Abstract
Purpose.
Fusarium is a major cause of microbial keratitis, and its ability to form biofilms was suggested as a contributing factor in recent outbreaks. We investigated the ability of outbreak Fusarium isolates (F. solani species complex [FSSC] and F. oxysporum species complex [FOSC]) to form biofilms in vitro and in vivo, and evaluated their antifungal susceptibilities.
Methods.
Biofilm formation was assessed using our in vitro contact lens model and in vivo murine model. Biofilm architecture was assessed using confocal laser scanning microscopy (CLSM). Susceptibility against amphotericin B (AmB), voriconazole (VCZ), and natamycin (NAT) was determined using the CLSI-M38-A2 method and XTT metabolic assay.
Results.
FSSC strains formed more biofilms than FOSC, in a strain- and clade-dependent manner. CLSM analyses revealed that “high biofilm forming” (HBF) strains had denser and thicker biofilms than “low biofilm forming” (LBF) strains of both species (thickness 51 vs. 41 μm for FSSC and 61 vs. 45 μm for FOSC strains, P < 0.05 for both comparisons). Fusarium biofilms exhibited species-dependent antifungal susceptibilities (e.g., FSSC biofilms AmB minimal inhibitory concentrations [MIC] ≥16 μg/mL, while NAT or VCZ MICs were 2–8 μg/mL). FSSC-infected mice had severe corneal opacification independent of biofilm thickness, while FOSC infection resulted in moderate corneal opacification. Corneal fungal burden of mice infected with HBF strains was higher than those of the LBF strains. In contrast, the reference ATCC isolate was unable to cause infection.
Conclusions.
The ability to form biofilms is a key pathogenicity determinant of Fusarium, irrespective of the thickness of these biofilms. Further studies are warranted to explore this association in greater detail.
Fusarium is a major cause of microbial keratitis. Outbreak isolates of F. solani formed more biofilms than F. oxysporum. These biofilms are resistant to commonly used antifungals, and important determinants of Fusarium pathogenesis, likely playing a major role in keratitis outbreaks.
Introduction
Corneal ulcers caused by bacteria and fungi are a major cause of visual impairment and blindness worldwide,1 and keratitis caused by Fusarium solani and F. oxysporum is among the most refractory and common causes of fungal keratitis,2–4 and its incidence is increasing in many areas of the world, including the United States.1,2,5–7 Fusarium is a filamentous fungus that infests agricultural plants, and the incidence of Fusarium keratitis peaks during harvest seasons when farm workers at are more risk of corneal injury and exposure to airborne spores.5,8,9 However, another major risk factor for Fusarium keratitis is contact lens wear due to increased association with contaminated lenses, lens cases, and contact lens care solutions. The formation of biofilm by these fungi on contact lenses and lens cases is thought to have a major role in causing keratitis once the lens is placed on the ocular surface.10 Biofilms are defined as a structured community of microorganisms surrounded by self-produced extracellular matrix, and are adherent to an inert or living surface. These biofilms are resistant to most antimicrobials.11
From 2005–2007, more than 300 cases of Fusarium keratitis were associated with contact lens wear and use of the contact lens cleaning solution ReNu with MoistureLoc (Bausch and Lomb, Rochester, NY),12–14 with many patients requiring keratoplasty and some having to undergo removal of the whole eye. The severity of disease was due to misdiagnosis and inappropriate treatment, and to failure of antimycotic agents. Patients experiencing these outbreaks had no history of recent ocular trauma or prior application of corticosteroids. Further, when this cleaning solution was withdrawn from worldwide market in May 2006, the number of patients declined to normal levels in June 2006.12
Microbial biofilms have been observed on contact lenses,15–17 and the 2005–2006 outbreak was attributed partly to the ability of Fusarium to form biofilms.10,18,19 In a previous study, we established a Fusarium biofilm model on multiple types of contact lenses using a single F. oxysporum isolate, and characterized the structure and developmental phases of the formed biofilms.10 In the current study, we used the established contact lens biofilm model to investigate the ability of Fusarium isolates from the keratitis outbreak to form biofilm. In addition, the antifungal susceptibility of planktonic forms of four outbreak strains against amphotericin B (AmB), natamycin (NAT), and voriconazole (VCZ) was determined following the M38-A2 method.20 Additionally, the susceptibility of biofilm forms of the 4 strains also was determined using a metabolic activity assay (tetrazolium-based XTT).10,21 Our data showed that Fusarium isolates from the outbreak formed biofilms on soft contact lenses in vitro and in vivo, and that biofilm formation was elevated in F. solani compared to F. oxysporum isolates. These biofilms were susceptible to NAT, but exhibited species-dependent susceptibility to AmB and VCZ. Using an established murine model of Fusarium keratitis, we show elevated fungal burden in high biofilm versus low biofilm forming isolates. Together, our findings supported the contention that the ability of Fusarium to form biofilms is a critical determinant in resistance to antibiotics and to the pathogenesis of fungal keratitis, and should be taken into consideration when managing patients with this disease.
