Abstract
Multiple studies have shown that infection with the endosymbiotic bacterium Wolbachia pipientis confers Drosophila melanogaster and other insects with resistance to infection by RNA viruses. Studies investigating whether Wolbachia infection induces the immune system or confers protection against secondary bacterial infection have not shown any effect. These studies, however, have emphasized resistance against extracellular pathogens. Since Wolbachia lives inside the host cell, we hypothesized that Wolbachia might confer resistance to pathogens that establish infection by invading host cells. We therefore tested whether Wolbachia-infected D. melanogaster are protected against infection by the intracellular pathogenic bacteria Listeria monocytogenes and Salmonella typhimurium, as well as the extracellular pathogenic bacterium Providencia rettgeri. We evaluated the ability of flies infected with Wolbachia to suppress secondary infection by pathogenic bacteria relative to genetically matched controls that had been cured of Wolbachia by treatment with tetracycline. We found no evidence that Wolbachia alters host ability to suppress proliferation of any of the three pathogenic bacteria. Our results indicate that Wolbachia-induced antiviral protection does not result from a generalized response to intracellular pathogens.
Introduction
Wolbachia is a genus of maternally inherited, obligate intracellular bacteria that infect a wide range of arthropods and filarial nematodes. It has been estimated that as many as 70% of all insect species may be infected [1]. Extensive horizontal transfer is credited with introducing Wolbachia to such a large number of host species. Once introduced, the successful spread of Wolbachia throughout host populations can be explained in large part by the ability to act as reproductive parasites, manipulating or disrupting the host reproductive biology in such a way to promote their own transmission. In many species, Wolbachia induces cytoplasmic incompatibility (CI), which causes high egg mortality in crosses between infected males and uninfected females, resulting in a relative fitness advantage for infected females and driving Wolbachia spread once Wolbachia infection has reached a critical threshold in the population [2]. Natural selection could help Wolbachia reach that threshold and facilitate further spread if the bacterium provides an additional selective advantage to infected hosts.
In one example of such an advantage, Drosophila melanogaster infected with Wolbachia pipientis show dramatic resistance to infection by RNA viruses [3], [4]. This antiviral protection appears robust in D. melanogaster, having been observed across multiple host genotypes and Wolbachia strains [3], [4]. Similar antiviral protection is observed when D. simulans is infected with certain Wolbachia strains, although other Wolbachia strains infecting D. simulans do not alter resistance [5]. These observations indicate that Wolbachia infection can influence host immunity, but the mechanism of pathogen resistance remains unknown. Previous work in Drosophila suggesting that Wolbachia infection does not confer protection against secondary bacterial infection has focused on extracellular bacterial pathogens [6]. Like viruses, however, some pathogenic bacteria establish infection by invading host cells where Wolbachia is resident. To date, there have been no published tests of whether Wolbachia can confer resistance to intracellular bacterial infection.
It has been hypothesized that Wolbachia alters the systemic immune response of the host, increasing the ability to quickly detect and mount a response to the infection. In Aedes aegypti, for example, Wolbachia-induced resistance to a range of pathogens including filarial nematodes, Gram-negative bacteria, and Dengue virus is associated with increased basal expression of immune genes [7], [8], [9]. Microarray analysis of Drosophila S2 cells showed slight upregulation of some genes involved in the Toll and IMD pathways in the presence of Wolbachia infection [10], although other studies of selected immune genes in whole flies have found that Wolbachia does not alter expression in D. melanogaster [6] or D. simulans [11]. If Wolbachia is able to alter the systemic immune response of D. melanogaster, we would expect to see increased resistance against bacterial pathogens in addition to viruses. Wolbachia infection does not confer D. melanogaster or D. simulans with resistance against the pathogenic bacteria Pseudomonas aeruginosa, Serratia marcescens, or Erwinia carotovora [6] which are all extracellular pathogens. We hypothesized that Wolbachia infection might increase resistance specifically to intracellular pathogens. Intracellular pathogen surveillance could be heightened as a consequence of Wolbachia infection, allowing for rapid detection and elimination of pathogens invading the cytoplasm. Alternatively, since Wolbachia resides within host cells, it could limit the success of an intracellular pathogen through competition for resources within the host cytoplasm. In either of these cases, increased resistance would only be observed when Wolbachia-infected individuals are challenged with an intracellular pathogen.
