Abstract
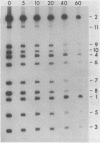
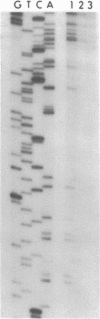
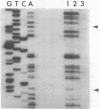
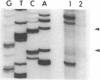
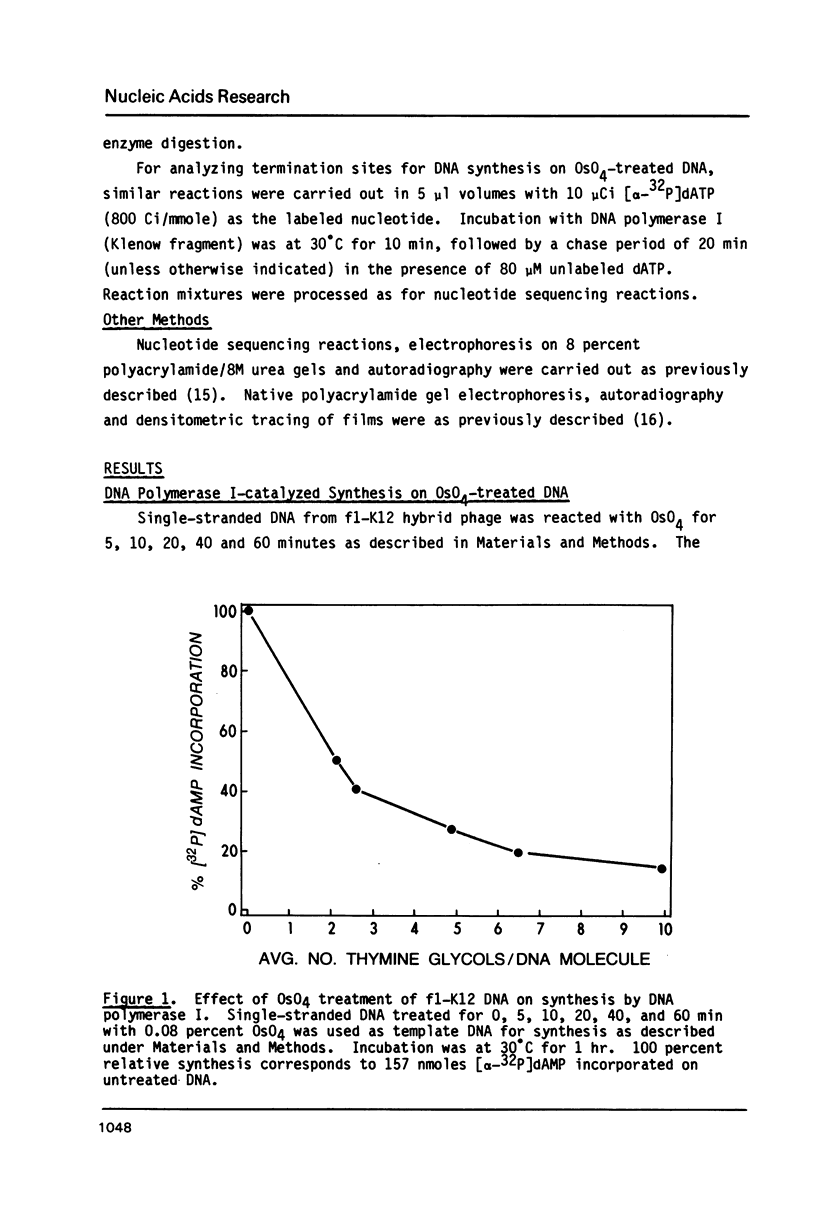
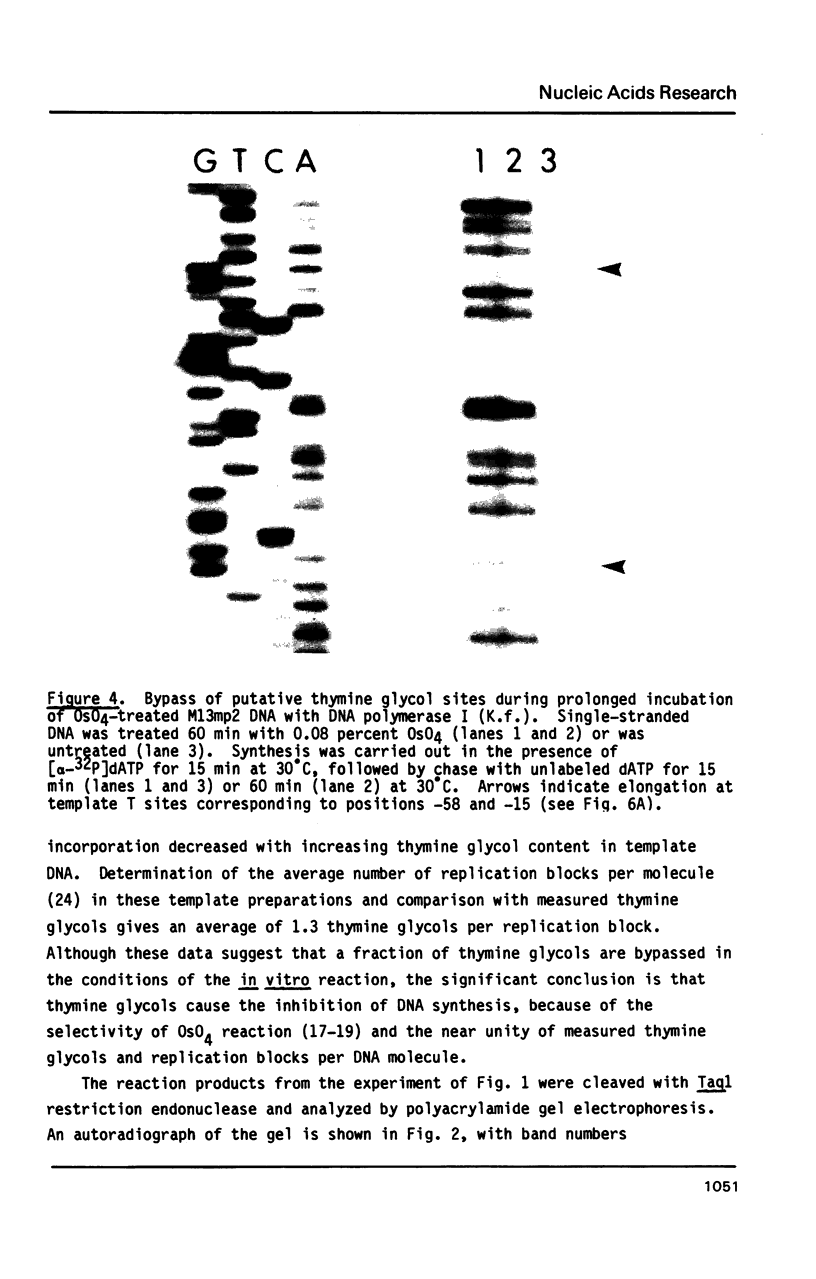
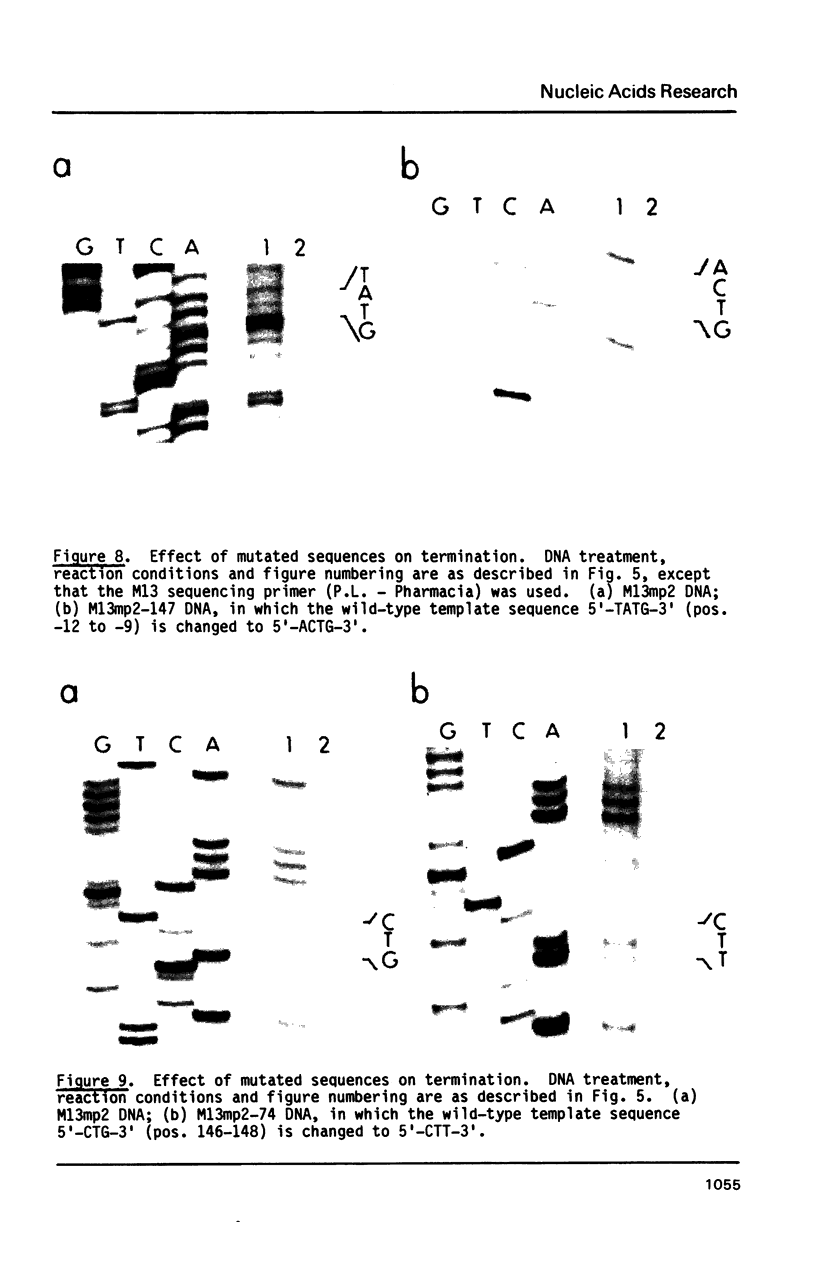
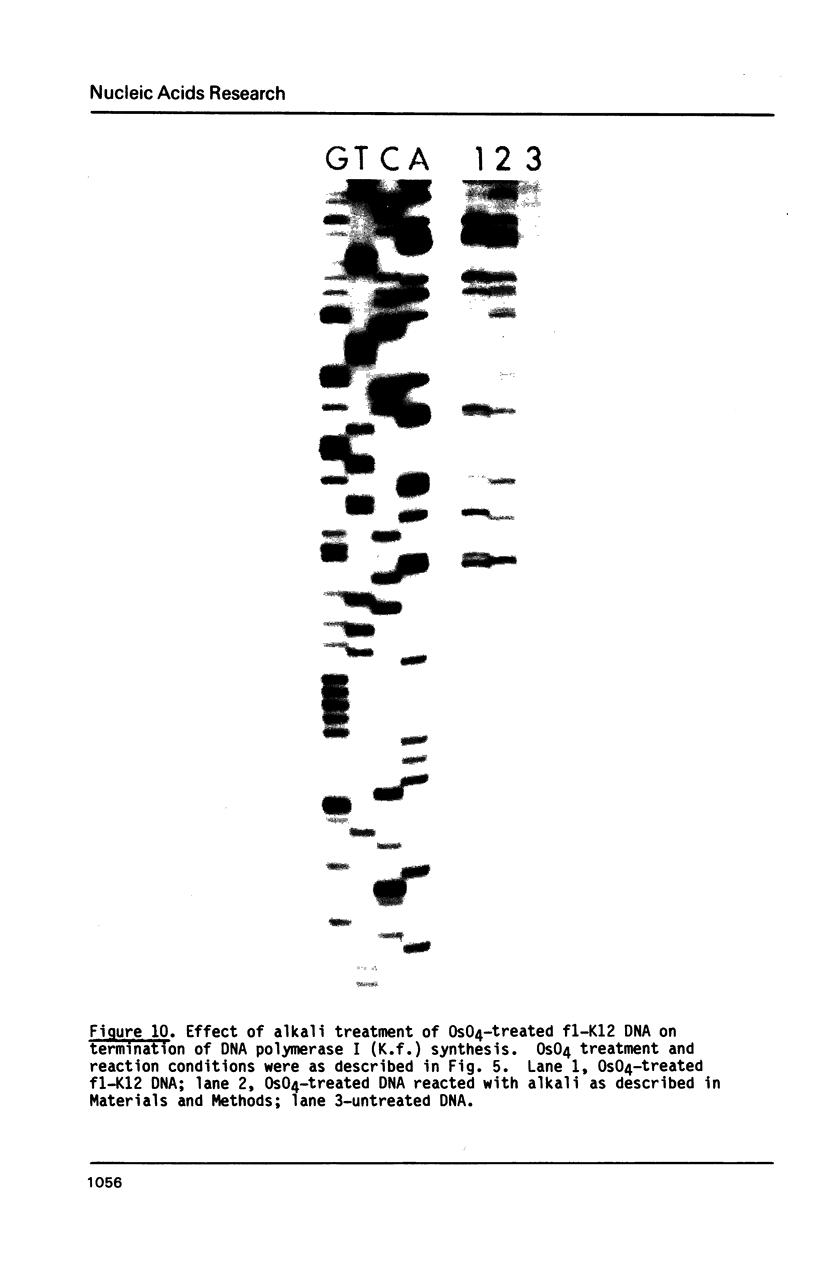
Single-stranded phage DNAs containing thymine glycols were prepared by oxidation with osmium tetroxide (OsO4) and were used as templates for DNA synthesis by E. coli DNA polymerase I. The induction of thymine glycol lesions in DNA, as measured by immunoassay, quantitatively accounted for an inhibition of in vitro DNA synthesis on modified templates. Analysis of termination sites for synthesis by DNA polymerase I (Klenow fragment) showed that DNA synthesis terminated at most template thymine sites in OsO4-treated DNA, indicating that incorporation occurred opposite putative thymine glycols in DNA. Nucleotides 5' and 3' to putative thymine glycol sites affect the reaction, however, since termination was not observed at thymines in the sequence 5'-CTPur-3'. Conversion of thymine glycols to urea residues in DNA by alkali treatment caused termination of DNA synthesis one nucleotide 3' to template thymine sites, including thymines in the 5'-CTPur-3' sequence, showing that the effect of surrounding sequence is on the elongation reaction by DNA polymerase rather than differential damage induction by OsO4.