Methods
Fungal Strains
Of the 27 Fusarium strains included in our study, 25 were generous gifts from the Centers for Disease Control (CDC), and the others were from our culture collection at the Center for Medical Mycology (CMM). All CDC strains were isolated from corneas of keratitis patients in the outbreak in the U.S., and were identified and characterized using DNA sequence-based multilocus genotyping.12,22,23 Detailed information regarding all the strains are listed in Table 1. Of these isolates 22 are members of the species-rich Fusarium solani species complex (FSSC), while the other five are members of the Fusarium oxysporum species complex (FOSC). Fusarium moniliforme ATCC MYA-3629 was used as a reference strain in the antifungal susceptibility tests conducted, and Candida krusei ATCC 6258 was used as a quality control isolate. We also obtained F. oxysporum environmental isolates MRL27845 and MRL27846 from Seogchan Kang, Penn State University.
Table 1. .
Fusarium Isolates Tested in the Current Study
|
Strain ID |
State |
Body Site |
USDA Organism ID |
| B6902 | NJ | Cornea | FSSC 1-a |
| B6904 | NJ | Cornea | FSSC 1-a |
| B6907 | CT | Cornea | FSSC 1-a |
| B6908 | CT | Cornea ulcer | FOSC 3-b |
| B6913 | OH | Cornea | FSSC 1-a |
| B6914 | OH | Cornea | FSSC 2-a |
| B6936 | VT | Cornea | FOSC 3-a |
| B6919 | PA | Cornea (L) | FSSC 1-a |
| B6921 | NJ | Cornea | FSSC 2-a |
| B6922 | PA | Cornea (R) | FSSC 3-a |
| B6926 | CT | Cornea | FSSC 2-d |
| B6927 | CT | Cornea (R) | FSSC 2-d |
| B6951 | IL | Cornea ulcer (L) | FSSC 2-e |
| B6952 | IL | Cornea (L) | FSSC 2-d |
| B6966 | IA | Cornea | FSSC 1-a |
| B6970 | MD | Cornea | FSSC 6-a |
| B6971 | MI | Right eye | FSSC 2-g |
| B6980 | OH | Cornea | FIESC 1-a |
| B6981 | OH | Eye ulcer (R) | FOSC 3-e |
| B6982 | TN | Eye | FSSC 2-d |
| B6983 | TN | Cornea | FSSC 1-a |
| B6984 | TN | Cornea (L) | FSSC 7-a |
| B6992 | MO | Cornea | FOSC 4-c |
| B7056 | FL | Cornea | FSSC 1-b |
| B7059 | FL | Cornea | FSSC 3-b |
| MRL8609 | OH | Cornea | FSSC 1-b |
| MRL8996 | OH | Cornea | FOSC 3-a |
L, left; R, right.
Antifungal Agents Used in the Susceptibility Testing
Three antifungal agents, AmB (ScienceLab, Houston, TX), NAT (SeqChem, Pangbourne, UK), and VCZ (Pfizer, New York, NY), were included in the tests and dissolved in dimethyl sulfoxide (DMSO) to 6.4 mg/mL as stock solutions, stored at −80°C. These antifungals were selected because they are used to treat patients with fungal keratitis.
Fungal Growth Conditions
Fusarium isolates were inoculated on potato dextrose agar (Becton, Dickinson and Company, Annapolis, MD), and incubated for 2 days at 35°C and then for 5 days at 26°C. Following incubation, conidia of the Fusarium strains were harvested and hyphae were removed by filtration through sterile gauze. Conidia were washed with PBS and standardized to 1 × 106 conidia/mL by counting spores in a hemacytometer for biofilm formation experiments, or washed with sterile 0.85% saline and standardized to 1 × 106 conidia/mL for testing the antifungal susceptibility of planktonically grown cells as described in the Clinical and Laboratory Standard Institute (CLSI) M38-A2 document.20 Culture suspension of planktonic F. moniliforme ATCCMYA-3629 was prepared under conditions similar to those used for the clinical strains, while suspension of Candida krusei cells (ATCC 6258) was prepared as recommended in the CLSI M27-A3 document.24
Biofilm Formation
The in vitro model of Fusarium biofilm formation on soft contact lens, established previously in our laboratory, was used to evaluate biofilm-forming abilities of the 27 Fusarium isolates.10 The XTT metabolic assay was proved useful in evaluating fungal biofilm formation on contact lenses.19 Lotrafilcon A soft contact lenses (Night & Day, Ciba Vision, 20% water, base curve 8.6, power +1.50, diameter 13.8, FDA Group 5; Duluth, GA) were selected in this study for biofilm formation because our previous study indicated that Fusarium strains formed the most abundant and hypha-richest biofilms on this type of lens.10 Briefly, Lotrafilcon A soft contact lenses were washed with PBS, placed in 12-well tissue culture plates containing 4 mL standardized cell suspension (1 × 106 conidia/mL), and incubated for 90 minutes at 37°C (adherence phase). These lenses were washed gently with 4 mL PBS to remove nonadherent cells. Next, lenses were immersed in Sabouraud dextrose broth (SDB; Difco Laboratories, Detroit, MI) medium and incubated for 48 hours at 37°C on a rocker. Biofilms were quantified using a tetrazolium XTT (2,3-bis-[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide) assay as described previously.10,21 Four strains, which formed the most and the least biofilm in the FSSC or FOSC group, were selected for further experiments to investigate their biofilm morphology and antifungal susceptibility patterns.