We investigated whether Wolbachia infection alters D. melanogaster defense against secondary bacterial infection, and in particular against pathogenic intracellular bacteria. We specifically focused in this paper on resistance, defined as the ability to minimize pathogen burden [12]. We compared the ability to suppress secondary pathogen infection of flies from five isofemale lines of D. melanogaster that are naturally infected with Wolbachia to the ability of those same lines to suppress pathogenic infection after removal of the Wolbachia with tetracycline. To control for the effect of the tetracycline, we also evaluated tetracycline treatment in five naturally Wolbachia-uninfected isofemale lines. In order to determine whether Wolbachia infection influences generalized resistance to multiple pathogens or a more specific response to intracellular pathogens, we tested infection with Salmonella typhimurium and Listeria monocytogenes, which are intracellular bacterial pathogens, and Providencia rettgeri, an extracellular pathogen. We found no evidence that Wolbachia alters resistance to any of the three bacterial pathogens tested.
Methods
Flies and Antibiotic Treatment
The D. melanogaster isofemale lines used in this experiment were established from field-inseminated females collected in Newfield, New York, USA, in 2005. Each individual female was placed in media-containing vials immediately after collection, and her resulting progeny were allowed to sib-mate. These isofemale lines have been maintained since then by recurrent mass sib-mating. Genetic variation observed among the isofemale lines therefore reflects variation in the natural population from which they were sampled. A diagnostic PCR which amplified wsp was used to determine Wolbachia infection status of the lines [13]. Antiviral protection has been observed in D. melanogaster infected with the Wolbachia strains wMel, wMelCS and wMelPop [3], [4]. There is evidence that wMel is the predominant variant infecting field populations [14], so it is likely to be the strain present in our recently founded lines, although we did not explicitly test this. We randomly chose 5 infected [WOLB(+)] and 5 uninfected [WOLB(–)] lines to use for the experiment. D. melanogaster can be experimentally cured of Wolbachia by treatment with the antibiotic tetracycline [15]. Flies from both naturally infected and naturally uninfected lines were treated with tetracycline as described below, resulting in four treatment groups to be contrasted for resistance to pathogenic bacterial infection: WOLB(+)TET(–), WOLB(+)TET(+), WOLB(–)TET(–), and WOLB(–)TET(+).
Flies were reared on the standard Cornell Drosophila medium (8.3% w/v glucose, 8.3% w/v brewer’s yeast, 1% w/v agar) throughout the experiment. For the antibiotic treatment, the flies were reared for three generations on the standard Cornell medium with 50 ug/ml tetracycline added [4]. After each generation on tetracycline supplemented medium, eight flies from each line were screened for the presence of Wolbachia using the PCR assay described above [13]. Approximately 50% of the flies screened after one generation of tetracycline treatment were cured of Wolbachia and approximately 90% were cured after two generations of treatment. After three generations of tetracycline treatment, Wolbachia was not detected in any of the flies tested and the isofemale lines were then returned to the standard medium without antibiotic for all subsequent generations. Flies in all treatments were maintained at 25°C with 12 h light, 12 h dark. Flies were infected 1–5 hours after “dawn”. All males used for infections were aged 3–5 days.
Bacterial Strains
Providencia rettgeri strain Dmel is a Gram-negative extracellular pathogen isolated from wild caught D. melanogaster that causes moderate mortality in the fly [16]. Salmonella enterica serotype Typhimurium S5520 (obtained from Dr. Martin Wiedmann, Cornell University) is a Gram-negative bacterium which is able to establish an intracellular infection causing mortality in D. melanogaster, although the bacteria do not replicate to high numbers [17]. Listeria monocytogenes 10403S (obtained from Dr. Martin Wiedmann, Cornell University) is a Gram-positive intracellular bacterium which is able to invade and replicate to high numbers within the cells of D. melanogaster, causing moderate mortality [18].
