Abstract
INTRODUCTION
Inflammation and infection are associated with premature birth and with activation of the fetal immune system. We hypothesized that exposure to microbial Toll-like receptor (TLR) ligands plays an important role in neonatal T-cell maturation and that early exposure to microbial products may result in early T-cell maturation and a tendency for these matured effector cells to change their homing receptor patterns.
RESULTS
Expression of the CD45RO marker was induced in term neonatal T cells after in vitro exposure to TLR ligands for 7 days. Interestingly, naive T cells from adult blood were unaffected by TLR ligand exposure. In addition, neonatal T cells had more cells with decreased expression of the α4β7 integrins and increased expression of CC R4 after in vitro exposure of TLR ligands—similar to the expression of these molecules in adult naive T cells.
DISCUSSION
These findings are relevant for the understanding of neonatal T-cell maturation and may contribute to our understanding of multiorgan inflammatory complications of prematurity.
METHODS
Cord blood was obtained from term and preterm infants. Using flow cytometry, we identified a mature (CD45RO+) phenotype in preterm infant cord blood (CB) T cells that had decreased expression of the α4β7 integrins and increased expression of the C-C chemokine receptor 4 (CC R4) as compared with term infant CB.
The limited functional capacity of the neonatal immune system increases the neonatal risk for infections (1), rendering the neonate at risk for certain complications (2–5). Preterm infants are especially susceptible to infections and morbidities that are linked to an unusual state of immune activation (2,6,7).
Although the determinants of immune activation in the premature infant are not well understood, in some cases, it may be related to in utero exposure to inflammation from chorioamnionitis. Microbial products (e.g., endotoxin and bacterial DNA) in the amniotic fluid have been detected in the presence of chorioamnionitis (8,9) and are related to a systemic inflammatory response in the fetus (10). Boggess et al. demonstrated in an animal model that chronic maternal exposure to Porphyromonas gingivalis resulted in maternal systemic dissemination and transplacental passage of bacterial products; fetal exposure was confirmed by detection of 16s DNA by PCR in both maternal and fetal compartments (11).
Antigen exposure is necessary to induce maturation of the adaptive immune system; thus, neonatal lymphocytes are predominantly naive and have a “resting” phenotype, as exposure to foreign antigen in utero is uncommon in uncomplicated pregnancies (1,12). Among preterm infants, however, T cells are often activated as reflected by increased proportions of cells expressing the activation markers CD25, CD69, and human leukocyte antigen D-related (HLA-DR) as compared with T cells of term infants (13).
In this study, we characterized the maturation phenotype and expression of homing receptors in T cells from the cord blood (CB) of term and preterm infants. T cells from preterm infants showed evidence of in vivo activation and evidence of maturation towards a memory phenotype. In addition, T cells from term infants (composed largely of naive T cells) but not naive T cells obtained from adult subjects can be activated in vitro by exposure to microbial Toll-like receptor (TLR) ligands to develop a more mature phenotype and to alter homing receptor expression. We suspect, therefore, that microbial products can play an important role in phenotypic maturation in the neonate but not in adults.
Results
The Proportion of CD45RO+ T Cells Is Higher in Preterm vs. Term CB
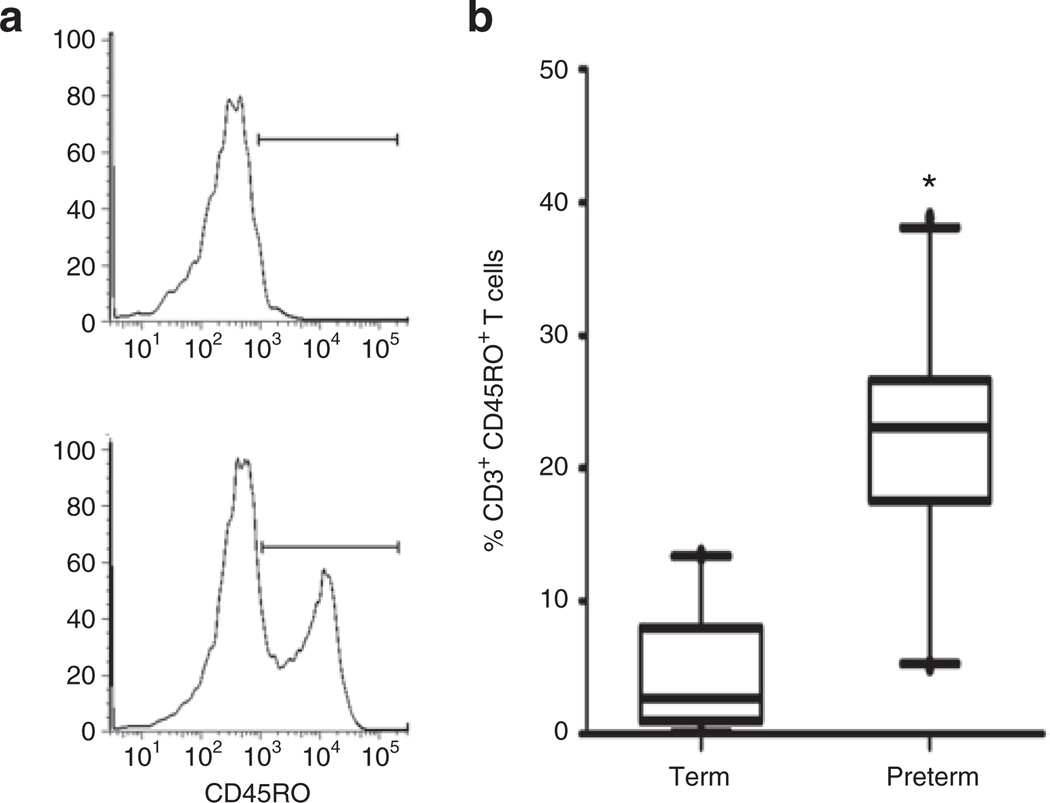
T cells were identified by size, granularity, and expression of CD45 and CD3 (Figure 1a,b). The proportion of CD3+CD45RO+ T cells (Figure 2a) was higher in preterm (median = 23.1%) vs. term CB (median = 2.7%) (Figure 2b). This difference was statistically significant (P = 0.0004).