Confocal Laser Scanning Microscopy (CLSM)
The architecture of biofilms formed on soft contact lenses by 4 strains was analyzed using CLSM, following our previously described method.21 Briefly, soft contact lenses containing biofilms were transferred to 12-well plates and incubated for 45 minutes at 37°C in 4 mL of PBS containing the fluorescent stains FUN-1 (10 mM) and concanavalin A-Alexa Fluor 488 conjugate (ConA, 25 mg/mL). After incubation with the dyes, the lenses were flipped and placed on a 35-mm-diameter glass-bottom Petri dish (MatTek Corp., Ashland, MA). Stained biofilms were observed using a Zeiss LSM510 confocal scanning laser microscope equipped with argon and HeNe lasers, and mounted on a Zeiss Axiovert100 M microscope (Carl Zeiss Microscopy GmbH, Hamburg, Germany). All observations were conducted with a water immersion C-apochromat objective (403; numerical aperture, 1.2).
Antifungal Susceptibility Testing of Planktonic Fusarium Cells
A broth microdilution method was used in accordance with the CLSI guidelines of M38-A2 document,20 except for the inocula being prepared by means of counting conidia.10,21 The standardized Fusarium suspension (1 × 106 conidia/mL in 0.85% saline) was diluted further with RPMI 1640 medium (Hardy Diagnostics, Criterion Dehydrated Culture Media, with MOPS), resulting in a suspension of 2 × 104 conidia/mL. The stock solutions of the three antifungal agents were diluted further with DMSO and RPMI 1640 medium to prepare working solutions with concentrations ranging from 0.25–128 μg/mL. Round-bottom 96-well microdilution plates were set up according to the M38-A2 methodology and were incubated at 35°C for 48 hours. For all three drugs, the minimum inhibitory concentrations (MICs) were read as the lowest drug concentration that prevented any discernable growth (100% inhibition) compared to growth control. After the MICs were read visually, the XTT metabolic assay also was used in reading MICs (final concentration of XTT 100 μg/mL and menadione 25 μM).25
Antifungal Susceptibility Testing of Fusarium Biofilms
Susceptibility of formed biofilms to different agents was evaluated as described previously.10 Briefly, stock solutions (6.4 mg/mL) of the drugs were diluted with DMSO and SDB medium to concentrations ranging from 0.5–256 μg/mL. Four mL of each drug solution were added to separate wells of a 12-well plate. The 11th and the 12th wells of every plate acted as growth control and negative control, respectively. Contact lens with mature (48-hour grown) Fusarium biofilms were transferred gently to the first to 11th wells and incubated at 37°C for 48 hours. At the end of the incubation period, contact lenses with biofilms were transferred gently to 12-well plates containing 4-mL of PBS, and their metabolic activities were determined by XTT assay. Next, percent inhibition induced in biofilms by each drug solution was evaluated by comparing their metabolic activities to that of biofilms grown in the absence of any drug.
Corneal Infection Model
For the biofilm model of infection, Fusarium isolates were incubated with contact lenses, and biofilms were formed as described above. The corneal epithelium of C57BL/6 mice was abraded and a 2 mm diameter contact lens punch was placed on the ocular surface for 2 hours.26 After this time, the lenses were removed, and the corneas were examined by microscopy on days 1 and 2 post-infection. Mice then were euthanized, eyes were homogenized, and colony-forming units (CFUs) were calculated.26 For the intrastromal model, 1 × 104 conidia were injected directly into the corneal stroma in 2 μL PBS as described previously.27 Corneal disease was examined and photographed on days 1 and 2 after infection, and on day 2 mice were euthanized, eyes were homogenized, and CFUs determined as before.26,27 All animal studies were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Statistical Analyses
All experiments were performed in duplicate. Statistical analysis was performed using ANOVA by the software SPSS 16.0 to assess the amount of biofilm formed by different Fusarium strain. A P value of < 0.05 was considered statistically significant. For antifungal susceptibility testing, difference of more than 3 dilutions between 2 MIC values was considered significant.
