Abstract
Immunohistochemistry (IHC) is an important tool used for diagnosis and prognosis of several hematological malignancies and is frequently used for quantitative and qualitative analysis of expression of different protein biomarkers in tissue sections. To understand the histopathological alterations in multiple myeloma (MM), IHC analysis of bone marrow (BM) biopsy is a commonly used method. However, due to the harsh decalcification process generally used for processing of bone marrow biopsies, protein epitopes are occasionally rendered unsuitable for IHC detection. We have developed a novel technique for processing BM spicule samples into a fibrin-clot matrix that allows for IHC detection of MM protein markers. This method does not require decalcification and results in a consistent, reliable assay. Using paired BM spicule-clot and BM core biopsies from patients diagnosed with multiple myeloma, we studied five MM specific antibodies including kappa and lambda immunoglobulin light chains, CD138, CYR61 and DKK1.
Keywords: Bone marrow spicule, immunohistochemistry, multiple myeloma
Multiple myeloma (MM) is a malignant B cell tumor originating from pre switched, follicle center B lymphocytes which differentiate into plasma cells that accumulate in the bone marrow (Bergsagel and Kuehl 2001). A combination of bone marrow biopsy and immunohistochemistry (IHC) is frequently used for diagnosis and monitoring disease progression (Beck et al. 2003). IHC is a widely available robust technique used to detect plasma cells or B cell monoclonality in histological sections (Chang et al. 2007). It is also used to detect many clinically significant diagnostic and prognostic markers of MM including surface molecules CD56, CD20, CXCR4 and other biomarkers such as FGFR3, cyclin D1, c-Maf and p53 whose expression may act as a surrogate for underlying genetic abnormalities (Yeung and Chang 2008). According to current guidelines, histopathological alterations in MM are studied by IHC using bone marrow trephine biopsy (Joshi et al. 2008). However, due to harsh decalcification processes (e.g. hydrochloric acid containing proprietary decalcifiers) generally used for processing bone marrow biopsies, protein biomarker epitopes are occasionally rendered unsuitable for IHC detection (Alers et al.1999). Thus in our current study, we have developed an alternative method for immunohistochemical analysis that does not require decalcification and results in a relatively more consistent and reliable assay, also allows for the investigation of histopathological alterations and protein biomarkers by IHC. To our knowledge, this is the first study for multiple myeloma which uses bone marrow spicule samples that have been processed into a fibrin clot matrix. In our study we have compared paired traditional bone marrow biopsy analysis method and our novel fibrin clot matrix bone marrow spicule method using five validated protein biomarkers of MM.
Material and methods
A total of nine MM patients were studied. Eight of the patients were male and one female, with a median age of 68 years. All patients were treated with high doses of chemotherapy followed by autologous stem cell transplant.
Bone marrow (BM) biopsy specimens were obtained from MM patients enrolled in trials at University of Arkansas for Medical Sciences (UAMS). The UAMS institutional review board approved the clinical trials and all patients provided informed written consent.
BM trephine biopsy specimens were fixed in 10% neutral buffered formalin and briefly decalcified by acid chelation and embedded in paraffin. Sequential 4 µm thick sections were cut and slides were prepared for BM biopsy immunostaining. One slide from each patient was stained using hematoxylin and eosin (H&E) to check cell morphology.
BM spicule samples were prepared by ficoll gradient separation of BM aspirates with BM spicules being collected from the top lipid layer. The BM spicule samples were then transferred into a 50 ml ClearSpin Filter (Novagen, Gibbstown, NJ) and centrifuged at 250 rpm for 2 minutes. If fluid still remained on the top of the filter, the sample was centrifuged for an additional 2 minutes or until filter clears from fluids. After centrifugation, BM spicules from the filter were transferred very carefully into a 1.5ml eppendorf tube appropriately labeled. Then 200 µl each of previously warmed (37°C) normal pooled human plasma with EDTA and Thromboplastin-DS (Pacific Hemostasis, Cape Town, South Africa) was added to the tube and mixed by hand. The tube was then placed at 37°C for 5 to 10 minutes to allow for clot formation. The tube was carefully removed and the clot and transferred into a HistoScreen™ mesh cassette (Perk Scientific Inc, Lansdowne, PA). The cassette was then placed into formalin (1:10 dilution, Fisher Scientific, Pittsburgh, PA) and fixed overnight followed by embedding in paraffin. For IHC staining, 4 µm sections of paraffin-embedded bone marrow biopsy cores or BM spicule fibrin-clot matrixes were cut and placed onto positively charged glass microscope slides and allowed to air-dry overnight prior to de-waxing.
