SUMMARY
The molecular components largely responsible for muscle attributes such as passive tension development (titin and collagen), active tension development (myosin heavy chain, MHC) and mechanosensitive signaling (titin) have been well studied in animals but less is known about their roles in humans. The purpose of this study was to perform a comprehensive analysis of titin, collagen and MHC isoform distributions in a large number of human muscles, to search for common themes and trends in the muscular organization of the human body. In this study, 599 biopsies were obtained from six human cadaveric donors (mean age 83 years). Three assays were performed on each biopsy – titin molecular mass determination, hydroxyproline content (a surrogate for collagen content) and MHC isoform distribution. Titin molecular mass was increased in more distal muscles of the upper and lower limbs. This trend was also observed for collagen. Percentage MHC-1 data followed a pattern similar to collagen in muscles of the upper extremity but this trend was reversed in the lower extremity. Titin molecular mass was the best predictor of anatomical region and muscle functional group. On average, human muscles had more slow myosin than other mammals. Also, larger titins were generally associated with faster muscles. These trends suggest that distal muscles should have higher passive tension than proximal ones, and that titin size variability may potentially act to ‘tune’ the protein's mechanotransduction capability.
KEY WORDS: titin, collagen, myosin heavy chain, fiber type, muscle design
INTRODUCTION
Skeletal muscle physiological studies have been carried out in many different species, including rat, rabbit, mouse, human, frog, fish, cat and bird. These studies provide the underlying basis of our understanding of how muscles function. Most studies have focused on specific functional groups of muscles (such as rotator cuff muscles, shank muscles or quadriceps), enabling investigators to concentrate their efforts and explore these systems in depth. However, using this approach makes it difficult to gain perspective across the overall musculoskeletal system of a particular animal. This situation is even more limited in humans. The invasive nature of many common muscle experiments and the difficulties associated with obtaining high quality tissue for research means that the majority of what is known about human muscle physiology is based on studies of large and superficial muscles (such as vastus lateralis, soleus and tibilais anterior (Bergstrom, 1962; Bergstrom, 1975; Edwards et al., 1975; Larsson and Salviati, 1992). The degree to which these are representative of overall human muscle physiology is unknown.
The purpose of this study was to perform a comprehensive analysis of human muscle biochemical properties to search for common themes and trends in the muscular organization of the body. We elected to study well-known components of active tension development (myosin heavy chain, MHC) (Schiaffino and Reggiani, 1994), passive tension development (titin and collagen) (Granzier and Irving, 1995; Prado et al., 2005) and mechanosensitive signaling (titin) (Lange et al., 2005). These components of muscle have been described in animal models (Prado et al., 2005; Eng et al., 2008) and in limited numbers of human muscles. By quantifying these components in a large number of muscles and revealing patterns using a variety of analytical methods, we have elucidated a number of formerly undescribed trends and characteristics. These data may also be useful in determining the extent to which animals provide useful model systems for studying human muscle, as well as providing a better understanding of how the human muscular system functions.
MATERIALS AND METHODS
Muscle biopsies
One-hundred biopsy sites from 92 individual muscles were sampled from six fresh cadaveric donors who were participants in the University of Minnesota's Anatomy Bequest Program. Exclusion criteria included history of spinal cord injury, muscular dystrophy, stroke, polio, myasthenia gravis or Guillain-Barré syndrome. For multi-headed muscles (biceps femoris, biceps brachii, triceps, gastrocnemius), biopsies were obtained from each head. Biopsies were taken from multiple locations for repeated muscles (1st and 4th lumbricals, 1st and 4th dorsal interossei, 1st and 3rd palmar interossei of the hand). For muscles with multiple tendinous insertions (extensor digitorum communis, flexor digitorum superficialis and flexor digitorum profundus), a single biopsy was obtained midway between the origin and the proximal aspect of the distal tendons. Extensor digiti quinti was analyzed distinct from extensor digitorum communis. All biopsies were obtained from all donors except for opponens digiti minimi in one donor.
Biopsies were approximately 5×5×20 mm in size. In muscles with linear or simple geometry, they were generally obtained from a position midway between the proximal and distal muscle–tendon junctions; in muscles with complex shapes (such as latissimus, pectoralis major and trapezius), biopsies were obtained from the approximate geometric midpoint of the muscle. For all biopsies, every effort was made to avoid inclusion of tendon, intermuscular septa or epimysial components of extracellular matrix that would confound muscle collagen quantification. Biopsies were flash frozen in liquid nitrogen (–196°C) after excision; the interval from official time of death to snap freezing ranged from 155 to 630 min, which was crucial to avoid titin degradation. Studies have revealed the presence of the T2 degradation isoform at times as early as 30-45 min postmortem (Bérard et al., 2008; Fritz and Greaser, 1991; Huff Lonergan et al., 2010), with different rates of T1 degradation across muscles. Biopsies were kept on dry ice or at –80°C until analysis.
Titin molecular mass
Titin molecular mass was quantified by a method developed previously (Warren et al., 2003). Briefly, muscle biopsies were pulverized and homogenized using an overhead stirrer (Caframo Limited, model BDC2002, Warton, ON, Canada) in a 40:1 sodium dodecyl sulfate-vertical agarose gel electrophoresis (SDS-VAGE) sample buffer to frozen tissue mass (v/w) ratio and stored at –80°C until analyzed by gel electrophoresis. SDS-VAGE sample buffer was composed of 8 mol l–1 urea, 2 mol l–1 thiourea, 30% glycerol v/v, 3% SDS w/v, 75 mmol l–1 DTT, 0.03% Bromophenol Blue and 0.05 mol l–1 Tris-Cl, pH 6.8 (Warren et al., 2003). An acrylamide plug (12.8% acrylamide, 10% v/v glycerol, 0.5 mol l–1 Tris-Cl, 2.34% N,N-diallyltartardiamide, 0.028% ammonium persulfate and 0.152% TEMED) was placed at the bottom of the gel to hold the agarose in place. Agarose gel [1% w/v Sea Kem Gold agarose (Lonza, Basel, Switzerland), 30% v/v glycerol, 50 mmol l–1 Tris-base, 0.384 mol l–1 glycine and 0.1% w/v SDS] was poured over the acrylamide plug and kept warm to prevent premature solidification.