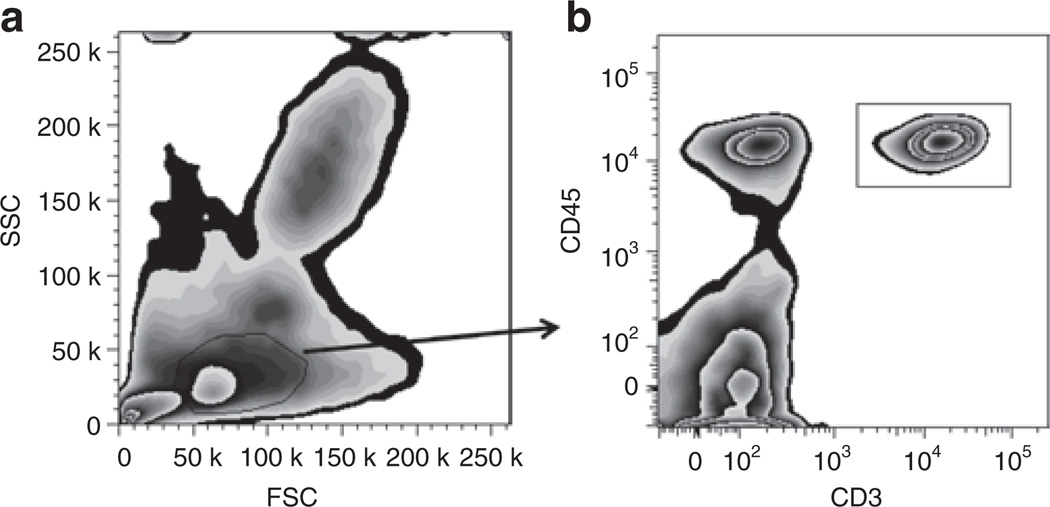
Figure 1.
Whole-blood flow cytometric analysis of lymphocytes. CB was stained with fluorochrome-labeled antibodies followed by red blood cell lysis and fixation. (a) Box-plot with lymphocytes gated based on size (forward scatter, FSC) and granularity (side scatter, SSC). (b) Neonatal T cells are selected based on CD45 and CD3 coexpression (box-plot). CB, cord blood.
Figure 2.
Increased proportion of CD3+CD45RO+ T cells in CB of preterm infants. (a) Whole-blood staining from term (upper histogram, 3.5%) and preterm CB (lower histogram, 35.9%). CD45+CD3+ cells were examined for expression of CD45RO. (b) Box and whiskers plot (median, minimum, and maximum values) of proportions of CD3+CD45+ cells expressing CD45RO in CB from term (n = 25) and preterm infants (n = 22). Statistical significance was determined by Mann–Whitney rank test (*P = 0.0004). CB, cord blood.
Microbial TLR Ligands Increase the Expression of CD45RO in Neonatal T Cells but Not in Adult Naive T Cells
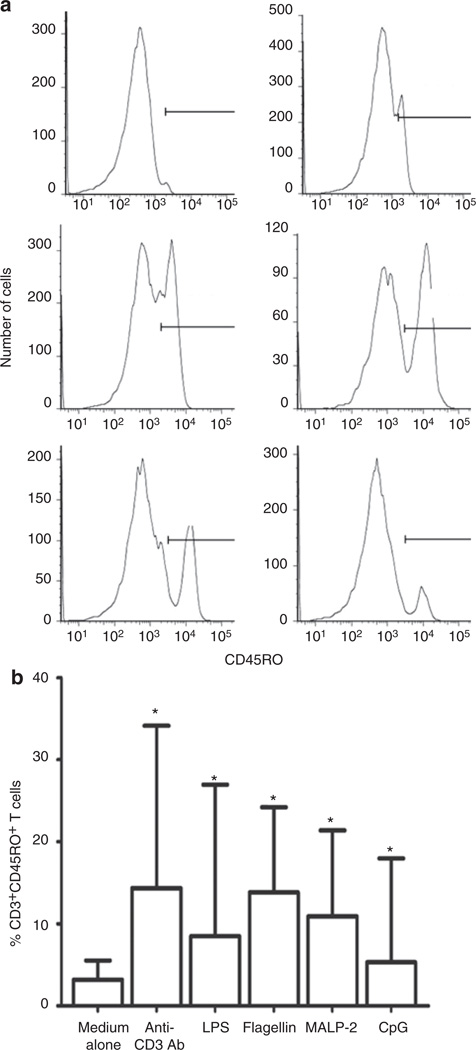
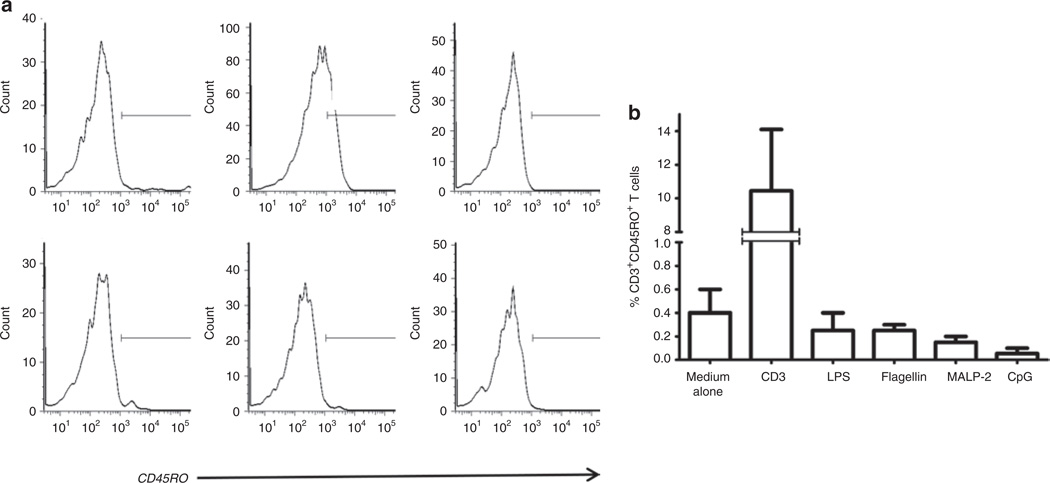
TLR ligands activate cells of the innate immune system in neonates and adults (14–16). As we previously described, TLR ligands also can drive T-cell activation. This effect that we described in adult cells was seen uniquely among memory (CD45RO+) T cells. No effect was observed in naive (CD45RA+) T cells (17). As we found an increased proportion of CD45RO+ T cells in CB of premature infants who are often exposed to microbial elements in the setting of chorioamnionitis, we hypothesized that bacterial TLR ligands might be activating neonatal CD45RA+ T cells to upregulate expression of CD45RO. TLR ligands were selected based on their expression by bacteria that commonly cause intrauterine infection and neonatal sepsis (18,19). Among CD45+ CD3+ lymphocytes, there was a significant increase in the proportion of CD3+ cells expressing CD45RO (Figure 3a). Incubation with flagellin resulted in the highest median percentage of CD45RO (14%), followed by macrophage-activating lipopeptide-2 (11%) and lipopolysaccharide (9%) (Figure 3b). By contrast, these TLR ligands did not induce CD45RO expression when incubated with naive T cells (CD45RO-depleted) from healthy adults (Figure 4a,b).
