Abstract
Purpose.
To derive a computerized measurement of optic disc volume from digital stereoscopic fundus photographs for the purpose of diagnosing and managing papilledema.
Methods.
Twenty-nine pairs of stereoscopic fundus photographs and optic nerve head (ONH) centered spectral domain optical coherence tomography (SD-OCT) scans were obtained at the same visit in 15 patients with papilledema. Some patients were imaged at multiple visits in order to assess their changes. Three-dimensional shape of the ONH was estimated from stereo fundus photographs using an automated multi-scale stereo correspondence algorithm. We assessed the correlation of the stereo volume measurements with the SD-OCT volume measurements quantitatively, in terms of volume of retinal surface elevation above a reference plane and also to expert grading of papilledema from digital fundus photographs using the Frisén grading scale.
Results.
The volumetric measurements of retinal surface elevation estimated from stereo fundus photographs and OCT scans were positively correlated (correlation coefficient r2 = 0.60; P < 0.001) and were positively correlated with Frisén grade (Spearman correlation coefficient r = 0.59; P < 0.001).
Conclusions.
Retinal surface elevation among papilledema patients obtained from stereo fundus photographs compares favorably with that from OCT scans and with expert grading of papilledema severity. Stereoscopic color imaging of the ONH combined with a method of automated shape reconstruction is a low-cost alternative to SD-OCT scans that has potential for a more cost-effective diagnosis and management of papilledema in a telemedical setting. An automated three-dimensional image analysis method was validated that quantifies the retinal surface topography with an imaging modality that has lacked prior objective assessment.
A computerized measurement of optic disc volume from stereo fundus photographs was validated in a sample of subjects with papilledema by correlating it with the same measurement derived from SD-OCT images and with expert Frisén grading, which would be of significant value for more cost-effective diagnosis and management of papilledema as well as other optic neuropathies.
Introduction
Raised intracranial pressure (ICP) is a potentially life threatening condition, which can also lead to visual loss and blindness.1 One of the only objective signs accessible during physical examination to confirm a suspicion of raised ICP is papilledema, or swelling of the optic nerve head (ONH). A relatively common cause of papilledema is idiopathic intracranial hypertension, which affects approximately 1 in 100,000 people in the United States and is more prevalent in women with a high body-mass index.2 Visual field tests may be normal in papilledema, and while medical tests such as lumbar puncture and neuroimaging can help in establishing elevated intracranial pressure, they can also give false-positive and false-negative results. Thus, reliable techniques to assess and quantify papilledema are of high interest and may help the diagnosis and management of disorders causing raised ICP,3 especially when expertise in ophthalmologic evaluation of the ONH may not be readily available.
Different imaging modalities have been used to evaluate the topography of ONH in patients with papilledema.4,5 From monocular color fundus photographs, papilledema can be quantified subjectively using the Frisén scale6 by taking into account visual features of the optic disc margin and the appearance of discontinuity of blood vessels as they course over the ONH.7 Stereo disc photography may increase the accuracy of the assessment of papilledema. However, its interpretation is subjective and qualitative and thus depends on the observer's expertise and bias. Early or subtle changes related to papilledema cannot be assessed precisely and objectively owing to the ordinal character of the Frisén scale, which remains one of its limitations, although it is in widespread clinical use.
Other attempts to quantify papilledema have included the use of confocal scanning laser (CSL) tomography,4 Heidelberg retina tomography (HRT),8 optical coherence tomography (OCT),7 and more recently, features extracted from monocular digital optic nerve photographs, which estimate the Frisén grade of papilledema.9 OCT10 is a noninvasive, high-resolution volumetric imaging technique that produces retinal cross-sectional images in three dimensions with an axial resolution of approximately 2 to 10 μm.3 Quantitative measurements of ONH morphology can be obtained by segmentation of OCT scans11 in patients with glaucoma,12 where performance of automated disc cupping measurement is comparable with that done by glaucoma specialists,13–16 and more recently, a similar comparison was made for papilledema (Wang JK, et al. IOVS 2011;323:ARVO E-Abstract 2986). Although the peripapillary retinal nerve fiber layer thickness and total retinal thickness increase in proportion to the severity of papilledema, the algorithm for determining their thickness may fail with more severe optic nerve edema.7
Considerable progress has been made during the past decades to develop new techniques for imaging retinal structures.13 Our group has recently published automated methods to quantify the ONH shape in patients with glaucoma using stereo fundus photographs, which show a high correspondence to OCT-based ONH shape metrics.17–19 In addition, we have developed automated methods that quantify color information from optic disc color photographs showing a high correspondence to glaucoma specialists' estimates of cup-to-disc ratio.20
The purpose of this pilot study was to evaluate the performance of our automated method of ONH shape reconstruction from stereo color fundus images in patients with papilledema, compared with expert grading of papilledema and to a reference standard from automated segmentation of the ONH from spectral domain (SD)-OCT images of the same patient. Both of the automated measurements objectively quantify ONH volume.