Titin standards were obtained from human soleus and rat cardiac muscle, which have known molecular masses of 3716 and 2992 kDa, respectively, based on sequence analysis of the 300 kilobase titin gene with a coding sequence contained within 363 exons (Labeit and Kolmerer, 1995; Freiburg et al., 2000). These tissues were also homogenized and stored at –80°C until analysis. Before loading onto the gel, a titin standards ‘cocktail’ was created by mixing 2 μl human soleus and 2 μl rat cardiac standards into 6 μl sample buffer. Sample wells were then loaded with both biopsy and rat cardiac homogenate. Four standard lanes, containing the human soleus and rat cardiac titin homogenates, were evenly distributed throughout the gel, to be later used for titin quantification. Gels were run on a dual slab gel chamber (C.B.S. Scientific, Del Mar, CA, USA) at 4°C for 5 h at 25 mA constant current.
Agarose gels were fixed and stained according to the Silver Stain Plus (BioRad, Hercules, CA, USA) procedure, except that gels were dried for ∼20 h at 40°C immediately after fixing. Gels were subsequently rinsed and stained as described in the Silver Stain Plus procedure (supplementary material Fig. S1). The relative mobility and intensity of each band was quantified using a GS-800 Calibrated Densitometer (BioRad) and Quantity One 1-D Analysis software (Bio-Rad). It has been demonstrated that using this method, relative mobilities of proteins on a gel are linearly related to the log of their molecular mass from 500 to 4000 kDa (Warren et al., 2003). This relationship was used to calculate molecular mass of the experimental titins based on their positions relative to the rat cardiac and human soleus titin standards [see fig. 1 of Ward et al. (Ward et al., 2009)]. Other investigators have reported multiple titin isoforms in single muscles (Prado et al., 2005; Ward et al., 2009). However, based on the large volumes loaded onto these gels as well as the large muscle samples homogenized, our methods were not sensitive enough to detect multiple isoforms. The values reported here should be considered weighted averages. Not all samples generated useable data with this titin assay. Thus, the sample size used for titin molecular mass analysis is 586 rather than the total sample size of 599.
MHC composition
Muscle tissue was homogenized using a cell disrupter (Bullet Blender, Next Advance, Averill Park, NY, USA). Homogenized protein solution was resuspended to 0.125 μg μl–1 protein (BCA protein assay, Pierce, Rockford, IL, USA) in a sample buffer consisting of 100 mmol l–1 DTT, 2% SDS, 80 mmol l–1 Tris-base pH 6.8, 10% glycerol and 0.01% w/v Bromophenol Blue. Samples were boiled (2 min) and stored at –80°C. Before protein was loaded onto the gel, it was further diluted 1:20 (0.006 μg μl–1) in the sample buffer; 10 μl of each sample were loaded in each lane, resulting in 0.06 μg protein in each lane. Total acrylamide concentration was 4% and 8% in the stacking and resolving gels, respectively (bisacrylamide, 1:50). Gels (16×22 cm, 0.75 mm thick) were run at a constant current of 10 mA for 1 h, and thereafter at a constant voltage of 275 V for 20 h at 4–6°C. Gels were silver stained (BioRad) and MHC bands were identified and quantified with densitometry (GS-800, BioRad) as previously described (Talmadge and Roy, 1993). The progression of the band was compared and identified based on its relative molecular mass to that of a human protein standard prepared (as described above) from a normal semitendinosus biopsy that showed all three human MHC bands (IIa, IIx and I). Serial dilutions demonstrated that protein could be detected at concentrations as low as 0.00154 μg μl–1.
Collagen content
Hydroxyproline (OH-P) content of muscle was used to determine collagen percentage using a modification of a previously published protocol (Edwards and O'Brien, 1980). Samples of tissue (20–25 mg) were hydrolyzed in 6 mol l–1 HCl at 110°C for 18 h and neutralized with NaOH to pH 6.98–7.04. Samples were then incubated with a chloramine T solution for 20 min at room temperature, followed by addition of a p-dimethylaminobenzaldehyde solution and incubation at 60°C for 30 min. OH-P concentration was determined by spectrophotometry at 550 nm and was normalized to the wet mass of the original tissue sample. OH-P standard solutions provided a calibration curve for spectrophotometry. OH-P content was used to calculate collagen amount using a constant (7.46) that corresponds to the average number of OH-P residues in a collagen molecule (Neuman and Logan, 1950).
Statistical analysis
Data are reported as means ± s.e.m. unless otherwise stated. Significance level (α) was set to 0.05 and all statistical tests were carried out using SPSS (version 19, IBM, Armonk, NY, USA) or Matlab (Mathworks, Natick, MA, USA). Data were screened for normality (by ensuring that skew and kurtosis were between –1.0 and 1.0) to justify the use of parametric statistical tests. OH-P data required the use of a log transformation to restore acceptable skew and kurtosis to <|1.0|. One- and two-way analyses of variance (ANOVA) were used to test for differences between groups. We measured a number of variables for each muscle, but the interrelationships (if any) among the variables and the anatomical organization (if any) are not known. Thus, we used the multivariate method of stepwise discriminant function analysis (DFA) to identify the variables that best predicted muscle group, using F-to-enter=3.84 and F-to-remove=2.71. These F-value limits were used because they are the default settings for DFA in SPSS. For DFA, muscles were classified by anatomical region (axial N=9, shoulder N=13, brachium N=7, antebrachium N=20, hand N=13, pelvic/gluteal N=10, thigh N=15, leg N=13), by primary function (flexor N=29, extensor N=34, abductor N=9, adductor N=13, internal rotator N=7, external rotator N=8), by antigravity (N=50) versus non-antigravity (N=50) status, and by joints crossed (single N=48, multiple N=52). A full list of muscles and their classifications is available in supplemental material Table S1. Cases without valid data for all independent variables cannot be included in DFA, so sample size for DFA was 586. Linear regression was used to define the relationship between titin mass and MHC percentage.
