete layer of outer cortex (violet), an incomplete layer of atypical cortex (grey), an incomplete layer of endodermis (red) and a vascular cylinder with a unilayered pericycle (yellow) and diarch arrangement of vascular tissues. (C, F) The mutant shr possesses a unilayered epidermis consisting of trichoblasts (dark blue) and atrichoblasts (light blue), a unilayered atypical cortex (grey) and a vascular cylinder with a unilayered pericycle (yellow) and diarch arrangement of vascular tissues; the endodermis is not developed. ac, atypical cortex (grey); ep, epidermis (blue); oc, outer cortex (violet); en, endodermis (red); pe, pericycle (yellow); x, xylem (brown); ph, phloem (green). Scale bars (A, B, D–F) = 20 µm; (C) = 10 µm.
The cortical initial daughter cells do not divide in scr-3 mutants to produce the outer cortical cells and the endodermal cells (Fig. 1B, E). Some divisions occur irregularly which results in a mosaic patterning, where the cortical region is composed either of two cell layers, outer cortical cells (oc in Fig. 1B, violet colour in Fig. 1E) and endodermal cells (en in Fig. 1B, red colour in Fig. 1E), or of a single cell layer of atypical cortical cells (ac in Fig. 1B, grey colour in Fig. 1E). As a result, the scr-3 mutant does not form a complete layer of endodermis on a cross-section, but it is usually arranged in two disconnected semi-circles consisting of 1–3 cells each. The files of the endodermal cells are not continual along the root axis from the base to the apex. The pattern of endodermal cells is not stable in the same root. It changes from three to six endodermal cells on the cross-sections at different distances from the root apex in the same root (whereas the wild-type Ler typically possesses eight files of endodermal cells). Additionally, a reduced number of cells is developed in the vascular cylinder when compared with the wild-type Ler.
The shr mutant roots are morphologically different from either the wild type or scr-3 mutants (Fig. 1C, F). The endodermal cells are not developed; thus the cortical layer consists only of a single layer of atypical cortical cells (ac in Fig. 1C, grey colour in Fig. 1F), and the vascular cylinder possesses a lower number of cells compared with the wild type. The reduced number of cells of the vascular cylinder and non-developed endodermal cells results in a smaller diameter of the primary root.
Casparian band development in the wild-type Ler
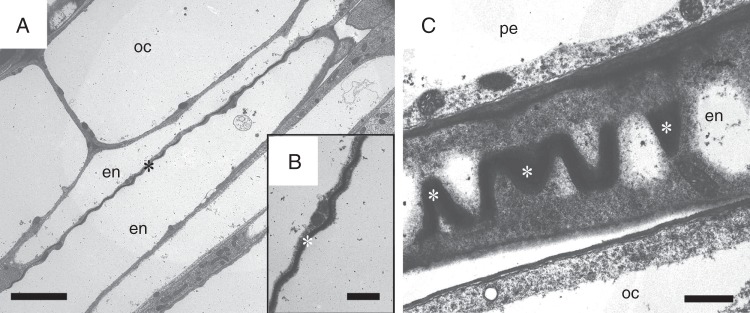
The endodermis develops in two stages in A. thaliana Ler primary roots. The first stage includes Casparian band formation (Fig. 2A). Based on the TEM and confocal laser scanning microscopy, it starts in Ler primary roots close to the root apex, at a distance of 1600 µm from the root cap junction, where the root hairs have already started to grow (Fig. 2B). The endodermal cells starting to form Casparian bands are usually highly vacuolated; however, abundant membranes of endoplasmic reticulum, numerous mitochondria, and dictyosomes of Golgi apparatus are present in the cytoplasm close to the developing Casparian bands (Fig. 2C3–C5). Casparian bands develop continuously in anticlinal endodermal cell walls. Cell wall components characteristic of developing Casparian bands are first deposited from the middle lamella. The early stage of this process can be seen by TEM as the appearance of an approx. 990 nm wide electron-dense area in the middle of the radial and transversal cell walls (Fig. 2C1–C5). The Casparian bands develop first at the proximal pole of the endodermal cell (oriented to the root base) and development continues to the distal pole of the same cell (oriented to the root apex). The process of deposition of Casparian band material continues and its volume not only extends into the middle lamella and within the primary cell wall, but deposits protrude slightly above the level of the primary cell wall. Material of Casparian bands is deposited from the centre of anticlinal endodermal cell walls and afterwards the electron-dense region expands centripetally and centrifugally until the Casparian bands reach their final size. At maturity they are approx. 1220 ± 125 nm wide in anticlinal cell walls when viewed on radial section. This occurs at a distance of 2100 µm from the root cap junction. Mature Casparian bands occupy approx. 0·15 ± 0·01 % of the volume of endodermis and <0·00018 ± 0·00003 % of the whole primary root at this distance from the root apex.
Fig. 2.
