Abstract
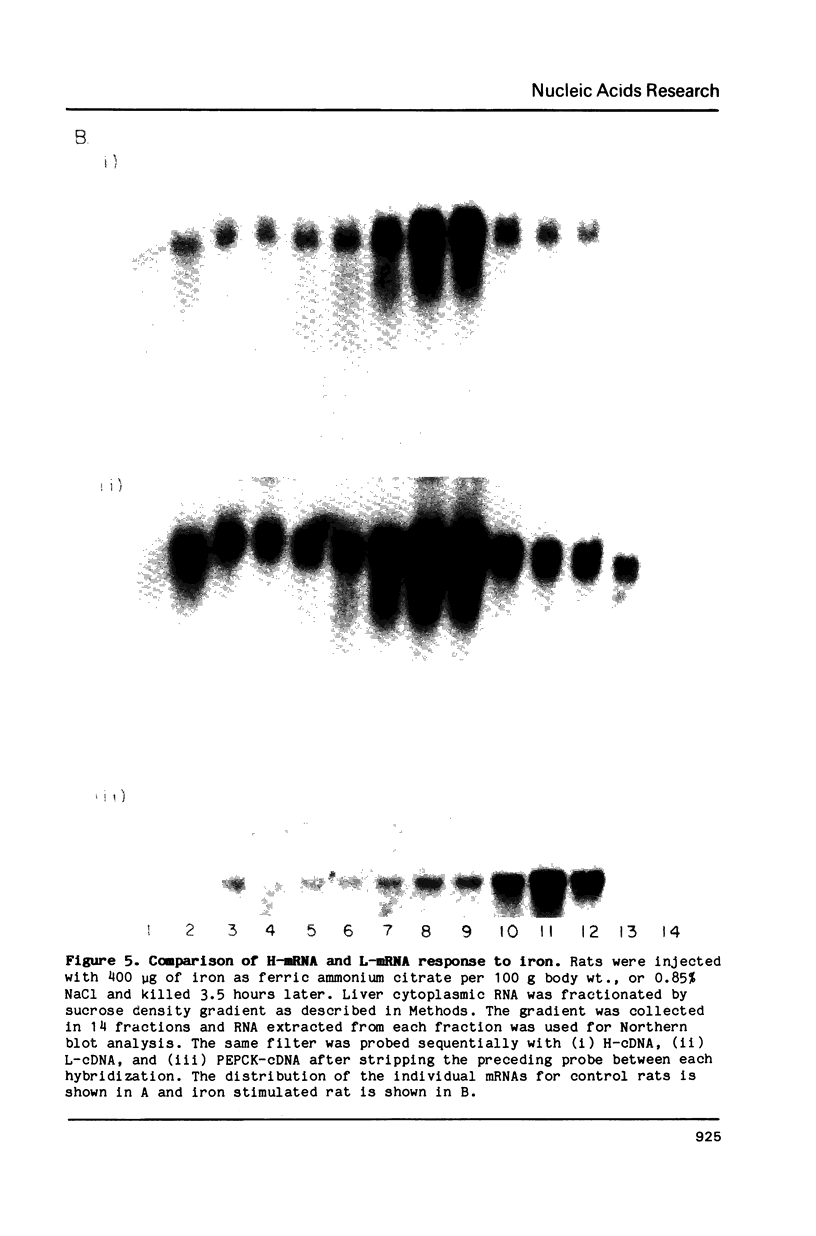
A few hours after administering iron to rats, liver ferritin synthesis increases several fold. However, Northern blot analysis with cDNA probes for ferritin light (L) and heavy (H) subunit mRNAs failed to show an increase in total population of either messenger. Cytoplasmic distribution of ferritin messages was therefore investigated in control and iron administered rats killed at 3.5 hours. The liver post-mitochondrial supernatant was fractionated on a sucrose gradient to separate polyribosomes, monosomes, ribosomal subunits and cell sap. RNA extracted from each fraction and analyzed using Northern blotting showed that 65% of the total mRNA population for each subunit was present in the cell sap of control rats, presumably as mRNP particles since ribosomal RNA was absent from this fraction. After iron administration, these reserves of free mRNA were recruited onto the polysomes, reducing the free mRNA pool to 15% of the total. We interpret this to be due to activation of blocked ferritin messages on entry of iron into the cell.