RESULTS
Donor characteristics
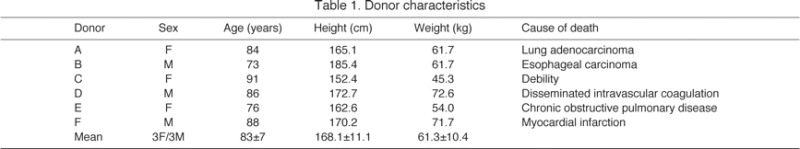
Biopsies were obtained from six donors (three male, three female) aged 83±3 years (Table 1). No major anatomical variants were noted and all donors had palmaris longus and plantaris muscles, which are often considered vestigial. Opponens digiti minimi could not be identified bilaterally on one donor.
Table 1.
Donor characteristics
Descriptive statistics
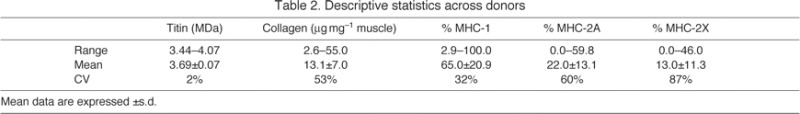
The grand mean ± s.d. of all samples was 3.69±0.07 MDa for titin molecular mass, 13.1±7.0 μg mg–1 for collagen wet mass, 65.0±20.9% for MHC-1, 22.0±13.1% for MHC-2A and 13.0±11.3% for MHC-2X (Table 2). Thus, humans should be considered relatively ‘large and slow’ mammals. The MHC distribution data are similar to previously published data from a smaller study (Johnson et al., 1973). Full data for all muscles are available in supplementary material Table S1.
Table 2.
Descriptive statistics across donors
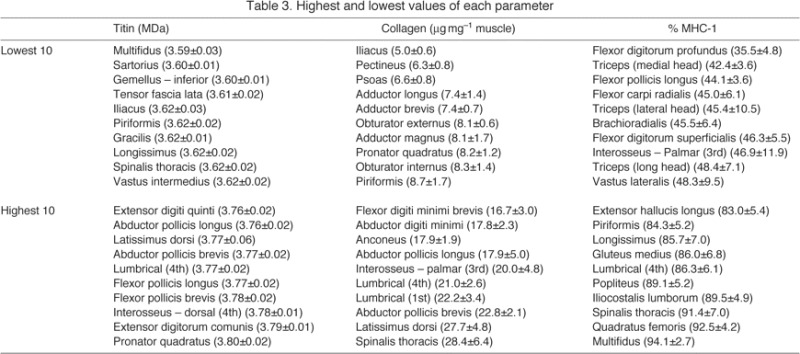
Muscles with the highest mean titin molecular mass values were generally upper extremity muscles, while those with the lowest values were more likely to be axial or pelvic/gluteal muscles (Table 3). Similar patterns were seen in collagen content (Table 3). The ‘slowest’ (highest percentage MHC-1) muscles were generally axial muscles, while the ‘fastest’ were generally found in the upper extremity (brachium and antebrachium, Table 3). Multifidus was a muscle of extremes, with both the shortest titins and the highest percentage MHC-1. Forearm muscles were also found at the boundaries, with pronator quadratus having the largest titin and flexor digitorum profundus having the lowest percentage MHC-1.
Table 3.
Highest and lowest values of each parameter
Grouping by anatomical region
One-way ANOVA using anatomical region as the factor demonstrated significant differences between anatomical regions for titin molecular mass, collagen content, and percentage MHC-1, MHC-2A and MHC-2X (P<0.001 for all parameters). Titin isoform size was higher in distal muscles of both upper and lower extremities (Fig. 1A). This trend was also observed for collagen (except for muscles in the shoulder region, Fig. 1B). Percentage MHC-1 data followed a pattern similar to collagen in the upper extremity but this was not consistent in the lower extremity (Fig. 1C).
Fig. 1.
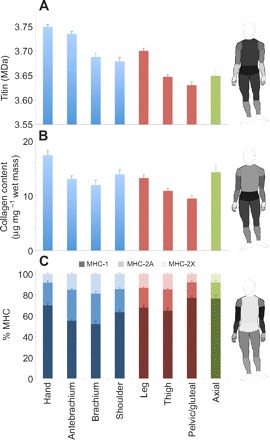
(A) Titin molecular mass, (B) collagen content and (C) percentage myosin heavy chain (% MHC) distribution in different anatomical regions of the human (represented by different colors). Shading in C indicates different MHC isoforms. Shading of regions on the human figures represents magnitude, where a lighter shade corresponds to a higher value (higher titin molecular mass, more collagen, greater percentage MHC-1). Note the significant variations across the body and the similarities in titin and collagen trends.
Two-way ANOVA of titin molecular mass, using proximity to body core (proximal: shoulder/pelvis, middle: brachium/thigh, distal: antebrachium/leg) and limb (upper extremity, lower extremity) as factors, revealed significant differences depending on proximity (P<0.001) and limb (P<0.001). It also revealed that these main effects of location were not different in upper versus lower extremities (P=0.69). These results thereby demonstrate a systematic proximal–distal variation in titin that is similar for upper and lower extremities.
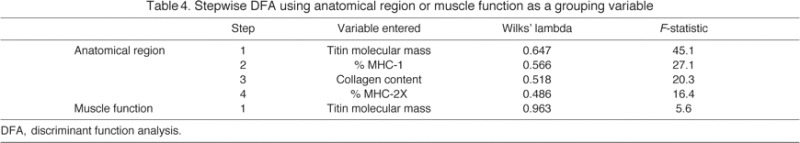
DFA using anatomical region as the grouping variable was conducted to determine the strongest discriminating variables among groups (the discriminant functions generated are shown in supplementary material Table S4). Box's M test indicated that the assumption of equality of covariance matrices was violated (P<0.001). However, given the large sample, this problem was not regarded as serious (Burns and Burns, 2008). The strongest predictive parameters, in order, were titin molecular mass, percentage MHC-1, collagen content and percentage MHC-2X (Table 4); cross-validated classification showed that 36.3% of muscles were classified in their correct anatomical region using these parameters. This was 2.9 times better than random classification, which would have correctly predicted group membership of 12.5% of cases.