Results
Biofilm Formation of Fusarium Clinical Isolates
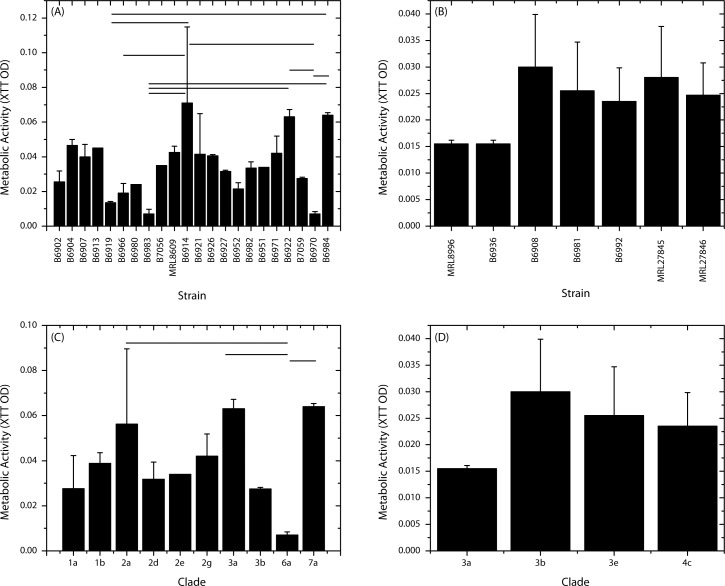
We used metabolic activity assay to quantify biofilm forming ability of 25 outbreak and 2 additional clinical isolates of Fusarium. We found that all tested isolates formed biofilms on Lotrafilcon A soft contact lenses after 48 hours of incubation. In general, the FSSC strains formed 1.5-fold more biofilms than the FOSC strains (mean XTT OD 0.035 ± 0.019 vs. 0.022 ± 0.007, respectively; P = 0.033). Among the FSSC strains, B6914 formed significantly more biofilms than B6919 (P = 0.009), B6966 (P = 0.03), B6983 (P = 0.002), and B6970 (P = 0.002; Fig. 1A). Moreover, metabolic activity of biofilms formed by strain B6919 was significantly more than that of biofilms formed by strain B6984 (P = 0.041), while strain B6922 formed significantly higher biofilms than B6982 and B6970 (P = 0.012 for both comparisons). In contrast, no significant difference in biofilm metabolic activity was observed for the FOSC strains (Fig. 1B). Furthermore, we tested the ability of two environmental isolates of F. oxysporum (MRL27845 and MRL278456), and found that that these strains did not differ in their biofilm forming ability from other FOSC strains (XTT OD 0.028 ± 0.01 and 0.025 ± 0.006, respectively; P > 0.05; Fig. 1B). Since the strains tested represented different clades, we determined whether the biofilm forming ability of Fusarium isolates differed by clades. As shown in Figure 1C, strains belonging to clade 6a formed more biofilms than 2a (P = 0.018) or clade 3a (P = 0.021), but less than clade 7a (P = 0.0054). No statistically significant differences were observed among FOSC clades (Fig. 1D). These results revealed that more of the FSSC strains formed biofilms than FOSC strains, and that the ability to form biofilms varied by strain and clade type.
Figure 1. .
Comparison of the ability of Fusarium isolates obtained from keratitis outbreak to form biofilms. Metabolic activity (XTT) assay was used to evaluate biofilm formation by isolates belonging to FSCC (A, C) or FOSC (B, D). Panels (A) and (B) show variation of biofilm forming ability by strain type, while panels (C) and (D) present results showing variation in biofilm formation by clade type. Horizontal bars: significantly different comparisons (P ≤ 0.05). MRL8609 and MRL8996 were non-outbreak isolates obtained from keratitis patients.
Biofilm Architecture
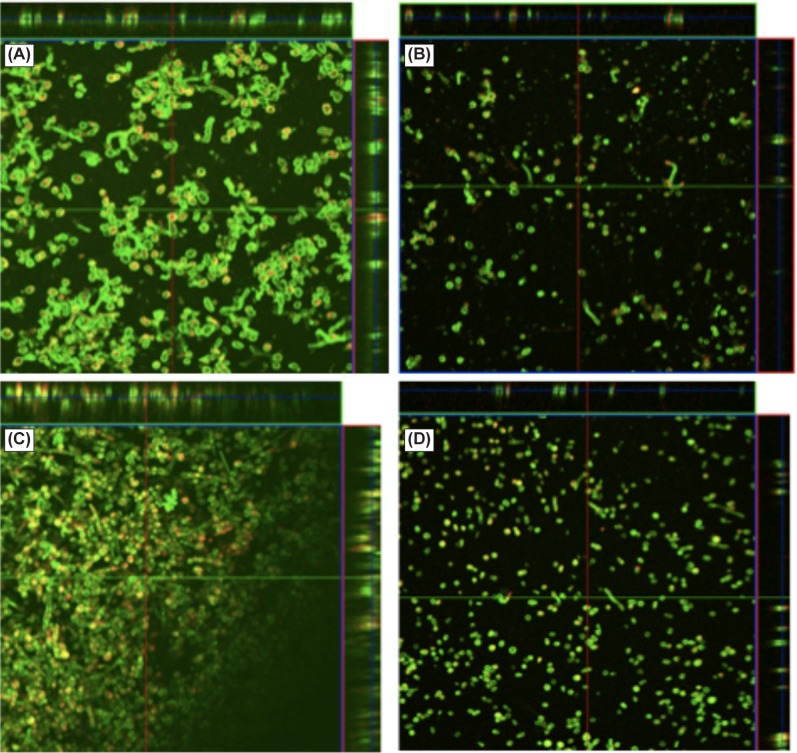
Next, we used CLSM and scanning electron microscopy (SEM) to characterize the architecture and ultrastructure of biofilms formed by two strains each of FSSC (B6914 and B6970) and FOSC (B6908 and B6936). These strains were selected as representative “low– ” and “high–” biofilm forming strains. CLSM analysis revealed that the “high biofilm forming” strains had denser and more compact biofilms than the “low biofilm forming” strains of both species (Figs. 2A, 2C). The “low biofilm former” strains (B6970 and B6936) formed biofilms in which the fungal conidia and hyphae were spread out, and the extracellular matrix was sparse (Figs. 2B, 2D). These analyses also revealed that thickness of FSSC 6914 biofilms was significantly higher than those formed by FSSC B6970 (thickness 51 vs. 41 μm, P < 0.05), and that those formed by FOSC B6908 were significantly thicker than B6936 (61 vs. 45 μm, P < 0.05).