Full text
PDF






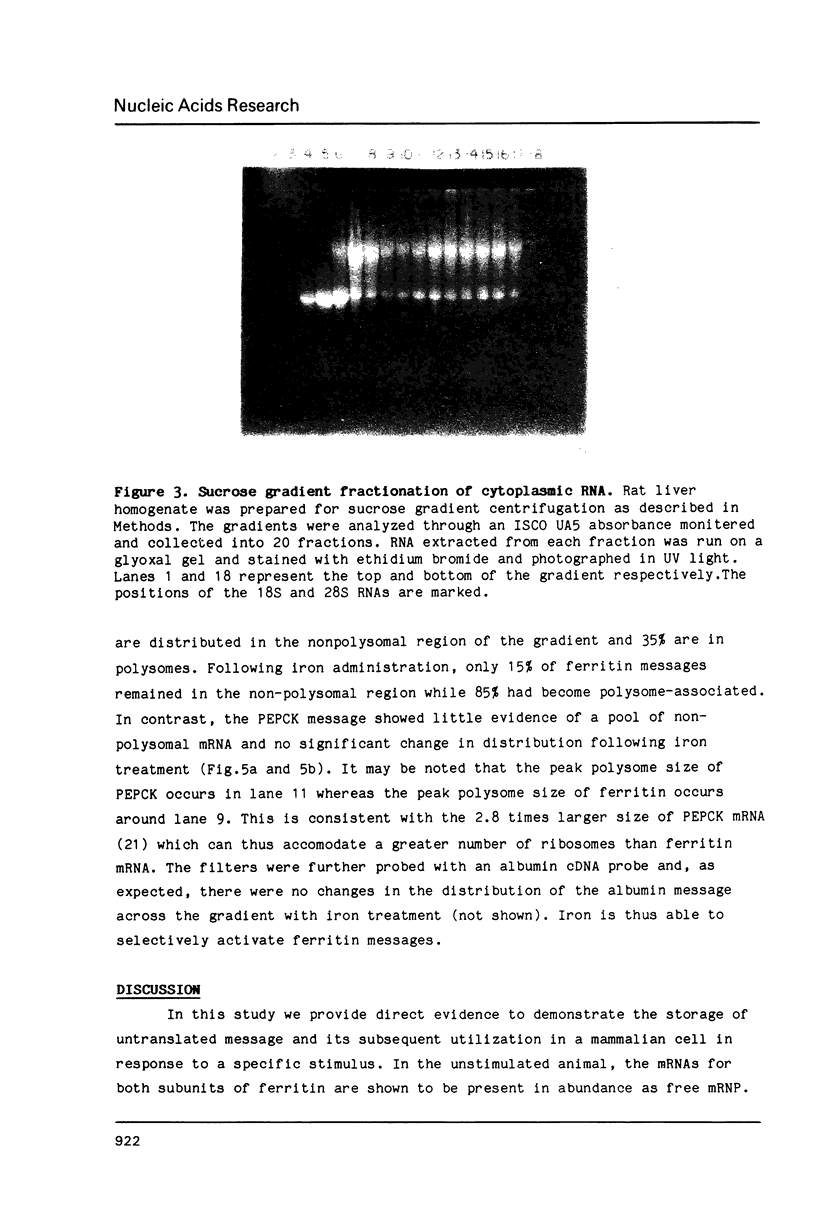
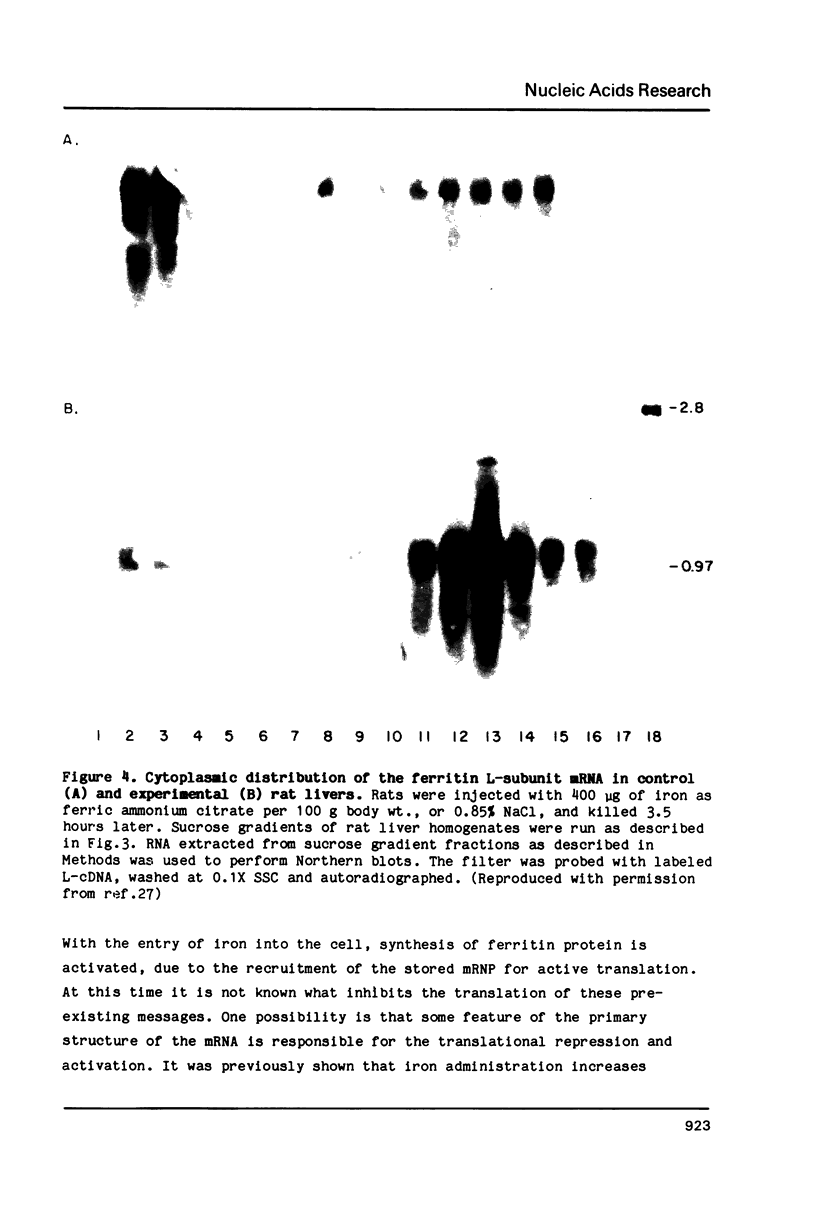
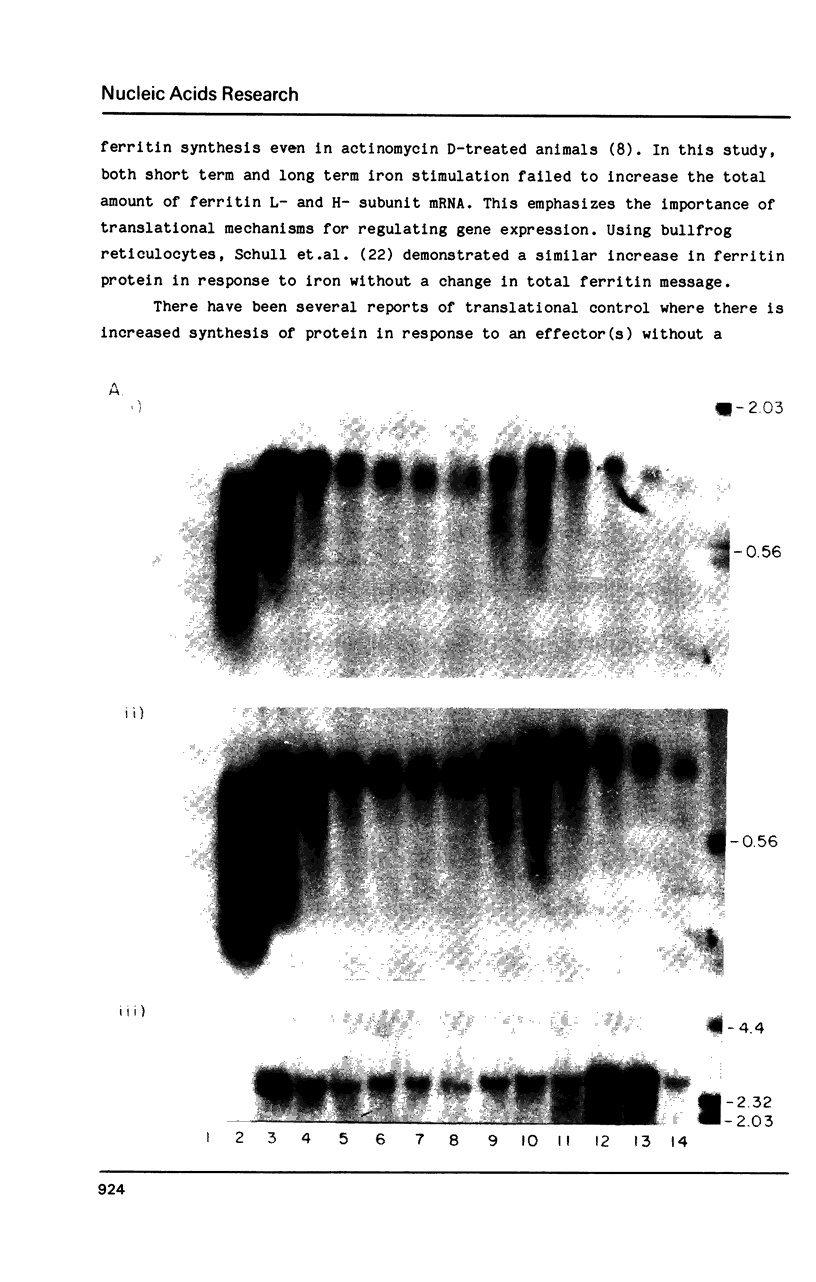


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. J., Leibold E. A., Munro H. N. Isolation of cDNA clones for the light subunit of rat liver ferritin: evidence that the light subunit is encoded by a multigene family. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1265–1269. doi: 10.1073/pnas.80.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cimbala M. A., Lamers W. H., Nelson K., Monahan J. E., Yoo-Warren H., Hanson R. W. Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney. Effects of insulin and cyclic AMP. J Biol Chem. 1982 Jul 10;257(13):7629–7636. [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Costanzo F., Santoro C., Colantuoni V., Bensi G., Raugei G., Romano V., Cortese R. Cloning and sequencing of a full length cDNA coding for a human apoferritin H chain: evidence for a multigene family. EMBO J. 1984 Jan;3(1):23–27. doi: 10.1002/j.1460-2075.1984.tb01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRYSDALE J. W., MUNRO