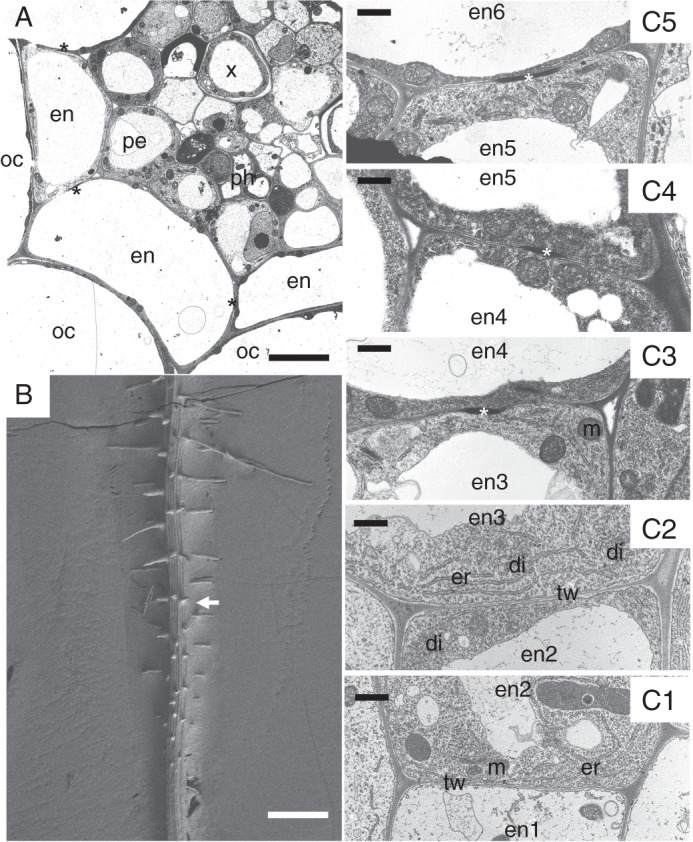
The development of Casparian bands in the primary root of A. thaliana wild-type Ler. (A) The cross-section of root with fully developed Casparian bands (asterisks) in endodermal cells in TEM. (B) Cryo-SEM image of a root with developing root hairs; the approximate region of the beginning of Casparian band development is indicated by an arrow. (C1–C5) The column of six successive endodermal cells (en1–en6) showing gradual development of Casparian bands. The cells closest to the root apex, en1 and en2, do not have Casparian bands. The transversal wall oriented to the root apex of the cell en3 is without a Casparian band, whereas the transversal wall oriented to the root base of the same cell starts to deposit a Casparian band. The width of Casparian bands is gradually increasing in successive endodermal cells, en4–en6. en, endodermis; di, dictyosome; er, endoplasmic reticulum; m, mitochondrion; oc, outer cortex; pe, pericycle; tw, transversal cell wall. Scale bars (A) = 5 µm; (B) = 250 µm; (C1–C5) = 0·5 µm.
The cell wall in the region of the Casparian band has a wavy shape (Fig. 3). This shape possesses specific waves in the space as it can be seen in both tangential (Fig. 3A, B) and radial sections (Fig. 3C). The length of the wave is irregular along the Casparian band and varies between 1417 and 2287 nm, with an amplitude of 260 ± 69 nm. Fully developed Casparian bands of all individual endodermal cells form a network of cylindrical shape. In the primary state of the root this network separates the apoplasm of the inner regions of the root (the vascular cylinder and centripetal part of the endodermis) from the apoplasm of the external regions of the root (centrifugal part of the endodermis, the rest of cortex and the epidermis).
Fig. 3.
Shape of Casparian bands. (A–C) The cell wall with a Casparian band has a specific wavy shape as can be seen in both tangential (A, B) and radial sections (C). oc, outer cortex; en, endodermis; pe, pericycle; asterisk, Casparian band. Scale bars (A) = 5 µm; (B, C) = 1 µm.
Endodermal cell–cell contact is required for the spatial control of Casparian band development
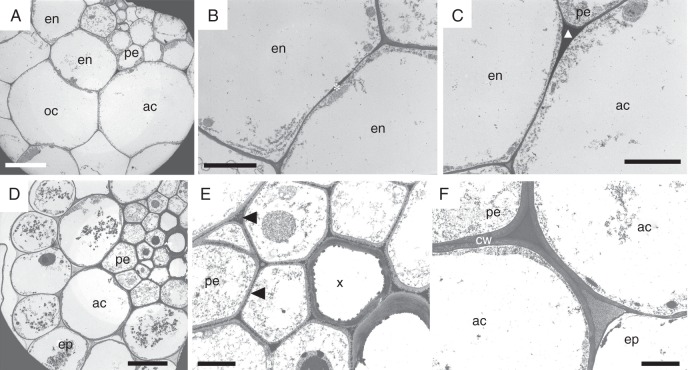
The cortex of the scr-3 mutant has a mosaic pattern consisting of typical endodermal cells, outer cortical cells and atypical cortical cells (Fig. 4A), resulting in a combination of two different localizations (typical and ectopic) of Casparian band-like material. The Casparian bands are deposited in the same position as in the wild type (in the centre of anticlinal cell walls) when two adjacent endodermal cells develop (Fig. 4B). In regions where no endodermal cells develop, the cortex comprises only a single layer of atypical cortical cells. In these cells the Casparian band-like material is deposited in this case in the centripetal junction of adjacent anticlinal cell walls of these atypical cortical cells (Fig. 4C). The same deposition pattern occurs when the developed endodermal cell and atypical cortical cell are attached by their anticlinal cell walls.
Fig. 4.
The deposition of Casparian band-like material in the primary root of A. thaliana scr-3 and shr mutants observed by TEM. (A–C) Cross-section of an scr-3 root with an incomplete endodermal layer; the cortex consists of a mosaic of typical endodermal cells, outer cortical cells and atypical cortical cells originating from the tangentially non-divided cortical initials. (B) Neighbouring endodermal cells develop Casparian bands (asterisk) in a typical position within radial cell walls. (C) Atypical cortical cells deposit Casparian band-like material (triangle) in the centripetal junctions of their anticlinal cell walls. (D–F) Cross-section of an shr root with non-developed endodermis. (E) The cortex consists of a single layer of atypical cortical cells without Casparian bands; however, Casparian band-like material (arrowheads) is deposited ectopically in the middle lamellae of vascular cylinder cells. (F) Atypical cortical cells form thicker cell walls in comparison with the wild-type Ler. ac, atypical cortical cell; cw, cell wall; en, endodermis; ep, epidermis; oc, outer cortex; pe, pericycle; x, xylem. Scale bars (A) = 5 µm; (B, C, E, F) = 2 µm; (D) = 10 µm.
