Abstract
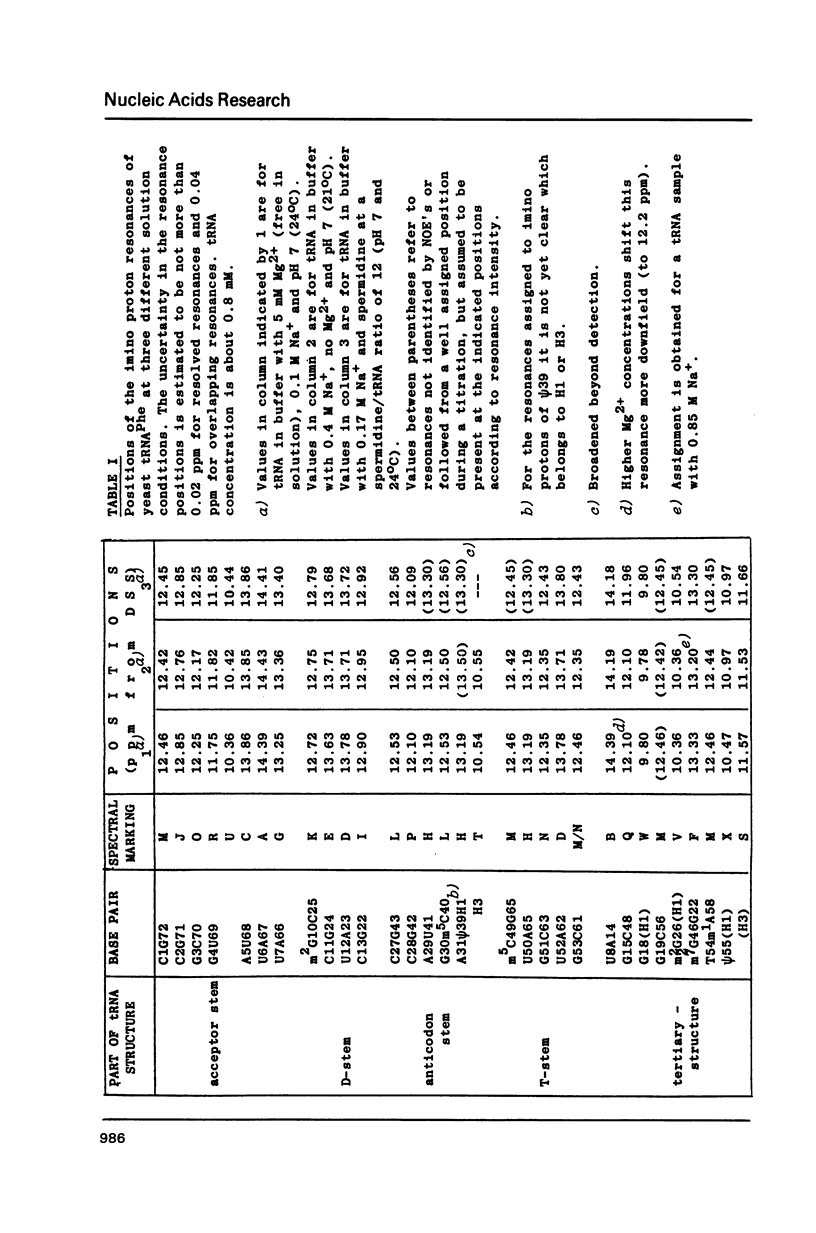
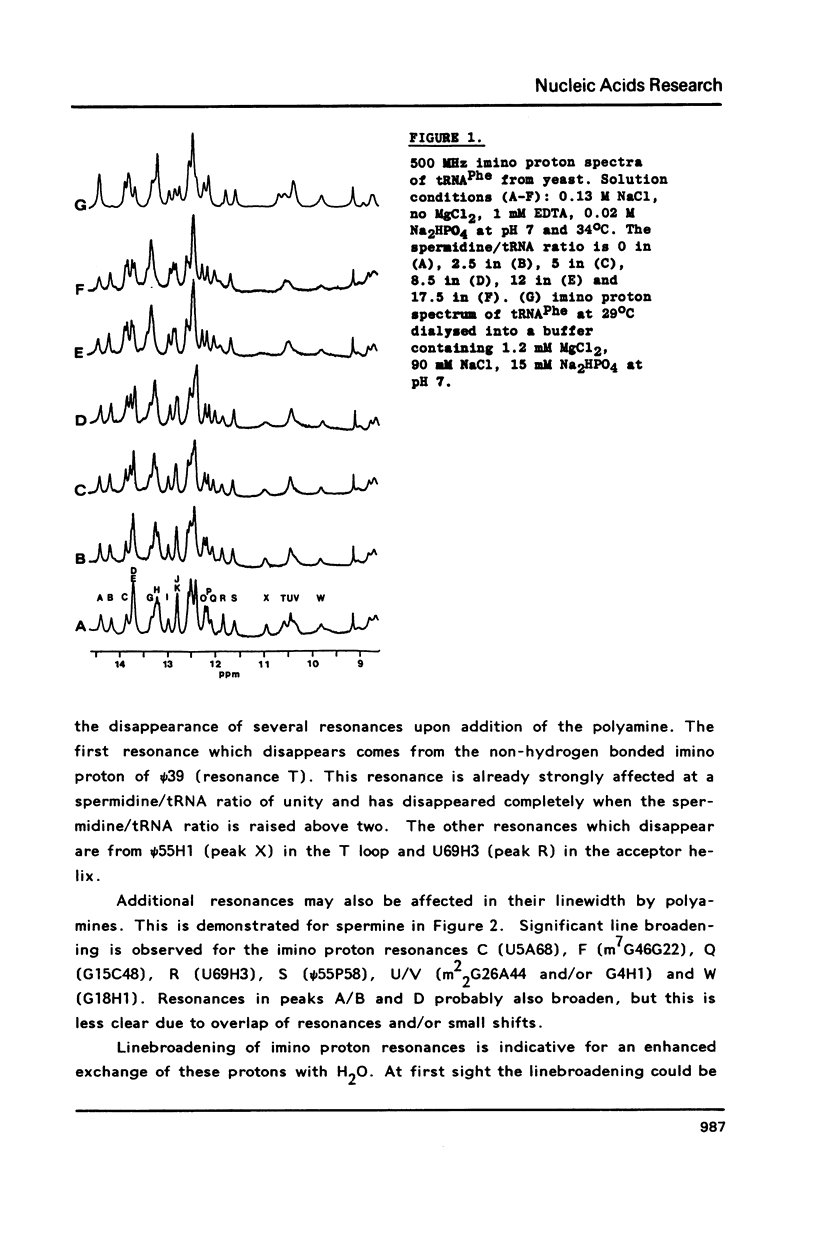
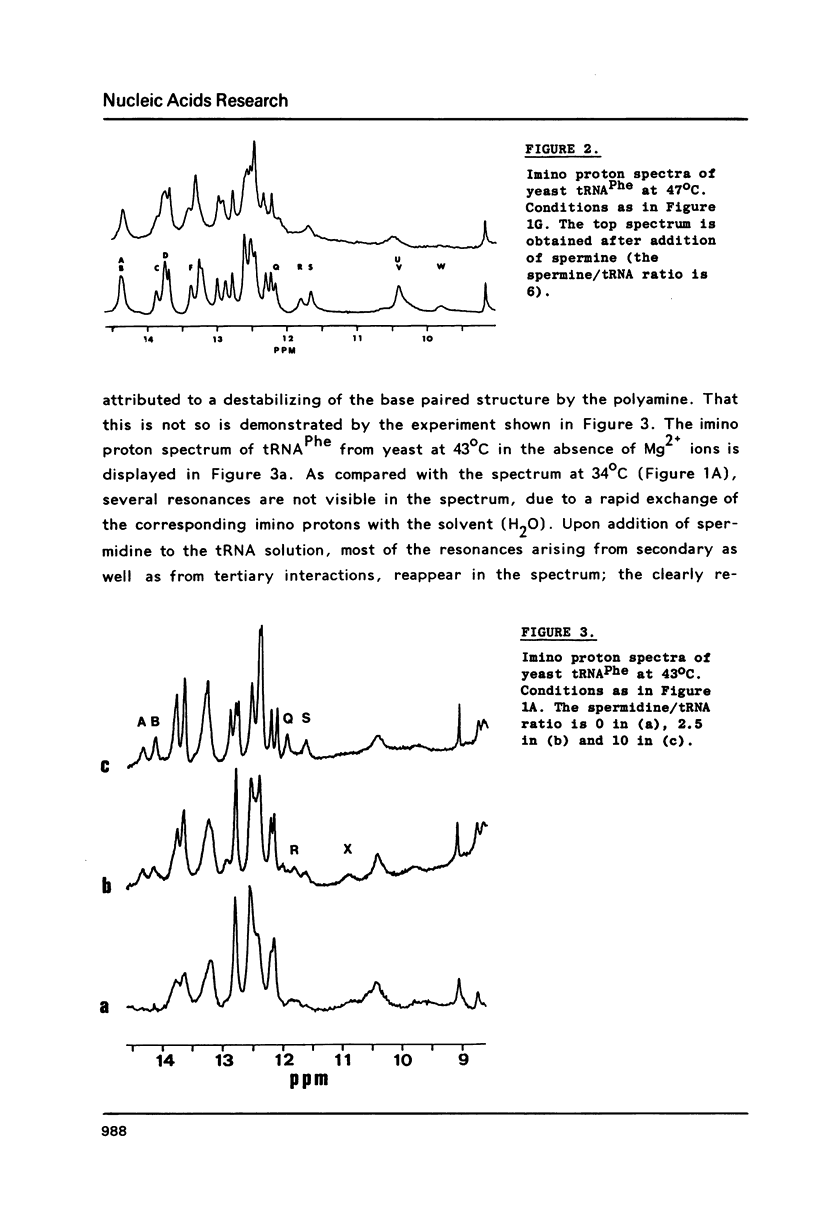
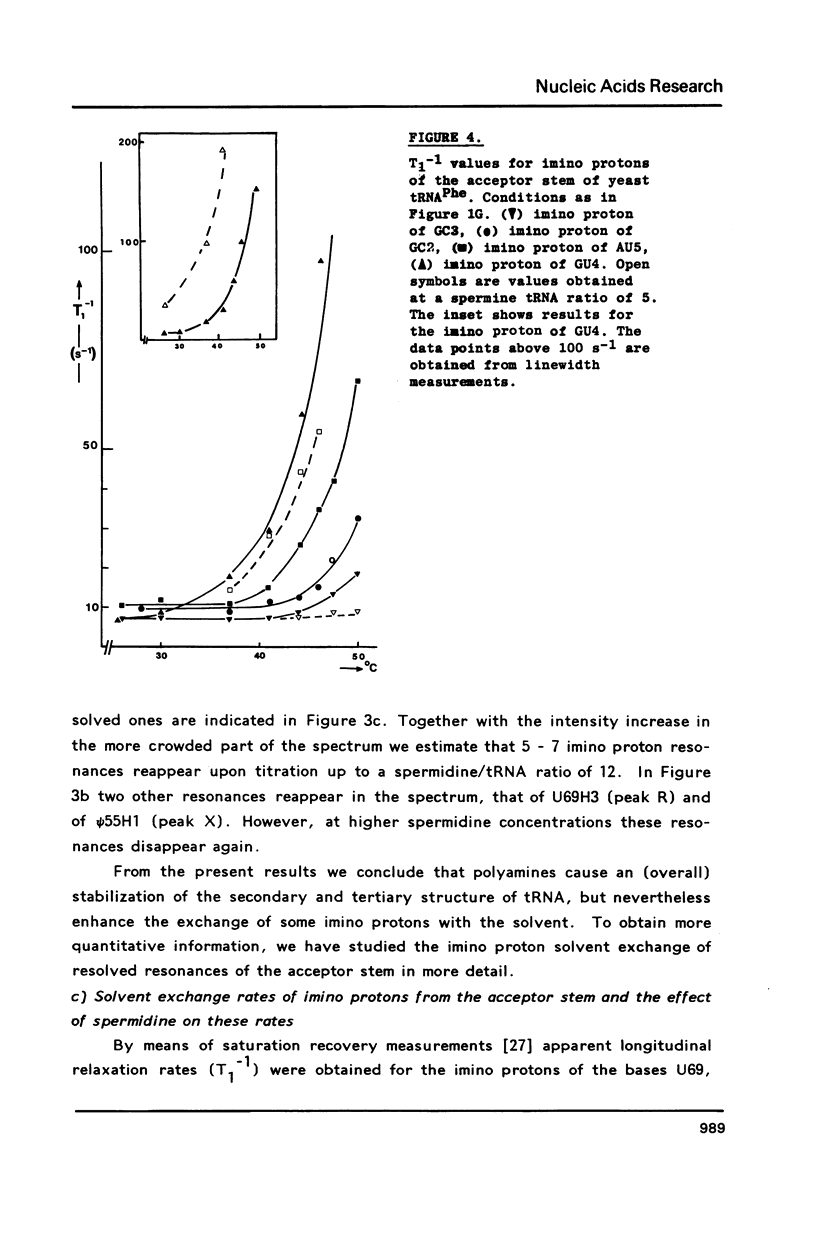
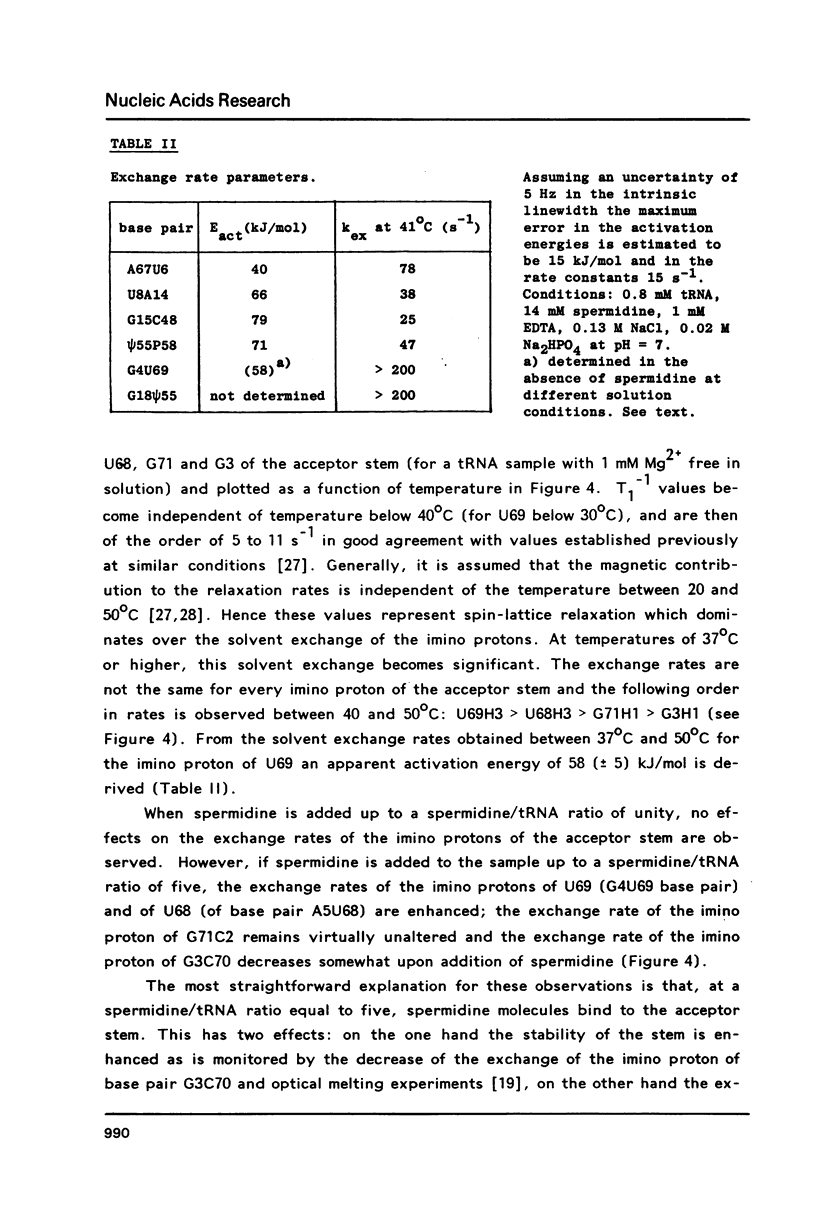
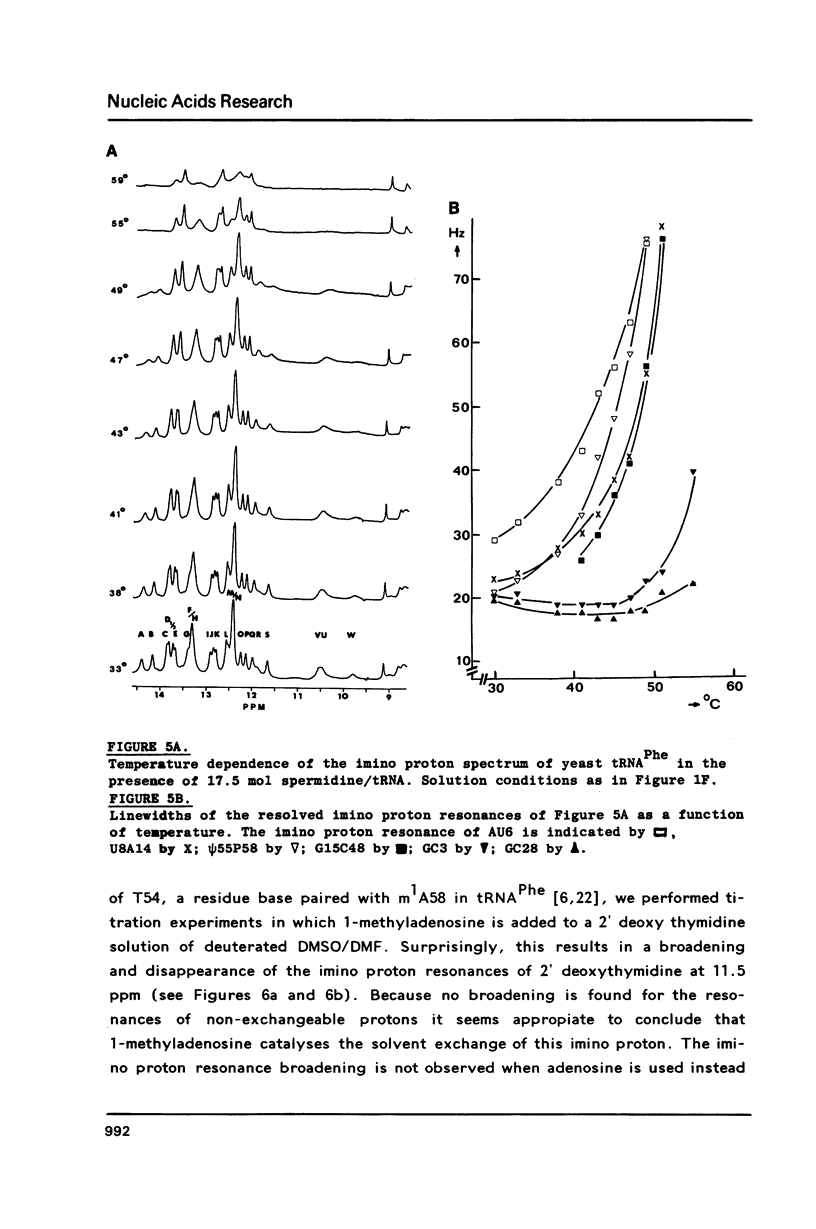
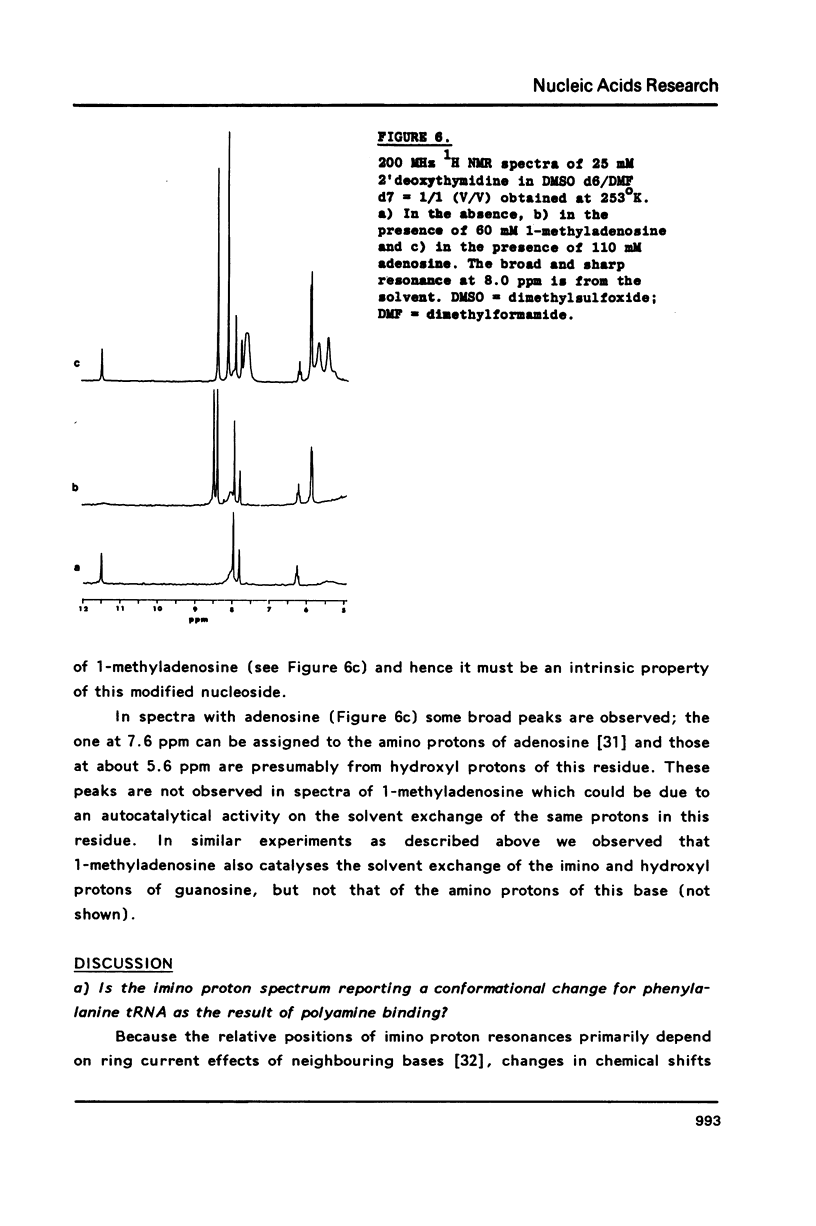
A comparison of imino proton NMR spectra of yeast tRNAPhe recorded at various solution conditions indicates, that polyamines have a limited effect on the structure of this tRNA molecule. Polyamines are found to catalyse the solvent exchange of several imino protons in yeast tRNAPhe not only of non hydrogen bonded imino protons, but also of imino protons of the GU and of some AU and tertiary base pairs. It is concluded that at low levels of catalysing components the exchange rates of the latter protons are not determined by the base pair lifetime. In the presence of high levels of spermidine the solvent exchange rates of imino protons of several base pairs in the molecule were assessed as a function of the temperature. Apparent activation energies derived from these rates were found to be less than 80 kJ/mol, which is indicative for (transient) independent opening of the corresponding base pairs. In the acceptor helix the GU base pair acts as a dynamic dislocation. The AU base