Table 4.
Stepwise DFA using anatomical region or muscle function as a grouping variable
Grouping by function
One-way ANOVA using primary function as the factor revealed significant differences between groups for titin molecular mass (P<0.001), collagen content (P=0.01) and percentage MHC-2X (P=0.01) (Fig. 2). However, only titin molecular mass had a large enough F-statistic to be included in stepwise DFA; the discriminant function generated using titin molecular mass (supplementary material Table S4) as the predictive parameter correctly classified 34.0% of cross-validated cases (∼2.0 times better than random classification, which would correctly predict 16.7% of cases) (Table 4).
Fig. 2.
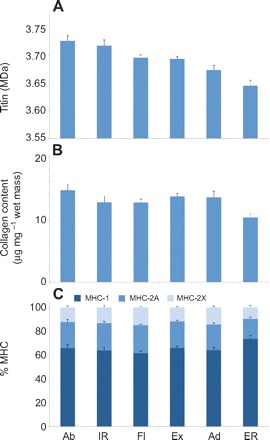
(A) Titin molecular mass, (B) collagen content and (C) percentage MHC distribution in muscles with different functions. In some cases, significant differences exist across muscles of different function but correlated trends between different molecular parameters are less clear than in Fig. 1. Abbreviations: Ab, abduction; IR, internal rotation; Fl, flexion; Ex, extension; Ad, adduction; ER, external rotation.
Antigravity versus non-antigravity muscles
Student's t-test between antigravity (N=50) and non-antigravity (N=50) muscles demonstrated significant differences for percentage MHC-1 (P=0.02) and percentage MHC-2X (P=0.01) (supplementary material Fig. S2). Percentage MHC-2X was the only parameter included in DFA (supplementary material Table S2); the discriminant function generated (supplementary material Table S4) using percentage MHC-2X as the predictive parameter correctly classified 54.8% of cross-validated cases (only marginally better than random classification, which would correctly predict 50.0% of cases).
Muscles that cross single versus multiple joints
Student's t-test between muscles that cross single (N=48) versus multiple (N=52) joints demonstrated significant differences for collagen content (P=0.03) and percentage MHC-2X (P=0.04) (supplementary material Fig. S3). Both parameters were included in DFA; collagen content was found to be a stronger predictive parameter (supplementary material Table S3). Using the function created by these parameters (supplementary material Table S4), 54.8% of cross-validated cases were correctly classified (again, only marginally better than random classification, which would correctly predict 50.0% of cases).
Interparameter relationships
A previous report of 37 rabbit muscles demonstrated a relationship between muscle speed and titin isoform (Prado et al., 2005). This is particularly interesting because a relationship between these two parameters suggests an interaction between active and passive tension development systems. In rabbits, slower muscles (higher average fractions of type-1 fibers by ATPase staining and higher percentage MHC-1 by SDS-PAGE) were found to have higher average titin molecular masses (Prado et al., 2005). When we performed a similar analysis on our dataset, we also found a significant linear relationship (P=0.001), but the correlation was opposite – slower muscles were found to have smaller titin molecular masses (Fig. 3A). A significant linear relationship was also observed between titin and percentage MHC-2A (P<0.001, Fig. 3B) but not between titin and percentage MHC-2X. The relationship between titin and collagen content was also significant (P<0.001, Fig. 3D). To determine whether the difference in the sign of the correlation was due to the limited number of muscles sampled by Prado and colleagues (Prado et al., 2005), we restricted our analysis to the 27 muscles in common between the two studies. Interestingly, we found that, when considering the same muscles, the relationship not only still had the opposite sign of the regression slope (P=0.004) but also explained a larger amount of titin molecular mass variability than when all muscles were considered (for all muscles R2=0.09, for these 27 muscles R2=0.33). In addition, these differences were enhanced when the muscles considered were restricted to ‘fast’ rabbit muscles (all except adductor brevis and soleus). Thus, we believe that the differences observed in the two studies are not due to selection bias but instead reflect a fundamental difference in muscular organization between the two species.
Fig. 3.
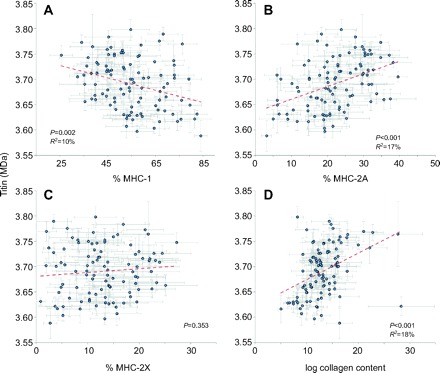
Relationships among titin molecular mass and (A) percentage MHC-1, (B) percentage MHC-2A, (C) percentage MHC-2X and (D) collagen content. Dashed lines are linear regression results; collagen content data (μg mg–1) were log transformed because independent variable data should be normally distributed for linear regression analysis. Note the significant negative correlation between titin mass and percentage MHC-1, which is opposite to that reported for rabbit muscle (Prado et al., 2005).
DISCUSSION
Although titin molecular mass, collagen content and MHC percentages have been measured in a few muscles of several animal species, comparatively little is known about their roles and relative contributions in human skeletal muscles. This study investigated organizational themes of these parameters across the human body. We sampled 100 muscles across the entire body with the exception of head and neck muscles and intrinsics of the foot. There are very few human muscle studies with sample sizes over 500; most are descriptive (Gaudy et al., 2001) or make use of non-invasive techniques (Kawakami et al., 2006). Our sample size of 599 is the largest dissection-based human muscle study. Further, other large studies have generally been carried out on a small group of muscles across a large population; our study reports data from many muscles across a few individuals. This reflects a difference in goals – whereas other studies typically attempt to obtain very accurate information about a few muscles, our goal was to gain general information about a wide population of muscles. Thus, the data reported here represent a robust human muscle study with a unique goal.