Figure 2. .
Confocal microscopy analysis of biofilms formed by FSSC (A, B) and FOSC (C, D) isolates. Formed biofilms were stained with fluorescent stains FUN-1 (red) and concanavalin A-Alexa Fluor 488 conjugate (green), and examined using a Zeiss LSM510 confocal scanning laser microscope equipped with argon and HeNe lasers, and mounted on a Zeiss Axiovert100 M microscope. All observations were conducted with a water immersion C-apochromat objective. Representative images are shown for (A) FSSC 6914, (B) FSSC 6970, (C) FOSC 6908, and (D) FOSC 6936. Magnification 20×.
Susceptibility of Fusarium Biofilms to Antifungal Agents
Next, we evaluated the susceptibility of biofilms formed by the selected isolates to three antimicrobial agents (NAT, VCZ, and AmB), and compared them to their planktonic forms. As shown in Table 2, planktonic forms of the tested isolates were susceptible to the three agents. The MICs were in the range of 1–8 μg/mL, with AmB exhibiting the lowest MIC (1 μg/mL). We also used XTT assay to determine the susceptibility of planktonically grown Fusarium isolates, and found that the MICs were in agreement between the two methods (data not shown). Compared to planktonically grown cells, FSSC biofilms exhibited reduced susceptibility to AmB (MIC 16 or 128 μg/mL), but were susceptible to NAT and VCZ (MIC range 2–8 μg/mL). In contrast, FOSC biofilms exhibited reduced susceptibility to VCZ (MIC 256 μg/mL), but were susceptible to AmB and NAT (MIC range 0.5–4 μg/mL, Table 2). These results demonstrated that Fusarium grown as a biofilm are more resistant to these antifungal agents than the planktonic form; however, biofilm forming organisms exhibited varying susceptibility to different antifungals in a species-specific manner.
Table 2. .
MIC (μg/mL) of Antimicrobial Agents against Planktonic and Biofilms Forms of Fusaria
|
Strain |
Planktonic MIC (μg/mL) |
Biofilms MIC (μg/mL)* |
||||
|
AmB |
NAT |
VCZ |
AmB |
NAT |
VCZ |
|
| F. solani B6914 | 1 | 4 | 8 | 128 | 4 | 2 |
| F. solani B6970 | 1 | 2 | 4 | 16 | 4 | 8 |
| F. oxysporum B6908 | 1 | 4 | 4 | 0.5 | 4 | 256 |
| F. oxysporum B6936 | 1 | 4 | 4 | 1 | 4 | 256 |
Biofilm MIC was defined as the drug concentration that led to reduction in metabolic activity by 50% compared to untreated control.