Infections
Since residual effects of tetracycline may persist multiple generations after treatment [19], we measured systemic bacterial load 2, 4, and 6 generations after ending tetracycline treatment. Three sets of infections were done in a day (one for each pathogen) and were repeated on three replicate days for each generation tested. For the infections, 15 males from each line and treatment were anesthetized on CO2 and pricked in the thorax with a 0.1 mm pin dipped into a bacterial culture. P. rettgeri cultures were grown in LB at 37°C with shaking overnight and diluted to A600 = 1 immediately before infections. L. monocytogenes cultures were grown in BHI liquid overnight at 37°C with shaking. To prepare the inocula, 2 ml of liquid culture with A600 = 1 was spun down and the supernatant removed, and the pellet was resuspended in 200 µl of BHI. S. typhimurium cultures were grown in BHI liquid overnight at 37°C without shaking. To prepare the inocula, 2 ml of liquid culture with A600 = 1 was spun down and the supernatant removed, and the pellet was resuspended in 200 µl of BHI.
To measure systemic bacterial load, 3 pools of 5 flies from each line were homogenized and plated approximately 24 hours after infection. Flies infected with P. rettgeri were homogenized in 500 µl LB, and the homogenate was diluted 1∶100 prior to plating on LB plates. Flies infected with L. monocytogenes and S. typhimurium were homogenized in 250 µl BHI. The L. monocytogenes homogenate was diluted 1∶10 in BHI prior to plating on BHI plates, and the S. typhimurium homogenate was not diluted prior to plating on BHI plates. A spiral plater (Don Whitley Scientific) was used to plate 50 µl of each sample over a continuous exponential dilution. Plates were grown at 37°C overnight. The bacteria used for experimental infections grow into visible colonies during this period, while gut commensal bacteria do not appear as visible colonies on the plates until approximately 24 hours later. Thus, we can be certain that the colonies we count reflect systemic pathogen load. Every plate was visually inspected to verify that the color and morphology of all colonies were consistent with that of the experimental bacteria, and any plates with contaminating colonies were discarded. The resulting colonies were counted using the ProtoCOL plate counter associated with the spiral plater to determine the systemic pathogen load of the flies.
Statistical Analysis
To assess the effect of Wolbachia infection, tetracycline treatment, and time since tetracycline treatment on resistance to each pathogen, we performed a mixed-model analyses of variance (ANOVA) on the natural log transformed bacterial load data using the following model:
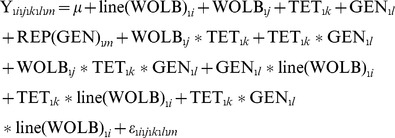 |
where Y is the natural log of the bacterial load, line(WOLB) (i = 1,5) represents the effect of Drosophila genetic line within each level of the model factor WOLB, WOLB (j = 1,2) represents the Wolbachia infection status of each line prior to antibiotic treatment, TET (k = 1,2) represents whether or not flies were treated with tetracycline, GEN (l = 1,3) represents whether the experiment was performed 2,4, or 6 generations after tetracycline treatment, and REP(GEN) (m = 1,3) is the random effect of the replicate day on which the data were collected within each generation. The factor WOLBj*TETk tests for differential effects of tetracycline treatment on Wolbachia-infected and Wolbachia-uninfected lines, which allows us to distinguish the effect of removing Wolbachia from the overall effect of tetracycline. The factor WOLBj*TETk*GENl tests whether effects of tetracycline on Wolbachia-infected and uninfected lines are consistent across the successive generations. The factor GENl*line(WOLB)i tests whether the lines within each WOLB level behave consistently across the generations. The factor TETk*line(WOLB)i tests whether the effect of tetracycline treatment varies among lines within each WOLB level. The factor TETk*GENl*line(WOLB)i tests whether tetracycline treatment has genotype-dependent effects that vary across generations.
To further elucidate the nature of the observed effect of the line(WOLB)i*TETk *GENl interaction on resistance to P. rettgeri (see Results), we performed an additional mixed ANOVA for each generation separately. This model takes the form:
where Y is the natural log of the bacterial load, line(WOLB) (i = 1,5) represents the effect of genotype nested within each level of the factor WOLB, WOLB (j = 1,2) represents the Wolbachia infection status of each line prior to antibiotic treatment, TET (j = 1,2) represents whether or not flies were treated with tetracycline, and REP (k = 1,3) is the random effect of the replicate day on which the experiment was performed. The factor WOLBj*TETk tests for differential effects of tetracycline treatment on Wolbachia-infected and Wolbachia-uninfected lines. The factor TETj*line(WOLB)i tests whether tetracycline treatment has genotype-dependent effects.