A single layer of cells forms in the place of the endodermis and cortical cell layers in the primary root of shr mutants (Fig. 4D). These cortical cells do not form typical Casparian bands like those found in the wild type. However, electron-dense material similar to the Casparian band material is present in the form of numerous punctate deposits in the middle lamellae of vascular cylinder cells (Fig. 4E). An additional feature of these mutant roots is an increased width of cortical cell walls (Fig. 4F) compared with the wild-type Ler.
Development of suberin lamellae
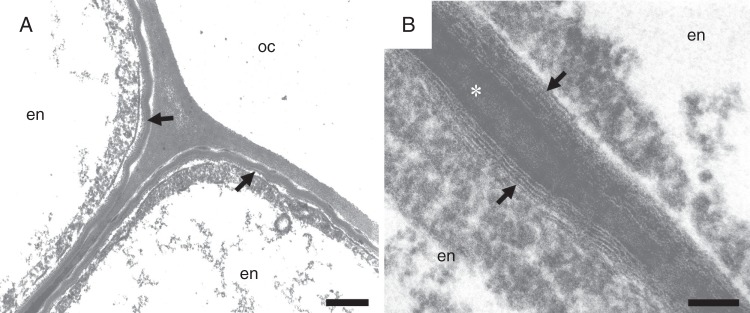
Cell wall modifications continue in wild-type Ler to a secondary stage where lamellar suberin is deposited on the inner surface of primary cell walls (Fig. 5A). This process usually starts at a distance of approx. 7500 µm from the root cap junction and approx. 5900 µm from the beginning of the site of Casparian band deposition. While this deposit appears as compact homogeneous material when imaged by cryo-scanning electron microscopy, by TEM it is seen to comprise alternating electron-dense and electron-translucent lamellae arranged parallel to the primary cell wall surface (Fig. 5B). The widths of the electron-dense and electron-translucent lamellae are 2·7–5·8 and 4·7 nm, respectively. The thickness of suberin deposits depends on the developmental stage of the cells. It usually differs not only in neighbouring endodermal cells, but also within the same cell. Suberin lamellae are first deposited in areas where the connection between tangential and anticlinal endodermal cell walls occurs. Suberin lamellae continue their development on the inner tangential cell wall, and after that on the outer tangential wall and on the anticlinal walls. The thinnest deposits are attached above the Casparian bands, and the thickest are in the regions of junctions of several cells. The thickness of suberin lamellae deposits increases with the increasing age of endodermal cells, i.e. with their increasing distance from the root apex. The maximum width of suberin lamellae deposits in 7-day-old Ler plants is 51 ± 7 nm.
Fig. 5.
Suberin lamellae covering endodermal cell walls of a primary root of A. thaliana wild-type Ler. (A, B) Suberin lamellae (arrows) possess the typical structure of alternating electron-dense and electron-translucent material. Arrow, suberin lamellae; asterisk, Casparian band; en, endoermis; oc, outer cortex. Scale bars (A) = 100 nm; (B) = 250 nm.
The process of suberin lamellae development does not start in all endodermal cells at the same distance from the apex. It starts first in the cells opposite the phloem elements in the vascular cylinder and thereafter in the cells adjacent to the xylem poles. At a distance of approx. 9800 µm from the root cap junction the whole cylinder of endodermal cells becomes covered by suberin lamellae and the endodermis is completely in the secondary state. At this distance from the root apex, suberin lamellae occupy approx. 2·39 ± 0·32 % of the endodermis and <0·00293 ± 0·00041 % of the whole primary root.
Endodermal cells of scr-3 mutants form suberin lamellae similar to the wild type. However, atypical cortical cells of scr-3 roots do not form any suberin lamellae. In the shr mutant, the cortical cells do not form suberin lamellae at all. Thus the typical endodermal apoplasmic barriers – formed in wild-type Ler by Casparian bands and suberin lamellae – are not developed in shr primary roots.
Development of endodermal cells in the vicinity of lateral root primordium
An early stage of the lateral root formation is represented by dividing pericycle cells. The endodermal cells surrounding this initiating root primordium respond early to this event. In the Ler genotype it occurs in primary roots at a distance of 7000–8000 µm from the root cap junction (the average length of the whole root is 45 300 ± 4100 µm). This process starts in the primary root within the area of the first stage of endodermal development, when endodermal cell walls possess only Casparian bands. However, the cells neighbouring the lateral root primordium preferentially proceed to the second stage, as shown by the deposition of suberin lamellae. This can be seen in the whole-mount roots cleared and stained by Fluorol yellow 088 and observed under fluorescence (Fig. 6A). The process of suberin lamellae deposition proceeds only gradually in the neighbouring cells as these cells mature. The endodermal cells surrounding the developing lateral root primordium form a pocket (Wurzeltasche) covered by suberin lamellae (Fig. 6B, C). This pocket covers and protects the primordium protruding through the peripheral tissues. The newly formed endodermal cells of the lateral root at its base are short and soon become protected by Casparian bands and suberin lamellae. At the base of a lateral root, a distinct collet (collar) of short endodermal cells is formed, which interconnects the endodermal network of the primary root with the network of a newly formed lateral root. This ‘safety zone’ is clearly visible after fluorescent staining at the basal parts of grown lateral roots, which are immersed in the tissues of the primary root (Fig. 6D).
Fig. 6.