Figure 3.
TLR ligands increased the proportion of neonatal T cells expressing CD45RO. (a) CBMCs from term infants were cultured for 7 days in the presence of anti-CD3 antibody or TLR ligands (LPS, flagellin, MALP-2, and CpG). Cells were then analyzed by flow cytometry, gated for the expression of CD45 and CD3, and further evaluated for the expression of CD45RO (histograms). (a) Representative histograms of expression of CD45RO in T cells in the presence of medium alone (1.2%) and anti-CD3 antibody (15.6%) (first row), LPS (35.2%) and flagellin (39.1%) (second row), and MALP-2 (27.8%) and CpG (8.9%) (third row). (b) Summary data representing median and interquartile ranges of CD3+CD45RO+ cells in CB of term infants (n = 18) after in vitro incubation in medium or medium supplemented with anti-CD3 or selected TLR ligands. Statistical significance for difference between medium alone and TLR groups (*P < 0.05) was determined by Mann–Whitney rank test. CB, cord blood; CBMC, cord blood mononuclear cell; CpG, –C–phosphate–G– site; LPS, lipopolysaccharide; MALP-2, macrophage-activating lipopeptide-2; TLR, Toll-like receptor.
Figure 4.
TLR ligand exposure does not increase the expression of CD45RO in naive T-cell–enriched adult PBMCs. (a) CD45RO-depleted PBMCs from healthy adults were cultured for 7 days in the presence of anti-CD3 or selected TLR ligands and then examined for expression of CD45RO (histograms). First row: medium alone (0.6%), anti-CD3 (14.1%), and LPS (0.1%). Second row: flagellin (0.7%), MALP-2 (0.5%), and CpG (0.2%). (b) Summary data representing three experiments. CpG, –C–phosphate–G– site; LPS, lipopolysaccharide; MALP-2, macrophage-activating lipopeptide-2; PBMC, peripheral blood mononuclear cell; TLR, Toll-like receptor.
Distinct Homing Receptor Patterns Manifested by Preterm, Term, and Adult Naive (CD45RA+) T Cells
The traffic of T lymphocytes from blood to tissues occurs via a series of interactions between lymphocytes and endothelial cells (20). Naive T cells typically traffic through secondary lymphoid organs in search of peptides bound to major histocompatibility complex molecules expressed by antigen-presenting cells. Upon encounter with antigen and T-cell-receptor triggering, these naive T cells gain distinct homing characteristics (21). Following priming, clonal expansion, and differentiation, these matured T cells leaving the lymph node acquire new migration capabilities (22). T cells activated and matured in Peyer’s patches or mesenteric lymph nodes typically express C-C chemokine receptor 9 (CCR9) and the integrin α4β7, whereas activation in peripheral lymph nodes draining nongastrointestinal tissues, such as the skin and the lung, typically results in upregulation of CCR4 (23).
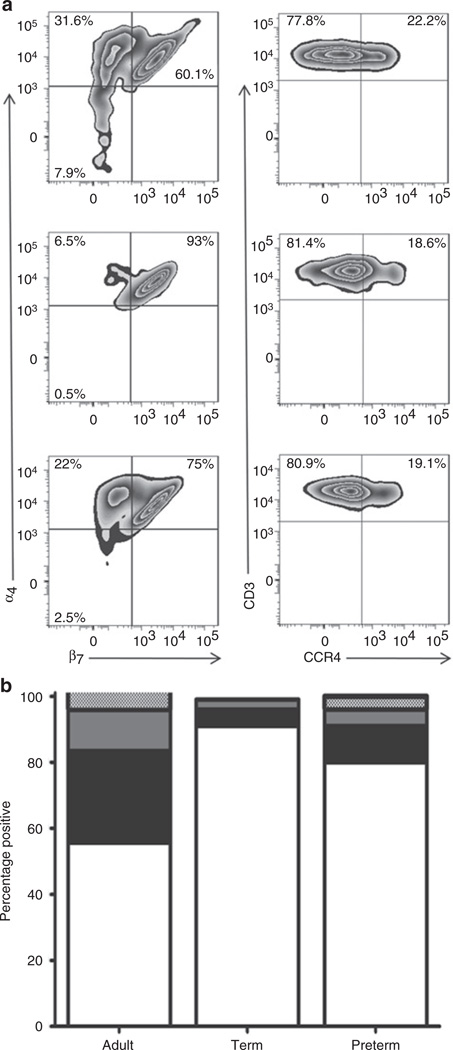
Cells were assessed for surface expression of CD3 and homing receptors α4β7 and CCR4 (Figure 5a). Among adult T cells (CD45+CD3+), 55% were α4+β7+, 27% were α4+β7−, 12% were α4−β7−, and only 4% were α4−β7+. In contrast, 92% of T cells from term infants were α4+β7+, 5% were α4+β7−, 3% were α4−β7−, and 0% were α4−β7+. Of note, the homing receptor pattern differed between preterm and term infant T cells (Figure 5b). Preterm infant T cells had a lower proportion of cells that were α4+β7+ (median values of 80% and 92% for preterm and term, respectively; P = 0.0034) and a higher proportion of cells that did not express the β7 homing receptor (median values of 16% and 8% in preterm and term infants, respectively; P = 0.0034). There was no difference in the expression of CCR4 on T cells between term and preterm infant cells.
Figure 5.
Differential homing receptor distribution in adult, term, and preterm T cells. (a) Adult peripheral blood (first row), term infant CB (second row), and preterm infant CB (third row). CD45+CD3+ cells were examined for expression of the homing receptors α4β7 and CCR4 (box-plots). (b) Stacked bars graph comparing the distribution of the α4β7 receptor among CD3+ T cells in adults (n = 10) and term (n = 22), and preterm infants (n = 26). White bars represent α4+β7+ T cells, black bars α4+β7− T cells, gray bars α4−β7− T cells, and dotted bars α4−β7+ T cells. Statistical significance (P < 0.0001) was determined by ANOVA. CB, cord blood; CCR, C-C chemokine receptor.