H. N. FAILURE OF ACTINOMYCIN D TO PREVENT INDUCTION OF LIVER APOFERRITIN AFTER IRON ADMINISTRATION. Biochim Biophys Acta. 1965 May 11;103:185–188. doi: 10.1016/0005-2787(65)90554-x. [DOI] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Dörner M. H., Salfeld J., Will H., Leibold E. A., Vass J. K., Munro H. N. Structure of human ferritin light subunit messenger RNA: comparison with heavy subunit message and functional implications. Proc Natl Acad Sci U S A. 1985 May;82(10):3139–3143. doi: 10.1073/pnas.82.10.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fruscoloni P., Al-Atia G. R., Jacobs-Lorena M. Translational regulation of a specific gene during oogenesis and embryogenesis of Drosophila. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3359–3363. doi: 10.1073/pnas.80.11.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Okamoto H. Translational control of proinsulin synthesis by glucose. Nature. 1980 Jan 3;283(5742):100–102. doi: 10.1038/283100a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire D. M., Olson C. D., Towle H. C., Dempsey M. E. Translational control of the circadian rhythm of liver sterol carrier protein. J Biol Chem. 1984 May 10;259(9):5368–5371. [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reue K. L., Quon D. H., O'Donnell K. A., Dizikes G. J., Fareed G. C., Lusis A. J. Cloning and regulation of messenger RNA for mouse apolipoprotein E. J Biol Chem. 1984 Feb 25;259(4):2100–2107. [PubMed] [Google Scholar]
- Rosenthal E. T., Tansey T. R., Ruderman J. V. Sequence-specific adenylations and deadenylations accompany changes in the translation of maternal messenger RNA after fertilization of Spisula oocytes. J Mol Biol. 1983 May 25;166(3):309–327. doi: 10.1016/s0022-2836(83)80087-4. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Theil E. C. Translational control of ferritin synthesis by iron in embryonic reticulocytes of the bullfrog. J Biol Chem. 1982 Dec 10;257(23):14187–14191. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Fitschen W. The mobilization of maternal histone messenger RNA after fertilization of the sea urchin egg. Cell Differ. 1978 Apr;7(1-2):103–114. doi: 10.1016/0045-6039(78)90011-8. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]