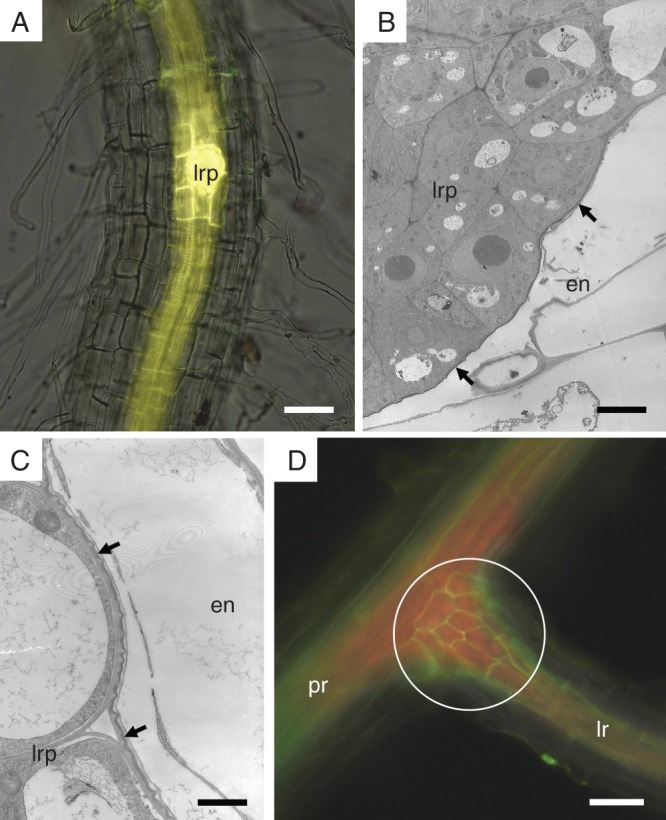
The deposition of suberin lamellae related to the development of the lateral root. (A) Endodermal cells surrounding the lateral root primordium are the first endodermal cells depositing suberin lamellae on their primary cell walls (fluorescent microscopy of a whole-mount root cleared and stained by Fluorol Yellow 088). (B and C) Lateral root primordium is covered by endodermal cells containing a protective layer of suberin lamellae (arrows); observed by TEM. (D) A collet of short cells (circle) develops at the base of the lateral root and interconnects the endodermal network of the primary root with the network of a newly formed lateral roots (fluorescent microscopy of whole-mount root cleared and stained by Fluorol Yellow 088). Arrow, suberin lamellae; circle, region of endodermal collet; en, endodermis; lr, lateral root; lrp, primordium of lateral root; pr, primary root. Scale bars (A) = 50 µm; (B) = 5 µm; (C) = 1 µm; (D) = 80 µm.
The development of Casparian bands and suberin lamellae in endodermal cells of A. thaliana Ler primary root related to the distance from the root apex and influenced by the lateral root primordium formation is summarized and shown schematically in Fig. 7.
Fig. 7.
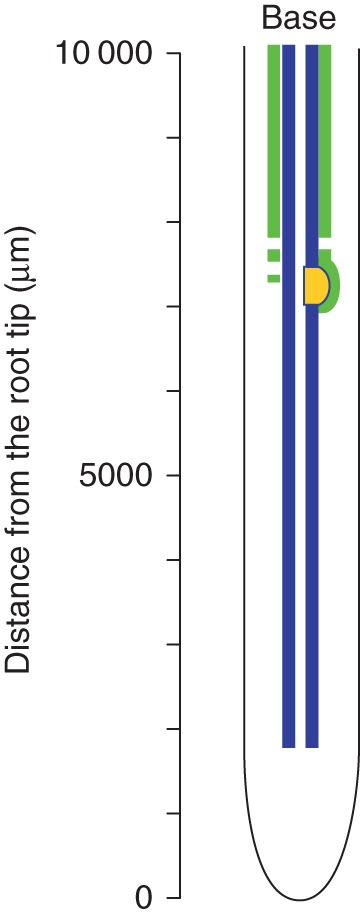
The schema indicating development of endodermal cells in the primary root of A. thaliana wild-type Ler. Casparian bands (blue) develop close to the root apex; suberin lamellae (green) develop first in endodermal cells surrounding the lateral root primordium (yellow).
DISCUSSION
The endodermal cells of the A. thaliana primary root develop in two stages – primary and secondary – which is consistent with observations in many other dicotyledonous species (von Guttenberg, 1968). Both stages of endodermal development are characterized by deposition of specific cell wall material. This helps to form the barrier controlling radial uptake and loss of water and solvents between the vascular cylinder and peripheral tissues, and the barrier against potential pathogen invasion (Schreiber et al., 1999; Strack et al., 2003).
In the primary stage, Casparian band development starts by forming a close contact between the cytoplasmic membrane and the cell wall, which results in specific band plasmolysis under hypertonic conditions (Ma and Peterson, 2001). A group of Casparian strip membrane domain proteins (CASPs) have been identified, which specifically mark a cytoplasmic membrane domain that predicts the formation of the Casparian band. Double mutants of CASPs exhibit disorganized Casparian bands (Roppolo et al., 2011). Similar to many other species (Ma and Peterson, 2001, 2003), Casparian band development in wild-type A. thaliana plants continues by deposition of electron-dense material in a typical position approximately at the centre of the anticlinal cell walls, and after that the bands are widened toward the tangential cell walls. Casparian bands in A. thaliana Ler, when first detected, are about 990 nm wide. The average width of Casparian bands in this early state in Zea mays is 920 nm (Uetake et al., 2001) and that of Pisum sativum is approx. 1000 nm (Karahara and Shibaoka, 1992). Casparian bands of A. thaliana are formed as wavy lamellae. The function of this feature is unknown. The same shape of Casparian bands was observed in the roots of several species, e.g. Iris germanica (Meyer et al., 2009) and P. sativum (Karahara and Shibaoka, 1992) in intact cells under fluorescent microscopy. Interestingly, isolated Casparian bands do not possess this specific shape (Karahara and Shibaoka, 1992). This shape may be maintained in intact cells to ensure the extensibility of endodermal cells to some degree.