In Vitro Exposure of Neonatal T Cells to Microbial TLR Ligands Alters the Expression of Homing Receptors
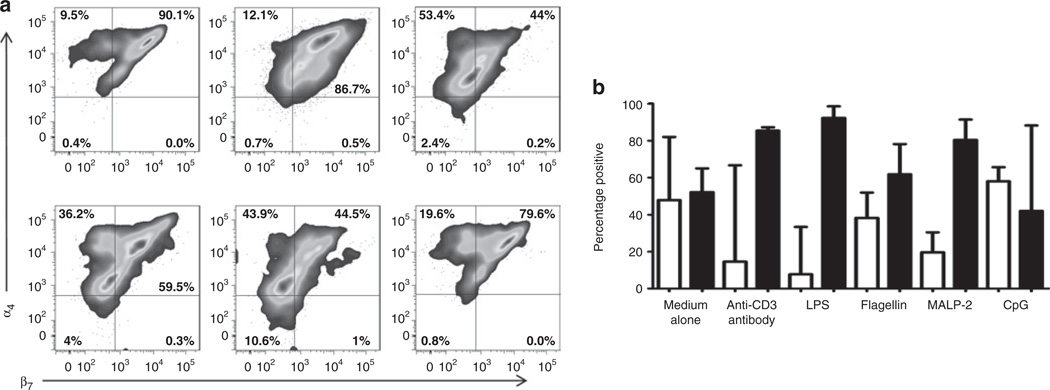
After incubation with selected TLR ligands, the percentage of CD3+α4+β7+ cells in term infant T cells decreased as compared with cells incubated with medium (Figure 6a,b); this decrease was primarily due to decreased expression of the β7 integrin (lipopolysaccharide 93% α4+β7−, flagellin 62% α4+β7−, macrophage-activating lipopeptide-2 80% α4+β7, –C–phosphate–G– site (CpG) 42% α4+β7−). We analyzed the expression of CCR4 in the CD3+α4+ subpopulation that did or did not express β7 (Figure 7a,b) and found that decreased expression of the β7 integrin was accompanied by increased expression of CCR4 after exposure to TLR ligands. This effect was especially striking in lipopolysaccharide-exposed cells (Figure 7b).
Figure 6.
TLR ligands change the proportion of neonatal T cells expressing the α4β7 homing receptor. (a) CBMCs from term infants were cultured for 7 days in the presence of medium alone, anti-CD3 antibody, and LPS (first row), and flagellin, MALP-2, and CpG (second row). Cells were analyzed by flow cytometry, gated for the expression of CD45 and CD3, and further evaluated for the expression of the α4β7 receptor (box-plots). (b) Column bar graph comparing cumulative data of the distribution of the α4β7 receptor in CD3+CD45RO+ cells in CB of term infants (n = 16) after exposure to TLR ligands. White bars represent percentage of cells positive for α4+β7+ and black bars represent percentage of cells positive for α4+β7−. Statistical significance (P < 0.0001) was determined by ANOVA. Median values are shown. CB, cord blood; CBMCs, cord blood mononuclear cells; CpG, –C–phosphate–G– site; LPS, lipopolysaccharide; MALP-2, macrophage-activating lipopeptide-2; TLR, Toll-like receptor.
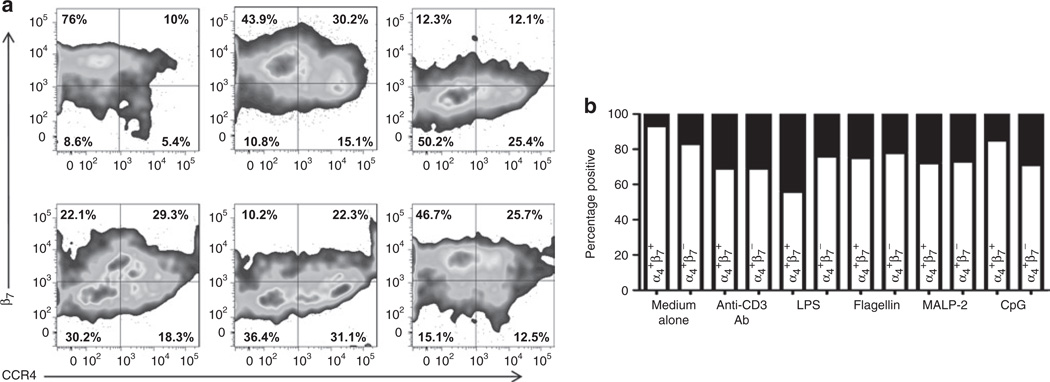
Figure 7.
Increased proportion of neonatal CD3+ T cells expressing CCR4 after exposure to TLR ligands. (a) CBMCs from term infants were cultured for 7 days in the presence of medium alone, anti-CD3 antibody, and LPS (first row), and flagellin, MALP-2, and CpG (second row). Cells were analyzed by flow cytometry; gated for the expression of CD45, CD3, α4; and further evaluated for the expression of β7 and CCR4 (box-plots). (b) Stacked bars graph comparing the distribution of the β7 subunit and CCR4 receptor among CD3+α4+ T cells in term infants (n = 16). White bars represent CCR4-negative cells and black bars represent CCR4-positive cells. Statistical significance (P < 0.0001) was determined by ANOVA. CBMC, cord blood mononuclear cell; CCR, C-C chemokine receptor; CpG, –C–phosphate–G– site; LPS, lipopolysaccharide; MALP-2, macrophage-activating lipopeptide-2.