Methods
Data Collection
In this pilot study, patients with elevated ICP and papilledema at initial presentation to the neuro-ophthalmology clinic at the University of Iowa Hospitals and Clinics were included if they underwent stereo digital fundus photography and OCT scans at the same visit. Some patients were also included who were re-imaged at more than one visit in order to assess change in their papilledema. Stereo images were not obtained according to a strict protocol using a fixed base; instead, the stereo base was not fixed but could vary, depending on the ophthalmic photographer who took the images. Ideally, a more prospective study would require photography with a standard, fixed base. Since fixed-base stereo fundus photography is not widely available in most ophthalmic photography units, the experiments presented in this study are likely to reflect the level of accuracy that one might expect in a clinical practice setting where stereo fundus photography is performed.
The study protocol was approved by the University of Iowa's Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Because of its retrospective nature, informed consent was not required from subjects.
Stereo digital photographs of the fundus were acquired with a Topcon RC 50-DX retinal camera with a Megavision 6 megapixel back (2392 × 2048 actual image size), which is a full-size (35 mm) charge-coupled device (CCD) sensor (OIS, Sacramento, CA). Three independent neuro-ophthalmologists assigned a six-stage Frisén grade to each stereo pair, and the Frisén reference standard grade for each case was obtained by using a winner-take-all majority rule. SD-OCT scans of the ONH were obtained using the Cirrus SD-OCT scanner (Zeiss-Meditec, Dublin, CA).
Twenty-nine pairs of stereo fundus photographs and SD-OCT scans obtained on the same visit date were included from 15 patients, based on the availability of both OCT and stereo color photographs of the optic disc on the same visit date, as well as our successful layer segmentation of 3-D OCT volumes. The dataset included cases with five levels of Frisén grade ranging from 0 to 4.
ONH Shape Estimate from Stereo Color Fundus Photographs
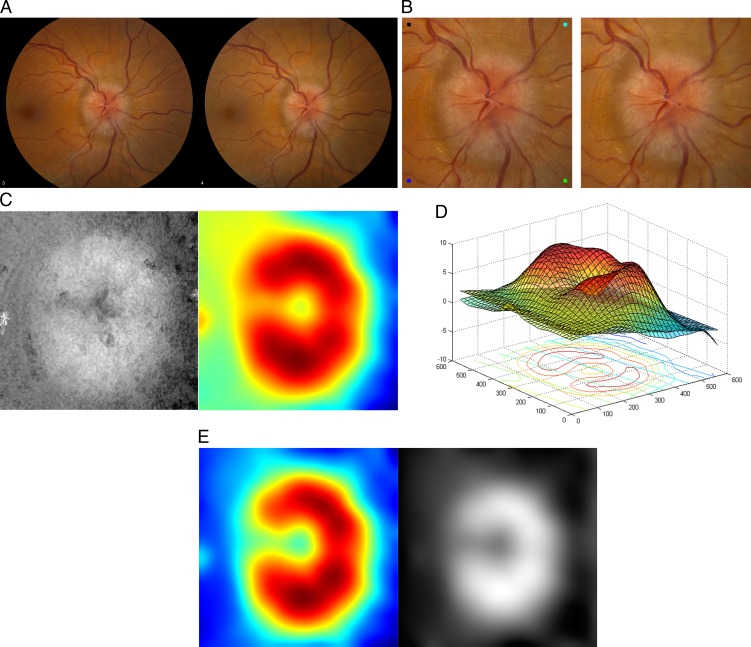
In a preprocessing step, the original stereo fundus photographs (Fig. 1A) were cropped to 1001 × 1001 pixels centered at optic disc and then rescaled to 668 × 668 pixels (Fig. 1B) for computational efficiency in the process of ONH shape reconstruction.