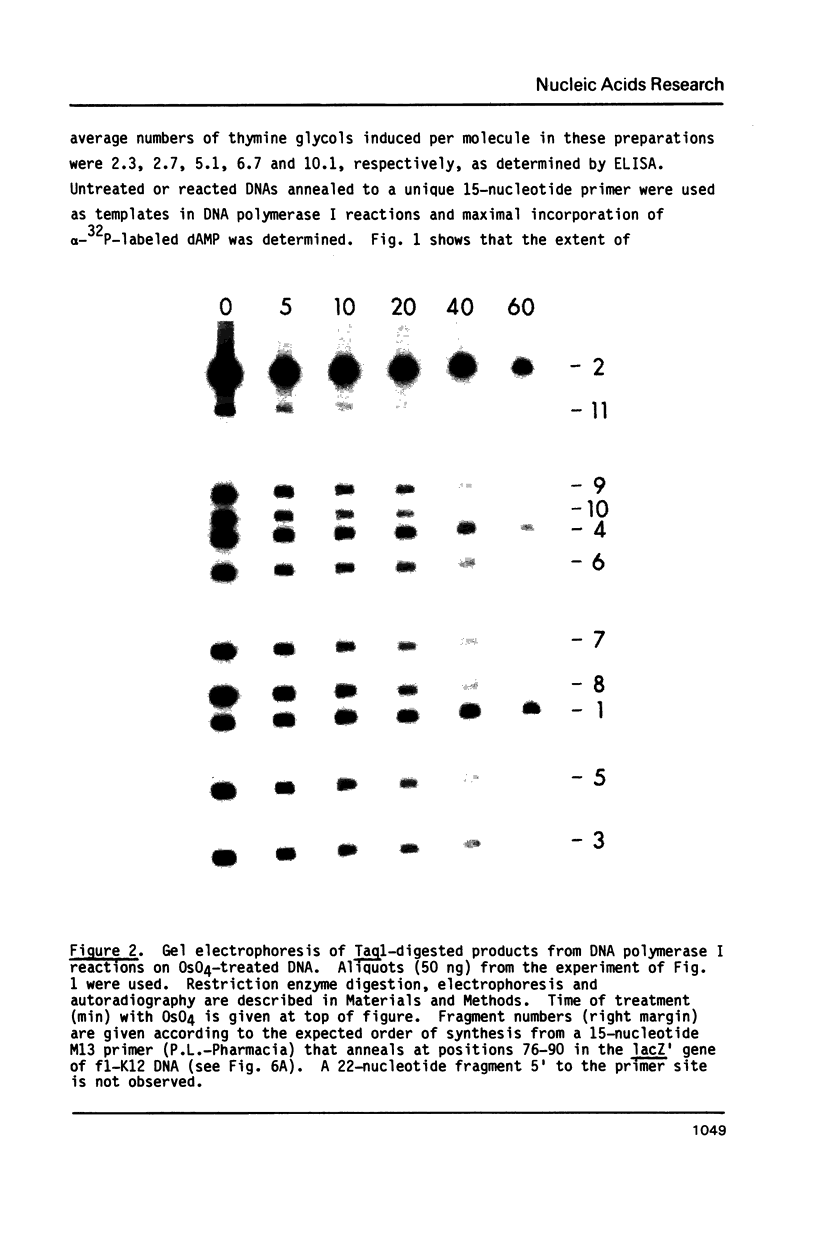
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achey P. M., Wright C. F. Inducible repair of thymine ring saturation damage in phi X174 DNA. Radiat Res. 1983 Mar;93(3):609–612. [PubMed] [Google Scholar]
- Beer M., Stern S., Carmalt D., Mohlhenrich K. H. Determination of base sequence in nucleic acids with the electron microscope. V. The thymine-specific reactions of osmium tetroxide with deoxyribonucleic acid and its components. Biochemistry. 1966 Jul;5(7):2283–2288. doi: 10.1021/bi00871a017. [DOI] [PubMed] [Google Scholar]
- Frenkel K., Goldstein M. S., Teebor G. W. Identification of the cis-thymine glycol moiety in chemically oxidized and gamma-irradiated deoxyribonucleic acid by high-pressure liquid chromatography analysis. Biochemistry. 1981 Dec 22;20(26):7566–7571. doi: 10.1021/bi00529a035. [DOI] [PubMed] [Google Scholar]
- HOFFMANN-BERLING H., MARVIN D. A., DUERWALD H. EIN FAEDIGER DNS-PHAGE (FD) UND EIN SPHAERISCHER RNS-PHAGE (FR), WIRTSSPEZIFISCH FUER MAENNLICHE STAEMME VON E. COLI. 1. PRAEPARATION UND CHEMISCHE EIGENSCHAFTEN VON FD UND FR. Z Naturforsch B. 1963 Nov;18:876–883. [PubMed] [Google Scholar]
- Hariharan P. V., Achey P. M., Cerutti P. A. Biological effect of thymine ring saturation in coliphage phiX174-DNA. Radiat Res. 1977 Feb;69(2):375–378. [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Excision of damaged thymine residues from gamma-irradiated poly(dA-dT) by crude extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3532–3536. doi: 10.1073/pnas.71.9.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan P. V. Determination of thymine ring saturation products of the 5,6-dihydroxydihydrothymine type by the alkali degradation assay. Radiat Res. 1980 Mar;81(3):496–498. [PubMed] [Google Scholar]
- Hayes R. C., LeClerc J. E. Preferential transfection with M13mp2 RF DNA synthesized in vitro. Gene. 1983 Jan-Feb;21(1-2):1–8. doi: 10.1016/0378-1119(83)90141-5. [DOI] [PubMed] [Google Scholar]
- Katcher H. L., Wallace S. S. Characterization of the Escherichia coli X-ray endonuclease, endonuclease III. Biochemistry. 1983 Aug 16;22(17):4071–4081. doi: 10.1021/bi00286a013. [DOI] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L., Saran B. R., Allen R., Jr Sequence analysis of ultraviolet-induced mutations in M13lacZ hybrid phage DNA. J Mol Biol. 1984 Dec 5;180(2):217–237. doi: 10.1016/s0022-2836(84)80001-7. [DOI] [PubMed] [Google Scholar]