Tissues were deparaffinized in xylene, dehydrated with ethanol, and rinsed in phosphate buffered saline (PBS). When necessary, heat-induced epitope retrieval was carried out on deparaffinized tissue sections using a modified pressure cooker (Decloaker™, Biocare Medical, Concord, CA) and a pH 6.0 citrate buffer (Bull’s Eye Retrieval solution, Biocare Medical). Slides were stained separately for expression of five antibodies: kappa (DakoCytomation, Carpenteria, CA), lambda (DakoCytomation), CD138 (clone B-B4, Biocare Medical), CYR61 (kindly provided by Dr. L.F. Lau, University of Illinois at Chicago) and DKK1 (Novus Biologicals, Littleton, CO). All reactions were performed using an automated immunostainer (DakoCytomation, Carpenteria, CA) in conjunction with the Envision+-HRP detection system (Dako). Appropriate negative controls were included for each case. Endogenous peroxidase was inhibited with methyl alcohol containing 0.01% H2O2 with additional blocking performed utilizing Background Sniper™ (Biocare Medical, Concord, CA) and/or Background Terminator™ (Biocare Medical). Nuclei were counter-stained with hematoxylin and sections evaluated by light microscopy using an Olympus BH-2 microscope (Olympus, Melville, NY) fitted with a SPOT2 digital camera (Diagnostic Instruments, Sterling Heights, MI). Scoring was done based on staining intensity of antibodies on a scale of 0 to +3 (0 scant, +1 mild, +2 moderate, +3 strong).
Results
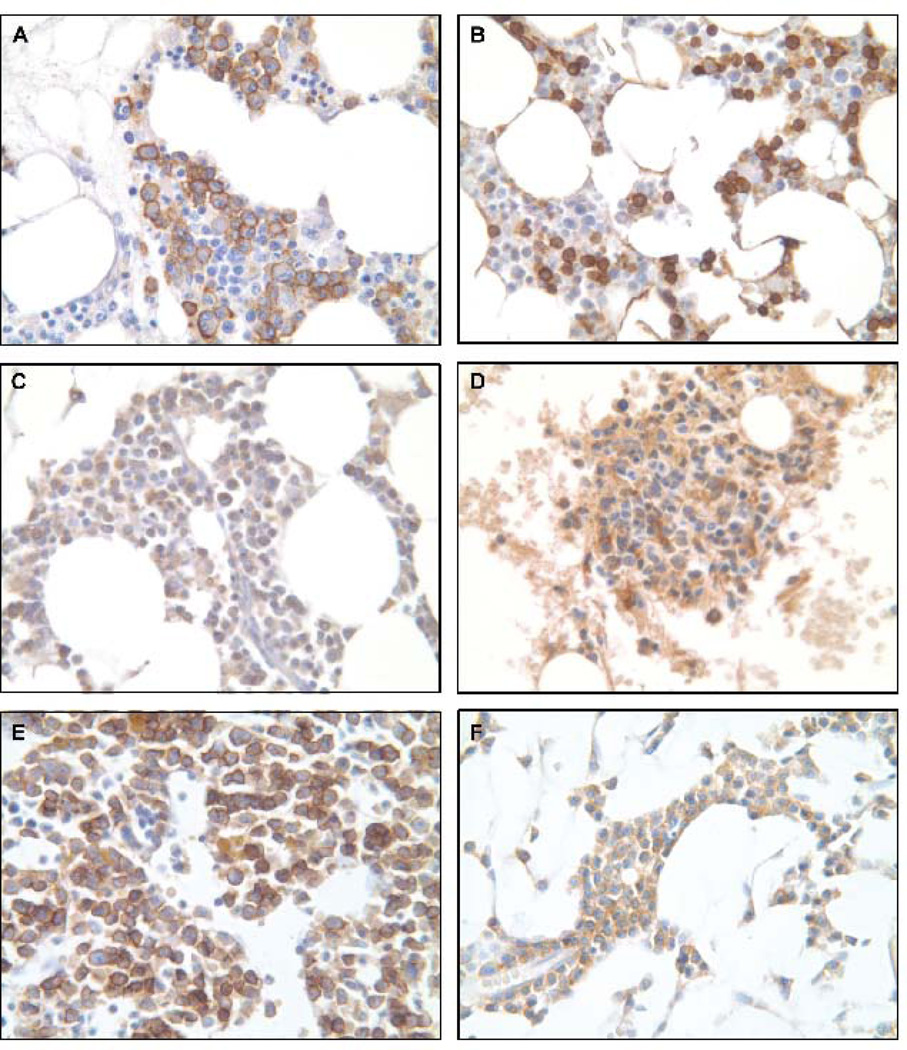
Studies have shown that CD138 (Syndecan 1) is expressed in 95% multiple myeloma cells by immunoreactivity. CD138 mediates myeloma cell adhesion and loss of CD138 from cell surface may contribute to myeloma proliferation and dissemination (Bayer-Garner et al. 2001). In our study, expression of CD138 was observed exclusively in plasma cells in both BM core biopsy and BM spicule clot samples. All plasma cells showed a strong staining (+3) of the cytoplasmic membrane (Fig. 1A, 1B). There were no differences in staining intensity and the cell morphology in BM spicule clot sections were comparable and in some cases superior when compared to the paired BM core biopsies.
Figure 1.
Immunohistochemical staining of paired BM spicule clot and BM core biopsy from same patient (400X). (A) CD138 staining of BM spicule clot demonstrating distinct cytoplasmic and membrane staining of plasma cells; (B) CD138 staining of BM core biopsy; (C) CYR61 staining of BM spicule clot demonstrating less background staining as compared to the paired BM core biopsy (D); (E) DKK1 staining of BM spicule clot demonstrating more intense cytoplasmic staining than the paired BM core biopsy (F).