Figure 1. .
One example of an ONH shape estimate from a pair of stereo fundus photographs. (A) Original stereo fundus photographs. (B) Cropped and resized stereo pair centered at optic disc, which can be fused by holding the page at an approximately 30-cm (12-inch) distance from the eye and gazing into infinity. (C) Left panel: disparity map represented as a gray-scale image, where the intensity of each pixel indicates the magnitude of retinal surface elevation at a specific location; Right panel: TPS-smoothed map displayed using color coding. (D) Reference plane placed by fitting an orthogonal regression to the optic disc margin. (E) Left panel: flattened disparity map, by horizontally aligning the reference plane, in which the color coding of each pixel represents the magnitude of retinal surface elevation at a specific location; Right panel: the same map represented as a gray-scale image.
To quantify the shape of the ONH, the retinal surface topography was derived using a multi-scale stereo matching algorithm by automatically finding dense correspondences between stereo pairs.17 This approach has demonstrated robust performance in the presence of spatially-varying reflectance, limited illumination, noise, and low contrast or low feature density, which is highly desirable in order to provide a robust estimate of ONH shape from stereo fundus photographs.17
The stereo derived depth map is the algorithm's estimation—in pixels, with sub-pixel accuracy—of the local shift caused by depth differences. The values of the depth map can be converted to brightness and displayed as a depth image (Fig. 1C, left panel).
The stereo based depth maps were interpolated to 36 × 36 pixels (Fig. 1C, right panel) using a thin plate spline (TPS) to smooth out noise, so that major shape variations from the anatomical structure of the ONH were retained. The shape of the ONH in papilledema usually appears as an elevation in its topography.4
The reference plane for the surface derived from the stereo image pair was determined by fitting an orthogonal regression to the retina peripheral to the optic disc margin using principal components analysis (PCA). The linear regression minimizes the perpendicular distances from the retinal surface defined within the optic disc margin to the fitted reference plane (Fig. 1D). The optic disc was masked out by a concentric circle. Only normal retina peripheral to the ONH was included within the mask to get a reference surface as flat as possible. Regions close to boundaries of the cropped fundus photographs were excluded to prevent potential artifacts.
The retinal surface was flattened by aligning the reference plane horizontally to correct for any “tilts” caused by misalignment of the camera optical axis with respect to the eye's optical axis (Fig. 1E).
ONH Shape Estimate from OCT Scans
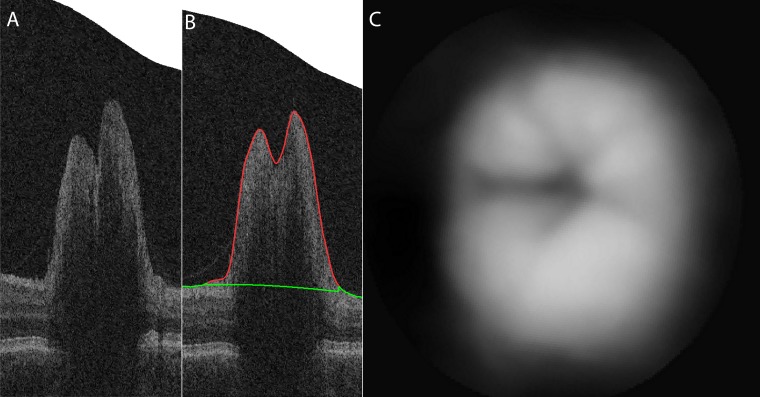
To determine the elevation of the ONH as a volumetric measure from OCT volumes, retinal surfaces are segmented in the raw OCT volume using our standard automated 3-D segmentation.11 An example OCT scan of an ONH, from the same patient and image on the same day as the ONH in Figure 1, and its segmentation is shown in Figure 2. The border of the inner retina and ONH facing the vitreous is segmented, shown in red (Fig. 2B). The inner border of the reference plane of the retina surrounding the optic disc, located similarly as in the case of stereo estimate, is shown in green. Peripherally sampled points on the segmented top surface are used to create an interpolated surface to be used as a reference surface: the outer surface of the retinal pigment epithelium (RPE) complex (Wang JK, et al. IOVS 2011;323:ARVO E-Abstract 2986).11
Figure 2. .