All of the model factors described in the text above are also listed in Table 1. Removal of various non-significant factors from the model does not change the qualitative outcome of any of our analyses, so we present here the full models in order to provide the most complete information. All analyses were performed using SAS 9.3 (SAS Institute).
Table 1. Description of Factors Tested in Analyses of Variance.
| Factor | Effect Type | Effect Measured |
| line(WOLB) | fixed | effect of each genetic line nested within the factor WOLB |
| WOLB | fixed | Wolbachia status of each line prior to tetracycline treatment |
| TET | fixed | whether or not flies were treated with tetracycline |
| GEN | fixed | number of generations since tetracycline treatment (2, 4, or 6) |
| REP(GEN) | random | replicate day on which the experiment was performed |
| WOLB*TET | fixed | differential effects of tetracycline on flies with and without Wolbachia |
| TET*GEN | fixed | differential effects of tetracycline across the generations tested |
| GEN*line(WOLB) | fixed | differential effects of line across the generations tested |
| TET*line(WOLB) | fixed | differential effects of tetracycline on flies of each line |
| WOLB*TET*GEN | fixed | different effects of tetracycline on flies with and without Wolbachia and across generations |
| TET*GEN*line(WOLB) | fixed | differential effects of tetracycline on flies of each line and across generations |
Results
When flies were infected with P. rettgeri, we observed significant differences in bacterial load across the isofemale lines (p<0.0001, Table 2), but no difference in bacterial load owing to the initial Wolbachia status of those lines (p = 0.0873). Systemic pathogen load of tetracycline-treated flies, considered across genotypes, did not differ from that of untreated flies (p = 0.2062). The effect of tetracycline treatment on Wolbachia-infected lines was not different from the effect of tetracycline treatment on Wolbachia-uninfected lines (WOLB*TET, p = 0.0860, Table 2 and Figure 1A), indicating that removal of Wolbachia does not influence ability to suppress P. rettgeri infection. Interestingly, we find a nearly significant TET*line(WOLB) interaction (p = 0.0610, Table 2), which suggests that the effects of tetracycline may be stronger in some genetic backgrounds than others. Additionally, the three-way TET*GEN* line(WOLB) interaction is significant (p = 0.0117, Table 2). This three way interaction indicates that the genotype-specific effect of tetracycline treatment varies across generations, but it does not provide any direct information about the nature of this complex interaction. We decided to investigate this three-way interaction further by running a separate analysis for each of the three generations tested. Interestingly, we find a significant TET*line(WOLB) interaction in response to P. rettgeri two generations after treatment (p = 0.0017, Table 3) whereas this interaction is not significant in the subsequent generations. Taken together, these results suggest that an effect of tetracycline may persist in some, but not other genetic backgrounds two generations after treatment, but that the effect does not persist for four or more generations in any of the genetic backgrounds.
Table 2. Analyses of variance for fixed effects relating genotype, Wolbachia status, tetracycline treatment, and generation to bacterial load.
| P. rettgeri | L. monocytogenes | S. typhimurium | |||||
| Factor | d.f. | F-ratio | P-value | F-ratio | P-value | F-ratio | P-value |
| line(WOLB) | 8 | 21.10 | <0.0001 | 14.01 | <0.0001 | 2.26 | 0.0222 |
| WOLB | 1 | 2.94 | 0.0873 | 1.13 | 0.2880 | 1.07 | 0.3020 |
| TET | 1 | 1.60 | 0.2062 | 1.03 | 0.3117 | 0.79 | 0.3740 |
| GEN | 2 | 3.93 | 0.0811 | 0.19 | 0.8324 | 0.02 | 0.9800 |
| WOLB*TET | 1 | 2.96 | 0.0860 | 0.12 | 0.7254 | 1.30 | 0.2548 |
| WOLB*TET*GEN | 2 | 0.01 | 0.9892 | 0.48 | 0.6206 | 0.09 | 0.9099 |
| TET*GEN | 2 | 2.11 | 0.1219 | 0.15 | 0.8623 | 0.56 | 0.5705 |
| GEN* line(WOLB) | 16 | 1.37 | 0.1543 | 0.71 | 0.7841 | 0.49 | 0.9514 |
| TET* line(WOLB) | 8 | 1.88 | 0.0609 | 1.42 | 0.1857 | 0.83 | 0.5767 |
| TET*GEN*line(WOLB) | 16 | 2.01 | 0.0117 | 1.30 | 0.1910 | 1.51 | 0.0910 |
Figure 1. Systemic bacterial load is not influenced by Wolbachia infection.