Demonstration of monoclonal plasma cells (PCs) in histological section by determination of immunoglobulin (Ig) light chain restriction is often necessary in the diagnosis of MM. Although IHC is the most frequently used method to detect kappa and lambda light chains in histologic sections, high background staining due to adsorbed Ig in the surrounding bone marrow stromal tissue can make interpretation of IHC difficult, particularly when small numbers of PCs are present (Beck et al. 2003). To overcome this problem, an alternative method, in situ hybridization (ISH) has been utilized more often in the clinical laboratory for Ig light chain staining. Unfortunately, the new BM spicule clot technique also showed high background staining for both light chains which is similar to BM core biopsies when compared with matched paired cases (Figure not shown).
CYR61, a member of CCN family is known to interact with heparin sulfate proteoglycans (HSPGs) and integrins, expressed in the developing skeleton, stimulate bone formation, and may have a role in bone metastasis progression (Perbal 2004, Clines et al. 2007). Our results showed that CYR61 is expressed predominantly in myeloid and lymphoid cells. Strong cytoplasmic staining (+2, +3) was observed in most of these cells with either BM spicule clots or BM core biopsies. Morphology and staining intensity was superior in the BM spicule clot compared to the BM core biopsy samples (Fig.1C, 1D).
Dickkopf-1 (DKK1), a soluble inhibitor of wingless/int (Wnt) signaling and osteoblastogenesis, is elevated in patients with MM and correlates with osteolytic bone disease (Heath et al. 2009). Studies have previously shown that DKK1 is produced by plasma cells in multiple myeloma which blocks the differentiation of osteoblasts and eventually diminishes bone formation (Tian et al. 2003). Our findings revealed a difference in staining intensity of DKK1 between BM spicule clots and BM core biopsies. In the current study, plasma cells in BM core biopsy samples demonstrated mild cytoplasmic DKK1 staining (+1) whereas plasma cells in BM spicule clot samples showed a strong staining intensity of DKK1 (+3) for all MM cases (Fig. 1E, 1F).