The same example of ONH shape estimate from the corresponding OCT scans. (A) Original ONH-centered OCT volume. (B) Segmented retinal surface (red line) and the reference plane (green line) overlaid on original OCT volume. (C) Estimated anatomic structure of ONH from OCT scans represented as a gray-scale image.
The entire volume is then flattened using the reference surface. The elevation is determined relative to the reference surface for each location, resulting in an OCT based depth map. The depth map can be represented as pixel intensities (Fig. 2C). This again results in a volumetric OCT-based shape estimate expressing the elevation of the ONH.
Volumetric Measure of Retinal Surface Elevation
Topographic maps were obtained using both the stereo based depth map and the OCT based depth map, which “wrapped” the retinal digital color image over the derived disc volume to provide an appearing optic nerve head topographic representation for illustration purposes.
To compare the OCT-derived and stereo-derived depth maps quantitatively, both of them were converted into a volumetric measure. The voxel size of the OCT scans used in this study is 30.15075 × 30.15075 × 1.955034 μm3, which can be readily used to convert the OCT derived depth map to cubic millimeters (mm3). An OCT projection image was produced by aggregating 3-D OCT volume along the depth dimension, which was then registered with the corresponding fundus image. With the scale factors obtained during registration, the areas of all pixels across fundus images were converted to squared millimeters (mm2) with a constant scaling. By integrating the intensities or magnitudes of pixel shift or disparity (dp) in the converted depth map, a measure (dp × mm2) was thus obtained from the stereo-derived depth map.
The volume of elevation of the ONH was quantified as a volumetric measure, expressed in (dp × mm2) within the optic disc region that is located above the reference plane (i.e., the horizontal plane after flattening). This is similar to the 3-D measurement of volume above reference (VAR) evaluated by HRT.21 A comparison of Figures 1C (right panel) and 1E (left panel) showed that after flattening, the asymmetry in the topography of the optic disc is apparent in this example and hence the possibility of quantified evaluation of the optic disc swelling in papilledema.
For comparison purposes, the volumetric measurement of retinal surface elevation estimated from the 29 pairs of stereo fundus photographs was normalized to have zero mean and unit variance. By translation and scaling, it using the mean and variance of the OCT estimates, both measurements were brought to the same range.
Statistical Analysis
The volumetric measurement of retinal surface elevation estimated from stereo fundus photographs is assessed by analyzing its correlation with that estimated from OCT scans. The correlation is shown in a scatter plot and Pearson's linear correlation coefficient r and the confidence interval (CI) were calculated. The agreement between the two measurements is also analyzed in a Bland-Altman plot by assigning the mean of the two measurements as the abscissa value, and the difference as the ordinate value.
The stereo derived volumetric measurements are compared to the expert estimate of Frisén grade of papilledema (using the winner-take-all reference standard obtained from 3 experts as above). Due to the ordinal character of the Frisén grade, Spearman's rank correlation coefficient ρ and the P values were calculated.
Results
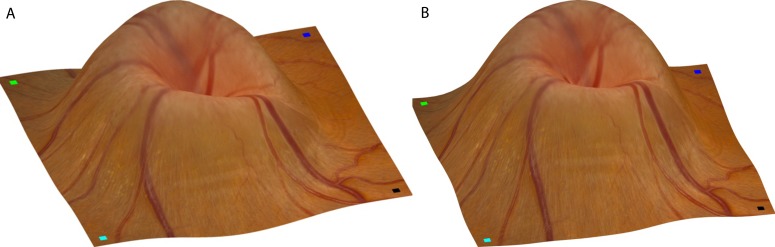
Figure 3 illustrates the topographic maps obtained from stereo fundus photographs and OCT scans. As one example of the typical cases of optic disc edema, it displayed a prominent elevation of the optic disc accompanied by an elevation in overall retinal surface topography. A circumferential halo is visible with major retinal arteries and veins along their length as they coursed over the optic disc and its border. Small segments of vessels were partly obscured by overlying swollen tissue due to decreased translucency (i.e., increased opacification of the RNFL). The major anatomical structures derived from both the stereo and OCT measurements were quite similar, allowing the two volumes derived to be directly compared.