- Masamune Y. Effect of ultraviolet irradiation of bacteriophage f1 DNA on its conversion to replicative form by extracts of Escherichia coli. Mol Gen Genet. 1976 Dec 22;149(3):335–345. doi: 10.1007/BF00268536. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Osborn A. L., King C. M., Strauss B. S. Effect of acetylated and deacetylated 2-aminofluorene adducts on in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7166–7170. doi: 10.1073/pnas.79.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Piette J. G., Hearst J. E. Termination sites of the in vitro nick-translation reaction on DNA that had photoreacted with psoralen. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5540–5544. doi: 10.1073/pnas.80.18.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin S. D., Moore P. D., Strauss B. S. In vitro bypass of UV-induced lesions by Escherichia coli DNA polymerase I: specificity of nucleotide incorporation. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1541–1545. doi: 10.1073/pnas.80.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin S. D., Strauss B. S. A role for DNA polymerase in the specificity of nucleotide incorporation opposite N-acetyl-2-aminofluorene adducts. J Mol Biol. 1984 Sep 25;178(3):569–594. doi: 10.1016/0022-2836(84)90239-0. [DOI] [PubMed] [Google Scholar]
- Rajagopalan R., Melamede R. J., Laspia M. F., Erlanger B. F., Wallace S. S. Properties of antibodies to thymine glycol, a product of the radiolysis of DNA. Radiat Res. 1984 Mar;97(3):499–510. [PubMed] [Google Scholar]
- Refolo L. M., Conley M. P., Sambamurti K., Jacobsen J. S., Humayun M. Z. Sequence context effects in DNA replication blocks induced by aflatoxin B1. Proc Natl Acad Sci U S A. 1985 May;82(10):3096–3100. doi: 10.1073/pnas.82.10.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagher D., Strauss B. Abasic sites from cytosine as termination signals for DNA synthesis. Nucleic Acids Res. 1985 Jun 25;13(12):4285–4298. doi: 10.1093/nar/13.12.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagher D., Strauss B. Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: uniqueness of adenine nucleotides. Biochemistry. 1983 Sep 13;22(19):4518–4526. doi: 10.1021/bi00288a026. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace S. S. Detection and repair of DNA base damages produced by ionizing radiation. Environ Mutagen. 1983;5(5):769–788. doi: 10.1002/em.2860050514. [DOI] [PubMed] [Google Scholar]