pairs at one side of the GU base pair exhibit faster transient opening than the GC base pairs on the other side of this wobble pair. The base pairs m2GC10 and GC11 from the D stem and GC28 from the anticodon stem show relatively slow opening up to high temperatures. Model studies suggest that 1-methyladenosine, an element of tRNA itself, catalyses imino proton solvent exchange in a way similar to polyamines.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assa-Munt N., Granot J., Behling R. W., Kearns D. R. 1H NMR relaxation studies of the hydrogen-bonded imino protons of poly(dA-dT). Biochemistry. 1984 Feb 28;23(5):944–955. doi: 10.1021/bi00300a023. [DOI] [PubMed] [Google Scholar]
- Bolton P. H., Kearns D. R. Effect of cations on tRNA structure. Biochemistry. 1977 Dec 27;16(26):5729–5741. doi: 10.1021/bi00645a013. [DOI] [PubMed] [Google Scholar]
- Cohen S. S. What do the polyamines do? Nature. 1978 Jul 20;274(5668):209–210. doi: 10.1038/274209a0. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Cole P. E., Hilbers C. W., Shulman R. G. The molecular mechanism of thermal unfolding of Escherichia coli formylmethionine transfer RNA. J Mol Biol. 1974 Jul 25;87(1):63–88. doi: 10.1016/0022-2836(74)90560-9. [DOI] [PubMed] [Google Scholar]