Figure 3. .
Comparison of ONH shape estimates from stereo fundus photographs and OCT scans using topographic maps. (A) Reference (left) fundus image wrapping onto reconstructed topography as output from stereo photographs. Small squares with different colors are marked at four corners of the reference image (left panel of Fig. 1B), indicating the orientation of retinal surface rendering. (B) Fundus image wrapping onto reconstructed topography as output from the OCT scans from the same view angle.
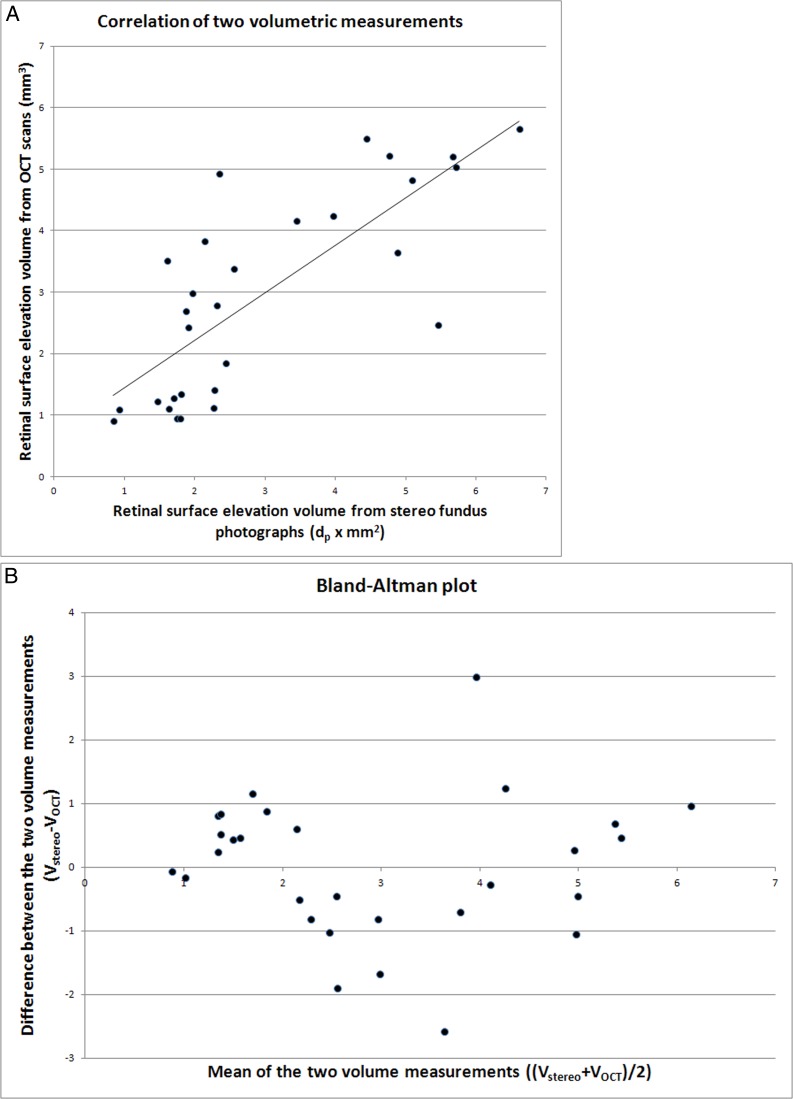
Both measurements was demonstrated in the scatter plot in Figure 4A, where the horizontal axis represented the normalized stereo volume in (dp × mm2) and the vertical axis the OCT volume in mm3. The correlation coefficient was r2 = 0.60 (95% CI, 0.57–0.89). The Bland-Altman plot in Figure 4B shows that the amount of elevation did not influence the association between the two methods.
Figure 4. .
Quantitative association of 29 volumetric measurements of retinal surface elevation derived from stereo fundus photographs and OCT scans. (A) Scatterplot of normalized stereo volume in (dp × mm2, x-axis) versus OCT volume in cubic millimeters (y-axis) with a correlation coefficient r2 = 0.60; P < 0.001 (see Methods section). A regression line is fitted to the data points. (B) Bland–Altman plot showing the difference between the disc volume estimated from OCT (VOCT) and that estimated from stereo fundus photographs, Vstereo (Vstereo − VOCT, y-axis), as a function of the average volume of the two methods: (Vstereo + VOCT)/2, x-axis.