Diversity of skeletal muscle
Titin molecular mass ranged from 3.4 to 4.1 MDa in our experimental sample; mean titin molecular mass for individual muscles ranged from 3.6 to 3.8 MDa. Prior to this study, human muscle titins were known to range in size from ∼3.0 MDa for cardiac titin to 3.70 MDa for soleus titin (Freiburg et al., 2000; Labeit and Kolmerer, 1995), and the genetic coding capacity for the largest titin is 4.2 MDa (Bang et al., 2001). The data reported here greatly expand upon the known size diversity of titin molecular mass in human skeletal muscle. In fact, 48 of the 100 muscles under study had titin molecular masses of over 3.70 MDa. When viewed in the context of average eukaryotic proteins, this size diversity seems large – approximately the size of 3.5–4 human proteins (Brocchieri and Karlin, 2005). However, this variability can be also viewed as small, as it is only ∼5% of the total mass of titin. Further, variability within a single muscle (average s.d. of each muscle=47 kDa) is on a par with the variability in the total sample population (total s.d. for all samples=69 kDa), which clearly demonstrates that titin molecular mass is tightly regulated. Similar variability patterns are seen in collagen content (average s.d. of log transformed data=0.18, total s.d.=0.22) and MHC percentage (MHC-1 average s.d.=17.4%, total s.d.=20.9%; MHC-2A average s.d.=10.6%, total s.d.=13.1%; MHC-2X average s.d.=9.8%, total s.d.=11.3%). Titin has the smallest average s.d.:total s.d. ratio, indicating that, of these properties, it is the most specific to each muscle. However, the similarity between average s.d. and s.d. of the entire sample population for each factor measured implies that there is likely not enough variability in any of these parameters to fully account for the larger degrees of variability observed in physiological measurements. It is likely that structural considerations such as muscle architecture are more strongly predictive of muscle function.
Grouping trends
When muscles were grouped by anatomical region, muscle function, antigravity versus non-antigravity function, or single versus multiple joint crossings, the most striking patterns were observed in anatomical region groupings (Fig. 1). In the upper and lower extremities, titin molecular mass and collagen content decreased from distal to proximal (except for shoulder collagen), increasing again for axial muscles.
When considered in the context of passive tension, the titin and collagen values predict opposite effects. When titin is discussed as a molecular spring responsible for development of passive tension in the myocyte (Granzier and Irving, 1995), its differing levels of stiffness are thought to arise from differential splicing of a single titin gene (Labeit and Kolmerer, 1995), resulting in longer, more compliant isoforms that give rise to lower passive tension while shorter isoforms are stiffer and yield higher passive tension (Horowits, 1992; Wang et al., 1991). The trend in titin molecular mass would therefore suggest that passive tension should be low in distal muscles and high in proximal muscles. However, increased collagen content is associated with increased passive tension; our data thus suggest that passive tension would be high in distal muscles and low in proximal muscles. Our data also suggest that, physiologically, the role of titin may not be to bear the majority of passive tension as the distal muscles, based on titin molecular mass, should be more compliant. We therefore claim that titin size variability is likely to have a different physiological role, potentially acting to ‘tune’ the protein's mechanotransduction capability. A similar relationship between titin size and collagen content has been observed in cardiac muscle, suggesting that stiffening (increased in passive tension) of cardiac muscle may occur independent of titin's contributions to passive tension (Neagoe et al., 2002).
It is important to note that most previous studies of titin's role in passive muscle stiffness were carried out using single fiber or even myofibrillar preparations (Prado et al., 2005; Horowits, 1992; Gollapudi and Lin, 2009; Granzier and Irving, 1995). A recent review summarizing several studies that scaled from single fibers to fiber bundles (thereby adding extracellular matrix, ECM), reported that the modulus changed by factors ranging from less than 1 to as high as 16-fold (Gillies and Lieber, 2011). This suggests two major points: both titin and ECM contribute to passive tension, and the relative contributions differ among muscles. Steps toward resolving the latter issue could have been taken in the present study by conducting similar passive tension experiments on the biopsies but as the effect of postmortem time on passive tension is not known, these experiments were not performed.
In addition to the trends observed for titin and collagen content, differences were seen in MHC content across different areas of the body. There are many possible organizing principles that could account for the trends observed; we present one such explanation here but it is important to note that this is merely one of many potentially viable interpretations of the data. These trends may reflect a combination of the importance of motor control and fatigue resistance. Muscles with a higher percentage MHC-1 are generally more fatigue resistant because of the reliance on oxidative phosphorylation for energy production, rather than glycolysis. In addition, slow motor units are generally composed of small axons that innervate few fibers, while the reverse is true for fast motor units. The high MHC-1 content of muscles of the hand may thereby reflect the importance of precise manual dexterity. However, dexterity must be augmented by a good range of motion and force production capability to maximize its utility. These parameters are related to muscle fiber length and physiological cross-sectional area (PCSA), respectively. The size of the antebrachium allows placement of muscles with fiber lengths and PCSAs large enough to provide sufficient excursion and force at the digital and carpal joints, enhancing the intrinsic dexterity of the hand. The lower amounts of slow myosin found in these muscles implies that force production is prioritized over fatigue resistance and that motor control of forearm muscles does not need to be as precisely regulated as those of the hand. This trend of lower percentage MHC-1 in muscles with large PCSA and fiber lengths is amplified in the brachium and then reversed in muscles of the shoulder. This may be because the shoulder is responsible for accurately positioning the hand in three-dimensional space while also supporting the weight of the brachium and antebrachium, for which fatigue resistance and good motor control are both paramount. The percentage MHC-1 of the muscles of the axial skeleton and lower extremity is higher than that of most of the upper extremity regions. Many of these muscles are frequently activated because of their postural and support function so the high percentage MHC-1 is likely a reflection of the importance of fatigue resistance of these muscle groups.