We observed a large number of mitochondria, vesicles of the endoplasmatic reticulum and dictyosomes in the cytoplasm surrounding the developing Casparian bands. This is in agreement with TEM observation of endodermal development in Allium cepa (Ma and Peterson, 2001). It can be assumed that those organelles are involved in the development of apoplasmic barriers. There is an assumption that lignin and suberin precursors synthesized inside endodermal cells are transported to the cytoplasmic membrane, released from protoplasts, oxidized by peroxidases bound to the cell walls and polymerized (Whetten and Sederoff, 1995; Franke and Schreiber, 2007).
The distance of Casparian band formation from the root apex is species specific, varies within species, and also depends on the rate of root growth (Lux et al., 2004; Martinka and Lux, 2004). The Casparian bands of A. thaliana Ler develop at a distance of 1600 µm from the root apex. It is similar to the results from the Columbia ecotype, where the membrane attachment and the lateral barrier for lipid tracer form at 11–12 endodermal cells from the boundary between the meristematic and elongation region (Alassimone et al., 2009), which means approx. 1500–1600 µm from the root cap junction. This distance can be much longer in other species: 10 000 µm in A. cepa (Barnabas and Peterson, 1992) and 13 000 µm in Z. mays (Uetake et al., 2001). Moreover the distance of Casparian band development is modified by external factors (e.g. Ma and Peterson, 2003; Lux et al. 2011; Vaculík et al., 2012) and it is also different for different categories of roots. Casparian bands mature closer to the root apex in the lateral roots compared with the primary root in Hordeum vulgare (Robards et al., 1973) and Camellia sinensis (Tanimoto et al., 2004). Their extremely early development (250 µm from the apex) was observed in non-growing lateral roots of Vicia faba compared with actively growing primary root of the same species (5000 µm from the apex) (Peterson and Lefcourt, 1990).
We assume that the endodermal cell–cell contact and presence of a position signal are required for spatial control of Casparian band localization. The evidence indicating this possibility results from the localization of Casparian band-like material in an atypical position in some mutant plants. Di Laurenzio et al. (1996) characterized the scr mutant of A. thaliana, where the root cortex consists of only one layer of cells with combined features of outer cortical and endodermal cells. According to these authors, this single layer of cortical cells possesses Casparian bands. It seems that in the scr-3 mutant observed by us there is not complete loss of function of the SCR product required for development of the endodermis (Cui et al., 2007), and therefore the mosaic composition of the root cortex is developed. The cortex of the scr-3 mutant consists of endodermal cells, outer cortical cells and atypical cortical cells. This results in the typical and ectopic localization of Casparian bands and Casparian band-like material in the same root. When two adjacent endodermal cells are developed, some hypothesized positional signal is present in the correct place, approximately in the middle of anticlinal cell walls. The Casparian bands develop in this case in the typical location. In the case when cortical initials do not divide into the endodermal and outer cortical cell, a file of atypical cortical cells is developed. These cells probably do not have the capacity to deposit the positional signal in the correct place and the result is the deposition of the Casparian band-like material ectopically in the centripetal junction of anticlinal cell walls. The deposition of Casparian bands closer to the inner tangential wall was also observed in P. sativum plants (Karahara and Shibaoka, 1992). However, to the best of our knowledge, there is no evidence about the localization of Casparian bands only in the junctions of adjacent anticlinal cell walls of cortex and outer tangential cell walls of the pericycle in any wild-type plants. We have also observed the ectopic localization of electron-dense material similar to Casparian band material in the shr mutant characterized by the absence of endodermis. Unlike in the scr-3 plants, the Casparian band-like material is distributed in a spotted pattern in the middle lamellae of vascular cylinder cells. This indicates that this cell wall modification is formed independently of cell identity, and a signal to initiate its formation is not endodermis dependent. These data also indicate that the formation of apoplasmic barriers in the cell walls is a crucial feature of the vascular plants to control the flow of water and solvents in the apoplasmic space, even when the endodermis is not developed.
After the primary state, the endodermal cells proceed to the secondary state and formation of suberin lamellae. The endodermis of A. thaliana develops suberin lamellae at first along the inner and later along the outer tangential cell walls. The opposite process of suberin lamellae deposition was found in A. cepa where they develop first along the outer and thereafter along the inner tangential cell walls (Ma and Peterson, 2001). The thickness of suberin depositions is not consistent within the same cell. Waduwara et al. (2008) have found that endodermal suberin lamellae are perforated by pores in onion roots, which could serve as areas for water and ions to enter the cytoplasm of the endodermis. Suberin deposits in the endodermis of A. thaliana root observed by TEM have a typical structure of alternating electron-dense and electron-translucent lamellae parallel with the surface of the primary cell wall. This structure can be formed due to the chemical nature of suberin, which consists of aliphatic and aromatic components (Bernards et al., 2002). Unlike electron-dense lamellae with variable width, the electron-translucent lamellae are of a stable width (Nawrath 2002). This may result from the variable size of aliphatic components and a relatively stable size of molecules of aromatic components (Compagnon et al., 2009).
The close contact between the cytoplasmic membrane and the cell wall, typical for the first state of endodermal development – Casparian bands – is disrupted after formation of suberin lamellae (Enstone and Peterson, 1997). The distance of suberin lamellae deposition from the root apex varies as in the case of Casparian bands. It depends on the plant species, age of the plant, the rate of individual root growth (Wilcox, 1962; Robards et al., 1973; Perumalla and Peterson, 1986), the growing conditions and the presence of environmental stressors such as drought, salinity, toxic metals, nitrate and oxygen supply (Soukup et al., 2002; Zelko and Lux, 2004; Martinka and Lux, 2004; Schreiber et al., 2005; Lux et al., 2011). Whereas the suberin lamellae of A. thaliana start to develop at a distance of 7000–8000 µm from the root apex as observed in our case, the suberin lamellae deposition starts at 80 000 µm in Z. mays (Zeier et al., 1999) and at 50 000 µm in C. sinensis (Tanimoto et al., 2004). Earlier development than we found in A. thaliana was observed in the root of Gentiana cultivated in soil, at a distance of 5000 µm from the root apex (Šottníková and Lux, 2003). The intermediate zone between state one and two of endodermal development of A. thaliana primary roots is relatively short. This is typical for many dicotyledonous plants where the outer cortex dies relatively early and endodermis temporarily takes the role of protective outer layer until the formation of periderm (von Guttenberg, 1968).