- Evans J. A., Deutscher M. P. Polyamine stimulation and cation requirements of rabbit liver tRNA nucleotidyltransferase. J Biol Chem. 1976 Nov 10;251(21):6646–6652. [PubMed] [Google Scholar]
- Heerschap A., Haasnoot C. A., Hilbers C. W. Nuclear magnetic resonance studies on yeast tRNAPhe I. Assignment of the iminoproton resonances of the acceptor and D stem by means of Nuclear Overhauser Effect experiments at 500 MHz. Nucleic Acids Res. 1982 Nov 11;10(21):6981–7000. doi: 10.1093/nar/10.21.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerschap A., Haasnoot C. A., Hilbers C. W. Nuclear magnetic resonance studies on yeast tRNAPhe. II. Assignment of the iminoproton resonances of the anticodon and T stem by means of nuclear Overhauser effect experiments at 500 MHz. Nucleic Acids Res. 1983 Jul 11;11(13):4483–4499. doi: 10.1093/nar/11.13.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerschap A., Haasnoot C. A., Hilbers C. W. Nuclear magnetic resonance studies on yeast tRNAPhe. III. Assignments of the iminoproton resonances of the tertiary structure by means of nuclear Overhauser effect experiments at 500 MHz. Nucleic Acids Res. 1983 Jul 11;11(13):4501–4520. doi: 10.1093/nar/11.13.