Chorioamnionitis Affects the Expression of CD45RO and the α4β7 Receptors in Neonatal T Cells
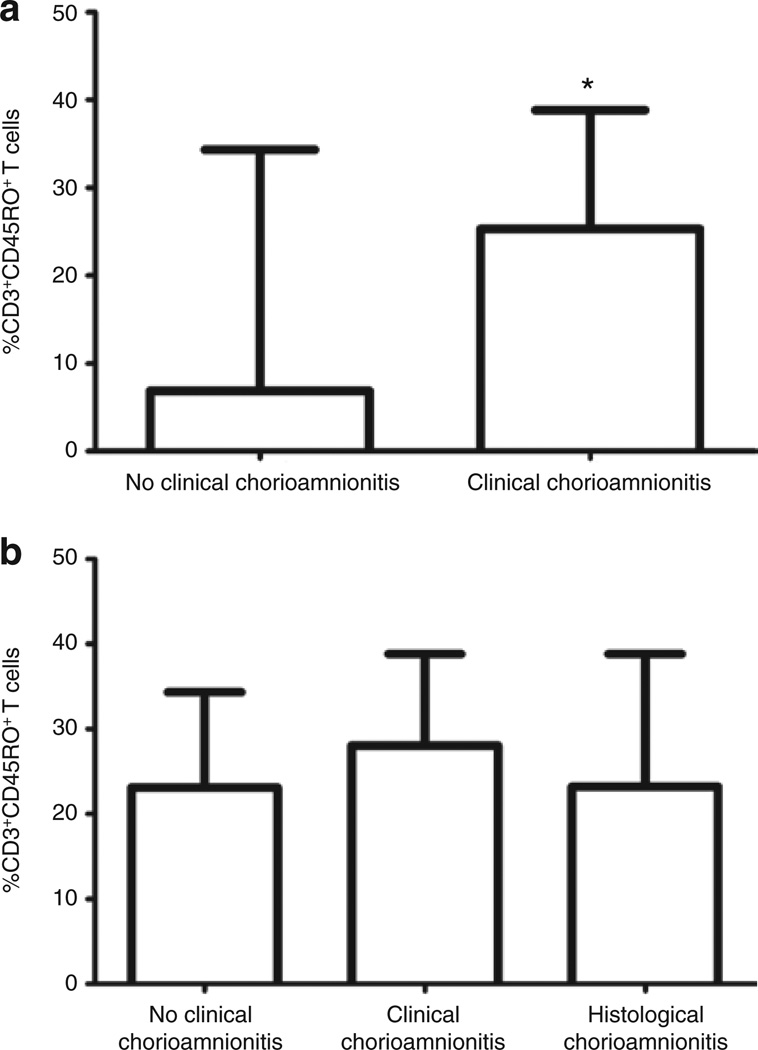
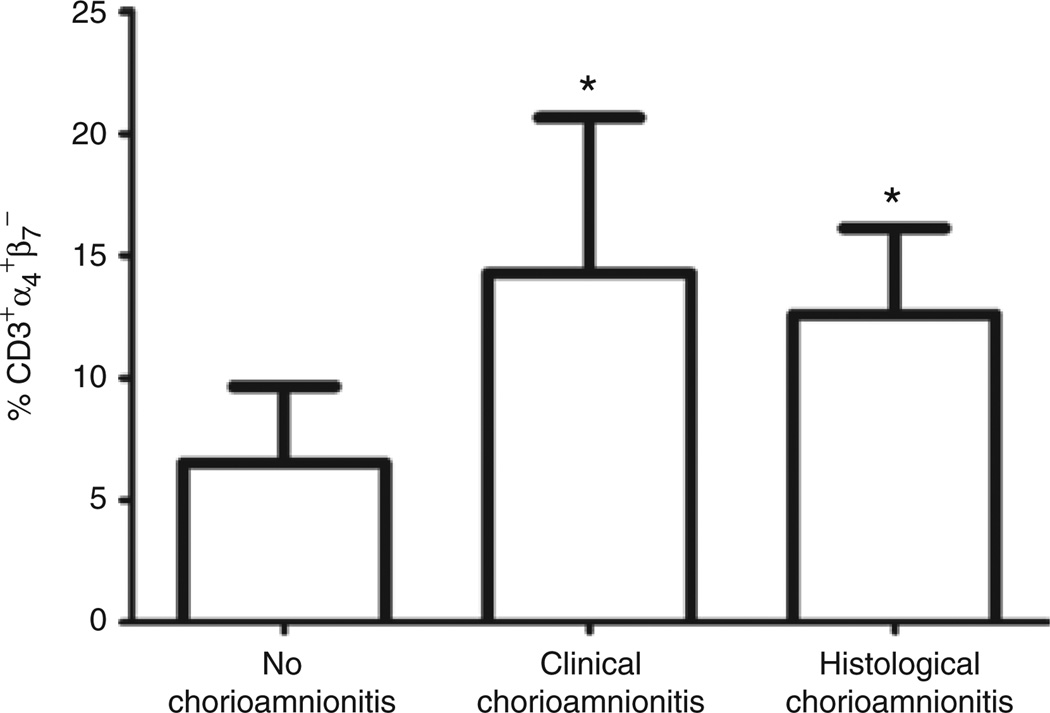
The proportion of T cells expressing CD45RO was compared between infants exposed to clinical chorioamnionitis (n = 5) and those without clinical chorioamnionitis (n = 41) (Figure 8a). The proportion of CD3+CD45RO+ was higher in the group with clinical chorioamnionitis (median = 25%) vs. no clinical chorioamnionitis (median = 6.9%). This difference was statistically significant (P = 0.008). There was no significant difference (P = 0.37) in the proportion of CD3+CD45RO+ T cells of preterm infants born to mothers without clinical chorioamnionitis (median = 23.1%), those born to mothers with clinical chorioamnionitis (median = 28.1%), and those born to mothers with histological chorioamnionitis (median = 23.2%) (Figure 8b).The proportion of CD3+α4+β7− was also significantly increased in infants exposed to clinical chorioamnionitis (median = 14.3%) and histological chorioamnionitis (median = 12.6%) as compared with those without exposure (median = 6.5%) (P ≤ 0.0001) (Figure 9).
Figure 8.
Increased proportion of CD3+CD45RO+ T cells in infants exposed to chorioamnionitis. (a) Column bar graph comparing proportion of CD3+CD45RO+ T cells in infants exposed to clinical chorioamnionitis (n = 5) with those not exposed (n = 41). This figure includes both term and preterm infants. (b) Column bar graph comparing proportion of CD3+CD45RO+ T cells in preterm infants exposed to clinical chorioamnionitis (n = 6) or histological chorioamnionitis (n = 12) with those not exposed (n = 11). Statistical significance was determined by Mann–Whitney rank test (*P = 0.003).
Figure 9.
Increased proportion of CD3+α4+β7− T cells in infants exposed to chorioamnionitis. Column bar graph comparing proportion of CD3+α4+β7− T cells in infants exposed to clinical chorioamnionitis (n = 5) or histological chorioamnionitis (n = 12) with those not exposed (n = 30). This figure includes both term and preterm infants. Statistical significance (*P < 0.0001) was determined by ANOVA.
Discussion
Although the neonatal immune system lacks memory of prior antigen exposure, it provides defense, albeit limited, against microbial pathogens while it is maturing and developing responses to newly encountered antigens. In this study, we report a novel characteristic of neonatal T cells that distinguishes them from adult T cells. We previously demonstrated that preterm labor is associated with neonatal T-cell activation, as evidenced by an increased proportion of cells expressing the activation markers CD25, CD69, and HLA-DR (13). In this study, we also report an increased proportion of T lymphocytes expressing the maturation antigen CD45RO in preterm CB and a distinct homing receptor pattern that distinguishes preterm infant T cells from term infant CB and adult T cells. Finally and most strikingly, we report that, in response to TLR ligand exposure, neonatal T cells have the capacity to acquire a maturation phenotype and that adult naive T cells lack this capacity. These observations provide novel insights into immune responsiveness of the neonate and the potential role of innate responses in the maturation of the adaptive immune system.