An interesting finding is the early development of suberin lamellae in endodermal cells surrounding developing lateral root primordia. The pocket surrounding a lateral root primordium protruding through the cortex of primary root was observed and described earlier as ‘Wurzeltasche’ (von Guttenberg, 1968). In the present study, it was found to be formed by endodermal cells in the secondary state covered by suberin lamellae and it may serve as a protection for lateral root primordia during their development and growth through the peripheral tissues of the primary root. This endodermal pocket may also protect the vascular cylinder of the primary root against an invasion of pathogens from the rhizosphere and it can help to control the radial transport of water and ions to and from the vascular cylinder. During ongoing growth of the lateral root its endodermis becomes interconnected with the endodermis of the primary root. This connection in the case of A. thaliana is formed by a collet (collar) consisting of short endodermal cells. The endodermal collet may represent ‘a safety zone’ for a mechanically weak region of the junction between the primary root and lateral root. This apoplasmic barrier safety zone seems to be in some aspects analogous to the hydraulic safety zone formed by short tracheal elements on the base of lateral roots (Luxová, 1990). Probably the main function of the endodermal collet is to provide the protection and safety of the vascular cylinder of the main root. The uncontrolled flow of water and solutes may occur after damage to the area of the lateral root primordium junction (Peterson et al., 1981).
The endodermal apoplasmic barriers represented by Casparian bands and suberin lamellae spatially occupy only <0·15 and 2·39 % of the endodermis, respectively. Despite this small volume, they can fulfil several important functions such as control of transport of ions, compounds and fluids between the middle cortex and vascular cylinder, protection against pathogens, regulation of oxygen supply, etc. (Peterson et al., 1981; Moore et al., 2002; Soukup et al., 2002; Strack et al., 2003).
From the results obtained, several questions have arisen and further investigations will be needed to study the hypothesized signal for endodermal apoplasmic barrier development, the function of the endodermal pocket and collet at the base of lateral roots, and the mechanisms controlling their development.
ACKNOWLEDGEMENTS
This work was supported by the Slovak Grant Agency [VEGA 1/0817/12]; and by the Slovak Research and Development Agency, contract No. APVV-0140-10. We thank members of the Laboratory of Electron Microscopy in České Budějovice for the possibility to work on the cryo-scanning electron microscope, and for their help and technical assistance. We thank Professor Thomas L. Rost for his critical comments and kind revision of this manuscript.
LITERATURE CITED
- Alassimone J, Naseer S, Geldner N. A developmental framework for endodermal differentiation and polarity. Proceedings of the National Academy of Sciences, USA. 2010;107:5214–5219. doi: 10.1073/pnas.0910772107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabas AD, Peterson CA. Development of Casparian bands and suberin lamellae in the endodermis of onion roots. Canadian Journal of Botany. 1992;70:2233–2237. [Google Scholar]
- Bernards MA. Demystifying suberin. Canadian Journal of Botany-Revue Canadienne de Botanique. 2002;80:227–240. [Google Scholar]
- Bonnett HTJ. The root endodermis: fine structure and function. Journal of Cell Biology. 1968;37:199–205. doi: 10.1083/jcb.37.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD. Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behaviour of roots. Plant Physiology. 2009;150:348–364. doi: 10.1104/pp.108.134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA. A berberine–aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma. 1988;146:133–142. [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA. Efficient lipid staining in plant material with Sudan red 7B or Fluoral yellow 088 in polyethylene glycol–glycerol. Biotechnic and Histochemistry. 1991;66:111–116. doi: 10.3109/10520299109110562. [DOI] [PubMed] [Google Scholar]
- Bryant AE. A demonstration of the connection of the protoplast of the endodermal cells with the Casparian strips in the roots of barley. New Phytologist. 1934;33:231. [Google Scholar]
- Compagnon V, Diehl P, Benveniste I, et al. CYP86B1 is required for very long chain ω-hydroxyacid and α,ω-dicarboxylic acid synthesis in root and seed suberin polyester. Plant Physiology. 2009;150:1831–1843. doi: 10.1104/pp.109.141408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa S, Dolan L. Development of the root pole and cell patterning in Arabidopsis roots. Current Opinion in Genetics and Development. 2000;10:405–409. doi: 10.1016/s0959-437x(00)00104-0. [DOI] [PubMed] [Google Scholar]
- Cui H, Levesque MP, Vernoux T, et al. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science. 2007;316:421–425. doi: 10.1126/science.1139531. [DOI] [PubMed] [Google Scholar]
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, et al. The SCARE-CROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86:423–433. doi: 10.1016/s0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, et al. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA. Suberin deposition and band plasmolysis in the corn (Zea mays L.) root exodermis. Canadian Journal of Botany. 1997;75:1188–1199. [Google Scholar]