Virulence of Fusarium Biofilms in a Murine Model of Contact Lens Associated Fungal Keratitis Model
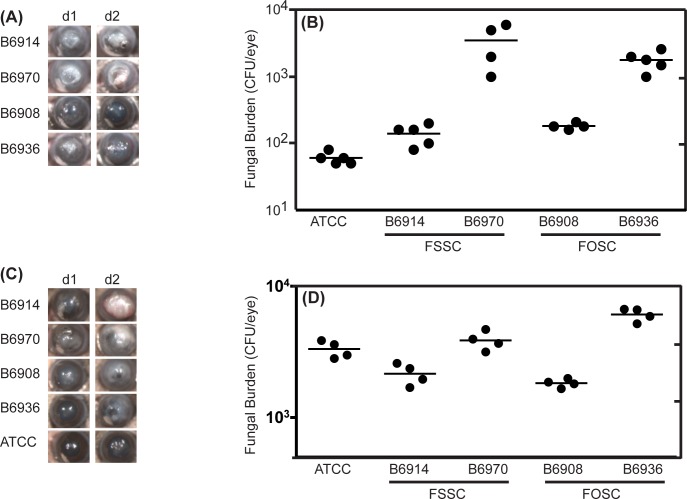
To determine if the ability to form biofilms relates to increased virulence of Fusarium, we examined the ability of outbreak isolates of F. solani and F. oxysporum to cause keratitis using our recently established murine model of contact lens-associated Fusarium keratitis.26 To ascertain if there are additional intrinsic differences in virulence among the tested strains, corneas also were infected by intrastromal injection.27 Mice infected by the biofilm route or intrastromally with either isolate of F. solani had severe corneal opacification, independent of whether they were “high-” or “low-” biofilm formers (Fig. 3). In contrast, mice infected with either isolate of F. oxysporum suffered moderate corneal opacification, and there was no difference between the high and low biofilm formers. Although corneal opacity was species dependent but not biofilm thickness dependent, we also found that corneal fungal burden as measured by CFU was associated with biofilm thickness. CFU from mice infected with “high-biofilm” forming strains were higher than those of the “low-biofilm” forming strains of both F. oxysporum and F. solani (Figs. 3A, 3B). Interestingly, the reference F. moniliforme ATCC isolate was unable to cause infection in contact lens biofilm infection model, resulting in the lowest fungal burden among all the isolates tested and minimal opacification (Figs. 3A, 3B). Results from the intrastromal injection model revealed a strain-dependent disease pattern similar to those from the corneal biofilm model, with F. solani causing more severe disease than F. oxysporum (Figs. 3C, 3D). The difference in CFU between high and low biofilm producing strains also was observed after intrastromal injection of either F. oxysporum or F. solani. Taken together, these findings demonstrate that the ability to form biofilms (comparing ATCC with clinical isolates) is a key determinant of Fusarium pathogenesis in vivo, that F. solani is more virulent than F. oxysporum regardless of biofilm thickness, and that the ability to form biofilms may contribute to survival of both species.
Figure 3. .
Evaluation of in vivo virulence of Fusarium keratitis outbreak isolates using contact lens-associated biofilms or intrasomal injection. Murine corneas were infected with F. solani or F. oxysporum strains either by (A, B) adding a biofilm-contaminated contact lens to abraded cornea, or (C, D) intrastromal injection with conidia. Corneas of C57BL/6 mice were infected with isolates belonging to FSSC (B6914, B6970), FOSC (B6908, B6936), or ATCC reference strains by abrading the cornea and adding a contact lens containing biofilms or intrasomally. (A, C) Corneal opacification in infected mice, showing representative eyes from two repeat experiments with 5 mice per group. (B, D) Corneal fungal burden of infected mice, expressed as CFUs/eye. Data points represent individual corneas.
Discussion
In our study, we compared the ability of Fusarium isolates obtained from the fungal keratitis outbreak to form biofilms, susceptibility of representative isolates to different antimicrobial agents, and their virulence in a mouse model of biofilm fungal keratitis. We found that all tested outbreak isolates, unlike the reference ATCC isolate, formed biofilms to varying degrees.
Our studies revealed that overall, the ability to form biofilms is higher in F. solani isolates compared to F. oxysporum isolates, which is consistent with the report by Chang et al. suggesting that the ability of Fusarium to form biofilms on contact lenses or lens cases may have had a role in the previous outbreak.12 Interestingly, F. solani species were the most commonly isolated (77%) in this outbreak, followed by F. oxysporum species (18%). The fact that F. solani isolates in our study formed more robust biofilms than F. oxysporum isolates (including the tested environmental isolates) supports the notion that the ability to form biofilms is an important determinant in the pathogenesis of fungal keratitis. This observation is similar to other studies comparing invasive and less invasive Candida species, showing that the more invasive C. albicans strains were more prone to form biofilms than potentially invasive but less commonly pathogenic species, such as C. parapsilosis, C. glabrata, and C. tropicalis.28 The finding that outbreak isolates varied in their ability to form biofilms also is similar to previous reports that demonstrated species-dependent biofilm formation for Candida.29,30 Estivill et al. investigated biofilm formation by five species of Candida on three clinical materials, and reported strain- and species-dependent biofilm formation.29 Lattif et al. characterized the biofilms formed by C. parapsilosis, C. metapsilosis, and C. orthopsilosis, and reported strain-dependent biofilm forming ability of these three species.30 Our results also showed that the ability to form biofilms varied by clade type of F. solani species. The implication of the difference between clades in their ability to form biofilms is not clear, and remains to be investigated using larger number of strains for each clade.
Our results showed that NAT exhibited similar MICs against planktonic and biofilm forms of F. solani and F. oxysporum, indicating that biofilm formation has no apparent effect on NAT susceptibility. In contrast, AmB and VCZ were active against planktonic forms of the two Fusarium species, while exhibiting species-dependent activity against Fusarium biofilms. In this regard, VCZ was active against biofilms formed by F. solani, whereas AmB was active against F. oxysporum biofilms. Although the underlying basis for these differences has yet to be determined, we showed that while efflux pumps have a critical role in fluconazole resistance in early-phase Candida biofilms, alteration in sterol composition contributed to resistance at the intermediate and mature phases of biofilm formation.31 In a separate study, Walsh et al. demonstrated that depletion of ergosterol contributed substantially to diminished binding of AmB to the fungal cytoplasmic cell membrane and, hence, result in polyene resistance.32 Based on these studies, we speculate that the resistance to AmB in F. solani biofilms may be due to depletion of membrane ergosterol, whereas resistance to VCZ in F. oxysporum biofilms may be due partly to upregulation of efflux pumps. The clinical implication of this finding is that species identification is critical for optimal management of Fusarium keratitis. Since NAT exhibited activity against both species, this agent may have use as the first line of treatment, especially in cases where biofilm formation is suspected. More detailed analysis must be performed using a large panel of organisms to ascertain the pattern of antifungal susceptibility with respect to different Fusarium species.