In adults, the principal stimulus for naive T-cell activation and maturation is considered to be T-cell-receptor triggering by neoantigenic peptides. In this study, however, we demonstrate that neonatal naive T cells can upregulate expression of the maturation antigen CD45RO through TLR-dependent mechanisms. Using selected TLR ligands, we found consistent upregulation of CD45RO by neonatal T cells. Of note, this response is lost in adult naive T cells. In the murine model, neonatal and adult naive T cells exhibit phenotypical and functional differences whereby neonatal naive T cells exhibit a higher level of effector Th1/Th2 cytokine production and an early proliferation response to stimulation with IL-7 (24). Our study indicates there is an additional difference between neonatal and adult cells. We speculate that the apparent maturation phenotype of neonatal T cells that we found in preterm CB may be driven by in vivo exposure to bacterial products. In utero exposure to plasmodial products that cross the placenta have been linked to altered neonatal immune responses to immunizations (25,26). We found a significant difference in the pattern of maturation and homing receptors in infants exposed to chorioamnionitis. This in utero maturation process could be related to placental inflammation. Of note, the analysis restricted to only preterm infants showed no differences between infants exposed to chorioamnionitis (either clinical or histological) and those not exposed to chorioamnionitis. These results should be interpreted very carefully because of the relatively small preterm group. Other factors should be taken into consideration that may increase the passage of bacterial products to the fetus, such as the duration of the rupture of membranes, presence of bacteria in maternal urine and/or blood, maternal periodontal disease, and presence of bacteria that are difficult to grow in cultures and whose presence could be detected only by PCR. Future studies should include measurement of microbial products in CB samples. We are currently investigating whether the maturation effect is mediated directly through TLR receptors in T cells or if antigen-presenting cells are needed.
To our knowledge, this is the first demonstration of a distinct homing receptor pattern in preterm infant CB T cells that differs from those of term infant and adult T cells. Lymphocyte migration is highly regulated, which ensures that distinct cell populations are delivered to intended site(s) in the proper physiological or pathological context (27). Homing receptors that are important for T-cell homeostasis include α4β7 and CCR4. The homing molecule α4β7 promotes T-cell entry into intestinal sites and CCR4 supports T-cell chemotaxis and entry into nongastrointestinal sites through interaction with its ligand induced in the setting of inflammation (28). The high proportion of α4β7 cells in CB may reflect the critical role of intestinal homing—and perhaps the importance of exposure to microbial elements in the gut—in neonatal T-cell development (29). In this study, we showed that in vitro exposure of CB cells to microbial TLR ligands alters the expression of these homing receptors, decreasing expression of α4β7 and increasing the expression CCR4. Our finding of fewer α4+β7+ T cells in preterm CB may reflect an earlier (premature) maturation of these cells in response to premature exposure to microbial elements. A consequence of this exposure may be increased migration of these cells to nongastrointestinal sites (e.g., lungs, skin, or brain) that may underlie the systemic inflammatory organ dysfunction often seen in premature infants. Studies are under way to better characterize the effector function and cytokine potential of these cells.
What are the possible clinical implications of T-cell activation/maturation and alteration in homing receptor patterns in the preterm infant? Premature infant morbidities (e.g., bronchopulmonary dysplasia, necrotizing enterocolitis, respiratory distress syndrome, periventricular leukomalacia, and intraventricular hemorrhage) appear to be related to a state of immune activation and inflammation (6,7,30–32). Evidence for this relationship includes direct associations between placental pathology and these morbidities in preterm infants (33) and an increased proportion of T lymphocytes migrating to lungs and brain in addition to the presence of CD45RO+ T cells (6,7,32). Here, we demonstrate alterations in T-cell maturation and homing receptor expression in premature infants that may contribute to these morbid events.
Most striking in this study was our finding of a novel and qualitative difference in the way neonatal and adult naive T cells respond to TLR ligand exposure. The apparent maturation response of neonatal T cells to these microbial products suggests either that microbial elements are necessary for physiologic maturation of the adaptive immune defenses (34) or that neonates can respond to microbial challenges using mechanisms that are not used by adults with more mature adaptive immune systems. These differences between neonatal and adult cells may relate to several factors including the developmental age of the cells, a history of previous exposure to antigen, differences in the nature of TLR interactions with their ligands, or function of antigen-presenting cells. These possibilities will be important to explore in further investigations.
Methods
Subjects
CB samples were obtained from subjects delivered at Case Medical Center/MacDonald’s Women’s Hospital and Tampa General Hospital after obtaining informed consent from parent(s). Healthy adult donor samples were obtained from the Center for AIDS Research at Case Western Reserve University after obtaining informed consent. These studies were performed in accordance with the policies of and approved by the institutional review boards at Case Western Reserve University/University Hospitals of Cleveland and the University of South Florida/Tampa General Hospital. CB samples were obtained from healthy term infants (gestational age ≥37 weeks, n = 26) and preterm infants (gestational age ≤36 weeks, n = 22); and peripheral blood was obtained from healthy adults (n = 10). Demographic and clinical details for the infants were obtained from the medical record (Table 1).
Table 1.
Subject characteristics
| Term (n = 26) |
Preterm (n = 22) |
P value | |
|---|---|---|---|
| Mean gestational age in weeks (SD) | 38.7 (±1.1) | 30.1 (±3.5) | <0.0001 |
| Race (%) | NS | ||
| AA | 56 | 36 | |
| C | 28 | 50 | |
| H | 16 | 14 | |
| Delivery mode (%) | NS | ||
| V | 40 | 32 | |
| C/S | 60 | 68 | |
| Prenatal antibiotics (%) | None | 100 | |
| Prenatal steroids (%) | None | 59 | |
| Prenatal magnesium sulfate exposure (%) | None | 32 | |
| Clinical chorioamnionitisa (%) | None | 27 | |
| Histological chorioamnionitisb (%) | N/A | 69 | |
| Stage 1 (%) | 27 | ||
| Stage 2 (%) | 36 | ||
| Stage 3 (%) | 36 |
AA, African American; bpm, beats per minute; C, Caucasian; C/S, Cesarean section; H, H ispanic; N/A, not applicable; V, vaginal.
Defined by fever (an intrapartum temperature >100.4 °F or >37.8 °C), significant maternal tachycardia (>12 bpm), fetal tachycardia (>160–180 bpm), purulent or foul-smelling amniotic fluid or vaginal discharge, uterine tenderness, and maternal leukocytosis (total blood leukocyte count >15,000–18,000 cells/µl).
Stage 1: neutrophils in placental chorionic plate only; stage 2: neutrophils throughout chorionic plate and subamniotic connective tissue; stage 3: necrotizing inflammation or multifocal abscesses.