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerschap A., Mellema J. R., Janssen H. G., Walters J. A., Haasnoot C. A., Hilbers C. W. Imino-proton resonances of yeast tRNAPhe studied by two-dimensional nuclear Overhauser enhancement spectroscopy. Eur J Biochem. 1985 Jun 18;149(3):649–655. doi: 10.1111/j.1432-1033.1985.tb08973.x. [DOI] [PubMed] [Google Scholar]
- Heerschap A., Walters J. A., Hilbers C. W. Interactions of some naturally occurring cations with phenylalanine and initiator tRNA from yeast as reflected by their thermal stability. Biophys Chem. 1985 Aug;22(3):205–217. doi: 10.1016/0301-4622(85)80044-2. [DOI] [PubMed] [Google Scholar]
- Heus H. A., van Kimmenade J. M., van Knippenberg P. H., Haasnoot C. A., de Bruin S. H., Hilbers C. W. High-resolution proton magnetic resonance studies of the 3'-terminal colicin fragment of 16 S ribosomal RNA from Escherichia coli. Assignment of iminoproton resonances by nuclear Overhauser effect experiments and the influence of adenine dimethylation on the hairpin conformation. J Mol Biol. 1983 Nov 15;170(4):939–956. doi: 10.1016/s0022-2836(83)80197-1. [DOI] [PubMed] [Google Scholar]
- Hirschman S., Leng M., Felsenfield G. Interaction of spermine and DNA. Biopolymers. 1967 Feb;5(2):227–233. doi: 10.1002/bip.1967.360050209. [DOI] [PubMed] [Google Scholar]