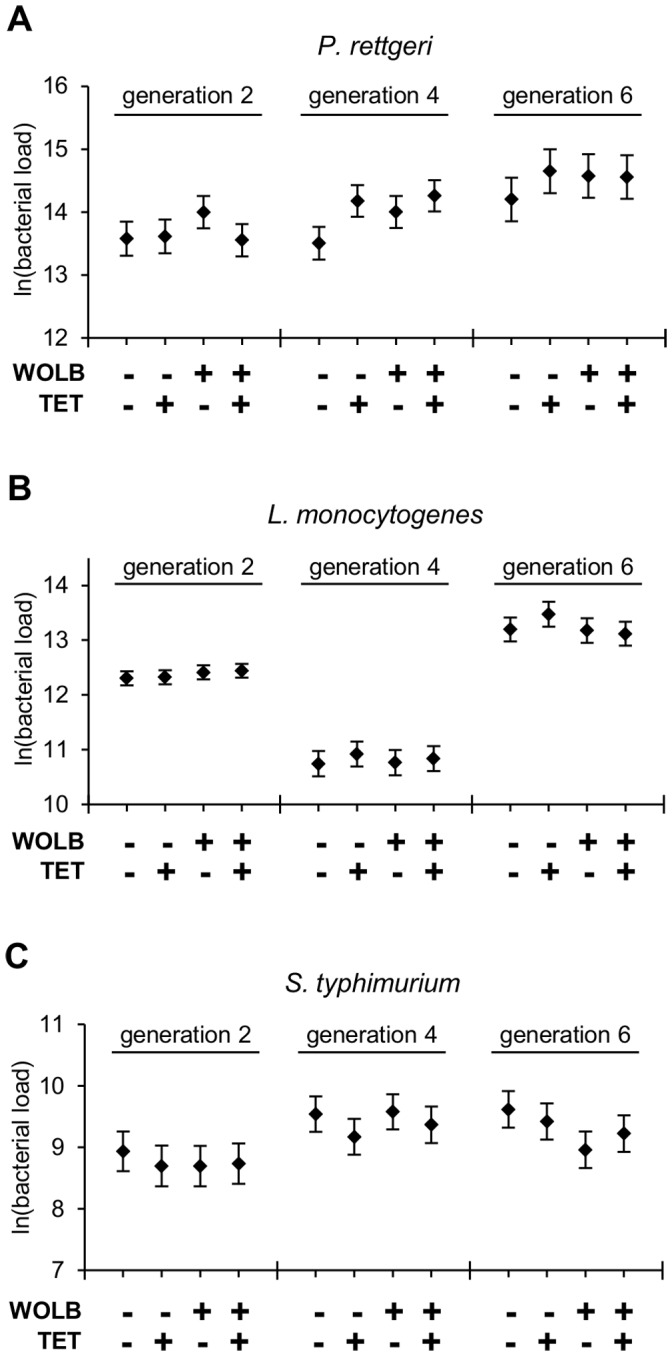
Least squares means for bacterial load (±1SE) of five Wolbachia-infected lines [WOLB(+)TET(-)] and genetically matched lines that have been cured of Wolbachia [WOLB(+)TET(+)], as well as five Wolbachia-uninfected lines [WOLB(-)TET(-)] and genetically paired tetracycline treated lines[WOLB(-)TET(+)]. Note that the WOLB category on the x-axis refers to initial Wolbachia-infection status prior to antibiotic treatment, rather than infection status at the time of experimental infections. Bacterial load was measured 24 hours after infection with the pathogenic bacteria (A) P. rettgeri (B) L. monocytogenes and (C) S. typhimurium. Assays were performed 2, 4, and 6 generations after ending tetracycline treatment, with three replicates in each generation. For each replicate, bacterial load was measured in 3 pools of 5 flies from every line.