Cell Preparation and Flow Cytometry
CB from term and preterm pregnancies was collected according to the National Cord Blood Program protocol (35). Peripheral blood was collected from adults by venipuncture and transferred into heparin-coated tubes. Cell-surface-molecule expression was assessed in whole blood within 6 h. This rapid analysis permits accurate assessment of the in vivo phenotype state.
Whole blood (200 µl) samples were stained for 15 min in the dark at room temperature with fluorochrome-labeled antibodies (Table 2) and then incubated for 15 min with FACS Lysing Solution (BD Bioscience, San Diego, CA). Cells were washed in wash buffer (phosphate-buffered saline with 1% bovine serum albumin and 0.1% sodium azide). Cells were then placed in wash buffer and fixed in 1% formaldehyde. Cells were analyzed using an LSRII Flow Cytometer (Becton Dickinson, San Jose, CA); FACSDIVA software (version 6.1.1 Bioscience, San Diego, CA) was used to organize the data, and FlowJo software (version 7, Tree Star, Ashland, OR) and Prism 5.0 Graphpad software (La Jolla, CA) were used to analyze the data. Nucleated red blood cells were excluded from the analysis by gating on cells expressing CD45 (36) (Figure 1a). T cells were identified by size, granularity, and expression of CD3 and CD45 (Figure 1b). An average of 6,000 CD3+ events were collected for analysis.
Table 2.
Cell-surface markers, fluorochrome labeling, and function
| Marker | Fluorochromes, isotypes, clones, and company | Description |
|---|---|---|
| CD3 | Pacific Blue, Ms IgG1,k, UCHT1, BD Biosciences | Identifies T lymphocytes associated with the TCR. |
| CD45 | Peridinin chlorophyll protein complex, Ms IgG1,k, 2D1, BD Biosciences | Type I transmembrane protein that is in various forms present on all differentiated hematopoietic cells except erythrocytes and plasma cells. |
| CD45RA | Fluorescein isothiocyanate, Ms IgG2b,k, Hl100, BD Biosciences | Expressed on naive T lymphocytes; antigen density decreases upon in vitro activation. |
| CD45RO | Allophycocyanin, Ms IgG2a,k, UCHL1, BD Biosciences | Expressed by most thymocytes, activated memory T cells, granulocytes, and monocytes. |
| α4 | Phycoerythrin-cyanine 5, Ms IgG1,k, 9F10, BD Biosciences | Integrin subunit is expressed on the cell membrane associated with the β1 or the β7 integrin chains. α4β7 is expressed in T cells that preferentially localize to the gut-associated lymphoid tissue. |
| β7 | Phycoerythrin, Rat IgG2a,k, FIB27, Biolegend | Integrin subunit; can associate with one of several different subunits. |
| CCR4 | Phycoerythrin-cyanine 7, Ms IgG1,k, 1G1, BD Biosciences | Homing of T cells to sites of cutaneous and lung inflammation. |
TCR, T-cell receptor.
T-Cell Exposure to TLR Ligands
CB mononuclear cells from term infants were isolated over a Ficoll-Hypaque cushion. Density sedimentation was performed twice to improve the purity of the mononuclear cells (Supplementary Figure S1 online) (37). Cell viability was examined using trypan blue. Cell viability after Ficoll-Hypaque was always higher than 95%. Cells were cultured in RPMI 1640 (Cambrex BioWhitaker; Walkersville, MD) supplemented with 10% fetal bovine serum (Hyclone; Logan, Utah), 1% 2 mmol/l L-glutamine (Cambrex BioWhitaker), and 1% streptomycin (Cambrex BioWhitaker). Cells were cultured for 7 days with anti-CD3 monoclonal antibody alone (BD Pharmigen, San Diego, CA; 100 ng/ml) or individual TLR, as indicated. Lipopolysaccharide (E. coli, lipopolysaccharide 5 µg/ml, TLR 4 ligand), flagellin (S. tyhimurium, 100 ng/ml, TLR 5 ligand), macrophage-activating lipopeptide-2 (MALP-2 isolated from Mycoplasma fermentans, 100 ng/ml, TLR 2–6 dimer), and CpG oligodeoxynucleotide 2006 (5 µg/ml, TLR 9 ligand) were purchased from Imgenex (San Diego, CA). After 7 days, cells were stained with fluorochrome-labeled antibodies to characterize T-cell populations (naive CD45RA+, memory CD45RO+, α4β7+ T cells, and CCR4+ T cells) (Table 2) and analyzed by flow cytometry, as described above. In addition, cells were stained with propidium iodide for viability at day 4 and day 6 (Supplementary Figure S2 online). Viability remained higher than 92%. Adult peripheral blood mononuclear cells were depleted of CD45RO+ cells by magnetic bead separation and examined for the presence of antigen-presenting cells (Supplementary Figure S3 online). Peripheral blood mononuclear cells were suspended in 80 µl of magnetic-activated cell sorting depletion buffer (phosphate-buffered saline, 0.5% bovine serum albumin, and 2 mmol/l EDTA) per 107 total cells. Then 20 µl of CD45RO microbeads (Miltenyi Biotec no. 130-046-001; Miltenyi Biotec, Auburn, CA) were added per 107 total cells. Cells were mixed and refrigerated for 15 min (4–8 °C). Cells were washed by adding 1–2 ml of depletion buffer per 107 cells and centrifuged for 10 min. Cells were then resuspended in 500 µl of buffer and run in the AutoMACS Magnetic Separator (Miltenyi Biotec) using the “Depletes” separation program. Depletion of CD45RO+ cells was confirmed by flow cytometry and the resulting T-cell populations were determined to be >95% CD45RA+CD45RO−. Neonatal T-cell populations were examined for the presence of CD45RO+ T cells before culture. CD45RO+ T cells in CB samples were <5%.
Placental Pathology
A histological placental diagnosis was also used because chorioamnionitis could still be present even in the absence of clinical symptoms. This diagnosis is routinely performed in placentas of preterm infants born at Tampa General Hospital and MacDonald’s Women’s Hospital. Results are summarized in Table 1.
Statistical Methods
Group medians were compared for statistically significant differences using a Mann–Whitney rank test or ANOVA; a P value of 0.05 was considered statistically significant. We analyzed the samples and generated graphs using GraphPad Prism 5 (La Jolla, CA).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the Cleveland Immunopathogenesis Consortium, the research nurses, and the fellows at Rainbow Babies and Children’s Hospital and Tampa General Hospital.
STATEMENT OF FINANCIAL SUPPORT
This work has been supported by grants from the Rainbow Babies and Children’s Foundation Fellowship Research Award, the NICHD Research Career Development in Child Health (K12 HD057581), the Case Western Reserve University Center for AIDS Research (AI-36219), the National Institutes of Health (AI 076174), and the Tampa General Hospital Office of Clinical Research Award.