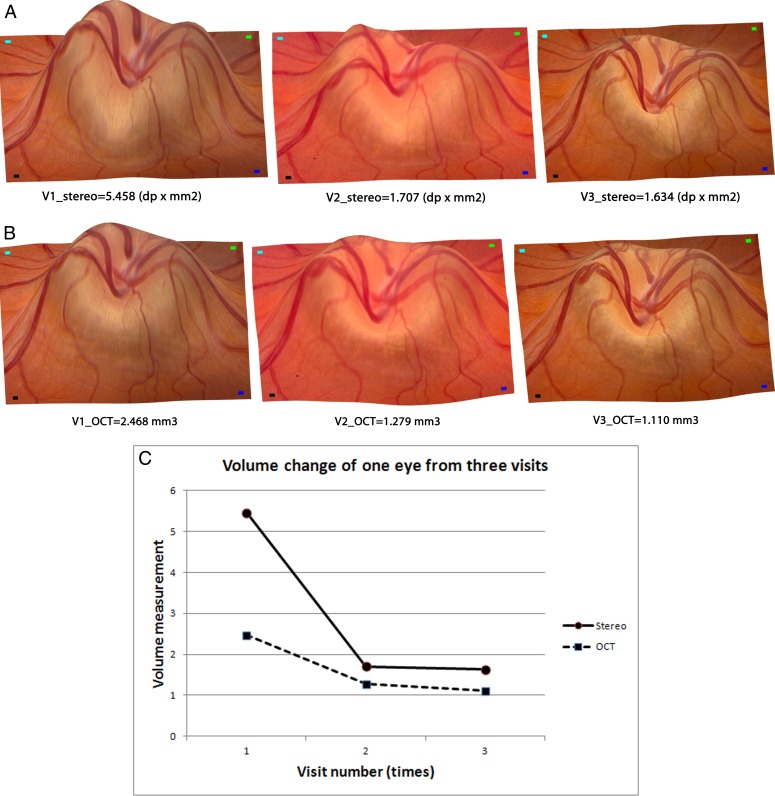
For 1 of 15 subjects, image data were available from three consecutive visits. We have illustrated the ONH volume changes for this subject, in the same eye, derived from both stereo fundus photographs and OCT scans in Figures 5A and 5B using topographic maps. Figure 5C illustrates the changes quantitatively in the derived disc volumes for both OCT and stereo on these three visits, showing similar reductions in optic disc swelling over time with medical treatment.
Figure 5. .
ONH shape volume comparisons between both measurements of the same patient at three visits. (A) Reference fundus image wrapping onto reconstructed topography as output from stereo photographs at the three visits from the same view angle. (B) Reconstructed topography of the same eye from OCT scans at the three visits from the same view angle. (C) Volume changes during these three visits (x-axis) from both measurements (y-axis) show similar tendency of a decrease in optic disc swelling over time with medical treatment.
A comparison of the expert estimate of Frisén grade of papilledema (winner-take-all estimate from three experts), with the stereo-derived volumetric measurements showed a significant correlation between the expert qualitative grade of papilledema and the stereo estimate of disc volume determined quantitatively (Spearman correlation coefficient r = 0.59; P < 0.001).
Discussion
Retinal surface elevation estimated from stereo color fundus photographs using our automated method of optic nerve head shape reconstruction correlated significantly with the reference standard volume derived from SD-OCT images and with expert Frisén grading in a sample of subjects with papilledema. This validates our hypothesis that our automated shape from stereo method has the potential of being a low cost alternative to analyze ONH elevation and shape objectively and quantitatively, and assess progression and efficacy of treatment directed toward lowering ICP and improving papilledema.
Because ophthalmoscopic evaluation and subjective grading of papilledema can show significant variability among observers and requires specialized clinical expertise, the estimate of ONH shape and quantification of optic disc swelling from stereo fundus photographs by three-dimensional (3-D) image analysis methodology may improve reproducibility and reliability of the assessment of papilledema and thus improve clinical decision-making regarding its diagnosis and treatment.