Our results showed that biofilm thickness did not correlate with antifungal resistance, which is consistent with a report that fungal strains that form thicker biofilms are not necessarily more resistant than those with low biofilm forming ability. In this regard, Baillie and Douglas reported that thin biofilms (with little extracellular matrix, ECM) did not exhibit significant differences in drug susceptibility compared to thicker biofilms, indicating that drug resistance is unrelated to biofilm thickness or matrix formation.33
Our finding that, in contrast to the reference ATCC strain, all Fusarium outbreak isolates tested formed biofilms in vitro and in vivo, suggested that the ability of Fusarium isolates to form biofilms could have been a contributing factor in the keratitis outbreak. This observation agrees with our previous finding that the ATCC isolate does not form biofilms in vitro.10 The lack of in vivo virulence of this isolate may be due to loss of virulence due to multiple subculturing during long-term storage. Therefore, although this ATCC isolate is recommended by the ISO guidelines for evaluating the antimicrobial effects of lens disinfectants, a recent clinical isolate should be used for testing against Fusarium biofilms.
Given that F. oxysporum and F. solani were isolated from the outbreak, we tested the hypothesis that biofilm thickness has a determining effect on severity of disease. However, we also found that F. solani isolates caused more severe corneal opacification than F. oxysporum isolates regardless of biofilm thickness. As F. solani keratitis also was more severe than F. oxysporum keratitis in the model in which conidia are injected intrastromally, it is likely that this difference may be attributable to additional virulence factors. Of particular interest was the observation that the low biofilm forming F. oxysporum and F. solani isolates had increased CFU compared to high biofilm isolates. This suggested either an inverse role for biofilm in fungal survival, or as this difference also was observed in the intrastromal model, that genes associated with biofilm formation also have a role in inhibiting fungal killing by infiltrating neutrophils.26,27 Therefore, the ability to cause keratitis in a murine model appears to be associated with biofilm formation, but independent of the biofilm thickness. Further studies using these strains may identify novel fungal virulence factors.
In summary, we found that Fusarium isolates from the contact lens-related outbreak all formed biofilm on soft contact lenses, but that the ability to form thicker biofilms was higher in F. solani isolates compared to F. oxysporum isolates. However, the role of biofilm formation on resistance to antifungal agents varied, where all were susceptible to NAT but exhibited species-dependent susceptibility to AmB and VCZ. The ability of Fusarium to form biofilms is a critical determinant in the pathogenesis of fungal keratitis and may contribute to the difficulty of clinical management of this disease.
Footnotes
Supported by NIH Grants R21EY021303 and R21AI074077 (PKM), RO1DE17846 (MAG), RO1EY18612 and P30EY11373 (EP), and with support from The Research to Prevent Blindness Foundation and the Ohio Lions Eye Research Foundation.
Disclosure: P.K. Mukherjee, None; J. Chandra, None; C. Yu, None; Y. Sun, None; E. Pearlman, None; M.A. Ghannoum, None
References
- 1. Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 2003;16:730–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosa RH, Jr, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in south Florida. Ophthalmology. 1994;101:1005–1013 [DOI] [PubMed] [Google Scholar]
- 3. Hu SBS, Fan VC, Koonapareddy C, Du TT, Asbell PA. Contact lens-related Fusarium infection: case series experience in New York City and review of fungal keratitis. Eye Contact Lens. 2007;33:322–328 [DOI] [PubMed] [Google Scholar]
- 4. Iyer SA, Tuli SS, Wagoner RC. Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens. 2006;32:267–271 [DOI] [PubMed] [Google Scholar]
- 5. Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J Ophthalmol. 2003;51:315–321 [PubMed] [Google Scholar]
- 6. Wong TY, Fong KS, Tan DT. Clinical and microbial spectrum of fungal keratitis in Singapore: a 5-year retrospective study. Int Ophthalmol. 1997;21:127–130 [DOI] [PubMed] [Google Scholar]
- 7. Bhartiya P, Daniell M, Constantinou M, Islam FM, Taylor HR. Fungal keratitis in Melbourne. Clin Experiment Ophthalmol. 2007;35:124–130 [DOI] [PubMed] [Google Scholar]
- 8. Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14:61–69 [DOI] [PubMed] [Google Scholar]
- 9. Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113:1943–1948 [DOI] [PubMed] [Google Scholar]