- Hurd R. E., Reid B. R. Helix-coil dynamics in RNA: the amino acid acceptor helix of Escherichia coli phenylalanine transfer RNA. J Mol Biol. 1980 Sep 15;142(2):181–193. doi: 10.1016/0022-2836(80)90044-3. [DOI] [PubMed] [Google Scholar]
- Jelenc P. C., Kurland C. G. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. D., Figueroa N., Redfield A. G. Real-time solvent exchange studies of the imino and amino protons of yeast phenylalanine transfer RNA by Fourier transform NMR. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3130–3134. doi: 10.1073/pnas.76.7.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. D., Redfield A. G. Study of transfer ribonucleic acid unfolding by dynamic nuclear magnetic resonance. Biochemistry. 1981 Jul 7;20(14):3996–4006. doi: 10.1021/bi00517a008. [DOI] [PubMed] [Google Scholar]
- Leroy J. L., Bolo N., Figueroa N., Plateau P., Guérón M. Internal motions of transfer RNA: a study of exchanging protons by magnetic resonance. J Biomol Struct Dyn. 1985 Feb;2(5):915–939. doi: 10.1080/07391102.1985.10507609. [DOI] [PubMed] [Google Scholar]
- Loftfield R. B., Eigner E. A., Pastuszyn A. The role of spermine in preventing misacylation by phenylalanyl-tRNA synthetase. J Biol Chem. 1981 Jul 10;256(13):6729–6735. [PubMed] [Google Scholar]
