Abstract
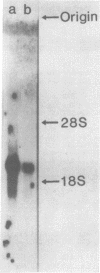
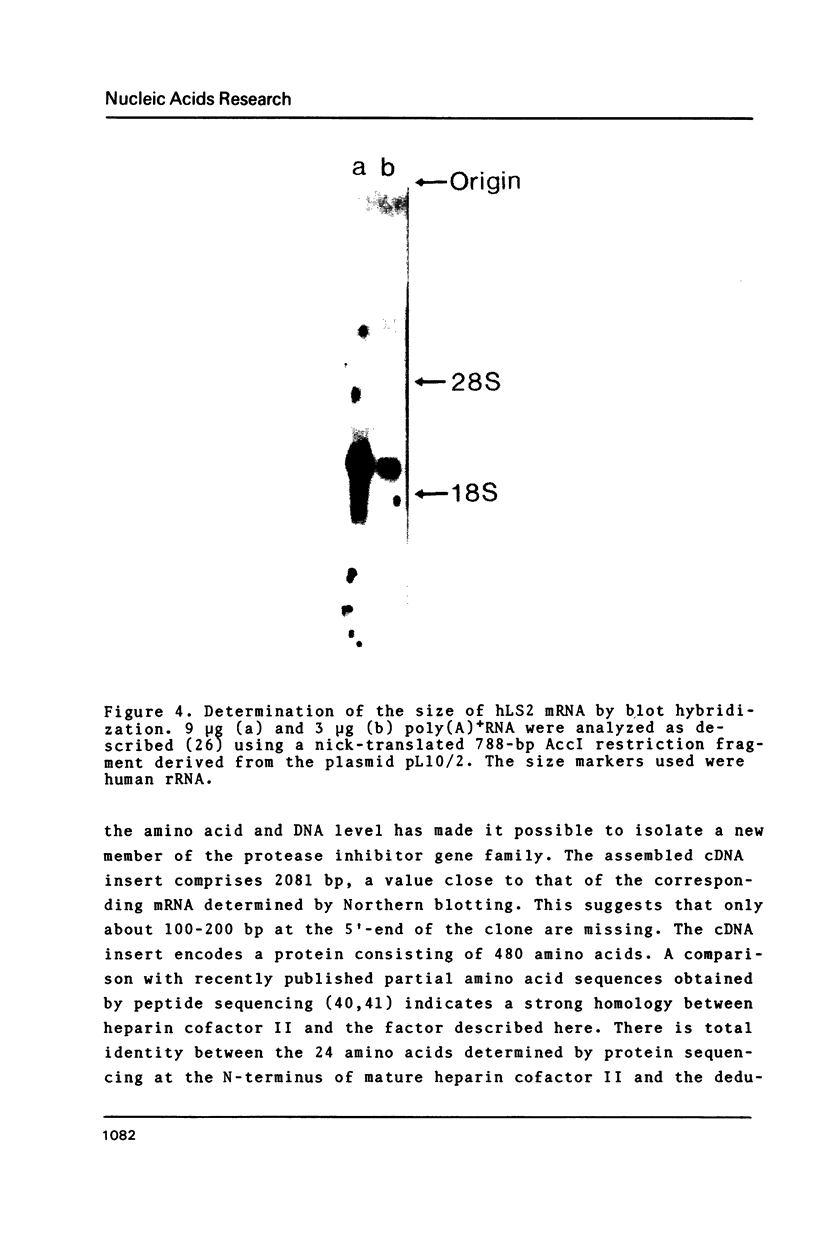
A 2.1-kb cDNA clone representing a new member of the protease inhibitor family was isolated from a human liver cDNA library. The inhibitor, named human Leuserpin 2 (hLS2), comprises 480 amino acids and contains a leucine residue at its putative reactive center. HLS2 is about 25-28% homologous to three human members of the plasma protease inhibitor family: antithrombin III, alpha 1-antitrypsin and alpha 1-antichymotrypsin. A comparison with published partial amino acid sequences shows that hLS2 is closely related to the thrombin inhibitor heparin cofactor II.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balldin G., Laurell C. B., Ohlsson K. Increased catabolism of alpha-Macroglobulins after intravenous infusion of trypsin-alpha1-antitrypsin complexes in dogs. Hoppe Seylers Z Physiol Chem. 1978 Jun;359(6):699–708. doi: 10.1515/bchm.1978.359.1.699. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Wallace D. M., Puskas R. S., Eschenfeldt W. H. Reverse transcriptase and its associated ribonuclease H: interplay of two enzyme activities controls the yield of single-stranded complementary deoxyribonucleic acid. Biochemistry. 1983 May 10;22(10):2365–2372. doi: 10.1021/bi00279a010. [DOI] [PubMed] [Google Scholar]
- Bock S. C., Wion K. L., Vehar G. A., Lawn R. M. Cloning and expression of the cDNA for human antithrombin III. Nucleic Acids Res. 1982 Dec 20;10(24):8113–8125. doi: 10.1093/nar/10.24.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen A., Herzog A., Cravador A., Hérion P., Chuchana P., Vander Straten A., Loriau R., Jacobs P., van Elsen A. Cloning and expression in Escherichia coli of full-length complementary DNA coding for human alpha 1-antitrypsin. DNA. 1983;2(4):255–264. doi: 10.1089/dna.1983.2.255. [DOI] [PubMed] [Google Scholar]
- Bouhnik J., Clauser E., Strosberg D., Frenoy J. P., Menard J., Corvol P. Rat angiotensinogen and des(angiotensin I)angiotensinogen: purification, characterization, and partial sequencing. Biochemistry. 1981 Nov 24;20(24):7010–7015. doi: 10.1021/bi00527a036. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Chandra T., Stackhouse R., Kidd V. J., Robson K. J., Woo S. L. Sequence homology between human alpha 1-antichymotrypsin, alpha 1-antitrypsin, and antithrombin III. Biochemistry. 1983 Oct 25;22(22):5055–5061. doi: 10.1021/bi00291a001. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Damus P. S., Hicks M., Rosenberg R. D. Anticoagulant action of heparin. Nature. 1973 Dec 7;246(5432):355–357. doi: 10.1038/246355a0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Angiotensinogen is related to the antitrypsin-antithrombin-ovalbumin family. Science. 1983 Oct 28;222(4622):417–419. doi: 10.1126/science.6604942. [DOI] [PubMed] [Google Scholar]