- Fernandez-Garcia N, Lopez-Perez L, Hernandez M, Olmos E. Role of phi cells and the endodermis under salt stress in Brassica oleracea. New Phytologist. 2009;181:347–360. doi: 10.1111/j.1469-8137.2008.02674.x. [DOI] [PubMed] [Google Scholar]
- Franke R, Schreiber L. Suberin – a biopolyester forming apoplastic plant interfaces. Current Opinion in Plant Biology. 2007;10:252–259. doi: 10.1016/j.pbi.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Franke R, Höfer R, Briesen I, et al. The DAISY gene from Arabidopsis thaliana encodes a fatty acid elongase condensing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza–micropyle region of seeds. The Plant Journal. 2009;57:80–95. doi: 10.1111/j.1365-313X.2008.03674.x. [DOI] [PubMed] [Google Scholar]
- von Guttenberg H. Der primäre Bau der Angiospermenwurzel. Handbuch der Pflanzenanatomie. Berlin/Stuttgart: Gebrüder Borntraegern; 1968. 472. [Google Scholar]
- Hattori T, Inanaga S, Tanimoto E, Lux A, Luxová M, Sugimoto Y. Silicon-induced changes in viscoelastic properties of Sorghum root cell walls. Plant and Cell Physiology. 2003;44:743–749. doi: 10.1093/pcp/pcg090. [DOI] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root trough radial signalling. Cell. 2000;101:555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- Höfer R, Briesen I, Beck M, Pinot F, Schreiber L, Franke R. The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid ω-hydroxylase involved in suberin monomer biosynthesis. Journal of Experimental Botany. 2008;59:2347–2360. doi: 10.1093/jxb/ern101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, et al. Role of a single aquaporin isoform in root water uptake. The Plant Cell. 2003;15:509–522. doi: 10.1105/tpc.008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Knipfer T, Steudle E. Effects of water storage in the stele on measurements of the hydraulics of corn and barley. New Phytologist. 2009;184:631–643. doi: 10.1111/j.1469-8137.2009.02994.x. [DOI] [PubMed] [Google Scholar]
- Karahara I, Shibaoka H. Isolation of Casparian strip in pea roots. Plant and Cell Physiology. 1992;33:555–561. [Google Scholar]
- Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Schreiber L, Mathew MK. The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.) Planta. 2009;230:119–134. doi: 10.1007/s00425-009-0930-6. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Ranathunge K, Nayak S, Schreiber L, Mathew MK. Root apoplastic barriers block Na(+) transport to shoots in rice (Oryza sativa L.) Journal of Experimental Botany. 2011;62:4215–4228. doi: 10.1093/jxb/err135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer K. Wurzelhaube, Hypodermis und Endodermis der Angiospermenwurzel. Bibliotheca Botanica. 1903;59:49–54. [Google Scholar]
- Lee S-B, Jung S-J, Go Y-S, et al. Two Arabidopsis 3-ketoacyl CoA synthase genes, KCS20 and KCS2/DAISY, are functionally redundant in cuticular wax and root suberin biosynthesis, but differentially controlled by osmotic stress. The Plant Journal. 2009;60:462–475. doi: 10.1111/j.1365-313X.2009.03973.x. [DOI] [PubMed] [Google Scholar]
- Lux A. A rapid method for staining of semi-thin sections of plant material. Biologia (Bratislava) 1981;36:753–757. (in Slovak) [Google Scholar]
- Lux A, Luxová M, Abe J, Morita S. Root cortex: structural and functional variability and responses to environmental stress. Root Research. 2004;13:117–131. [Google Scholar]
- Lux A, Morita S, Abe J, Ito K. An improved method for clearing and staining free-hand sections and whole-mount samples. Annals of Botany. 2005;96:989–996. doi: 10.1093/aob/mci266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Martinka M, Vaculík M, White PJ. Root responses to cadmium in the rhizosphere: a review. Journal of Experimental Botany. 2011;62:21–37. doi: 10.1093/jxb/erq281. [DOI] [PubMed] [Google Scholar]
- Luxová M. Effect of lateral root formation on the vascular pattern of barley roots. Botanica Acta. 1990;103:305–310. [Google Scholar]
- Ma F, Peterson CA. Development of cell wall modifications in the endodermis and exodermis of Allium cepa roots. Canadian Journal of Botany. 2001;79:621–634. [Google Scholar]
- Ma F, Peterson CA. Current insights into the development, structure, and chemistry of the endodermis and exodermis of roots. Canadian Journal of Botany. 2003;81:405–421. [Google Scholar]
- Martinka M, Lux A. Response of roots of three populations of Silene dioica to cadmium treatment. Biologia. 2004;59(Suppl. 13):185–189. [Google Scholar]
- Meyer CJ, Seago JL, Jr, Peterson CA. Environmental effects on the maturation of the endodermis and multiseriate exodermis of Iris germanica roots. Annals of Botany. 2009;103:687–702. doi: 10.1093/aob/mcn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell HJ, Hall JL, Barber MS. Elicitor-induced cinnamyl alcohol dehydrogenase activity in lignifying wheat (Triticum aestivum L.) leaves. Plant Physiology. 1994;104:551–556. doi: 10.1104/pp.104.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Bowen HC, Scrase-Field S, Knight MR, White PJ. The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevations in root endodermal cells subjected to cooling. The Plant Journal. 2002;30:457–465. doi: 10.1046/j.1365-313x.2002.01306.x. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Nawrath C. The biopolymers cutin and suberin. In: Somerville CR, Meyerowitz EM, editors. The Arabidopsis book. Rockville, MD: American Society of Plant Biologists; 2002. pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D, Shi JX, Bocobza S, Franke RB, Schreiber L, Aharoni A. The Arabidopsis DSO/ABCG11 transporter affects cutin metabolism in reproductive organs and suberin in roots. Molecular Plant. 2010;3:563–575. doi: 10.1093/mp/ssp103. [DOI] [PubMed] [Google Scholar]