- Macon J. B., Wolfenden R. 1-Methyladenosine. Dimroth rearrangement and reversible reduction. Biochemistry. 1968 Oct;7(10):3453–3458. doi: 10.1021/bi00850a021. [DOI] [PubMed] [Google Scholar]
- Mandal C., Kallenbach N. R., Englander S. W. Base-pair opening and closing reactions in the double helix. A stopped-flow hydrogen exchange study in poly(rA).poly(rU). J Mol Biol. 1979 Dec 5;135(2):391–411. doi: 10.1016/0022-2836(79)90443-1. [DOI] [PubMed] [Google Scholar]
- Nilsson L., Rigler R., Wintermeyer W. The influence of spermine on the structural dynamics of yeast tRNAPhe. Biochim Biophys Acta. 1983 Sep 9;740(4):460–465. doi: 10.1016/0167-4781(83)90095-7. [DOI] [PubMed] [Google Scholar]
- Nöthig-Laslo V., Weygand-Durasević I., Kućan Z. Structural changes of yeast tRNA(Tyr) caused by the binding of divalent ions in the presence of spermine. J Biomol Struct Dyn. 1985 Feb;2(5):941–951. doi: 10.1080/07391102.1985.10507610. [DOI] [PubMed] [Google Scholar]
- Nöthig-Laslo V., Weygand-Durasević I., Zivković T., Kućan Z. Binding of spermine to tRNATyr stabilizes the conformation of the anticodon loop and creates strong binding sites for divalent cations. Eur J Biochem. 1981 Jul;117(2):263–267. doi: 10.1111/j.1432-1033.1981.tb06332.x. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Ikuta S., Kozlowski S., Itakura K. Sequence dependence of hydrogen exchange kinetics in DNA duplexes at the individual base pair level in solution. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2184–2188. doi: 10.1073/pnas.80.8.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. Initial stages of the thermal unfolding of yeast phenylalanine transfer RNA as studied by chemical modification: the effect of magnesium. Eur J Biochem. 1977 Nov 15;81(1):91–101. doi: 10.1111/j.1432-1033.1977.tb11930.x. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Robison B., Zimmerman T. P. A conformational study of yeast phenylalanine transfer ribonucleic acid. J Biol Chem. 1971 Jan 10;246(1):110–117. [PubMed] [Google Scholar]
- Roy S., Redfield A. G. Assignment of imino proton spectra of yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1983 Mar 15;22(6):1386–1390. doi: 10.1021/bi00275a010. [DOI] [PubMed] [Google Scholar]
- Sakai T. T., Cohen S. S. Effects of polyamines on the structure and reactivity of tRNA. Prog Nucleic Acid Res Mol Biol. 1976;17:15–42. doi: 10.1016/s0079-6603(08)60064-1. [DOI] [PubMed] [Google Scholar]
- Schreier A. A., Schimmel P. R. Interaction of polyamines with fragments and whole molecules of yeast phenylalanine-specific transfer RNA. J Mol Biol. 1975 Apr 5;93(2):323–329. doi: 10.1016/0022-2836(75)90136-9. [DOI] [PubMed] [Google Scholar]