Titin as a predictive factor
When grouped by anatomical region or function, DFA demonstrated that titin was the factor that best discriminated between different groups. DFA also revealed that percentage MHC-2X and collagen content were the factors that best predicted antigravity versus non-antigravity function and single versus multiple joint crossings, respectively. However, use of these two parameters correctly classified 54.8% of muscles, barely better than the 50% correct classification that would be expected based upon random chance. So, we do not feel that conclusions based on these results are very powerful or predictive of function. The very high discriminating ability of titin is partly due to its low variability within a specific muscle [average titin molecular mass coefficient of variation (CV) for individual muscles=1%], which is dramatically less than either the MHC or collagen content data (average CV of 28% for MHC-1 and 44% for collagen content). The finding that titin molecular mass is a good predictor of anatomical location or muscle function suggests that titin's mechanotransduction capability (to the extent that it depends on molecular mass) is muscle specific. There is no question that titin can function as a mechanotransducer (Lange et al., 2005) but the precise molecular nature or even the protein subdomains responsible for mechanotransduction are not yet well defined.
Comparison with other mammals
Animals are frequently used as models to better understand human physiology. Thus, it was relevant to examine trends observed in animal systems and determine whether similar trends exist in humans. Understanding how physiology differs between humans and other mammals is important in terms of knowing how to properly interpret animal studies.
A previous study that examined similar parameters in the rat hindlimb found that plantarflexors were ‘slower’ than dorsiflexors, ascribing this to the greater antigravity function of the plantarflexors (Eng et al., 2008). However, we found that, on average, human dorsiflexors were slower, although this relationship was not statistically significant (P=0.10, 1–β=0.37). Further, we found opposite relationships at each joint in the lower extremity [Fig. 4; cf. fig. 4 from Eng et al. (Eng et al., 2008)]. The differences across the ankle are likely due to the fact that rodents are digigrade while humans are plantargrade. Differences across the knee may be due to the fact that rat knee extensor activity is relatively higher than that of humans as their gait pattern relies on a much more flexed knee compared with humans.
Fig. 4.
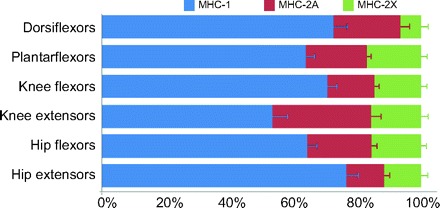
Comparison of MHC percentages for each functional muscle group in the lower extremity. Note that at each joint of the lower extremity there are differences between flexors and extensors opposite to those seen in the rat hindlimb (Eng et al., 2008).
In addition, previous work in rabbits has suggested that there may be a link between the active and passive tension development systems, as observed in the relationship between titin molecular mass and percentage MHC-1 (Prado et al., 2005). However, across our human study population, we observed a relationship opposite to that reported for rabbit muscle [Fig. 3A; cf. figs 7a and 7d from Prado et al. (Prado et al., 2005)]. Therefore, it appears that if any interaction between active and passive tension production systems exists, the nature of this interaction is fundamentally different in human versus rabbit muscle systems.
Limitations
Despite the greatest of care being taken during study design and experimental execution, this study has several limitations. We understand that the values obtained in our assays do not represent the general human population. Rather, they are most representative of an elderly Caucasian population living in Minnesota in the area surrounding Minneapolis.
It is exceedingly difficult to obtain biopsies of multiple muscles from donors who are not deceased. In addition, most young, healthy deceased tissue donors are justifiably prioritized to organ and tissue donation for transplant. Further, the time constraints created by the rapid post-mortem degradation of titin meant that the great majority of donors eligible for our study were of advanced age. The effects of aging on MHC isoform distribution have been studied in humans (Lexell et al., 1983; Lexell, 1995; Lexell et al., 1988; Larsson, 1983) and rodents (Caccia et al., 1979; Kanda and Hashizume, 1989; Kadhiresan et al., 1996). It is understood that aging brings about a loss in fibers (Lexell et al., 1988) and a decrease in type II/type I fiber number (Caccia et al., 1979; Larsson, 1983; Kanda and Hashizume, 1989) and fiber area ratio (Larsson, 1983; Arbanas et al., 2010); this age-related remodeling of motor units appears to involve denervation of fast fibers with reinnervation from nerves that innervate slow fibers (Kadhiresan et al., 1996). We thereby expect the MHC percentages in our study population to be skewed towards higher percentage MHC-1 amounts. A recent study using the same MHC determination method reported values of ∼30% MHC-1 for both gracilis and semitendinosus muscles in a population of normal children with an average age of 16 (Smith et al., 2011). For comparison, the percentage MHC-1 in this study for gracilis and semitendinosus was 65.5±8.5% and 60.5±9.3%, respectively. However, the focus of our study places a higher priority on relative MHC values between muscles, not between people. While it might be possible that the ‘slowing’ of muscles with age happens differentially between muscles (thereby limiting the application of our conclusions), the lack of studies investigating multiple muscle regions makes it impossible to adequately address this concern.
MHC-1, -2A and -2X proteins are all encoded by different genes in the human genome. This is different from titin, which is encoded by a single gene; different isoforms are generally thought to arise as products of alternative splicing (Labeit and Kolmerer, 1995). Because of this difference, upregulation or downregulation of a single gene can modify MHC isoform distribution within a muscle but will not affect the titin molecular mass. Factors controlling the degree of splicing (and thereby titin molecular mass) are not yet known and are likely to be more complex than changes in levels of genetic expression. Therefore, the effects of age on titin molecular mass would be expected to be less dramatic than percentage MHC changes unless titin molecular mass differences are sensitive to epigenetic changes. The study of Smith et al. (Smith et al., 2011) reported titin molecular masses that were not significantly different from those reported in our study (gracilis: P=0.20, 1–β=0.52; semitendinosus: P=0.07, 1–β=0.52), implying that the effects of age on titin molecular mass are small.
Another important limitation is our sample size. Although six donors may be considered a small sample population, the study focus was to find trends in overall human muscle organization, not necessarily to obtain data that were completely representative of the average human population. We were therefore more concerned with relative values between muscles and believe our study population was large enough to accomplish our goals of finding organizational trends.
There are some aspects of the experimental execution that also merit discussion. Each muscle was sampled at one location, and this biopsy was considered representative of the entire muscle, thereby assuming that regional variation within a muscle is smaller than variation between muscles. This assumption is supported by past work in rhesus monkeys, where fiber-type percentage variability between subjects was demonstrated to be much greater than between biopsies within a muscle in a single subject (Roy et al., 1991), and in human paraspinal muscles, where fiber-type distribution was independent of biopsy depth (Regev et al., 2010).