Discussion
IHC for Ig light chain has been used routinely to evaluate the presence of monoclonality in plasma cell dyscrasias (Beck et al. 2003). CD 138 was found to be a specific marker for plasma cells and was not present in association with other hematopoetic cells or endothelial cells (Bayer-Garner et al. 2001). Both of these two widely used markers for MM diagnosis were detected in our BM spicule clot samples processed from MM patients using this novel fibrin clot matrix thus demonstrating that this technique is suitable for IHC staining of MM specimens. The other two markers CYR61 and DKK1 are currently used by various laboratories studying MM. Both of these markers also demonstrate that this new method may be utilized for IHC evaluation of their expression as well. In general, BM spicule clot cases display equal or better morphology and staining when compared to matched BM core biopsy samples. While BM core biopsies had areas of non specific staining for most of the antibodies, this was not generally observed in the BM spicule samples with the exception of immunoglobulin light chains which displayed an element of non-specific staining with both sample types. Moreover, we observed a consistently superior morphology in the BM spicule samples than in matched bone marrow core biopsies. In conclusion, our study demonstrates that BM spicule samples processed from MM patients using this novel fibrin-clot matrix technique were suitable for IHC and had generally lower background and non-specific staining coupled with improved morphology when compared to matched core biopsies. The use of this methodology obviates the need for harsh decalcification methods that may destroy antigenic epitopes while preserving much of the anatomical microarchitecture of the bone marrow core.
Acknowledgment
The authors would like to thank Ryan Williams for his help in sample collection and the Experimental Pathology Shared Resource Laboratory for providing the embedding and sectioning services.
References
- Alers JC, Krijtenburg PJ, Vissers KJ, Dekken HV. Effect of bone decalcification procedures on DNA in situ hybridization and comparative genomic hybridization: EDTA is highly preferable to a routinely used acid decalcifier. J. Histochem. Cytochem. 1999;47(5):703–709. doi: 10.1177/002215549904700512. [DOI] [PubMed] [Google Scholar]
- Bayer-Garner IB, Sanderson RD, Dhodapkar MV, Owens RB, Wilson CS. Syndecan-1 (CD138) immunoreactivity in bone marrow biopsies of multiple myeloma: shed syndecan-1 accumulates in fibrotic regions. Mod. Pathol. 2001;14(10):1052–1058. doi: 10.1038/modpathol.3880435. [DOI] [PubMed] [Google Scholar]
- Beck RL, Tubbs RR, Hussein M, Pettay J, Hsi ED. Automated colorimetric in situ hybridization (CISH) detection of immunoglobulin (Ig) light chain MRNA expression in plasma cell (PC) dyscrasias and non-hodgkin lymphoma. Diag. Mol. Pathol. 2003;12(1):14–20. doi: 10.1097/00019606-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma. Oncogene. 2001;20:5611–5622. doi: 10.1038/sj.onc.1204641. [DOI] [PubMed] [Google Scholar]
- Chang H, Yeung J, Qi C, Wei X. Aberrant nuclear p53 protein expression detected by immunohistochemistry is associated with hemizygous P53 deletion and poor survival for multiple myeloma. BrJHaematol. 2007;138:324–329. doi: 10.1111/j.1365-2141.2007.06649.x. [DOI] [PubMed] [Google Scholar]
- Clines GA, Mohammad KS, Bao Y, Stephens OW, Suva LJ, Shaughnessy JD, Fox JW, Chirgwin JM, Guise TA. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol. Endocrin. 2007;21(2):486–498. doi: 10.1210/me.2006-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD, Jr., Evans HR, Snowden JA, Stover DR, Vanderkerken K, Croucher PI. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J. Bone Miner. Res. 2009;24(3):425–436. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- Joshi R, Horncastle D, Elderfield K, Lampert I, Rahemtulla A, Naresh KN. Bone marrow trephine combined with immunohistochemistry is superior to bone marrow aspirate in follow-up of myeloma patients. J. Clin. Pathol. 2008;61:213–216. doi: 10.1136/jcp.2007.049130. [DOI] [PubMed] [Google Scholar]
- Perbal B. CNN proteins: multifunctional signaling regulators. The Lancet. 2004;363(9402):62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesion in multiple myeloma. N. Engl. J. Med. 2003;349(26):2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- Yeung J, Chang H. Genomic aberrations and immunohistochemical markers as prognostic indicators in multiple myeloma. J. Clin. Pathol. 2008;61:832–836. doi: 10.1136/jcp.2007.049585. [DOI] [PubMed] [Google Scholar]