Low OCT signal strength often occurs in advanced stages of papilledema and can significantly reduce data quality.7 Swelling of the ONH and the surrounding retinal tissue has been reported to impair OCT cross-sectional analysis.3 This might be attributed to changes in the structural properties of the retinal tissue, which could affect the OCT scan image by changing the tissue reflectivity patterns and causing a more homogeneous, undifferentiated scan image.3 A few cases have also been observed in our study where the 3-D layer segmentation algorithm failed in OCT scans from eyes with severe papilledema because the examiner did not include the entire depth of the disc within the confines of the z-measurement window. Those few cases were excluded from correlation calculation in our experiments, which indicates potential limitations of existing SD-OCT–based imaging of severe papilledema.
Moreover, OCT cannot image spectral reflectance and transparency properties of ONH tissue and, thus, is not yet a complete replacement for evaluation of papilledema using the Frisén scale, as some features of the ONH are only visible in color photographs.13 Stereo fundus imaging is more cost-effective than OCT imaging, is more widely available, and encompasses decades of historical data that are not available from OCT.
To our knowledge, this is the first study to assess the topography of the ONH from stereoscopic fundus photographs by validating its reliability using SD-OCT scans in patients with elevated ICP. In addition, stereo fundus photographs contain multiple features to assist in the differential diagnosis of ONH elevation, such as rim area and color, small hemorrhages of the retinal nerve fiber layer (RNFL), disc hyperemia, and vessel obscuration, which may present useful information in addition to disc volume for assessing the presence, severity, and cause of optic disc swelling.9,20 Measurements such as the volume normalized by the size of the optic disc may also be more accurate than the absolute volume itself in assessing the degree of papilledema. A collection of such 3-D shape and volumetric parameters may be more discriminative than 2-D parameters alone in differentiating various stages and causes of disc swelling. Our future efforts will be directed towards incorporating such features into the disc volume measurement to further improve upon the analysis of papilledema.
Even with ever wider availability of SD-OCT, stereo fundus photographs may become an important and reliable alternative for quantifying ONH swelling because of the substantially lower cost, especially in a screening and/or telemedicine setting, remote from ophthalmologic expertise of the fundus camera compared with the SD-OCT scanner.
There were several issues with this pilot study. Because of its retrospective nature, stereo images were not obtained according to a strict protocol using a fixed base; instead, the stereo base could vary, depending on the ophthalmic photographer who took the images. This may explain several cases of under- or overestimates when the base may have been less due to a small pupil opening or variation between photographers (see Fig. 4). Obviously, an even more quantitative prospective study will require photography with a standard, fixed base. Likely, depth maps derived from those will correlate even better with SD-OCT–derived ONH elevation.
The differential interactions of visible (in stereo photographs) and near infrared light (in SD-OCT) with swollen optic nerve tissue also may have affected the comparison between the two measures. In some cases, because the pupil was not well dilated, the separation of two stereo images was more limited in order to accommodate the small pupil size and still provide high-quality images. In addition, unintended shifts of the images can also be caused by eye movements or fixation instabilities. Those are potential limitations of the stereo-based method.
Given the close associations of volumetric estimates derived from stereo fundus photographs and OCT scans, our future work may include exploring potentials to calibrate the stereo fundus camera so that OCT volumes may be predicted from fundus photographs, which would be of significant value for more cost-effective diagnosis and management of papilledema as well as other optic neuropathies.
In summary, our results show that the retinal surface elevation estimated from stereo color fundus photographs correlated significantly with the reference standard volume derived from SD-OCT images and with expert Frisén grading of papilledema in a sample of subjects with papilledema.
Footnotes
Supported by the Department for Veterans Affairs, National Eye Institute Grants R01 EY017066 and R01 EY018853; Research to Prevent Blindness, New York, New York; the Carver Center for Macular Degeneration, and the Marlene S. and Leonard A. Hadley Glaucoma Research Fund.