- Ferguson W. S., Finlay T. H. Localization of the disulfide bond in human antithrombin III required for heparin-accelerated thrombin inactivation. Arch Biochem Biophys. 1983 Feb 15;221(1):304–307. doi: 10.1016/0003-9861(83)90147-9. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Rennard S. I., Crystal R. G. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981 Oct;68(4):889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M. J., Noyes C. M., Church F. C. Reactive site peptide structural similarity between heparin cofactor II and antithrombin III. J Biol Chem. 1985 Feb 25;260(4):2218–2225. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Heilig R., Muraskowsky R., Kloepfer C., Mandel J. L. The ovalbumin gene family: complete sequence and structure of the Y gene. Nucleic Acids Res. 1982 Jul 24;10(14):4363–4382. doi: 10.1093/nar/10.14.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig R., Perrin F., Gannon F., Mandel J. L., Chambon P. The ovalbumin gene family: structure of the X gene and evolution of duplicated split genes. Cell. 1980 Jul;20(3):625–637. doi: 10.1016/0092-8674(80)90309-8. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Shaw P. H., Boyd P. A., Baumann H., Hastie N. D. Plasma protease inhibitors in mouse and man: divergence within the reactive centre regions. Nature. 1984 Sep 13;311(5982):175–177. doi: 10.1038/311175a0. [DOI] [PubMed] [Google Scholar]
- Hunt L. T., Dayhoff M. O. A surprising new protein superfamily containing ovalbumin, antithrombin-III, and alpha 1-proteinase inhibitor. Biochem Biophys Res Commun. 1980 Jul 31;95(2):864–871. doi: 10.1016/0006-291x(80)90867-0. [DOI] [PubMed] [Google Scholar]
- Kageyama R., Ohkubo H., Nakanishi S. Primary structure of human preangiotensinogen deduced from the cloned cDNA sequence. Biochemistry. 1984 Jul 31;23(16):3603–3609. doi: 10.1021/bi00311a006. [DOI] [PubMed] [Google Scholar]
- Koide T., Odani S., Takahashi K., Ono T., Sakuragawa N. Antithrombin III Toyama: replacement of arginine-47 by cysteine in hereditary abnormal antithrombin III that lacks heparin-binding ability. Proc Natl Acad Sci U S A. 1984 Jan;81(2):289–293. doi: 10.1073/pnas.81.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Leicht M., Long G. L., Chandra T., Kurachi K., Kidd V. J., Mace M., Jr, Davie E. W., Woo S. L. Sequence homology and structural comparison between the chromosomal human alpha 1-antitrypsin and chicken ovalbumin genes. Nature. 1982 Jun 24;297(5868):655–659. doi: 10.1038/297655a0. [DOI] [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Fritsch E. F., Lauer J., Lawn R. M. The molecular genetics of human hemoglobins. Annu Rev Genet. 1980;14:145–178. doi: 10.1146/annurev.ge.14.120180.001045. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morii M., Travis J. Amino acid sequence at the reactive site of human alpha 1-antichymotrypsin. J Biol Chem. 1983 Nov 10;258(21):12749–12752. [PubMed] [Google Scholar]