However, even if a single biopsy is representative of the entire muscle, each muscle was considered equivalent in our analysis. This may have a confounding effect, as small muscles may be over-represented in our analysis. This effect could have been mitigated by normalizing to muscle mass or PCSA, the only known muscle parameter to be directly related to maximal force production (Powell et al., 1984). However, PCSA and muscle mass vary much more across different regions of the body than do titin molecular mass, collagen content or MHC distribution. The coefficients of variance across different anatomical regions are 71% for PCSA and 87% for muscle mass [84 muscles considered (Jacobson et al., 1992; Delp et al., 2001; Brown et al., 2011; Peterson and Rayan, 2011; Lieber, 2010)]. When compared against CV of 1% for titin molecular mass, 18% for collagen content and 14% for percentage MHC-1, it becomes apparent that the results of normalizing data from biopsies to PCSA (or muscle mass) primarily reflect the differences in average PCSA (or average muscle mass) between different body regions rather than providing further insight with regards to the relative titin molecular mass, collagen content and MHC distribution throughout the body.
A final limitation is the matter of categorization. By necessity of the assumptions that underlie our statistical analyses, each muscle had to be placed in a single category despite the physiological reality that many muscles have multiple functions or could be considered to exist in multiple locations. Muscles were categorized according to their primary function and location to the best of our ability (a full list is available in supplementary material Table S1). We recognize that this is an imperfect system but feel that it is a necessity for reduction of data into manageable groups.
CONCLUSIONS
This study provides several new insights into the primary components of organizational schemata of muscles in the human body. Titin molecular mass is variable across muscles but appears to differ in anatomical regions in a proximal–distal manner. Titin was also the single most useful factor for categorizing muscles by anatomical region or function. Collagen content and percentage MHC-1 also exhibited anatomical specialization, likely reflecting their physiological roles in active and passive tension production. These parameters were more variable across muscles than titin molecular mass, with MHC isoform distribution accounting for the greatest amount of variability across all samples. Comparison of our work with similar studies carried out in other animals demonstrates, unsurprisingly, that organizational schemes that govern quadrupeds are different from those that we observe in our bipedal system. We foresee trends reported here guiding future investigations to more fully understand specific musculoskeletal systems and to better appreciate the roles that they play in overall body physiology and function.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Tony Choi and Mary Esparza for their technical assistance with biochemical assays and Dr Marion Greaser for teaching us the SDS-VAGE technique used to measure titin molecular mass. We also thank the staff members of the anatomy bequest program at the University of Minnesota, especially Angie McArthur, Jake Anderson, Andy Ashton, Paul Hill and John Straub.
FOOTNOTES
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/215/15/2551/DC1
FUNDING
This work was supported by National Institutes of Health [R24HD050837 to R.L.L.]. Deposited in PMC for release after 12 months.
REFERENCES
- Arbanas J., Klasan G. S., Nikolic M., Cvijanovic O., Malnar D. (2010). Immunohistochemical analysis of the human psoas major muscle with regards to the body side and aging. Coll. Antropol. 34 Suppl. 2, 169-173 [PubMed] [Google Scholar]
- Bang M. L., Centner T., Fornoff F., Geach A. J., Gotthardt M., McNabb M., Witt C. C., Labeit D., Gregorio C. C., Granzier H. L., et al. (2001). The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ. Res. 89, 1065-1072 [DOI] [PubMed] [Google Scholar]
- Bérard J., Kreuzer M., Bee G. (2008). Effect of litter size and birth weight on growth, carcass and pork quality, and their relationship to postmortem proteolysis. J. Anim. Sci. 86, 2357-2368 [DOI] [PubMed] [Google Scholar]
- Bergström J. (1962). Muscle electrolytes in man. Determined by neutron activation analysis on needle biopsy specimens. A study on normal subjects, kidney patients, and patients with chronic diarrhoea. Scand. J. Clin. Lab. Invest. 14, Suppl. 68, 110 [Google Scholar]
- Bergström J. (1975). Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Invest. 35, 609-616 [PubMed] [Google Scholar]
- Brocchieri L., Karlin S. (2005). Protein length in eukaryotic and prokaryotic proteomes. Nucleic Acids Res. 33, 3390-3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. H. M., Ward S. R., Cook M. S., Lieber R. L. (2011). Architectural analysis of human abdominal wall muscles: implications for mechanical function. Spine 36, 355-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R., Burns R. (2008). Discriminant Analysis. In Business Research Methods and Statistics Using SPSS, pp. 589-608 London: SAGE Publications Ltd. [Google Scholar]
- Caccia M. R., Harris J. B., Johnson M. A. (1979). Morphology and physiology of skeletal muscle in aging rodents. Muscle Nerve 2, 202-212 [DOI] [PubMed] [Google Scholar]
- Delp S. L., Suryanarayanan S., Murray W. M., Uhlir J., Triolo R. J. (2001). Architecture of the rectus abdominis, quadratus lumborum, and erector spinae. J. Biomech. 34, 371-375 [DOI] [PubMed] [Google Scholar]
- Edwards C. A., O’Brien W. D., Jr (1980). Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin. Chim. Acta 104, 161-167 [DOI] [PubMed] [Google Scholar]
- Edwards R. H. T., Jones D. A., Maunder C., Batra G. J. (1975). Needle biopsy for muscle chemistry. Lancet 305, 736-740 [DOI] [PubMed] [Google Scholar]
- Eng C. M., Smallwood L. H., Rainiero M. P., Lahey M., Ward S. R., Lieber R. L. (2008). Scaling of muscle architecture and fiber types in the rat hindlimb. J. Exp. Biol. 211, 2336-2345 [DOI] [PubMed] [Google Scholar]
- Freiburg A., Trombitas K., Hell W., Cazorla O., Fougerousse F., Centner T., Kolmerer B., Witt C., Beckmann J. S., Gregorio C. C., et al. (2000). Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ. Res. 86, 1114-1121 [DOI] [PubMed] [Google Scholar]