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/pr
REFERENCES
- 1.Trivedi HN, HayGlass KT, Gangur V, Allardice JG, Embree JE, Plummer FA. Analysis of neonatal T cell and antigen presenting cell functions. Hum Immunol. 1997;57:69–79. doi: 10.1016/s0198-8859(97)00202-4. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293–301. doi: 10.1016/s0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 3.Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 4.Hamada S, Vearncombe M, McGeer A, Shah PS. Neonatal group B streptococcal disease: incidence, presentation, and mortality. J Matern Fetal Neonatal Med. 2008;21:53–57. doi: 10.1080/14767050701787474. [DOI] [PubMed] [Google Scholar]
- 5.Yurdakök M. Antibiotic use in neonatal sepsis. Turk J Pediatr. 1998;40:17–33. [PubMed] [Google Scholar]
- 6.Duggan PJ, Maalouf EF, Watts TL, et al. Intrauterine T-cell activation and increased proinflammatory cytokine concentrations in preterm infants with cerebral lesions. Lancet. 2001;358:1699–1700. doi: 10.1016/s0140-6736(01)06723-x. [DOI] [PubMed] [Google Scholar]
- 7.Turunen R, Vaarala O, Nupponen I, et al. Activation of T cells in preterm infants with respiratory distress syndrome. Neonatology. 2009;96:248–258. doi: 10.1159/000220764. [DOI] [PubMed] [Google Scholar]
- 8.Jalava J, Mäntymaa ML, Ekblad U, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol. 1996;103:664–669. doi: 10.1111/j.1471-0528.1996.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Roslansky P, Oyarzun E, et al. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol. 1988;158:1044–1049. doi: 10.1016/0002-9378(88)90216-5. [DOI] [PubMed] [Google Scholar]
- 10.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 11.Boggess KA, Madianos PN, Preisser JS, Moise KJ, Jr, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol. 2005;192:554–557. doi: 10.1016/j.ajog.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Michie CA. Jewell A CD45 and exploring neonatal immunocompromise. J Pediatr Infect Dis. 2009;4:193–195. [Google Scholar]
- 13.Luciano AA, Yu H, Jackson LW, Wolfe LA, Bernstein HB. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS ONE. 2011;6:e16698. doi: 10.1371/journal.pone.0016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 15.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Fleer A, Krediet TG. Innate immunity: toll-like receptors and some more. A brief history, basic organization and relevance for the human newborn. Neonatology. 2007;92:145–157. doi: 10.1159/000102054. [DOI] [PubMed] [Google Scholar]
- 17.Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS ONE. 2008;3:e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan MH, Steel J, Kennea N, Feldman RG, Edwards AD. The role of intrauterine bacteria in brain injury. Acta Paediatr Suppl. 2004;93:4–5. doi: 10.1111/j.1651-2227.2004.tb03039.x. [DOI] [PubMed] [Google Scholar]
- 19.Kristóf K, Kocsis E, Nagy K. Clinical microbiology of early-onset and late-onset neonatal sepsis, particularly among preterm babies. Acta Microbiol Immunol Hung. 2009;56:21–51. doi: 10.1556/AMicr.56.2009.1.2. [DOI] [PubMed] [Google Scholar]
- 20.Sackstein R. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr Opin Hematol. 2005;12:444–450. doi: 10.1097/01.moh.0000177827.78280.79. [DOI] [PubMed] [Google Scholar]
- 21.Dudda JC, Martin SF. Tissue targeting of T cells by DCs and microenvironments. Trends Immunol. 2004;25:417–421. doi: 10.1016/j.it.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Kremmer E, Palermo B, et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037–2045. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 23.Campbell JJ, Haraldsen G, Pan J, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 24.Opiela SJ, Koru-Sengul T, Adkins B. Murine neonatal recent thymic emigrants are phenotypically and functionally distinct from adult recent thymic emigrants. Blood. 2009;113:5635–5643. doi: 10.1182/blood-2008-08-173658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra I, Dent A, Mungai P, et al. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000116. e1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steiner K, Myrie L, Malhotra I, et al. Fetal immune activation to malaria antigens enhances susceptibility to in vitro HIV infection in cord blood mononuclear cells. J Infect Dis. 2010;202:899–907. doi: 10.1086/655783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 28.Grindebacke H, Stenstad H, Quiding-Järbrink M, et al. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J Immunol. 2009;183:4360–4370. doi: 10.4049/jimmunol.0901091. [DOI] [PubMed] [Google Scholar]
- 29.Conroy ME, Shi HN, Walker WA. The long-term health effects of neonatal microbial flora. Curr Opin Allergy Clin Immunol. 2009;9:197–201. doi: 10.1097/ACI.0b013e32832b3f1d. [DOI] [PubMed] [Google Scholar]
- 30.von Bismarck P, Claass A, Schickor C, Krause MF, Rose-John S. Altered pulmonary interleukin-6 signaling in preterm infants developing bronchopulmonary dysplasia. Exp Lung Res. 2008;34:694–706. doi: 10.1080/01902140802389693. [DOI] [PubMed] [Google Scholar]
- 31.Emami CN, Petrosyan M, Giuliani S, et al. Role of the host defense system and intestinal microbial flora in the pathogenesis of necrotizing enterocolitis. Surg Infect (Larchmt) 2009;10:407–417. doi: 10.1089/sur.2009.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petito CK, Adkins B. Choroid plexus selectively accumulates T-lymphocytes in normal controls and after peripheral immune activation. J Neuroimmunol. 2005;162:19–27. doi: 10.1016/j.jneuroim.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Ogunyemi D, Murillo M, Jackson U, Hunter N, Alperson B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Matern Fetal Neonatal Med. 2003;13:102–109. doi: 10.1080/jmf.13.2.102.109. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubinstein P, Stevens CE. The New York Blood Center’s Placental/Umbilical Cord Blood Program. Experience with a ‘new’ source of hematopoietic stem cells for transplantation. Ernst Schering Res Found Workshop. 2001;33:47–70. doi: 10.1007/978-3-662-04469-8_4. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji T, Sakata T, Hamaguchi Y, Wang F, Houwen B. New rapid flow cytometric method for the enumeration of nucleated red blood cells. Cytometry. 1999;37:291–301. doi: 10.1002/(sici)1097-0320(19991201)37:4<291::aid-cyto6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.Yang MH, Lin SJ. Effect of two-round Ficoll-Hypaque density gradient centrifugation on lymphocyte subsets and natural killer activity of umbilical cord blood mononuclear cells. Pediatr Hematol Oncol. 2001;18:57–63. doi: 10.1080/088800101750059864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.