- Owen M. C., Brennan S. O., Lewis J. H., Carrell R. W. Mutation of antitrypsin to antithrombin. alpha 1-antitrypsin Pittsburgh (358 Met leads to Arg), a fatal bleeding disorder. N Engl J Med. 1983 Sep 22;309(12):694–698. doi: 10.1056/NEJM198309223091203. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ragg H., Weissmann C. Not more than 117 base pairs of 5'-flanking sequence are required for inducible expression of a human IFN-alpha gene. Nature. 1983 Jun 2;303(5916):439–442. doi: 10.1038/303439a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D. Chemistry of the hemostatic mechanism and its relationship to the action of heparin. Fed Proc. 1977 Jan;36(1):10–18. [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sie P., Dupouy D., Pichon J., Boneu B. Constitutional heparin co-factor II deficiency associated with recurrent thrombosis. Lancet. 1985 Aug 24;2(8452):414–416. doi: 10.1016/s0140-6736(85)92737-0. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Innis R. B. Peptide neurotransmitters. Annu Rev Biochem. 1979;48:755–782. doi: 10.1146/annurev.bi.48.070179.003543. [DOI] [PubMed] [Google Scholar]
- Stead N., Kaplan A. P., Rosenberg R. D. Inhibition of activated factor XII by antithrombin-heparin cofactor. J Biol Chem. 1976 Nov 10;251(21):6481–6488. [PubMed] [Google Scholar]
- Tanaka T., Ohkubo H., Nakanishi S. Common structural organization of the angiotensinogen and the alpha 1-antitrypsin genes. J Biol Chem. 1984 Jul 10;259(13):8063–8065. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus D. W., Blank M. K. Heparin cofactor II. Purification and properties of a heparin-dependent inhibitor of thrombin in human plasma. J Biol Chem. 1982 Mar 10;257(5):2162–2169. [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Tran T. H., Marbet G. A., Duckert F. Association of hereditary heparin co-factor II deficiency with thrombosis. Lancet. 1985 Aug 24;2(8452):413–414. doi: 10.1016/s0140-6736(85)92736-9. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Weber R., Ryffel G. U. Comparative analysis of the structural organization of two closely related vitellogenin genes in X. laevis. Cell. 1980 May;20(1):107–117. doi: 10.1016/0092-8674(80)90239-1. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Nagata S., Boll W., Fountoulakis M., Fujisawa A., Fujisawa J. I., Haynes J., Henco K., Mantei N., Ragg H. Structure and expression of human IFN-alpha genes. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 24;299(1094):7–28. doi: 10.1098/rstb.1982.0102. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Witt I., Kaiser C., Schrenk W. J., Wunderwald P. Amino acid composition and N-terminal sequence of antithrombin BM. Thromb Res. 1983 Dec 1;32(5):513–518. doi: 10.1016/0049-3848(83)90261-x. [DOI] [PubMed] [Google Scholar]
- Wunderwald P., Schrenk W. J., Port H. Antithrombin BM from human plasma: an antithrombin binding moderately to heparin. Thromb Res. 1982 Feb 1;25(3):177–191. doi: 10.1016/0049-3848(82)90237-7. [DOI] [PubMed] [Google Scholar]