Table 3. Analyses of variance relating fixed effects of genotype, Wolbachia status, and tetracycline treatment to bacterial load when infected with P. rettgeri 2, 4, and 6 generations after tetracycline treatment.
| generation 2 | generation 4 | generation 6 | |||||
| Factor | d.f. | F-ratio | P-value | F-ratio | P-value | F-ratio | P-value |
| line(WOLB) | 8 | 8.33 | <0.0001 | 6.69 | <0.0001 | 9.24 | <0.0001 |
| WOLB | 1 | 1.02 | 0.3146 | 1.64 | 0.2029 | 0.39 | 0.5311 |
| TET | 1 | 0.68 | 0.4117 | 3.91 | 0.0497 | 1.12 | 0.2918 |
| TET*WOLB | 1 | 1.08 | 0.2999 | 0.83 | 0.3646 | 1.01 | 0.3176 |
| TET* line(WOLB) | 8 | 3.30 | 0.0017 | 0.61 | 0.7707 | 1.91 | 0.0619 |
When flies were infected with L. monocytogenes, we observed significant differences in bacterial load across the isofemale lines (p<0.0001, Table 2), but no difference in L. monocytogenes load owing to the initial Wolbachia status of those lines (p = 0.2880) or to tetracycline treatment (p = 0.3117). The effect of tetracycline treatment on Wolbachia-infected lines was not different from the effect of tetracycline treatment on Wolbachia-uninfected lines (WOLB*TET, p = 0.7254, Table 2 and Figure 1B), indicating that removal of Wolbachia does not influence ability to suppress L. monocytogenes infection. In contrast to infection with P. rettgeri, there was no genotype-by-treatment interaction in response to L. monocytogenes infection (p = 0.1857), nor was there any indication of a three way genotype-by-treatment-by-generation interaction (p = 0.1910).
When flies were infected with S. typhimurium, we observed significant differences in bacterial load across the isofemale lines (p = 0.0222, Table 2), but no effect of initial Wolbachia status (p = 0.3020) or tetracycline treatment (p = 0.3740). The effect of tetracycline treatment on Wolbachia-infected lines was not different from the effect of tetracycline treatment on Wolbachia-uninfected lines (WOLB*TET, p = 0.2548, Table 2 and Figure 1C), indicating that removal of Wolbachia does not influence ability to suppress infection by S. typhimurium. As with infection by L. monocytogenes, there was no genotype-by-treatment interaction in response to S. typhimurium infection (p = 0.5767) and no three way genotype-by-treatment-by-generation interaction (p = 0.0910).
Discussion
In this experiment we used two intracellular bacterial pathogens and one extracellular bacterial pathogen to investigate whether Wolbachia infection influences D. melanogaster resistance to pathogenic bacteria. Unfortunately there are no known natural intracellular bacterial pathogens of D. melanogaster, so for this experiment we used the human pathogens Listeria monocytogenes and Salmonella typhimurium. We did not find evidence that Wolbachia confers protection against either of the intracellular bacteria. Although these are not natural pathogens of D. melanogaster, both are able to invade and replicate within the cells of D. melanogaster and have been used to study intracellular infection in D. melanogaster [17], [18]. When Wolbachia-infected D. melanogaster are infected with DCV or Nora virus, both of which are natural pathogens, survival is increased and viral proliferation is inhibited [4]. Increased survival is also observed in Wolbachia-infected flies infected with the non-natural pathogen FHV, but in this case viral proliferation does not appear to be inhibited [4]. This observed disconnection between virus proliferation and host mortality suggests that the mechanisms by which Wolbachia confers protection involve both host immunity and host tolerance, the effects of which may be specific to particular pathogens or natural host-pathogen pairs. In future studies it may be interesting to test whether similar infection phenotypes are observed with natural intracellular bacterial pathogens.
Likewise, it would also be of interest to investigate the effects of Wolbachia on host fitness over the course of an infection. In this experiment we measured resistance, defined as the ability to minimize pathogen burden [12], because we were specifically interested in whether the presence of Wolbachia influences the host ability to suppress secondary bacterial infection. Wolbachia infection could conceivably also increase host tolerance of infection, such that Wolbachia-infected flies might survive longer or have higher reproductive success than uninfected flies despite similar pathogen infection loads. However, Wolbachia infection has previously been reported to have no effect on mortality in D. melanogaster after infection with extracellular bacterial pathogens [6].