- 10. Imamura Y, Chandra J, Mukherjee PK, et al. Fusarium and Candida albicans biofilms on soft contact lenses: model development, influence of lens type and susceptibility to lens care solutions. Antimicrob Agents Chemother. 2008;52:171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mukherjee PK, Chandra J. Candida biofilm resistance. Drug Resist Updat. 2004;7:301–309 [DOI] [PubMed] [Google Scholar]
- 12. Chang DC, Grant GB, O'Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296:953–963 [DOI] [PubMed] [Google Scholar]
- 13. Khor WB, Aung T, Saw SM, et al. An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. JAMA. 2006;295:2867–2873 [DOI] [PubMed] [Google Scholar]
- 14. Donnio A, Van Nuoi DN, Catanese M, Desbois M, Ayeboua L, Merle H. Outbreak of keratomycosis attributable to Fusarium solani in the French West Indies. Amer J Ophthalmol. 2007;143:356–358 [DOI] [PubMed] [Google Scholar]
- 15. Elder MJ, Stapleton F, Evans E, Dart JK. Biofilm-related infections in ophthalmology. Eye. 1995;9:102–109 [DOI] [PubMed] [Google Scholar]
- 16. Gorlin AI, Gabriel MM, Wilson LA, Ahearn DG. Binding of acanthamoeba to hydrogel contact lenses. Curr Eye Res. 1996;15:151–155 [DOI] [PubMed] [Google Scholar]
- 17. McLaughlin-Borlace L, Stapleton F, Matheson M, Dart JK. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol. 1998;84:827–838 [DOI] [PubMed] [Google Scholar]
- 18. Dyavaiah M, Ramani R, Chu D, et al. Molecular characterization, biofilm analysis and experimental biofouling study of Fusarium isolates from recent cases of fungal keratitis in New York State. BMC Ophthalmology. 2007;7:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Behlau I, Gilmore MS. Microbial biofilms in ophthalmology and infectious disease. Arch Ophthalmol. 2008;126:1572–1581 [DOI] [PubMed] [Google Scholar]
- 20. Clinical Laboratory Standards Institute Reference Method for Broth Dilution Antifungal Susceptibility Testing of Conidium-Forming Filamentous Fungi: Approved Standard M38-A. Wayne, PA:CLSI, 2002. [Google Scholar]
- 21. Chandra J, Mukherjee PK, Ghannoum MA. In vitro growth and analysis of Candida biofilms. Nat Protoc. 2008;3:1909–1924 [DOI] [PubMed] [Google Scholar]
- 22. O'Donnell K, Sarver BAJ, Brandt M, et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria, including isolates from the 2005-06 multistate contact lens-associated U.S. keratitis outbreaks. J Clin Microbiol. 2007;45:2235–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Donnell K, Sutton DA, Rinaldi MG, et al. Genetic diversity of human pathogenic members of the Fusarium oxysporum complex inferred from multilocus DNA sequence data and amplified fragment length polymorphism analyses: evidence for the recent dispersion of a geographically widespread clonal lineage and nosocomial origin. J Clin Microbiol. 2004;42:5109–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clinical Laboratory Standards Institute Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard – Second Edition. CLSI Document M27-A3. Wayne, PA:CLSI;, 2002. [Google Scholar]
- 25. Shehata AS, Mukherjee PK, Ghannoum MA. Comparison between the standardized clinical and laboratory standards institute M38-A2 method and a 2,3–bis(2-methoxy-4-nitro-5-[(sulphenylamino)carbonyl]-2H-tetrazolium hydroxide-based method for testing antifungal susceptibility of dermatophytes. J Clin Microbiol. 2008;46:3668–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun Y, Chandra J, Mukherjee PK, et al. A murine model of contact lens associated Fusarium keratitis. Invest Ophthalmol Vis Sci. 2010;51:1511–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tarabishy AB, Aldabagh B, Sun Y, Szczotka-Flynn L, Ghannoum MA, Pearlman E. MyD88 regulation of Fusarium keratitis is dependent on TLR4 and IL-1R1 but not TLR2. J Immunol. 2008;181:593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuhn DM, Chandra J, Mukherjee PK, Ghannoum MA. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002;70:878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Estivill D, Arias A, Torres-Lana A, Carrillo-Muñoz AJ, Arévalo MP. Biofilm formation by five species of Candida on three clinical materials. J Microbiol Methods. 2011;86:238–242 [DOI] [PubMed] [Google Scholar]
- 30. Lattif AA, Mukherjee PK, Chandra J, et al. Characterization of biofilms formed by Candida parapsilosis, C. metapsilosis, and C. orthopsilosis. Int J Med Microbiol. 2010;300:265–270 [DOI] [PubMed] [Google Scholar]
- 31. Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71:4333–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walsh TJ, Petraitis V, Petraitiene R, et al. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J Infect Dis. 2003;188:305–319 [DOI] [PubMed] [Google Scholar]
- 33. Baillie GS, Douglas LJ. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother. 2000;46:397–403 [DOI] [PubMed] [Google Scholar]