Disclosure: L. Tang, P; R.H. Kardon, None; J.-K. Wang, None; M.K. Garvin, P; K. Lee, None; M.D. Abràmoff, P
References
- 1. Cameron AJ. Marked papilloedema in pulmonary emphysema. Br J Ophthalmol. 1933;17:167–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Binder DK, Horton JC, Lawton MT, McDermott MW. Idiopathic intracranial hypertension. Neurosurgery. 2004;54:538–551 [DOI] [PubMed] [Google Scholar]
- 3. Menke MN, Feke GT, Trempe CL. OCT measurements in patients with optic disc edema. Invest Ophthalmol Vis Sci. 2005;46:3807–3811 [DOI] [PubMed] [Google Scholar]
- 4. Trick GL, Vesti E, Tawansy K, Skarf B, Gartner J. Quantitative evaluation of papilledema in pseudotumor cerebri. Invest Ophthalmol Vis Sci. 1998;39:1964–1971 [PubMed] [Google Scholar]
- 5. Rebolleda G, Munoz-Negrete FJ. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:5197–5200 [DOI] [PubMed] [Google Scholar]
- 6. Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982;45:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scott CJ, Kardon RH, Lee AG, Frisen L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010;128:705–711 [DOI] [PubMed] [Google Scholar]
- 8. Catrambone JE, He W, Prestigiacomo CJ, Carmel PW. Monitoring papilledema with Heidelberg Retina Tomograph in a patient with ruptured aneurysm: a case report. Surg Neurol. 2008;70:79–81 [DOI] [PubMed] [Google Scholar]
- 9. Echegaray S, Zamora G, Yu H, Luo W, Soliz P, Kardon R. Automated analysis of optic nerve images for detection and staging of papilledema. Invest Ophthalmol Vis Sci. 2011;52:7470–7478 [DOI] [PubMed] [Google Scholar]
- 10. Schuman JS, Hee MR, Arya AV, et al. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995;6:89–95 [DOI] [PubMed] [Google Scholar]
- 11. Garvin MK, Abramoff MD, Kardon R, Russell SR, Wu X, Sonka M. Intraretinal layer segmentation of macular optical coherence tomography images using optimal 3-D graph search. IEEE Trans Med Imaging. 2008;27:1495–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Budenz DL, Fredette MJ, Feuer WJ, Anderson DR. Reproducibility of peripapillary retinal nerve fiber thickness measurements with stratus OCT in glaucomatous eyes. Ophthalmology. 2008;115:661–666 [DOI] [PubMed] [Google Scholar]
- 13. Abramoff MD, Garvin MK, Sonka M. Retinal imaging and image analysis. IEEE Rev Biomed Eng. 2010;3:169–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee K, Niemeijer M, Garvin MK, Kwon YH, Sonka M, Abramoff MD. Segmentation of the optic disc in 3-D OCT scans of the optic nerve head. IEEE Trans Med Imaging. 2010;29:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu Z, Abramoff MD, Kwon YH, Lee K, Garvin M. Automated Segmentation of Neural Canal Opening and Optic Cup in 3-D Spectral Optical Coherence Tomography Volumes of the Optic Nerve Head. Invest Ophthalmol Vis Sci. 2010;51:5708–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu J, Ishikawa H, Wollstein G, et al. Automated volumetric evaluation of stereoscopic disc photography. Opt Express. 2010;18:11347–11359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang L, Garvin MK, Lee K, Alward WL, Kwon YH, Abramoff MD. Robust multi-scale stereo matching from fundus images with radiometric differences. IEEE Trans Pattern Anal Mach Intell. 2011;33:2245–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang L, Scheetz TE, Mackey DA, et al. Automated quantification of inherited phenotypes from color images: a twin study of the variability of optic nerve head shape. Invest Ophthalmol Vis Sci. 2010;51:5870–5877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang L, Kwon YH, Alward WLM, et al. 3D reconstruction of the optic nerve head using stereo fundus images for computer-aided diagnosis of glaucoma. SPIE Medical Imaging. 2010;7624:76243D–76243D-8 [Google Scholar]
- 20. Abramoff MD, Alward WL, Greenlee EC, et al. Automated segmentation of the optic disc from stereo color photographs using physiologically plausible features. Invest Ophthalmol Vis Sci. 2007;48:1665–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salgarello T, Falsini B, Tedesco S, Galan ME, Colotto A, Scullica L. Correlation of optic nerve head tomography with visual field sensitivity in papilledema. Invest Ophthalmol Vis Sci. 2001;42:1487–1494 [PubMed] [Google Scholar]