In addition to investigating the effect of Wolbachia on resistance to bacterial pathogens, we examined the residual effect of tetracycline on flies multiple generations after treatment. Reduced mitochondrial metabolism and increased mtDNA density have been reported in D. simulans two generations after treatment with tetracycline [19], and antibiotic treatment additionally eliminates commensal gut microbes. Gut microbes have important regulatory effects on the immune system in the gut, and the presence or absence of individual microbes can disrupt gut homeostasis [20]. For example, aseptically reared Anopheles gambiae are more susceptible to Plasmodium falciparum infection than are non-sterile mosquitoes [21]. Two generations after cessation of tetracycline treatment, we found a significant line-by-tetracycline interaction on the ability of flies to suppress infection by the Gram-negative extracellular pathogen P. rettgeri. This suggests that residual effects of tetracycline may persist in some, but not other, genetic backgrounds for multiple generations. We speculate that there may be genetic variation for the number of generations required to recover from the effects of tetracycline treatment, perhaps resulting from differences in the ability to reacquire commensal gut microbes and return gut homeostasis or to differences in the rate of mitochondrial recovery. Additional research is required to elucidate the nature of this interaction.
In summary, it is well established that Wolbachia provides protection against RNA viruses in Drosophila [3], [4] so we sought to determine whether the Wolbachia-induced resistance to viruses could be generalized to other intracellular pathogens. We measured the abilities of Wolbachia-infected and uninfected D. melanogaster to suppress infection by the intracellular pathogenic bacteria L. monocytogenes and S. typhimurium and the extracellular pathogenic bacterium P. rettgeri, but we observed no effect of Wolbachia on resistance to infection by any of the three, irrespective of how they colonize the host.
Acknowledgments
We are thankful to Mark Jandricic and Chloe Ota for help collecting the data, and to Sarah Short and Madeline Galac for helpful discussion and comments on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Institutes of Health grant R01 AI083932. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jeyaprakash A, Hoy MA. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 2.Siozios S, Sapountzis P, Ioannidis P, Bourtzis K. Wolbachia symbiosis and insect immune response. Insect Sci. 2008;15:89–100. [Google Scholar]
- 3.Hedges LM, Brownlie JC, O’Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:e1000002. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborne SE, Leong YS, O'Neill SL, Johnson KN. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 2009;5:e1000656. doi: 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong ZS, Hedges LM, Brownlie JC, Johnson KN. Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila. PLoS ONE. 2011;6:e25430. doi: 10.1371/journal.pone.0025430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The endosymbiotic bacterium Wolbachia induces resistance to Dengue virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Xi ZY, Gavotte L, Xie Y, Dobson SL. Genome-wide analysis of the interaction between the endosymbiotic bacterium Wolbachia and its Drosophila host. BMC Genomics. 2008;9:1. doi: 10.1186/1471-2164-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourtzis K, Pettigrew MM, O’Neill SL. Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Mol Biol. 2000;9:635–639. doi: 10.1046/j.1365-2583.2000.00224.x. [DOI] [PubMed] [Google Scholar]
- 12.Raberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W, Rousset F, O’Neil S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riegler M, Sidhu M, Miller WJ, O'Neill SL. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biol. 2005;15:1428–1433. doi: 10.1016/j.cub.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann AA, Turelli M, Simmons GM. Unidirectional incompatibility between populations of Drosophila simulans. Evolution. 1986;40:692–701. doi: 10.1111/j.1558-5646.1986.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 16.Galac M, Lazzaro BP. Comparative pathology of bacteria in the genus Providencia to a natural host, Drosophila melanogaster. Microbes Infect. 2011;13:673–683. doi: 10.1016/j.micinf.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandt SM, Dionne MS, Khush RS, Pham LN, Vigdal TJ, et al. Secreted Bacterial Effectors and Host-Produced Eiger/TNF Drive Death in a Salmonella-Infected Fruit Fly. PLoS Biol. 2004;2:e418. doi: 10.1371/journal.pbio.0020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansfield BE, Dionne MS, Schneider DS, Freitag NE. Exploration of host-pathogen interactions using Listeria monocytogenes and Drosophila melanogaster. Cell Microbiol. 2003;5:901–911. doi: 10.1046/j.1462-5822.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- 19.Ballard JW, Melvin RG. Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in Drosophila. Insect Mol Biol. 2007;16:799–802. doi: 10.1111/j.1365-2583.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 20.Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]