- Fritz J., Greaser M. L. (1991). Changes in titin and nebulin in postmortem bovine muscle revealed by gel-electrophoresis, western blotting and immunofluorescence microscopy. J. Food Sci. 56, 607-610 [Google Scholar]
- Gaudy J.-F., Zouaoui A., Bri P., Charrier J.-L., Laison F. (2002). Functional anatomy of the human temporal muscle. Surg. Radiol. Anat. 23, 389-398 [DOI] [PubMed] [Google Scholar]
- Gillies A. R., Lieber R. L. (2011). Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44, 318-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S. K., Lin D. C. (2009). Experimental determination of sarcomere force-length relationship in type-I human skeletal muscle fibers. J. Biomech. 42, 2011-2016 [DOI] [PubMed] [Google Scholar]
- Granzier H. L., Irving T. C. (1995). Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys. J. 68, 1027-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R. (1992). Passive force generation and titin isoforms in mammalian skeletal muscle. Biophys. J. 61, 392-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff Lonergan E., Zhang W., Lonergan S. M. (2010). Biochemistry of postmortem muscle – lessons on mechanisms of meat tenderization. Meat Sci. 86, 184-195 [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Raab R., Fazeli B. M., Abrams R. A., Botte M. J., Lieber R. L. (1992). Architectural design of the human intrinsic hand muscles. J. Hand Surg. Am. 17, 804-809 [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Polgar J., Weightman D., Appleton D. (1973). Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J. Neurol. Sci. 18, 111-129 [DOI] [PubMed] [Google Scholar]
- Kadhiresan V. A., Hassett C. A., Faulkner J. A. (1996). Properties of single motor units in medial gastrocnemius muscles of adult and old rats. J. Physiol. 493, 543-552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K., Hashizume K. (1989). Changes in properties of the medial gastrocnemius motor units in aging rats. J. Neurophysiol. 61, 737-746 [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Abe T., Kanehisa H., Fukunaga T. (2006). Human skeletal muscle size and architecture: variability and interdependence. Am. J. Hum. Biol. 18, 845-848 [DOI] [PubMed] [Google Scholar]
- Labeit S., Kolmerer B. (1995). Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 270, 293-296 [DOI] [PubMed] [Google Scholar]
- Lange S., Xiang F., Yakovenko A., Vihola A., Hackman P., Rostkova E., Kristensen J., Brandmeier B., Franzen G., Hedberg B., et al. (2005). The kinase domain of titin controls muscle gene expression and protein turnover. Science 308, 1599-1603 [DOI] [PubMed] [Google Scholar]
- Larsson L. (1983). Histochemical characteristics of human skeletal muscle during aging. Acta Physiol. Scand. 117, 469-471 [DOI] [PubMed] [Google Scholar]
- Larsson L., Salviati G. (1992). A technique for studies of the contractile apparatus in single human muscle fibre segments obtained by percutaneous biopsy. Acta Physiol. Scand. 146, 485-495 [DOI] [PubMed] [Google Scholar]
- Lexell J. (1995). Human aging, muscle mass, and fiber type composition. J. Gerontol. A Biol. Sci. Med. Sci. 50, 11-16 [DOI] [PubMed] [Google Scholar]
- Lexell J., Henriksson-Larsén K., Winblad B., Sjöström M. (1983). Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve 6, 588-595 [DOI] [PubMed] [Google Scholar]
- Lexell J., Taylor C. C., Sjöström M. (1988). What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 84, 275-294 [DOI] [PubMed] [Google Scholar]
- Lieber R. L. (2010). Skeletal Muscle Structure, Function, and Plasticity, 3rd edn. Baltimore: Lippincott Williams and Wilkins; [Google Scholar]
- Neagoe C., Kulke M., del Monte F., Gwathmey J. K., de Tombe P. P., Hajjar R. J., Linke W. A. (2002). Titin isoform switch in ischemic human heart disease. Circulation 106, 1333-1341 [DOI] [PubMed] [Google Scholar]
- Neuman R. E., Logan M. A. (1950). The determination of collagen and elastin in tissues. J. Biol. Chem. 186, 549-556 [PubMed] [Google Scholar]
- Peterson S. L., Rayan G. M. (2011). Shoulder and upper arm muscle architecture. J. Hand Surg. Am. 36, 881-889 [DOI] [PubMed] [Google Scholar]
- Powell P. L., Roy R. R., Kanim P., Bello M. A., Edgerton V. R. (1984). Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J. Appl. Physiol. 57, 1715-1721 [DOI] [PubMed] [Google Scholar]
- Prado L. G., Makarenko I., Andresen C., Krüger M., Opitz C. A., Linke W. A. (2005). Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J. Gen. Physiol. 126, 461-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev G. J., Kim C. W., Thacker B. E., Tomiya A., Garfin S. R., Ward S. R., Lieber R. L. (2010). Regional Myosin heavy chain distribution in selected paraspinal muscles. Spine 35, 1265-1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. R., Bodine-Fowler S. C., Kim J., Haque N., de Leon D., Rudolph W., Edgerton V. R. (1991). Architectural and fiber type distribution properties of selected rhesus leg muscles: feasibility of multiple independent biopsies. Acta Anat. (Basel) 140, 350-356 [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Reggiani C. (1994). Myosin isoforms in mammalian skeletal muscle. J. Appl. Physiol. 77, 493-501 [DOI] [PubMed] [Google Scholar]
- Smith L. R., Lee K. S., Ward S. R., Chambers H. G., Lieber R. L. (2011). Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J. Physiol. 589, 2625-2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge R. J., Roy R. R. (1993). Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J. Appl. Physiol. 75, 2337-2340 [DOI] [PubMed] [Google Scholar]
- Wang K., McCarter R., Wright J., Beverly J., Ramirez-Mitchell R. (1991). Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension. Proc. Natl. Acad. Sci. USA 88, 7101-7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. R., Tomiya A., Regev G. J., Thacker B. E., Benzl R. C., Kim C. W., Lieber R. L. (2009). Passive mechanical properties of the lumbar multifidus muscle support its role as a stabilizer. J. Biomech. 42, 1384-1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren C. M., Krzesinski P. R., Greaser M. L. (2003). Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis 24, 1695-1702 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