- Shoup R. R., Miles H. T., Becker E. D. NMR evidence of specific base-pairing between purines and pyrimidines. Biochem Biophys Res Commun. 1966 Apr 19;23(2):194–201. doi: 10.1016/0006-291x(66)90527-4. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Hilbers C. W. Ring-current shifts in the 300 MHz nuclear magnetic resonance spectra of six purified transfer RNA molecules. J Mol Biol. 1973 Jun 25;78(1):57–69. doi: 10.1016/0022-2836(73)90428-2. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Onishi T. Binding of transfer RNA to polyamines in preference to Mg-2+. Biochem Biophys Res Commun. 1975 Apr 7;63(3):611–617. doi: 10.1016/s0006-291x(75)80428-1. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Samejima K., Nagano K., Watanabe M., Sugeta H., Kyogoku Y. Determination of protonation sites in thermospermine and in some other polyamines by 15N and 13C nuclear magnetic resonance spectroscopy. Eur J Biochem. 1983 Feb 1;130(2):383–389. doi: 10.1111/j.1432-1033.1983.tb07164.x. [DOI] [PubMed] [Google Scholar]
- Tropp J. S., Redfield A. G. Proton exchange rates in transfer RNA as a function of spermidine and magnesium. Nucleic Acids Res. 1983 Apr 11;11(7):2121–2134. doi: 10.1093/nar/11.7.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]