- Perumalla CJ, Peterson CA. Deposition of Casparian bands and suberin lamellae in the exodermis and endodermis of young corn and onion roots. Canadian Journal of Botany. 1986;64:1873–1878. [Google Scholar]
- Peterson CA, Lefcourt BEM. Development of endodermal Casparian bands and xylem in lateral roots of broad bean. Canadian Journal of Botany. 1990;68:2729–2735. [Google Scholar]
- Peterson CA, Emanuel ME, Humphreys GB. Pathway of movement of apoplastic fluorescent dye tracers through the endodermis at the site of secondary root formation in corn (Zea mays) and broad bean (Vicia faba) Canadian Journal of Botany. 1981;59:618–625. [Google Scholar]
- Ranathunge K, Schreiber L. Water and solute permeabilities of Arabidopsis roots in relation to the amount and composition of aliphatic suberin. Journal of Experimental Botany. 2011;62:1961–1974. doi: 10.1093/jxb/erq389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, Steudle E, Lafitte R. A new precipitation technique provides evidence for the permeability of Casparian bands to ions in young roots of corn (Zea mays L.) and rice (Oryza sativa L.) Plant, Cell and Environment. 2005;28:1450–1462. [Google Scholar]
- Ranathunge K, Lin JX, Steudle E, Schreiber L. Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCl permeabilities in rice (Oryza sativa L.) roots. Plant, Cell and Environment. 2011;34:1223–1240. doi: 10.1111/j.1365-3040.2011.02318.x. [DOI] [PubMed] [Google Scholar]
- Robards AW, Jackson SM, Clarkson DT, Sanderson J. The structure of barley roots in relation to the transport of ions into the stele. Protoplasma. 1973;77:291–311. [Google Scholar]
- Roppolo D, De Rybel B, Tendon VD, et al. A novel protein family mediates Casparian strip formation in the endodermis. Nature. 2011;473:380–383. doi: 10.1038/nature10070. [DOI] [PubMed] [Google Scholar]
- Roschzttardtz H, Conéjéro G, Curie C, Mari S. Identification of the endodermal vacuole as the iron storage compartment in the Arabidopsis embryo. Plant Physiology. 2009;151:1329–1338. doi: 10.1104/pp.109.144444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost TL, Baum SF, Nichol S. Root apical organization in Arabidopsis thaliana ecotype ‘WS’ and a comment on root cap structure. Plant and Soil. 1996;187:91–95. [Google Scholar]
- Schreiber L, Hartmann K, Skrabs M, Zeier J. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. Journal of Experimental Botany. 1999;50:1267–1280. [Google Scholar]
- Schreiber L, Franke R, Hartmann K. Effects of NO3 deficiency and NaCl stress on suberin deposition in rhizo- and hypodermal (RHCW) and endodermal cell walls (ECW) of castor bean (Ricinus communis L.) roots. Plant and Soil. 2005;269:333–339. [Google Scholar]
- Sottnikova A, Lux A. Development, dilation and subdivision of cortical layers of gentian (Gentiana asclepiadea) root. New Phytologist. 2003;160:135–143. doi: 10.1046/j.1469-8137.2003.00863.x. [DOI] [PubMed] [Google Scholar]
- Soukup A, Votrubová O, Čížková H. Development of anatomical structure of roots of Phragmites australis. New Phytologist. 2002;153:277–287. [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Strack D, Fester T, Hause B, Schlieman W, Walter MH. Arbuscular mycorrhiza: biological, chemical and molecular aspects. Journal of Chemical Ecology. 2003;29:1955–1979. doi: 10.1023/a:1025695032113. [DOI] [PubMed] [Google Scholar]
- Tanimoto E, Homma T, Matsuo K, Hoshino T, Lux A, Luxova M. Root structure and cell-wall extensibility of adventitious roots of tea (Camellia sinensis L. cv. Yabukita) Biologia. 2004;59(Suppl. 13):57–66. [Google Scholar]
- Ubeda-Tamás S, Federici F, Casimiro I, et al. Gibberellin signalling in the endodermis controls Arabidopsis root meristem size. Current Biology. 2009;19:1194–1199. doi: 10.1016/j.cub.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Uetake Y, Ikeda A, Kondo F, Karahara I. The 6th Symposium of the International Society of Root Research, 11–15 November 2001. Nagoya: Japan; 2001. Radial widening of the Casparian strip in primary roots of maize under salt stress. [Google Scholar]
- Vaculík M, Lux A, Luxová M, Tanimoto E, Lichtscheidl I. Silicon mitigates cadmium inhibitory effects in young maize plants. Environmental and Experimental Botany. 2009;67:52–58. [Google Scholar]
- Vaculík M, Konlechner C, Langer I, et al. Root anatomy and element distribution vary between two Salix caprea isolates with different Cd accumulation capacities. Environmental Pollution. 2012;163:117–126. doi: 10.1016/j.envpol.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Kirk TK, Sherwood RT. Lignification as a mechanism of disease resistance. Annual Review of Phytopathology. 1980;18:259–288. [Google Scholar]
- Waduwara CI, Walcott SE, Peterson CA. Suberin lamellae of the onion root endodermis: their pattern of development and continuity. Botany-Botanique. 2008;86:623–632. [Google Scholar]
- Whetten R, Sederoff R. Lignin biosynthesis. The Plant Cell. 1995;7:1001–1013. doi: 10.1105/tpc.7.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox H. Growth studies of the root of incense cedar, Libocedrus decurrens. 1. The origin and development of primary tissues. American Journal of Botany. 1962;49:221–236. [Google Scholar]
- Zeier J, Ruel K, Ryser U, Schreiber L. Chemical analysis and immunolocalisation of lignin and suberin in endodermal and hypodermal/rhizodermal cell walls of developing maize (Zea mays L.) primary roots. Planta. 1999;209:1–12. doi: 10.1007/s004250050601. [DOI] [PubMed] [Google Scholar]
- Zelko I, Lux A. Effect of cadmium on Karwinskia humboldtiana roots. Biologia. 2004;59(Suppl. 13):205–209. [Google Scholar]